SUMMARY
Adipocyte progenitors (APs) express platelet-derived growth factor receptors, PDGFRα and PDGFRβ. Elevated PDGFRα signaling inhibits adipogenesis and promotes fibrosis, however the function of PDGFRs in APs remains unclear. We combined lineage tracing and functional analyses in a sequential dual-recombinase approach that creates mosaic Pdgfr-mutant cells by Cre/lox recombination with a linked Flp/frt reporter to track individual cell fates. Using mosaic lineage labeling, we show that adipocytes are derived from the Pdgfra lineage during postnatal growth and adulthood. In contrast, adipocytes are only derived from the mosaic Pdgfrb lineage during postnatal growth. Functionally, postnatal mosaic deletion of PDGFRα enhances adipogenesis and adult deletion enhances β3-adrenergic receptor induced beige adipocyte formation. Mosaic deletion of PDGFRβ also enhances white, brown and beige adipogenesis. These data show both PDGFRs are cell-autonomous inhibitors of adipocyte differentiation and implicate downregulation of PDGF signaling as a critical event in the transition from AP to adipocyte.
Graphical Abstract
eTOC Blurb
Platelet-derived growth factor receptors are markers for adult mesenchymal progenitor cells. Olson et al. analyze mice with induced functional genetic mosaicism for Pdgfra or Pdgfrb and demonstrate that both receptors cell-autonomously inhibit adipocyte differentiation, which implicates downregulation of PDGF signaling as a critical event in adipogenesis.
INTRODUCTION
Adipocyte progenitors (APs) are a mesenchymal cell type that resides in connective tissues throughout the body with potential to differentiate into different kinds of adipocytes or myofibroblasts. White adipocytes (WAs) are specialized to store lipid. Brown and beige adipocytes (BAs and bAs) convert lipid stores into heat. Platelet-derived growth factor (PDGF) receptors PDGFRα and PDGFRβ are markers for APs, but they are also expressed on other cells. PDGFRα is expressed in embryonic neural crest and mesoderm and remains highly expressed in many adult fibroblastic cells and mesenchymal progenitors. PDGFRβ is expressed in some of the same embryonic and adult cells as PDGFRα, and also highly expressed in mural cells including vascular smooth muscle cells (VSMCs) and pericytes (Andrae et al., 2008; Armulik et al., 2011; Hoch and Soriano, 2003). Several questions remain about PDGFR expression in APs, as different studies have emphasized one receptor or the other as an AP marker. There is agreement that the Pdgfra-lineage includes most or all types of APs (Berry and Rodeheffer, 2013; Gao et al., 2018; Lee et al., 2015; Lee et al., 2012). In contrast, studies of the Pdgfrb-lineage reached different conclusions. The first study to implicate PDGFRβ in the adipocyte lineage used Pdgfrb-Cretg to label WAs from development through postnatal day 30 (P30) (Tang et al., 2008). Since PDGFRβ is a well-known marker for mural cells, this aligns with the expectation that blood vessels are a niche for APs (Nishimura et al., 2007). However, a different Pdgfrb-Cretg did not label WAs in development or in young mice and only labeled them in mice older than 8 weeks of age (Gao et al., 2018). Inducible systems have also been tested: tamoxifen-inducible Pdgfrb-CreERtg labeled embryonic APs (Hong et al., 2015), and a doxycycline inducible Pdgfrb-rtTA;TRE-Cre approach labeled adult WAs in response to long-term high fat diet (HFD) (Vishvanath et al., 2016). The Pdgfrb-lineage studies have all used different transgenes for lineage tracing, which may underlie different results. Single-cell RNA sequencing has emphasized that APs are heterogeneous and may express both PDGFRs (Burl et al., 2018; Hepler et al., 2018; Merrick et al., 2019; Schwalie et al., 2018). A parallel comparison of the Pdgfra- and Pdgfrb-lineages using a consistent genetic approach would improve understanding of AP identity.
Aside from Pdgfr expression and lineage analysis, an important question relates to the in vivo function of PDGFRs in mesenchymal progenitors. PDGF regulates cell proliferation, migration, and differentiation by binding to its receptors. This leads to dimerization and autophosphorylation on tyrosine residues in the cytoplasmic domain, which triggers recruitment of kinases and adaptor proteins that activate downstream signaling pathways (Heldin and Westermark, 1999). Gain-of-function mutation of mouse PDGFRα causes APs to become myofibroblast-like cells that secrete ECM and generate fibrosis in adipose tissue (Iwayama et al., 2015). A similar mutation in mouse PDGFRβ also causes adipose tissue fibrosis in some contexts (He et al., 2017). It is not clear if PDGF signaling blocks adipogenesis cell-autonomously by inhibiting AP differentiation into fat cells or indirectly via production of profibrotic ECM. It is intriguing that PDGFRs are downregulated during adipocyte differentiation as this may suggest an important function for PDGFR in the transition from progenitor to mature adipocyte. It remains to be tested whether PDGFR loss-of-function might increase adipogenesis.
The complicated expression pattern of PDGFRα/PDGFRβ and the potential for confounding cell non-autonomous effects (like fibrosis) make delineation of in vivo functions very challenging. Here, we created new conditional knockin mice that allow Cre/lox-mediated mutation of Pdgfra or Pdgfrb with genetically linked Flp/frt-mediated lineage labeling. This dual-recombinase system labels single mutant cells and their progeny with a fluorescent reporter. Utilizing this system, we perform lineage labeling to compare the adipogenic potential of the Pdgfra and Pdgfrb cell lineages during embryonic, postnatal, and young adult growth. We also used this system to perform mosaic mutant analysis, which allowed us to investigate the in vivo function of PDGFRα/PDGFRβ in adipogenesis across different fat depots while minimizing confounding indirect effects of widespread mutation.
RESULTS
Characterization of Conditional PDGFR-Flp Knockin Mice
The genetic mosaic approach generates mutations in a few scattered cells while leaving most cells in the tissue unchanged. For this to succeed, it is crucial to unambiguously distinguish rare mutant cells from non-mutant cells. To ensure that only mutant cells acquire a lineage label, we generated mice to combine the Cre/lox and Flp/frt recombination systems in a sequential dual-recombinase approach. Here, Cre is used to create mutations and permit expression of an optimized Flp recombinase (Flpo) (Raymond and Soriano, 2007) from the mutant allele, which activates a Tomato reporter (Fig. 1a). This configuration creates a genetic link between gene mutation and cell labeling. Here we describe three new knockin alleles. The Pdgfra conditional loss-of-function allele is called aR-Flp and expresses PDGFRα protein; following Cre recombination it becomes aR-ΔFlp and expresses Flpo (Fig. 1a’). Similarly, the Pdgfrb conditional loss-of-function allele, called bR-Flp, switches from PDGFRβ expression to Flpo expression (bR-ΔFlp; Fig. 1a’’). The Pdgfra conditional gain-of-function allele, called aR-K.Flp, is silent in the absence of Cre, and switches to express PDGFRαK and Flpo following Cre recombination (Fig. 1a’’’). K denotes constitutively active PDGFRαK, which has a D842V mutation in the kinase domain (Heinrich et al., 2003; Olson and Soriano, 2009). See Supplementary Figure 1 and the Methods section for detailed information regarding targeting vectors. Correctly targeted ES cell clones were used to generate chimeras for germline transmission of the knockin alleles.
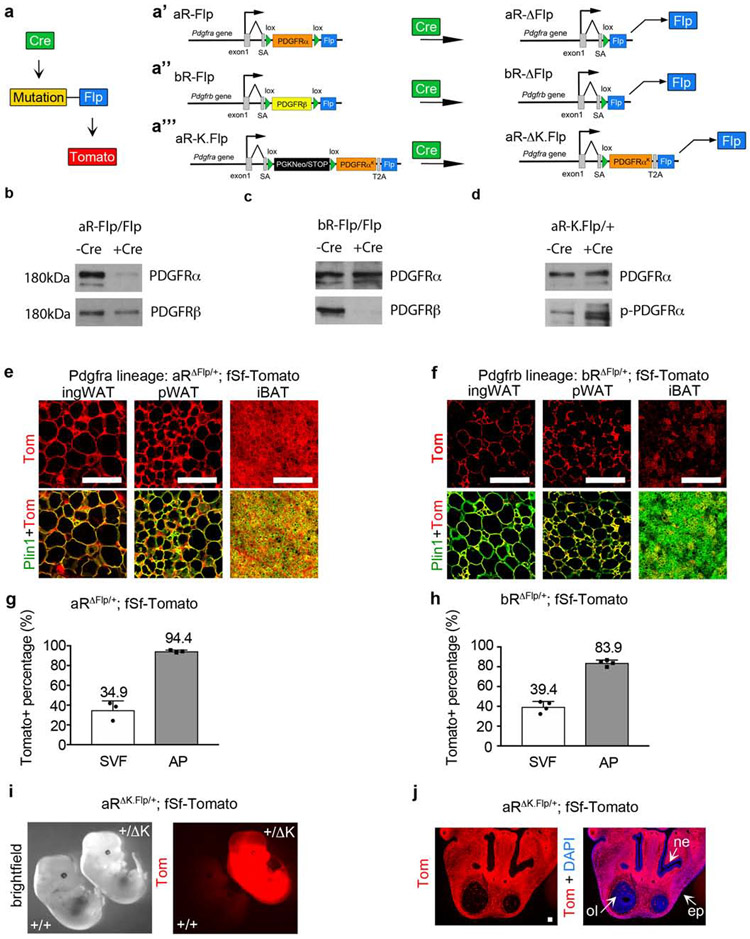
Figure 1. PDGFR-Flp mice create conditional Pdgfra/Pdgfrb mutations with genetically linked lineage tracing.
(a) The sequential dual-recombinase approach: Cre induces a mutation and Flp expression, and Flp induces Tomato expression. Flp is controlled by the endogenous Pdgfra or Pdgfrb promoters and is linked to a conditional Pdgfr mutation: (a’) aR-Flp becomes aR-ΔFlp. (a’’) bR-Flp becomes bR-ΔFlp. (a’’’) aR-K.Flp becomes aR-ΔK.Flp. (b-d) Primary dermal fibroblasts were infected with Cre-expressing lentivirus or -Cre control, followed by Western blot: (b) Blot from aRFlp/Flp cells, showing Cre-dependent deletion of PDGFRα. (c) Blot from bRFlp/Flp cells, showing Cre-dependent deletion of PDGFRβ. (d) Blot from aRK.Flp/+ cells, showing Cre-dependent increased phosphorylation of PDGFRα. (e and f) Lineage tracing of embryonic Pdgfra- (e) or Pdgfrb- (f) lineages in inguinal WAT, perigonadal WAT, or interscapular BAT at P21, by immunofluorescence for Tomato and Perilipin-1. (g and h) Flow cytometry of Tomato+ cells in the stromal vascular fraction (SVF) or adipocyte progenitor (AP) fraction at P21, with AP defined as Lin− (CD31− CD45− Ter119−), CD29+, CD34+. Tomato+ % in each fraction is shown + SD: (n = 3 or 4). (i) aR+/+;fSf-Tomato or aRΔK.Flp/+;fSf-Tomato embryos at E13.5. (j) Lineage tracing of the PDGFRαK-lineage at E13.5 in the frontonasal prominence by Tomato epifluorescence with DAPI. Tomato is expressed in mesenchymal cells but is absent from epithelial and neuronal cells: nasal epilthelium (ne), olfactory lobe (ol) and epidermis (ep). All Scale bars: 100μm.
Detailed descriptions of the conditional alleles (a’-a’’’) are shown in Supplementary Fig. 1.
Western blots from adult tissue and embryos are shown in Supplementary Fig. 2.
Absence of Tomato in Cre-naïve mice is shown in Supplementary Fig. 3.
Representative FACS plots and controls for g and h are shown in Supplementary Fig. 4.
Cre-naïve PdgfraFlp/Flp (aRFlp/Flp), PdgfrbFlp/Flp (bRFlp/Flp), and PdgfraK.Flp/+ (aRK.Flp/+) mice were viable and phenotypically normal. They expressed PDGFR proteins at a level similar to wild type or slightly reduced in the case of aRK.Flp/+ (Supplementary Fig. 2a-c). This indicates that PDGFRα and PDGFRβ cDNA knockins can substitute for the endogenous Pdgfra and Pdgfrb genes in most situations (Klinghoffer et al., 2001). Sox2-Cretg expresses Cre in the epiblast and globally targets all cell lineages including the germline. By crossing Sox2-Cre with aRFlp/Flp or bRFlp/Flp mice, we generated aRΔFlp/+;Sox2-Cre and bRΔFlp/+;Sox2-Cre mice where one Pdgfr allele is replaced by Flp (Supplementary Fig. 2d,e). Backcrossing resulted in aRΔFlp/ΔFlp or bRΔFlp/ΔFlp embryos that do not survive (Soriano, 1994, 1997). To verify that PDGFRα/PDGFRβ protein is eliminated, we used timed-pregnancies to obtain aRΔFlp/ΔFlp embryos at E11.5 and bRΔFlp/ΔFlp fetuses at E17.5. Tissue lysates showed complete absence of PDGFRα or PDGFRβ in the respective homozygotes compared to heterozygotes (Supplementary Fig. 2g,h). PDGFRα was hyperphosphorylated in aRΔK.Flp/+;Sox2-Cre embryos at E13.5 (Supplementary Fig. 2f,i). Cre-dependence was also shown in vitro by transduction of Cre-expressing virus or control virus into dermal fibroblasts from aRFlp/Flp, bRFlp/Flp, or aRΔK.Flp/+ mice, followed by Western blotting (Fig. 1b-d).
Lineage Labeling with Pdgfr-Flp Knockin Mice
The aRΔFlp/+ and bRΔFlp/+ mice provided an opportunity to trace the fates of embryonic PDGFRα+ and PDGFRβ+ cells by crossing with the R26-frt.Stop.frt-tdTomato reporter (denoted as fSf-Tomato). We verified that Cre-naïve aRFlp/Flp, bRFlp/Flp, and aRK.Flp/+ mice could not activate fSf-Tomato (Supplementary Fig. 3a-c). However, in aRΔFlp/+Sox2-Cretg mice with fSf-Tomato, all detectable subcutaneous and visceral WAs and interscapular BAs were Tomato+ (Fig. 1e). This is consistent with a previous study that used Pdgfra-Cretg transgenic mice (Berry and Rodeheffer, 2013). We also found that bRΔFlp/+Sox2-Cretg mice with fSf-Tomato achieved partial Tomato-labeling of WAs and BAs at P21 (Fig. 1f), consistent with previous studies using Pdgfrb-Cretg transgenic mice (Tang et al., 2008).
Adipocyte progenitors (APs) reside locally in the stromal-vascular fraction (SVF) of adipose tissue, which also includes immune cells, endothelial cells, and non-adipogenic fibroblasts. APs can be identified by flow cytometry based on the expression of mesenchymal and progenitor cell markers (CD29 and CD34), while negative for erythroid, lymphoid, and endothelial cell markers (Ter119, CD45, and CD31) (Supplementary Fig. 4). In white adipose tissue (WAT) from P21 aRΔFlp/+ mice and bRΔFlp/+ mice, Tomato+ cells were 35-40% of the total live cellular SVF and 84-95%% of APs (Fig. 1g,h). Therefore, the embryonic Pdgfra- and Pdgfrb-lineages here label portions of the total SVF, and both are enriched in APs, consistent with previous studies using transgenic mice (Berry and Rodeheffer, 2013; Gao et al., 2018; Tang et al., 2008).
Lastly, we created timed pregnancies between aRK.Flp/+;fSf-Tomato mice and Sox2-Cretg mice to obtain aRΔK.Flp/+ embryos, which were viable at E13.5 with Tomato labeling of PDGFRαK-expressing cells (Fig. 1i). Tomato was broadly expressed in mesenchymal cells of the frontonasal prominence but not in cells of the nasal epithelium, the olfactory lobe, or the overlying epidermis (Fig. 1j). Since viable aRΔK.Flp/+ embryos could not survive later than E13.5 and the earliest fat depots do not appear until E14.5 (Han et al., 2011; Schulz and Tseng, 2013), lineage tracing aR-ΔK.Flp mutant cells in adipose tissue requires an inducible system.
Different Adipogenic Properties of Mosaic PDGFRα+ and PDGFRβ+ Cells in Adult Mice
To examine adult PDGFRα+ and PDGFRβ+ cell labeling with an inducible system, we used Ubc-CreERtg to globally express Tamoxifen (Tmx)-regulated Cre under control of the Ubiquitin C promoter. As there is no perfect Cre driver for APs, we chose Ubc-CreERtg to be as inclusive as possible in our analysis, relying on the endogenous Pdgfra- and Pdgfrb-promoters to control Flpo expression. Hence, we generated mice with Ubc-CreERtg and fSf-Tomato plus heterozygosity for the Cre-naïve knockin cassette (i.e. aRFlp/+ or bRFlp/+). After verifying Tomato expression only in the presence of Ubc-CreER and Tmx (Supplementary Fig. 3d-f), we administered a single dose of Tmx (100mg/kg) to 6-week-old mice and analyzed them one week later (Fig. 2a). This approach generated mosaic tissues where most cells remain Cre-naïve but a subset of cells convert to the aRΔFlp/+ or bRΔFlp/+ genotype, which allows them to become Tomato+ if they are a cell type with a sufficiently active Pdgfr promoter to drive Flpo expression.
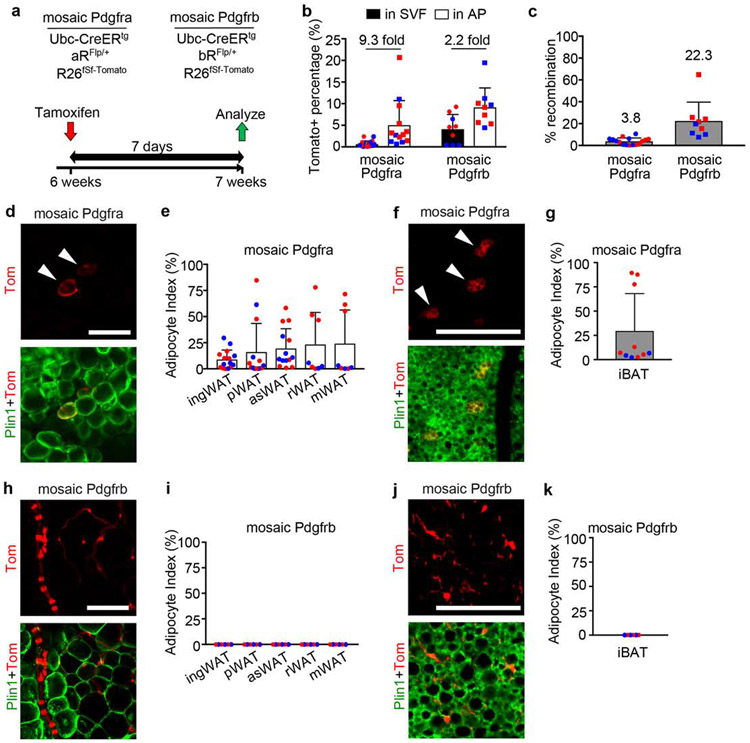
Figure 2. Short term adult mosaic labeling: Pdgfra-lineage labels adipocytes and Pdgfrb-lineage labels mural cells.
(a) Mosaic Pdgfra and Pdgfrb genotypes and experimental scheme for labeling induced at 6-weeks old with 1x Tmx (100mg/kg) and analysis after 7 days. (b) Flow cytometry of Tomato+ cells in the SVF or AP fraction of ingWAT. Tomato+ % shown + SD, and fold enrichment of Tomato+ AP within Tomato+ SVF (n = 13 Pdgfra, 9 Pdgfrb). (c) qPCR for recombination efficiency in Pdgfra (n=13) or Pdgfrb (n=9) mosaics. Average recombination + SD. (d-k) Whole mount adipose tissue with Tomato lineage labeling and Plin1 immunofluorescence with quantification of adipocyte index + SD for each tissue. (d) Pdgfra-lineage labeling in ingWAT (arrowheads = WA). (e) Adipocyte index for mosaic Pdgfra WAT (n = 8-15). (f) Pdgfra-lineage labeling in iBAT (arrowheads = BA). (g) Adipocyte index for mosaic Pdgfra iBAT (n = 10). (h) Pdgfrb-lineage labeling in ingWAT (no WA labeling). (i) Zero adipocyte index for mosaic Pdgfrb WAT (n = 6). (j) Pdgfrb-lineage labeling in iBAT (no BA labeling). (i) Zero adipocyte index for mosaic Pdgfrb iBAT (n = 6). (b,c,e,g,i,k) Blue represents male and red represents female. All Scale bars: 100μm.
Histological controls for mosaic WAT/BAT and antibody stains for PDGFRa/PDGFRb are shown in Supplementary Fig. 5.
By flow cytometry, Tomato was enriched in WAT-derived APs compared to total SVF from mosaic Pdgfra and Pdgfrb mice regardless of sex (Fig. 2b). We also assessed Cre/lox recombination at the genomic DNA level using quantitative PCR to measure deletion of the PDGFRα/PDGFRβ cDNAs. The average recombination rate was lower in mosaic Pdgfra compared to Pdgfrb (Fig. 2c), indicating that the Pdgfra locus is less accessible to Cre. Importantly, the same PCR primers are used to detect PDGFRα or PDGFRβ cDNA deletion because of sequences common to both targeting vectors (Supplementary Fig. 1a,b).
Adipose tissues from mosaic mice were histologically indistinguishable from tissues of mice without Tmx (Supplementary Fig. 5a,b). By fluorescence microscopy, scattered Tomato+ cells in mosaic Pdgfra WAT exhibited a fibroblast-like appearance and were co-stained with PDGFRα antibody that was validated on aRΔFlp/ΔFlp tissue (Supplementary Fig. 5c,e). Also scattered throughout the tissue were Tomato+ cells with a balloon-like morphology and attached nucleus, which were confirmed as adipocytes with Plin1 antibody staining (Fig. 2d). As PDGFRα is downregulated during adipogenesis and is not expressed in mature adipocytes, Tomato+ adipocytes are derived from PDGFRα+ APs. Labeled adipocytes occurred as single cells or as clusters, consistent with stochastic labeling of APs along a proliferation/differentiation trajectory (Burl et al., 2018). For quantification, we devised an adipocyte index as the number of Tomato+ WA divided by the total number of Tomato+ cells (WA + stromal cells). We generated this measurement in five different depots: inguinal WAT (ingWAT), perigonadal WAT (pWAT), anterior subcutaneous WAT (asWAT), retroperitoneal WAT (rWAT), and mesenteric WAT (mWAT). For reasons that remain unclear, there was variability in adipocyte index with some mice having more than 50% adipocytes among their Tomato+ cells and others labeled only stromal cells (Fig. 2e). The average index ranged from 10% to 25% across different fat depots. Therefore, some PDGFRα+ cells in adult WAT undergo adipogenesis within 7 days of Tmx, most likely reflecting variations in basal adipogenesis. We obtained similar results in BAT 1 week after Tmx, with many Tomato+ BAs in mosaic Pdgfra BAT and high inter-animal variability (Fig. 2f).
Unlike Pdgfra, most Tomato+ cells in adult mosaic Pdgfrb WAT were mural cells, either vascular smooth muscle cells (VSMCs) or pericytes (Fig. 2h) and were co-stained with PDGFRβ antibody that was validated on bRΔFlp/ΔFlp tissue (Supplementary Fig. 5d,f). However, Tomato+ adipocytes were not observed in any WAT at 7 days after Tmx (Fig. 2i). The result was similar in mosaic Pdgfrb BAT (Fig. 2j,k). Therefore, the adult population labeled in mosaic Pdgfrb fat consists mainly of mural cells that do not differentiate into adipocytes within 7 days of Tmx.
Expression of PDGFRα and PDGFRβ in the vascular adventitia
Intriguingly, we occasionally saw single Tomato+ cells in adult mosaic Pdgfrb WAT that were positioned in the perivascular adventitia outside the VSMC layer. We suspected that these cells might be inefficiently labeled in the mosaic setting. To address this we administered 100mg/kg Tmx for 5 days and then analyzed after 7 more days (Fig. 3a). Although higher Tmx still did not generate Tomato+ adipocytes, it did produce robust labeling of adventitial fibroblasts (Fig. 3b). Moreover, when mosaic Pdgfra mice were administered high Tmx there was a strikingly similar pattern of labeled adventitial fibroblasts (Fig. 3a,c). To investigate potential co-expression of Pdgfra/Pdgfrb-reporters, we crossed bRFlpFlp/+ mice (genotype PdgfrbFlp/+;Ubc-CreERtg;fSf-Tomato) with PdgfraH2BEGFP/+ knockin mice to create a double reporter (Fig. 3d). Here, PDGFRα+ cells are continually marked with a nuclear-localized EGFP, and cells with current or prior Pdgfrb expression are mosaically labeled with Tomato. Note that H2B-EGFP is a stable protein and dim expression was detectable in some adipocyte nuclei (Supplemental Fig. 6a), consistent with PDGFRα expression in APs prior to terminal differentiation (Lee et al., 2012). At one week after 1x Tmx, most Tomato+ cells were mural cells wrapped around CD31+ capillaries and arterioles, and EGFP+ nuclei could be distant from vessels or juxtaposed to vessels but nearly always distinct from Tomato+ cells (Fig. 3e), indicating that mosaic bR-Flp/Tomato and constitutive aR-H2BEGFP reporters are largely non-overlapping. Nevertheless, when we used confocal microscopy to create z-stacks of adipose tissue vasculature, instances of co-expression (red cytoplasm/yellow nucleus) were seen when Tomato labeling occurred in adventitial fibroblasts (Fig. 3f)(Supplementary Videos 1-3).
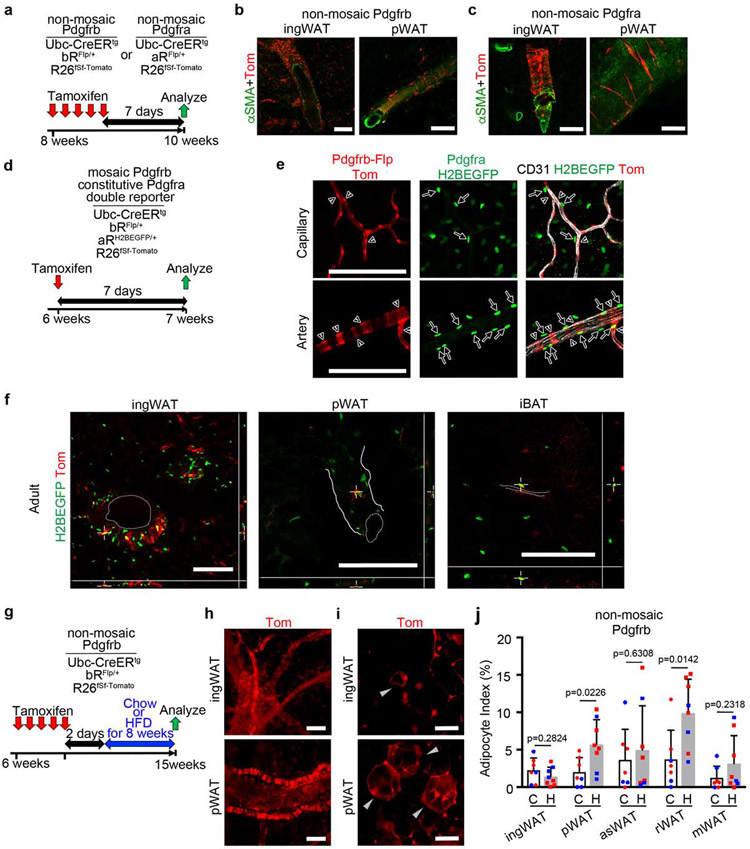
Figure 3. PDGFRα and PDGFRβ are expressed in the vascular adventitia under non-mosaic labeling conditions.
(a) Non-mosaic Pdgfra and Pdgfrb genotypes and experimental scheme for labeling at 8-weeks old with 5x Tmx (100mg/kg) and analysis after 7 more days. (b and c) Thick section WAT with Tomato labeling of adventitial fibroblasts and αSMA immunofluorescence of VSMCs. Representative of 1 male and 3 females (Pdgfrb) or 5 males and 6 females (Pdgfra). (d) Double reporter genotypes and experimental scheme for labeling at 6-weeks old with 1x Tmx (100mg/kg) and analysis after 7 days. (e) Thick section capillary or artery with Pdgfrb-lineage labeling (mural cells, red), PDGFRα+ nuclei (green), and CD31 immunofluorescence (endothelial cells, white). Some GFP+ cells are adjacent to blood vessels (arrows), but they are not co-labeled with Tomato+ (arrowheads). Representative of 4 mice. (f) Confocal z-stack of rare adventitial fibroblasts co-labeled with PDGFRα-H2BGFP and Pdgfrb-lineage/Tomato. Dotted lines indicate vessel lumens and solid lines indicate perivascular outline. Representative of 3 male and 1 female. See also Supplementary Videos 1-3. (g) Non-mosaic Pdgfrb genotypes and experimental scheme for labeling at 6-weeks old with 5x Tmx (100mg/kg) and analysis after 8 weeks of chow diet or HFD feeding. (h-i) Whole mount WAT with non-mosaic Pdgfrb-lineage/Tomato labeling of (h) adventitial fibroblasts or (i) adipocytes (gray arrowheads). (j) Adipocyte index + SD for non-mosaic Pdgfrb WAT after 8 weeks of chow diet (C) or HFD (H) (n = 7-8 mice). Blue represents male and red represents female. HFD differences are significant for pWAT and rWAT. All Scale bars: 100μm.
Additional data for HFD are shown in Supplementary Fig. 6.
A doxycycline inducible Pdgfrb-rtTA;TRE-Cre system can lineage trace a significant portion of WAs in pWAT after 8 weeks, and this labeling can be further increased by feeding HFD (Vishvanath et al., 2016). It is currently controversial whether this labeling reflects PDGFRβ+ mural cells or adventitial fibroblasts as a long-term source of WAs (Guimaraes-Camboa and Evans, 2017). To further investigate this question with our model, we generated mosaic Pdgfrb mice with 1x Tmx and then fed HFD for 8 weeks, but we could only detect rare Tomato+ WAs comprising <1% of the Tomato+ cell population in any tissue (Supplementary Fig. 6b,c). Next we generated Pdgfrb mice with 5x Tmx for adventitial fibroblast labeling and fed normal chow or HFD for 8 weeks (Fig. 3g). HFD caused obesity with a significant increase in % body fat and WAT expansion (Supplemental Fig. 6e-g). There was strong Tomato labeling of adventitial fibroblasts and many WAs independent of diet (Fig. 3h,i). Furthermore, in mice that were fed HFD, the adipocyte index was significantly increased in pWAT and rWAT, two visceral depots susceptible to HFD-induced hyperplasia (Fig. 3j)(Shao et al., 2018). Tomato+ BAs were also detected in BAT with widespread labeling, but HFD did not increase the index (Supplemental Fig. 6h). Therefore, adult PDGFRβ+ cells can give rise to new adipocytes with time, and this lineage tracing requires widespread (non-mosaic) labeling conditions that efficiently target adventitial fibroblasts. Taken together, these data suggest an adult adipogenic niche composed of CD31+ blood endothelial cells, PDGFRβ+ mural cells, and adventitial APs expressing both PDGFRα/PDGFRβ.
Inactivation of PDGFRα or PDGFRβ in Postnatal Progenitors Enhances Adipogenesis
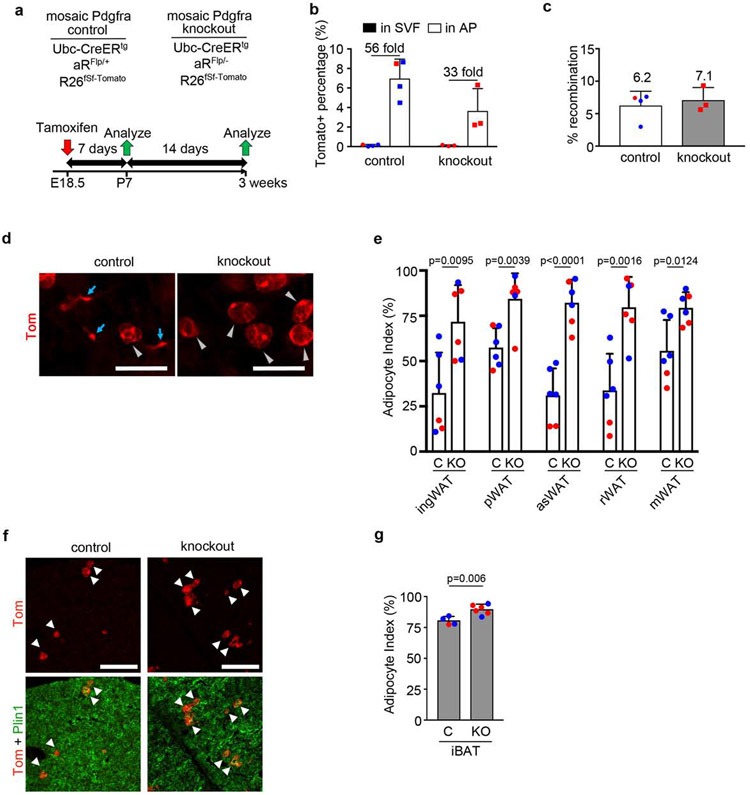
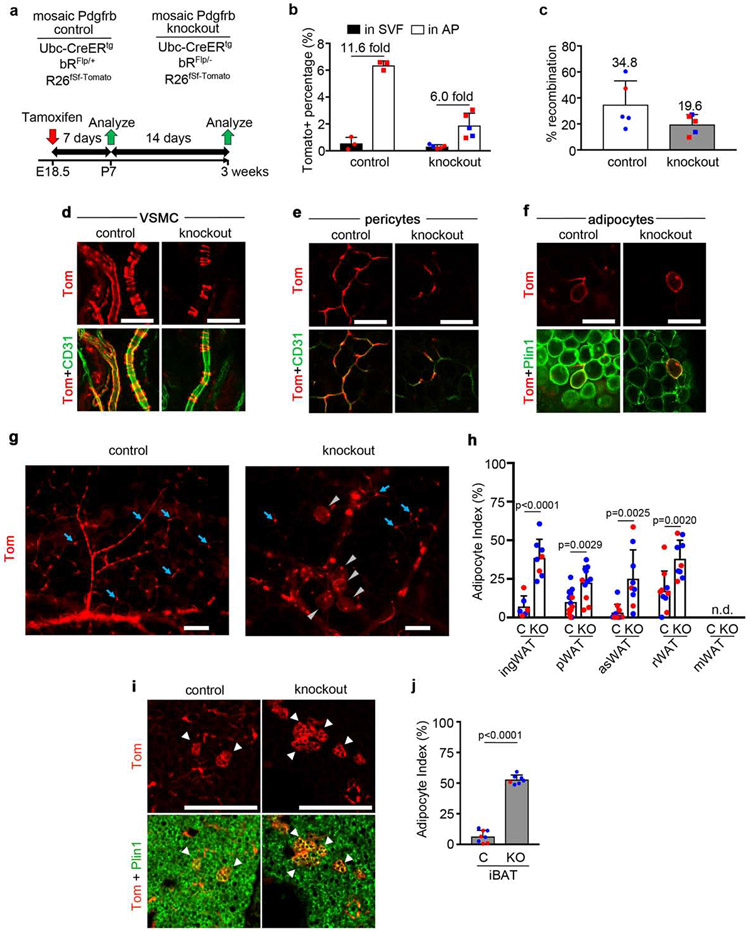
Next we turned to the question of whether the PDGFRs have a functional role in adipogenesis is not known. To investigate PDGFRα, we designed experiments to produce mosaic knockout mice by pairing a conditional Flp allele with a null allele (e.g. aRFlp/−) so that Ubc-CreERtg induces Tomato+ cells with no PDGFRα (e.g. aRΔFlp/−). Mosaic control mice have a conditional Flp allele paired with a wild-type allele (e.g. aRFlp/+), which Cre converts to Tomato+ cells that retain PDGFRα (e.g. aRΔFlp/+) (Supplementary Fig. 7). We gave a single Tmx dose of 50mg/kg to pregnant females to induce recombination in the fetus at E18.5 when WAT is still immature, for analysis at P7 or P21 (Fig. 4a). At P7, Tomato+ cells were only 0.1% of the total SVF in WAT, but they were highly enriched in the AP population (Fig. 4b). Mosaicism was similar between genotypes at the gDNA level (Fig. 4c). Pdgfra control and knockout mice grew normally and there was no histological difference in WAT/BAT at P21. In both genotypes there were abundant Tomato+ stromal cells and WAs (Fig. 4d). As shown in Fig. 4e, the adipocyte index was increased in knockout WAT compared to control. Most of the Tomato+ cells in control BAT were brown adipocytes with only a minor population of stromal cells (Fig. 4f). There was an increase in Tomato+ BAs in knockout BAT compared to control (Fig. 4g). Therefore, PDGFRα is not required for adipocyte differentiation, but instead removal of PDGFRα increases differentiation.
Figure 4. Mosaic inactivation of Pdgfra increases adipogenesis.
(a) Mosaic Pdgfra control or knockout genotypes and experimental scheme for labeling at E18.5 with 1x Tmx (50mg/kg) to pregnant dams, with analysis at P7 or P21. (b) Flow cytometry of Tomato+ cells in the SVF or AP fraction of ingWAT at P7. Tomato+ % shown + SD, and fold enrichment of Tomato+ AP within Tomato+ SVF (n = 3-4). (c) qPCR for recombination efficiency at P7. Average recombination + SD (n = 3-4). (d) Whole mount ingWAT at P21 with Tomato+ stromal cells (blue arrows) and WA (gray arrowheads). (e) Adipocyte index + SD for mosaic Pdgfra control (C) or knockout (KO) WAT (n = 6). (f) iBAT at P21 with mosaic Pdgfra labeling in BA (arrowheads). (g) Adipocyte index + SD for mosaic Pdgfra control (C) or knockout (KO) iBAT (n = 4-6). (b,c,e,g) Blue represents male and red represents female. All scale bars: 100μm.
More description of the experimental scheme is shown in Supplementary Fig. 7.
Using the same approach for PDGFRβ (Fig. 5a, Supplementary Fig. 7), Tomato labeling in P7 WAT from Pdgfrb control and knockout was 0.5% or less of the total SVF and was enriched in the AP population (Fig. 5b). There was lower gDNA mosaicism in knockouts (Fig. 5c). Both genotypes grew normally with no histological difference in WAT/BAT at P21. Differences were immediately apparent in Tomato+ cell labeling, however, with a striking reduction in Tomato+ mural cells in the knockout (Fig. 5d,e). This was expected because PDGFRβ is required for proliferation and maintenance of VSMCs and pericytes (Crosby et al., 1998; Lindahl et al., 1997). Since Tomato+ cells in knockout mice lack PDGFRβ (genotype: bRΔFlp/−), they are at a competitive disadvantage compared to cells in their milieu that retain PDGFRβ. Adipocytes were also labeled in control and knockout mice lineage traced from E18.5 to P21 (Fig. 5f), and in contrast to the mural cell compartment where PDGFRβ deletion leads to fewer Tomato+ mural cells, there was a dramatic increase in Tomato+ WAs in knockout WATs (Fig. 5g,h), Therefore, PDGFRβ is not required for postnatal adipogenesis, but instead, inactivation of PDGFRβ facilitates WA differentiation. There were also many Tomato+ BAs in both Pdgfrb genotypes at P21 (Fig. 5i), and the index was increased in knockouts (Fig. 5j). From these data we conclude that both receptors oppose adipogenesis, potentially by controlling downstream signaling pathways that inhibit progression towards differentiation.
Figure 5. Mosaic inactivation of Pdgfrb increases adipogenesis.
(a) Mosaic Pdgfrb control and knockout genotypes and experimental scheme for labeling at E18.5 with 1x Tmx (50mg/kg) to pregnant dams, with analysis at P7. (b) Flow cytometry of Tomato+ cells in the SVF or AP fraction of ingWAT at P7. Tomato+ % in each fraction + SD, and fold enrichment of Tomato+ AP within the Tomato+ SVF (n = 3-5). (c) qPCR for recombination efficiency at P7. Average recombination + SD (n = 5). (d-f) Whole mount ingWAT at P21: (d) Tomato+ vascular smooth muscle cells (VSMC) with CD31+ endothelial cells (green). (e) Tomato+ pericytes with CD31. (f) Tomato+ WA with Plin1. (g) Whole mount ingWAT at P21 with Tomato+ mural cells (blue arrows) and WA (gray arrowheads). (h) Adipocyte index + SD for mosaic Pdgfrb control (C) or knockout (KO) WAT (n = 6-11). mWAT was not analyzed. (i) iBAT at P21 with mosaic Pdgfrb labeling in BA (arrowheads). (j) Adipocyte index + SD for mosaic Pdgfrb control (C) or knockout (KO) iBAT (n = 7-8). (b,c,h,j) Blue represents male and red represents female. All scale bars: 100μm.
More description of the experimental scheme is shown in Supplementary Fig. 7.
PDGFRαK Inhibits Differentiation but Progenitors Retain Adipogenic Potential
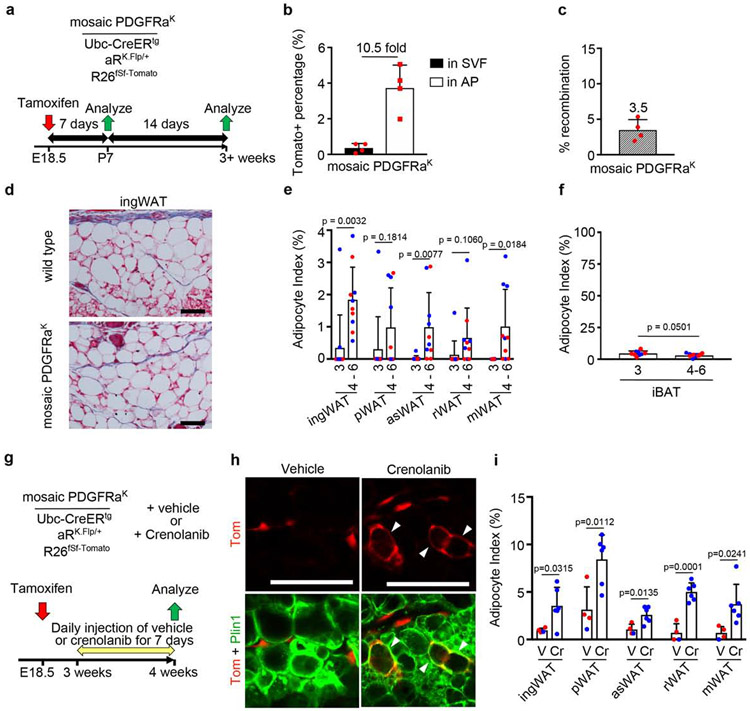
Elevated PDGFRα signaling causes APs to become myofibroblast-like cells that secrete excess ECM (Iwayama et al., 2015). Although this causes WAT lipoatrophy (Sun et al., 2017), it is unclear whether PDGFRα signaling directly inhibits adipogenesis from APs in vivo. To investigate adipogenesis in the absence of overt tissue fibrosis, we crossed Ubc-CreERtg mice with Cre-naïve aRK.Flp/+;fSf-Tomato mice and administered Tmx at E18.5 to obtain mosaic PDGFRαK mice (Fig. 6a,c). Tmx dosage was reduced to 10mg/kg to avoid fibrosis. Tomato labeling in the overall SVF was only 0.4%, but almost 4% of the APs were labeled (Fig. 6b). At P21, aRK.Flp/+ WAT was indistinguishable from Tmx-treated wild-type mice, with no evidence of fibrosis (Fig. 6d). These mice were nearly devoid of Tomato+ WAs, strongly suggest that PDGFRαK signaling inhibits adipogenesis directly and cell-autonomously. However, Tomato+ WAs began to emerge by 4-6 weeks of age, albeit at a rate far lower than mosaic Pdgfra control WAT (compare y-axis of Fig. 4e and Fig. 6e). Tomato+ BAs were <10% of the total Tomato+ cells in aRK.Flp/+ BAT at P21, again in contrast with the high adipocyte index in mosaic Pdgfra control BAT (compare Fig. 4g and Fig. 6f). Together, these results strongly suggest that PDGFRαK signaling inhibits adipogenesis directly and cell-autonomously, but not permanently. To further investigate the reversibility of PDGFRαK effects, we used a PDGFR-selective kinase inhibitor, Crenolanib (Heinrich et al., 2012). We generated mosaic aRK.Flp/+ mice with Tmx at E18.5 and administered Crenolanib or vehicle from P21 until P28 (Fig. 6g). Crenolanib significantly increased adipogenesis compared with vehicle-treated counterparts (Fig. 6h,i), further supporting the conclusion that PDGFRαK does not erase adipogenic potential.
Figure 6. Mosaic constitutive PDGFRαK signaling blocks adipogenesis and is reversible.
(a) Mosaic PDGFRαK genotype and experimental scheme for labeling induced at E18.5 with 1x Tmx (10mg/kg) to pregnant dams, with analysis at P7 and P21 or later. (b) Flow cytometry of Tomato+ cells in the SVF or AP fraction of ingWAT at P7. Tomato+ % + SD, and fold enrichment of Tomato+ AP within the Tomato+ SVF (n = 4). (c) qPCR for recombination efficiency + SD (n = 4). (d) Trichrome-stained ingWAT from Tmx-treated wild type or mosaic PDGFRαK littermate at P21, showing no evidence of fibrosis. (e-f) Adipocyte index + SD for mosaic PDGFRαK WAT at P21 or 4-6 weeks. (e) Most WAT has adipocyte index of zero at 3 weeks, and a significantly higher index from 4-6 weeks. (f) iBAT adipocyte index decreases after 3 weeks (n = 6-10). (g) Mosaic PDGFRαK genotype +/− crenolanib and experimental scheme for labeling at E18.5 with 1x 10mg/kg Tmx to pregnant dams. Vehicle or crenolanib were administered from 3 to 4 weeks, followed by analysis. (h) Whole mount ingWAT with mosaic PDGFRαK labeling at 4 weeks. Tomato+ WA (arrowheads) appear in crenolanib-treated mice. (i) Adipocyte index + SD for mosaic PDGFRαK WAT treated with vehicle (V) or crenolanib (Cr) (n = 4-6). (b,c,e,f,i) Blue represents male and red represents female. All scale bars: 100μm.
Inactivation of PDGFRα/PDGFRβ Enhances Adrb3-induced Beige Adipogenesis
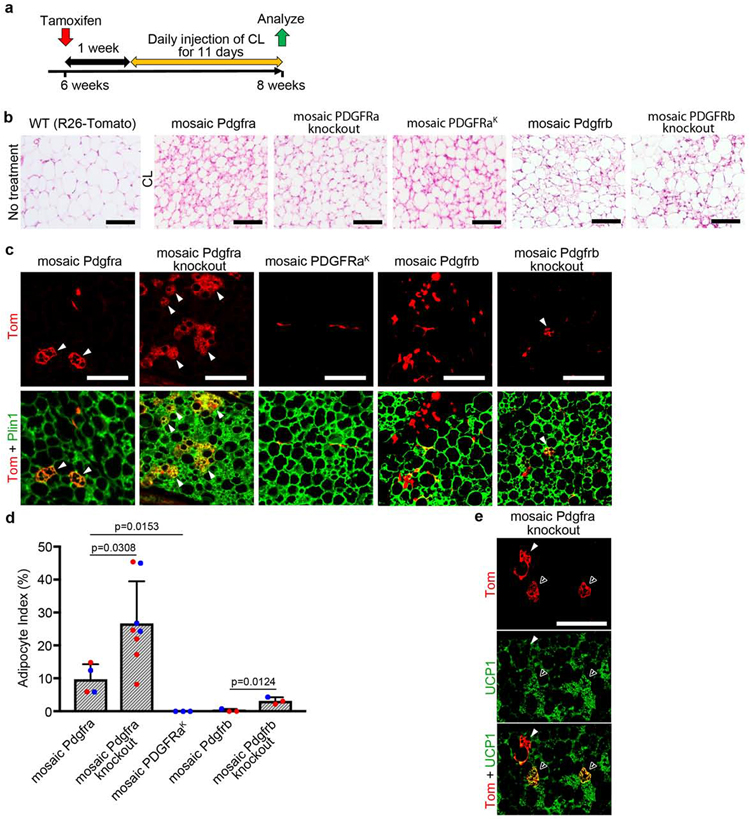
Beige adipocytes (bAs) have functional similarities to BAs in generating heat but they are induced in WAT in response to cold temperature or stimulation with β3-adrenergic receptor (Adrb3) agonists (Ikeda et al., 2018). The Adrb3 agonist CL316,243 (CL) can induce de novo beige adipogenesis from PDGFRα+ progenitors specifically in pWAT (Lee et al., 2013; Lee et al., 2012). It has been suggested in cell lines that PDGFRα promotes bA differentiation and PDGFRβ counteracts it (Gao et al., 2018). If this operates in vivo and occurs cell-autonomously, then mosaic Pdgfra knockout should impair bA differentiation and mosaic expression of PDGFRαK or Pdgfrb knockout might enhance it. We generated a cohort including each of the five adult mosaic lines described in this study: aRFlp/+ controls, aRFlp/Flp knockouts, aRK.Flp/+ mice with elevated PDGFRα signaling, bRFlp/+ controls, and bRFlp/− knockouts. Tmx was administered at 6 weeks of age and then after 1 week of recovery we injected CL for 11 days (Fig. 7a). Here we used aRFlp/Flp mice instead of aRFlp/− mice because we noticed that by 6 weeks old some aRFlp/− mice exhibited runting and poor survival irrespective of Tmx treatment, suggestive of an age-dependent hypomorphic phenotype in aRFlp/− mice. Histological analysis confirmed pWAT beiging in all Tmx/CL-treated mice (Fig. 7b). Tomato+ bAs were most frequently seen in aRFlp/Flp knockouts, followed by aRFlp/+ controls (Fig. 7c). aRK.Flp/+ mice and bRFlp/+ controls exhibited near-zero bA labeling. A small number of Tomato+ bAs were seen in bRFlp/− knockouts (Fig. 7c). There were also Tomato+ WAs in aRFlp/+ and aRFlp/Flp mice (not shown), consistent with basal white adipogenesis in the week between Tmx and the initiation of CL. We calculated an index for bAs by dividing the number of Tomato+ bAs by the total number of Tomato+ cells counted (bA + WA + stromal cells). aRFlp/Flp and bRFlp/− knockouts showed a significantly higher bA index compared with their respective aRFlp/+ and bRFlp/+ controls (Fig. 7d). Some Tomato+ multilocular cells that we scored as bAs were Ucp1+, but most were negative (Fig. 7e), consistent with the finding that many bAs in pWAT are indeed Ucp1 negative (Bertholet et al., 2017). In conclusion, the reciprocal increase in bA labeling with mosaic Pdgfra knockout and absence of labeling with PDGFRαK shows that PDGFRα directly opposes CL-induced beige adipogenesis. And although adult PDGFRβ+ cells do not contribute to de novo bAs under these conditions, mosaic Pdgfrb knockout allows some lineage contribution to bAs. Therefore, PDGFRα or PDGFRβ downregulation facilitates lineage contribution to CL-induced beige adipogenesis.
Figure 7. Mosaic inactivation of Pdgfra/Pdgfrb increases beige adipogenesis.
(a) Experimental scheme for labeling at 6 weeks with 1x Tmx, 1-week chase, 11 days of treatment with CL-316,243 (CL), and analysis at about 8 weeks. (b) H&E-stained pWAT without CL treatment, or different mosaic pWAT after 11-days CL showing browning response to Adrb3 activation. (c) Mosaic pWAT after CL treatment showing multilocular Tomato+ beige adipocytes (bA) with Plin1 (arrowheads). Immunofluorescence on paraffin. (d) Beige adipocyte index + SD for mosaic pWAT after CL, which is the number of Tomato+ bA divided by the total number of Tomato+ cells counted (bA + WA + stromal) (n = 3-8). Blue represents male and red represents female. (e) Mosaic Pdgfra knockout pWAT after CL treatment, showing multilocular Tomato+ bA with Ucp1 (open arrowhead) or without Ucp1 (filled arrowhead). All scale bars: 100μm.
DISCUSSION
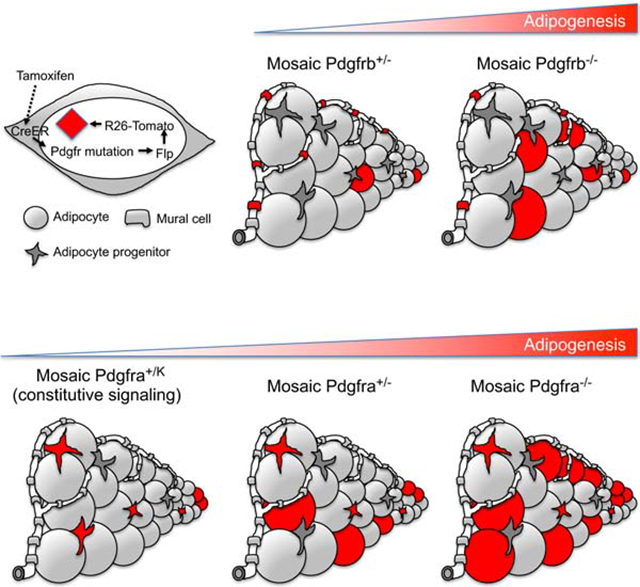
In this study we show that the progenitor cell markers PDGFRα and PDGFRβ function as gatekeepers of adipocyte differentiation and their depletion facilitates adipogenesis in functional genetic mosaics in vivo. Conversely, constitutive activation of PDGFRα cell-autonomously blocks adipogenesis in the absence of fibrosis, and this block is reversible. Our major finding applies to postnatal development of WAs and BAs, and bA differentiation induced by Adrb3 activation in adult mice. These results imply that downregulation of PDGF signaling regulates the transition from progenitor to mature adipocyte.
The elucidation of gene function in vivo depends on identifying direct, cell-autonomous consequences of gene manipulation. This is sometimes difficult because mutations that affect entire lineages can trigger homeostatic mechanisms that mask the direct effects. As a relevant example, conditional Pdgfrb knockout in adult mice, either globally or in PPARγ+ adipocyte progenitors, has significant physiological consequences, including deleterious effects on the vasculature and reduced adipose tissue mass, but with an overall improvement in glucose tolerance (Jiang et al., 2017; Onogi et al., 2017). But whether Pdgfrb perturbations directly affect adipocyte differentiation in vivo cannot be elucidated from such a complex phenotype. Similarly, constitutive PDGFRαK signaling caused progressive adipose tissue fibrosis, but it was unclear whether the in vivo effects on adipocytes were direct or secondary to fibrosis (Iwayama et al., 2015). We adopted a functional genetic mosaic approach to more precisely define Pdgfra/Pdgfrb gene function. The genetic mosaic approach creates a few mutant cells while leaving most cells as non-mutant. Consequently, non-mutant cells provide a normal environment to protect against secondary effects on organ function. Non-mutant cells can also alleviate homeostatic mechanisms that mask weaker phenotypes. Mosaic analysis has been performed for many genes in the Drosophila genome (Xu and Rubin, 2012), and several different approaches have been explored in mammalian models (Lao et al., 2012; Pontes-Quero et al., 2017; Zong et al., 2005).
The three knockin constructs described in this study uniquely combine Cre/lox-mutagenesis of the endogenous Pdgfra/Pdgfrb genes with expression of Flpo recombinase, which can be used to lineage label mutant cells. Flpo expression is dependent on the endogenous Pdgfr promoter, and needs to be expressed at a sufficiently high level to induce recombination of its reporter target. Our experimental design generates Cre recombination in some cell types that do not become labeled because they are negative or low for Pdgfr expression. Therefore, while all labeled cells are mutant, the label is expected to underestimate the overall prevalence of mutant cells. The use of Ubc-CreERtg was intended to maximize the diversity of the founder cell population, capturing any cells with a sufficiently active Pdgfr promoter at the time of Cre/loxP recombination, plus any cells that acquire Pdgfr promoter activity during the experiment. A final technical point relates to cell labeling with these constructs in heterozygous cells, which have reduced Pdgfr expression by 50% compared to neighboring cells. It is possible that the relative difference in Pdgfr expression could influence the behavior of labeled control cells. The potential for allele reduction to influence cell behavior is a consideration for any knockin/knockout model.
A portion of this work focused on lineage labeling to characterize adipogenic potential. We found that nearly all APs develop from an embryonic Pdgfra-lineage, while the Pdgfrb-lineage shows partial labeling in development. Because we used Sox2-Cretg to target the epiblast, we interpret this labeling to reflect maximal contribution from each lineage, and our results are consistent with previous studies using Pdgfr-Cre transgenes (Berry and Rodeheffer, 2013; Tang et al., 2008). In adult mice, we found that Pdgfra-lineage cells became adipocytes within 7 days of Tmx in all tissues examined. We interpret this as reflecting continual basal adipogenesis because we used low Tmx to minimize drug-induced adipocyte turnover (Ye et al., 2015). We found that the frequency of Pdgfra-lineage AP differentiation was highly variable between animals, with some having more than 50% adipocytes in Tomato+ cells and others displaying almost exclusively stromal cells. Further studies are needed to elucidate variables that influence basal adipogenesis, as indirect measurements have similarly shown that adipocyte hyperplasia is highly variable between individual humans (Arner et al., 2010). In contrast, mosaic Pdgfrb-lineage cells in adult mice did not become adipocytes within 7 days of Tmx. Further exploration of Pdgfrb-lineage cells under non-mosaic conditions revealed labeling of adventitial cells on medium and large-sized blood vessels. These cells are distinct from VSMCs and reside in a separate layer surrounding the vascular media. Again, adipocytes were not labeled by the Pdgfrb-lineage within 7 days of high Tmx treatment. However, when these mice were aged for 8 weeks, we could find lineage contribution to adipocytes. Adipogenesis was significantly increased in visceral fat with HFD feeding. This finding of long-term adipogenesis from the Pdgfrb-lineage is congruent with lineage tracing with Pdgfrb-rtTA;TRE-Cre mice (Vishvanath et al., 2016), but since we could only label adipocytes under conditions where adventitial fibroblasts were also highly labeled, our result suggests that PDGFRβ+ APs are adventitial fibroblasts, not mural cells. Moreover, we found that non-mosaic conditions generated adventitial cell labeling from the Pdgfra-lineage. Co-labeling of adventitial fibroblasts with constitutive Pdgfra-H2BGFP and mosaic Pdgfrb-lineage together suggest that double-positive adventitial fibroblasts are long-term APs. Our results are consistent with recent studies where mural cell derived adipogenesis was carefully examined using Tbx18CreER for lineage tracing. Unlike PDGFRα+ cells, Tbx18+ mural cells scarcely differentiated into adipocytes (Cattaneo et al., 2020; Guimaraes-Camboa et al., 2017). Together with our findings, these independent results demonstrate that adventitial fibroblasts are the primary PDGFRβ+ cell type that contributes to adipogenesis rather than PDGFRβ+ mural cells.
Constitutive PDGFRα signaling generates fibrosis by causing APs to become matrix secreting myofibroblast-like cells (Iwayama et al., 2015), but it was unknown whether the opposite could be true: that reduced signaling could enhance adipogenesis. Here we show that mosaic deletion of Pdgfra or Pdgfrb significantly increased the proportion of Tomato+ cells that differentiated into WAs or BAs in vivo. This regulatory effect was not limited to postnatal adipogenesis but also affected bA formation in adulthood. Together, these findings suggest that extinguishing PDGFRα or PDGFRβ signaling is an important regulated event necessary for adipocyte differentiation. Future studies may focus on the events that lead to PDGFR downregulation, and which PDGFR signaling pathways oppose adipogenesis. Increasing the overall number of white and brown/beige adipocytes is predicted to confer health benefits by increasing lipid storing capacity and energy expenditure (Kajimura et al., 2015; Kim et al., 2007; Wang et al., 2013). Promoting adipogenesis through PDGFR inhibition could also allow tissues to escape from fibrosis by channeling myofibroblasts into adipocytes. Therefore, knowledge about the mechanisms that regulate adipocyte populations in the body might be manipulated for therapeutic purposes (Cawthorn et al., 2012; Kusminski et al., 2016).
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to the Lead Contact, Lorin E. Olson (Lorin-Olson@omrf.org). Unique mice generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee at the Oklahoma Medical Research Foundation. Mice were maintained in a 12 hr light/dark cycle and housed in groups of two to five with unlimited access to water and food. All strains were maintained on a mixed C57BL/129 genetic background at room temperature. The lines Sox-2Cretg (JAX:008454)(Hayashi et al., 2002) and Ubc-CreERtg (JAX:007001)(Ruzankina et al., 2007) were purchased from the Jackson Laboratories. PDGFRaH2B:EGFP (JAX:007669)(Hamilton et al., 2003) was a gift from Dr. Philippe Soriano. The line Rosa26LSL-fSf-tdTomato (JAX:021875)(Madisen et al., 2015) was purchased from the Jackson Laboratories and crossed with Sox2-Cretg to eliminate the loxP-flanked stop cassette, rendering it a Flp/frt-regulated reporter. The lines Pdgfrafloxed and Pdgfrbfloxed (JAX:006492, 010977)(Schmahl et al., 2008; Tallquist et al., 2003) were gifts from Philippe Soriano and were crossed with Sox2-Cretg to generate null alleles. Following these genetic modifications, Sox2-Cretg was eliminated by breeding, and the resulting Rosa26fSf-tdTomato, Pdgfra+/−, and Pdgfrb+/− mice were used for our studies.
The aR-Flp, aR-K.Flp and bR-Flp cassettes were knocked into the endogenous Pdgfra or Pdgfrb loci by homologous recombination in ES cells. The aR-Flp targeting vector (Figure S1A) was designed to insert a SA.lox.PDGFRα.lox-Flpo-attB-Neo-attP cassette into the endogenous Pdgfra gene. We modified the previous targeting vector (Olson and Soriano, 2009) by replacing the lox-Neo/Stop-lox cassette with a lox-PDGFRα-lox cassette, and the PDGFRαK cDNA was replaced with Flpo cDNA. Furthermore, a new neomycin-resistance cassette was inserted downstream of Flpo with flanking attB/attP sequences to allow removal of the resistance cassette by PhiC31. The aR-K.Flp targeting vector (Figure S1C) was designed to insert a SA.lox.Neo/Stop.lox-PDGFRαK.T2A.Flp cassette in place of exons 2-4 of the endogenous Pdgfra gene. Thus, aR-K.Flp was based on a previously described vector (Olson and Soriano, 2009) with modification to replace the PDGFRαK stop codon with nucleotides encoding a self-cleaving T2A sequence and a codon-optimized Flpo recombinase (Kranz et al., 2010; Raymond and Soriano, 2007). The PDGFRαK protein has a point mutation, D842V, which renders it constitutively active. The bR-Flp targeting vector (Figure S1B) was designed to insert a SA.lox.PDGFRβ.lox-Flpo-attB-Neo-attP cassette in place of exons 2-4 the endogenous Pdgfrb gene. Thus, bR-Flp was based on a pervious vector (Olson and Soriano, 2011) with modification to replace the lox-Neo/Stop-lox cassette with a lox.PDGFRβ.lox cassette, and the PDGFRβ cDNA was replaced with Flpo cDNA. A neomycin-resistance cassette was inserted downstream of Flpo with flanking attB/attP sequences. Correctly targeted ES cells were identified by PCR and confirmed by Southern blotting as previously described (Olson and Soriano, 2009, 2011). The attB-Neo-attP cassettes were removed from ES cells by transiently expressing codon-optimized PhiC31o (Raymond and Soriano, 2007). Chimeric males were generated by standard blastocyst injection and transplantation procedures. For aR-Flp and aR-K.Flp, PCR genotyping of genomic DNA was performed using three primers, aex2, ain2, and aex3, which generates a 306bp band for wild type Pdgfra and a 166bp band for either knockin. For bR-Flp, PCR genotyping was performed with three primers, bex2, bin2, and bex3, which generates a 346bp band for wild type Pdgfrb and a 160bp band for the knockin.
Tmx (Sigma T5648) was prepared as a 20mg/ml stock in corn oil. For mosaic Cre-recombination at 6 weeks old, mice were ip injected or gavaged with 100mg Tmx/kg bw, except for mosaic PDGFRαK mice, which received only 10mg/kg. Mosaic mice were fed normal chow diet (Purina 5053) or high fat diet (Research Diets D12492), as indicated in the text. For non-mosaic Cre-recombination, 8 week old mice were gavaged with 100 mg Tmx/kg bw for 5 consecutive days. For mosaic Cre-recombination in the fetus, pregnant dams were gavaged with 50 mg Tmx/kg body weight (bw) at E18.5. The resulting offspring were analyzed at P7 or P21. For Crenolanib treatments (Selleck S2730), P21 mosaic PDGFRαK mice mice were randomly allocated into drug or vehicle groups, which were ip injected daily with 15mg Crenolanib/kg bw or vehicle for 7 days. Crenolanib was prepared as a 50 mg/ml stock, which was diluted in glycerol formal (vehicle) to 1 mg/ml. For CL316,243 (CL) treatments (Cayman 17499), mice were injected with 100mg Tam/kg bw, followed by 1-week wash out period, followed by ip injection of 1 mg CL/kg bw for 11 days. Both males and females were analyzed.
METHOD DETAILS
Viral Transduction of Dermal Fibroblasts
Lentiviral constructs were created to constitutively express Cre-IRES-iRFP720 or IRES-iRFP720 (no Cre) from the hybrid promoter mCMV/EF1alpha/HTLV (InVivoGen), which is highly active in mouse cells. Generation of lentiviral particles and transductions were performed as previously described (Berry et al., 2014). Following transductions, dermal fibroblasts were cultured in DMEM + 10% FBS, 2mM L-glutamine, and 2mM pen/strep were selected with 1μg/mL puromycin, and then subjected to Western blot as described below.
Western Blotting
Protein was extracted from embryonic/fetal tissue or adult lung by mincing tissue in 1ml ice cold RIPA buffer (50mM Tris pH7.4, 150mM NaCl, 1% NP-40, 0.1% SDS, 0.25% sodium deoxycholate) with the addition of protease and phosphatase inhibitors (Complete protease inhibitor cocktail (Sigma 11836170001), 1mM EDTA, 1mM sodium orthovanadate, 1mM NaF, 1mM PMSF). After 10 minutes incubation on ice, lysates were sonicated for 30 seconds, followed by incubation on ice for one hour. The lysate was cleared by centrifugation. Protein concentration was determined by BCA assay (Pierce 23225). Similar procedures were used to isolate protein from viral transduced cells. For Western blotting, equal amounts of each protein sample (40 μg from embryo or 75 μg from lung) were separated by 8% gel SDS-PAGE, using two gels run in parallel. Proteins were transferred from gel to nitrocellulose membranes, blocked with 5% BSA, and then subjected to Western blotting with antibodies against total PDGFRα (Santa Cruz 338), total PDGFRβ (Santa Cruz 432), or phospho-PDGFRα (Cell Signaling 24188). Membranes were then probed with horseradish-peroxidase conjugated anti-rabbit secondary antibody (Jackson 711-035-152) diluted in 5% milk. Primary and secondary antibodies were used at 1:2000 and 1:5000, respectively. Blots were developed with ECL Western blotting substrate (Pierce 32106) and autoradiography film.
Measurement of Recombination Efficiency by qPCR
gDNA was extracted from embryos, from pup ear punches, or from intestine sampled from necropsied adults. Minced tissue was incubated overnight at 55oC in tissue digestion buffer (100mM Tris pH 7.4, 100mM NaCl, 25mM EDTA, 0.5% SDS, 200 micrograms/ml proteinase K). DNA was then extracted with phenol/chloroform, precipitated with 0.5 volumes 7.5M ammonium acetate and 3 volumes 100% ethanol, washed with 70% ethanol, and resuspended in 0.1X TE buffer (1mM Tris pH7.4, 0.1mM EDTA). Quantitative PCR was performed in duplicate using 50-100ng gDNA on a Bio-Rad iCycler with iTaq SYBR Green Supermix (Bio-Rad 1725122). To measure recombination efficiency, which is the % conversion of aR-Flp into aR-ΔFlp, and bR-Flp into bR-ΔFlp, primers SAF and FlpR were used to amplify the junction of the splice acceptor and the Flpo cDNA, which is created by deletion of the lox-Pdgfra-lox or lox-Pdgfrb-lox cassettes (Figure S1A, S1B). To measure the recombination of aR-K.Flp into aR-ΔKFlp, primers SAF and aRR were used to amplify the junction of the splice acceptor and PDGFRaK cDNA, which is created by deletion of the lox-Stop-lox cassette (Figure S1C). In all cases, normalization was achieved with primers Flp1 and Flp2, which amplify the Flpo cDNA. PCR efficiency was determined by comparison to a standard curve of gDNA from aRΔK.Flp/+;Sox2-Cre, aRΔFlp/+;Sox2-Cre, or bRΔFlp/+;Sox2-Cre embryos.
Flow Cytometry
Adipose tissue was excised, minced with scissors, and digested in Hank’s Balanced Salt Solution (HBSS) containing 0.3 mg/ml collagenase Type I, 3% BSA, 1.2 mM CaCl2 and 1 mM MgCl2 for 75 min with shaking at 37 °C. The suspension was filtered with 40 μm cell strainer and centrifuged at 500 g. Supernatant and the floating layer including adipocytes were removed and then the pellet was washed with FACS buffer (PBS with 2% FBS and 1mM EDTA). The pellet was stained with AF700 anti-mouse CD29 (1:100)(#102218), APC anti-mouse CD34 (1:20)(# 119310), BV421 anti-mouse CD45.2 (1:40)(#109831), PerCP/Cy5.5 anti-mouse CD31 (1:20)(# 102420), PE/Cy7 anti-mouse TER-119 (1:100)(#116221) and Zombie Green (1:1000)(#423111) (all from BioLegend). The staining was performed on ice for 10 min and then washed with FACS buffer. Stained samples were analyzed on a BD LSRII and data analysis was performed utilizing BD FACS Diva and FlowJo. Cells isolated from adipose tissue of Tomato-negative mice, along with primary-minus-one antibody combinations, and Tomato-positive mice, along with all antibodies, were used as the negative controls to determine the gates (see Supplementary Fig. 4).
Immunofluorescence Staining
Embryos were fixed in 4% paraformaldehyde for 16 h. After cryo embedding and sectioning (10-14 μm), sections were washed in PBS for 5 min and then incubated in PBS containing DAPI for 5 min. Samples were mounted with Fluoro Gel with DABCO (Electron Microscopy Sciences 17985-02). Adipose tissue was excised and fixed in 4% paraformaldehyde for 16 h. After paraffin embedding and sectioning (8 μm), sections were deparaffinized and rehydrated. For PDGFRα staining, antigen retrieval was performed by incubating in 10mM sodium citrate buffer pH6.0 with 0.05% Tween 20 for 30 min at 95-100 °C. All sections were blocked with 5% donkey serum for 1h at room temperature and incubated with primary antibodies for 24 h at 4 °C. The following primary antibodies were diluted in PBS containing 5% donkey serum and used: goat anti-Plin1 (1:300)(Novus 100-60554), rabbit anti-RFP (1:100)(Rockland 600-401-379), rabbit anti-UCP1 (1:100)(Abcam 10983) and goat anti-PDGFRα (1:250)(R&D AF1062). After washing in PBS, sections were incubated with secondary antibodies for 1 h at room temperature. The following secondary antibodies were diluted in PBS containing 5% donkey serum and used: donkey anti-goat AF488 (1:100)(Jackson 705-545-003), donkey anti-rabbit Cy3 (1:100)(Jackson 711-545-003) and donkey anti-rabbit AF488 (1:100)(Jackson 711-545-152). After incubating in PBS containing DAPI, sections were mounted with Fluoro Gel with DABCO. For staining of PDGFRβ and αSMA, thick frozen sections (120 μm) were made. Sections were washed in PBS for 5 min and then incubated with primary antibodies for 24 h at 4 °C. The following primary antibodies were diluted in PBS containing 5% donkey serum and used: rat anti-PDGFRβ (1:200)(eBioscience 14-1402-82) and αSMA (1:200) (Cell signaling 19245). After incubating in PBS containing DAPI, sections were mounted with Fluoro Gel with DABCO. Sections were imaged by Nikon ECLIPSE 80i and analysed by Nikon NIS-Elements D3.2 or Nikon C2+ Confocal microscope and analysed by Nikon NIS-Elements AR. For wholemount staining, tissues were fixed in 4% paraformaldehyde/PBS overnight. Then samples approximately 2 mm3 were washed in PBS and followed by x2 wash in PBS with 0.2% TritonX-100 for 1 hr. Samples were incubated in PBS with 0.2% TritonX-100 and 20% DMSO for 24 h and then incubated in PBS with 0.1% Tween-20, 0.1% TritonX-100, 0.1% NP40, 0.1% sodium deoxycholate and 20% DMSO for 24h. After x2 washes in PBS with 0.2% TritonX-100 for 1 hr, samples were incubated in PBS with 0.2% TritonX-100, 20% DMSO and 0.3M glycine for 24 h and followed by block in PBS with 0.2% TritonX-100, 10% DMSO and 6% serum for 24h. Samples were washed x2 in PBS with 0.2% Tween-20 and 10 μg/ml heparin (PTwH) for 1 hr and then incubated with rabbit anti-Plin1 (Cell Signaling 9349) or armenian hamster anti-CD31 (Abcam 10983) in PTwH with 5% DMSO and 3%serum for 48 hr. Samples were washed twice with PTwH for 1 hr and incubated with secondary antibodies (donkey anti-rabbit AF488 or goat anti-armenian hamster AF488 or donkey anti-rabbit AF647 or goat anti-armenian hamster AF647) diluted in PTwH with 3% donkey or goat serum for 24 hr. Then samples were washed in PTwH for 24 h and then mounted on a slide with mounting medium (Fluoro Gel with DABCO) and a barrier of Vaseline to seal the coverslip. All whole mount staining steps were performed at room temperature. Stained samples were imaged with a ZEISS LSM 710 confocal laser-scanning microscope and analysed by ZEISS ZEN microscope software.
Body Composition Analysis
Live mice were analyzed by EchoMRI Body Composition Analyzer (EchoMRI) and % body fat was calculated based on body weight. For tissue weight, one side of ingWAT or pWAT was weighed and divided by total body weight for % tissue weight.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical Analysis
Data are presented as means + SD. Differences were analyzed by unpaired two-tailed Student’s t test between two groups using Graphpad Prism 7. All measurements were from distinct biological samples. Statistical parameters are found in the figure legends, including exact n and number of biological repeats. Exact P-values are shown.
Quantification of Adipocyte Index for Tomato+ Cells
Adipose tissue was fixed in 4% paraformaldehyde for 24 h and washed in PBS. Thin-sliced adipose tissue was mounted between a coverslip and slide. Scattered Tomato+ WAs in mosaic whole mount WAT could be distinguished from non-adipocytes by their distinct spherical morphology and attached nucleus, such that adipocyte marker co-staining was not routinely needed for manual quantification using a Nikon ECLIPSE 80i fluorescence microscope. A given WAT from an individual mouse was considered a biological replicate. Between 100 and 600 Tomato+ cells were scored per WAT (depending on depot). For Pdgfra mosaics, all Tomato+ cells were scored as adipocytes or stromal cells. For Pdgfrb mosaics, only adipocytes and pericytes were scored because it was difficult to unambiguously count single VSMCs. For each sample, the % Tomato+ WAs was calculated by dividing the number of Tomato+ WAs by the total number of Tomato+ cells (WA + stromal cells). This index served to normalize for variable mosaicism from mouse to mouse. For BAT, and bAs in pWAT, paraffin tissue sections were used for quantification after staining with RFP/Tomato antibody. For BAT, the % Tomato+ BAs was calculated by dividing the number of Tomato+ BAs by the total number of Tomato+ cells (BA + stromal cells). For bAs in pWAT, scattered bAs and WAs were distinguishable based on unambiguous multilocular or unilocular morphology, and the % Tomato+ bAs was calculated by dividing the number of Tomato+ bAs by the total number of Tomato+ cells (bA + WA + stromal cells). Experiments were not blinded, as the mouse genotypes were known prior to analysis. Two independent investigators replicated the method of quantification (C.S. and H.S.).
DATA AND CODE AVAILABILITY
The quantitative datasets supporting the current study have been deposited in the “Mendeley Data” repository, dataset “Mosaic mutant analysis identifies PDGFRα/PDGFRβ as negative regulators of adipogenesis”, https://doi:10.17632/26r263sm6w.1.
Supplementary Material
Supplementary Video 1. bR-Flp/Tomato; aR-H2BEGFP double reporter ingWAT related to Fig 3f
Supplementary Video 2. bR-Flp/Tomato; aR-H2BEGFP double reporter pWAT related to Fig 3f
Supplementary Video 3. bR-Flp/Tomato; aR-H2BEGFP double reporter iBAT related to Fig 3f
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Plin1 | Cell Signaling | Cat# 9349 |
| Rabbit polyclonal anti-UCP1 | Abcam | Cat# 10983 |
| Goat polyclonal anti-Plin1 | Novus | Cat# 100-60554 |
| Rabbit polyclonal anti-RFP | Rockland | Cat# 600-401-379 |
| Armenian hamster polyclonal anti-CD31 | Herzog 2013 | Abcam #119341 |
| Rabbit polyclonal anti-PDGFRα | Santa Cruz | Cat# 338 |
| Goat polyclonal anti-PDGFRα | R&D | Cat# AF 1062 |
| Rabbit polyclonal anti-PDGFRβ | Santa Cruz | Cat# 432 |
| Rat monoclonal anti-PDGFRβ | eBioscience | Cat# 14-1402-82 |
| Rabbit monoclonal anti-pY762 PDGFRα | Cell Signaling | Cat# 24188 |
| Rabbit monoclonal anti-α-Smooth Muscle Actin | Cell Signaling | Cat# 19245 |
| Donkey anti-rabbit HRP conjugate | Jackson ImmunoResearch | Cat# 711-035-152 |
| Donkey anti-rabbit AF647 conjugate | Jackson ImmunoResearch | Cat# 711-605-152 |
| Goat anti-Armenian hamster AF647 conjugate | Jackson ImmunoResearch | Cat# 127-605-099 |
| Donkey anti-Goat AF488 conjugate | Jackson ImmunoResearch | Cat# 705-545-003 |
| Donkey anti-Rabbit Cy3 conjugate | Jackson ImmunoResearch | Cat# 711-165-152 |
| Donkey anti-Rabbit AF488 conjugate | Jackson ImmunoResearch | Cat# 711-545-152 |
| Donkey anti-Rat AF488 conjugate | Jackson ImmunoResearch | Cat# 712-545-150 |
| AF 700 anti-mouse/rat CD29 | BioLegend | Cat# 102218 |
| APC anti-mouse CD34 | BioLegend | Cat# 119310 |
| BV 421 anti-mouse CD45.2 | BioLegend | Cat# 109831 |
| PerCP/Cy5.5 anti-mouse CD31 | BioLegend | Cat# 102420 |
| PE/Cy7 anti-mouse TER-119/Erythroid Cells | BioLegend | Cat# 116221 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Tamoxifen (Tmx) | Sigma Aldrich | Cat# T5648 |
| Crenolanib | Selleck Chemicals | Cat# S2730 |
| CL316,243 (CL) | Cayman Chemical | Cat# 17499 |
| Complete protease inhibitor cocktail | Sigma Aldrich | Cat# 11836170001 |
| Collagenase Type 1 | Gibco | Cat# 17100-017 |
| Collagenase Type 2 | Worthington | Cat# LS004176 |
| DMEM | Corning | Cat# 10-017-CV |
| Fetal Bovine Serum | Atlanta Biologicals | S11550 |
| L-glutamine | Gibco | 25030 |
| Pen/strep | Gibco | 18140 |
| Critical Commercial Assays | ||
| ECL Western blotting substrate | Pierce | Cat# 32106 |
| BCA assay | Pierce | Cat# 23225 |
| iTaq SYBR Green Supermix | Bio-Rad | Cat# 1725122 |
| Fluoro Gel with DABCO | Electron Microscopy Sciences | Cat# 17985-02 |
| Zombie Green | BioLegend | Cat# 423111 |
| DAPI | Sigma Aldrich | Cat# D9542 |
| Masson’s Trichrome Stain Kit | Electron Microscopy Sciences | Cat# 26367-series |
| Deposited Data | ||
| Quantitative Datasets | This paper | doi:10.17632/26r263sm6w.1 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Ubc-CreERtg | Ruzankina 2007 | JAX:007001 |
| Mouse: Sox2-Cretg | Hayashi 2002 | JAX:008454 |
| Mouse: PdgfraH2B:GFP | Hamilton 2003 | JAX:007669 |
| Mouse: R26LSL-fSf-tdTomato | Madisen 2015 | JAX:021875 |
| Mouse: Pdgfrafloxed | Tallquist & Soriano 2003 | JAX:006492 |
| Mouse: Pdgfrbfloxed | Schmahl 2008 | JAX:010977 |
| Mouse: PdgfraK.Flp | This paper | N/A |
| Mouse: PdgfraFlp | This paper | N/A |
| Mouse: PdgfrbFlp | This paper | N/A |
| Oligonucleotides | ||
| aex2: ACCTCCCACCAGGTCTTTCT | This paper | N/A |
| ain2: CTGTAAGCCCATCCCAGAGA | This paper | N/A |
| aex3: GCCAGCTCACTTCACTCTCC | This paper | N/A |
| bex2: GGGCTTCCAGGAGTGATACC | This paper | N/A |
| bin2: CCAGCTGGACTGAAGAGGAG | This paper | N/A |
| bex3: CCGAGCAGGTCAGAACAAAG | This paper | N/A |
| SAF: CAAACTCTTCGCGGTCTTTC | This paper | N/A |
| aRR: CCCCATAGCTCCTGAGACCT | This paper | N/A |
| FlpR: CTTGCACAGGATGTCGAACTG | This paper | N/A |
| Flp1: GGCAGTTCGTGGAGAGATT | This paper | N/A |
| Flp2: GCCTTCTGGGTCTTGTACTT | This paper | N/A |
| Recombinant DNA | ||
| pPGKPhiC31obpA | Raymond & Soriano 2007 | Addgene Plasmid #13795 |
| pFlpo | Raymond & Soriano 2007 | Addgene Plasmid #13792 |
| aR-K.Flp SA.lox.Neo/Stop.lox-PDGFRαK.T2A.Flp targeting vector for the mouse Pdgfra gene |
This paper | N/A |
| aR-Flp sa-lox-Pdgfra-lox-Flpo-attB-Neo-attP targeting vector for the mouse Pdgfra gene |
This paper | N/A |
| bR-Flp sa-lox-Pdgfrb-lox-Flpo-attB-Neo-attP targeting vector for the mouse Pdgfrb gene |
This paper | N/A |
| Lenti-mCMV/EF1alpha/HTLV-Cre-IRES-iRFP720-PGK-Puro | This paper | N/A |
| Lenti-mCMV/EF1alpha/HTLV-IRES-iRFP720-PGK-Puro | This paper | N/A |
| Software and Algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| GraphPad Prism 7 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| BD FACS Diva | BD Biosciences | https://www.bdbiosciences.com/enus/instruments/research-instruments/research-software/flow-cytometry-acquisition/facsdiva-software |
| FlowJo | FlowJo | https://www.flowjo.com/ |
| NIS-Elements D3.2 | Nikon | https://www.microscope.healthcare.nikon.com/products/software/nis-elements |
| NIS-Elements AR | Nikon | https://www.microscope.healthcare.nikon.com/products/software/nis-elements |
| ZEN | ZEISS | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html |
| Other | ||
| LabDiet 5053 (normal chow) | Purina | LabDiet 5053 |
| High Fat Diet (HFD) | Research Diets | D12492 |
HIGHLIGHTS.
Three new Pdgfra/Pdgfrb knockin mice for mosaic mutant analysis were made.
Pdgfra- and Pdgfrb-lineages make distinct contributions to adipocyte differentiation
Differentiation is transiently blocked by constitutive PDGFRα signaling
Mosaic Pdgfra or Pdgfrb knockout enhances white/beige/brown adipogenesis
ACKNOWLEDGEMENTS
We thank Linda Thompson, Sathish Srinivasan, Philippe Soriano, Juan Sanchez-Gurmaches, and members of the Olson lab for helpful discussions, and Kevin Kelley at the Icahn School of Medicine at Mt. Sinai Mouse Genetics and Gene targeting core for assistance in generating chimeric mice. We also thank the Flow Cytometry Core and Imaging Core Facilities (associated with P20-GM103636) and the Microscopy Core and Mouse Phenotyping Core Facilities (associated with P30-GM114731) of the Oklahoma Medical Research Foundation Centers of Biomedical Research Excellence. This study was supported by US National Institutes of Health (NIH) grants R01-AR070235 and P20-GM103636, and grants from the Oklahoma Center for Adult Stem Cell Research - a program of TSET (L.E.O). C.S. was supported by a predoctoral fellowship from the American Heart Association. L.E.O. is a Pew Scholar in Biomedical Research and this work was supported in part by the Pew Charitable Trusts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS
The authors declare no competing or financial interests.
REFERENCES
- Andrae J, Gallini R, and Betsholtz C (2008). Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22, 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, and Betsholtz C (2011). Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21, 193–215. [DOI] [PubMed] [Google Scholar]
- Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, Bernard S, and Arner P (2010). Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, and Rodeheffer MS (2013). Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 15, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry WL, Kim TD, and Janknecht R (2014). Stimulation of beta-catenin and colon cancer cell growth by the KDM4B histone demethylase. International journal of oncology 44, 1341–1348. [DOI] [PubMed] [Google Scholar]
- Bertholet AM, Kazak L, Chouchani ET, Bogaczynska MG, Paranjpe I, Wainwright GL, Betourne A, Kajimura S, Spiegelman BM, and Kirichok Y (2017). Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab 25, 811–822 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burl RB, Ramseyer VD, Rondini EA, Pique-Regi R, Lee YH, and Granneman JG (2018). Deconstructing Adipogenesis Induced by beta3-Adrenergic Receptor Activation with Single-Cell Expression Profiling. Cell Metab 28, 300–309 e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo P, Mukherjee D, Spinozzi S, Zhang L, Larcher V, Stallcup WB, Kataoka H, Chen J, Dimmeler S, Evans SM, et al. (2020). Parallel Lineage-Tracing Studies Establish Fibroblasts as the Prevailing In Vivo Adipocyte Progenitor. Cell reports 30, 571–582 e572. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, and MacDougald OA (2012). Adipose tissue stem cells: the great WAT hope. Trends in endocrinology and metabolism: TEM 23, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby JR, Seifert RA, Soriano P, and Bowen-Pope DF (1998). Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet 18, 385–388. [DOI] [PubMed] [Google Scholar]
- Gao Z, Daquinag AC, Su F, Snyder B, and Kolonin MG (2018). PDGFRalpha/PDGFRbeta signaling balance modulates progenitor cell differentiation into white and beige adipocytes. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, et al. (2017). Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 20, 345–359 e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes-Camboa N, and Evans SM (2017). Are Perivascular Adipocyte Progenitors Mural Cells or Adventitial Fibroblasts? Cell Stem Cell 20, 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TG, Klinghoffer RA, Corrin PD, and Soriano P (2003). Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol 23, 4013–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, and Koh GY (2011). The spatiotemporal development of adipose tissue. Development 138, 5027–5037. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, and McMahon AP (2002). Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev 119 Suppl 1, S97–S101. [DOI] [PubMed] [Google Scholar]
- He C, Medley SC, Kim J, Sun C, Kwon HR, Sakashita H, Pincu Y, Yao L, Eppard D, Dai B, et al. (2017). STAT1 modulates tissue wasting or overgrowth downstream from PDGFRbeta. Genes Dev. [DOI] [PMC free article] [PubMed]
- Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, et al. (2003). PDGFrA activating mutations in gastrointestinal stromal tumors. Science 299, 708–710. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Griffith D, McKinley A, Patterson J, Presnell A, Ramachandran A, and Debiec-Rychter M (2012). Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin Cancer Res 18, 4375–4384. [DOI] [PubMed] [Google Scholar]
- Heldin CH, and Westermark B (1999). Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79, 1283–1316. [DOI] [PubMed] [Google Scholar]
- Hepler C, Shan B, Zhang Q, Henry GH, Shao M, Vishvanath L, Ghaben AL, Mobley AB, Strand D, Hon GC, et al. (2018). Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch RV, and Soriano P (2003). Roles of PDGF in animal development. Development 130, 4769–4784. [DOI] [PubMed] [Google Scholar]
- Hong KY, Bae H, Park I, Park DY, Kim KH, Kubota Y, Cho ES, Kim H, Adams RH, Yoo OJ, et al. (2015). Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development 142, 2623–2632. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Maretich P, and Kajimura S (2018). The Common and Distinct Features of Brown and Beige Adipocytes. Trends in endocrinology and metabolism: TEM 29, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, and Olson LE (2015). PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev 29, 1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, Jo A, Tang W, Arpke RW, Kyba M, and Graff JM (2017). A PPARgamma transcriptional cascade directs adipose progenitor cell-niche interaction and niche expansion. Nat Commun 8, 15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, and Seale P (2015). Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab 22, 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, et al. (2007). Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117, 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M, and Soriano P (2001). The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Molecular cell 7, 343–354. [DOI] [PubMed] [Google Scholar]
- Kranz A, Fu J, Duerschke K, Weidlich S, Naumann R, Stewart AF, and Anastassiadis K (2010). An improved Flp deleter mouse in C57Bl/6 based on Flpo recombinase. Genesis 48, 512–520. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, Bickel PE, and Scherer PE (2016). Targeting adipose tissue in the treatment of obesity-associated diabetes. Nature reviews Drug discovery 15, 639–660. [DOI] [PubMed] [Google Scholar]
- Lao Z, Raju GP, Bai CB, and Joyner AL (2012). MASTR: a technique for mosaic mutant analysis with spatial and temporal control of recombination using conditional floxed alleles in mice. Cell reports 2, 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, and Granneman JG (2013). Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab 18, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Konkar AA, and Granneman JG (2015). Cellular origins of cold- induced brown adipocytes in adult mice. FASEB J 29, 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, and Granneman JG (2012). In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 15, 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, and Betsholtz C (1997). Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242–245. [DOI] [PubMed] [Google Scholar]
- Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, et al. (2015). Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, Percec I, and Seale P (2019). Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, et al. (2007). Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56, 1517–1526. [DOI] [PubMed] [Google Scholar]
- Olson LE, and Soriano P (2009). Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, and Soriano P (2011). PDGFRbeta Signaling Regulates Mural Cell Plasticity and Inhibits Fat Development. Dev Cell 20, 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onogi Y, Wada T, Kamiya C, Inata K, Matsuzawa T, Inaba Y, Kimura K, Inoue H, Yamamoto S, Ishii Y, et al. (2017). PDGFRbeta Regulates Adipose Tissue Expansion and Glucose Metabolism via Vascular Remodeling in Diet-Induced Obesity. Diabetes 66, 1008–1021. [DOI] [PubMed] [Google Scholar]
- Pontes-Quero S, Heredia L, Casquero-Garcia V, Fernandez-Chacon M, Luo W, Hermoso A, Bansal M, Garcia-Gonzalez I, Sanchez-Munoz MS, Perea JR, et al. (2017). Dual ifgMosaic: A Versatile Method for Multispectral and Combinatorial Mosaic Gene-Function Analysis. Cell 170, 800–814 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, and Soriano P (2007). High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS One 2, e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, and Brown EJ (2007). Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl J, Rizzolo K, and Soriano P (2008). The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev 22, 3255–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, and Tseng YH (2013). Brown adipose tissue: development, metabolism and beyond. Biochem J 453, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalie PC, Dong H, Zachara M, Russeil J, Alpern D, Akchiche N, Caprara C, Sun W , Schlaudraff KU, Soldati G, et al. (2018). A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. [DOI] [PubMed]
- Shao M, Vishvanath L, Busbuso NC, Hepler C, Shan B, Sharma AX, Chen S, Yu X, An YA, Zhu Y, et al. (2018). De novo adipocyte differentiation from Pdgfrbeta(+) preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat Commun 9, 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P (1994). Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8, 1888–1896. [DOI] [PubMed] [Google Scholar]
- Soriano P (1997). The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124, 2691–2700. [DOI] [PubMed] [Google Scholar]
- Sun C, Berry WL, and Olson LE (2017). PDGFRalpha controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development 144, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, French WJ, and Soriano P (2003). Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS biology 1, E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, and Graff JM (2008). White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, and Gupta RK (2016). Pdgfrbeta+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab 23, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, and Scherer PE (2013). Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19, 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, and Rubin GM (2012). The effort to make mosaic analysis a household tool. Development 139, 4501–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Wang QA, Tao C, Vishvanath L, Shao M, McDonald JG, Gupta RK, and Scherer PE (2015). Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Molecular metabolism 4, 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Espinosa JS, Su HH, Muzumdar MD, and Luo L (2005). Mosaic analysis with double markers in mice. Cell 121, 479–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1. bR-Flp/Tomato; aR-H2BEGFP double reporter ingWAT related to Fig 3f
Supplementary Video 2. bR-Flp/Tomato; aR-H2BEGFP double reporter pWAT related to Fig 3f
Supplementary Video 3. bR-Flp/Tomato; aR-H2BEGFP double reporter iBAT related to Fig 3f
Data Availability Statement
The quantitative datasets supporting the current study have been deposited in the “Mendeley Data” repository, dataset “Mosaic mutant analysis identifies PDGFRα/PDGFRβ as negative regulators of adipogenesis”, https://doi:10.17632/26r263sm6w.1.