Abstract
Background:
Individuals with ADHD and other externalizing psychopathologies tend to display poor behavioral performance on the go/no-go task, which is thought to reflect deficits in inhibitory control. However, clinical neuroimaging studies using this task have yielded conflicting results, raising basic questions about what the task measures and which aspects of the task relate to clinical outcomes. We used computational modeling to provide a clearer understanding of how neural activations from this task relate to the cognitive mechanisms that underlie performance and probe the implications of these relationships for clinical research.
Methods:
143 young adults (ages 18–21) performed the go/no-go task during fMRI scanning. We used the diffusion decision model (DDM), a cognitive modeling approach, to quantify distinct neurocognitive processes that underlie go/no-go performance. We then assessed correlations between DDM parameters and brain activation from standard go/no-go contrasts and assessed relationships of DDM parameters and associated neural measures with clinical ratings.
Results:
Right-lateralized prefrontal activations on correct inhibition trials, which are generally assumed to isolate neural processes involved in inhibition, were unrelated to DDM parameters (and other performance indices). However, responses to failed inhibitions in brain regions associated with error monitoring were strongly related to more efficient task performance, and correlated with externalizing behavior and ADHD symptoms.
Conclusions:
Our findings cast doubt on conventional interpretations of go/no-go task-related activations as reflecting the neural basis of inhibitory functioning. We instead find that error-related contrasts provide clinically-relevant information about neural systems involved in monitoring and optimizing the efficiency of cognitive performance.
Keywords: computational psychiatry, diffusion decision model, externalizing, ADHD, error monitoring systems, inhibitory control
Introduction
The go/no-go task, in which participants are asked to make a motor response following stimulus presentations but withhold their response after a subset of “no-go” stimuli, is one of the most ubiquitous experimental paradigms in clinical neuroscience. Commonly assumed to index an individual’s ability to inhibit pre-potent or impulsive responses, behavioral metrics from the task, including false alarm (FA) rate and response times, indicate generally poorer task performance in attention-deficit/hyperactivity disorder (ADHD), substance use disorders, and externalizing psychopathology more broadly (1–3). Such findings are often cited as evidence in support of the hypothesis that poor inhibitory control is a trans-diagnostic risk factor for externalizing disorders (4–8). In turn, functional magnetic resonance imaging (fMRI) measures of go/no-go task-related neural activations have played a fundamental role in research on the neurodevelopmental mechanisms of inhibitory control (9,10) and on aberrant brain processes in clinical conditions associated with disinhibition (11–14).
Go/no-go neuroimaging studies typically focus on several contrasts. Analyses in which activity during successful inhibitions (SIs: “no-go” trials where a response is inhibited) is contrasted against activity during “go” trials or a baseline are typically assumed to isolate neural activity related to inhibitory processes (15,16; cf. 17), and tend to reveal right-lateralized activation in prefrontal and parietal structures, including the inferior frontal gyrus (IFG) most prominently (17,18). This approach is based on the “pure insertion” assumption that intact inhibitory processes are present during SIs, but not other trials, and hence that individuals’ magnitude of activation during SIs should correspond to individual or clinical differences in the integrity of response inhibition (13,16,19,20). In addition, analyses in which activity during failed inhibitions (FIs) is contrasted against that during other trials or baseline may be conducted with the goal of indexing neural activity related to error monitoring. Indeed, such contrasts typically reveal activation in regions associated with the processing of errors, including the anterior cingulate (ACC) and insula (19–24).
Despite the go/no-go paradigm’s success in reliably eliciting patterns of neural activation, associations between these activations and clinical outcomes are sometimes difficult to interpret. Across a variety of studies using this paradigm, clinical groups with behavioral disinhibition symptomatology have alternately been found to display reduced (12,19,20,25) or increased (13,26,27,28,29) activation in the brain structures assumed to implement inhibitory processes. Furthermore, significant clinical differences in go/no-go task-related activation are often found when behavioral performance differences are absent (12,14,23,26,28,29). Taken together, these trends in the literature challenge the conventional assumption that the magnitude of neural responses from go/no-go task contrasts indexes the same underlying construct of inhibitory control that is assumed to be indexed by the task’s behavioral measures.
This variability in the go/no-go neuroimaging literature, despite reliable evidence for worse task performance in clinical conditions, could be explained if activations from traditional contrasts do not, despite common assumptions, index fundamental neural mechanisms that underlie individual differences in inhibitory performance. One promising way to assess this possibility is to apply well-validated computational models of the go/no-go, which are formal theories of the data-generating process in the task and yield quantitative measures of individual differences in latent mechanisms that underlie inhibitory performance metrics (e.g., FA rate). Such models can be used to test whether task activations display evidence of relationships with individual differences in one or more of these latent mechanisms.
In this study, we apply the diffusion decision model (DDM), a well-validated computational model of two-choice decisions (30,31) that has been extended to explain performance in the go/no-go paradigm (32–34). Although the go/no-go may not be widely viewed as a “decision” task, experimental work has demonstrated that the mechanisms of deciding whether to respond or withhold from responding are functionally comparable to those of decisions between two response options (32, 34). Indeed, DDM and related decision-making models have consistently provided excellent accounts of behavioral data from the go/no-go (32–35). The DDM frames the decision of whether to respond or withhold as a noisy evidence accumulation process that drifts between boundaries which represent each of the two possible decision outcomes (Figure 1). Although the process typically drifts towards the correct boundary (e.g., the “withhold” boundary on trials with “no-go” stimuli), errors occur when it terminates at the other boundary due to noise. In the DDM framework, several cognitive mechanisms can explain differences in go/no-go task performance: 1) individuals’ efficiency of evidence accumulation towards the correct choice, as indexed by drift rate (v) parameters, 2) response biases, which are indexed by the start point (z) parameter and tend to favor decisions with higher probabilities, such as responding in standard go/no-go paradigms (31), 3) caution, as indexed by a parameter for the separation between boundaries (a), with lower values indicating a faster but more error-prone decision-making style, and 4) time taken up by processes peripheral to the decision (e.g., encoding, motor responses), indexed by a non-decision time (Ter) parameter.
Figure 1.

Simplified schematic of the diffusion decision model explanation of the go/no-go task, as outlined by Ratcliff, Huang-Pollock & McKoon (2018) and Huang-Pollock et al. (2017). On each trial, the decision process drifts between an upper boundary for the decision to respond, set at parameter a, and a lower (implicit) boundary for the decision to withhold from responding, set at 0. The process begins at the location determined by the start point (z) parameter and moves over time according to a stochastic process (similar to a random walk) that generally terminates at the correct decision boundary, but occasionally terminates at the incorrect decision boundary due to noise (i.e., on error trials). The rate at which the process drifts toward the correct boundary (v) is estimated separately for trials with “go” stimuli (v.go) and trials with “no-go” stimuli (v.nogo). RT on a given trial is determined by the “decision time”, which is the amount of time it takes the process to reach one of the two boundaries, and the “non-decision time” (Ter parameter), which accounts for time taken up by processes peripheral to the decision (e.g., stimulus encoding, motor response latency). Note that the start point (z) is closer to the upper boundary for the decision to respond. This reflects a bias that is expected in a go/no-go task, or any other task where one type of decisional outcome is more likely to be correct than the other (e.g., when “go” stimuli are much more common than “no-go” stimuli), because participants develop an a priori expectation for which outcome is most likely to be correct.
The current study applied the DDM to data from a large sample of individuals at risk for externalizing psychopathology to provide a clearer understanding of whether neural activations from the go/no-go paradigm relate to the latent mechanisms that underlie task performance, and implications of these relationships for the study of psychopathology. Under the assumption that the latent mechanisms indexed by the DDM are the main determinants of go/no-go performance metrics (FA rates, response times), evidence for (or against) relationships between task activations and individual differences in these mechanisms would validate (or invalidate) the idea that activations reflect fundamental determinants of inhibitory performance.
Methods
Participants
An initial sample of 147 participants, ages 18–21, was recruited from the Michigan Longitudinal Study (MLS) to participate in a neuroimaging study. The MLS is an ongoing prospective study that follows a community sample of families with a history of alcohol use disorder (AUD) and low-risk families from the same neighborhoods (36,37). Participants were excluded from participating in the larger MLS if they displayed signs of fetal alcohol syndrome, and were excluded from the neuroimaging study if they 1) displayed contraindications to MRI, 2) were left-handed, 3) suffered from a neurological, acute or chronic medical illness, 4) had a personal, or first-degree relative, history of psychosis or schizophrenia, or 5) were prescribed psychoactive medications in the past 6 months, with the exception of psychostimulants prescribed for attention difficulties. Participants using prescribed psychostimulants (n=4) were asked to discontinue their medication at least 48 hours before scanning. All procedures were approved by an Institutional Review Board, and participants provided written informed consent.
Three participants were excluded because their behavioral data did not meet quality control criteria for model-based analyses (>=200 available trials and >.55 overall accuracy) and one was excluded because they did not commit any FAs, precluding analysis of FI contrasts. This left a final sample of 143 participants (Table 1).
Table 1.
Characteristics of the sample and descriptive statistics for cognitive and behavioral measures. For continuous variables, numbers indicate the mean with standard deviations in parentheses. Substance use data (drinks, binge drinking days and marijuana use days per year) reflect averages across all available measurements from individuals at ages 18–21. ASR summary statistics do not include the 5 subjects with missing data on these measures (all were male). AUD = alcohol use disorder; SUD = substance use disorder; FH-AUD = family history of alcohol use disorder (either parent)
| Gender (Male/Female) | 87/56 |
| Age at scan | 19.66 (1.22) |
| Race/Ethnicity | |
| Caucasian | 128 |
| Hispanic/Latino | 5 |
| African American | 6 |
| Other/bi-racial | 4 |
| FH-AUD (Positive/Negative/Unknown) | 108/34/1 |
| Average drinks/year | 363(540) |
| Average binge drinking days/year | 36(63) |
| Number that ever used marijuana between ages 18–21 | 77 |
| Average marijuana use days/year | 38(83) |
| Current AUD Diagnosis | 20 |
| Current other SUD Diagnosis | 16 |
| AUD + other SUD Diagnosis | 7 |
| Current ADHD Diagnosis | 15 |
| Currently prescribed stimulant medications | 4 |
| ASR Externalizing - raw score | 9.43 (7.90) |
| ASR Externalizing - T score | 48.98 (10.13) |
| ASR ADHD Symptoms - raw score | 5.02 (4.26) |
| ASR ADHD Symptoms - T score | 54.33 (6.56) |
| Drift rate for “go” stimuli (v.go) | 2.77 (1.03) |
| Drift rate for “no-go” stimuli (v.nogo)* | 1.90 (0.88) |
| Average drift rate for all stimuli (v.avg) | 2.34 (0.88) |
| Boundary separation (a) | 1.00 (0.21) |
| Non-decision time (Ter)** | .317 (.032) |
| Response bias (z) | 0.62 (0.07) |
| Hit RT mean (MRT)** | .448 (.061) |
| Hit RT standard deviation (SDRT)** | .105 (.055) |
| False alarm (FA) rate | 0.28 (0.16) |
| Number of FAs | 16.41(9.66) |
Multiplied by −1 for comparability to v.go;
seconds
Go/No-Go Task
Participants completed an event-related go/no-go task (10) during fMRI. They were presented with a series of letters for 500ms at a time (interstimulus fixation interval of 3500ms) and were asked to press a button for every letter other than “X” (“go” trials) but to withhold their response when “X” was presented (“no-go” trials). Participants completed 5 182-second imaging runs of 49 trials, for a total of 245 trials, 60 (25%) of which were “no-go” trials. Neuroimaging acquisition parameters and pre-processing steps (38,39) are reported in Supplemental Materials.
Psychopathology Measures
Participants completed the neuroimaging study in between MLS data collection waves. At waves 6–8 (ages 18–24), participants filled out the Adult Self Report (ASR: 40) measure. For each participant, the administration of the ASR closest in time to the scan was identified (mean days between ASR administration and scan=421, SD=401), and raw scores from this administration were used. Five participants (all male) did not have ASR data available and were therefore not included in these analyses.
DDM Analysis
The go/no-go version of the DDM outlined by (32,33) was fit to data using functions from the R (41) package rtdists (42) following the chi-square minimization procedure described in these studies. As between-trial variability parameters are difficult to estimate without massive trial numbers (43) and “simple” versions of the DDM, without these parameters, provide estimates of the main model parameters that appear to be comparably informative to those of the “full” DDM (44), only the main DDM parameters were estimated: drift rates for “go” and “no-go” stimuli, (v.go, v.nogo), starting point (z), boundary separation (a) and non-decision time (Ter). The upper boundary was assumed to trigger responses, while the lower boundary was the non-response boundary. Start point (z) was estimated as a proportion of a, and z values greater than .50 therefore indicate the expected bias towards the upper “go” boundary. Additional information on model specification, estimation, and fit is available in Supplemental Materials.
Individual-level fMRI Analyses
An individual-level general linear model was fit with three regressors convolved with the hemodynamic response function: 1) correct go trials (hereafter referred to as the GO condition), 2) SI trials, and 3) FI trials. Motion parameters from realignment and average white matter signal intensity for each volume were included as nuisance regressors. Statistical maps for each subject were then generated for two contrasts of interest: 1) the standard “response inhibition” contrast (SI>GO), and 2) an “error monitoring” contrast (FI>GO).
Data Analytic Plan
In order to conduct a comprehensive search for fMRI correlates of DDM parameters, we completed both 1) whole-brain regression analyses and 2) region of interest (ROI) correlation analyses using ROIs from previous studies. Although we focused on DDM parameters, we also used the same methods to identify fMRI correlates of summary statistics (Supplemental Materials), including FA rate and the mean and standard deviation of “go” response times (MRT and SDRT).
In two whole-brain analyses, all DDM parameters were entered as predictors of neural activation in the SI>GO and FI>GO contrast maps, respectively. Results were thresholded at a familywise error (FWE) rate of .05, and further corrected for multiple comparisons using false discovery rate (FDR: cluster-level q<.05). One-sample t-tests of activation in each contrast were conducted using the same thresholds to ensure that univariate activations were consistent with prior work.
In the ROI analysis, we first identified sets of ROI coordinates that prior research suggested would likely correspond to activations in each contrast. For the “response inhibition” (SI>GO) contrast, we used coordinates of the 11 regions found to be active during no-go>go contrasts in a 2013 meta-analysis of event-related go/no-go studies (17). Coordinates were transformed from Talairach to MNI space using the Lancaster (45) method. For the “error monitoring” (FI>GO) contrast, there did not appear to be any previous meta analyses of error-related activations in the go/no-go task. We thus used Neurosynth (46) to identify coordinates for regions linked to error processing. Specifically, we conducted a term-based meta-analysis with the term “error” on 6/4/2019 and downloaded the resulting statistical map for the “association test”, which tests whether activation in each voxel occurs more consistently in studies that mention the term than in those that do not (see: neurosynth.org/faq). We excluded clusters with <10 voxels from this map and identified peak coordinates for the 8 clusters that remained. Once ROI coordinates for both contrasts were identified, parameter estimates, averaged within 8mm-radius spheres centered about each coordinate, were extracted using MarsBaR (47).
Next, we conducted correlation analyses between each DDM parameter and activation estimates from each ROI in JASP (48), a statistical package which allows for both frequentist and Bayesian versions of common tests. Bayesian analyses allow estimation of 95% posterior credible intervals, which indicate the range in which there is a .95 probability that each r value falls, as well as Bayes factors (BF10)1. BF10 is intuitively interpreted as an odds ratio for the research hypothesis; a value of 5, for example, indicates the data are 5 times more likely under the research hypothesis than under the null hypothesis. We used BF10 for inference due to its ability to quantify evidence for both the research and null hypotheses (49,50) but also report frequentist p-values, corrected for multiple comparisons using FDR (q<.05, families defined by DDM parameter) (50), to corroborate BF10 inferences and assess whether correlations may have resulted from multiple testing.
Once associations between DDM parameters and neural activations were identified, we tested whether the parameters and activations that were part of these associations displayed evidence of clinical relevance. To avoid bias associated with “double dipping”, we focused on neural correlates identified in the ROI analysis. We selected any parameter which displayed a robust relationship (defined as both BF10 >3 and having a significant p-value after FDR correction) with at least one ROI, and then selected the individual ROIs most strongly related to that parameter in the SI>GO and FI>GO contrasts, respectively. Next, we assessed correlational relationships between these measures and raw scores from two ASR scales: externalizing behavior and DSM-IV ADHD symptoms. The former was selected because of previously-reported associations between go/no-go activations and risk for substance use and other externalizing behaviors (14,23,28,29,53). The latter was selected because of well-established associations between DDM parameters and ADHD (33,54–56).
Results
DDM Parameter Estimates
Plots comparing empirical RT and accuracy data to predictions of the DDM suggested that the model generally described behavioral data well (Supplemental Materials). Notably, most participants’ start point (z) values were above .50 (Table 1), indicating that they were biased toward the decision to respond, as would be expected for a task with a greater proportion of “go” than “no-go” stimuli.
Table 2 displays Pearson correlation (r) values and CIs for relationships between all DDM parameters and traditional behavioral summary statistics. Higher FA rates were related to lower caution (a) and greater bias toward “go” (z), as expected, but were most strongly related to lower drift rates. Notably, the correlation of FA rate with v.go was almost identical to its correlation with v.nogo. This finding, combined with the fact the v.go and v.nogo are also strongly correlated (r=.73), suggests that FA rates on the task are largely determined by individuals’ general efficiency of evidence accumulation, which influences behavior across all trials of the task rather than being specific to “no-go” trials. Therefore, for all subsequent analyses, v.go and v.nogo were averaged to provide a general index of evidence accumulation efficiency (v.avg). Hit MRT was most strongly related to a and Ter, while hit SDRT was strongly related to drift rate, consistent with previous simulation studies (57). Overall, these analyses suggest that traditional summary statistics are determined by various DDM parameters, although there are no clear one-to-one mappings between parameters and traditional metrics.
Table 2.
Bayesian Pearson correlation values for relationships between commonly-used behavioral summary statistics from the go/no-go task and all DDM parameters. For each correlation, the first number indicates the posterior median, representing the most likely r value, while the second and third numbers (in italics) indicate the upper and lower bounds of the 95% credible interval (CI) for the r value. FA = false alarm, MRT = mean response time, SDRT = standard deviation of response time, v.go = drift rate on “go” trials, v.nogo = drift rate on “no go” trials, v.avg = mean of v.go and v.nogo, z = start point (with higher values), a = boundary separation, Ter = non-decision time.
| Bayesian Pearson Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|
| FA rate | Hit MRT | Hit SDRT | v.go | v.nogo | v.avg | z | a | |
| FA rate | — | |||||||
| — | ||||||||
| — | ||||||||
| Hit MRT | −.10 | — | ||||||
| .07 | — | |||||||
| −.25 | — | |||||||
| Hit SDRT | .23 | .76 | — | |||||
| .38 | .82 | — | ||||||
| .07 | .67 | — | ||||||
| v.go | −.67 | −.42 | −.64 | — | ||||
| −.56 | −.27 | −.53 | — | |||||
| −.75 | −.54 | −.73 | — | |||||
| v.nogo | −.66 | −.33 | −.53 | .73 | — | |||
| −.55 | −.18 | −.39 | .79 | — | ||||
| −.74 | −.47 | −.63 | .63 | — | ||||
| v.avg | −.71 | −.41 | −.63 | .94 | .92 | — | ||
| −.62 | −.26 | −.52 | .96 | .94 | — | |||
| −.78 | −.53 | −.72 | .92 | .88 | — | |||
| z | .53 | −.29 | −.19 | −.14 | −.07 | −.12 | — | |
| .63 | −.13 | −.02 | .02 | .10 | .05 | — | ||
| .39 | −.43 | −.34 | −.30 | −.23 | −.27 | — | ||
| a | −.34 | .55 | .38 | .14 | −.10 | .03 | −.08 | — |
| −.18 | .65 | .51 | .29 | .06 | .19 | .09 | — | |
| −.47 | .42 | .23 | −.03 | −.26 | −.14 | −.24 | — | |
| Ter | −.21 | .50 | −.03 | .11 | .12 | .12 | .02 | .10 |
| −.05 | .61 | .13 | .26 | .28 | .28 | .18 | .26 | |
| −.36 | .36 | −.19 | −.06 | −.04 | −.04 | −.14 | −.06 |
Univariate fMRI Analyses
Whole-brain maps for univariate contrasts (Figure 2a; Table 3) revealed neural responses that were consistent with previous literature. The SI>GO contrast revealed right-lateralized activity in regions commonly inferred to be involved in top-down inhibitory control, such as the IFG (18,58,59). The FI>GO contrast identified areas previously associated with error processing and performance monitoring, including the ACC and bilateral clusters spanning the IFG and anterior insula (22,24). Conjunction images (Supplemental Materials) indicated that there was considerable overlap between the two contrasts in prefrontal, insular and parietal regions and the ACC.
Figure 2.

Whole-brain t-statistic maps of neural activations in the main fMRI contrasts of interest (a) and results from whole-brain regression analyses involving DDM parameters (b) at selected axial slices. All statistical maps were thresholded at FWE<.05 and further corrected for multiple comparisons using FDR (cluster-level q<.05). White numbers in the bottom right corner indicate the z-coordinate for each slice. SI = successful inhibition; FI = failed inhibition; GO = correct go trial; v.avg = mean of drift rates on “go” and “no go” trials.
Table 3.
Peak MNI coordinates, anatomical region, Brodmann area (BA), and size (in voxels) of all clusters that survived thresholding at FWE<.05 and correction for multiple comparisons using FDR (cluster-level q<.05) in the whole-brain one-sample t-tests aimed at identifying activation in the SI>GO and FI>GO contrasts. Coordinates for up to two additional local maxima (>8mm apart) within each cluster are also reported in italics.
| Contrast | Region (BA) | x | y | z | Size |
|---|---|---|---|---|---|
| SI>GO Activation | R. Inferior Parietal (40) | 58 | −44 | 28 | 27574 |
| R. Middle Frontal (9) | 40 | 42 | 22 | ||
| R. Insula/IFG (13) † | 34 | 20 | 0 | ||
| L. Supramarginal (39) | −58 | −48 | 34 | 5764 | |
| L. Superior Temporal (39) | −62 | −50 | 24 | ||
| L. Superior Temporal (22) | −62 | −44 | 12 | ||
| L. Middle Frontal (10) | −34 | 38 | 26 | 1062 | |
| L. Middle Frontal (10) | −34 | 46 | 24 | ||
| L. Middle Frontal (*) | −40 | 38 | 40 | ||
| L. Orbitofrontal (10) | −22 | 58 | −14 | 126 | |
| Posterior Cingulate (*) | 8 | −10 | 28 | 114 | |
| Posterior Cingulate (*) | 4 | −22 | 24 | ||
| Posterior Cingulate (*) | −10 | −22 | 30 | ||
| L. Precentral (4) | −36 | −20 | 50 | 487 | |
| L. Precentral (6) | −32 | −12 | 56 | ||
| L. Postcentral (1) | −44 | −26 | 48 | ||
| R. Orbitofrontal (11) | 12 | 20 | −22 | 68 | |
| FI>GO Activation | Anterior Cingulate (32) | 4 | 30 | 26 | 20070 |
| R. Insula (13) | 44 | 14 | −4 | ||
| L. Middle Frontal (10) | −28 | 50 | 22 | ||
| L. Insula (13) | −44 | 14 | −6 | 4518 | |
| L. Insula (13) | −32 | 18 | −12 | ||
| L. Inferior Parietal (39) | −60 | −42 | 30 | ||
| R. Inferior Parietal (40) | 60 | −42 | 26 | 3525 | |
| R. Middle Temporal (21) | 52 | −30 | −8 | ||
| R. Middle Temporal (21) | 54 | −40 | −2 | ||
| L. Cerebellum (*) | −32 | −60 | −28 | 218 | |
| L. Cerebellum (*) | −30 | −48 | −32 | ||
| R. Cerebellum (*) | 38 | −52 | −32 | 858 | |
| Cerebellum (*) | 2 | −72 | −20 | ||
| Cerebellum (*) | 2 | −60 | −8 | ||
| R. Middle Temporal (20) | 46 | 2 | −40 | 118 | |
| R. Precuneus (7) | 12 | −68 | 36 | 638 | |
| L. Precuneus (7) | −12 | −68 | 32 | ||
| R. Cuneus (17) | 12 | −76 | 8 | 514 | |
| L. Cuneus (17) | −6 | −74 | 6 | ||
| Brainstem (*) | 10 | −36 | −34 | 62 | |
| L. Cerebellum (*) | −10 | −48 | −32 | ||
| R. Orbitofrontal (10) | 26 | 54 | −16 | 139 |
Although this local maximum coordinate falls in the insula (BA 13), activation extends into the nearby IFG, suggesting that both structures contribute to activation in this cluster;
coordinate is outside of defined BAs
Associations with Cognitive Mechanisms
The only clusters from whole-brain regression analyses that survived corrections for multiple comparisons were activations from the FI>GO contrast that were positively related to v.avg (Figure 2b; Table 4). These activations included multiple regions previously linked to error monitoring, such as the ACC, IFG and anterior insula, as well as two areas in middle temporal gyrus.
Table 4.
Peak MNI coordinates, anatomical region, Brodmann area (BA), and size (in voxels) of all clusters that survived thresholding at FWE<.05 and correction for multiple comparisons using FDR (cluster-level q<.05) in the whole-brain regression analyses involving DDM parameters. Coordinates for up to two additional local maxima (>8mm apart) within each cluster are also reported in italics. The first column indicates the contrast map used, the DDM parameter involved, and the direction of the relationships (positive vs. negative). v.avg = mean of drift rates on “go” and “no go” trials;
| Contrast / Parameter / Relationship Direction | Region (BA) | x | y | z | Size |
|---|---|---|---|---|---|
| FI>GO / v.avg / Positive | R. Inferior Frontal (45) | 50 | 24 | 0 | 348 |
| R. Insula (47) | 32 | 24 | −10 | ||
| R. Insula (13) | 34 | 16 | −6 | ||
| R. Anterior Cingulate (32) | 8 | 38 | 18 | 382 | |
| R. Anterior Cingulate (10) | 4 | 48 | 8 | ||
| L. Anterior Cingulate (*) | −10 | 34 | 14 | 65 | |
| L. Anterior Cingulate (*) | −10 | 40 | 2 | ||
| R. Middle Temporal (21) | 62 | −30 | −8 | 94 | |
| R. Middle Temporal (22) | 58 | −16 | −10 | 37 |
coordinate is outside of defined BAs
Consistent with whole-brain analyses, the strongest associations identified in ROI analyses (Tables 5–6) were positive correlations between v.avg and activations from the FI>GO contrast in regions previously linked to error processing. FI-related activations in the bilateral insula/IFG were the most strongly correlated with v.avg and displayed decisive evidence (BF10>100) for a relationship. The only correlational relationship involving ROI activations from the SI>GO contrast to survive FDR correction was a negative relationship between v.avg and activation in the right superior temporal gyrus (STG), although BF10 suggested that evidence was preliminary (BF10=2.40). No other DDM parameters displayed relationships that survived correction, and BF10 generally suggested that evidence for these relationships was moderate at best, and typically against their presence. Follow-up analyses focusing on ROI correlations with v.avg (Supplemental Materials) indicated that activations of ROIs in the insula and IFG were significantly more strongly correlated with v.avg in the FI>GO contrast than in the SI>GO contrast, suggesting that, although these regions appear to be active in both contrasts, their relationship with v.avg is driven by error-related activity.
Table 5.
Most likely correlation (r) values, 95% posterior credible intervals (CIs), Bayes factors (BF10) and frequentist p-values for correlational relationships between ROI activations and the DDM parameters indexing efficiency (v.avg) and bias (z). The first column lists the contrast, anatomical region, and MNI coordinates (x, y and z in parentheses) for each ROI. For p-values: bolded = survives FDR correction for multiple comparisons within each family of tests (families defined by DDM parameters)
| Efficiency (v.avg) | Bias (z) | |||||
|---|---|---|---|---|---|---|
| Region | r [CI] | BF10 | p | r [CI] | BF10 | p |
| SI/GO L. Inferior Frontal (−35,21,−9) | −.11[−.27,.05] | 0.26 | 0.179 | −.02[−. 18,14] | 0.11 | 0.808 |
| SI/GO L. Middle Frontal (−46,25,25) | −.10[−.26,.06] | 0.22 | 0.227 | −. 10[−.26,.06] | 0.21 | 0.230 |
| SI/GO L. Supramarginal (−59,−50,35) | −.02[−.18,.15] | 0.11 | 0.833 | .08[−.08,.24] | 0.17 | 0.321 |
| SI/GO Medial Frontal (2,22,41) | .11[−.06,.27] | 0.24 | 0.196 | −.02[−.18,.14] | 0.11 | 0.809 |
| SI/GO L. Medial Frontal (−38,59,3) | .04[−.13,.20] | 0.11 | 0.680 | .01[−.16,.17] | 0.11 | 0.938 |
| SI/GO R. Inferior Frontal (51,14,25) | .13[−.04,.28] | 0.33 | 0.130 | −.18[−.33,−.02] | 1.12 | 0.029 |
| SI/GO R. Inferior Frontal (39,24,−10) | .01[−.15,.17] | 0.11 | 0.920 | .06[−.10,.22] | 0.14 | 0.462 |
| SI/GO R. Inferior Parietal (51,−50,41) | .18[.02,.33] | 1.03 | 0.032 | .04[−.13,.20] | 0.11 | 0.676 |
| SI/GO R. Middle Frontal (46,39,24) | .11[−.05,.27] | 0.25 | 0.184 | .01[−.16,.17] | 0.11 | 0.948 |
| SI/GO R. Superior Frontal (13,18,58) | .13[−.04,.29] | 0.34 | 0.121 | .04[−.13,.20] | 0.11 | 0.678 |
| SI/GO R. Superior Temporal (63,−20,−5) | −.21[−.36,−.05] | 2.40 | 0.012 | .08[−.08,.24] | 0.17 | 0.321 |
| FI/GO Anterior Cingulate (0,22,38) | .21[.05,.36] | 2.75 | 0.010 | −. 10[−.26,.06] | 0.21 | 0.231 |
| FI/GO L. Insula/IFG (−38,20,−6) | .33[.18,.47] | 360.70 | 5.0E-05 | −.06[−.22,.11] | 0.13 | 0.501 |
| FI/GO L. Parietal (−62,−44,34) | .20[.03,.35] | 1.67 | 0.018 | −. 14[−.29,.03] | 0.40 | 0.101 |
| FI/GO L. Striatum (−12,10,−10) | .07[−.10,.23] | 0.14 | 0.433 | −.09[−.24,.08] | 0.17 | 0.314 |
| FI/GO R. Insula/IFG (42,18,−6) | .42[.28,.55] | 9.5E+04 | 1.4E-07 | −.06[−.22,.11] | 0.13 | 0.515 |
| FI/GO R. Parietal (58,−44,30) | .26[. 10,.40] | 13.98 | 0.002 | −. 14[−.29,.03] | 0.39 | 0.102 |
| FI/GO R. Striatum (14,10,−10) | .04[−.12,.20] | 0.12 | 0.640 | −.03[−.19,.14] | 0.11 | 0.758 |
| FI/GO R. pre-SMA (4,30,54) | .20[.04,.35] | 1.81 | 0.016 | −.20[−.35,.−.04] | 1.91 | 0.015 |
Table 6.
Most likely correlation (r) values, 95% posterior credible intervals (CIs), Bayes factors (BF10) and frequentist p-values for correlational relationships between ROI activations and the DDM parameters indexing caution (a) and non-decision time (Ter). The first column lists the contrast, anatomical region, and MNI coordinates (x, y and z in parentheses) for each ROI. For p-values: bolded=survives FDR correction for multiple comparisons within each family of tests (families defined by DDM parameters)
| Caution (a) | Non-decision (Ter) | |||||
|---|---|---|---|---|---|---|
| Region | r [CI] | BF10 | p | r [CI] | BF10 | p |
| SI/GO L. Inferior Frontal (−35,21,−9) | −.09[−.25,.07] | 0.19 | 0.281 | −.09[−.25,.08] | 0.18 | 0.296 |
| SI/GO L. Middle Frontal (−46,25,25) | −.00[−. 16,. 16] | 0.11 | 0.994 | −.01 [−. 17,. 15] | 0.11 | 0.910 |
| SI/GO L. Supramarginal (−59,−50,35) | −.12[−.27,.05] | 0.26 | 0.172 | −.08[−.24,.08] | 0.17 | 0.320 |
| SI/GO Medial Frontal (2,22,41) | −.11 [−.26,.06] | 0.23 | 0.211 | −.07[−.23,.10] | 0.15 | 0.419 |
| SI/GO L. Medial Frontal (−38,59,3) | −.08[−.24,.09] | 0.16 | 0.345 | −.01 [−. 17,. 16] | 0.11 | 0.955 |
| SI/GO R. Inferior Frontal (51,14,25) | −.06[−.22,.11] | 0.13 | 0.483 | −.06[−.22,.10] | 0.14 | 0.473 |
| SI/GO R. Inferior Frontal (39,24,−10) | −.09[−.25,.08] | 0.18 | 0.305 | −.24[−.38,−.08] | 5.96 | 0.004 |
| SI/GO R. Inferior Parietal (51,−50,41) | −.12[−.28,.04] | 0.30 | 0.146 | −.17[−.32,−.01] | 0.82 | 0.041 |
| SI/GO R. Middle Frontal (46,39,24) | −.07[−.23,.10] | 0.14 | 0.431 | −.07[−.23,.10] | 0.14 | 0.428 |
| SI/GO R. Superior Frontal (13,18,58) | −.12[−.27,.05] | 0.28 | 0.161 | −.06[−.22,.10] | 0.13 | 0.478 |
| SI/GO R. Superior Temporal (63,−20,−5) | −. 18[−.33,.−.02] | 1.07 | 0.030 | −.12[−.27,.05] | 0.28 | 0.161 |
| FI/GO Anterior Cingulate (0,22,38) | −.01 [−. 17,. 15] | 0.11 | 0.883 | .07[−.09,.23] | 0.15 | 0.385 |
| FI/GO L. Insula/IFG (−38,20,−6) | .02[−.14,.18] | 0.11 | 0.801 | . 16[−.00,.32] | 0.68 | 0.052 |
| FI/GO L. Parietal (−62,−44,34) | .07[−.10,.22] | 0.14 | 0.442 | .08[−.08,.24] | 0.17 | 0.327 |
| FI/GO L. Striatum (−12,10,−10) | .14[−.03,.29] | 0.38 | 0.106 | . 14[−.02,.30] | 0.45 | 0.087 |
| FI/GO R. Insula/IFG (42,18,−6) | .03[−.13,.19] | 0.11 | 0.710 | .13[−.04,.28] | 0.33 | 0.127 |
| FI/GO R. Parietal (58,−44,30) | .14[−.02,.30] | 0.42 | 0.093 | .06[−.10,.22] | 0.14 | 0.457 |
| FI/GO R. Striatum (14,10,−10) | .10[−.06,.26] | 0.22 | 0.220 | . 18[.02,.33] | 1.04 | 0.031 |
| FI/GO R. pre-SMA (4,30,54) | .04[−.12,.20] | 0.12 | 0.629 | −.03 [−. 19,. 14] | 0.11 | 0.755 |
Analyses of relationships between behavioral summary statistics (FA rate, MRT, SDRT) and task activations (Supplemental Materials) did not reveal any associations that were robust between the whole-brain and ROI analyses. Notably, SI>GO activations in the IFG and other regions typically assumed to underlie inhibitory ability displayed little evidence of a relationship with FA rate, the standard measure of inhibitory performance.
Prediction of Psychopathology
As v.avg was the only DDM parameter that displayed robust evidence of relationships with activations, we focused on this parameter and its strongest ROI correlates in the SI>GO and FI>GO contrasts: the right STG and right insula/IFG, respectively. Although BF10 indicated that evidence for prediction was modest (Supplemental Table 5; Figure 3), FI-related activation in the right insula/IFG displayed preliminary evidence of a negative relationship with externalizing behavior (BF10=2.06) and ADHD symptoms (BF10=2.77), while v.avg displayed preliminary evidence of a negative relationship with ADHD symptoms (BF10=2.37), only. SI-related activation in the right STG displayed evidence against the presence of a relationship with both scales. Commonly-used behavioral summary statistics (FA rate, hit MRT and hit SDRT) failed to predict symptom ratings in frequentist tests and BF10 indicated that evidence for these correlations was weak (BF10<=1.12).
Figure 3.
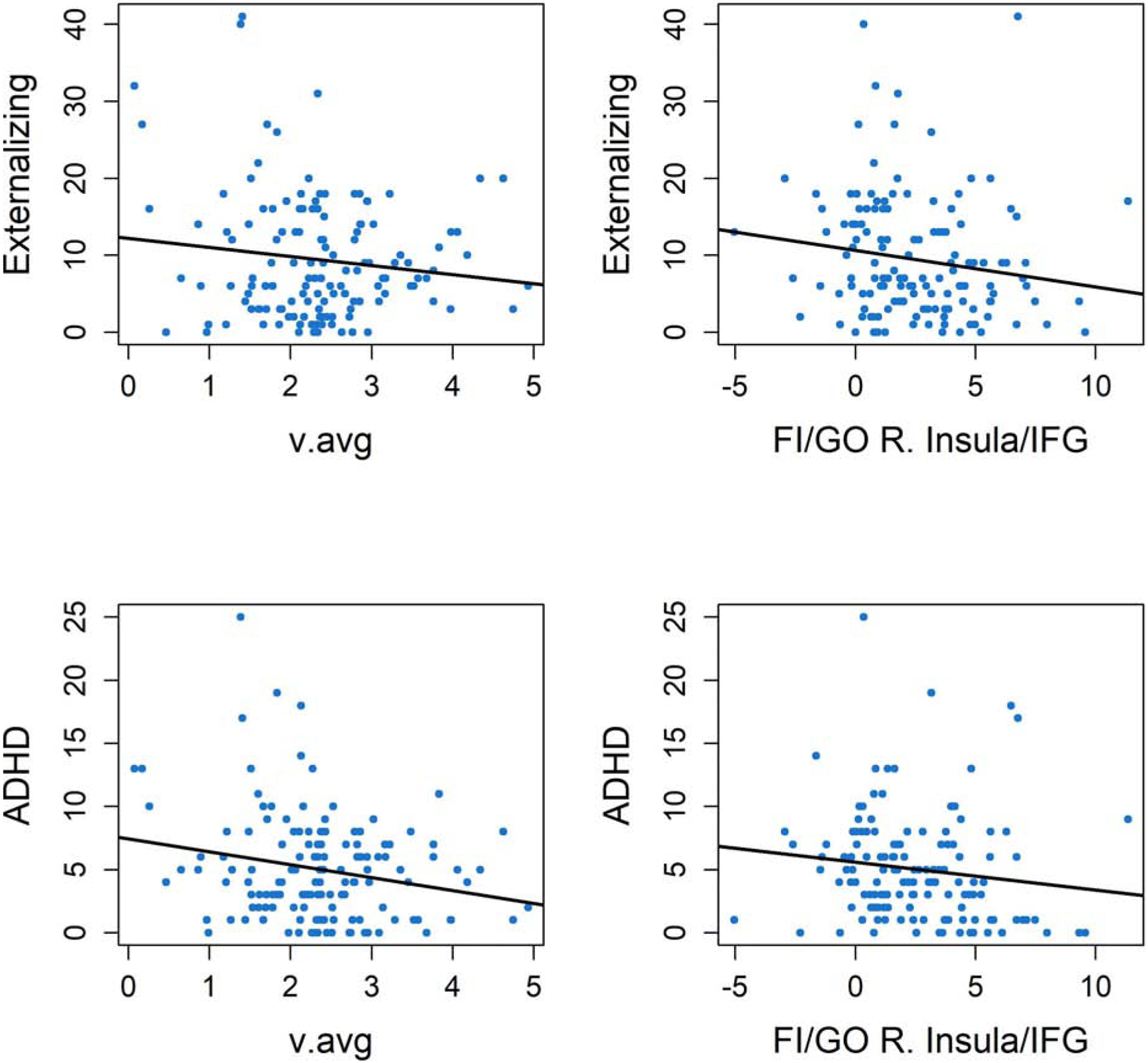
Scatterplots of DDM parameters and neural correlates of DDM parameters which displayed evidence of correlational relationships with psychopathology ratings, including average drift rates (left panels) and FI>GO activations in the right insula/inferior frontal gyrus (IFG) (right panels). Plots display their relationships with raw scores of the DSM-ADHD and externalizing behavior subscales from the ASR, as well as regression lines for these relationships. For histograms of T-scores from these clinical measures, which demonstrate the range of severity in symptoms relative to clinical norms, see Supplemental Figure 6.
Discussion
We used the DDM, a well-validated computational model (30,31), to test the common assumption that individual differences in go/no-go task-related neural activations index the integrity of neurocognitive mechanisms that underlie individual and clinical differences in inhibitory performance.
FI-related activations in the ACC, IFG and insula, regions associated with error processing (22,24), were consistently related to individuals’ drift rate (v.avg) across whole-brain and ROI-based analyses, suggesting that neural systems involved in performance monitoring are crucial for optimizing the ability to efficiently decide whether to initiate or withhold responses. FI-related activation in the strongest ROI correlate of v.avg, the right insula/IFG, also displayed modest, but promising, evidence for negative relationships with externalizing behaviors and ADHD symptoms. In contrast, activation in only one region from the SI>GO contrast, the right STG, displayed evidence of a relationship with a DDM parameter (v.avg). However, this relationship was only identified in the ROI-based analysis, suggesting it may not be robust, and SI-related activation in this ROI displayed evidence for a lack of relationship with both clinical criterion measures, suggesting that it displays limited clinical relevance.
Taken together with the body of behavioral research linking ADHD and externalizing behaviors to poorer performance on the go/no-go task (1–3,60), these results suggest that the task does indeed tap into a neurocognitive construct of clinical relevance. However, as individuals’ inhibition ability (defined functionally as FA rate) is similarly related to drift rate (v) on both “go” and “no-go” trials, and as SI-related activations in the “inhibition” network show little evidence of relationships with DDM parameters that underlie task performance, this construct does not appear to be selectively related to the inhibition of responses. Rather, our findings suggest that this construct may be better-defined as a task-general efficiency of evidence accumulation, which both determines individuals’ observed inhibition ability (FA rate) and has broader clinical relevance. Hence, investigations into fundamental neural mechanisms of clinical differences in go/no-go task performance may benefit from a focus on neural correlates of evidence accumulation efficiency, such as performance monitoring systems, rather than on SIs.
Even if SI-related neural activations are not selective probes of the latent mechanisms measured by the DDM, it is nonetheless possible that they have utility for measuring the combination of neural processes involved in response inhibition. However, our supplemental analyses indicated that SI-related activations, surprisingly, did not show robust relationships with any index of behavioral performance (including FA rate), which casts serious doubt on their utility for measuring neural determinants of inhibition. One possible reason these activations may be poor probes is that the assumption of “pure insertion” - that neural processes necessary for inhibition are present on “no-go”, but not “go”, trials - is incorrect. Indeed, this explanation is consistent with the DDM, which posits that the same core decision-making mechanisms are present across “go” and “no-go” trials. Therefore, efforts to identify neural correlates of v.avg or other mechanistic determinants of go/no-go performance may benefit from methods that eschew “pure insertion” assumptions, such as data-driven network-based approaches (61–63) or intrasubject regression of trial-to-trial changes in model parameters on features of the fMRI time series (64,65).
Our findings are consistent with recent accounts of cognitive deficits in ADHD. Although response inhibition has long been considered a core deficit in the disorder (66), individuals with ADHD display less efficient evidence accumulation across a variety of tasks with different response inhibition demands (55,56,67–69). These findings have recently been interpreted (67,68) as suggesting that individuals with ADHD display dysfunction related to the locus coeruleus-norepinephrine (LC-NE) system, which is posited (70) to optimize arousal and efficiency of processing in response to perceived task utility. As brain regions involved in monitoring performance lapses are thought to provide top-down input to this system (70), the current study’s findings on the neural correlates of drift rate are broadly consistent with this theory, although additional work is needed to rigorously evaluate links between error-related activations, drift rate and the LC-NE system.
Findings are also relevant to studies that probe clinical differences in neural responses to the stop-signal task (SST), another classic inhibition measure (71). Individuals with ADHD diagnoses and symptoms display blunted responses in the IFG and other prefrontal regions to both successful and failed inhibitions during the SST (72–77). As the SST typically involves a dynamic tracking algorithm that elicits a roughly 50% inhibition failure rate, this paradigm is arguably better-suited for the investigation of error-related activations than the go/no-go. Hence, future work involving the SST is warranted to further evaluate the implications of our study.
This study has several limitations. First, the psychopathology ratings were not gathered at the same time as the fMRI session, and were, on average, more than a year removed. Given that these criterion measures may be state-dependent, this large interval may have made estimates of predictive validity less reliable. Second, a large portion of participants were male (61%) and displayed family risk factors for externalizing psychopathology (76%); thus, results may not generalize to samples without these features. As the diagnosis rates and distributions of clinical measures (Table 1, Supplemental Figure 6) indicate that the sample spans the range from relatively healthy individuals to those at risk for psychopathology, our sample was likely well-suited for the identification of neural correlates of externalizing symptoms. Third, we assumed that DDM parameters measured the primary mechanistic processes that determine individual differences in task behavior (e.g., FA rates), and our inferences therefore depend on the integrity of the DDM account of the task. Finally, although we would, given this assumption, expect DDM parameters to predict clinical outcomes better than summary measures, future work in larger data sets is necessary to test this hypothesis by providing reliable estimates of the predictive power of both types of measures.
In conclusion, the current study assessed relationships between neural activations from common go/no-go task contrasts and parameter estimates from the DDM to test the assumption that neural responses in these contrasts index individual differences in the integrity of clinically-relevant neurocognitive processes. Surprisingly, activations associated with successful inhibitions (including those in the right IFG) were unrelated to DDM mechanisms and other performance metrics. In contrast, activity during inhibitory errors in the ACC, IFG and insula was strongly related to efficiency of processing on the task. These results call common mechanistic interpretations of go/no-go task-related activations into question and suggest instead that error-related activations from the task can inform clinical neuroscience by providing information about neural systems that monitor and optimize task performance.
Supplementary Material
Acknowledgements
This project was supported by NIAAA grants R01 AA07065 and R01 AA025790. Alexander Weigard was supported by NIAAA T32 AA007477. A draft version of this manuscript was submitted to bioRxiv.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have no biomedical financial interests or potential conflicts of interest to report.
Priors for r in all Bayesian tests were uniform distributions bounded at −1 and 1.
References
- 1.Endres MJ, Rickert ME, Bogg T, Lucas J, & Finn PR (2011). Externalizing psychopathology and behavioral disinhibition: Working memory mediates signal discriminability and reinforcement moderates response bias in approach–avoidance learning. Journal of Abnormal Psychology, 120(2), 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metin B, Roeyers H, Wiersema JR, van der Meere J, & Sonuga-Barke E (2012). A meta-analytic study of event rate effects on Go/No-Go performance in attention-deficit/hyperactivity disorder. Biological psychiatry, 72(12), 990–996. [DOI] [PubMed] [Google Scholar]
- 3.Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, & Schachar R (2014). Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. Journal of Abnormal Psychology, 123(2), 429. [DOI] [PubMed] [Google Scholar]
- 4.Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde AL, … & Frouin V (2014). Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. American journal of psychiatry, 171(12), 1310–1319. [DOI] [PubMed] [Google Scholar]
- 5.Saunders B, Farag N, Vincent AS, Collins FL Jr, Sorocco KH, & Lovallo WR (2008). Impulsive errors on a Go-NoGo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcoholism: Clinical and Experimental Research, 32(5), 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdejo-García A, Lawrence AJ, & Clark L (2008). Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews, 32(4), 777–810. [DOI] [PubMed] [Google Scholar]
- 7.Winstanley CA, Eagle DM, & Robbins TW (2006). Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clinical psychology review, 26(4), 379–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JL, Mattick RP, Jamadar SD, & Iredale JM (2014). Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug and alcohol dependence, 145, 1–33. [DOI] [PubMed] [Google Scholar]
- 9.Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, … & Forman SD (1997). A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of cognitive neuroscience, 9(6), 835–847. [DOI] [PubMed] [Google Scholar]
- 10.Durston S, Thomas KM, Yang Y, Uluğ AM, Zimmerman RD, & Casey BJ (2002). A neural basis for the development of inhibitory control. Developmental Science, 5(4), F9–F16. [Google Scholar]
- 11.Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, … & Casey BJ (2003). Differential patterns of striatal activation in young children with and without ADHD. Biological psychiatry, 53(10), 871–878. [DOI] [PubMed] [Google Scholar]
- 12.Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, & Tapert SF (2011). Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and alcohol dependence, 119(3), 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, & Halperin JM (2004). Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related FMRI study. American Journal of Psychiatry, 161(9), 1650–1657. [DOI] [PubMed] [Google Scholar]
- 14.Wetherill RR, Squeglia LM, Yang TT, & Tapert SF (2013). A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology, 230(4), 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garavan H, Ross TJ, & Stein EA (1999). Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences, 96(14), 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng D, Oka T, Bokura H, & Yamaguchi S (2008). The key locus of common response inhibition network for no-go and stop signals. Journal of Cognitive Neuroscience, 20(8), 1434–1442. [DOI] [PubMed] [Google Scholar]
- 17.Criaud M, & Boulinguez P (2013). Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience & biobehavioral reviews, 37(1), 11–23. [DOI] [PubMed] [Google Scholar]
- 18.Swick D, Ashley V, & Turken U (2011). Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage, 56(3), 1655–1665. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadi A, Pearlson GD, Meda SA, Dager A, Potenza MN, Rosen R, … & Wood RM (2013). Influence of alcohol use on neural response to go/no-go task in college drinkers. Neuropsychopharmacology, 38(11), 2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claus ED, Feldstein Ewing SW, Filbey FM, & Hutchison KE (2013). Behavioral control in alcohol use disorders: relationships with severity. Journal of studies on alcohol and drugs, 74(1), 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garavan H, Ross TJ, Murphy K, Roche RAP, & Stein EA (2002). Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage, 17(4), 1820–1829. [DOI] [PubMed] [Google Scholar]
- 22.Stevens MC, Kiehl KA, Pearlson GD, & Calhoun VD (2009). Brain network dynamics during error commission. Human brain mapping, 30(1), 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, & Zucker RA (2014). Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug and Alcohol Dependence, 141, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huster RJ, Eichele T, Enriquez-Geppert S, Wollbrink A, Kugel H, Konrad C, & Pantev C (2011). Multimodal imaging of functional networks and event-related potentials in performance monitoring. Neuroimage, 56(3), 1588–1597. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen J, Casey BJ, van Erp TG, Tamm L, Epstein JN, Buss C, … & Somerville L (2016). ADHD and cannabis use in young adults examined using fMRI of a Go/NoGo task. Brain imaging and behavior, 10(3), 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czapla M, Baeuchl C, Simon JJ, Richter B, Kluge M, Friederich HC, … & Loeber S (2017). Do alcohol-dependent patients show different neural activation during response inhibition than healthy controls in an alcohol-related fMRI go/no-go-task?. Psychopharmacology, 234(6), 1001–1015. [DOI] [PubMed] [Google Scholar]
- 27.Dillo W, Göke A, Prox-Vagedes V, Szycik GR, Roy M, Donnerstag F, … & Ohlmeier MD (2010). Neuronal correlates of ADHD in adults with evidence for compensation strategies–a functional MRI study with a Go/No-Go paradigm. GMS German Medical Science, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding WN, Sun JH, Sun YW, Chen X, Zhou Y, Zhuang ZG, … & Du YS (2014). Trait impulsivity and impaired prefrontal impulse inhibition function in adolescents with internet gaming addiction revealed by a Go/No-Go fMRI study. Behavioral and Brain Functions, 10(1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, & Frank LR (2007). Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology, 194(2), 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratcliff R (1978). A theory of memory retrieval. Psychological review, 85(2), 59. [Google Scholar]
- 31.Ratcliff R, Smith PL, Brown SD, & McKoon G (2016). Diffusion decision model: current issues and history. Trends in cognitive sciences, 20(4), 260–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratcliff R, Huang-Pollock C, & McKoon G (2018). Modeling individual differences in the go/no-go task with a diffusion model. Decision, 5(1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang-Pollock C, Ratcliff R, McKoon G, Shapiro Z, Weigard A, & Galloway-Long H (2017). Using the diffusion model to explain cognitive deficits in attention deficit hyperactivity disorder. Journal of abnormal child psychology, 45(1), 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez P, Ratcliff R, & Perea M (2007). A model of the go/no-go task. Journal of Experimental Psychology: General, 136(3), 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endres MJ, Donkin C, & Finn PR (2014). An information processing/associative learning account of behavioral disinhibition in externalizing psychopathology. Experimental and clinical psychopharmacology, 22(2), 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zucker RA, Ellis DA, Fitzgerald HE, Bingham CR, & Sanford K (1996). Other evidence for at least two alcoholisms II: Life course variation in antisociality and heterogeneity of alcoholic outcome. Development and Psychopathology, 8(4), 831–848. [Google Scholar]
- 37.Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, & Ellis DA (2000). The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy In: Fitzgerald HE, Lester BM, & Zucker RA (Eds), Children of Addiction: Research, Health and Policy Issues. (pp. 109–141). New York: Routledge Falmer Publishers. [Google Scholar]
- 38.Glover GH, & Law CS (2001). Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic resonance in medicine, 46(3), 515–522. [DOI] [PubMed] [Google Scholar]
- 39.Fessler JA, Lee S, Olafsson VT, Shi HR, & Noll DC (2005). Toeplitz-based iterative image reconstruction for MRI with correction for magnetic field inhomogeneity. IEEE Transactions on Signal Processing, 53(9), 3393–3402. [Google Scholar]
- 40.Achenbach TM, & Rescorla L (2003). Manual for the ASEBA adult forms & profiles: for ages 18–59: adult self-report and adult behavior checklist. ASEBA. [Google Scholar]
- 41.R Core Team (2018). R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Austria, 2015. [Google Scholar]
- 42.Singmann H, Brown S, Gretton M, & Heathcote A (2016). rtdists: Response time distributions. R package version 0.4–9. URL http://CRAN.R-project.org/package=rtdists. [Google Scholar]
- 43.Voss A, Nagler M, & Lerche V (2013). diffusion Models in Experimental Psychology: A Practical Introduction. Experimental Psychology, 60(6), 385–402. [DOI] [PubMed] [Google Scholar]
- 44.Dutilh G, Annis J, Brown SD, Cassey P, Evans NJ, Grasman RP, … & Kupitz CN (2016). The quality of response time data inference: A blinded, collaborative assessment of the validity of cognitive models. Psychonomic bulletin & review, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, … & Fox PT (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human brain mapping, 28(11), 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, & Wager TD (2016). Neurosynth.
- 47.Brett M, Anton JL, Valabregue R, & Poline JB (2002, June). Region of interest analysis using an SPM toolbox. In 8th international conference on functional mapping of the human brain (Vol. 16, No. 2, p. 497). [Google Scholar]
- 48.JASP Team. (2018). JASP (Version 0.9. 0.1)[Computer software].
- 49.Dienes Z (2016). How Bayes factors change scientific practice. Journal of Mathematical Psychology, 72, 78–89. [Google Scholar]
- 50.Wagenmakers EJ, Marsman M, Jamil T, Ly A, Verhagen J, Love J, … & Matzke D (2018). Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychonomic bulletin & review, 25(1), 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- 52.Rosseel Y, Oberski D, Byrnes J, Vanbrabant L, Savalei V, Merkle E, … & Chow M (2018). Package ‘lavaan’.
- 53.Martz ME, Zucker RA, Schulenberg JE, & Heitzeg MM (2018). Psychosocial and neural indicators of resilience among youth with a family history of substance use disorder. Drug and alcohol dependence, 185, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metin B, Roeyers H, Wiersema JR, van der Meere JJ, Thompson M, & Sonuga-Barke E (2013). ADHD performance reflects inefficient but not impulsive information processing: A diffusion model analysis. Neuropsychology, 27(2), 193. [DOI] [PubMed] [Google Scholar]
- 55.Weigard A, & Huang-Pollock C (2017). The role of speed in ADHD-related working memory deficits: a time-based resource-sharing and diffusion model account. Clinical Psychological Science, 5(2), 195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziegler S, Pedersen ML, Mowinckel AM, & Biele G (2016). Modelling ADHD: a review of ADHD theories through their predictions for computational models of decision-making and reinforcement learning. Neuroscience & Biobehavioral Reviews, 71, 633–656. [DOI] [PubMed] [Google Scholar]
- 57.Matzke D, & Wagenmakers EJ (2009). Psychological interpretation of the ex-Gaussian and shifted Wald parameters: A diffusion model analysis. Psychonomic bulletin & review, 16(5), 798–817. [DOI] [PubMed] [Google Scholar]
- 58.Chambers CD, Garavan H, & Bellgrove MA (2009). Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience & biobehavioral reviews, 33(5), 631–646. [DOI] [PubMed] [Google Scholar]
- 59.Stevens MC, Kiehl KA, Pearlson GD, & Calhoun VD (2007). Functional neural networks underlying response inhibition in adolescents and adults. Behavioural brain research, 181(1), 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bohlin G, Eninger L, Brocki KC, & Thorell LB (2012). Disorganized attachment and inhibitory capacity: Predicting externalizing problem behaviors. Journal of abnormal child psychology, 40(3), 449–458. [DOI] [PubMed] [Google Scholar]
- 61.Sripada C, Angstadt M, Rutherford S, Kessler D, Kim Y, Yee M, & Levina E (2019). Basic Units of Inter-Individual Variation in Resting State Connectomes. Scientific reports, 9(1), 1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dubois J, Galdi P, Paul LK, & Adolphs R (2018). A distributed brain network predicts general intelligence from resting-state human neuroimaging data. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1756), 20170284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sripada C, Rutherford S, Angstadt M, Thompson WK, Luciana M, Weigard A, … & Heitzeg M (2019). Prediction of neurocognition in youth from resting state fMRI. Molecular psychiatry, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frank MJ, Gagne C, Nyhus E, Masters S, Wiecki TV, Cavanagh JF, & Badre D (2015). fMRI and EEG predictors of dynamic decision parameters during human reinforcement learning. Journal of Neuroscience, 35(2), 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Maanen L, Brown SD, Eichele T, Wagenmakers EJ, Ho T, Serences J, & Forstmann BU (2011). Neural correlates of trial-to-trial fluctuations in response caution. Journal of Neuroscience, 31(48), 17488–17495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological bulletin, 121(1), 65. [DOI] [PubMed] [Google Scholar]
- 67.Karalunas SL, Geurts HM, Konrad K, Bender S, & Nigg JT (2014). Annual research review: Reaction time variability in ADHD and autism spectrum disorders: Measurement and mechanisms of a proposed trans-diagnostic phenotype. Journal of Child Psychology and Psychiatry, 55(6), 685–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weigard A, Huang-Pollock C, Brown S, & Heathcote A (2018). Testing formal predictions of neuroscientific theories of ADHD with a cognitive model–based approach. Journal of abnormal psychology, 127(5), 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shapiro Z, & Huang-Pollock C (2019). A diffusion-model analysis of timing deficits among children with ADHD. Neuropsychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aston-Jones G, & Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci, 28, 403–450. [DOI] [PubMed] [Google Scholar]
- 71.Logan GD, & Cowan WB (1984). On the ability to inhibit thought and action: A theory of an act of control. Psychological review, 91(3), 295. [DOI] [PubMed] [Google Scholar]
- 72.Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, … & Fauth-Bühler M (2012). Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature neuroscience, 15(6), 920. [DOI] [PubMed] [Google Scholar]
- 73.Rubia K, Smith AB, Brammer MJ, Toone B, & Taylor E (2005). Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. American Journal of Psychiatry, 162(6), 1067–1075. [DOI] [PubMed] [Google Scholar]
- 74.Cubillo A, Smith AB, Barrett N, Giampietro V, Brammer MJ, Simmons A, & Rubia K (2012). Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cerebral Cortex, 24(1), 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, & Rubia K (2010). Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of psychiatric research, 44(10), 629–639. [DOI] [PubMed] [Google Scholar]
- 76.Janssen TW, Heslenfeld DJ, van Mourik R, Logan GD, & Oosterlaan J (2015). Neural correlates of response inhibition in children with attention-deficit/hyperactivity disorder: A controlled version of the stop-signal task. Psychiatry Research: Neuroimaging, 233(2), 278–284. [DOI] [PubMed] [Google Scholar]
- 77.Hart H, Chantiluke K, Cubillo AI, Smith AB, Simmons A, Brammer MJ, … & Rubia K (2014). Pattern classification of response inhibition in ADHD: toward the development of neurobiological markers for ADHD. Human Brain Mapping, 35(7), 3083–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


