Abstract
The rapidly rising incidence of neurodevelopmental disorders with social deficits is raising concern that developmental exposure to environmental contaminants may be contributory. Firemaster 550 (FM 550) is one of the most prevalent flame-retardant (FR) mixtures used in foam-based furniture and baby products and contains both brominated and organophosphate components. We and others have published evidence of developmental neurotoxicity and sex specific effects of FM 550 on anxiety-like and exploratory behaviors. Using a prosocial animal model, we investigated the impact of perinatal FM 550 exposure on a range of socioemotional behaviors including anxiety, attachment, and memory. Virtually unknown to toxicologists, but widely used in the behavioral neurosciences, the prairie vole (Microtus ochrogaster) is a uniquely valuable model organism for examining environmental factors on sociality because this species is spontaneously prosocial, biparental, and displays attachment behaviors including pair bonding. Dams were exposed to 0, 500, 1000, or 2000 μg of FM 550 via subcutaneous (sc) injections throughout gestation, and pups were directly exposed beginning the day after birth until weaning. Adult offspring of both sexes were then subjected to multiple tasks including open field, novel object recognition, and partner preference. Effects were dose responsive and sex-specific, with females more greatly affected. Exposure-related outcomes in females included elevated anxiety, decreased social interaction, decreased exploratory motivation, and aversion to novelty. Exposed males also had social deficits, with males in all three dose groups failing to show a partner preference. Our studies demonstrate the utility of the prairie vole for investigating the impact of chemical exposures on social behavior and support the hypothesis that developmental FR exposure impacts the social brain. Future studies will probe the possible mechanisms by which these effects arise.
Keywords: social, anxiety, brain, neurodevelopment, neural, endocrine disruptors, endocrine disrupting chemicals, flame retardants
Graphical Abstract
Introduction
Chemical flame retardants (FRs) are extensively applied to a wide range of products including electronics, building materials, foam-based furniture, and infant products (Stapleton et al., 2005; Stapleton et al., 2011). They are also prevalent pollutants in the wild and built environment (Dodson et al., 2012; Ma et al., 2012; Roze et al., 2009; Stapleton et al., 2008; van der Veen and de Boer, 2012). In the early 2000s, as polybrominated diphenyl ethers (PBDEs) were being phased out, alternatives were introduced including FireMaster® 550 (FM 550), a mixture of brominated flame retardants (BFRs) and organophosphate ester flame retardants (OPFRs), (Stapleton et al., 2008; Stapleton et al., 2014a; van der Veen and de Boer, 2012). In 2011, FM 550 was the second most commonly detected flame retardant in US furniture (Stapleton et al., 2012). Widespread use and exposure constitute a pressing need to understand the potential risks exposure to this relatively new FR mixture poses to human health. Work by us and others has identified FM 550 as potentially endocrine disrupting and developmentally neurotoxic (Baldwin et al., 2018; Baldwin et al., 2017; Belcher et al., 2014; Hoffman, K. et al., 2014; McGee et al., 2013; Patisaul et al., 2013; Peng et al., 2017; Phillips et al., 2016; Saunders et al., 2013; Saunders et al., 2015; Tung et al., 2017). Most significantly, there is speculation that chemical exposures, including FRs, may be contributing to rapidly rising rates of neurodevelopmental disorders, such as Autism Spectrum Disorder (ASD) (Grandjean and Landrigan, 2014; Kalkbrenner et al., 2014). However, experimental evidence of relevant effects is lacking, particularly on the behavioral hallmarks of ASD including the Systems for Social Processing Domain as defined by the National Institute of Mental Health (NIHM) Research Domain Criteria (RDoC) (Mittal and Wakschlag, 2017). Here we used a uniquely suitable prosocial rodent species to test the hypothesis that developmental exposure to FM 550 alters socioemotional behaviors including affiliation, attachment, and social anxiety.
FM 550 is comprised of two brominated compounds, 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (BEH-TEBP), triphenyl phosphate (TPHP) and numerous TPHP analogs with varying degrees of aryl isopropylation (collectively named ITPs) (Phillips et al., 2017; Stapleton et al., 2014a; van der Veen and de Boer, 2012). Like PBDE predecessors, FM 550 can readily escape from products and accumulate in the environment. Indoors, this includes contamination of house dust (Dodson et al., 2012; Stapleton et al., 2008; van der Veen and de Boer, 2012), the inhalation or ingestion of which is likely the most significant route of human exposure, especially for children. Because of rapidly rising use, levels of FM 550 components in US house dust are similar to those of the now phased out PBDEs (Butt et al., 2014; Stapleton et al., 2008; Stapleton et al., 2014b). FM 550 components are also found in aquatic environments (Ma et al., 2012; Roze et al., 2009) and air particles in the European Arctic (Salamova et al., 2014), demonstrating environmental mobility, persistence, and bioaccumulation. Additionally, the OPFR components have been used for decades as plasticizers and bear a structural resemblance to organophosphate insecticides, which are known to have adverse effects on neurodevelopment. In particular, TPHP is a high production volume chemical commonly applied to polyvinyl chloride (PVC), circuit boards, adhesives, rubbers, and other products, including personal care products like nail polish (Mendelsohn et al., 2016).
FM 550 components are consistently detected in human tissues including breast milk, blood, hair, fingernails, and urine (Hoffman, Kate et al., 2014; Mendelsohn et al., 2016; Zhou et al., 2014). BEH-TEBP and EH-TBB have been found in hair at concentrations of 20–240 and 11–350 ng/g, respectively, and < 17–80 and < 9.2–71 ng/g in nails, respectively (Liu et al., 2015). BEH-TEBP and EH-TBB have been identified in breast milk with one study detecting EH-TBB in 44% of the samples with concentrations ranging between < 0.01 and 0.48 ng/g lw, and BEH-TEBP in 50% of the samples with concentrations ranging between < 0.01 and 0.73 ng/g lw (Tao et al., 2017). While the OPFR components are quickly metabolized and excreted in urine (Van den Eede et al., 2013), some studies have detected TPHP in human breast milk at a median concentration of 0.28 ng/g ww (Kim et al., 2014). Additionally, as with many chemicals, children appear to have higher FR exposures than adults (Butt et al., 2014; Butt et al., 2016; Cequier et al., 2015; Gibson et al., 2019). The urinary metabolite of EH-TBB, 2,3,4,5-tetrabromobenzoic acid (TBBA), is more frequently detected in children than adults with children having geometric mean levels of approximately 7.4 pg/mL (Butt et al., 2014). Collectively, these studies suggest that human FM 550 exposure is widespread and that children may be a particularly sensitive population.
There is growing evidence that FM 550 and its individual components can be endocrine disrupting and developmentally neurotoxic. Our group was the first to show in vivo that FM 550 acts as an endocrine disruptor with developmental exposure in Wistar rats producing weight gain, glucose intolerance, elevated anxiety-like behavior in females and thickening of the lateral ventricle in males at an oral dose as low as 100 μg/day. We have also identified evidence of thyroid hormone disruption both in vivo and in vitro (Belcher et al., 2014; Patisaul et al., 2013). The full mixture has been identified as possibly cardiotoxic (McGee et al., 2013) and obesogenic (Patisaul et al., 2013; Pillai, Hari K. et al., 2014). Adult zebrafish exposed to TPHP exhibited reduced fecundity, altered sex steroid levels (estradiol, testosterone, vitellogenin) and changes in gene expression related to the HPG axis (Liu et al., 2013). TPHP and ITPs interact with Peroxisome Proliferator-Activated Receptor (PPARγ) and several nuclear receptors, including the androgen receptor (AR), in vitro (Fang et al., 2015; Honkakoski et al., 2004; Pillai, H. K. et al., 2014) suggesting multiple modes of action. A screening assay testing a variety of BFRs and OPFRs for effects on neuronal differentiation and growth found that the OPFRs had comparable activity to BFRs in most of the assays (Behl et al., 2015). Studies in zebrafish, cell-based assays and other model systems have also revealed potential developmental neurotoxic and behavioral effects comparable to PBDEs (Behl et al., 2015; Jarema et al., 2015; Kim et al., 2015).
The present studies build on our prior work showing that oral FM 550 exposure at concentrations (100, 300 or 1000 μg/day) well below the purported no observed adverse effect level (NOAEL) of 50 mg/kg/day induces anxiety-like behaviors and hyperactivity in rats (Baldwin et al., 2017; Patisaul et al., 2013). Here we used a more prosocial animal model to explore effects on social behaviors. The fact that traditional rodent models, such as rats and mice, do not spontaneously display prosocial traits including affiliation, paternal care, and pair bonding, is a critical obstacle to experimentally testing for potential linkages between early life chemical exposures and human social disorders. Additionally, ASD mouse models often have severe neuropathology not seen in human patients. Thus, for the present studies, we used the prairie vole (Microtus ochrogaster), a prosocial animal model used for decades in the neurosciences to explore the biological basis of social cognition, affiliation and communication, as well as the etiology of social disorders such as ASD (McGraw and Young, 2010; Modi and Young, 2012).
Decades of transformative work in the prairie vole and related species has linked pro-social traits to the oxytocin/vasopressin (OT/AVP) system and its interactions with serotonergic and mesolimbic dopamine pathways (McGraw and Young, 2010; Modi and Young, 2012). Additionally, the model has been used to develop intranasal OT therapy for ASD patients (Modi and Young, 2012). Although rarely leveraged in toxicology, we have previously used voles to show that developmental exposure to Bisphenol A (BPA) can induce sex-specific outcomes relevant to ASD including disrupted OT/AVP neuron numbers, altered socioemotional behaviors, and regional changes in microglial density (Rebuli et al., 2016; Sullivan et al., 2014); thus demonstrating the functional utility of prairie voles in toxicological studies.
For the present study, sex was considered as a biological variable because exploratory and social behaviors are sexually dimorphic and toxicant exposure, including FM 550 exposure, can cause sex-specific changes in these behaviors (Baldwin et al., 2017; Palanza et al., 2008; Palanza et al., 1999; Patisaul and Adewale, 2009; Rebuli et al., 2016; Schantz and Widholm, 2001). Exposure was by subcutaneous (sc) injection because, although we have previously published information regarding the pharmacokinetics and pharmacodynamics of FM 550 in rats, nothing is known about either in voles. The three doses used (500, 1000 or 2000 μg/day) are within the range of what we have previously administered to rats and internal levels were measured in a small cohort of voles to maximize human translation. We also included 5-Methoxytryptamine (5MT), a global serotonin agonist, as a positive control for chemically-induced social impairment because prior work in male prairie voles showed that perinatal exposure can produce behavioral and neural hallmarks of ASD including reduced sociality, reduction in OT/AVP neuron numbers, and decreased density of 5-HT fibers in the amygdala (Martin et al., 2012). In summary, the present study tested the hypothesis that FM 550 exposed voles would display less exploratory behavior and increased anxiety, as we have previously shown in the rat (Baldwin et al., 2017; Patisaul et al., 2013), plus deficits in sociality and affiliative behaviors.
Methods
The ARRIVE (Animal Research: Reporting of In Vivo Experiments) Guidelines Checklist for Reporting Animal Research was used in the construction of this manuscript with all elements met (Kilkenny et al., 2010). The ARRIVE guidelines were developed in consultation with the scientific community as part of an NC3Rs (National Centre for the Replacement Refinement and Reduction of Animals in Research) initiative to improve the standard of reporting of research using animals.
Animals
Animal care, maintenance and experimental protocols met the standards of the Animal Welfare Act and the U.S. Department of Health and Human Services ‘Guide for the Care and use of Laboratory Animals’ and were approved by the North Carolina State University (NCSU) Institutional Animal Care and Use Committee (IACUC). A supervising veterinarian approved and monitored all procedures throughout the duration of the project. Prairie voles (Microtus ochrogaster) were obtained from founders generously gifted by Bruce S. Cushing at the University of Texas El Paso and bred in house as indicated in humidity- and temperature-controlled rooms at 22°C and 30% average humidity, each with 12-h:12-h light:dark cycles (lights on at 6AM EST) in the Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved Biological Resource Facility at NCSU. Food (Lab Diet 5326 high fiber rabbit diet) and water were provided ad libitum. As in our prior studies (Patisaul et al., 2013; Patisaul et al., 2009) and in accordance with recommended practices for endocrine disrupting chemical (EDC) research (Cannizzaro et al., 2008; Hunt et al., 2009; Richter et al., 2007), all animals were housed in conditions specifically designed to minimize unintended EDC exposure including use of glass water bottles with metal sippers, woodchip bedding, and thoroughly washed polysulfone caging. The diet is not a low phytoestrogen diet (content varies by lot) but high fiber and at least some phytoestrogen content is required to maximize health and fertility of this herbivorous species (National Research Council (U.S.). Subcommittee on Laboratory Animal Nutrition., 1995).
Dose preparation
As we have done previously (Baldwin et al., 2018; Patisaul et al., 2013), the sesame oil-based dosing solutions were prepared and coded by the Stapleton lab at Duke University and transferred to the Patisaul lab at NCSU where dosing and subsequent testing was performed. Briefly, a commercial mixture of FM 550 was obtained from Great Lakes Chemical (West Lafayette, IN, USA) (Stapleton et al., 2008) and each dosing solution (0, 500, 1000 and 2000 μg/20 μl) was prepared by weighing the appropriate amount of FM 550 and diluting it in sesame oil (Sigma) with stirring for 6 h, and then stored in amber bottles at 4°C until use. FM 550 doses were selected based on our prior work in rats and well below the purported NOAEL of 50 mg/kg/day (Baldwin et al., 2017; Patisaul et al., 2013). Additionally, the top dose was thought most likely to produce detectible levels of the parent compounds and primary metabolites in serum. Based on our human exposure data the 500 μg/day dose is estimated to be just above maximum human exposure levels (Hoffman et al., 2014; Butt et al., 2016). However, because human exposure levels are dynamic and rising, and most risk assessments include a 100 fold safety factor for interspecies comparisons, it is important to evaluate exposures to FM550 at doses that bracket environmentally relevant exposures and levels above current estimates of human exposure.
A small aliquot of the mixtures were analyzed by gas chromatography mass spectrometry in the Stapleton lab to confirm doses were on target prior to exposure. The global serotonin agonist, 5MT, was used as a positive control for chemical disruption of social behavior because a prior study found perinatal exposure to alter social behavior and its underlying neurochemistry in male prairie voles (Martin et al., 2012). A commercial stock of 5MT was obtained from Aldrich chemistry (Lot number BCBT9524, 97% pure, 286583–1G, St Louis, MO) and dosing solution was prepared by weighing the appropriate amount of 5MT and diluting it in 100% ethanol to give the final concentration of 50 μg 5MT / 20 μl. Ethanol was the vehicle chosen because, although prior studies used tonic saline as a vehicle, we found it to go into suspension rather than solution. 5MT also did not dissolve in sesame oil. Dosing solution was prepared every 3 days to prevent evaporation and stored in amber bottles at 4°C until use.
Exposure
Dams from the breeding colony were randomly assigned to an exposure group: FM 550 (0, 500, 1000, 2000 μg) or 5MT (50 μg). Exposure to the dam via sc injection occurred from the day after parturition of the previous litter, designated as gestational day (GD) 0, through the day of birth the experimental offspring birth. No dosing occurred on the day of birth. Offspring were then dosed directly by sc injection from the day after parturition, postnatal day (PND) 1, until weaning, PND 21. Offspring exposed to 5MT started to develop skin irritation at the injection sites, thus 5MT exposure ceased on PND 7. Dosing occurred daily beginning at 2:00 pm for all animals. Although oral exposure is considered more human relevant, sc injection was chosen because the pharmacokinetics of FM 550 in prairie voles is completely unknown and not assumed to be similar to other rodents.
Litter size and composition was recorded at weaning. After weaning, offspring were housed by sex at 2 to 3 per cage with littermates or similarly aged conspecifics. Final numbers of litters per group were 13 control, 10 500 μg FM 550, 9 1000 μg FM 550, 11 2000 μg FM 550, and 8 5MT. Up to three male (M) and female (F) pups per litter were randomly selected for behavioral testing, with their siblings reserved for different experiments. Although litter is the preferred statistical unit for toxicological studies, the individual pups were used here for logistical reasons (this species produces small litters and must remain pair bonded which limits breeding options) and because the colony is wild-derived and thus considerably more genetically diverse than typical mouse and rat strains. However, to limit any confounding effects due to litter, no more than 3 same sex pups per litter were selected. The number of litters represented by offspring sex in each group is: control (13 F, 11 M), 500 μg FM 550 (10 F, 9 M), 1000 μg FM 550 (8 F, 9 M), 2000 μg FM 550 (7 F, 11 M), 5MT (7 F, 8 M).
Behavioral Testing
All behavioral testing was conducted in a room at the NCSU Biological Resources Facility specifically dedicated and equipped for this purpose. Since lab-housed prairie voles are diurnal all testing was conducted under white light between 10:00 am and 3:30 pm and video recorded by a camera suspended overhead for later analysis using TopScan (Clever Sys Inc) software with no people or other distractions in the room. Behavioral testing occurred in either a high walled (43 cm) blue, opaque open box area (58 cm × 58 cm) or a 3-chambered arena made of plexiglass with a total length of 198.12 cm, 30.48 cm deep and 30.48 cm wide and divided into three chambers roughly equal in size with small compartments (17.78 × 30.48 × 30.48 cm) on either side. Wired cups were used to restrain animals (Stoelting, Wood Dale, IL, item number 60451). The room contained multiple arenas, but males and females were never tested in the room at the same time, and all equipment was thoroughly cleaned with ethanol between testing.
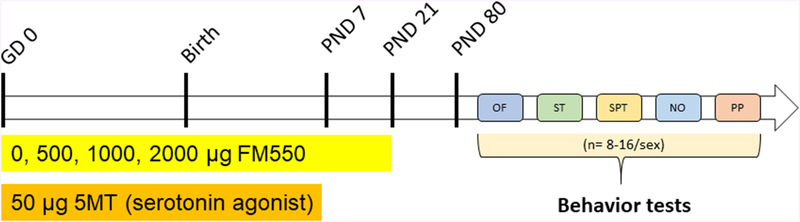
Behavioral studies began on PND 80 and conducted in a sequence considered least to most intensive for the animals: open field, sociability, social preference, novel object, and partner preference. Animals were tested in only one behavioral test each day and completed no later than 9 months of age. For experiments using “strangers,” these animals were approximately the same size or smaller, and sexually naive. Of the test animals, 1 animal had malocclusion and 1 animal had no hind left leg, thus these animals were excluded from the study. The overall study design is shown in Figure 1.
Figure 1.
Study design.
Open Field Test
The open field test (OF) tracks investigation of a novel environment and has been used to examine anxiety and exploration in a variety of rodents (Prut and Belzung, 2003), including prairie voles (Lee et al., 2019; Olazábal and Young, 2005; Stowe et al., 2005). Adult prairie voles were subjected to a standard 30-minute open field test as described previously (Baldwin et al., 2017). Briefly, the test animal was gently placed in the center of an empty open arena and allowed to freely explore for 30 minutes. The center was defined digitally by dividing the task floor into a 3×3 square grid of equal size using the TopScan software. Endpoints were distance traveled in the entire arena and center, number of entries, and time in the center. Duration in the center and total distance traveled were binned into 5-min intervals for further analysis. All videos were individually reviewed by an observer blind to exposure to exclude any trials in which an error or some other erroneous factor occurred. Nine test animals, 6 females and 3 males, were excluded from the analysis due to technical or other issues. Final animal numbers were as follows: control (15 F, 14 M); 500 μg FM 550 (13 F, 11 M); 1000 μg FM 550 (10 F, 8 M); 2000 μg FM 550 (8 F, 15 M); 5MT (9 F, 16 M).
Sociability Test (ST)
Using a three-chambered arena, the subject animal is given the opportunity to explore a novel “stranger” animal or a novel object to access social and exploratory motivation, with prairie voles typically being highly prosocial (Stetzik et al., 2018). Two wired cups were positioned on opposite ends of the 3-chambered arena; one empty and one holding a same sex stranger. The wired cup allows the animals to interact but not harm each other. All strangers were given time to acclimate to the restrainer cup prior to testing. The test animal was gently placed in the center of the middle chamber and given 10 minutes to explore. Time spent in contact with the stranger cup or empty cup chamber, total investigation time, and total distance traveled were analyzed. A sociability index was calculated by subtracting time spent with the inanimate object from time spent with the stranger animal, divided by the total time spent with both. A sociability index of 1.0 indicates 100% preference for the stranger and −1.0 indicates 100% preference for the empty cup. One female video was excluded due to camera failure. Final animal numbers were as follows: control (19 F, 15 M); 500 μg FM 550 (12 F, 12 M); 1000 μg FM 550 (11 F, 9 M); 2000 μg FM 550 (9 F, 15 M); 5MT (9 F, 16 M).
Social Preference Test (SPT)
The social preference test assesses preference for a familiar versus a novel animal, where prairie voles typically spend more time with the familiar animal (DeVries, A.C. et al., 1997). The task and assessed endpoints were the same as the sociability test except the cups contain a stranger and a familiar (cage mate) same sex animal. A social preference index was calculated by subtracting the time spent with the stranger animal from the time spent with the familiar animal, divided by the total time spent with both. A social preference index of 1.0 indicates 100% preference for the familiar animal and −1.0 indicates 100% preference for the stranger. One female did not interact with either the stranger or the familiar animal and thus excluded from the analysis. Four male trials were excluded due to technical issues. Final animal numbers were as follows: control (19 F, 14 M); 500 μg FM 550 (13 F, 8 M); 1000 μg FM 550 (10 F, 9 M); 2000 μg FM 550 (9 F, 15 M); 5MT (9 F, 16 M).
Novel Object Recognition Test (NOR)
Novel object recognition tests are used to assess short- and long-term memory (Ennaceur and Delacour, 1988). Animals able to recognize novelty are expected to spend more time investigating a novel object over a familiar one. Objects used were glass jars varying in volume, shape and color. Objects occupied relatively the same amount of floor space and had similar shapes. Two identical test objects were placed inside the open field arena equally spaced within two digitally defined quadrants on one end of the arena to ensure free range of motion around the objects and minimize capacity to hide behind the objects. The test animal was gently placed in the arena close to the middle of the wall on the opposite side of the objects, allowed to explore the area for five minutes, and then removed. One of the objects was then replaced with another distinct test object. Thirty minutes later, the test animal was placed back into the arena and allowed to re-explore the area for five minutes then returned to the home cage. The next day, another distinct object replaced the previously used novel object and the test animal was again allowed to explore the arena for five minutes. The novel objects were introduced in a designated corner throughout the sessions to maintain consistency and avoid novel spatial recognition. Objects were thoroughly cleaned between test sessions. Total investigation time, time exploring each object, and total distance traveled were analyzed for each trial. Investigation ratio was calculated by dividing the time spent with the novel object by the total time spent with both (Madularu et al., 2014). Five females and 8 males were euthanized before NO testing for other purposes, and thus not available for this test. One 500 μg FM 550 female was excluded due to a technical error with one of the recordings. Final animal numbers were as follows: control (14 F, 13 M); 500 μg FM 550 (13 F, 11 M); 1000 μg FM 550 (11 F, 7 M); 2000 μg FM 550 (9 F, 13 M); 5MT (9 F, 15 M).
Partner Preference Test (PPT)
Prairie voles spontaneously display social monogamy and a partner preference test (PPT) assesses the strength of pair bond formation. (Modi and Young, 2012; Williams et al., 1992) Test animals were cohabitated for 24 hours with an opposite sex, unrelated, sexually naïve “partner” of reproductive age. This length of time is sufficient to induce a pair bond even if mating does not occur (Williams et al., 1992). As in the previous tests, two cups were positioned on opposite ends of the 3-chambered arena. One cup held the partner of the test animal and the other held a stranger animal. The stranger was a novel, opposite sex, unrelated, sexually naïve animal. The animals in the cups were given time to acclimate to the new arena. The test animal was gently placed in middle chamber and given 10 minutes to explore. Time spent in proximity to the stranger cup or partner cup, was recorded. To gain more resolution, time spent with the partner and stranger were also binned in minute intervals. A partner preference index was calculated by subtracting the time spent with the stranger animal from the time spent with the partner animal, divided by the total time spent with both. One female climbed on top of the cup and was therefore excluded. Seven females and 10 males had been euthanized for other purposes and were thus unavailable for this test. Final animal numbers were as follows: control (13 F, 12 M); 500 μg FM 550 (13 F, 11 M); 1000 μg FM 550 (9 F, 6 M); 2000 μg FM 550 (9 F, 13 M); 5MT (9 F, 15 M).
Internal dose assessment
To estimate internal exposure levels, a separate cohort of sexually naïve adult voles (4–5 per sex; PND 86–112) were exposed to FM 550 (2000 μg) daily via sc injection for 5 days. The animals were euthanized 4 hours after dosing on the 5th day and serum collected for analysis. Serum samples were processed according to (Butt et al., 2016). Samples (approximately 0.1 mL) were allowed to thaw and then were spiked with the internal standards 13C-TPHP, 13C-EH-TBB, and 13C-BEH-TEBP (Wellington Laboratories). Deionized water (0.1 mL) and formic acid (0.05 mL) were added to deproteinate the serum. Samples were vortexed and subsequently sonicated for 15 minutes. Serum extracts were purified in two rounds of solid phase extraction (SPE). The first SPE purification step used Waters Oasis PRiME HLB extraction cartridges (500 mg, 6 cc). Briefly, cartridges were conditioned with 5 mL of dichloromethane, 5 mL of methanol, and 5 mL of deionized water. After samples were loaded, the cartridges were washed with 5 mL deionized water and vacuumed to dryness. Analytes were eluted from the cartridges using 10 mL of 1:1 dichlormethane/ethyl acetate. Extracts were then concentrated to near dryness under a gentle stream of nitrogen and reconstituted in 1 mL of hexane. The second SPE purification step used Waters Sep-Pak Ca Certified Silica cartridges (1 g, 6 cc). Briefly, cartridges were conditioned with 10 mL of hexane and sample extracts were loaded. Analytes were eluted from the cartridges using 10 mL of 1:1 hexane/ethyl acetate. Samples were concentrated under a gentle stream of nitrogen, reconstituted in hexane, and spiked with the recovery standards dTPHP (Sigma Aldrich) and 4-Fluoro-2,3,4,6-tetrabromodiphenylether (FBDE-69, Wellington Laboratories). Analytes were quantified using previously described gas chromatography/mass spectrometry methods and six-point calibration curves (Phillips et al., 2017; Stapleton et al., 2008). Average recoveries of 13C-TPHP, 13C-TBB, and 13C-TBPH were 90 ± 13%, 94 ± 6%, and 99 ± 11%, respectively. Means were calculated for each component analyzed if more than 50% of the samples were above the minimum detection level (MDL). MDL varied by analyte (Table 1). Individual sample values below the MDL were estimated by dividing the MDL by 2. For no instance or analyte was more than one value replaced in this regard.
Table 1.
Summary of internal serum levels for virgin adult prairie voles following 5-day sc exposure to 2000 μg FM 550. The percentage of each analyte in the original FM 550 mixture and corresponding minimum detection levels are reported. Serum levels are reported as mean ± SEM.
| Mean ± SEM (ng/mL) | ||||
|---|---|---|---|---|
| FM 550 Components | % Analyte in Original FM 550 mixture | Minimum Detection Level (ng) | Female (n = 5) | Male (n = 4) |
| EH-TBB | 36% | 0.47 | 14.59 ± 3.73 | 24.92 ± 12.03 |
| BEH-TEBP | 14% | 0.41 | <MDL | <MDL |
| TPHP | 18% | 0.51 | 13.51 ± 2.22 | 14.23 ± 2.88 |
| 2IPPDPP | 32% | 0.30 | 15.69 ± 6.57 | 13.58 ± 5.79 |
| 3IPPDPP | 0.03 | <MDL | <MDL | |
| 4IPPDPP | 0.21 | 13.64 ± 3.31 | 18.74 ± 7.26 | |
| 2,4-DIPPDPP | 0.19 | 4.55 ± 1.42 | 20.19 ± 4.43 | |
| B2IPPPP | 0.62 | <MDL | <MDL | |
| B3IPPPP | 0.27 | <MDL | <MDL | |
| B4IPPPP | 0.05 | <MDL | <MDL | |
| B-2,4-DIPPPP | 0.87 | <MDL | <MDL | |
| T3IPPPP | 0.07 | <MDL | <MDL | |
| T4IPPPP | 0.03 | <MDL | <MDL | |
Statistical analysis
Statistical analyses were performed using Graph Prism, version 6 (La Jolla, CA, USA). A ROUT outliers test (Q=1%) was used to identify and remove statistical outliers. For all analyses, statistical significance was defined as α ≤ 0.05. Characteristics of litters (numbers of male, female, and total pups, and male pup percentage) were compared between dosed groups and controls using Mann-Whitney tests. Deviation from a predicted equal sex ratio (50 %) was tested within each dose group using Wilcoxon signed ranks test. For behavioral endpoints, unexposed males and females were compared using a student’s one-tailed t-test to check for known and hypothesized sex differences, as we have done previously for similar studies (Baldwin et al., 2017; Rebuli and Patisaul, 2016) with the identification of known sex differences interpreted as confirmation that the studies were sufficiently powered to detect biologically meaningful effect sizes. The full data sets, with the exception of the 5MT group, were then evaluated within sex by one-way ANOVA to test for a main effect of exposure and followed up with a Holms-Sidak post hoc test for multiple comparisons when significant. The 5MT group was considered separately because it was used as a positive control for effect, thus inclusion with the FM 550 groups would bias the ANOVA (Haseman et al., 2001). A two-tailed t-test was used to compare 5MT and unexposed controls animals of the same sex. For the FM 550 groups, a linear trend test was used to analyze dose responsivity. The 5-min binned OF data were analyzed by a two-way repeated measures ANOVA and Fisher’s protected Least Significant Difference (LSD) test within sex. For all tasks where an investigation index was calculated, animals identified as statistical outliers, or that had total investigation times less than 2% of the total testing time (and thus considered non-participatory) were excluded. The behavioral indices were analyzed by a one sample t-test to determine if they were significantly different from chance (sociability, social preference and partner preference of 0.0; and investigation ratio of 0.5). For the PPT, time spent with the partner animal and stranger animal were compared within each dose group at each 1-min time interval by student’s t-test to determine how preference changed over the course of the test. For each outcome, effect size was calculated as recommended by multiple behavioral neuroscience groups including The American Psychological Association (Fritz et al., 2012; Lakens, 2013). ANOVA effect size was determined by calculating Eta squared (η2) and partial Eta squared (ηp2). Effects are defined as small at 0.01, medium at 0.06, and large at 0.14. T-test effect size was calculated by Cohen’s d which are defined as small at 0.2, medium at 0.5, and large at 0.81.
Results
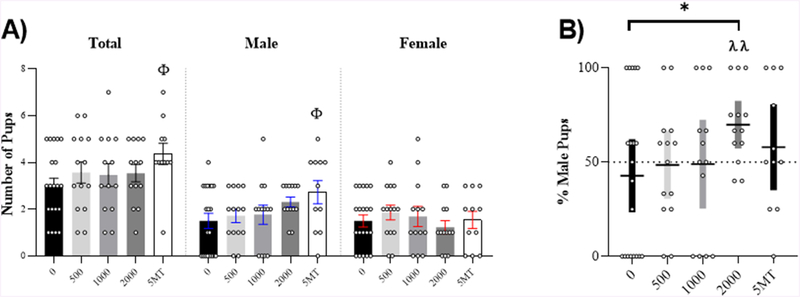
No evidence of overtly toxic effects of exposure, such as gross morphological defects or heightened offspring mortality were observed. Dams exposed to 5MT had a larger litter size (U = 59, p ≤ .03, d = 0.91) and more male pups (U = 61, p ≤ .04, d = 0.82) compared to control dams (Fig 2.A). Dams exposed to 2000 μg FM 550 had a significantly greater percentage of males per litter (significantly higher than a sex ratio of 50 %) (W = 68, p = .005, d = 0.95). This proportion was also significantly greater than control dams (U = 77, p ≤ .05, d = 0.78) (Fig 2.B).
Figure 2.
Litter outcomes. (A) Number of total, male, and female pups per litter. Dams exposed to 5MT had significantly larger litters and more male pups. (B) Dams exposed to 2000 μg FM 550 had a significantly greater percentage of males per litter compared to control dams and the expected, equal sex ratio (50%). Bar graphs depict mean ± SEM; box and whiskers plots depict mean ± 95 % CI; individual litters are represented by circles. For each dose (n = 11–20); *significant effect of FM 550; Φ significant effect of 5MT; (* and Φp ≤ .05). Significant difference from equal sex ratio (50% dotted line) is indicated by λ (λλp ≤ .01).
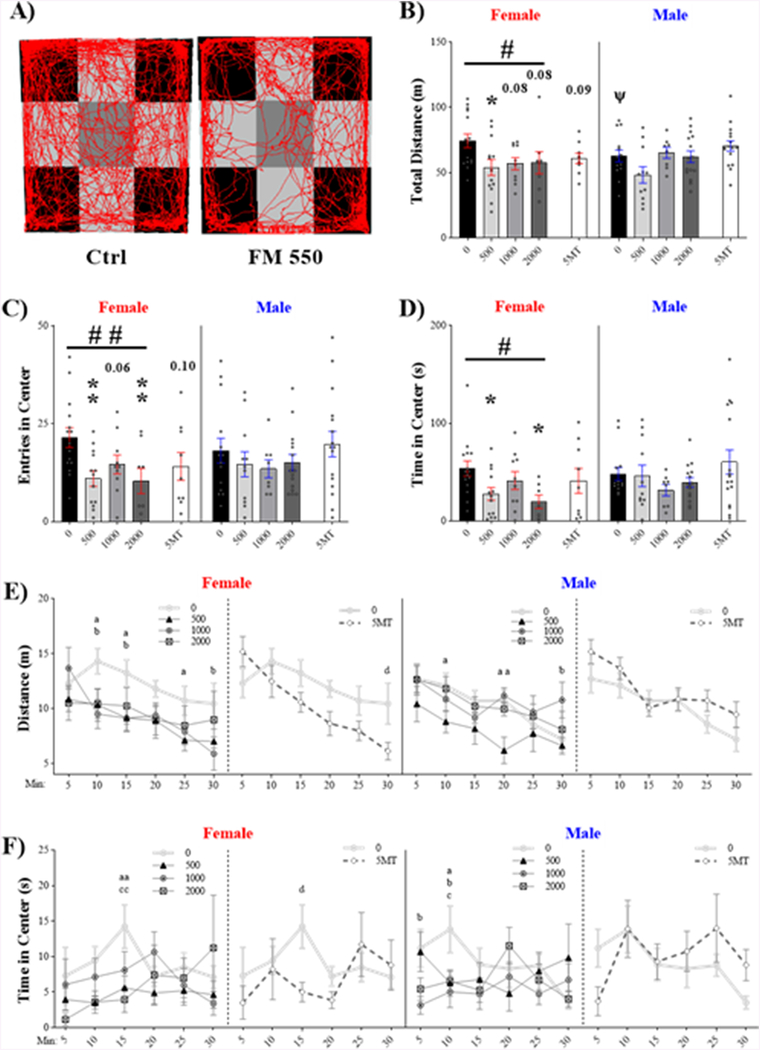
Open Field
Effects of FM 550 exposure on OF behavior were sex specific with only females affected (Fig 3, representative trace in Fig 3.A). As expected, total distance traveled was sexually dimorphic (Fig 3.B, t27 = 2.73; p ≤ .05, d = 0.62), with females traveling more than males. Within FM 550 exposed females, there was a significant effect of exposure on distance traveled (F3, 42 = 2.85, p ≤ .05, η2 = 0.17) and a dose-dependent decrease across groups (F1, 42 = 4.11, p ≤ .05), with 500 μg FM 550 females (p ≤ .03, d = 0.97) traveling less than control females. There was a perceptible decrease in the 1000 μg FM 550 (p ≤ .08, d = 0.95) and 2000 μg FM 550 females (p ≤ .08, d = 0.77), but neither reached statistical significance. To further investigate FM 550 effects across the 30-min session, total distance traveled was binned into 5-min intervals (Fig 3.E) and analyzed within sex. As expected, for both males (F5, 220 = 8.86, p ≤ .0001, ηp2 = 0.17) and females (F5, 210 = 6.88, p ≤ .0001, ηp2 = 0.14) there was a significant effect of time, with activity decreasing over time. No significant interaction between time and exposure was found for either sex. Within females, effect of exposure did not quite reach statistically significance (F3, 42 = 2.70, p = .06, ηp2 = 0.16) but had a large effect size. Significant differences at the individual time points were sporadic (Fig. 2.E).
Figure 3.
Open field outcomes were sex specific with effects most profound in females. (A) Representative activity traces from a control and FM 550 exposed female superimposed on the arena digital layout used for analysis. (B) Total distance traveled was sexually dimorphic with females traveling more than males. There was a significant, dose-responsive effect of FM 550 exposure in females, with exploration decreasing with dose. (C) In females, there was a main effect of FM 550 exposure on center entries with entries significantly decreased at the lowest and highest dose levels and suggestive at the mid-level dose. (D) Time in the center was also significantly lower in the lowest and highest female dose groups. (E) Distance traveled and (F) duration in center was binned into 5-min intervals to evaluate activity and exploration patterns across the 30-min test. Significant group differences at individual time points are depicted by letters (a: 500 μg; b: 1000 μg; c: 2000 ug FM 550; d: 5MT). Graphs depict mean ± SEM; circles represent individual animals (B-D); circles = control, triangles = 500 μg, hexagons = 1000 μg, squares = 2000 μg, diamonds = 5MT (E-F). For each dose (n= 8–16), * denotes statistically significant exposure effects and # denotes a statistically significant dose-response effect, within sex, while ψ denotes significant sex differences between controls. For binned data (E-F), letters identify group differences from control at individual time intervals: a = 500 μg, b = 1000 μg, c = 2000 μg, d = 5MT. A single symbol represents p ≤ .05 and a double symbol represents p ≤ .01.
Effects on center entries were also female specific. A main effect of exposure (Fig 3.C, F3, 42 = 4.63, p ≤ .007, η2 = 0.25) was observed in females, with entries decreasing with dose (F1, 42 = 7.84, p ≤ .008). The 500 μg FM 550 (p ≤ .007, d = 1.21) and 2000 μg FM 550 females (p ≤ .01, d = 1.16) entered the center less often than controls. The 1000 μg FM 550 females also made fewer entries but, although the effect size was medium, the effect did not quite reach statistical significance (p = .06, d = 0.76). Exposure also affected the time females spent in the center (Fig 3.D, F3, 41 = 3.57, p ≤ .02, η2 = 0.21). Time in center was dose-dependently decreased (F1, 41 = 5.91, p ≤ .02), with 500 μg (p ≤ .03, d = 0.96) and 2000 μg (p ≤ .03, d = 1.26) FM 550 females spending less time in the center than controls. Time in the center was binned into 5-min intervals and analyzed within sex (Fig 3.F). Within females, no main effects of time (F5, 210 = 1.05, p = .39, ηp2 = 0.02), exposure (F3, 42 = 1.75, p = .17, ηp2 = 0.06), or any interaction (F15, 210 = 1.20, p = .28, ηp2 = 0.08) were found. By contrast, in FM 550 males, a significant interaction between time and exposure on duration in the center was found (F15, 220 = 1.77, p ≤ .04, ηp2 = 0.11). This effect was driven by decreased time in the center at the 10-minute time point in all FM 550 exposed male groups compared to controls (Fig 3.F).
5MT had no significant main effects on any OF behavior in either sex. Within the binned, 5-min intervals, there was a significant effect of time on distance traveled in 5MT males (F5, 140 = 12.49, p ≤ .0001, ηp2 = 0.31) and females (F5, 110 = 7.61, p ≤ .0001, ηp2 = 0.26), with activity decreasing over time. Within females, there was a significant interaction between 5MT exposure and time (F5, 110 = 2.43, p ≤ .04, ηp2 = 0.10) with significantly less traveling observed in the final time block. A main effect of time on duration in the center was only observed in 5MT males (F5, 140 = 2.55, p ≤ .03, ηp2 = 0.08), with duration in the center decreasing over time. In females, there was a significant time by exposure interaction (F15, 220 = 1.77, p ≤ .04, d = 0.06) with activity only affected in the 15-minute block.
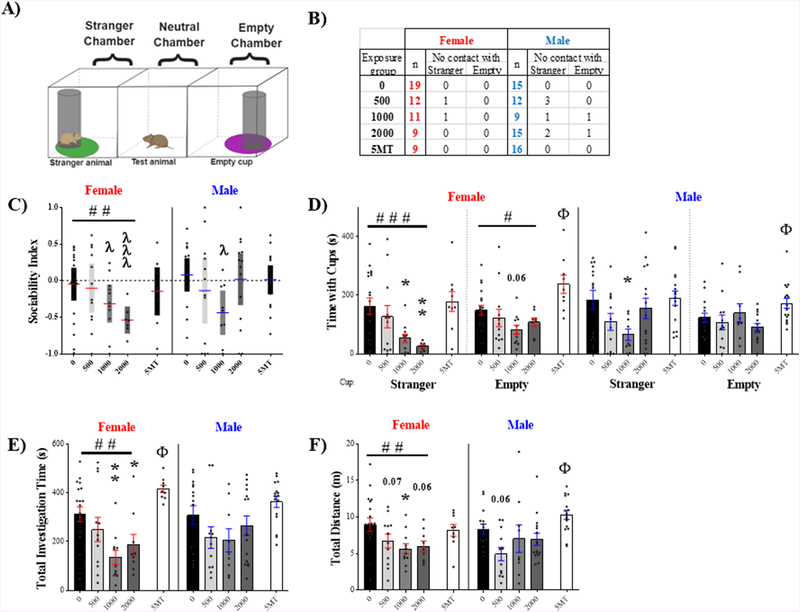
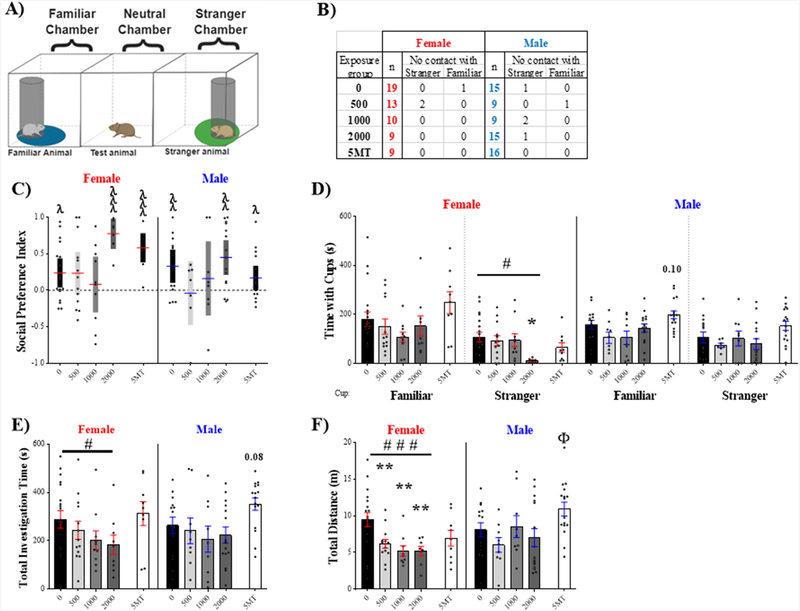
Sociability Test
All animals interacted with at least one cup during the test (Fig 4.A). However, there were instances in the FM 550 groups where the test animal visited one but not the other (Fig 4.B). The total number of animals not making contact with both cups (stranger or empty) was compared to the controls using Fisher’s exact test. Frequency of single contact (25%) was only marginally higher in males exposed to 500 μg FM 550 (p ≤ .08) compared to same sex controls. All animals were included in subsequent analyses. No endpoints were found to be sexually dimorphic, but effects of FM 550 were primarily observed in females.
Figure 4.
Sociability test outcomes were sex specific, with effects primarily in females. (A) Arena schematic depicting placement of each element and the zone around each cup in which the test animal was considered to be in contact with the cup. (B) Number of individuals per group that failed to interact with either the stranger or empty cup. The 500 μg FM 550 males had a marginally higher incidence rate of single interaction compared to same sex controls. (C) Sociability index revealed no preference for either cup in the unexposed controls but greater preference for the empty cup in the 1000 μg FM 550 group of both sexes and the 2000 μg FM 550 female dose group. (D) FM 550 dose-dependently reduced time spent with either the stranger or empty cup in females with the 1000 μg and 2000 μg dose groups spending significantly less time in contact with the stranger. A main effect of exposure was not found for males but the 1000 μg FM 550 group spent significantly less time with the stranger. In both sexes, the 5MT group spent significantly more time with the empty cup. (E) Total investigation time in females dose-dependently decreased with FM 550 exposure and was significantly lower in the 1000 μg and 2000 μg dose groups. Conversely, investigation time was higher in the 5MT exposed females. (F) In FM 550 exposed females, total distance traveled was dose-dependently reduced. The 1000 μg FM 550 group was statistically different from same sex controls and decreases were suggestive in the other two dose groups. The serotonin agonist 5MT significantly increased distance traveled only in males. Bar graphs depict mean ± SEM; box and whisker plots depict mean ± 95 % CI; individual animals are depicted by circles. For each dose (n= 9–19); *significant effects of FM 550; Φ significant effect of 5MT; # significant dose-response effect within sex (*, #, and Φp ≤ .05; ** and ##p ≤ .01; ###p ≤ .001). In the sociability index, a significant difference from chance (SI= 0, dotted line) is indicated by λ (λp ≤ .05, λλλp ≤ .001). A sociability index of 1.0 indicates preference for stranger animal and an index of −1.0 indicates preference for the empty cup.
To assess preference for a stranger versus an inanimate object, a sociability index (SI) was calculated for each animal and compared to equal preference (SI = 0) by one-sample t-test (Fig 4.C). Neither control females (t18 = 0.44¸ p = .66, d = 0.10) nor control males (t18 = .47¸ p = .47, d = 0.19) displayed a significant preference for the stranger or empty cup. There was a significant effect of FM 550 exposure on sociability index in females (F3, 47 = 2.85, p ≤ .05, d = 0.15), with a dose dependent (F1,47 = 8.07, p ≤ .007) aversion to the stranger. Females exposed to 1000 μg (t10 = 2.69, p ≤ .02, d = 0.81) or 2000 μg (t8 = 5.35, p ≤ .0007, d = 1.78) FM 550 spent significantly more time with the empty cup. In males, exposure had no main effect on preference (F3, 47 = 0.94, p ≤ .43, η2 = 0.06), however the 1000 μg FM 550 males spent significantly more time with the empty cup compared to the stranger (t8 = 3.31, p ≤ .01, d = 1.10).
Similarly, time spent in proximity to the stranger cup was significantly affected by FM 550 exposure in females (Fig 4.D, F3,46 = 4.88, p ≤ .005, η2 = 0.24) and strongly dose dependent, with time decreasing with dose (F1,46 = 14.24, p ≤ .0005). Females exposed to 1000 μg (p ≤ .01, d = 1.10) and 2000 μg (p ≤ .007, d = 1.35) FM 550 spent significantly less time in proximity to the stranger than same sex controls. In males, a main effect of exposure did not reach statistical significance but had a large effect size (F3,47 = 2.46, p = .07, η2 = 0.14). Thus, post hoc analysis was performed and the 1000 μg males were found to spend less time with the stranger (p ≤ .005, d = 1.16). Time spent in proximity to the empty cup was not significantly affected by FM 550 exposure in females (F3, 46 = 2.08, p = .12, η2 = 0.12), however there was a dose-responsive decrease (F1,46 = 4.25, p = .04). Difference from controls was only suggestive for the 1000 μg (p ≤ .06, d = 1.04) and the 2000 μg FM 550 (p ≤ .34, d = 0.64) females. There were no significant effects of FM 550 on time spent with the empty cups in males.
Total investigation time was calculated by adding time spent in proximity to the stranger cup and empty cup. There was a main effect of FM 550 exposure on total investigation time in females (Fig 4.E, F3,47 = 4.45, p ≤ .007, η2 = 0.22), with investigation time decreasing with dose (F1,47 = 10.05, p ≤ .003). The 1000 μg (p ≤ .004, d = 1.46) and 2000 μg (p ≤ .05, d = 0.95) FM 550 exposed females traveled less than controls. No main effect of FM 550 exposure was found in males (F3, 47 = 1.24, p = .31, η2 = 0.07).
Finally, within females, there was a main effect of FM 550 exposure on total distance traveled (Fig 4.F, F3, 47 = 3.16, p ≤ .03, η2 = 0.17) with activity decreasing with dose (F1,47 = 7.63, p ≤ .008). Females exposed to 1000 μg FM 550 traveled less than controls (p ≤ .03, d = 0.94) and there were marginal decreases in the 500 μg (p = .07, d = 0.60) and 2000 μg (p = .06, d = 0.83) females. No main effect on distance traveled was found for males (Fig 4.F, F3,47 = 1.98, p = .13, η2 = 0.11), but, because the effect size was medium, follow up analysis revealed a potential decrease in the 500 μg FM 550 group (p = .06, d = 1.18).
As anticipated, 5MT exposure significantly affected male behavior in this task (Martin et al., 2012), with distance traveled significantly increased (t29 = 2.13, p ≤ .04, d = 0.77) along with time in proximity to the empty cup (t29 = 2.12, p ≤ .04, d = 0.76). 5MT males showed no sociability preference (t15 = 0.13, p = .88, d = 0.03). Female behavior was also significantly affected by 5MT exposure. Total investigation time (t26 = 2.26, p ≤ .03, d = 0.91) and time in proximity to the empty cup (t26 =2.77, p ≤ .01, d = 1.12) were increased in 5MT females compared to same sex controls. Females exposed to 5MT displayed no social preference (t8 = 1.03, p = .33, d = 0.34).
Social Preference Test
As in the ST, there were instances in the SPT (Fig 5.A) where some animals interacted with only one cup, but incidence did not significantly differ between groups (Fig 5.B). Uniquely, one 500 μg FM 550 female contacted only a single cup in both tasks, otherwise all these instances occurred in different animals. None of the endpoints in this task were found to be sexually dimorphic, and the exposure related outcomes were almost exclusively in females.
Figure 5.
Social preference test. (A) Social preference arena schematics showing the placement of each element. FM 550 exposure affected social preference in both sexes. (B) Exposure did not impact the number of animals failing to interact with either the stranger or empty cup. (C) Preference for the familiar animal was not displayed by the 500 and 1000 μg groups of both sexes. (D) FM 550 exposure impacted time with the stranger, with exposed females spending less time with the stranger animal. (E) Total investigation time in females decreased with FM 550 exposure. (F) Total distance traveled was decreased by FM 550 exposure in females. Social preference behavior was also affected by 5MT, with exposed males traveling more compared to controls. Both males and females exposed to 5MT displayed a social preference for the familiar animal over the stranger. Bar graphs depict mean ± SEM; box and whisker plots depict mean ± 95 % CI; individual animals are depicted by circles. For each dose (n= 9–19), significant differences due to FM 550 exposure denoted by * and Φ for 5MT. # denotes a dose-response effect within sex (*, #, and Φp ≤ .05; **p ≤ .01; ###p ≤ .001). Significant difference from chance (SI= 0, dotted line) for social preference indicated by λ (λp ≤ .05, λλp ≤ .01, λλλp ≤ .001). A social preference index of 1.0 indicates maximal preference for familiar animal while an index of −1.0 indicates maximal preference for the stranger.
Preference for the familiar or stranger animal was assessed by calculating a social preference index (Fig 5.C). Control males (t13 = 3.16, p ≤ .008, d = 0.84) and females (t16 = 2.52, p ≤ .02, d =.61) displayed a significant preference for the familiar animal, and this preference was also displayed by the 5MT males (t15 = 2.12, p ≤ .05, d = 0.53) and females (t8 = 6.73, p ≤ .0001, d = 2.24). Within males, FM 550 exposure was only suggested to impact social preference (F3,40 = 2.02, p = .13, η2 = 0.13) with only the 2000 μg group displaying a significant preference for the familiar animal (t14 = 4.04, p ≤ .001, d = 1.04) and no preference in the lower two dose groups. In females, there was a significant effect of FM 550 exposure on social preference (F3,43 = 3.80, p = .02, η2 = 0.21) with only the 2000 μg FM 550 group displaying a strong preference for the familiar animal (t8 = 5.11, p ≤ .0009, d = 1.70) and no preference observed in the two lower doses. Time spent in proximity the stranger animal was also significantly affected by exposure in females (Fig 5.D, F3, 45 = 3.01, p ≤ .04, η2 = 0.17), with contact decreasing with dose (F1,45 = 6.12, p ≤ .02). Females exposed to 2000 μg spent significantly less time with the stranger (p ≤ .02, d = 1.34) compared to controls. In males, there was no main effect of FM 550 exposure on time spent with either animal.
There was no main effect of FM 550 exposure on total investigation time in females (Fig 5.E, F3,47 = 1.50, p ≤ .23, η2 = 0.09) but the overall effect was dose-responsive (F1, 47 = 4.41, p ≤ .04). Total investigation in the highest dose group (p = .19, d = 0.72) had a medium effect size but did not reach statistical significance. Similarly, there was no effect of FM 550 exposure in males, but a suggestive increase in total investigation time was observed in the 5MT males (p = .08, d = .77) with a medium effect size.
Total distance traveled was only affected by FM 550 in females (Fig 5.F, F3, 45 = 6.41, p ≤ .001, η2 = 0.30). Distance traveled decreased with dose (F1, 45 = 15.56, p ≤ .0003) with females exposed to 500 μg (p ≤ .005, d = 0.95), 1000 (p ≤ .004, d = 1.18), and 2000 μg (p ≤ .004, d = 1.19) FM 550 all traveling significantly less compared to controls. 5MT exposure significantly increased distance traveled but only in in males (t28 = 2.10, p ≤ .05, d = 0.77).
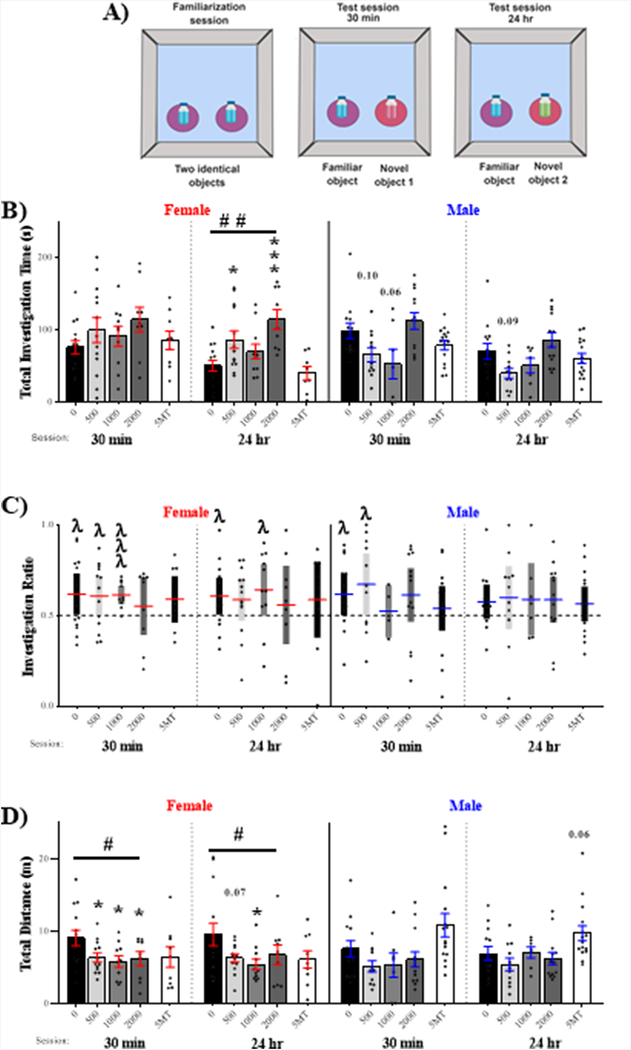
Novel Object Recognition Test
Exploration and recognition of a novel object were examined at 30 min (short-term memory test) and 24 hrs (long-term memory test) after the familiarization session (Fig 6.A). Recognition of the novel object was assessed by object preference (Fig 6.B); calculated by using a one sample t-test to compare investigation ratios to chance (IR = 0.5, dotted line). Control females displayed novel object recognition during both the short-term (t13 = 2.24, p ≤ .04, d = 0.60) and long-term (t13 = 2.29, p ≤ .04, d = 0.61) memory tests. Of the FM 550 exposed females, recognition was achieved only in the 1000 μg group at 30 minutes (t9 = 5.05, p ≤ .0007, d = 1.60) and 24 hrs (t10 = 2.19, p ≤ .05, d = 0.66) but impaired in the 500 and 2000 μg females in both tests. Females exposed to 5MT showed a significant preference for the novel object in the long term test (t7 = 2.58, p ≤ .04, d = 0.91), although there was some suggestion of a preference at 30 min (t8 = 1.61, p = .15, d =0.54). Within control males, novel object preference was only observed during the short-term memory test (t12 = 2.20, p ≤ .05, d = 0.61). Object recognition was impaired in all but the 500 μg FM 550 males (t10 = 2.25, p ≤ .05, d = 0.68). None of the male groups displayed object preference after 24 hours.
Figure 6.
Effects on novel object recognition were sex specific and more pronounced in females. (A) Schematics depicting the placement of the objects and the circular zones in which investigation was measured during the familiarization and subsequent short-term (30 min) and long-term (24 hr) memory test sessions (B). In females, FM 550 dose-dependently increased total investigation time in the 24 hr recollection session. In males, a main effect of FM 550 was found for both the 30 min and 24 hr session with investigation decreased in exposed animals. 5MT had no effect in either sex (C). Novel object recognition was impaired at both time points in the 2000 μg FM 550 females. Males exposed to 1000 and 2000 μg FM 550 did not display recognition during the short-term memory test and none of the male groups appeared to achieve novel object recognition in the long-term memory test. None of the 5MT groups displayed a preference for the novel object. (D) In females, total distance traveled significantly decreased with dose in both sessions. For each dose (n= 7–15), significant differences due to exposure of FM 550 denoted by *, while # denotes a dose-response effect within sex (* and #p ≤ .05; ##p ≤.01; ***p ≤ .001). Significant difference from chance (IR= 0.5, dotted line) is indicated by λ (λp ≤ .05, λλλp ≤ .001). An investigation ratio of 1.0 indicates maximal preference for novel object while a ratio of 0 indicates maximal preference for the familiar object.
Total investigation time was suggested to be sexually dimorphic in the short-term (t25 = 1.63, p = .06, d = 0.63) and long-term (t25 = 1.52, p = .07, d = 0.59) memory tests, with control males spending more time investigating both objects than control females in each trial (Fig 6.C). Within females, there was no main effect of FM 550 exposure on total investigation time at 30 min (F3,42 = 1.21, p = .32, η2 = .08). After 24 hours, there was a significant effect of FM 550 exposure (F3, 43 = 5.96, p ≤ .002, η2 = .29) with a dose-dependent increase in total investigation time (F1,43 = 12.29, p = .001). Females exposed to 500 (p ≤ .03, d = 0.99) or 2000 μg (p ≤ .0006¸ d = 1.91) FM 550 spent significantly more time investigating both objects. A similar effect was suggested for the 1000 μg FM 550 females (p = .19, d = 0.65). Within males, there was a main effect of FM 550 exposure on total investigation time at both the short-term (F3,40 = 4.76, p ≤ .006, η2 = .26) and long term (F3,40 = 4.44, p ≤ .009, η2 = .25) time points, with total investigation time lower compared to controls. During the short-term memory test, none of groups statistically differed from controls but decreased investigation in the 500 (p = .10, d = 0.93) and 1000 μg (p = .06, d = 1.04) FM 550 groups had a large effect size. Similarly, in the 24 hr test, investigation time was somewhat lower in the 500 (p = .09, d = .93) and 1000 μg (p = .38, d = 0.54) FM 550 males. 5MT had no effect in either sex.
Distance traveled was suggested to be sexually dimorphic during the long term memory test (t25 = 1.41, p = .09, d = 0.54), with females traveling more than males. In females, distance traveled was significantly affected by FM 550 exposure (Fig 6.D) during both the short-term (F3, 42 = 2.99, p ≤ .04, η2 = .18) and long-term (F3, 43 = 2.64, p = .06, η2 = .16) memory tests. Decreased activity was dose-dependent iat 30 min (F1,42 = 5.79, p ≤ .02), with 500 (p ≤ .05, d = 0.82), 1000 (p ≤ .04, d = 0.93), and 2000 μg (p ≤ .05, d = 0.84) FM 550 females traveling less than controls. There was also a dose-dependent decrease in activity during the long term memory test (F1,43 = 4.06, p ≤ .05), with 500 (p = .08, d = 0.73), 1000 (p ≤ .04, d = 0.89), and 2000 μg (p = .11, d = 0.54) FM 550 females traveling less than controls. Within males, there were no significant effects of FM 550 exposure on distance traveled at either time point. 5MT had no effects in either sex.
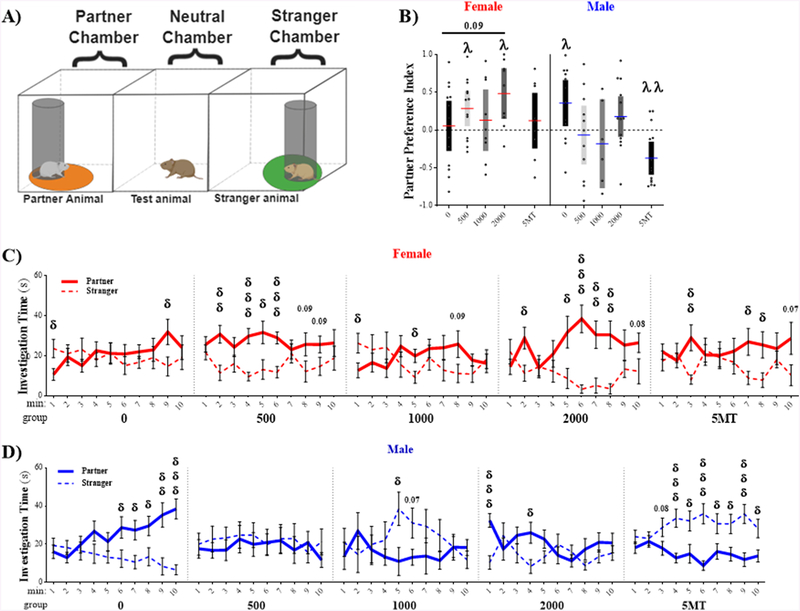
Partner Preference Test
Strength of the pair bond was assessed via PPT in the three chamber arena (Fig 7.A). Partner preference was determined by calculating a partner preference index, and comparing that to equal preference using a one-sample t-test (Fig 7.B.). As anticipated, control males displayed a strong partner preference (t11 = 2.58, p ≤ .03, d = 0.75). Unexpectedly, however, control females did not display any preference (t12 = 0.36, p ≤ .72, d = 0.10). A main effect of FM 550 was not found for partner preference in males (F3, 38 = 2.11, p = .12, η2 = 0.14), although the effect size was large. None of the male FM 550 groups displayed a partner preference and the 5MT males preferred the stranger (t13 = 3.68, p ≤ .003, d = 0.98). Within females, the 500 μg (t12 = 2.65, p ≤ .02, d = 0.73) and 2000 μg FM 550 (t8 = 3.33, p ≤ .01, d = 1.11) groups displayed a preference for the partner.
Figure 7.
Effects on partner preference were male specific. (A) Schematics depicting the placement of the animals and the circular zones where contact between the test animal and the cups was recorded. (B) Control males, but not control females, displayed a significant partner preference. In females, the 500 μg and 2000 μg FM 550 groups displayed preference for the partner. By contrast no partner preference was observed in any male FM 550 group and the 5MT males preferred the stranger. (C-D). Time with the partner (solid line) and stranger (dotted line) at 1 min intervals across the test revealed rapidly emerging partner preference in 500 μg and 2000 μg FM 550 females as well as control males. 5MT males developed a rapid preference for the stranger. Control females only developed a preference for their partner toward the end of the test. For each dose (n = 6–15), significant difference from equal preference (PPI= 0, dotted line) is indicated by λ (λp ≤ .05). A partner preference index of 1.0 indicates maximal preference for partner while an index of −1.0 indicates maximal preference for the stranger. Significant differences in time spent with the stranger and familiar animal is indicated by δ at each minute interval. A single symbol represents p ≤ .05, double symbol represents p ≤ .01 and triple symbol represents p ≤ .001.
Because the female controls did not show a significant partner preference, activity in the 10 minute test was binned into 1-min intervals to assess partner preference over time with the hypothesis that a preference may have emerged late in the female controls. The data were assessed at each time point by t-test. Control males developed a significant preference by the 6th minute and spent significantly more time with the partner thereafter (Fig 7.D). By contrast, a partner preference did not emerge in the control females until 9 min (Fig 7.C). Females exposed to 500 μg or 2000 μg FM 550 displayed a partner preference early, and consistently over time. Preference in 1000 μg FM 550 group was more sporadic. Males exposed to 500 μg FM 550 displayed no preference at any time point, while preference for the stranger was observed at multiple time points in 1000 μg FM 550 and 5MT male groups.
Internal serum levels
Serum levels of the FM 550 components measured in male and female adults are summarized in Table 1. In general the relative abundance of the FM 550 components in serum were similar to the commercial mixture with a few exceptions. BEH-TEBP accounts for ~14% of FM 550 (Belcher et al., 2014), but was not detected in the serum, likely due to its rapid clearance from the body (Knudsen et al., 2017). EH-TBB was the most abundant analyte measured in the serum with a concentration ranging from 15 to 25 ng/mL. TPHP and several of the ITP isomers were also detected in serum and ranged in concentration from 0.2 to 20 ng/mL. Generally levels were similar between males and females; however, several ITP isomers were detected in female serum and not male serum, including 3IPPDPP, B2iPPPP, B4IPPP and Bis-2,4-DIPPPP. B3IPPPP was detected at 0.95 ng/mL in male serum but not female serum. These sex differences may suggest sex-specific differences in toxicokinetics, although a more detailed study would be needed to confirm.
Discussion
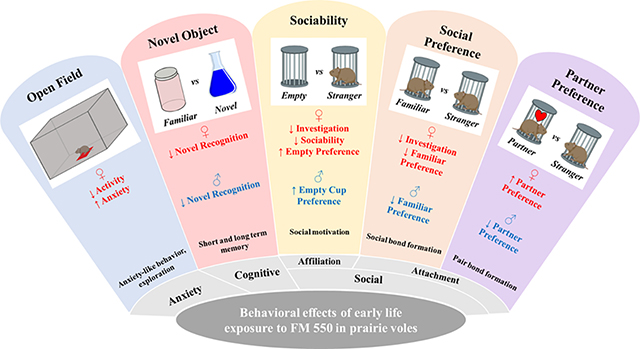
This is the first study to examine the behavioral effects of FM 550 in a prosocial animal, and the results (summarized in Table 2) reveal that developmental FM 550 exposure had sex-specific effects on aspects of sociability, anxiety, attachment, and memory. Overall, females were more appreciably affected than males, with many of the socioemotional effects dose responsive. Exposed females displayed more anxiety-like behaviors and were aversive to novel situations and individuals. This combination of generalized and social anxiety likely contributed to the heightened attachment for the familiar animal in the SPT and PPT. By contrast, effects in males were specific to dose and task but suggestive of impaired affiliation and short term memory. Most notably, exposure eliminated partner preference in all dose groups which suggests impairment of pair bond formation. Critically, the internal exposure data supports prior work showing rapid metabolism of OPFRs (Knudsen et al., 2017), and will aid in estimating human risk as more exposure data become available. The outcomes of this study provide further evidence that developmental exposure to FM 550 can sex-specifically impact socioemotional behaviors and underscore the utility of prairie vole model for investigating the impact of chemical exposures on social behavior.
Table 2.
Summary of behavioral effects.
| Females | Males | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | Vehicle (sesame oil) | 500 μg FM 550 | 1000 μg FM 550 | 2000 μg FM 550 | 5MT | Vehicle (sesame oil) | 500 μg FM 550 | 1000 μg FM 550 | 2000 μg FM 550 | 5MT |
| OF | • F>M activity | • ↑ Anxiety • ↓ Activity |
• ns | • ↑ Anxiety | • ns | • F>M activity | • ns | • ns | • ns | • ns |
| ST | • No preference | • No preference | • Empty preference • ↓ Investigation • ↓ Stranger • ↓ Activity |
• Empty preference • ↓ Investigation • ↓ Stranger |
• No preference • ↑ Investigation • ↑ Empty |
• No preference | • No preference | • Empty preference • ↓ Stranger |
• No preference | • No preference • ↑ Empty • ↑ Activity |
| SPT | • Familiar preference | • No preference • ↓ Activity |
• No preference • ↓ Activity |
• ↑ Familiar preference • ↓ Stranger • ↓ Activity |
• ↑ Familiar preference | • Familiar preference | • No preference | • No preference | • Familiar preference | • Familiar preference • ↑ Activity |
| NO | • Novel recognition (30m/24h) | • No recognition (30m/24h) • ↑ Investigation (24h) • ↓ Activity (30m) |
• Novel recognition (30m/24h) • ↓ Activity (30m/24h) |
• No recognition (30m/24h) • ↑ Investigation (24h) • ↓ Activity (30m) |
• No recognition (30m) • Novel recognition (24h) |
• Novel recognition (30m) • No recognition (24h) F>M |
• Novel recognition (30m) • No recognition (24h) |
• No recognition (30m/24h) | • No recognition (30m/24h) | • No recognition (30m/24h) |
| PPT | • No preference | • Partner preference | • No preference | • Partner preference | • No preference | • Partner preference | • No preference | • No preference | • No preference | • Stranger preference |
The most robust behavioral phenotype observed across all tests was heightened anxiety in females, both in social and non-social situations. Exploration consistently decreased with dose in the OF, ST, SPT, and NOR, suggesting reluctance to explore a novel environment regardless of the context. Similarly, exposed females displayed higher avoidance of the center region in the OF, a phenotype diagnostic of heightened anxiety (Carola et al., 2002; Goma and Tobena, 1978; Olazabal and Young, 2005; Osako et al., 2018); again, the effect was dose-responsive. In the sociability task, exposed females displayed a strong dose-responsive aversion to a novel animal, but also an aversion to the empty cup which constitutes a novel object in this context. Thus, in this task, females displayed both social and general anxiety. Albeit rare and sporadic, that a few exposed females made no contact with the stranger animals during the ST and SPT further supports the conclusion that developmental FM 550 exposure is anxiogenic in females.
By comparison, evidence for higher anxiety in the FM 550 exposed males was only found in the social tasks, and not in all dose groups. In the ST, significant aversion to a stranger conspecific occurred only in the mid-level dose group although, as in females, there were instances in the ST and SPT where some animals made no social contact, particularly with strangers. In contrast to the exposed females, there was no evidence of aversion to novelty in the ST or changes in exploratory behavior in the OF or the social tests observed in males.
Heightened anxiety following developmental FM 550 exposure has previously been observed by us and others. We first identified the potential effects of developmental FM 550 exposure on anxiety in both sexes in a pilot study using Wistar rats (Patisaul et al., 2013). This was corroborated in males in a subsequent, more robustly powered study (Baldwin et al., 2017). That the high anxiety phenotype is more apt to appear in males in rats but females in prairie voles likely reflects a species difference. In addition to profound differences in prosocial behaviors, the hormone-dependent mechanisms by which the brains of prairie voles masculinize differs from rats and mice, and they are not as physically sexually dimorphic (De Vries and Simerly, 2002; Lonstein et al., 2005), thus sex-specific replication of exposure-related phenotypes across rodent species should not be assumed, nor expected. Our data are also consistent with a large body of work showing that adverse early life experience, including social isolation, can produce similarly high-anxiety phenotypic outcomes in voles (Pan et al., 2009). Similarly, we have shown that female prairie voles exposed to the estrogen disrupting chemical BPA during the second week of life also displayed heightened anxiety as adults in the OF, while males were unaffected (Sullivan et al., 2014). Collectively, these data support the conclusion that early life FM 550 exposure can induce socioemotional deficits that may differ by sex, with the most vulnerable sex likely depending on the timing of exposure, dose, and species. These sex differences are critical to understand given the strong sex-bias for many psychoemotional disorders including depression, anxiety, and ASD (McCarthy, 2016).
Deficits in sociality were observed in both sexes. The most striking was the absence of a partner preference in any of the three male FM 550 exposure groups. By contrast, two of the three female exposure groups displayed a partner preference, suggesting that exposure did not affect the bond and, if anything, enhanced it because these females took less time than unexposed controls to display a partner preference. Interpretation of the female partner preference results is complicated by the lack of evident partner attachment in the controls. This was likely because the test was not run long enough to fully capture that preference. Prior studies have shown that while preferences emerge in the first hour, they become stronger over time (Beery et al., 2018; Williams et al., 1992). During the SPT, however, unexposed controls of both sexes displayed the expected preference for the familiar individual (Beery et al., 2018; DeVries, A.C. et al., 1997). In females, this inclination was intensified in the 2000 μg FM 550 group with a clear aversion to the stranger. In the lower two dose groups, no social preference emerged but overall social and arena exploration were lower. Collectively these results further support the interpretation of heightened anxiety, with comfort coming from opportunities to interact with familiarity, and aversion to novel situations. Similarly, in males, two of the three FM 550 groups failed to display a preference for the familiar animal, but overall exploration time was unaffected supporting the conclusion that exposure adversely impacted affiliation and attachment in males, but not necessarily anxiety.
It has long been surmised that environmental factors, including chemical exposures, are contributing to rising rates of ASD and other neurodevelopmental disorders with social deficits, but there remains a paucity of supporting experimental evidence, and a long list of suspect chemicals including multiple FRs (Fujiwara et al., 2016; Gore et al., 2014; Kalkbrenner et al., 2014; Raznahan, 2014; Sealey et al., 2016; Ye et al., 2017). While it cannot be concluded, and we do not suggest, that perinatal FM 550 exposure enhances ASD risk, our results are consistent with impairments within the Systems for Social Processing Domain of the Research Domain Criteria (RDoC); a framework developed by NIMH to improve interrogation of the basic dimensions of human behavior by emphasizing quantified measures of behavior and cognition, instead of comparatively subjective and vaguely defined phenotypes and symptoms (Mittal and Wakschlag, 2017; Simmons and Quinn, 2014). The translational value of our data is also strengthened by the use of the prairie vole because aspects of their spontaneous social behavior are more aligned with human social traits than rats or mice. For example, prairie voles show a preference for familiar conspecifics, including their mated partner (DeVries, A.C. et al., 1997; Williams et al., 1992), which contrasts with other rodent species that often live in colonies and thus prefer social novelty (Moy et al., 2004). The adverse outcomes on socioemotional behaviors reported herein are also consistent with linkages reported in humans. Exposure to OPFRs has been associated with poorer social skills in pre-school age children (Lipscomb et al., 2017). Similarly, maternal exposure to OPFRs, including TPHP, is associated with higher behavioral and externalizing problems at 36 months including withdrawal, attention problems, depression and aggression (Doherty et al., 2019a).
In addition to effects on affiliation and attachment, this is the first study to show that developmental FM 550 exposure may induce memory deficits. While effects on short and long term memory were specific to sex and dose, deficits, particularly in short term memory, were observed in both sexes. Female results are potentially complicated by the fact that all of the exposed groups traveled less over the course of the test; likely a consequence of higher anxiety. However, in contrast to the tests with a social stimulus, investigation time was unaffected or even heightened at 24 hours. Future studies should further probe this phenomenon to better distinguish anxiety-related effects from memory deficits. While the NOR assay provides an advantage over other memory tests in that it relies on the spontaneous tendency of rodents to explore a novel object, requiring no reinforcement to motivate the behavior, levels of exploration can be inconsistent or motivated by stimuli other than the novel object. Thus the potential impairments reported herin require replication.
In humans PBDEs are well known to impair cognitive function, learning, and memory (Eriksson et al., 2001; Viberg et al., 2003a, 2004a, b, 2007; Viberg et al., 2003b; Viberg et al., 2006), but less is known about their alternative brominated or OPFR replacements. There is a small but growing body of observational evidence suggesting that prenatal OPFR exposure may adversely affect human cognitive development. For example, prenatal exposure to isopropylated triarylphosphate isomers, the presumed parent compounds of ip-PPP, has been associated with impaired cognitive development, including fine motor skills and early language abilities (Doherty et al., 2019b). Maternal exposure to the OPFRs in FM 550 during pregnancy has also been linked to reduced cognitive performance, especially working memory, in kids approximately 7 years of age (Castorina et al., 2017).
The non-selective 5-HT agonist 5MT was used as a positive control because decades of work using this compound has shown that developmental exposure impairs a range of socioemotional behaviors in rats (Whitaker-Azmita et al., 1990; Whitaker-Azmitia, 2005) including ultrasonic vocalizations, responsiveness to auditory and tactile stimuli, and decreased spontaneous alternations (Kahne et al., 2002; Shemer et al., 1991). Most significantly, a prior study by Martin and colleagues showed that, at this same dose, perinatally exposed male voles display decreased affiliation and increased anxiety-like behaviors (Martin et al., 2012). Female voles were not tested, thus the present studies both reproduce and extend these findings. In the ST, 5MT exposed voles of both sexes spent more time near the empty cup, an outcome consistent with Martin et al. (Martin et al., 2012) which reported decreased contact with the stimulus animal in this task. Additionally, investigation time and distance traveled in the social tasks were heightened, particularly in males, and 5MT exposed males displayed a significant preference for a novel female rather than their mate in the PPT. We did not, however, see the expected increase in male anxiety, and outcome that may be due to the shortened postnatal exposure adopted herein (dosing ceased on PND 7 as opposed to PND 21). Female anxiety was also unaffected. Collectively, our data show that serotonin agonism during perinatal development impacts social behavior in both sexes of prairie voles. 5MT exposure also impaired novel object recognition at 30 min suggesting some impairment of short-term memory. Serotonin plays a critical role in fetal brain development (Aitken and Törk, 1988; Lauder and Krebs, 1978; Patel and Zhou, 2005; Whitaker-Azmitia and Azmitia, 1986; Whitaker-Azmitia et al., 1995) and elevated blood levels of serotonin have been found in a subset of autistic children (Anderson et al., 1987; McBride et al., 1998). Although we have previously shown in rats that FM 550 may disrupt serotonin production and signaling in the placenta (Rock et al., 2018), we are not positing that disruption of 5-HT is necessarily the mode of action underlying the behavioral alterations described herein. Ongoing work will explore that possibility in more depth.
The mechanisms by which FM 550 sex-specifically affects socioemotional behavior remain to be elucidated. It is well established that OT and AVP fundamentally contribute to pair bonding, affiliation, and other pro-social behaviors (Reviewed in (Young, 2003)), and that differences in social behaviors between prairie voles and other rodent species (including polygynous vole species) is largely due to the distribution of oxytocin, vasopressin and estrogen receptors, and their interactions with the mesolimbic dopamine system (Cushing and Wynne-Edwards, 2006; Lei et al., 2010; McGraw and Young, 2010; Ploskonka et al., 2016; Young et al., 2011). Estrogen plays an important role in regulating OT and AVP signaling in the brain (Gimpl and Fahrenholz, 2001; Richard and Zingg, 1990). OT also sex-specifically regulates estrogen receptor expression and can induce permanent organizational effects on estrogen receptor expression and, consequently, social behavior (Cushing et al., 2004; Kramer et al., 2007; Yamamoto et al., 2006). Thus disruption of either of these critical hormone systems has the potential to change socioemotional behavior. That chemical exposures can disrupt estrogen signaling is well known (Gore et al., 2015; Patisaul and Belcher), and there is emerging evidence that EDCs can also disrupt OT/AVP pathways and associated behaviors in a sex-specific manner (Reviewed in (Patisaul, 2017)). Additionally, corticosterone (CORT) and the hypothalamic pituitary adrenal (HPA) axis also influence affiliative behavior in prairie voles (DeVries, 2002). In naive female voles, exposure to an unfamiliar male vole rapidly decreases CORT levels (DeVries et al., 1995). Additionally, in female voles, exposure to swim stress or CORT injections inhibits partner preference while adrenalectomy enhances it (DeVries et al., 1995; Devries, C. et al., 1997). In males, the effect of swim stress or CORT is opposite, and adrenalectomy increases preference for a novel female (DeVries et al., 1995; Devries, C. et al., 1997). These studies show the environment can influence the HPA axis in a sex-specific manner, with robust effects on social behavior, often via interactions with OT and AVP (DeVries, 2002). Ongoing studies will probe these and other potential mechanisms by which FM 550 may have disrupted the social brain.
Internal levels of the FM 550 components were quantified in virgin animals to obtain an estimate of maternal exposure in the study animals and internal dosing levels. Because it is not the route by which humans are exposed, and it bypases first-pass liver metabolism, use of subcutaneous injection could be viewed as a limitation. This route was necessary, however, because nothing is known about FR metabolism in voles. Generally the profile of compounds suggested more rapid metabolism of the the OPFRs than the BRFs. Followup work will help establish the pharmacokinetics of FM 550 in this unique species.
Conclusions
These data reveal that developmental exposure to FM 550 adversely impacts socioemotional behaviors and, to some degree, memory in a prosocial species. Heightened anxiety in females was the most robust outcome, which is concordant with our prior work in rats, that also found evidence of elevated anxiety (Baldwin et al., 2017; Patisaul et al., 2013). Pair bond formation in males was also adversely impacted by all three doses of FM 550 but future work will be required to confirm this effect, and more comprehensively probe for effects in females. Deficits in sociality have also been reported in zebrafish exposed to FM 550 0–5 days post fertilization or in adolescence (45 days post fertilization) at doses as low as 0.01 mg/L (Bailey and Levin, 2015), further suggesting that adverse outcomes on sociality likely occur well below the purported NOAEL. Because the prairie vole model has been used extensively to study the neurobiology of social behaviors including paternal care, pair bonding, and social cognition, we assert that it is a particularly valuable for understanding the neurobehavioral effects of toxicant exposure.
Highlights.
There is concern that early life exposure to flame retardants (FRs) is contributing to rising rates of neurodevelopmental disorders.
Prairie voles were used to test the impact of the FR mixture FM 550 on socioemotional behaviors and memory.
Females were most strongly affected with developmental exposure to human-relevant levels affecting anxiety, exploration and aspects of sociality.
Partner preference was impaired in FM 550 exposed males.
These data support the hypothesis that chemical FRs adversely impact neurodevelopment and socioemotional behavior.
Acknowledgements
The authors gratefully acknowledge Kylie Rock, Shannah Witchey, and Genevieve St. Armour for assistance with dosing. We also thank Ruta Grinceviciute, a visiting summer undergraduate student from the University of Texas El Paso, for assistance with behavioral testing and scoring, and Bruce S. Cushing for supplying the animals that founded our colony and supporting Ruta. Great appreciation is also extended to the staff of the Biological Resources Facility at NC State University, particularly Sandy Elliott, Holly, and “Slim,” for expert animal care and husbandry. And thank you to Samantha Hall for assisting with the chemical extractions. Visual illustrations were created with BioRender. Dr. A.L. Philips can now be reached at Risk Assessment and Natural Resource Sciences, Arcadis U.S., Inc., Raleigh, NC 27607.
Funding
This work was supported by a Department of Defense Autism Research Program, Idea Development Award to HB Patisaul (AR160055), and NIEHS CHHE grant P30ES025128 to RC Smart. AL Phillips and HM Stapleton were supported by NIEHS R01ES016099. M Ruis was supported by NIEHS R01 ES028110 and NIEHS T32-ES021432.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitken AR, Törk I, 1988. Early development of serotonin-containing neurons and pathways as seen in wholemount preparations of the fetal rat brain. Journal of Comparative Neurology 274(1), 32–47. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Freedman DX, Cohen DJ, Volkmar FR, Hoder EL, McPhedran P, Minderaa RB, Hansen CR, Young JG, 1987. Whole Blood Serotonin in Autistic And Normal Subjects. Journal of Child Psychology and Psychiatry 28(6), 885–900. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Levin ED, 2015. Neurotoxicity of FireMaster 550® in zebrafish (Danio rerio): Chronic developmental and acute adolescent exposures. Neurotoxicology and teratology 52(Pt B), 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin KR, Horman B, Phillips AL, McRitchie SL, Watson S, Deese-Spruill J, Jima D, Sumner S, Stapleton H, Patisaul H, 2018. EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocrine connections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, Patisaul HB, 2017. Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Sci Rep 7(1), 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Christensen JD, Lee NS, Blandino KL, 2018. Specificity in Sociality: Mice and Prairie Voles Exhibit Different Patterns of Peer Affiliation. Frontiers in behavioral neuroscience 12, 50–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M, Hsieh J-H, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES, Jarema KA, Padilla S, Tice RR, 2015. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicology and Teratology 52, 181–193. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM, 2014. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol. Lett. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM, 2014. Metabolites of Organophosphate Flame Retardants and 2-Ethylhexyl Tetrabromobenzoate in Urine from Paired Mothers and Toddlers. Environmental Science & Technology 48(17), 10432–10438. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM, 2016. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environment International 94, 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Gagliano M, Cannizzaro G, Mantia G, La Barbera M, Provenzano G, Cannizzaro E, 2008. Perinatal exposure to 5-metoxytryptamine, behavioural-stress reactivity and functional response of 5-HT1A receptors in the adolescent rat. Behavioural Brain Research 186(1), 98–106. [DOI] [PubMed] [Google Scholar]
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P, 2002. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behavioural Brain Research 134(1), 49–57. [DOI] [PubMed] [Google Scholar]
- Castorina R, Bradman A, Stapleton HM, Butt C, Avery D, Harley KG, Gunier RB, Holland N, Eskenazi B, 2017. Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere 189, 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marcé RM, Becher G, Thomsen C, 2015. Human exposure pathways to organophosphate triesters — A biomonitoring study of mother–child pairs. Environment International 75, 159–165. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le W-W, Hoffman GE, 2004. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Research 1016(2), 247–254. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Wynne-Edwards KE, 2006. Estrogen receptor-α distribution in male rodents is associated with social organization. Journal of Comparative Neurology 494(4), 595–605. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB, 2002. Anatomy, development and function of sexually dimorphic neural circuits, in: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT (Eds.), Hormones Brain and Behavior. Academic Press, San, Diego, pp. 137–191. [Google Scholar]
- DeVries AC, 2002. Interaction among Social Environment, the Hypothalamic–Pituitary–Adrenal Axis, and Behavior. Hormones and Behavior 41(4), 405–413. [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS, 1995. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc Natl Acad Sci U S A 92(17), 7744–7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS, 1997. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Canadian Journal of Zoology 75(2), 295–301. [Google Scholar]
- Devries C, Taymans SE, Carter CS, 1997. Social Modulation of Corticosteroid Responses in Male Prairie Voles. Annals of the New York Academy of Sciences 807(1), 494–497. [DOI] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA, 2012. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environmental science & technology 46(24), 13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, Olshan AF, Daniels JL, 2019a. Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study. Neurotoxicology 73, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, Olshan AF, Daniels JL, 2019b. Prenatal exposure to organophosphate esters and cognitive development in young children in the Pregnancy, Infection, and Nutrition Study. Environ. Res. 169, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J, 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research 31(1), 47–59. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A, 2001. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environmental health perspectives 109(9), 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Webster TF, Ferguson PL, Stapleton HM, 2015. Characterizing the peroxisome proliferator-activated receptor (PPARgamma) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environ. Health Perspect. 123(2), 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, Richler JJ, 2012. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 141(1), 2–18. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Morisaki N, Honda Y, Sampei M, Tani Y, 2016. Chemicals, Nutrition, and Autism Spectrum Disorder: A Mini-Review. Front Neurosci 10, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EA, Stapleton HM, Calero L, Holmes D, Burke K, Martinez R, Cortes B, Nematollahi A, Evans D, Anderson KA, Herbstman JB, 2019. Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere 219, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F, 2001. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiological Reviews 81(2), 629–683. [DOI] [PubMed] [Google Scholar]
- Goma M, Tobena A, 1978. Reliability of various measures obtained in open-field test. Psychol. Rep 43(3 Pt 2), 1123–1128. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev 36(6), E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Martien KM, Gagnidze K, Pfaff D, 2014. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr. Rev 35(6), 961–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ, 2014. Neurobehavioural effects of developmental toxicity. Lancet Neurol 13(3), 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K, 2001. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol. Sci. 61(2), 201–210. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Fang M, Horman B, Patisaul HB, Garantziotis S, Birnbaum LS, Stapleton HM, 2014. Urinary Tetrabromobenzoic Acid (TBBA) as a Biomarker of Exposure to the Flame Retardant Mixture Firemaster 550. Environ. Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Fang M, Horman B, Patisaul HB, Garantziotis S, Birnbaum LS, Stapleton HM, 2014. Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster® 550. Environmental health perspectives 122(9), 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkakoski P, Palvimo JJ, Penttilä L, Vepsäläinen J, Auriola S, 2004. Effects of triaryl phosphates on mouse and human nuclear receptors. Biochemical Pharmacology 67(1), 97–106. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Susiarjo M, Rubio C, Hassold TJ, 2009. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biology of reproduction 81(5), 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S, 2015. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicology and teratology 52(Pt B), 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahne D, Tudorica A, Borella A, Shapiro L, Johnstone F, Huang W, Whitaker-Azmitia PM, 2002. Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiol Behav 75(3), 403–410. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, Schmidt RJ, Penlesky AC, 2014. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Current problems in pediatric and adolescent health care 44(10), 277–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, 2010. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1(2), 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-W, Isobe T, Muto M, Tue NM, Katsura K, Malarvannan G, Sudaryanto A, Chang K-H, Prudente M, Viet PH, Takahashi S, Tanabe S, 2014. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 116, 91–97. [DOI] [PubMed] [Google Scholar]
- Kim S, Jung J, Lee I, Jung D, Youn H, Choi K, 2015. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquatic Toxicology 160, 188–196. [DOI] [PubMed] [Google Scholar]
- Knudsen GA, Sanders JM, Birnbaum LS, 2017. Disposition of the emerging brominated flame retardant, bis(2-ethylhexyl) tetrabromophthalate, in female Sprague Dawley rats: effects of dose, route and repeated administration. Xenobiotica 47(3), 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Yoshida S, Papademetriou E, Cushing BS, 2007. The organizational effects of oxytocin on the central expression of estrogen receptor alpha and oxytocin in adulthood. BMC Neurosci 8, 71–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D, 2013. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Krebs H, 1978. Serotonin as a Differentiation Signal in Early Neurogenesis. Developmental Neuroscience 1(1), 15–30. [DOI] [PubMed] [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, Beery AK, 2019. Affiliation, Aggression, and Selectivity of Peer Relationships in Meadow and Prairie Voles. Frontiers in Behavioral Neuroscience 13(52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Cushing BS, Musatov S, Ogawa S, Kramer KM, 2010. Estrogen receptor-alpha in the bed nucleus of the stria terminalis regulates social affiliation in male prairie voles (Microtus ochrogaster). PloS one 5(1), e8931–e8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb ST, McClelland MM, MacDonald M, Cardenas A, Anderson KA, Kile ML, 2017. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ Health 16(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Wang Q, Liang K, Liu J, Zhou B, Zhang X, Liu H, Giesy JP, Yu H, 2013. Effects of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquatic Toxicology 128–129, 147–157. [DOI] [PubMed] [Google Scholar]
- Liu L-Y, Salamova A, He K, Hites RA, 2015. Analysis of polybrominated diphenyl ethers and emerging halogenated and organophosphate flame retardants in human hair and nails. Journal of Chromatography A 1406, 251–257. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, De Vries GJ, 2005. Unexpected effects of perinatal gonadal hormone manipulations on sexual differentiation of the extrahypothalamic arginine-vasopressin system in prairie voles. Endocrinology 146(3), 1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Venier M, Hites RA, 2012. 2-Ethylhexyl Tetrabromobenzoate and Bis(2-ethylhexyl) Tetrabromophthalate Flame Retardants in the Great Lakes Atmosphere. Environmental Science & Technology 46(1), 204–208. [DOI] [PubMed] [Google Scholar]
- Madularu D, Athanassiou M, Yee JR, Kenkel WM, Carter CS, Mumby DG, 2014. Oxytocin and object preferences in the male prairie vole. Peptides 61, 88–92. [DOI] [PubMed] [Google Scholar]
- Martin MM, Liu Y, Wang Z, 2012. Developmental exposure to a serotonin agonist produces subsequent behavioral and neurochemical changes in the adult male prairie vole. Physiol Behav 105(2), 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride PA, Anderson GM, Hertzig ME, Snow ME, Thompson SM, Khait VD, Shapiro T, Cohen DJ, 1998. Effects of Diagnosis, Race, and Puberty on Platelet Serotonin Levels in Autism and Mental Retardation. Journal of the American Academy of Child & Adolescent Psychiatry 37(7), 767–776. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, 2016. Sex differences in the developing brain as a source of inherent risk. Dialogues Clin Neurosci 18(4), 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SP, Konstantinov A, Stapleton HM, Volz DC, 2013. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicological sciences : an official journal of the Society of Toxicology 133(1), 144–156. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ, 2010. The prairie vole: an emerging model organism for understanding the social brain. Trends in neurosciences 33(2), 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, Webster TF, Stapleton HM, 2016. Nail polish as a source of exposure to triphenyl phosphate. Environment international 86, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Wakschlag LS, 2017. Research domain criteria (RDoC) grows up: Strengthening neurodevelopment investigation within the RDoC framework. J. Affect. Disord. 216, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Young LJ, 2012. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Hormones and behavior 61(3), 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN, 2004. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain and Behavior 3(5), 287–302. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). Subcommittee on Laboratory Animal Nutrition., 1995. Nutrient requirements of laboratory animals, 4th rev. ed. National Academy of Sciences, Washington, D.C. [Google Scholar]
- Olazabal DE, Young LJ, 2005. Variability in “spontaneous” maternal behavior is associated with anxiety-like behavior and affiliation in naive juvenile and adult female prairie voles (Microtus ochrogaster). Dev Psychobiol 47(2), 166–178. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Young LJ, 2005. Variability in “spontaneous” maternal behavior is associated with anxiety-like behavior and affiliation in Naïve juvenile and adult female prairie voles (Microtus ochrogaster). Developmental Psychobiology 47(2), 166–178. [DOI] [PubMed] [Google Scholar]
- Osako Y, Nobuhara R, Arai Y-CP, Tanaka K, Young LJ, Nishihara M, Mitsui S, Yuri K, 2018. Partner Loss in Monogamous Rodents: Modulation of Pain and Emotional Behavior in Male Prairie Voles. Psychosom Med 80(1), 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S, 2008. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environmental Research 108(2), 150–157. [DOI] [PubMed] [Google Scholar]
- Palanza P, Morellini F, Parmigiani S, vom Saal FS, 1999. Prenatal exposure to endocrine disrupting chemicals: effects on behavioral development. Neuroscience & Biobehavioral Reviews 23(7), 1011–1027. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z, 2009. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci. Lett. 454(1), 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TD, Zhou FC, 2005. Ontogeny of 5-HT1A receptor expression in the developing hippocampus. Developmental Brain Research 157(1), 42–57. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, 2017. Endocrine Disruption of Vasopressin Systems and Related Behaviors. Front Endocrinol (Lausanne) 8, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Adewale HB, 2009. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Frontiers in behavioral neuroscience 3, 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Belcher SM, Endocrine disruptors, brain, and behaviors. [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM, 2013. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. Journal of biochemical and molecular toxicology 27(2), 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Todd KL, Mickens JA, Adewale HB, 2009. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 30(3), 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Sun J, Saunders DMV, Codling G, Wiseman S, Jones PD, Giesy JP, 2017. Hydroxylated 2-Ethylhexyl tetrabromobenzoate isomers in house dust and their agonistic potencies with several nuclear receptors. Environ Pollut 227, 578–586. [DOI] [PubMed] [Google Scholar]
- Phillips AL, Chen A, Rock KD, Horman B, Patisaul HB, Stapleton HM, 2016. Editor’s Highlight: Transplacental and Lactational Transfer of Firemaster(R) 550 Components in Dosed Wistar Rats. Toxicol. Sci. 153(2), 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Konstantinov A, Stapleton HM, 2017. Characterization of Individual Isopropylated and tert-Butylated Triarylphosphate (ITP and TBPP) Isomers in Several Commercial Flame Retardant Mixtures and House Dust Standard Reference Material SRM 2585. Environ Sci Technol 51(22), 13443–13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ, 2014. Ligand binding and activation of PPARgamma by Firemaster(R) 550: effects on adipogenesis and osteogenesis in vitro. Environ. Health Perspect. 122(11), 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ, 2014. Ligand binding and activation of PPARγ by Firemaster® 550: effects on adipogenesis and osteogenesis in vitro. Environmental health perspectives 122(11), 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploskonka SD, Eaton JL, Carr MS, Schmidt JV, Cushing BS, 2016. Developmental expression of estrogen receptor beta in the brain of prairie voles (Microtus ochrogaster). Developmental Psychobiology 58(2), 223–230. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology 463(1), 3–33. [DOI] [PubMed] [Google Scholar]
- Raznahan A, 2014. Sizing up the search for autism spectrum disorder (ASD) risk markers during prenatal and early postnatal life. J. Am. Acad. Child Adolesc. Psychiatry 53(10), 1045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Gibson P, Rhodes CL, Cushing BS, Patisaul HB, 2016. Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain. General and comparative endocrinology 238, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Patisaul HB, 2016. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. The Journal of steroid biochemistry and molecular biology 160, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S, Zingg HH, 1990. The human oxytocin gene promoter is regulated by estrogens. Journal of Biological Chemistry 265(11), 6098–6103. [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS, 2007. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive toxicology (Elmsford, N.Y.) 24(2), 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KD, Horman B, Phillips AL, McRitchie SL, Watson S, Deese-Spruill J, Jima D, Sumner S, Stapleton HM, Patisaul HB, 2018. EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocrine connections 7(2), 305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KNJA, Sauer PJJ, Bos AF, 2009. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environmental health perspectives 117(12), 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamova A, Hermanson MH, Hites RA, 2014. Organophosphate and Halogenated Flame Retardants in Atmospheric Particles from a European Arctic Site. Environmental Science & Technology 48(11), 6133–6140. [DOI] [PubMed] [Google Scholar]
- Saunders DM, Higley EB, Hecker M, Mankidy R, Giesy JP, 2013. In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO. Toxicol Lett 223(2), 252–259. [DOI] [PubMed] [Google Scholar]
- Saunders DM, Podaima M, Codling G, Giesy JP, Wiseman S, 2015. A mixture of the novel brominated flame retardants TBPH and TBB affects fecundity and transcript profiles of the HPGL-axis in Japanese medaka. Aquatic toxicology (Amsterdam, Netherlands) 158, 14–21. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, 2001. Cognitive effects of endocrine-disrupting chemicals in animals. Environmental health perspectives 109(12), 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey LA, Hughes BW, Sriskanda AN, Guest JR, Gibson AD, Johnson-Williams L, Pace DG, Bagasra O, 2016. Environmental factors in the development of autism spectrum disorders. Environ Int 88, 288–298. [DOI] [PubMed] [Google Scholar]
- Shemer AV, Azmitia EC, Whitaker-Azmitia PM, 1991. Dose-related effects of prenatal 5-methoxytryptamine (5-MT) on development of serotonin terminal density and behavior. Developmental Brain Research 59(1), 59–63. [DOI] [PubMed] [Google Scholar]
- Simmons JM, Quinn KJ, 2014. The NIMH Research Domain Criteria (RDoC) Project: implications for genetics research. Mamm. Genome 25(1–2), 23–31. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF, 2008. Alternate and New Brominated Flame Retardants Detected in U.S. House Dust. Environmental Science & Technology 42(18), 6910–6916. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA, 2005. Polybrominated Diphenyl Ethers in House Dust and Clothes Dryer Lint. Environmental Science & Technology 39(4), 925–931. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A, 2011. Identification of flame retardants in polyurethane foam collected from baby products. Environmental science & technology 45(12), 5323–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF, 2014a. Flame retardant associations between children’s handwipes and house dust. Chemosphere 116, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF, 2014b. Flame retardant associations between children’s handwipes and house dust. Chemosphere 116, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A, 2012. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environmental science & technology 46(24), 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetzik LA, Sullivan AW, Patisaul HB, Cushing BS, 2018. Novel unconditioned prosocial behavior in prairie voles (Microtus ochrogaster) as a model for empathy. BMC Research Notes 11(1), 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe JR, Liu Y, Curtis JT, Freeman ME, Wang Z, 2005. Species differences in anxiety-related responses in male prairie and meadow voles: The effects of social isolation. Physiol Behav 86(3), 369–378. [DOI] [PubMed] [Google Scholar]
- Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, Patisaul HB, 2014. A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster). Endocrinology 155(10), 3867–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, Abou-Elwafa Abdallah M, Ashworth DC, Douglas P, Toledano MB, Harrad S, 2017. Emerging and legacy flame retardants in UK human milk and food suggest slow response to restrictions on use of PBDEs and HBCDD. Environment International 105, 95–104. [DOI] [PubMed] [Google Scholar]
- Tung EWY, Ahmed S, Peshdary V, Atlas E, 2017. Firemaster(R) 550 and its components isopropylated triphenyl phosphate and triphenyl phosphate enhance adipogenesis and transcriptional activity of peroxisome proliferator activated receptor (Ppargamma) on the adipocyte protein 2 (aP2) promoter. PLoS One 12(4), e0175855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eede N, Maho W, Erratico C, Neels H, Covaci A, 2013. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett 223(1), 9–15. [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J, 2012. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88(10), 1119–1153. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P, 2003a. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicology and Applied Pharmacology 192(2), 95–106. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P, 2004a. Investigations of Strain and/or Gender Differences in Developmental Neurotoxic Effects of Polybrominated Diphenyl Ethers in Mice. Toxicological Sciences 81(2), 344–353. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P, 2004b. Neonatal exposure to the brominated flame-retardant, 2,2′,4,4′,5-pentabromodiphenyl ether, decreases cholinergic nicotinic receptors in hippocampus and affects spontaneous behaviour in the adult mouse. Environmental Toxicology and Pharmacology 17(2), 61–65. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P, 2007. Changes in spontaneous behaviour and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209). NeuroToxicology 28(1), 136–142. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Jakobsson E, Örn U, Eriksson P, 2003b. Neurobehavioral Derangements in Adult Mice Receiving Decabrominated Diphenyl Ether (PBDE 209) during a Defined Period of Neonatal Brain Development. Toxicological Sciences 76(1), 112–120. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G.r., Eriksson P, 2006. Neonatal Exposure to Higher Brominated Diphenyl Ethers, Hepta-, Octa-, or Nonabromodiphenyl Ether, Impairs Spontaneous Behavior and Learning and Memory Functions of Adult Mice. Toxicological Sciences 92(1), 211–218. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmita PM, Shemer AV, Caruso J, Molino L, Azmitia EC, 1990. Role of High Affinity Serotonin Receptors in Neuronal Growtha. Annals of the New York Academy of Sciences 600(1), 315–330. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, 2005. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? International Journal of Developmental Neuroscience 23(1), 75–83. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Azmitia EC, 1986. Autoregulation of fetal serotonergic neuronal development: Role of high affinity serotonin receptors. Neurosci Lett 67(3), 307–312. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM, 1995. Serotonin as a developmental signal. Behavioural Brain Research 73(1), 19–29. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T, 1992. Partner Preference Development in Female Prairie Voles Is Facilitated by Mating or the Central Infusion of Oxytocina. Annals of the New York Academy of Sciences 652(1), 487–489. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Carter CS, Cushing BS, 2006. Neonatal manipulation of oxytocin affects expression of estrogen receptor alpha. Neuroscience 137(1), 157–164. [DOI] [PubMed] [Google Scholar]
- Ye BS, Leung AOW, Wong MH, 2017. The association of environmental toxicants and autism spectrum disorders in children. Environmental pollution 227, 234–242. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z, 2011. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol 32(1), 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, 2003. The Neural Basis of Pair Bonding in a Monogamous Species: A Model for Understanding the Biological Basis of Human Behavior In: National Research Council (US) Panel for the Workshop on the Biodemography of Fertility and Family Behavior; Washington (DC): National Academies Press (US); 2003 4. [Google Scholar]
- Zhou SN, Buchar A, Siddique S, Takser L, Abdelouahab N, Zhu J, 2014. Measurements of selected brominated flame retardants in nursing women: implications for human exposure. Environmental science & technology 48(15), 8873–8880. [DOI] [PMC free article] [PubMed] [Google Scholar]