Abstract
Opioid use disorder is a major public health crisis. While effective treatments are available, outcomes vary widely across individuals and relapse rates remain high. Understanding neural mechanisms of treatment response may facilitate the development of personalized and/or novel treatment approaches. Methadone-maintained, polysubstance-using individuals (n=53) participated in fMRI scanning before and after substance-use treatment. Connectome-based predictive modeling (CPM)—a recently developed, whole-brain approach—was used to identify pre-treatment connections associated with abstinence during the 3-month treatment. Follow-up analyses were conducted to determine the specificity of the identified opioid abstinence network across different brain states (cognitive vs. reward task vs. resting-state) and different substance use outcomes (opioid vs. cocaine abstinence). Post-treatment fMRI data were used to assess network changes over time and within-subject replication. To determine further clinical relevance, opioid abstinence network strength was compared to healthy subjects (n=38). CPM identified an opioid abstinence network (p=.018), characterized by stronger within-network motor/sensory connectivity, and reduced connectivity between the motor/sensory network and medial frontal, default mode and frontoparietal networks. This opioid abstinence network was anatomically distinct from a previously identified cocaine abstinence network. Relationships between abstinence and opioid and cocaine abstinence networks replicated across multiple brain states but did not generalize across substances. Network connectivity measured at post-treatment related to abstinence at 6-month follow-up (p<.009). Healthy comparison subjects displayed intermediate network strengths relative to treatment responders and non-responders. These data indicate dissociable anatomical substrates of opioid versus cocaine abstinence. Results may inform the development of novel opioid-specific treatment approaches to combat the opioid epidemic.
Introduction
The opioid epidemic has led to a dramatic rise in opioid-related mortality1, 2, highlighting an urgent need for improved prevention and intervention efforts based on known biological mechanisms. Although evidence-based treatments are available for opioid use disorder (OUD)3, there is substantial individual variability in response to different treatments, and multiple quit attempts are standard. As the risk of opioid overdose is highest following unsuccessful treatment, in part due to reduced tolerance4, more targeted intervention approaches to reduce relapse rates are urgently needed. Several prior studies have examined neural correlates of treatment response, and there is preliminary evidence to suggest that reduced neural response to drug cues at baseline is associated with better treatment outcomes (for review, see5, 6). However, few studies have examined how neural functioning outside the context of drug cue reactivity relates to risk for relapse, and no prior studies have applied machine learning to identify whole brain patterns of functional connectivity that predict opioid use.
In addition, polysubstance use is common and undertreated. Approximately half of individuals seeking treatment for OUD have comorbid cocaine use, which is associated with poorer outcomes7, including higher overdose risk8, 9. Therefore, elucidation of the neural mechanisms of opioid and other substance use among individuals in treatment for OUD is needed to stimulate the development of novel treatment approaches, and may ultimately facilitate the identification of individuals who are likely to benefit from additional resources to achieve and/or maintain abstinence10. However, neurobiological features conferring vulnerability for opioid relapse remain poorly understood; see11 for systematic review.
Here we use a recently developed whole-brain, machine-learning approach— connectome-based predictive modeling (CPM)—to identify neural network connections associated with future opioid relapse. Unlike traditional regression/correlation approaches, CPM with leave-one-out (LOO) cross-validation (CV) is designed to protect against overfitting, increasing the likelihood that identified brain-behavior relationships will generalize in novel samples12, 13. As CPM is entirely data-driven and allows one-to-one mapping back to brain anatomy13, it is a powerful tool for identifying complex networks subserving multifaceted behaviors, which we have previously used to identify a network associated with cocaine abstinence14.
We first applied CPM to identify neuromarkers of opioid abstinence among methadone-maintained, polysubstance-using individuals. Given evidence suggesting that distinct personality and neurocognitive profiles underlie cocaine versus opioid dependence15—in addition to differential affective and environmental precipitants to craving and use of each substance16, 17—we hypothesized that connections identified via CPM as associated with opioid use would be largely dissociable from those previously identified as predictive of cocaine use14. Additionally, as recent advances in brain-behavior modeling demonstrate that individual differences in functional connectivity may be maximized by examining data acquired during performance of different tasks (i.e., brain state manipulation)18–22, we also examined the impact of brain state on network identification and brain-behavior associations. Finally, as addiction remission is hypothesized to arise from multiple neurobiological processes—i.e., some networks may return to premorbid levels of functioning when drug use is discontinued, whereas others may be upregulated to support the active maintenance of abstinence23—we compared opioid abstinence network strength within our patient sample to an independent sample of healthy comparison (HC) participants to determine whether individuals who respond well to treatment display network strengths that are comparable to or increased relative to HCs.
Materials
Participants and recruitment
Patients were recruited from a larger randomized controlled trial (RCT) of treatment for cocaine use disorder ( NCT00809835; see Supplemental Materials for details on RCT). As reported previously14, 74 patients from the parent RCT enrolled in the current neuroimaging protocol, who were already engaged in methadone maintenance treatment for OUD, and also had cocaine use disorder (see Supplemental Materials for additional information, Figures S1). A final sample of 53 participants were included in the CPM analyses (3 excluded for incomplete data, 18 excluded for excessive motion, Table S1). While no formal power analysis was conducted for the neuroimaging component of the RCT, our prior work in this population found that this sample size was sufficiently powered to develop a CPM of cocaine abstinence that replicated in an external sample14. Demographic and clinical characteristics for all participants are shown in Table 1. All participants provided written informed consent approved by the Yale School of Medicine IRB following description of study procedures. Abstinence during treatment was determined using biweekly urine testing and defined as the percentage of urine specimens testing negative for opioids (excluding methadone) during treatment. A subsample of participants (n=41) also completed a post-treatment scan. Additionally, 38 HCs were included who underwent fMRI scanning as part of ongoing research protocols at Yale University (details in Supplemental Materials).
Table 1–
Demographic and clinical characteristics of clinical trial (n=53) and healthy control (n=38) participants
| Clinical trial participants (n=53) | Healthy control participants (n=38) | F or X2 | p-value | |
|---|---|---|---|---|
| Female. No. (%) | 14 (26.4) | 16 (42.1) | 2.5 | 0.116 |
| Age, mean (SD) | 35.2 (9.4) | 38.5 (9.1) | 2.8 | 0.097 |
| Educational attainment (some college or above, %) | 18 (34) | 35 (92.1) | 30.8 | <0.001 |
| Methadone dose at baseline (mg), mean (SD) | 74.7 (23.1) | - | - | - |
| Years of regular cocaine use, mean (SD) | 8.1 (6.5) | - | - | - |
| Years of regular opioid use, mean (SD) | 9.2 (7.4) | - | - | - |
| Lifetime cannabis use disorder, No. (%) | 37 (69.8) | - | - | - |
| Lifetime alcohol use disorder, No. (%) | 30 (56.6) | - | - | - |
| No. prior outpatient drug treatments, mean (SD) | 3.0 (3.8) | - | - | - |
| No. prior inpatient drug treatments, mean (SD) | 3.0 (4.7) | - | - | - |
| Lifetime No. of arrests, mean (SD) | 5.3 (6.1) | - | - | - |
| Estimated IQ (Shipley), mean (SD) | 90.1 (12.8) | - | - | - |
| Days in treatment at initial fMRI scan, mean (SD) | 1.5 (6.7) | - | - | - |
| Percent opioid negative urines, mean (SD) | 64.9 (37.1) | - | - | - |
| Percent cocaine negative urines, mean (SD) | 23.0 (28.0) | - | - | - |
Neuroimaging data acquisition
fMRI data were acquired during cognitive control (Stroop) and reward (Monetary Incentive Delay; MID)24 tasks (all participants), as well as during resting state (n=36). CPM analyses related to reward task data and subsequent cocaine use have been published previously14. Task, acquisition, preprocessing, and calculation of functional connectivity matrices are described in the Supplemental Materials.
Connectome-based predictive modeling (CPM) for opioid abstinence
To identify patterns of connectivity linked with opioid abstinence, CPM was conducted with cognitive control task data in MATLAB using validated custom scripts12, available at https://www.nitrc.org/projects/bioimagesuite. CPM generates a brain-behavior model using behavioral data (here, percentage of opioid-negative urines) and whole brain connectomes as inputs12. This approach identifies positive and negative predictive features in a training dataset using regression analysis (here, either Pearson’s correlation or partial correlation). The sum of positive and negative edge weights is then calculated for each individual and entered into predictive models with behavioral data assuming linear relationships. Resultant coefficients are then applied to the test dataset to generate behavioral predictions and model performance is quantified as the correspondence between actual and predicted values (here, using Spearman’s rho to account for the non-normal distribution of opioid abstinence). Consistent with current recommendations for predictive modeling in modestly sized neuroimaging samples25, the current analyses use LOOCV. Only edges that are shared across every iteration are retained in the final positive and negative opioid abstinence networks. Permutation testing was used for significance testing (details in Supplemental Materials). As LOOCV may in some instances overfit the data26, CPM was repeated using 5-fold CV and this was iterated 50 times.
Abstinence network strength across substance use outcomes
To assess the specificity of the opioid abstinence network and the previously identified cocaine abstinence network14 to predict opioid versus cocaine abstinence, associations between opioid abstinence network strength and cocaine abstinence (defined as the percentage of cocaine negative urine drug screens collected during treatment), as well as between cocaine abstinence network strength and opioid abstinence, were tested using Spearman correlations.
Abstinence network strength across brain states
To assess the replicability of the opioid and cocaine abstinence networks across different brain states, CPM analyses were repeated using connectomes from reward task data to predict opioid use, and using connectomes from cognitive control task data to predict cocaine use. Furthermore, edge weights corresponding to each network were extracted from connectomes generated from reward task (opioid abstinence network) or cognitive control task (previously identified cocaine abstinence network) and resting state data (further details in Supplemental Materials, Table S2). Associations between network strengths across brain states and opioid and cocaine abstinence were tested using Spearman correlations.
Network change and replication over time
Post-treatment fMRI data were used to assess changes from pre- to post-treatment and to determine replicability of brain-behavior associations over time. Post-treatment abstinence was defined as the percentage of self-reported days of opioid abstinence during a 6-month follow-up period.
Network strength relative to HCs
Finally, we were also interested in assessing how HC network strength compares to patients as a function of treatment response. Edge weights corresponding to the opioid and cocaine abstinence networks were extracted from HC cognitive control and reward task connectomes. Participants were classified into groups of treatment responders and non-responders for opioids and cocaine separately (details in Supplemental Materials). Independent samples t-tests were used to compare mean network strength between (i) responders versus HCs and (ii) non-responders versus HCs (i.e., as networks were defined based on dimensional treatment responses, we did not compare network strengths between responders and non-responders).
Results
Opioid abstinence network
CPM analysis of baseline cognitive control task data successfully identified a network associated with subsequent within-treatment opioid abstinence, including motion as a covariate (r(df=52)=0.32, p=0.018; Figure 1A; see Supplemental Materials for more on motion controls). Across 50 iterations, the CPM analysis with 5-fold cross-validation yielded consistent results (initial: r=.33, mean±st.dev: r=.27±.10, median: r=.27).
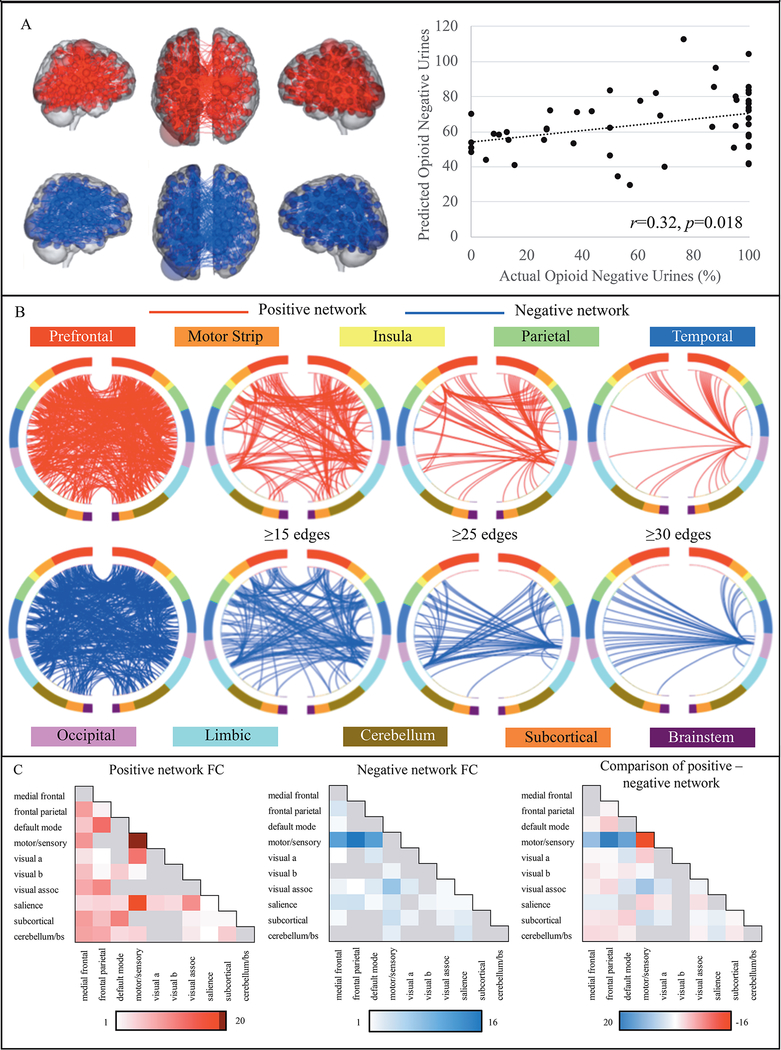
Figure 1 –
Positive and negative opioid abstinence networks
Figure 1A displays the positive opioid abstinence network (red), for which stronger connectivity predicts more opioid abstinence during treatment and the negative opioid abstinence network (blue), in which decreased connectivity is associated with more opioid abstinence. Larger spheres correspond to higher degree nodes. There was a significant association between CPM-predicted opioid abstinence (y-axis) and the observed percentage of opioid-negative urines during treatment (x-axis; p=.018). Figure 1B displays circle plots summarizing positive (red) and negative (blue) opioid abstinence network anatomy based on overlap with macroscale brain regions. Regions are organized according to their anatomical location, with more anterior regions are at the top of each plot, and more ventral and posterior regions displayed towards the bottom. The first set of plots contain all network edges, subsequent plots are thresholded to include only higher degree nodes. Figure 1C summarizes positive and negative opioid abstinence network anatomy based on overlap with canonical neural networks. Cells along the diagonal represent within-network connectivity and the remaining cells correspond to between-network connectivity. Color bars correspond to the percentage of each network accounted for by edges within/between each canonical neural network. Darker colors indicate a higher percentage of edges. The last figure shows the percentage of positive versus negative edges that correspond to different canonical neural networks. Red cells indicate relatively more positive network edges and blue cells indicate relatively more negative network edges.
Opioid abstinence network anatomy
Consistent with prior connectome-based work, the opioid abstinence network identified via CPM was complex and included connections within and between multiple large-scale canonical neural networks (opioid and cocaine abstinence networks available at https://www.nitrc.org/projects/bioimagesuite/). Nonetheless, it contained only 2.7% of possible connections (985 total edges: 520 positive, 465 negative). Figure 1B summarizes networks based on connectivity between macroscale brain regions, which included connections between frontal, parietal, temporal, and occipital lobes (details in Supplemental Materials).
Figure 1C summarizes connectivity based on the number of connections between canonical networks for positive and negative edges— hereafter referred to as the positive and negative networks. The positive network was predominantly characterized by within-network connections of the motor/sensory network, and between-network connections of the motor/sensory, salience and visual networks, as well as the default mode and frontoparietal networks. The negative network was largely comprised of connections between the motor/sensory network and frontoparietal, medial frontal, and default mode networks. To determine the relative specificity of different network subcomponents, edges corresponding to each of these five canonical networks were iteratively ‘knocked-out’ and CPM analyses were rerun. In all cases, CPMs still successfully predict opioid abstinence (details in Supplemental Materials; Table S3).
Network change and replication over time
Opioid abstinence network strength did not change across treatment (t(df=40)=−.587, p=.56), and post-treatment opioid abstinence network strength was positively associated with subsequent opioid abstinence across the 6-month follow-up period (rho(df=40)=.404, p<.009).
Comparison with cocaine abstinence network anatomy
Figure 2A–E shows anatomical overlap between the opioid abstinence network and the previously identified cocaine abstinence network14. Notably, there was almost no overlap between cocaine and opioid abstinence networks (details in Supplemental Materials).
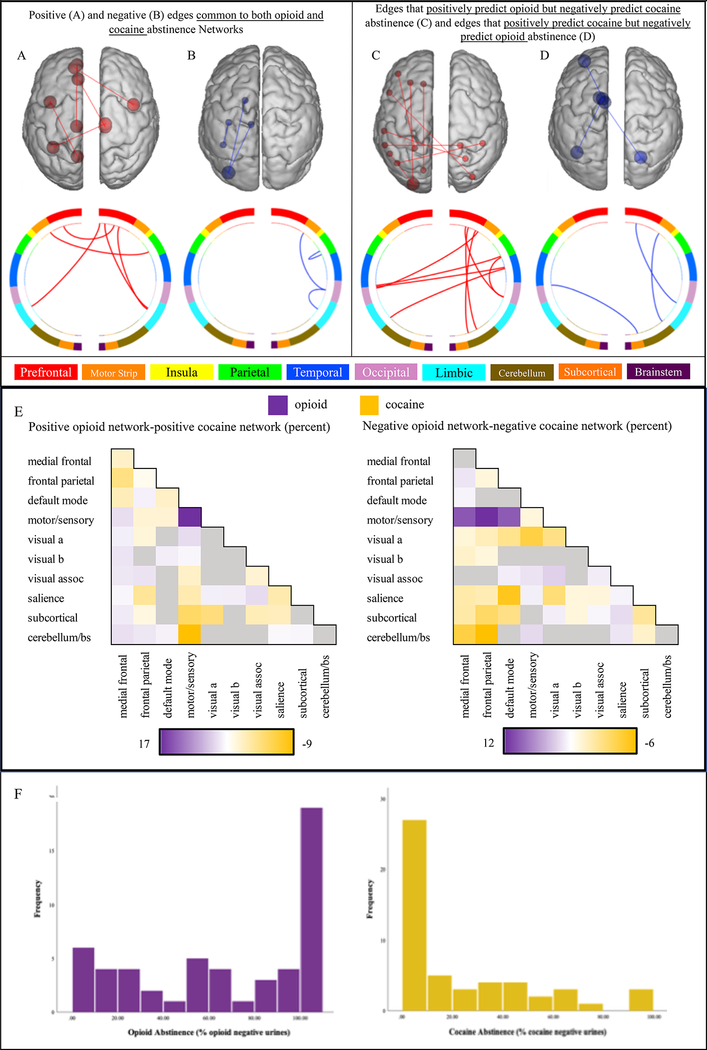
Figure 2 –
Anatomical specificity of opioid and cocaine abstinence networks
Figure 2A-D compares the anatomy of the opioid and cocaine abstinence networks based on their overlap with macroscale brain regions. Figure 2A depicts edges that belong to both the positive opioid and positive cocaine abstinence networks, and figure 2B shows edges that are common to both the negative opioid and the negative cocaine abstinence network. Figure 2C shows edges that positively predict opioid but negatively predict cocaine abstinence, and figure 2D displays edges that positively predict cocaine but negatively predict opioid abstinence. Figure 2E compares the anatomy of the opioid and cocaine abstinence networks based on their overlap with canonical neural networks. Color bars correspond to the difference between the percentage of the positive and negative opioid versus cocaine abstinence network accounted for by edges within and between each canonical network. Purple cells indicate relatively more edges in the opioid abstinence network and orange cells indicate relatively more edges in the cocaine abstinence network. Figure 2F displays frequency distributions of the percent of opioid and cocaine negative urines collected across the 12-week treatment period. Percent of opioid negative urines was not significantly correlated with the percent of cocaine negative urines (Spearman rho=.082, p=.565).
Specificity of opioid and cocaine abstinence networks across substance use outcomes
There were no significant associations between within-treatment cocaine abstinence and opioid abstinence network strength during cognitive control task performance (rho(df=52)=0.07, p=0.606), reward task performance (rho(df=52)=0.06, p=0.674) or resting-state (rho(df=52)=0.12, p=.47). Similarly, there were no significant associations between within-treatment opioid abstinence and cocaine abstinence network strength during reward task performance (rho(df=52)=0.11, p=0.425).
CPM analyses controlling for concurrent cocaine and opioid use
We repeated both opioid and cocaine abstinence CPM analyses controlling for cocaine and opioid use, respectively. The CPM model predicting opioid abstinence from Stroop data remained significant after controlling for concurrent cocaine use (r=.30, p=.025). Similarly, our CPM model predicting cocaine abstinence from reward task data also remained significant after controlling for concurrent opioid use (r=.45, p<.001).
Correspondence between opioid and cocaine use
Across individuals there was no significant association between cocaine and opioid abstinence (as indicated by % negative urines; rho=.132, p=.352). In addition, prediction errors (i.e., actual abstinence – predicted abstinence for each drug) for cocaine and opioid CPMs were not significantly correlated (rho = .169, 1-tailed p = .113).
Replication of opioid abstinence network across brain states and in relation to cocaine abstinence
CPM analysis of reward task data did not significantly predict within-treatment opioid abstinence (r(df=52)=0.17, p=0.10; Figure 3A). Alternatively, extracting the opioid abstinence network generated using cognitive control data from reward task and resting-state data indicated significant positive associations between within-treatment opioid abstinence and opioid abstinence network strength during reward task performance (rho(df=52)=0.71, p<.001) and during resting state (rho(df=52)=0.46, p=.005; Figure 3B), indicating replication across brain states. Applying the opioid abstinence network to predict cocaine abstinence across brain states, Spearman’s correlation analyses indicated no significant associations between within-treatment cocaine abstinence and opioid abstinence network strength during cognitive control task performance (rho(df=52)=0.07, p=0.606), reward task performance (rho(df=52)=0.06, p=0.674) or resting state (rho(df=52)=0.12, p=.472; Figure 3B). Therefore, cognitive control, but not reward task, data could be used to identify an opioid abstinence network, and the opioid abstinence network was specific for predicting opioid, not cocaine abstinence.
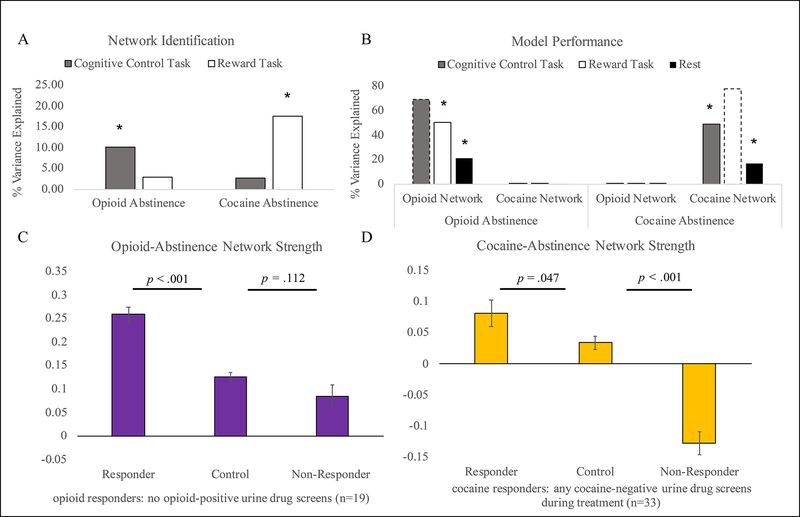
Figure 3 –
Brain state manipulation and comparison with healthy controls
Figure 3A demonstrates that CPM analyses successfully predict opioid abstinence using cognitive control - but not reward task - data, whereas cocaine abstinence is successfully predicted by reward task – but not cognitive control task – data. Figure 3B demonstrates that both opioid and cocaine abstinence networks can predict substance use outcomes (opioid or cocaine use, respectively) across brain states, and perform better with task versus resting-state data. Associations between the strength of each network in the brain state used for network generation (opioid abstinence network strength in cognitive control task data and cocaine abstinence network strength in reward task data) and abstinence are plotted for reference using dotted lines. Figure 3C-D compares opioid (3C) and cocaine (3D) network strength (y-axis) between treatment responders, treatment non-responders, and HCs. Because patients were stably maintained on methadone at the time of study enrollment, patients were classified as opioid treatment responders if they had no opioid positive urines during treatment. In contrast, patients were actively using cocaine at the time of study enrollment, so patients were classified as cocaine treatment responders if they had any cocaine negative drug screens during treatment. Error bars indicate the standard error of the mean.
CPM analysis of cognitive control task data did not predict within-treatment cocaine abstinence (r(df=52)=0.16, p=0.13; Figure 3A). However, extracting edge weights for the cocaine abstinence network (that was identified using reward task data) from cognitive control task and resting-state connectomes demonstrated significant positive associations between within-treatment cocaine abstinence and cocaine abstinence network strength during cognitive control task performance (rho(df=52)=0.70, p<.001) and during rest (rho(df=35)=0.41, p=.014; Figure 3B), indicating replication across brain states. In contrast, applying the cocaine abstinence network to predict opioid abstinence across brain states, no significant associations between within-treatment opioid abstinence and cocaine abstinence network strength were found during cognitive control task performance (rho(df=52)=0.003, p=0.983) or during resting state (rho(df=35)=0.091, p=.597; Figure 3B). Therefore, reward, but not cognitive control task, data could be used to identify a cocaine abstinence network, and the cocaine abstinence network was specific for predicting cocaine, but not opioid abstinence (Figure 3A–B).
Connectivity strength relative to HCs
Overall, network strength of the cocaine and opioid abstinence networks did not differ between HCs and patients (cocaine abstinence network t(df=75.5)=−1.36, p=0.178; opioid abstinence network t(df=72.9)=.91, p=369). However, HCs demonstrated an intermediate level of network strength relative to treatment responders and non-responders (Figure 3C–D). Specifically, opioid abstinence network strength was significantly decreased in HCs relative to opioid treatment responders (t(df=55)=7.5, p<.001), and non-significantly increased relative to opioid non-responders (t(df=43.1)=−1.6, p=.112). Similarly, HCs demonstrated significantly decreased cocaine abstinence network strength relative to cocaine treatment responders (t(df=47.1)=2.04, p=.047), and significantly greater cocaine abstinence network strength relative to cocaine treatment non-responders (t(df=56)=−8.3, p<.001).
Discussion
We applied CPM to identify a neural network associated with opioid abstinence during treatment among methadone-maintained, polysubstance-using individuals and compared this to a previously identified cocaine abstinence network14. Consistent with prior evidence for distinct neural mechanisms of opioid and stimulant use17, 27, opioid and cocaine networks were largely anatomically distinct and were specific for predicting opioid or cocaine abstinence only (respectively). Across-task comparisons revealed that while distinct brain states were optimal for identifying each network, once identified, both networks successfully predicted substance-specific abstinence across multiple brain states.
Findings of dissociable neural substrates of different substance use behaviors are consistent with recent machine-learning work that identified distinct profiles of neurocognitive, personality, psychiatric, and demographic characteristics in association with opioid versus stimulant dependence15. Congruently, recent evidence suggests that different mood states16 and contexts17, 27 precipitate craving and relapse for heroin versus cocaine, and that these processes may be driven by distinct neural mechanisms17. Similarly, a growing literature suggests that a significant proportion of the variance in substance use behavior is accounted for by substance-specific genetic and neurocognitive risk factors, in addition to characteristics that confer vulnerability to substance use more generally (for review, see28). Collectively, these data suggest there are substance-specific neural substrates of opioid use. Tailoring interventions according to individual risk factors has been proposed as a relatively unexplored approach for improving the precision and efficacy of addiction treatment28. Thus, targeting opioid-specific clinical and neurobiological mechanisms may represent a promising approach for designing more effective treatments for OUD.
Primarily, opioid abstinence was associated with increased within-network motor/sensory connectivity and increased between-network connectivity of motor/sensory, salience, default mode, and frontoparietal networks, as well as decreased connectivity between motor/sensory and medial frontal, default mode, and frontoparietal networks. Figure 4 presents a theoretical working network model highlighting these key aspects of the opioid abstinence network. Nonetheless, our virtual lesion analysis indicated that brain-behavior associations remained significant following removal of any one of these networks (see Supplemental Materials), indicating that no single network alone was required to support abstinence. Despite this generality, a lesion analysis eliminating the entire opioid abstinence network was unable to predict opioid abstinence altogether, demonstrating the centrality of the identified connections for predicting opioid abstinence.
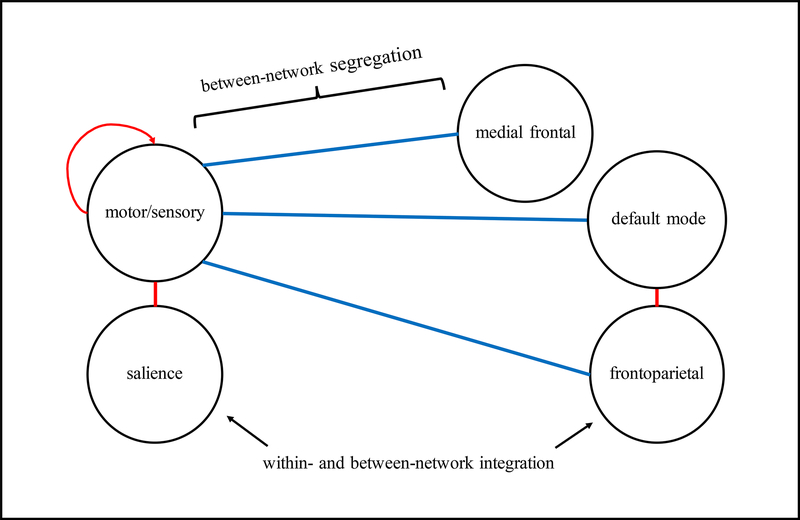
Figure 4 –
Network model of opioid abstinence network
Figure 4 presents a network model summarizing the dominant connections of the opioid abstinence network. Stronger connectivity (red) within the motor/sensory network and between motor/sensory and salience, and default mode and frontoparietal networks predicted greater within-treatment opioid abstinence, and reduced connectivity (blue) between the motor/sensory and medial frontal, default mode, and frontoparietal networks predicted greater within-treatment opioid abstinence.
Speculatively, motor/sensory connectivity may be related to opioid abstinence via its role in sensorimotor learning and acquired automaticity of opioid use behaviors30. In particular, the automatization of actions related to substance use is an important component of the addiction process, and individuals with more automatized drug use behaviors may be more vulnerable to relapse30. Congruently, in addition to the significant contribution of sensorimotor connectivity, 14% of the positive opioid abstinence network was comprised of between-network cerebellar connections and 12% consisted of between-network subcortical connections (including caudate and putamen nodes; see Figure 1). Collectively, these results demonstrate that a large proportion of the opioid abstinence network is comprised of connections between regions implicated in automatic motor movements. Future research is needed to assess how opioid abstinence network characteristics relate to sensorimotor processing of drug-relevant stimuli, as well as to explore the possibility of targeting sensorimotor components of addiction to improve treatment outcomes. For additional discussion of network anatomy, see Supplemental Materials (Figure S2).
Notably, network identification—but not model performance—was dependent on brain state, supporting previous work18 suggesting that linking brain function to behavior is critically dependent on selecting the optimal brain state for data acquisition and subsequent feature selection22. Future studies utilizing FC data collected while participants view drug-relevant stimuli or make drug-related decisions may further improve our ability to predict abstinence and enhance our understanding of the neural mechanisms of addiction recovery.
The neurobiology of successful addiction remission is theorized to involve complex interactions between multiple neural circuits23. In particular, certain circuits may return to premorbid functioning once substance exposure is discontinued, whereas other neural processes may be upregulated to maintain abstinence by supporting self-monitoring, cognitive control, and/or new skill acquisition. The current finding that HCs display intermediate network strength relative to treatment responders and non-responders suggests that the opioid abstinence network contributes to the maintenance of abstinence, such that individuals who are successful in treatment are characterized by ‘hyper-normal’ network strength relative to HCs23. Furthermore, these results suggest that novel interventions targeting this circuitry prior to treatment—such as neurofeedback, neuromodulation, and novel pharmacological and behavioral approaches—may be helpful in promoting subsequent within-treatment abstinence.
Limitations
This study has several limitations. While CPM with LOOCV is more robust than the whole-brain correlation or regression approaches employed in prior studies of OUD11–13, this study did not include an external replication sample25. Thus, additional replication in an external sample is needed. However, this limitation should be considered within the context of the urgent need for research to address the current opioid epidemic31 and the current scarcity of neuroimaging data from clinical OUD samples in the United States11. Additionally, given the modest sample of female participants included in our analyses, we did not have sufficient power to run separate CPM analyses within male and female subgroups (see Supplemental Materials for more on sex differences).
Opioid abstinence network strength did not differ across the 12-week period of treatment, rather the ‘predictive’ ability of the opioid abstinence network replicated over time, with connectivity patterns measured at post-treatment relating to abstinence measured during 6-month follow-up. However, as participants were already maintained on methadone at the time of study entry, additional research is needed to assess whether network strength increases during the early stages of successful opioid treatment or else represents a stable predictor of treatment response, such that individuals with increased pre-treatment opioid abstinence network strength may be more likely to achieve abstinence. Moreover, although opioid network strength was a significant predictor of opioid abstinence, it accounted for only ~10% of the variance in opioid use during treatment. It is likely that functional connectivity data collected during more drug-relevant tasks (i.e. drug-cue reactivity paradigms) could yield networks that account for an even greater proportion of the variance in treatment outcome. Nonetheless, an important advantage of predictive modelling approaches, such as CPM, is to decrease the likelihood of overfitting, and thus yield smaller, but more realistic effect size estimates25. Additionally, it will be important to replicate the current analyses comparing network strength to HCs using better matched control samples, as well as examining whether network strength continues to increase with ongoing skill building in extended abstinence. Furthermore, a more comprehensive understanding of the neurobiological basis for successful addiction treatment will also require future studies examining neural networks of treatment compliance as well as treatment outcome10. Several prior studies have identified neural correlates of adherence to addiction treatment32, 33, and there is evidence to suggest that neural mechanisms of treatment compliance may be distinct from neural mechanisms of treatment response34, 35. Therefore, future research applying machine learning to identify neural networks associated with treatment compliance (rather than abstinence) could elucidate the extent to which successful treatment engagement and response involve common versus distinct neural networks. Finally, future work is needed to assess how opioid abstinence network characteristics relate to clinical facets of OUD symptomatology and recovery, and whether novel treatment approaches aimed at targeting these systems could improve treatment outcomes for individuals with OUD.
Conclusions
The current study utilized a recently developed, connectome-based approach to identify a distinct neural network associated with opioid versus cocaine abstinence in a sample of treated individuals with both opioid and cocaine use disorders. The opioid abstinence network was specifically related to opioid (but not cocaine) abstinence across multiple brain states and over time. In addition, our data suggest that HCs show an intermediate level of network strength relative to treatment responders and non-responders, suggesting that the opioid abstinence network is involved in successfully achieving and maintaining of abstinence. Future research is needed to extend these results across diverse clinical settings, to identify intermediate clinical correlates of opioid abstinence network strength, and to assess the utility of developing novel treatments targeting this circuitry to improve treatment outcomes in OUD.
Supplementary Material
Acknowledgements
This work was supported by grants K01DA039299, R21DA045969, P50DA09241 T32DA022975, and R01DA035058 from the National Institute on Drug Abuse. Data reported here have been presented at the American College of Neuropsychopharmacology’s 57th Annual Meeting, at the Collaborative Perspectives on Addiction 2019 Annual Meeting, and are scheduled for additional oral presentation at the Society for Biological Psychiatry’s 74th Annual Scientific Meeting, and the College on Problems of Drug Dependence (CPDD) 81st Annual Scientific Meeting.
Disclosures
Drs. Lichenstein, Scheinost and Yip report no financial relationships with commercial interest. Dr. Carroll has received multiple grants from NIDA and NIAAA. She is a member of CBT4CBT LLC; this is managed through University. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised RiverMend Health and Opiant/Lakelight Therapeutics; has received unrestricted research support from Mohegan Sun Casino and grant support from the National Center for Responsible Gaming; and has consulted for legal and gambling entities on issues related to addictive disorders.
Footnotes
Supplementary information is available at MP’s website
References
- 1.McCarty D, Priest KC, Korthuis PT. Treatment and Prevention of Opioid Use Disorder: Challenges and Opportunities. Annu Rev Public Health 2018; 39: 525–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCabe SE, West BT, Jutkiewicz EM, Boyd CJ. Multiple DSM-5 substance use disorders: A national study of US adults. Hum Psychopharmacol 2017; 32(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Brink W, Haasen C. Evidenced-based treatment of opioid-dependent patients. Can J Psychiatry 2006; 51(10): 635–646. [DOI] [PubMed] [Google Scholar]
- 4.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry 2015; 23(2): 63–75. [DOI] [PubMed] [Google Scholar]
- 5.Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology 2019; 44(2): 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart JL, May AC, Aupperle RL, Bodurka J. Forging Neuroimaging Targets for Recovery in Opioid Use Disorder. Front Psychiatry 2019; 10: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castells X, Kosten TR, Capella D, Vidal X, Colom J, Casas M. Efficacy of opiate maintenance therapy and adjunctive interventions for opioid dependence with comorbid cocaine use disorders: A systematic review and meta-analysis of controlled clinical trials. Am J Drug Alcohol Abuse 2009; 35(5): 339–349. [DOI] [PubMed] [Google Scholar]
- 8.Lorvick J, Browne EN, Lambdin BH, Comfort M. Polydrug use patterns, risk behavior and unmet healthcare need in a community-based sample of women who use cocaine, heroin or methamphetamine. Addict Behav 2018; 85: 94–99. [DOI] [PubMed] [Google Scholar]
- 9.Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction 2003; 98(1): 7–22. [DOI] [PubMed] [Google Scholar]
- 10.Moeller SJ, Paulus MP. Toward biomarkers of the addicted human brain: Using neuroimaging to predict relapse and sustained abstinence in substance use disorder. Prog Neuropsychopharmacol Biol Psychiatry 2018; 80(Pt B): 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protocols 2017; 12(3): 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 2015; 18(11): 1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yip SW, Scheinost D, Potenza MN, Carroll KM. Connectome-based prediction of cocaine abstinence. American Journal of Psychiatry 2019; 175(2): 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn WY, Vassileva J. Machine-learning identifies substance-specific behavioral markers for opiate and stimulant dependence. Drug Alcohol Depend 2016; 161: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry 2009; 66(1): 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Pirro S, Galati G, Pizzamiglio L, Badiani A. The Affective and Neural Correlates of Heroin versus Cocaine Use in Addiction Are Influenced by Environmental Setting But in Opposite Directions. J Neurosci 2018; 38(22): 5182–5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene A, Gao S, Scheinost D, Constable R. Task-induced brain state manipulation improves prediction of individual traits. Nature communications 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci 2016; 19(1): 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg MD, Hsu WT, Scheinost D, Todd Constable R, Chun MM. Connectome-based Models Predict Separable Components of Attention in Novel Individuals. J Cogn Neurosci 2018; 30(2): 160–173. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg MD, Zhang S, Hsu WT, Scheinost D, Finn ES, Shen X et al. Methylphenidate Modulates Functional Network Connectivity to Enhance Attention. J Neurosci 2016; 36(37): 9547–9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finn ES, Scheinost D, Finn DM, Shen X, Papademetris X, Constable RT. Can brain state be manipulated to emphasize individual differences in functional connectivity? Neuroimage 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garavan H, Brennan KL, Hester R, Whelan R. The neurobiology of successful abstinence. Curr Opin Neurobiol 2013; 23(4): 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P et al. Individuals Family History Positive for Alcoholism Show Functional Magnetic Resonance Imaging Differences in Reward Sensitivity That Are Related to Impulsivity Factors. Biol Psychiatry 2011; 69(7): 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheinost D, Noble S, Horien C, Greene AS, Lake EMR, Salehi M et al. Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage 2019; 193: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varoquaux G, Raamana PR, Engemann DA, Hoyos-Idrobo A, Schwartz Y, Thirion B. Assessing and tuning brain decoders: Cross-validation, caveats, and guidelines. NeuroImage 2017; 145: 166–179. [DOI] [PubMed] [Google Scholar]
- 27.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 2011; 12(11): 685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassileva J, Conrod PJ. Impulsivities and addictions: a multidimensional integrative framework informing assessment and interventions for substance use disorders. Philos Trans R Soc Lond B Biol Sci 2019; 374(1766): 20180137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George O, Koob GF. Individual differences in the neuropsychopathology of addiction. Dialogues Clin Neurosci 2017; 19(3): 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yalachkov Y, Kaiser J, Naumer MJ. Sensory and motor aspects of addiction. Behav Brain Res 2010; 207(2): 215–222. [DOI] [PubMed] [Google Scholar]
- 31.Volkow ND, Boyle M. Neuroscience of Addiction: Relevance to Prevention and Treatment. Am J Psychiatry 2018: appiajp201817101174. [DOI] [PubMed] [Google Scholar]
- 32.Shi Z, Jagannathan K, Wang AL, Fairchild VP, Lynch KG, Suh JJ et al. Behavioral and Accumbal Responses During an Affective Go/No-Go Task Predict Adherence to Injectable Naltrexone Treatment in Opioid Use Disorder. Int J Neuropsychopharmacol 2019; 22(3): 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang AL, Elman I, Lowen SB, Blady SJ, Lynch KG, Hyatt JM et al. Neural correlates of adherence to extended-release naltrexone pharmacotherapy in heroin dependence. Transl Psychiatry 2015; 5: e531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreno EM, Dominguez-Salas S, Diaz-Batanero C, Lozano OM, Marin JAL, Verdejo-Garcia A. Specific aspects of cognitive impulsivity are longitudinally associated with lower treatment retention and greater relapse in therapeutic community treatment. J Subst Abuse Treat 2019; 96: 33–38. [DOI] [PubMed] [Google Scholar]
- 35.Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD et al. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol Addict Behav 2013; 27(2): 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.