Abstract
Purpose:
The goal of this study was to determine the volumetric vessel density (VVD) in the intraretinal layers, and its relations with visual function and disability in patients with multiple sclerosis (MS).
Design:
Cross-sectional study
Methods:
Eighty patients with relapsing-remitting MS (RRMS) and 99 age- and gender-matched healthy controls (HC) were recruited. The retinal microvascular network in the macular area was imaged using optical coherence tomography angiography in 123 eyes without a history of optic neuritis (MSNON) and 36 eyes with a history of ON (MSON). The VVD was calculated as the vessel densities in the retinal vascular network (RVN), superficial vascular plexus (SVP) or deep vascular plexus (DVP) of an annulus (0.6 – 2.5 mm diameter), divided by the corresponding tissue volume of the intraretinal layers respectively.
Results:
The VVD of RVN and DVP in MSNON were significantly higher than in HC (P < .05). The VVD of RVN, SVP, and DVP in MSON were significantly higher than in MSNON and HC (P < .05). The VVD in both RVN and SVP were positively related to EDSS and disease duration, but negatively related to low contrast letter acuity (P < .05). The VVD measurements were also negatively and strongly related to the corresponding tissue volumes (P < .05).
Conclusions:
This is the first study to reveal increased retinal VVD in patients with RRMS. The measurements of VVD in the RVN and SVP are related to disability and visual function, which may be developed as image markers for tracking disease progression.
Keywords: multiple sclerosis, volumetric vessel density (VVD), low contrast visual acuity (LCVA) and disability
INTRODUCTION
Multiple sclerosis (MS) is a demyelinating central nervous system disease characterized by diffuse inflammation and related neural damage.1–3 Vascular pathology, such as cerebral hypoperfusion,4–9 is considered to be related to disease progression. As a proxy of the cerebral vasculature, alterations of the retinal microvasculature may represent changes in the cerebral vascular system.10,11 Reduced parafoveal microvascular density and increased foveal microvascular density were reported in a mixed group of patients with relapsing-remitting multiple sclerosis (RRMS) and progressive MS.12 In addition, parafoveal vessel density was increased in a follow-up study from the same group of the patients.13 In contrast, another study reported no alterations of parafoveal vessel density in patients with early stage RRMS.14
Retinal neurodegeneration (i.e., loss of retinal nerve fibers and ganglion cells) has been used as a potential biomarker in MS clinical trials.15–18 The thinning of the retinal nerve fiber layer (RNFL) and ganglion cell-inner plexiform layer (GCIPL) in patients with MS may affect the microvasculature due to changed metabolic demand secondary to neurodegeneration or simply tissue structural alterations. Therefore, the alterations of both the retinal vasculature and its corresponding tissue volume are expected to coexist and relate with the immune-mediated inflammatory process. Analysis of the alterations in both the retinal vasculature and neural structure may provide a better understanding of the interaction between these two key components in the vasculo-neuronal tissue. However, previous studies did not take into account of tissue volume while measuring the vessel density in the intraretinal tissue,12,14 which may explain the discrepant results among these studies.12,14 Hence, the intraretinal tissue volume is needed as a common denominator for estimating vessel density, here referred to as volumetric vessel density (VVD).19 Furthermore, the microvascular network responsible for distributing blood throughout the tissue is not evenly distributed in the intraretinal layers,20 indicating the different metabolic demands for maintaining physiological activities in various intraretinal layers. The goal of the present study was to determine the VVD in intraretinal layers and its relation with visual function and disability in patients with RRMS.
METHODS
The study was approved by the Institutional Review Board at the University of Miami. All study subjects were informed about the methods, and an informed consent form was signed by each participant. The tenets of the Declaration of Helsinki were followed. Eighty patients with RRMS were recruited from an ongoing observational study at the Departments of Neurology and Ophthalmology of the University of Miami, Miller School of Medicine from July 2015 to April 2019. Prior optic neuritis (ON) was recorded based on a history of acute vision loss and pain on eye movements. Based on the history of ON, the eyes of the MS group was divided into 2 groups: eyes without prior ON (MSNON) and eyes with a history of ON (MSON). Meanwhile, age- and sex-matched healthy controls (HCs) were recruited at the Bascom Palmer Eye Institute. Their demographic and clinical characteristics are summarized in Table 1.
Table 1.
Characteristics of the patients and controls
| MS | HC | P Value | |
|---|---|---|---|
| Subjects | 80 | 99 | |
| - Subjects with ON | 32 | ||
| Eye | 159* | 198 | |
| - Eye with ON | 36 | ||
| Age (yr) | 40.4 ± 10.4 | 38.6 ± 13.8 | 0.17 |
| Sex | 15M:65F | 31M:68F | 0.08 |
| EDSS | 2.2 ± 1.8 | ||
| Disease Duration (yr) | 8.1 ± 7.0 | ||
| LCLA (2.5%) | 51.1 ± 8.5 | ||
| LCLA (1.25%) | 26.2 ± 9.0 |
The results are presented as the mean ± standard deviation.
one eye was excluded due to low image quality of OCTA. MS: multiple sclerosis; EDSS: expanded disability status scale; LCLA: low contrast letter acuity.
All patients with MS had their diagnoses confirmed by their treating neurologists according to the 2010 Revised McDonald Criteria.21 They were on a stable disease-modifying treatment (DMT) and did not experience any relapses in the past six months. Subjects with ophthalmologic or neurologic disorders (other than MS), such as macular edema, macular degeneration, glaucoma, diabetic and hypertensive retinopathy or a refractive error greater than ± 6 diopters, were excluded from the study. Each patient underwent a complete neurological and ophthalmic examination, such as best-corrected visual acuity, low contrast letter acuity (LCLA, 1.25%, and 2.5%), intraocular pressure (IOP), and slit lamp biomicroscopy of anterior and posterior segments. The LCLA was tested with a low-contrast letter acuity chart (low-contrast Sloan letter chart, Precision Vision, LaSalle, IL) set up in a retro-illuminated light box, and the scores were calculated as the numbers of letters correctly read by the subject.22
Custom ultra-high resolution optical coherence tomography (UHR-OCT) was used for measuring tissue volumes of intraretinal layers.23,24 This spectral domain OCT (SD-OCT) has an axial resolution of ~3 μm. The system adapted a commercial segmentation software program (Orion, Voxeleron LLC, Pleasanton, CA, USA), which can automatically segment up to 6 intraretinal layers and export their thickness maps and tissue volumes.23,24 A scanning protocol of 512 × 128 pixels was used to scan an area of 6 × 6 mm centered on the fovea. Six intraretinal layers were segmented from the volumetric dataset using Orion software.23,24 The segmented intraretinal layers were the retinal nerve fiber layer (RNFL), ganglion cell-inner plexiform layer (GCIPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), and retinal photoreceptor (PR). With the setting of 2.5 mm centered on the fovea in the Orion software, the tissue volume of each intraretinal layer was exported.19,19,25
OCTA (Zeiss Angioplex™ OCTA, Carl Zeiss Meditec, Dublin, CA) was used for measuring the density of the retinal vasculature.26 OCTA detects the motion of red blood cells with depth information, which provides an opportunity to study vessel distribution in the intraretinal layers.20 In the present study, an area of 3 × 3 mm2 centered on the fovea was scanned using the Zeiss OCTA device. Both eyes of each study participant were imaged. OCTA signal strength > 7 was used as the quality control for analysis. Two retinal vascular layers were extracted as the superficial vascular plexus (SVP) located in the RNFL and GCIPL, and the deep vascular plexus (DVP), located in the INL and OPL.27 In addition, the total retinal vascular network (RVN) in the layers from the RNFL to OPL was also extracted since the RVN appeared not to be the simple sum of SVP and DVP. Some vessels in the DVP appeared to be hidden behind the vessel of SVP.23
Similar to previous studies,23,26 fractal analysis was performed to obtain the fractal dimension (Dbox) representing the vessel density of the microvasculature. Fractal analysis is commonly used to analyze the density of tree-like networks, such as the retinal vessels from the fundus photography28 and angiography from OCTA.26,29 The fractal approach measures the vessel density according to the vessel length per area unit using skeletonized images,30–32 while commonly used vessel density in previous MS studies measures the density according to vessel length and area (sometime referring to vessel coverage per unit area).12–14 While the measurement in commercial OCTA devices does not use skeletonized images,12–14 Agemy et al. converted OCTA enface images into skeletonized images for calculating the vessel density referring to capillary perfusion density.27 The details of image processing and fractal analysis have been well described in previous studies.11,19,23 The same image processing used in previous studies19,23,26 for the OCTA en face images of the microvasculature in an annulus zone (0.6–2.5 mm in diameter) was used here. To analyze the microvessels and avoid the image artifact referred to as OCTA projection errors caused by large vessels in the superficial layer projected into the deep vascular plexus,19,23,26 large vessels with a width > 25 μm were removed. OCTA images were then skeletonized for fractal analysis.
Because the en face view image of the OCTA angiography is the sum of vessels projected into a two-dimensional image from the three-dimensional angiography, the vessel density calculated from the en face OCTA image can be regarded as the total vessels in the tissue. Monofractal analysis was used to process segmented microvessels of the OCTA en face vessel images. Fractal dimension (Dbox representing vessel density, VD) was processed and measured using the fractal analysis toolbox (TruSoft Benoit Pro 2.0, TruSoft International, Inc., St. Petersburg, FL), which applied the box-counting technique with a maximum size of 104 and a rotation of 15 in the fractal analysis settings.19,23,26 The repeatability of measuring fractal dimension (Dbox) was tested previously and the coefficients of repeatability were ~2%.33 The VVD was then calculated as the vessel density (i.e., fractal dimension: Dbox) divided by the corresponding tissue volume measured using UHR-OCT.
The retinal vasculature was recently proposed to have 2 to 4 distinct vascular layers depending on the location.20 However, the proposed detailed segregation is not widely used. In the present study, the SVP and DVP and the RVN for the macular region were used and exported as the en face OCTA images. The analysis of VD was performed in a circular area (ϕ = 2.5 mm) centered on the fovea. Retinal vessel density (RVN) was the VD of RVN. Superficial vessel density (SVD) was the VD of SVP, and deep vessel density (DVD) was the VD of DVP. The VVD was calculated as the VD divided by the corresponding tissue volume.19 The VVD of the retina (VVDr) was the VD of the retinal vascular network (RVN) divided by the tissue volume (the same circular area) from the RNFL to OPL. Similarly, the VVD measurements of the superficial (VVDs) and deep (VVDd) inner retina were the VD measurements of the SVP and DVP divided by these corresponding tissue volumes (RNFL + GCIPL for the VVDs; INL + OPL for the VVDd) with a diameter of 2.5 mm.
Statistical Analysis
All data were analyzed with SPSS (Statistical Package for the Social Sciences, IBM, Armonk, New York, Ver. 25). Generalized estimating equation (GEE) models were used to account for the inter-correlation of eyes within subjects. Eyes (left or right) were set as within-subject variables in the GEE models. The OCT measurements were dependent variables, while age, sex and eye were set as covariates. Pearson correlation was used to test the relationships among OCT measurements (both eyes) and between OCT measurements and clinical manifestations (with one eye per subject). The eye with ON in patients with a history of ON was selected. The right eye of patients without a history of ON was selected for analysis of relations between OCT measurements and clinical manifestations. Eyes with a history of ON All data were presented as the mean ± standard deviation and a P-value less than .05 was considered statistically significant. Stepwise regression of all measurements was conducted to determine significant parameters for discriminating the groups. The receiver-operating characteristics (ROC) were calculated as the area under the ROC curves (AUCs) of the OCT measurements. Statistical analysis of the AUCs of the single parameter and multivariate AUCs of the combined parameters were analyzed using MedCalc Software (ver. 19.1.3, MedCalc Software bv, Belgium). The combined parameters were the significant parameters yielded from the stepwise regression.
RESULTS
There were no significant differences in age or sex between groups (P > .05). MS eyes had normal visual function: bilateral 20/20 or better of best corrected visual acuity, full confrontational visual field, and normal anterior and posterior segment exams.
Compared to HC, MS eyes (i.e., MSNON + MSON) had significantly higher vessel densities in the RVN and SVP (P < .05) and greater volumetric vessel densities in all VVD measurements (VVDr, VVDs, and VVDd, all P < .05). In contrast, the tissue volumes of 4 layers (RNFL+GCIPL+INL+OPL), RNFL + GCIPL and INL + OPL were significantly lower in MS (P < .05).
When MS eyes were divided into MS without a history of ON (MSNON) and MS with a history of ON (MSON), VVDr and VVDd in MSNON eyes were significantly higher than HC (P < .05, Fig. 1). VVDr, VVDs, and VVDd in MSON eyes were significantly higher than MSNON and HC (P < .05). In addition, the VD measurements of the RVN and SVP were significantly higher in MSNON than HC (P < .05). However, the VD measurements in the RVN and DVP in MSON were significantly lower than in MSNON (P < .05). No significant differences in VD measurements were found in the RVN and SVP between MSON and HC (P > .05). Tissue volumes in 4 layers and combined RNFL and GCIPL were significantly lower in MSNON compared to HC and in MSON compared to HC and MSNON (P < .05). In addition, the tissue volume of INL + OPL was significantly lower in MSON than HC (P < .05).
Fig. 1. Volumetric vessel densities, vessel densities and tissue volumes depending on history of ON.
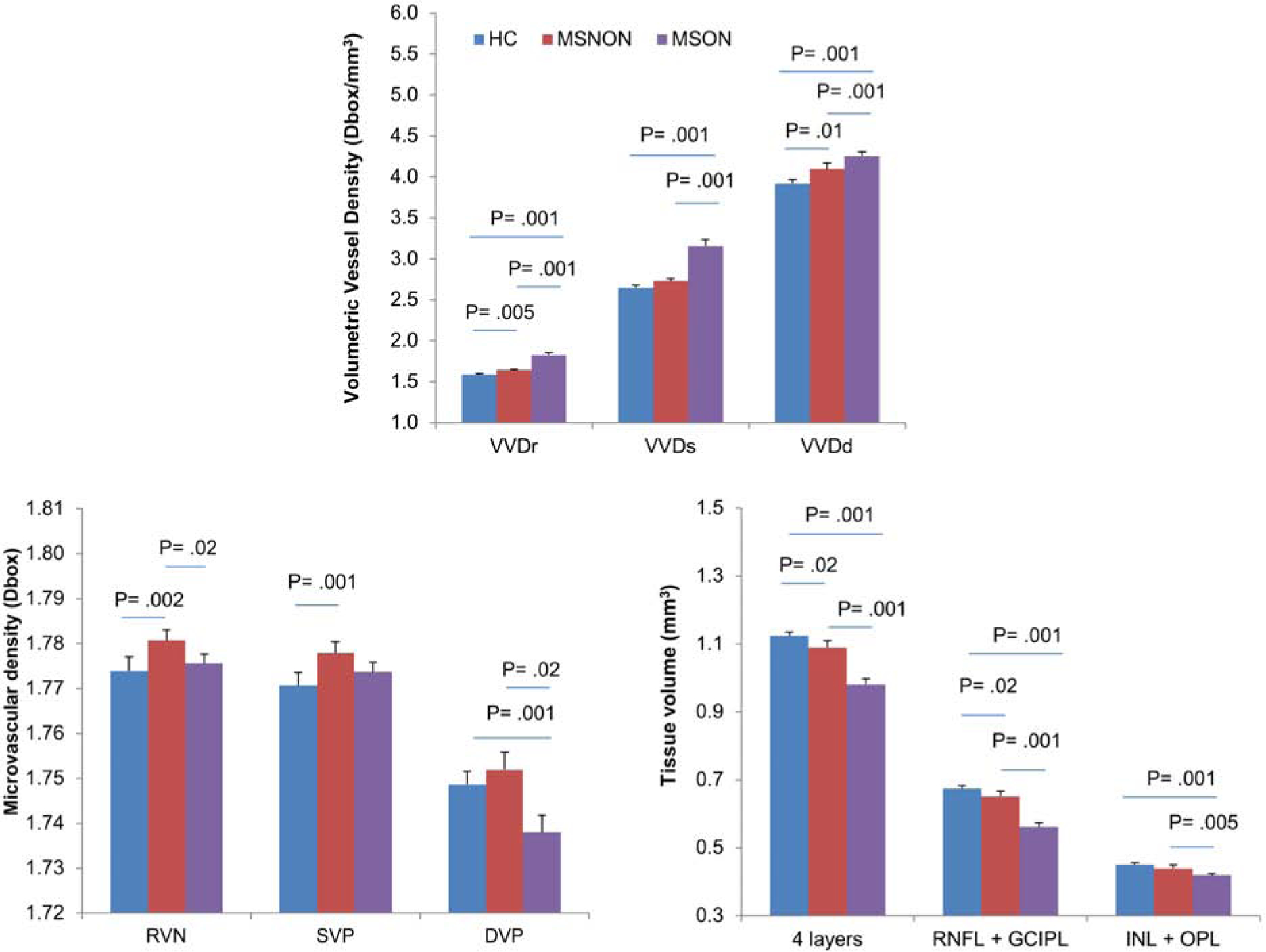
MS eyes were divided into MS without a history of ON (MSNON) and MS with a history of ON (MSON), VVD measurements of VVDr and VVDd in MSNON eyes were significantly higher than HC (Top, P < .05). VVD measurements in VVDr, VVDs, and VVDd in MSON eyes were significantly higher than MSNON and HC (P < .05). In contrast, vessel densities of the RVN and SVP were significantly higher in MSNON than HC (Bottom left, P < .05). However, vessel densities in the RVN and DVP in MSON were significantly lower than in MSNON. No significant differences in vessel densities were found in the RVN and SVP between MSON and HC (P > .05). Tissue volumes in 4 layers and combined RNFL and GCIPL were significantly lower in MSNON compared to HC and in MSON compared to HC and MSNON (Bottom right, P < .05). VVDr: volumetric vessel density in the retinal vascular network (RVN); VVDs: volumetric vessel density in the superficial vascular plexus (SVP); VVDd: volumetric vessel density in the deeper vascular plexus (DVP); RNFL: retinal nerve fiber layer; GCIPL: Ganglion cell-inner plexiform layer. Bars = standard errors.
Both VVDr and VVDs were positively related to EDSS and disease duration and negatively related to LCLA (P < .05, Fig. 2). However, VVDd was not related to any of these clinical measurements (P > .05). VVD measurements were negatively and strongly related to the corresponding tissue volumes (P < .05, Fig. 3). VVDr and VVDs were also negatively related to RVD and SVD, respectively (P < .05). RVD and SVD were positively related to corresponding tissues (P < .05). However, VD measurements were not related to their corresponding tissue volumes (P > .05). The AUC of the VVDr was ranked the highest in discriminating MSON from HC at the sensitivity of 83.3% and specificity of 79.3% with the cut off value of 1.673.
Fig. 2. Relations between volumetric vessel densities and clinical manifestations.
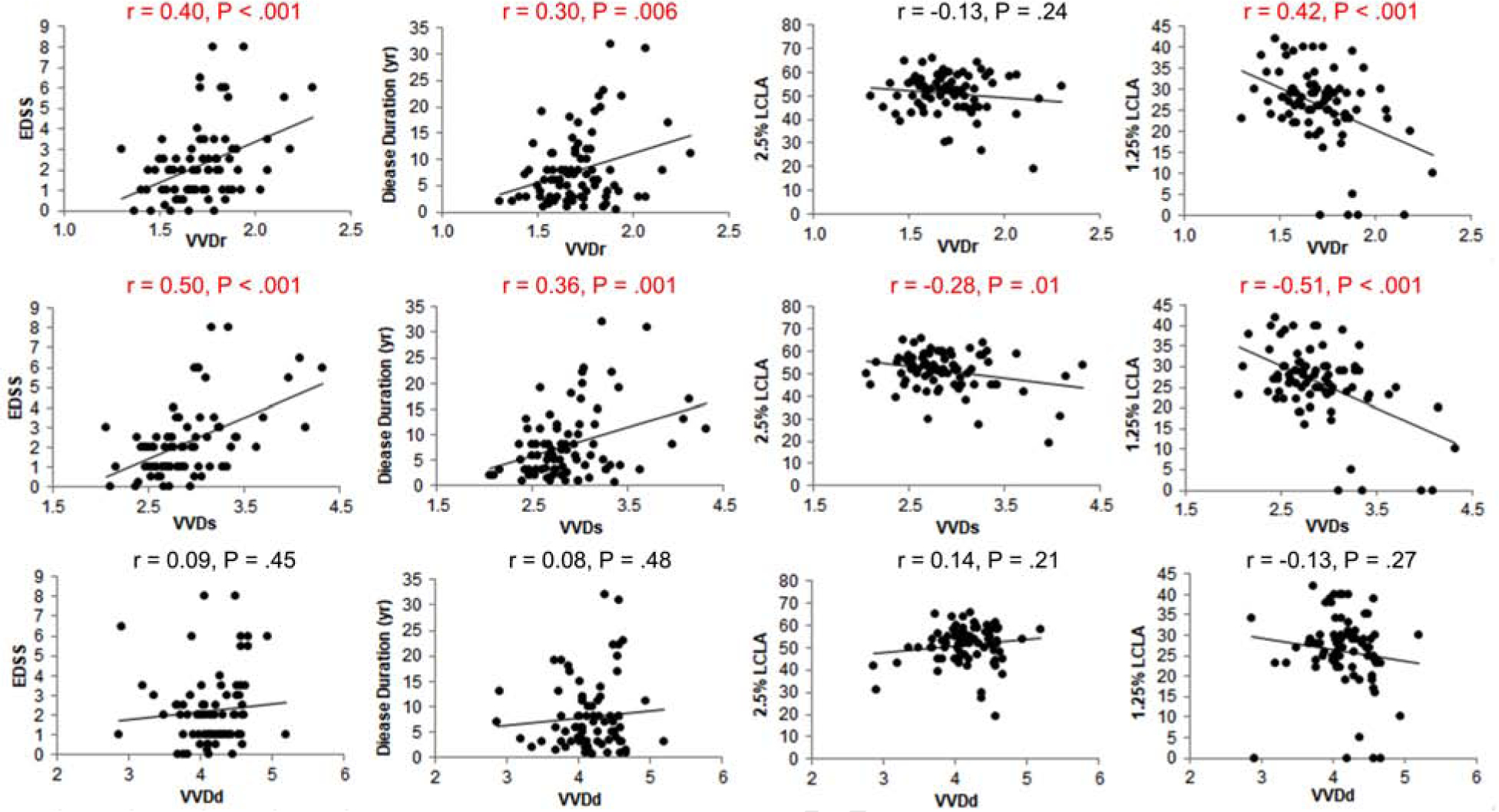
Both VVDr (Top row) and VVDs (Middle row) were positively related to EDSS and disease duration and negatively related to LCLA (P < .05), except for VVDr. However, VVDd (Bottom row) was not related to any of these clinical measurements.
Fig. 3. Relations between VVD and vessel densities and tissue volumes.
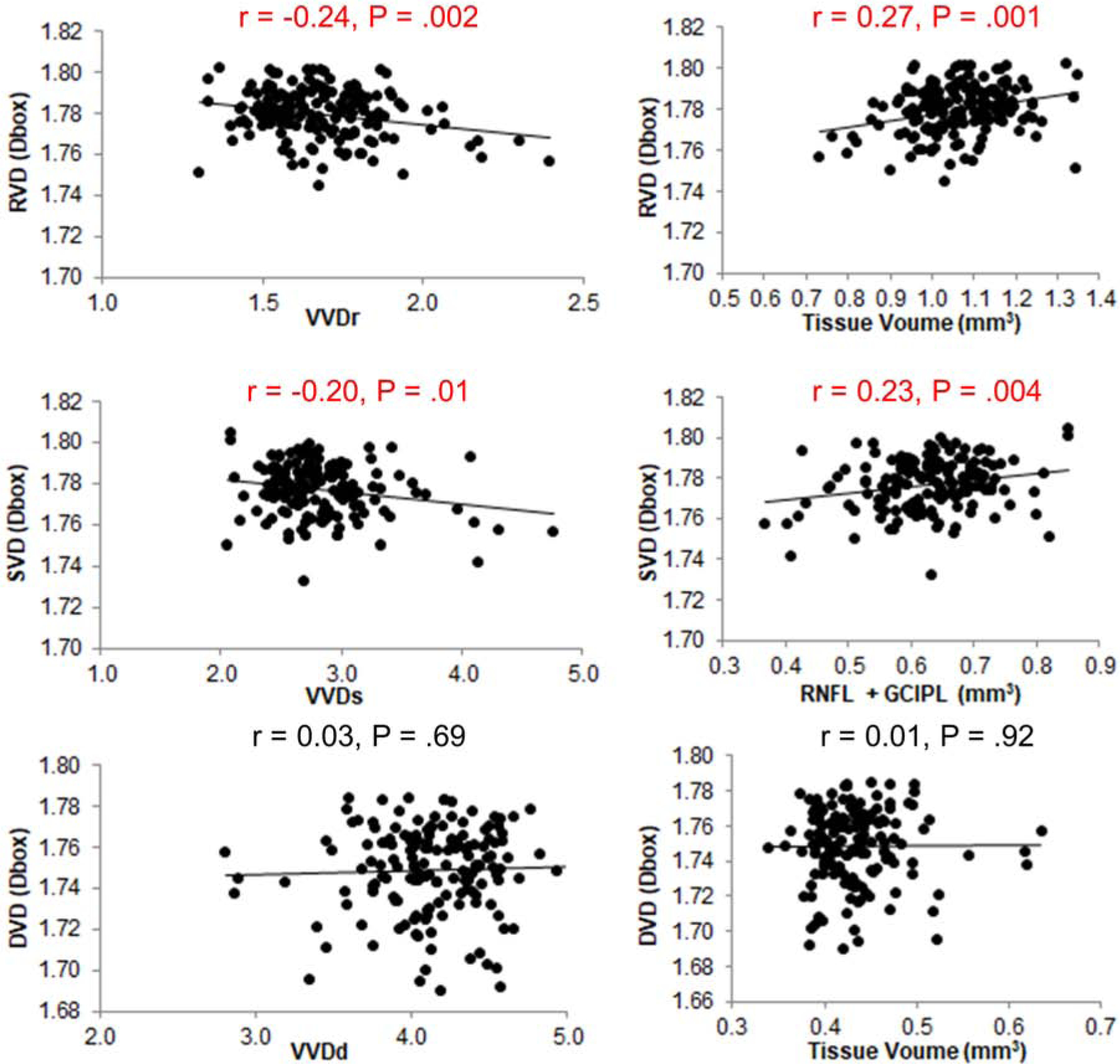
VVDr and VVDs were negatively related to RVD (Top left) and SVD (Middle left), respectively (P < .05). RVD (Top right) and SVD (Middle right) were positively related to their corresponding tissue volumes (P < .05). However, DVD was not related to VVDd (Bottom left) and the tissue volumes (Bottom right) (P > .05).
ROC analyses showed that the AUCs of the VVD were similar to the tissue volume measurements in differentiating MSON from HC and MSNON (all P < .05, Figure 4, Table 3). While the AUCs of the tissue volumes of the inner retina, combined RNFL and GCIPL, VVDr and VVDs ranged from 0.84 to 0.87 in discriminating MSON from HC, the AUCs of these parameters ranged from 0.78 to 0.80 in discriminating MSON from MSNON (all P < .05). The AUCs of all measurements except for DVD were from 0.58 to 0.65 in discriminating MSNON from HC (P < .05).
Fig. 4. The discrimination power.
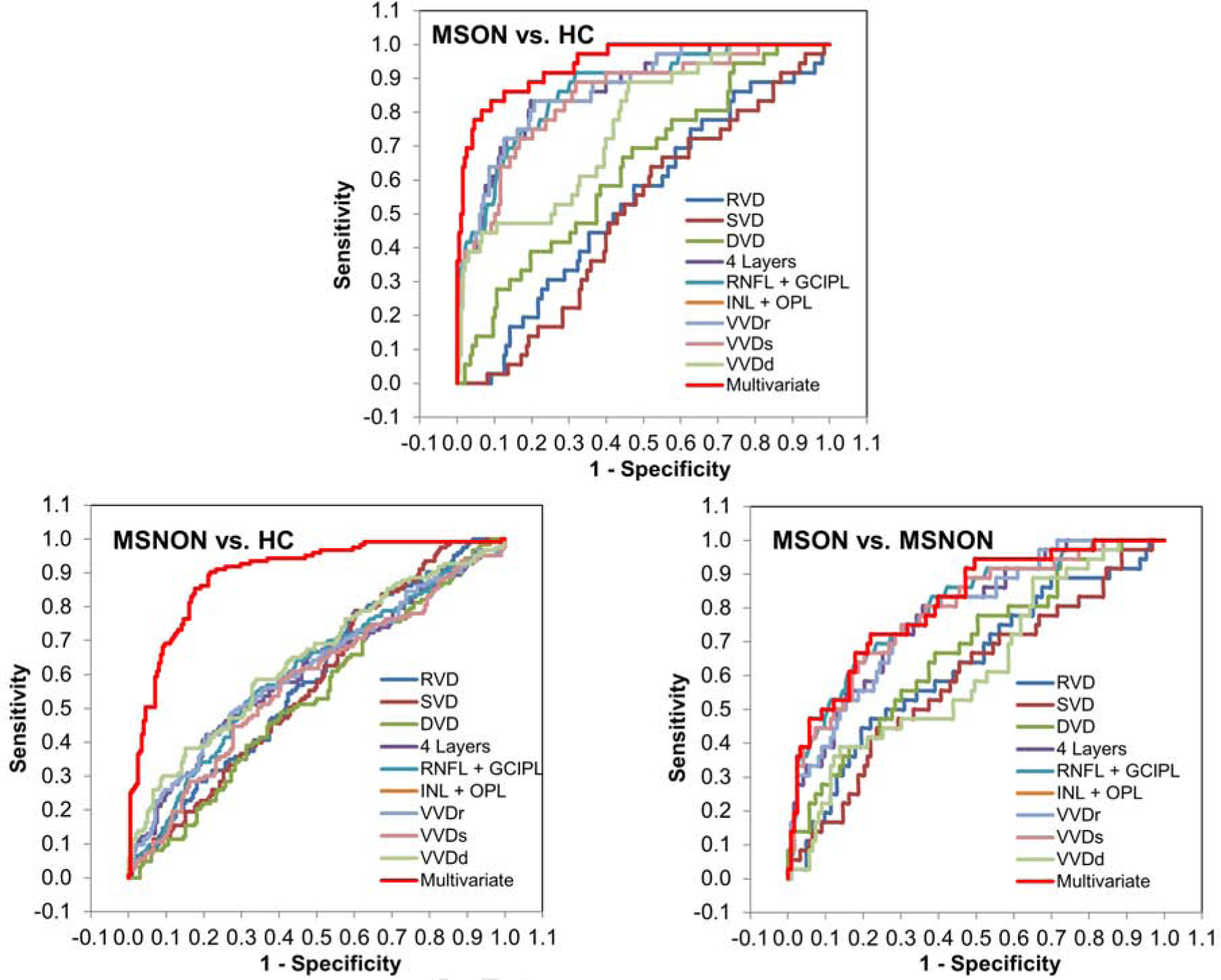
ROC analyses showed that the AUCs of the VVD were similar to the tissue volume measurements in differentiating MSON from HC (Top) and MSNON (Bottom right) (all P < .05). While the AUCs of the tissue volumes of the inner retina, combined RNFL and GCIPL, VVDr and VVDs ranged from 0.84 to 0.87 in discriminating MSON from HC. The AUCs of all measurements except for DVD were from 0.58 to 0.65 in discriminating MSNON from HC (Bottom left) (P < .05). The AUCs of these parameters ranged from 0.78 to 0.80 in discriminating MSON from MSNON (Bottom right) (all P < .05). With combined significant parameters determined using the stepwise regression, the multivariate AUC of VVDr, INL+OPL, VVDs, RVD and SVD was 0.94 in discriminating MSON from HC, which was significantly higher than any single AUC (Top) (all P < .05). The multivariate AUC of VVDd, INL+OPL, DVD and VVDs was 0.89 in discriminating MSNON from HC, which was significantly higher than any single AUC (Bottom left) (all P < .05). The multivariate AUC of RNFL+GCIPL and DVD was 0.81 in discriminating MSON from MSNON, which was higher than the single AUC of RVD, SVD, DVD, INL+OPL and VVDd (Bottom right) (P < .05). MSON: multiple sclerosis with a history of optical neuritis; MSNON: multiple sclerosis without a history of optical neuritis; HC: healthy control. RVD: vessel density (Dbox) of retinal vascular network; SVD: vessel density (Dbox) of superficial vascular plexus; DVD: vessel density (Dbox) of deep vascular plexus; RNFL: retinal nerve fiber layer; GCIPL: ganglion cell-inner plexiform layer; INL: inner nuclear layer; OPL: Outer plexiform layer.
Table 3:
Discrimination powers calculated as the area under the curve (AUC) and pair-wise comparisons
| MSON vs. HC | AUC (P Value) | RVD | SVD | DVD | 4 Layers | RNFL+GCIPL | INL+OPL | VVDr | VVDs | VVDd |
|---|---|---|---|---|---|---|---|---|---|---|
| RVD | 0.54 (P= .477) | |||||||||
| SVD | 0.50 (P= .972) | P= .133 | ||||||||
| DVD | 0.63 (P= .006) | P= .009 | P= .001 | |||||||
| 4 Layers | 0.87 (P< .001) | P< .001 | P< .001 | P< .001 | ||||||
| RNFL+GCIPL | 0.87 (P< .001) | P< .001 | P< .001 | P< .001 | P= .972 | |||||
| INL+OPL | 0.71 (P< .001) | P= .005 | P= .001 | P= .237 | P< .001 | P= .001 | ||||
| VVDr | 0.87 (P<.001) | P< .001 | P< .001 | P< .001 | P= .400 | P= .841 | P< .001 | |||
| VVDs | 0.84 (P< .001) | P< .001 | P< .001 | P< .001 | P=.113 | P= .001 | P= .004 | P= .072 | ||
| VVDd | 0.76 (P< .001) | P= .001 | P< .001 | P= .057 | P= .002 | P= .011 | P< .001 | P= .001 | P= .051 | |
| Multivariatea | 0.94 (P< .001) | P< .001 | P< .001 | P< .001 | P= .002 | P= .003 | P< .001 | P= .002 | P= .001 | P< .001 |
| MSNON vs. HC | AUC (P Value) | RVD | SVD | DVD | 4 Layers | RNFL+GCIPL | INL+OPL | VVDr | VVDs | VVDd |
| RVD | 0.59 (P= .007) | |||||||||
| SVD | 0.58 (P= .015) | P= .419 | ||||||||
| DVD | 0.54 (P= .282) | P= .054 | P= .127 | |||||||
| 4 Layers | 0.61 (P= .001) | P= .641 | P= .510 | P= .134 | ||||||
| RNFL+GCIPL | 0.61 (P= .001) | P= .681 | P= .545 | P= .161 | P= .868 | |||||
| INL+OPL | 0.59 (P= .007) | P= .945 | P= .795 | P= .238 | P= .328 | P= .603 | ||||
| VVDr | 0.61 (P= .001) | P= .557 | P= .429 | P= .100 | P= .169 | P= .649 | P= .222 | |||
| VVDs | 0.58 (P= .018) | P= .874 | P= .975 | P= .377 | P= .061 | P< .001 | P= .733 | P= .027 | ||
| VVDd | 0.65 (P< .001) | P= .189 | P= .129 | P= .011 | P= .072 | P= .225 | P< .001 | P= .112 | P= .036 | |
| Multivariateb | 0.89 (P< .001) | P< .001 | P< .001 | P< .001 | P< .001 | P< .001 | P< .001 | P< .001 | P< .001 | P< .001 |
| MSON vs. MSNON | AUC (P Value) | RVD | SVD | DVD | 4 Layers | RNFL+GCIPL | INL+OPL | VVDr | VVDs | VVDd |
| RVD | 0.63 (P= .015) | |||||||||
| SVD | 0.59 (P= .085) | P= .147 | ||||||||
| DVD | 0.67 (P= .001) | P= .312 | P= .063 | |||||||
| +4 Layers | 0.78 (P< .001) | P= .011 | P= .003 | P= .082 | ||||||
| RNFL+GCIPL | 0.80 (P< .001) | P= .003 | P= .001 | P= .028 | P= .169 | |||||
| INL+OPL | 0.62 (P= .023) | P= .889 | P= .697 | P= .489 | P< .001 | P< .001 | ||||
| VVDr | 0.78 (P< .001) | P= .016 | P= .005 | P= .097 | P= .506 | P= .132 | P< .001 | |||
| VVDs | 0.79 (P< .001) | P= .009 | P= .003 | P= .061 | P= .484 | P= .071 | P= .001 | P= .380 | ||
| VVDd | 0.62 (P= .027) | P= .836 | P= .762 | P= .456 | P< .001 | P< .001 | P= .384 | P< .001 | P= .001 | |
| Multivariatec | 0.81 (P< .001) | P< .001 | P< .001 | P= .002 | P= .270 | P= .701 | P< .001 | P= .250 | P= .460 | P< .001 |
MSON: multiple sclerosis with a history of optical neuritis; MSNON: multiple sclerosis without a history of optical neuritis; HC: healthy control. RVD: vessel density (Dbox) of retinal vascular network; SVD: vessel density (Dbox) of superficial vascular plexus; DVD: vessel density (Dbox) of deep vascular plexus; RNFL: retinal nerve fiber layer; GCIPL: ganglion cell-inner plexiform layer; INL: inner nuclear layer; OPL: Outer plexiform layer.
Multivariate: VVDr, INL+OPL, VVDs, RVD and SVD;
Multivariate: VVDd, INL+OPL, DVD, VVDs and VVDr.
Multivariate: RNFL+GCIPL and DVD.
With combined significant parameters determined using the stepwise regression, the multivariate AUC of VVDr, INL+OPL, VVDs, RVD and SVD was 0.94 in discriminating MSON from HC, which was significantly higher than any single AUC (all P < .05, Figure 4, Table 3). The multivariate AUC of VVDd, INL+OPL, DVD and VVDs was 0.89 in discriminating MSNON from HC, which was significantly higher than any single AUC (all P < .05). The multivariate AUC of RNFL+GCIPL and DVD was 0.81 in discriminating MSON from MSNON, which was higher than the single AUC of RVD, SVD, DVD, INL+OPL and VVDd (P < .05).
DISCUSSION
To the best of our knowledge, this is the first study to determine the retinal VVD in patients with RRMS. Because MS is a chronic inflammatory disease, alterations of vessel density in concert with tissue loss may more pertinently represent the vascular alterations and their interaction with the perfused tissue. The present study provides strong evidence that the changes in the microvasculature coexist and are related to neurodegeneration, resulting in the increased VVD. Most importantly, VVDr and VVDs showed an increased trend from MSNON to MSON, and were related to disability (i.e., EDSS) and visual function (i.e., LCLA).
The increases VVD in and of VVD in all the MS eyes in the present study could be the result of diffuse chronic inflammation and related angiogenesis.34,35 Widespread and subtle inflammation in MS has been confirmed by histopathologic studies in both the brain36 and retina.37 In addition, angiogenesis, the formation of new vessels, is found in MS demyelinating lesions.34,35 In addition to inflammation, the increased VVD could be due to a compensatory response to retinal tissue hypoperfusion.19 Tissue hypoperfusion can impair tissue oxygenation38 and induce hypoxia-like changes.7–9 Such hypoxia can form increased microvascular density39 through the increased levels of Vascular Endothelial Growth Factor (VEGF) release and the production of several other angiogenic molecules. In that case, an overshoot of vascular density can lead to a structure with a higher vascular density.40
In contrast to the clear trend toward increased VVD in patients with MS in the present study, alterations of VD showed variations in both directions (i.e., increased or decreased) in previous studies, resulting in a lack of consensus in the literature (Table 2).12–14,41 Although this could be due to different study cohorts with different disease severity, it may also be due to the lack of consideration of the effect of intraretinal layer thinning. As mentioned above, inflammation can result in angiogenesis. Indeed, increased VD at foveal areas in patients with MS, including eyes with a history of ON has been reported, albeit the parafoveal VD was lower compared to normal controls.12 Interestingly, at the one-year follow-up of the same group of patients,13 parafoveal VD was increased, which was explained as an improvement of the disease condition.13 In contrast, no significant difference of parafovea VD between MSNON and HC was reported by Feucht et al.14 In the present study, the trend of increased VVD (HC to MSNON to MSON) coexisting with the decreased tissue volume (HC to MSNON to MSON) may indicate that the microvascular networks appear as the collective outcome of a myriad of responses to tissue functional demands and/or diseased conditions.42
Table 2.
Macular vascular measurement in patients with MS
| Author | Date | Device | Scan (mm) | Area (mm) | Cohort (subject) | Cohort (Eye) | Measurement | Key findings |
|---|---|---|---|---|---|---|---|---|
| Jiang et al. | present | Zeiss Angioplex | 3×3 | 0.6–2.5 | MSNON=48 | MSNON = 123 | RVD* | Increased in MSNON, decreased in MSON compared to MSNON |
| Annulus | MSON=32 | MSON = 36 | SVD* | Increased in MSNON compared to HC | ||||
| HC=99 | HC=198 | DVD* | Decreased in MSON compared to MSNON and HC | |||||
| VVDr* | Increased in MSNON and MSON | |||||||
| VVDs* | Increased in MSON | |||||||
| VVDd* | Increased in MSNON and MSON | |||||||
| Feucht et al.14 | 2019 | Optovue Angiovue | 6×6 | 3×3 | MSNON=25 | MSNON = 62 | SVD | NS between MSNON vs. HC, Decrease in MSON |
| MSON=17 | MSON = 21 | DVD | NS between MSNON vs. HC, Decrease in MSON | |||||
| HC=50 | HC=100 | VD of Choriocapillaries | NS among groups | |||||
| Lanzillo et al.12 | 2018 | Optovue Angiovue | 6×6 | 3×3 | MSNON=27 | MSNON = 77 | SVD | Decreased parafoveal SVD in MSNON/MSON vs. HC |
| 6×6 | MSON=23 | MSON = 23 | Increased foveal SVD in MSNON/MSON vs. HC | |||||
| HC=46 | HC=92 | |||||||
| Lanzillo et al.13 | 2019 | Optovue Angiovue | 6×6 | 3×3 | MSNON=27 | MSNON = 77 | SVD | Parafoveal increase in MSNON/MSON vs. HC after 1 year follow-up |
| 6×6 | MSON=23 | MSON = 23 | ||||||
| HC=46 | HC=92 | |||||||
| Wang et al.41 | 2014 | Prototype SS-OCT | 3×3 | 0.6– 2.6 | MSNON=25 | MSNON = 38 | FI | NS among groups. |
| Annulus | MSON=10 | MSON = 14 | ||||||
| HC=21 | HC=21 |
MSNON: multiple sclerosis without history of optic neuritis; MSON: multiple sclerosis with history of optic neuritis; HC: healthy control; RVD: vessel density of the retinal vascular network; SVD: vessel density of the superficial vascular plexus; DVD: vessel density of the deep vascular plexus; VVDr: volumetric vessel density in the RVN; VVDs: volumetric vessel density in the SVP; VVDd: volumetric vessel density in the DVP; SS-OCT: swept light source optical coherence tomography; NS: not significant.
based on fractal dimension (Dbox) for VD and VVD.
More intriguingly, increased VVD appeared to be more clinically relevant to EDSS, disease duration and visual function compared to VD. The VVDs was positively correlated with disease duration and EDSS but negatively related to LCLA. In other words, the increased VVD was associated with poorer visual function, longer disease duration and worse neurologic disability. One possible reason could be that VVDs represent microvessel structure and blood flow distribution in the retinal neuronal tissue (RNFL + GCIPL). Increased vessel density (the numerator) and decreased neuronal tissue volume (the denominator) both contribute to the rising VVD, especially VVDs, while the disease progresses. Furthermore, the increased VVD appeared to be mostly attributed by the changes in tissue volume, not the fluctuation of vessel density, which was supported by the ROC analyses. The discrimination powers of the VVDr and VVDs were similar to their corresponding tissue volumes. On the other hand, in eyes with MSON, the vessel density of the DVP and the tissue volume of INL + OPL were both decreased. The increase of VVDd indicates that the change of tissue volume carried heavier weight in contributing to the change of VVD. Another explanation could be that tissue loss is one-directional (i.e., no neural re-generation), while the change of vessel density could be a two-way direction. This is supported by the finding of higher vessel density in MSNON in the SVP and lower vessel density in MSON compared to HC. In contrast, VVD showed an ascending trend from HC to MSNON to MSON. Therefore, VVD measurements, especially VVDr and VVDs, could be a good candidate for development into an imaging biomarker for vascular tissue interaction and disease progression.
Furthermore, the good discrimination powers of VVDr and VVDs may also facilitate better diagnosis of MSON and MSNON. Although AUCs of the VVD measurements did not outbid the structural measurements, vascular measurements appeared to add valuable diagnostic information on retinal vascular changes in patients with MS. The outstanding multivariate AUCs provided excellent performances compared to the single parameter. This may indicate both vascular and structural parameters, representing different features in the neurovascular system, contribute to the improved discrimination powers.
Because the two-way fluctuation of VD occurs in patients with MS, the relationship between retinal VD and clinical manifestations has not been well established,11,12,14,41 which may prevent VD from being developed as an imaging biomarker for disease progression and treatment efficacy. Additionally, previous measurements of vessel density based on OCTA were performed without the consideration of the tissue volume supplied by the vascular network.11,12,14,41 In the event of decreased tissue volume in RNFL and GCIPL, the vessel density per tissue cube may not decrease in terms of volumetric vessel density. Indeed, the en face view of OCTA is based on the vessels (with fast or slow blood flow in the arterioles, venules, and capillaries) of the volumetric tissue of interest where the vessels are segmented. Only counting the vessel density may not accurately reflect the vessel density in the given tissue volume, which is also altered in MS. This may explain why these previous studies failed to establish a relationship between retinal vessel density and clinical manifestation.11,12,14,41 Lanzillo et al. established a relationship between retinal vessel density and EDSS (r = −0.27) but not multiple sclerosis severity scores (MSSS) or disease duration.12 In contrast, relationships between VVDr and clinical manifestations (EDSS, disease duration and LCVA) were established in the present study, indicating that this parameter may be better as a disease-specific biomarker in determining changes in the retinal tissue and vasculature in MS. Feucht 201914Lanzillo 201812Lanzillo 201913Wang X, 201441
There are several limitations to the present study. First, no longitudinal studies were conducted to determine VVD over time, which prevents the study from interpreting whether neurodegeneration or vascular impairment occurs first. However, this is the first attempt to understand retinal vessel density changes concerning the tissue volume by a cross-sectional study with a relatively larger sample size. Second, cerebral perfusion was not measured in the same study cohort, and the link between the eye and brain in tissue perfusion cannot be established. Third, we used fractal analysis to represent the vessel density of microvessels, while previous studies used vessel density (vessel coverage) in percentage by relying on the portion of the pixels of the vessels, including the large vessels.12,14,41 We removed the large vessels and only analyzed the microvessels, while the previous studies analyzed all vessels, including the large vessels. In addition, we used fractal analysis of skeletonized vessel images to estimate the vessel density (i.e., how many vessels per unit area), while previous studies used commercial algorithms to calculate the vessel density on how large the area covered by the vessels including large and small vessels.12–14 The differences in the methods of analyzing vessel density prevent a direct comparison between our study and previous studies.12,14,41 Currently, the commercial algorithms to calculate the vessel density in the Zeiss OCTA device is not available and we were not able to compare our fractal measurement with these commercial density metrics. Further studies to compare the fractal dimension and vessel density based on the commercial density measurements are needed. Fourth, a previous study by Bhaduri et al. demonstrated both vessel numbers and diameters of the large retinal vessels in the peripapillary region were related to MS severity (not a history of ON).43 We did not measure vessel diameters of the small vessels in the macular area. While the alteration of the fractal dimension indicates the changes in vessel numbers, whether the small vessels become narrower remains unknown. Fifth, the variation of the VD was rather small, possibly due to the two-way fluctuation. Considering the measurement repeatability (~2%) reported previously,33 the measurement of the VD may not be practically translated into the clinic. In contrast, the one-way variation of the VVD ranged from 9% to 19%, which may be further developed into a marker for the clinic. However, further measurements of the repeatability of VVD are needed. Lastly, it may be worth noting that different fields of view were used in previous studies.12,14,41 Although the scanning areas may not be a key factor in explaining the discrepancies of the findings, studying the right area may help to determine pathologically and clinically meaningful markers for tracking disease progression. Several studies used a large field of view (6 × 6 mm) for OCTA scanning (304 A-scan per B-scan × 304 B-scan by Optovue system),12–14 resulting in the pixel interval of 20 μm, which may not be sufficient to image capillaries (with vessel width ~10 μm). Interestingly, these previous studies mainly analyzed retinal vasculature in a field of view of 3 mm in diameter, possibly due to the capability of the inherent analysis software in their OCT systems.12–14 In contrast, a previous study using swept light source OCT (fast scanning rate) used 3 × 3 mm as the field of view to study retinal microvasculature in patients with MS,41 which provided better resolution in resolving the angiograph of capillaries. This field of view is similar to the setting in the present study. Our scan pattern with a field of view of 3 × 3 mm provides a scan interval of ~10 μm between 2 A-scans, which may better resolve the capillaries.
In summary, this is the first study to reveal increased retinal VVD in patients with RRMS. The changes of VVD in the RVN and SVP are related to disability and visual function, which may be able to be developed as an image marker for tracking disease progression.
Supplementary Material
ACKNOWLEDGMENTS
-
Funding / Support
Grant/financial support: The work has been supported by the National Multiple Sclerosis Society (RG-1506-04890), NIH Center Grant P30 EY014801, and a grant from Research to Prevent Blindness (RPB).
-
Financial Disclosures
Dr. Gregori has research support from Carl Zeiss Meditec. All other authors have no proprietary interest in any materials or methods. All other authors have proprietary interest in any materials or methods. Dr. Delgado received honoraria for consulting service from Novartis (Ad Board Meeting).
-
Other Acknowledgments
Design of the study (HJ, YL, SD, JW); data collection (HJ, GRG, YL, YL, HJ, YD, GC, SD, JW); analysis and interpretation of the data (HJ, GRG, YL, HJ, YL, YD, GC, SD, JW); preparation, review and approval of the manuscript (HJ, GRG, YL, YL, HJ, YD, GC, SD, JW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herranz E, Gianni C, Louapre C, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol 2016;80(5):776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawlitzki M, Neumann J, Kaufmann J, et al. Loss of corticospinal tract integrity in early MS disease stages. Neurol Neuroimmunol Neuroinflamm 2017;4(6):e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azevedo CJ, Overton E, Khadka S, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol Neuroinflamm 2015;2(3):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’haeseleer M, Hostenbach S, Peeters I, et al. Cerebral hypoperfusion: a new pathophysiologic concept in multiple sclerosis? J Cereb Blood Flow Metab 2015;35(9):1406–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapointe E, Li DKB, Traboulsee AL, Rauscher A. What Have We Learned from Perfusion MRI in Multiple Sclerosis? AJNR Am J Neuroradiol 2018;39(6):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monti L, Morbidelli L, Rossi A. Impaired Cerebral Perfusion in Multiple Sclerosis: Relevance of Endothelial Factors. Biomark Insights 2018;13:1177271918774800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol 2009;8(3):280–291. [DOI] [PubMed] [Google Scholar]
- 8.Juurlink BH. The multiple sclerosis lesion: initiated by a localized hypoperfusion in a central nervous system where mechanisms allowing leukocyte infiltration are readily upregulated? Med Hypotheses 1998;51(4):299–303. [DOI] [PubMed] [Google Scholar]
- 9.D’haeseleer M, Cambron M, Vanopdenbosch L, De Keyser J. Vascular aspects of multiple sclerosis. Lancet Neurol 2011;10(7):657–666. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Delgado S, Tan J, et al. Impaired retinal microcirculation in multiple sclerosis. Mult Scler 2016;22(14):1812–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Delgado S, Liu C, et al. In vivo characterization of retinal microvascular network in multiple sclerosis. Ophthalmology 2016;123(2):437–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanzillo R, Cennamo G, Criscuolo C, et al. Optical coherence tomography angiography retinal vascular network assessment in multiple sclerosis. Mult Scler 2018;24(13):1706–1714. [DOI] [PubMed] [Google Scholar]
- 13.Lanzillo R, Cennamo G, Moccia M, et al. Retinal vascular density in multiple sclerosis: a 1-year follow-up. Eur J Neurol 2019;26(1):198–201. [DOI] [PubMed] [Google Scholar]
- 14.Feucht N, Maier M, Lepennetier G, et al. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult Scler 2019;25(2):224–234. [DOI] [PubMed] [Google Scholar]
- 15.Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2017;16(10):797–812. [DOI] [PubMed] [Google Scholar]
- 16.Nolan RC, Akhand O, Rizzo JR, Galetta SL, Balcer LJ. Evolution of Visual Outcomes in Clinical Trials for Multiple Sclerosis Disease-Modifying Therapies. J Neuroophthalmol 2018;38(2):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 2007;69(16):1603–1609. [DOI] [PubMed] [Google Scholar]
- 18.Villoslada P, Sepulcre J, Toledo J, Bejarano B. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 2008;71(21):1747–1748. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Jiang H, Liu Y, et al. Age-Related Alterations in Retinal Tissue Perfusion and Volumetric Vessel Density. Invest Ophthalmol Vis Sci 2019;60(2):685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell JP, Zhang M, Hwang TS, et al. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep 2017;7:42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balcer LJ, Baier ML, Cohen JA, et al. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology 2003;61(10):1367–1373. [DOI] [PubMed] [Google Scholar]
- 23.Wei Y, Jiang H, Shi Y, et al. Age-Related Alterations in the Retinal Microvasculature, Microcirculation, and Microstructure. Invest Ophthalmol Vis Sci 2017;58(9):3804–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao Y, Jiang H, Wei Y, et al. Visualization of Focal Thinning of the Ganglion Cell-Inner Plexiform Layer in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis 2018;64(4):1261–1273. [DOI] [PubMed] [Google Scholar]
- 25.Gameiro GR, Jiang H, Liu Y, et al. Retinal tissue hypoperfusion in patients with clinical Alzheimer’s disease. Eye and Vision 2018;5(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H, Wei Y, Shi Y, et al. Altered Macular Microvasculature in Mild Cognitive Impairment and Alzheimer Disease. J Neuroophthalmol 2018;38(3):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography in normals and diabetic retinopathy patients. Retina 2015;35(11):2353–2363. [DOI] [PubMed] [Google Scholar]
- 28.Grauslund J, Green A, Kawasaki R, Hodgson L, Sjolie AK, Wong TY. Retinal vascular fractals and microvascular and macrovascular complications in type 1 diabetes. Ophthalmology 2010;117(7):1400–1405. [DOI] [PubMed] [Google Scholar]
- 29.Hu R, Ding C. Quantification of Vessel Density in Retinal Optical Coherence Tomography Angiography Images Using Local Fractal Dimension. Invest Ophthalmol Vis Sci 2016;57(4):2262. [DOI] [PubMed] [Google Scholar]
- 30.Ong YT, De Silva DA, Cheung CY, et al. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke 2013;44(8):2121–2127. [DOI] [PubMed] [Google Scholar]
- 31.Talu S Multifractal geometry in analysis and processing of digital retinal photographs for early diagnosis of human diabetic macular edema. Curr Eye Res 2013;38(7):781–792. [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Debuc DC, Rundek T, et al. Automated segmentation and fractal analysis of high-resolution non-invasive capillary perfusion maps of the human retina. Microvasc Res 2013;89:172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Wang J, Jiang H, et al. Retinal Microvasculature Alteration in High Myopia. Invest Ophthalmol Vis Sci 2016;57(14):6020–6030. [DOI] [PubMed] [Google Scholar]
- 34.Girolamo F, Coppola C, Ribatti D, Trojano M. Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol Commun 2014;2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk S, Frank JA, Karlik S. Angiogenesis in multiple sclerosis: is it good, bad or an epiphenomenon? J Neurol Sci 2004;217(2):125–130. [DOI] [PubMed] [Google Scholar]
- 36.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009;132(Pt 5):1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 2010;133(Pt 6):1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks DJ, Beaney RP, Powell M, et al. Studies on cerebral oxygen metabolism, blood flow, and blood volume, in patients with hydrocephalus before and after surgical decompression, using positron emission tomography. Brain 1986;109 (Pt 4):613–628. [DOI] [PubMed] [Google Scholar]
- 39.Reglin B, Secomb TW, Pries AR. Structural adaptation of microvessel diameters in response to metabolic stimuli: where are the oxygen sensors? Am J Physiol Heart Circ Physiol 2009;297(6):H2206–H2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pries AR, Secomb TW. Making microvascular networks work: angiogenesis, remodeling, and pruning. Physiology (Bethesda) 2014;29(6):446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Jia Y, Spain R, et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol 2014;98(10):1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Secomb TW, Pries AR. Microvascular Plasticity: Angiogenesis in Health and Disease--Preface. Microcirculation 2016;23(2):93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhaduri B, Nolan RM, Shelton RL, et al. Detection of retinal blood vessel changes in multiple sclerosis with optical coherence tomography. Biomed Opt Express 2016;7(6):2321–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


