Abstract
Angelman syndrome is a neurodevelopmental disorder presenting with severe deficits in motor, speech, and cognitive abilities. The primary genetic cause of Angelman syndrome is a maternally transmitted mutation in the Ube3a gene, which has been successfully modeled in Ube3a mutant mice. Phenotypes have been extensively reported in young adult Ube3a mice. Because symptoms continue throughout life in Angelman syndrome, we tested multiple behavioral phenotypes of male Ube3a mice and WT littermate controls at older adult ages. Social behaviors on both the 3-chambered social approach and male-female social interaction tests showed impairments in Ube3a at 12 months of age. Anxiety-related scores on both the elevated plus-maze and the light↔dark transitions assays indicated anxiety-like phenotypes in 12 month old Ube3a mice. Open field locomotion parameters were consistently lower at 12 months. Reduced general exploratory locomotion at this age prevented the interpretation of an anxiety-like phenotype, and likely impacted social tasks. Robust phenotypes in middle-aged Ube3a mice appear to result from continued motor decline. Motor deficits may provide the best outcome measures for preclinical testing of pharmacological targets, towards reductions of symptoms in adults with Angelman syndrome.
Keywords: Angelman, Ube3a mice, 12 month old, social behavior, anxiety-related, exploration
INTRODUCTION
Angelman syndrome is a rare genetic neurodevelopmental disorder characterized by severe intellectual disabilities, impaired speech, developmental delays, microcephaly, seizures, anxiety, motor dysfunctions, ataxic gait, social communication deficits, and a happy demeanor with excessive laughter (Angelman, 1965; Williams et al., 2010; Bird, 2014; Wheeler et al., 2017; den Bakker et al., 2018; www.angelman.org). The primary genetic cause of Angelman syndrome resides in the deletion of a sequence of imprinted genes at chromosomal locus 15q11-q13. Maternal transmission of the deletion, particularly the reduced expression of the ubiquitin ligase UBE3A gene within this locus, results in Angelman syndrome, whereas paternal transmission results in another distinct neurodevelopmental disorder, Prader-Willi syndrome (Knoll et al., 1989; Nicholls, 1993; Buiting et al., 2016). No medical treatments currently exist for the underlying causes of Angelman syndrome (Wheeler et al., 2017).
Mouse models incorporating the loss of maternal Ube3a have been generated and well-characterized for several behavioral features relevant to the symptoms of Angelman syndrome, including motor and cognitive deficits (Jiang et al., 1999; 2010; Heck et al., 2008; Mabb et al., 2011; Baudry et al., 2012; Kaphzan et al., 2012; Jana, 2012; Huang et al., 2013; Santini et al., 2015; Leach and Crawley, 2018; Sonzogni et al., 2018). Ube3a mutant mouse models provide a preclinical research tool for the discovery of effective therapeutics for Angelman syndrome (van Woerden et al., 2007; Huang et al., 2011; Egawa et al., 2012; Margolis et al., 2015; Beaudet and Meng, 2016; Bi et al, 2016; Tan and Bird, 2016; Ciarloni et al., 2017; Stoppel and Anderson, 2017; Guzzetti et al., 2018; Rotaru et al., 2018; Lee et al, 2018).
Here we focus on a relatively unexplored aspect of Ube3a mice. Although diagnosed in childhood, Angelman syndrome is a lifetime disorder. Adults with Angelman syndrome continue to display severe symptoms (Smith et al., 2001; Larson et al., 2015; Prasad et al., 2018). In contrast, most behavioral characterizations of Ube3a mutant mouse phenotypes have employed young mice, in the 8-14 week old range, paralleling the early stages of this neurodevelopmental disorder. At these younger ages, deficits have been consistently reported on motor assays including rotarod (Miura et al., 2002; Heck et al., 2008; Jiang et al., 2010; Daily et al., 2011; Egawa et al., 2012; Huang et al., 2013; Ciarlone et al., 2017; Leach and Crawley, 2018; Sonzogni et al., 2018) and open field exploratory locomotion (Allensworth et al., 2011; Huang et al., 2013; Ciarlone et al., 2017; Sonzogni et al., 2018). Higher anxiety-related behaviors (Jiang et al., 2010; Ciarlone et al, 2017 and others), impaired water maze spatial learning (Miura et al., 2002; Jiang et al., 2010; Huang et al., 2013; Leach and Crawley, 2018) with slower swim speeds in some genetic backgrounds (Huang et al., 2013; Leach and Crawley, 2018), and impaired fear conditioned learning and memory (Miura et al., 2002; Jiang et al., 2010; Huang et al., 2013) have been reported for Ube3a mice at younger ages. To our knowledge, the oldest ages of Ube3a mice tested behaviorally have been 17-23 weeks for water maze and at 31 weeks for rotarod (Huang et al. 2013). The present studies evaluated behavioral phenotypes of older Ube3a mice as compared to their wildtype littermates (WT) on two anxiety-related tests and two social tests, conducted primarily at age 12 months. In addition, open field locomotion was quantified to detect decreases or increases in activity at the older age, which could confound the interpretations of anxiety-related and social assay results.
EXPERIMENTAL METHODS
Mice
Conventional Ube3a knockout mice, derived from maternal transmission and generated on a C57BL/6J background, were purchased from The Jackson Laboratory (JAX) in Bar Harbor, Maine (JAX catalogue #016590), along with their WT littermates. Males were used for the present experiments, to achieve the full Ns required for evaluation of male-female social interactions in mice, while ensuring that all subject mice had identical past experience in the other behavioral assays. Attrition across the aging period reduced the numbers of originally purchased mice to N=8 WT and N=13 Ube3a. Female target mice used for the social tests were sex-matched 129/SvImJ (JAX #2448) for 3-chambered social approach, and C57BL/6J (B6, JAX #000664) for male-female interactions, purchased from JAX and housed by strain. Mice were housed in ventilated Tecniplast cages in an AAALAC approved temperature-controlled vivarium on a 12:12 circadian cycle with lights on at 7 AM. All husbandry, breeding, behavioral testing, and drug treatment procedures were approved by the University of California Davis Institutional Animal Care and Use Committee, and were conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Methodological considerations
Behavioral testing was conducted during the light phase of the circadian cycle, between 8:30 AM and 5:30 PM. On the day of each experiment, mice in their home cages were habituated to the testing room for one hour before the start of the behavioral test. Order of testing was (1) elevated plus-maze, (2) light↔dark transitions, (3) open field, (4) 3-chambered social approach, (5) male-female social interactions. For all assays, surfaces of the testing chamber were cleaned with 70% ethanol after each subject mouse was tested, with sufficient time for the ethanol odor to dissipate before the start of the next test session.
Mice were coded by ear notch pattern to ensure that investigators remained uninformed of genotype and treatment condition. Automated equipment was used for most behavioral assays. For the male-female social interaction assay, in which human scoring of videos was required, investigators remained blind to genotype through the use of numerically coded videos and coded mouse identification numbers.
Behavioral assays
Elevated plus-maze
The elevated plus-maze task was employed to assess anxiety-like conflict behavior in rodents, by titrating the tendency of mice to explore a novel environment versus the tendency of mice to avoid open, raised, brightly lit areas. The four arms are elevated one meter from the floor, with two closed arms surrounded by walls and two open arms in which the drop-off is detectable. Methods used were previously described (Bailey et al., 2007; Silverman et al., 2011; Brielmaier et al., 2012; Kazdoba et al., 2016; Rhine et al., 2019). The photocell-equipped automated plus-maze (Med Associates model ENV-560A, St. Albans, VT) consisted of two open arms (35.5 cm × 6 cm) and two closed arms (35.5 cm × 6 cm). An 0.5 cm high lip surrounded the edges of the open arms. The two closed arms were surrounded by black Plexiglas walls 20 cm high. Room illumination was 300 lux. Each subject mouse was gently placed into the central start area (6 cm × 6 cm), facing an open arm. The mouse was allowed to freely explore for a 5 minute test session. Time spent in the open arms and in the closed arms, number of entries into the open arms, and total number of entries into all four arms, were quantified by the Med Associates software.
Light dark transitions
The lights↔dark transitions test, also termed the light/dark box, assesses anxiety-like conflict behavior in mice by evaluating the tendency of mice to explore a novel environment versus the tendency of mice to avoid brightly lit open areas. The light↔dark transitions test was performed as previously described (Silverman et al. 2011; Brielmaier et al., 2012; Rhine et al., 2019). The photocell-equipped automated 2-chambered apparatus and software were developed by George Dold and coworkers, Research Services Branch, National Institute of Mental Health, Bethesda, MD (Crawley and Goodwin, 1980). The test began by placing the mouse in the light chamber (28 cm × 27.5 cm × 27 cm, 300 lux), made of clear Plexiglas walls and open at the top. The enclosed dark chamber (28 cm × 27.5 cm × 19 cm, ~5 lux), made of black Plexiglas walls with a black Plexiglas ceiling, was reached by traversing the photocell beam in the opening in the partition between the two chambers. The mouse was allowed to explore freely for 10 minutes. Time in the dark chamber and total number of transitions between the light and dark chambers were automatically recorded by Labview 8.5.1 software (National Instruments, Austin, TX).
Open field
Open field activity served as a control assay to detect motor abnormalities that could introduce artifacts into the interpretations of social and/or anxiety-related abnormalities. Methods used were previously described (Silverman et al. 2011; Brielmaier et al., 2012; Rhine et al., 2019). Exploratory locomotion was assessed for 30 minutes in individual mice placed in a novel open field arena, 42 cm × 42 cm × 31 cm high, 30 lux. The open field arenas were equipped with photocell detectors in the x and y (horizontal), and z (vertical) dimensions, interfaced with beam break detection software (AccuScan, Omnitech Electronics, Columbus, OH). Total distance traveled, horizontal movements, vertical movements, and time spent in the center of the arena were automatically recorded and quantified with Accuscan VersaMax 400 software.
3-Chambered social approach
Sociability was tested in four identical 3-chambered social approach chambers, using methods previously described (Yang et al., 2013; Brielmaier et al. 2012; Silverman et al. 2011, 2012; Kazdoba et al., 2016; Rhine et al., 2019). Each apparatus included a side chamber into which an empty inverted wire pencil cup (novel object) was placed, an empty middle chamber, and a side chamber into which an inverted wire pencil cup containing an age- and sexed-matched novel 129/SvImJ mouse (novel mouse) was placed. The target 129/SvImJ mice were previously habituated to the wire cup for 15 minutes/day during the two days preceding testing, to enhance their inactivity during the test session. Testing was conducted under low light, 30 lux. An initial 10 minute habituation session confined to the center chamber was followed by a 10 minute habituation session in all three empty chambers. Time in the two empty side chambers was similar, confirming absence of an innate side bias. After the two 10 minute habituation sessions, the 10 minute social approach session was conducted. Locations of the novel object wire cup and the novel 129/SvImJ target mouse were randomized between the two side chambers across test sessions. Time spent in each chamber (chamber time parameter), time spent within a 2 cm radius of each wire cup (sniff time parameter), and number of entries between compartments (control for exploratory locomotion), were videotracked and quantified by Noldus Ethovision software. Three body point detection tracking was employed to confirm that the subject mouse was facing the target mouse, to maximize accurate scoring of time spent sniffing the novel object and time spent sniffing the novel mouse.
Male-female social interactions
Male female reciprocal social interaction testing was conducted as previously described (Brielmaier et al. 2012; Silverman et al. 2011, 2012; Kazdoba et al., 2016; Stoppel et al., 2018; Rhine et al., 2019). Male subject mice were group housed and sexually naive at the time of testing. B6 females in proestrus or estrus (open vagina with pink or reddish pink surrounding tissue) were used as partner mice. Approximately synchronous estrus was induced by placing male urine and bedding from male cages into the cages of the partner B6 females on each of the three days preceding testing. A 5 minute interaction session was conducted in a clean empty mouse cage within a sound attenuating chamber (ENV-018V; Med Associations, St. Albans, VT), with interior walls covered with convoluted foam sheets (Uline, Pleasant Prairie, WI), under dim red lighting conditions (10 lux). Each female partner was given at least 30 minutes of rest time between re-use in another testing session. Male-female interactions were videorecorded with a digital closed-circuit television camera (Panasonic, Secaucus, NJ). Parameters scored in the male subject mice included nose-to-nose sniffing, nose-to-anogenital sniffing, and following. Total duration and number of bouts of each parameter were quantified from numerically coded videos by a well-trained investigator uninformed of genotype, using Noldus Observer event recording software (Noldus Information Technology, Leesburg, VA). Ultrasonic vocalization calls in the 40-120 Hz range were recorded during the test session using an Avisoft ultrasonic microphone and software (Avisoft Bioacoustics, Avisoft SASLabPro version 4.40). Previous work indicates that almost all calls during male-female interactions in laboratory mice are emitted by the male (Whitney et al. 1973; White et al. 1998; Wang et al. 2008; Sugimoto et al. 2011). Total number of calls were counted as previously described (Brielmaier et al., 2012; Yang et al., 2013, 2015).
Statistics
GraphPad Prism version 7 was used to conduct statistical analyses of the data and to generate graphs. Elevated plus-maze, light↔dark transitions, and male-female interactions were analyzed for genotype differences using unpaired Student’s t-test statistics. Open field exploratory activity was analyzed with a Two-Way ANOVA, using time and genotype as factors, for each parameter (total distance, horizontal activity, vertical activity, and center distance). In cases of a significant ANOVA, a Sidak’s multiple comparison test was used to compare open field activity between genotypes for specific 5 minute time bins within the 30 minute open field session. 3-chambered social approach, which provides a simple binary, yes-or-no measure of sociability within each genotype (yang et al., 2011; Kazdoba et al., 2016; Rhine et al., 2019), was defined as significantly more time spent in the chamber with the novel mouse than in the chamber with the novel object, and/or significantly more time spent sniffing the novel mouse than sniffing the novel object. Unpaired Student’s t-test was used to compare time spent in the chamber containing the novel mouse versus time in the chamber containing the novel object, and time spent sniffing the novel mouse versus time spent sniffing the novel object.
RESULTS
Anxiety-related behaviors in 12 month old Ube3a mice
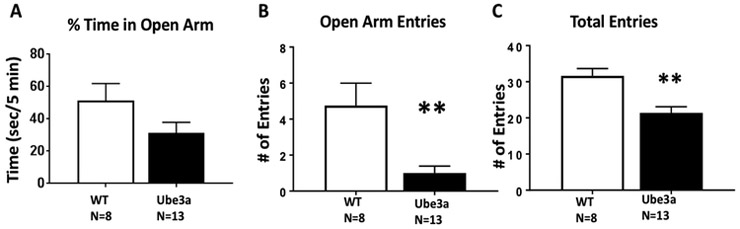
Figure 1 displays genotype differences in elevated plus-maze scores between Ube3a versus WT mice at age 12 months. A) Time spent in the open arms was not significantly different (t1,19 = 1.702, p=0.105, NS). B) Number of entries into the open arms was significantly less in the Ube3a mice as compared to WT (t1,19 = 3.45, p<0.01). C) Total entries into all four open and closed arms was significantly less in Ube3a as compared to WT (t1,19 = 3.81, p<0.01). Fewer total entries indicate significantly less exploratory activity in the Ube3a group. Less overall exploratory locomotion in these older mice represents a confound for this assay. The likely interpretation of these data is that the apparent anxiety-related genotype difference was actually the consequence of less general exploration.
Figure 1.
Elevated plus-maze behavior in 12 month old Ube3a mice and WT littermate controls. A) Time spent in the open arms was not significantly different. B) Number of entries into the open arms was significantly less in the Ube3a mice as compared to the WT mice (**p<0.01). C) Total entries into all four open and closed arms was significantly less in Ube3a than WT (*p<0.01), indicating less overall exploratory locomotion at the older age, rather than a specific anxiety-like phenotype. For all figures, data are expressed as mean + standard error of the mean, numbers of mice per group are displayed under the x-axis, and statistical results are more fully described in the Results text.
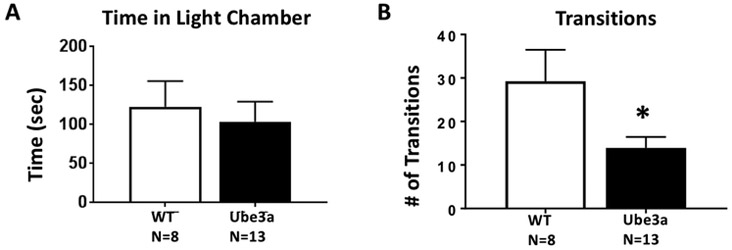
Figure 2 displays a genotype difference in light↔dark transitions between 12 month old Ube3a and WT. A) Time spent in the light chamber did not differ between genotypes (t1,19 = 0.460, p=0.651, NS). B) Ube3a made significantly fewer transitions between chambers (t1,19 = 2.37, p<0.05). Fewer movements between compartments could indicate an anxiety-like component, but is consistent with lower general exploratory locomotion in the older mice, as detected in fewer total arm entries in the elevated plus-maze assay, as shown in Figure 1C, and open field assay, shown in Figure 3.
Figure 2.
Light↔dark transitions in 12 month old Ube3a and WT mice. A) Time spent in the light chamber did not differ between genotypes. B) Ube3a made significantly fewer transitions between chambers as compared to WT (*p<0.05). Lower number of transitions is likely to be the result of reduced overall locomotion, as seen in the elevated plus-maze and open field assays, rather than a specific anxiety-like phenotype.
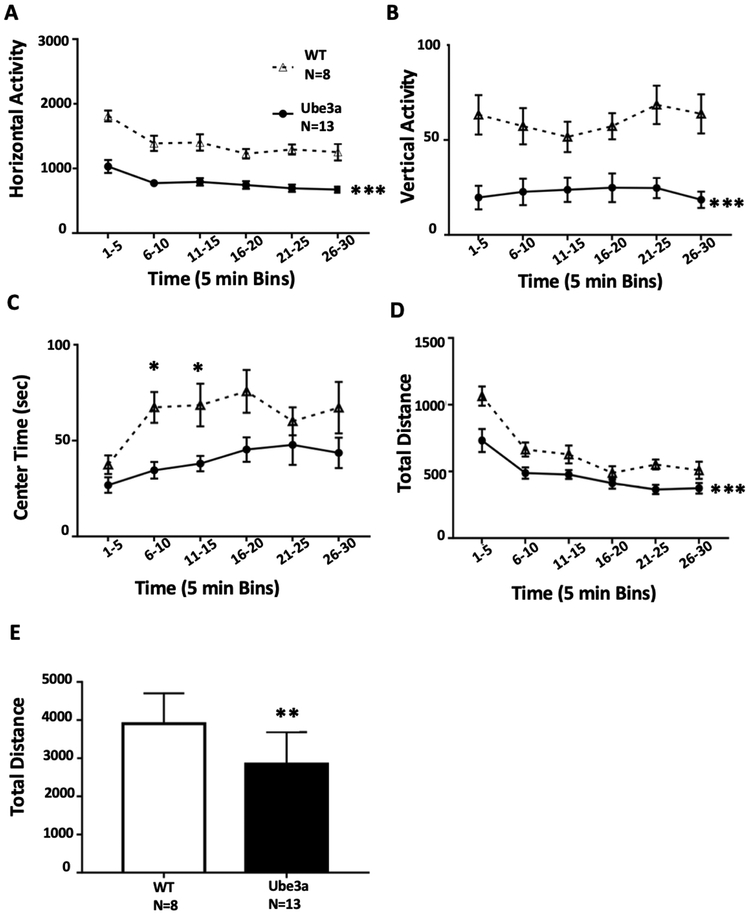
Figure 3.
Open field exploratory locomotion in 12 month old Ube3a and WT mice. A) Horizontal activity was lower in Ube3a than WT (***p<0.001). B) Vertical activity was lower in Ube3a mice at most time points (*p=0.05). C) Center time was lower in Ube3a (*p<0.05). D) Total distance traveled was lower in Ube3a (***p<0.001). E) Summed total distance was lower in Ube3a (**p<0.001), supporting the interpretation of reduced general exploratory locomotion in the older 12 month old mice.
General exploratory locomotion in 12 month old Ube3a mice
Figure 3 displays open field exploratory locomotion in 12 month old Ube3a and WT mice. A) Horizontal activity was significantly lower in Ube3a as compared to WT throughout the test session (Two-Way ANOVA: Time: F5,95 = 19.9, p<0.001; Genotype: F1,19 = 44.2, p<0.001; Interaction: F5,95 = 1.58, p<0.05; Sidak’s multiple comparison test p<0.001 at all time points). B) Vertical activity was significantly lower in Ube3a than WT at most time points (Two-Way ANOVA: Time: F5,95 = 1.66, NS; Genotype: F1,19 = 14.7, p<0.001; Interaction: F5,95 = 2.54, p<0.054; Sidak’s p<0.05 at all time points except 11-15 minutes). C) Center time was lower in Ube3a as compared to WT (Two-Way ANOVA: Time: F5,95 = 4.48, p<0.001; Genotype: F1,19 = 9.44, p<0.001; Interaction: F5,95 = 1.10, p=0.364; Sidak’s p<0.05 at 6-10 and 11-15 minutes). D) Total distance traveled was lower in Ube3a than WT during the first five minute time bin (Two-Way ANOVA: Time: F5,95 = 44.0, p<0.001; Genotype: F1,19 = 8.23, p<0.01; Interaction: F5,95 = 2.68, p<0.05; Sidak’s p<0.001 at 1-5 minutes). E) Total distance summed across the 30 minute test session was lower in Ube3a than WT (t1,19 = 2.87, p<0.01).
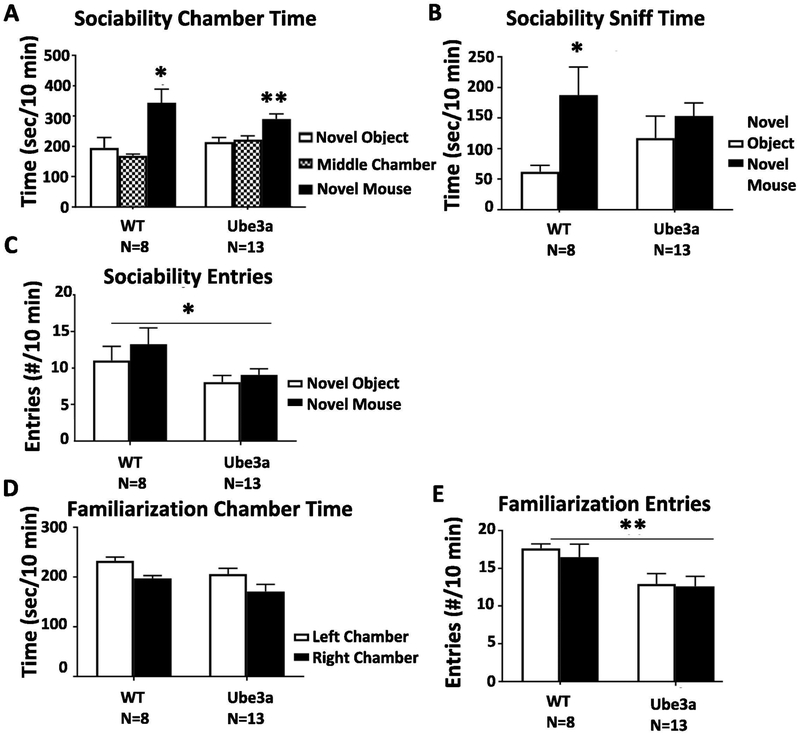
Social behavior assays in 12 month old Ube3a mice
Figure 4 presents evidence for a partial absence of sociability in 12 month old Ube3a and WT mice on the 3-chambered social approach assay, which may have been caused by less overall exploration of the three chambers. A) WT displayed normal sociability on the chamber time parameter, spending significantly more time in the side chamber with the novel mouse than in the side chamber with the novel object (t1,14 = 2.61, p<0.05). Ube3a displayed significant sociability on the chamber time parameter, spending significantly more time in the side chamber with the novel mouse than in the side chamber with the novel object (t1,24 = 3.31, p<0.01). B) WT displayed normal sociability in the 3-chambered social approach task on the sniff time parameter, spending significantly more time sniffing the novel mouse than sniffing the novel object (t1,14 = 2.59, p<0.05). Ube3a failed to display sociability on the sniff time parameter (t1,24 = 0.501, NS). C) Number of entries into the side chambers, the internal control measure for general exploration, showed fewer total entries by Ube3a than WT (F1,38 = 5.269, p< 0.05), indicating lower exploratory locomotion. D) During the familiarization phase before the start of the sociability session, time spent in the two side chambers was similar, confirming lack of innate side bias, for both genotypes (F1,38 = 0.256, NS). E) During the familiarization session, the total number of entries into the two side chambers was lower in Ube3a than WT, again indicating lower general exploratory locomotion at this age (F1,38 = 9.202, p < 0.01), which creates a potential confound for interpreting the results of this assay.
Figure 4.
Sociability in 12 month old Ube3a and WT mice. A) WT displayed normal sociability in the 3-chambered social approach task on the chamber time parameter, spending significantly more time in the side chamber with the novel mouse than in the side chamber with the novel object (*p<0.05). Ube3a displayed significant sociability on the chamber time parameter, spending significantly more time in the side chamber with the novel mouse than in the side chamber with the novel object (**p<0.01). B) WT displayed normal sociability in the 3-chambered social approach task on the sniff time parameter, spending significantly more time sniffing the novel mouse than sniffing the novel object (*p<0.05). Ube3a failed to display sociability on the sniff time parameter. C) Number of entries into the side chambers, the internal control measure for general exploration, was lower for Ube3a than WT (*p<0.05). D) During the familiarization phase before the start of the sociability session, time spent in the two side chambers was similar, confirming lack of innate side bias. E) During the familiarization session, number of entries into the two side chambers was lower for Ube3a than WT (**p<0.01), again indicating less exploratory locomotion at the 12 month age, which likely explains the apparent social deficit.
Figure 5 displays lower scores on all three parameters of male-female social interactions in 12 month old male Ube3a mice as compared to male WT. A) Number of bouts of nose-to-nose sniffing was fewer in Ube3a than WT (t1,19 = 3.73, p<0.01). B) Time spent in nose-to-nose sniffing was less in Ube3a than WT (t1,19 = 4.72, p<0.001). C) Number of bouts of nose-to-anogenital sniffing were fewer in Ube3a than WT (t1,19 = 2.90, p<0.01). D) Time spent in nose-to-anogenital sniffing was less in Ube3a than WT (t1,19 = 3.72, p<0.01). E) Number of bouts of following were fewer in Ube3a than WT (t1,19 = 3.08, p<0.01), which may be the result of less general exploratory locomotion. F) Time spent following showed a trend for less following in Ube3a than WT (t1,19 = 1.94, p=0.0674). G) Total number of ultrasonic vocalizations was lower in Ube3a than WT (t 1,19 = 2.78, p<0.05). Essentially no calls were emitted by Ube3a males during the 5 minute session with an estrous female.
Figure 5.
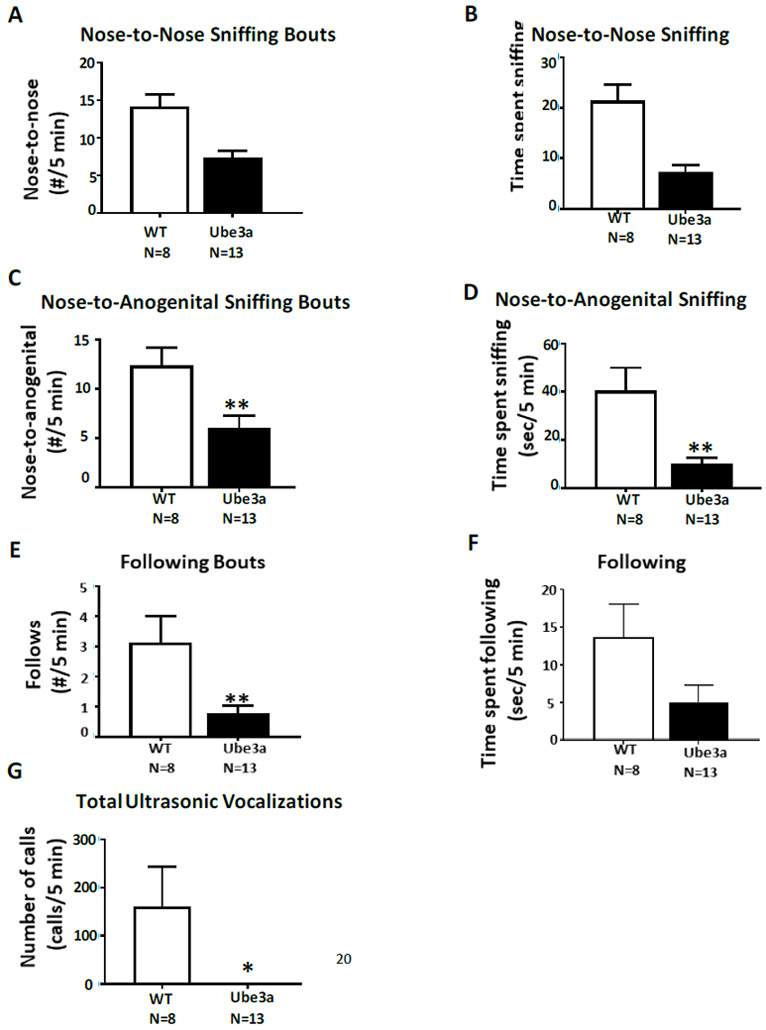
Male-female social interactions in 12 month old male WT and male Ube3a mice. A) Number of bouts of nose-to-nose sniffing were fewer in Ube3a than WT (**p<0.01). B) Time spent in nose-to-nose sniffing was less in Ube3a than WT (***p<0.001). C) Number of bouts of nose-to-anogenital sniffing were fewer in Ube3a than WT (**p<0.01). D) Time spent in nose-to-anogenital sniffing was less in Ube3a than WT (**p<0.01). E) Number of bouts of following were fewer in Ube3a than WT (**p<0.01). F) Time spent following showed a trend for less following in Ube3a than WT (p=0.0674), which may be the result of less general exploratory locomotion. G) Number of ultrasonic vocalizations emitted was lower in Ube3a than WT (*p<0.05).
DISCUSSION
Adults with Angelman syndrome present with a range of symptoms, including impaired locomotion and mobility, continuing severe cognitive impairments, limited speech, anxiety, sleep dysfunction, seizures, obesity, and gastrointestinal disruption (Smith et al., 2001; Larson et al., 2015; Prasad et al., 2018). The present studies employed the Ube3a mutant mouse model of Angelman syndrome to explore anxiety-related, social, and motor phenotypes of older adult male Ube3a mice as compared to their wild-type littermates. Establishing phenotypic abnormalities across the lifespan of Ube3a mice could enable preclinical discovery of interventions to ameliorate symptoms in adults with Angelman syndrome.
The present results replicate and extend previous reports of motor behavioral phenotypes in Ube3a mice. Lower open field locomotor activity on all parameters was highly significant in Ube3a mice at 12 months of age, extending previous findings at younger ages (Allensworth et al., 2011; Huang et al., 2013; Ciarlone et al., 2017; Sonzogni et al., 2018). Interpretations of anxiety-related phenotypes appear to be confounded by the motor deficits in older Ube3a mice. At 12 months of age, Ube3a mice made significantly fewer entries into the open arms of the elevated plus-maze. However, because total entries into all four arms were also significantly lower, it is likely that the open arm entries were impaired due to generally lower exploratory locomotion. Similarly, Ube3a mice at 12 months of age made significantly fewer transitions between the light and dark compartments of the light↔dark transitions apparatus. However, because time spent in the light did not differ between genotypes, it is possible that the fewer transitions between compartments were due to overall lower exploratory locomotion. A definitive interpretation of anxiety-related phenotypes in older Ube3a mice therefore cannot be made.
Evidence for impaired social behaviors was seen in 12 month old Ube3a mice in two social assays. On 3-chambered social approach, WT displayed normal sociability, i.e. time in the chamber with the novel mouse was significantly higher than time in the chamber with the novel object, and time spent sniffing the novel mouse was significantly higher than time spent sniffing the novel object, meeting the definition of sociability in this binary, yes-or-no assay (Yang et al., 2013; Rhine et al., 2019). The 12 month old Ube3a also met the definition for sociability on the chamber time parameter, spending significantly more time in the chamber with the novel mouse than in the chamber with the novel object, but failed to display significant sociability on the sniff time parameter. At this older age, the total number of entries into the side chamber was lower in Ube3a than WT, consistent with less exploratory locomotion in the open field, introducing a motor artifact into the interpretation of a sociability deficit in Ube3a mice at 12 months of age. Therefore, the partial absence of sociability in the 12 month Ube3a could be due to lower general exploration of the side chambers.
Previous investigations of 3-chambered social approach behavior in Ube3a mutant mice at younger ages have reported normal sociability at age 2-3 months (Allensworth et al., 2011), absence of sociability at age 2-3 months (Jamal et al., 2017), partial sociability on one parameter (Huang et al., 2013), and normal sociability during the first 5 minutes of the 10 minute test, with continuing sociability during the second 5 minutes whereas WT did not display continuing sociability (Stoppel and Anderson, 2017). These several studies were conducted at ages 6-13 weeks. It will be important to evaluate additional cohorts of older Ube3a and WT mice, to more fully understand the apparent social approach deficits and motor confounds.
Social interactions between a freely-moving subject male and an estrous female were significantly lower in 12 month old Ube3a than WT on all of the parameters measured, including time spent in nose-to-nose sniffing, number of bouts of nose-to-nose sniffing, time spent in nose-to-anogenital sniffing, number of bouts of nose-to-anogential sniffing, time spent in following, number of bouts of following, and number of vocalizations emitted. However, given the lower exploratory locomotion scores of 12 month old Ube3a on open field, on the exploratory locomotion components of elevated plus-maze, and on number of side chamber entries during 3-chambered social approach, a definitive interpretation of a social deficit in this assay cannot be made in the 12 month old group due to the likely confound of reduced general exploration.
Reporting artifacts and negative results is especially important in the preclinical phase of evaluating therapeutics for neurodevelopmental disorders. The present results indicate that motor deficits are prominent in 12 month old male Ube3a mice. The extent to which significantly lower general exploratory activity affected social behaviors is unclear. Particularly in the male-female social interaction test, the arena is an empty mouse cage, which is small enough to require minimal locomotion. While open field activity parameters were consistently lower in Ube3a mice, scores were within ranges generally displayed by many strains of mice. Further experiments will be needed, using additional motor assays and at intermediate ages, to determine the magnitude of motor deficits that artifactually affect performance on the social and anxiety-related assays in Ube3a mice.
Effective therapeutics for adults with Angelman syndrome could significantly improve quality of life. The remarkably diverse range of pharmacological interventions which have been evaluated preclinically in Ube3a mice (van Woerden et al., 2007; Huang et al., 2011; Baudry et al., 2012; Kaphzan et al., 2012; Meng et al., 2012, 2015; Powell et al., 2013; Godavarthi et al., 2014; Llewellyn et al., 2015; Mandel-Brehm et al., 2015; Silva-Santos et al., 2015; Sun et al., 2015, 2016; Bailus et al., 2016; Mabb et al., 2016; Ciarlone et al., 2016, 2017; Jamal et al., 2017; Guzzetti et al., 2018; Lee et al., 2018) employed juvenile and young adult ages of Ube3a mice, as appropriate for the discovery of cures early in life. Our findings suggest that robust motor phenotypes in Ube3a mice at older adult ages, including open field activity and entries into the side chambers during social approach, and possibly some social and anxiety-related phenotypes, may offer additional preclinical opportunities to discover pharmacological interventions that significantly improve aspects of the severe symptomatology present in adults with Angelman syndrome.
HIGHLIGHTS.
Angelman syndrome Ube3a mice have been characterized primarily at young ages
Twelve month old Ube3a mice were evaluated for relevant behavioral phenotypes
Anxiety-related, social, open field deficits were observed
Motor deficits likely contributed to the anxiety-related and social phenotypes
ACKNOWLEDGMENTS
We thank Drs. Gary Lynch, Christine Gall, and Julie Lauterborn, University of California Irvine, for discussions of the concepts underlying our investigations of mutant mouse models of neurodevelopmental disorders. We thank Maria Schultz and Prescott Leach for preliminary studies with Ube3a mice, which informed the present experimental design. This work was supported by NIH grants R01 NS085709 (JNC), U54 HD079125 (JNC), and a University of California Davis Medical Science Research Fellowship to medical student Rebecca Dutta (RD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
All authors report no financial interests or conflicts of interest. No industry involvement or funding supported these studies.
REFERENCES
- Allensworth M, Saha A, Reiter LT, Heck DH (2011) Normal social seeking behavior, hypoactivity and reduced exploratory range in a mouse model of Angelman syndrome. BMC Genet. 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelman H (1965) "'Puppet' Children: A report of three cases". Dev Med Child Neurol. 7:681–688. [DOI] [PubMed] [Google Scholar]
- Aytan N, Choi JK, Carreras I, Crabtree L, Nguyen B, Lehar M, Blusztajn JK, Jenkins BG, Dedeoglu A (2018) Protective effects of 7,8-dihydroxyflavone on neuropathological and neurochemical changes in a mouse model of Alzheimer's disease. Eur J Pharmacol 828:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN (2007) Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacology Biochemistry and Behavior 86: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Kramar E, Xu X, Zadran H, Moreno S, Lynch G, Gall C, Bi X (2012) Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol Dis 47:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird LM (2014) Angelman syndrome: review of clinical and molecular aspects. Appl Clin Genet. 7:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet AL, Meng L (2016) Gene-targeting pharmaceuticals for single-gene disorders. Hum Mol Genet 25:R18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Sun J, Ji AX, Baudry M (2016) Potential therapeutic approaches for Angelman syndrome. Expert Opin Ther Targets 20:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly Sm, Genestine M, Millonig JH, DiCicco-Bloom E, Crawley JN (2012) Autism-relevant social abnormalities and cognitive deficits in Engrailed-2 knockout mice. PLoS ONE 7:e40914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K, Williams C, Horsthemke B (2016) Angelman syndrome—insights into a rare neurogenetic disorder. Nat Rev Neurol 12:584–593. [DOI] [PubMed] [Google Scholar]
- Ciarlone SL, Grieco JC, D'Agostino DP, Weeber EJ (2016) Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiol Dis 96:38–46. [DOI] [PubMed] [Google Scholar]
- Ciarlone SL, Wang X, Rogawski MA, Weeber EJ (2017) Effects of the synthetic neurosteroid ganaxolone on seizure activity and behavioral deficits in an Angelman syndrome mouse model. Neuropharmacology 116:142–150. [DOI] [PubMed] [Google Scholar]
- Crawley JN and Goodwin FK (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13:167–170. [DOI] [PubMed] [Google Scholar]
- Daily JL, Nash K, Jinwal U, Golde T, Rogers J, Peters MM, Burdine RD, Dickey C, Banko JL, Weeber EJ (2011) Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for Angelman syndrome. PLoS ONE 6:e27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Bakker H, Sidorov MS, Fan Z, Lee DJ, Bird LM, Chu CJ, Philpot BD (2018) Abnormal coherence and sleep composition in children with Angelman syndrome: a retrospective EEG study. Mol Autism 27:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Ohno M (2012) 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer's disease. Neuropsychopharmacology 37:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa K, Kitagawa K, Inoue K, Takayama M, Takayama C (2012) Decreased tonic inhibition in cerebellar granule cells causes motor dysfunction in a mouse model of Angelman syndrome. Sci Transl Med 4:163ra157. [DOI] [PubMed] [Google Scholar]
- Godavarthi SK, Sharma A, Jana NR (2014) Reversal of reduced parvalbumin neurons in hippocampus and amygdala of Angelman syndrome model mice by chronic treatment of fluoxetine. J Neurochem 130:444–454. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Calzari L, Buccarello L, Cesari V, Toschi I, Cattaldo S, Mauro A, Pregnolato F, Mazzola SM, Russo S (2018) Taurine administration recovers motor and learning deficits in an Angelman syndrome mouse model. Int J Mol Sci 19: E1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DH, Zhao Y, Roy S, LeDoux MS, Reiter LT (2008) Analysis of cerebellar function in Ube3a-deficient mice reveals novel genotype-specific behaviors. Hum Mol Genet 17:2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Allen JA, Mabb AM, King IF, Miriyala J, Taylor-Blake B, Sciaky N, Dutton JW Jr, Lee HM, Chen X, Jin J, Bridges AS, Zylka MJ, Roth BL, Philpot BD (2011) Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature 481:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Burns AJ, Nonneman RJ, Baker LK, Riddick NV, Nikolova VD, Riday TT, Yashiro K, Philpot BD, Moy SS (2013) Behavioral deficits in an Angelman syndrome model: effects of genetic background and age. Behav Brain Res 243:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal I, Kumar V, Vatsa N, Shekhar S, Singh BK, Sharma A, Jana NR (2017) Rescue of altered HDAC activity recovers behavioural abnormalities in a mouse model of Angelman syndrome. Neurobiol Dis 105:99–108. [DOI] [PubMed] [Google Scholar]
- Jana NR (2012) Understanding the pathogenesis of Angelman syndrome through animal models. Neural Plast. 2012:710943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lev-Lehman E, Bressler J, Tsai TF, Beaudet AL (1999) Genetics of Angelman syndrome. Am J Hum Genet 6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Pan Y, Zhu L, Landa L, Yoo J, Spencer C, Lorenzo I, Brilliant M, Noebels J, Beaudet AL (2010) Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PloS ONE 5:e12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphzan H, Hernandez P, Jung JI, Cowansage KK, Deinhardt K, Chao MV, Abel T, Klann E (2012) Reversal of impaired hippocampal long-term potentiation and contextual fear memory deficits in Angelman syndrome model mice by ErbB inhibitors. Biol Psychiatry 72:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdoba TM, Hagerman RJ, Zolkowska D, Rogawski MA, Crawley JN (2016) Evaluation of the neurosteroid ganaxolone on social and repetitive behaviors in the BTBR mouse model of autism. Psychopharmacology 233:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll JH, Nicholls RD, Magenis RE, Graham JM, Lalande M, Latt SA (1989) Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet 32:285–290. [DOI] [PubMed] [Google Scholar]
- Larson AM, Shinnick JE, Shaaya EA, Thiele EA, Thibert RL (2015) Angelman syndrome in adulthood. Am J Med Genet 167A:331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach PT, Crawley JN (2018) Touchscreen learning deficits in Ube3a, Ts65Dn and Mecp2 mouse models of neurodevelopmental disorders with intellectual disabilities. Genes, Brain and Behavior 17:e12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Clark EP, Kuijer MB, Cushman M, Pommier Y, Philpot BD (2018) Characterization and structure-activity relationships of indenoisoquinoline-derived topoisomerase I inhibitors in unsilencing the dormant Ube3a gene associated with Angelman syndrome. Mol Autism 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Dodart JC, Aron L, Finley LW, Bronson RT, Haigis MC, Yankner BA, Harper JW (2013) Altered social behavior and neuronal development in mice lacking the Uba6-Use1 ubiquitin transfer system. Mol Cell 50:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn KJ, Nalbandian A, Gomez A, Wei D, Walker N, Kimonis VE. (2015) Administration of CoQ10 analogue ameliorates dysfunction of the mitochondrial respiratory chain in a mouse model of Angelman syndrome. Neurobiol Dis 76:77–86. [DOI] [PubMed] [Google Scholar]
- Mabb AM, Ehlers MD (2010) Ubiquitination in postsynaptic function and plasticity. Ann Rev Cell Dev Biol. 2010;26:179–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabb AM, Judson MC, Zylka MJ, Philpot BD (2011) Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci 34:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabb AM, Simon JM, King IF, Lee HM, An LK, Philpot BD, Zylka MJ (2016) Topoisomerase 1 regulates gene expression in neurons through cleavage complex-dependent and -independent mechanisms. PLoS One 11:e0156439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel-Brehm C, Salogiannis J, Dhamne SC, Rotenberg A, Greenberg ME (2015) Seizure-like activity in a juvenile Angelman syndrome mouse model is attenuated by reducing Arc expression. Proc Natl Acad Sci USA 112:5129–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis SS, Sell GL, Zbinden MA, Bird LM (2015) Angelman Syndrome. Neurotherapeutics 12:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Person RE, Beaudet AL (2012) Ube3a-ATS is an atypical RNA polymerase II transcript that represses the paternal expression of Ube3a. Hum Mol Genet 21:3001–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Ward AJ, Chun S, Bennett CF, Beaudet AL, Rigo F (2015) Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature 518:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Kishino T, Li E, Webber H, Dikkes P, Holmes GL, Wagstaff J (2002) Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol Dis. 9:149–59. [DOI] [PubMed] [Google Scholar]
- Nicholls RD (1993) Genomic imprinting and uniparental disomy in Angelman and Prader-Willi syndromes: a review. Am J Med Genet 46:16–25. [DOI] [PubMed] [Google Scholar]
- Powell WT, Coulson RL, Gonzales ML, Crary FK, Wong SS, Adams S, Ach RA, Tsang P, Yamada NA, Yasui DH, Chédin F, LaSalle JM (2013) R-loop formation at Snord116 mediates topotecan inhibition of Ube3a-antisense and allele-specific chromatin decondensation. Proc Natl Acad Sci USA 110:13938–13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Grocott O, Parkin K, Larson A, Thibert RL (2018) Angelman syndrome in adolescence and adulthood: A retrospective chart review of 53 cases. Am J Med Genet 176:1327–1334. [DOI] [PubMed] [Google Scholar]
- Rhine MA, Parrott JM, Schultz MN, Kazdoba TM, Crawley JN (2019) Hypothesis-driven investigations of diverse pharmacological targets in two mouse models of autism. Autism Research 12:401–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, van Woerden GM, Wallaard I, Elgersma Y (2018) Adult Ube3a gene reinstatement restores the electrophysiological deficits of prefrontal cortex layer 5 neurons in a mouse model of Angelman syndrome. J Neuroscience 38:8011–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Turner KL, Ramaraj AB, Murphy MP, Klann E, Kaphzan H (2015) Mitochondrial superoxide contributes to hippocampal synaptic dysfunction and memory deficits in Angelman syndrome model mice. J Neuroscience 35:16213–16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santos S, van Woerden GM, Bruinsma CF, Mientjes E, Jolfaei MA, Distel B, Kushner SA, Elgersma Y (2015) Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. J Clin Invest 125:2069–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Sukoff Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN (2012) Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Science Translational Medicine 4:131ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN (2011) Sociability and motor functions in Shank1 mutant mice. Brain Research 1380: 120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC (2001) Angelman syndrome: evolution of the phenotype in adolescents and adults. Dev Med Child Neurol 43:476–480. [DOI] [PubMed] [Google Scholar]
- Sonzogni M, Wallaard I, Santos SS, Kingma J, du Mee D, van Woerden GM, Elgersma Y (2018) A behavioral test battery for mouse models of Angelman syndrome: a powerful tool for testing drugs and novel Ube3a mutants. Mol Autism 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel DC, Anderson MP (2017) Hypersociability in the Angelman syndrome mouse model. Exp Neurol. 293:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel LJ, Kazdoba TM, Schaffler MD, Preza AR, Heynen A, Crawley JN, Bear MF (2018). R-baclofen reverses cognitive deficits and improves social interactions. Neuropsychopharmacology, 43:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Okabe S, Kato M, Koshida N, Shiroishi T, Mogi K, Kikusui T, Koide T (2011) A role for strain differences in waveforms of ultrasonic vocalizations during male-female interaction. PLoS One 6:e22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Liu Y, Moreno S, Baudry M, Bi X (2015) Imbalanced mechanistic target of rapamycin C1 and C2 activity in the cerebellum of Angelman syndrome mice impairs motor function. J Neurosci 35:4706–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Liu Y, Tran J, O'Neal P, Baudry M, Bi X (2016) mTORC1-S6K1 inhibition or mTORC2 activation improves hippocampal synaptic plasticity and learning in Angelman syndrome mice. Cell Mol Life Sci 73:4303–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WH, Bird LM (2016) Angelman syndrome: Current and emerging therapies in 2016. Am J Med Genet C Semin Med Genet 172:384–401. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, Jiang YH, Elgersma Y, Weeber EJ (2007) Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nature Neuroscience 10:280–282. [DOI] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J (2008) Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One 3:e1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AC, Sacco P, Cabo R (2017) Unmet clinical needs and burden in Angelman syndrome: a review of the literature. Orphanet J Rare Dis 12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG (1998) 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol Behav 63:467–473. [DOI] [PubMed] [Google Scholar]
- Whitney G, Coble JR, Stockton MD, Tilson EF (1973) Ultrasonic emissions: do they facilitate courtship of mice. J Comp Physiol Psychol 84:445–452. [DOI] [PubMed] [Google Scholar]
- Williams CA, Driscoll DJ, Dagli AI (2010) Clinical and genetic aspects of Angelman syndrome. Genet Med 12:385–395. [DOI] [PubMed] [Google Scholar]
- Yang M, Loureiro D, Kalikhman D, Crawley JN (2013) Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Front Behav Neurosci. 7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Mahrt EJ, Lewis F, Foley G, Portmann T, Dolmetsch RE, Portfors CV, Crawley JN (2015) 16p11.2 deletion syndrome mice display sensory and ultrasonic vocalization deficits during social interactions. Autism Research 8:507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN (2011) Automated three-chambered social approach task for mice. Current Protocols in Neuroscience 8:26.1–26.16. [DOI] [PMC free article] [PubMed] [Google Scholar]