To the Editor:
Acute myeloid leukemia (AML) encompassing translocation t(8;21)(q22;q22.1) results in the chimeric fusion protein AML1-ETO (AE), also known as RUNX1-RUNX1T1 transcript. The presence of AE defines a precursor stage of leukemia, however additional molecular events are required for transformation [1, 2]. Alternative splicing of the ETO gene introduces an additional exon adjacent to exon 8, namely exon 9a, spanning 155 bp. Inclusion of exon 9a alters the open reading frame of the AE gene leading to a carboxy-terminal truncated isoform of the AE protein, known as AML1-ETO9a (AE9a), which lacks Drosophila nervy homology regions (NHR) 3 and 4 [3]. In a retroviral transduced mouse model, co-expression of AE and AE9a induces a more immature leukemic phenotype with a rapid onset of AML [4]. The authors hypothesized that the relative AE9a allelic burden as compared to the full-length AE transcript might affect the transforming capacity of the protein [4]. As to now, there is only scarce data on the incidence of AE9a with limited evidence indicating that AE9a transcript levels (TL) impact on prognosis in t(8;21)-AML. In a previous study on 118 pediatric and adult t(8;21)-AML patients, elevated AE9a (n = 86) was correlated with the worsened clinical outcome as well as increased incidence of KIT mutations and higher KIT gene expression [5]. However, the two-step nested PCR approach used to detect AE9a does not allow accurate quantification and therefore possibly overestimates gene expression levels. In another study, Ommen et al. reported the presence of AE9a in 11/13 patients with t(8;21)-AML and observed lower decline of AE9a TL in relapsing as compared to non-relapsing patients during the course of the disease [6]. More recently, we performed a transcriptome study applying novel high-throughput sequencing technologies and detected the AE9a variant in 27/27 t(8;21)-AML cases [7].
We, therefore, sought to systematically assess the incidence and prognostic significance of AE9a co-expression in the context of clinical and genetic factors in a large clinically well-annotated cohort of patients with t(8;21)-AML. We complemented these analyses by the generation of a mouse model (Rosa26-LSL-AE9a-IRES-GFP x Vav1-Cre) with hematopoietic-specific AE9a-expression starting early on in development to determine the role of AE9a for leukemia initiation and progression.
In total, 129 patients based on the availability of a diagnostic bone marrow (BM) or peripheral blood (PB) sample were included; 93 patients were enrolled on one of five clinical trial protocols of the German-Austrian AML Study Group (AMLSG) (Supplemental Appendix); 36 patients were treated outside clinical studies. 127 patients received standard intensive chemotherapy and 2 patients were treated based on a non-intensive treatment protocol. The median follow up was 3.6 years (detailed patients characteristics are provided in the Supplemental Appendix, Table S1). AE9a mRNA expression was determined by qRT-PCR (Fig. S1a). Co-expression of AE9a as a fraction of the full-length AE transcript was reported as AE9a/AE ratio (%); ABL1 was used as housekeeping gene control [8]. Gene mutation status was available for KIT, FLT3 (ITD/TKD), NRAS and ASXL2 [9–11]. This study was conducted in accordance with the Declaration of Helsinki. Written informed consent for treatment and genetic testing was obtained from all patients.
The AE9a isoform was detectable in all 129 patients of our study cohort, which is in line with our recent finding and the data published by Ommen et al. [6, 7]. In contrast, Jiao et al. identified the AE9a splice variant only in a proportion of the patients [5]. This discrepancy is probably due to the varying techniques that have been applied; Jiao et al. determined the relative gene expression < 10−3 on a gradient dilution of Kasumi-1 cells as threshold for PCR-negativity [5], whereas our definition of PCR-negativity was set Ct > Y-intercept. In our data set, the median AE9a/AE ratio was 32% (range 3–77%) and did not significantly differ between BM (n = 116, range 8–77%, median 31%) and PB (n = 13, range 3–66%, median 52%). Interestingly, the allelic AE9a burden corresponded to our previous findings independently obtained by RNA-sequencing (13–64%) [7]. Median AE9a/AE ratio was neither correlated with clinical features (sex, age, WBC, platelets, BM blasts; Table S2) nor gene mutations affecting KIT, FLT3 or ASXL2.
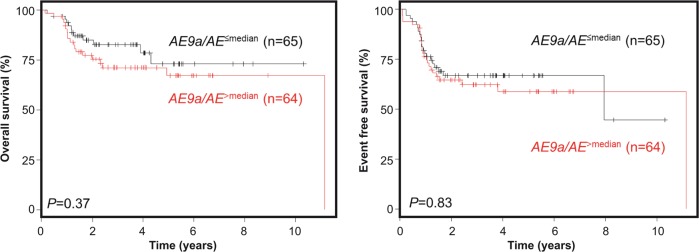
Using Cox regression analysis, AE9a/AE ratios were not associated with the clinical endpoints overall survival (OS), event-free survival (EFS) and cumulative incidence of relapse (CIR) (Table S3). The same was true when we performed univariate analyses comparing AE9a/AE ratios dichotomized at the median (AE9a/AE>median vs. AE9a/AE≤median): here, AE9a/AE ratios did not impact 4-yr OS (71 vs 79%; P = 0.37; Fig. 1 left, Table S4), 4-yr EFS (67 vs 59%; P = 0.83; Fig. 1 right, Table S4), and 4-yr CIR (32 vs 33%; P = 0.35; Table S4). Furthermore, we evaluated a possible correlation between AE9a/AE ratio and NRAS or KIT mutations that frequently co-occur in the t(8;21)-AML subtype. In a subgroup analysis, no significant differences with regard to the clinical endpoints OS, EFS and CIR were found between AE9a/AE>median and AE9a/AE≤median if stratified according to NRAS (Fig. S1b) or KIT (Fig. S1c) mutations. Finally, absolute AE9a quantification as ratio to the ABL housekeeping gene was performed to test its prognostic value independently of the AE transcript. AE9a/ABL ratios were dichotomized along the median (AE9a/ABL>median vs. AE9a/ABL≤median) but again were not associated with outcome: 4-yr OS (74 vs 89%; P = 0.33), 4-yr EFS (58 vs 72%; P = 0.18), and 4-yr CIR (40 vs 29%; P = 0.31).
Fig. 1.
Prognostic impact of AE9a/AE on clinical outcome. OS (left) and EFS (right) are shown according to dichotomization of AE9a/AE>median (red) and AE9a/AE≤median (black)
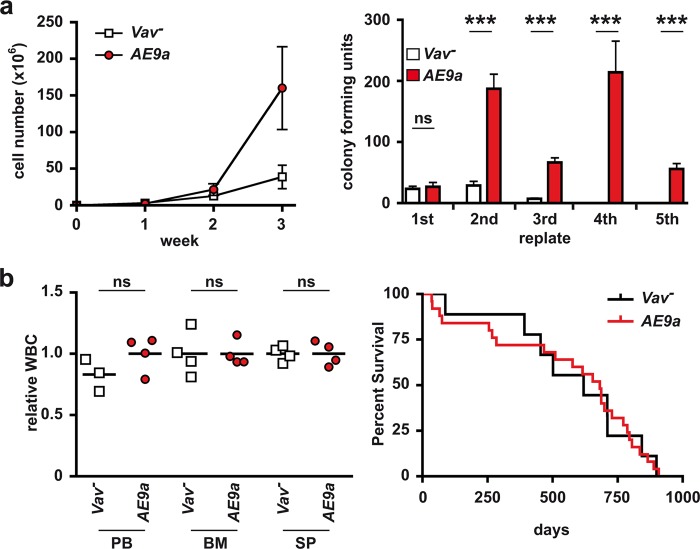
Recently, several studies showed that elevated expression of AE9a in primitive hematopoietic cells in mice via retroviral transduction protocols and subsequent transplantation of the transduced cells results in leukemia, even though with a long latency [12, 13]. These data imply a role for AE9a in leukemia initiation or progression, but do not exclude that additional mutations upon virus insertion might be necessary to contribute to the disease. In order to validate our clinical observations, we generated a novel mouse model for targeted expression of AE9a in the hematopoietic system (Rosa26-LSL-AE9a-IRES-GFP x Vav1-Cre, hereafter referred to as AE9a mice, Fig. S2a–c, Tables S5–S7) to further characterize the role of AE9a in leukemogenesis independent of viral transduction protocols. Expression of AE9a was verified at the level of mRNA (Fig. S2d), protein (Fig. S2e) as well as by GFP co-expression (Fig. S2f, g). As expected, isolated GFP+ BM cells from twelve weeks old AE9a mice (Fig. S3a) showed increased proliferation rates (Fig. 2a, left) and colony-forming capacity (Fig. 2a, right) compared to cells from control (Vav-) mice. In addition, numbers of short term hematopoietic stem cells (ST-HSCs, Fig. S3b, left) were significantly elevated, while there were no significant changes in the number of long term hematopoietic stem cells (LT-HSCs, Fig. S3b, middle) and LSK cells (Fig. S3b, right). In addition, the frequency of CMPs (common myeloid progenitors; CD16/32−, CD34+) and GMPs (granulocyte/macrophage progenitors, CD16/32+, CD34+), but not MEPs (megakaryocyte-erythrocyte progenitors, CD16/32−, CD34−) was elevated (Fig. S3c). Most interestingly, although there was a tendency for an elevated number of c-Kit+ cells in BM and spleen which usually correlates with pre-leukemia, [Fig. S3d [12, 13]], none of the AE9a-expressing mice showed signs of leukemia as revealed by WBC (Fig. 2b, left) or survival compared to the controls (Fig. 2b, right).
Fig. 2.
AE9a-expressing bone marrow cells exhibit enhanced stem cell characteristics but do not initiate leukemogenesis. a, left, AE9a-expressing cells show enhanced proliferation capacity. Proliferation potential of Lineage−, cKit+, GFP+ cells from 12 weeks old AE9a mice (red dots) and Lineage−, cKit+ cells isolated from Cre-negative littermate controls (Vav−, white squares) in suspension culture was estimated by cell number counts taken in seven days intervals over 3 weeks. MW ± SD, n = 2. a, right, AE9a-expressing cells show significant self-renewal capacity. Colony-forming potential of Lineage−, cKit+, GFP+ cells from 12 weeks old AE9a mice (red bars) and Lineage−, cKit+ cells isolated from Vav1− littermate controls (white bars) was measured by serial replating on semi-solid methylcellulose medium in seven days intervals over 5 weeks. MW ± SD (error bars) of the colony-forming units of triplicates from one representative experiment with n = 2 mice/group is shown. b, left, White blood cell counts (WBC) are not altered in 16 weeks old AE9a mice (red dots) compared to Vav− littermate controls (white squares). Individual and mean values of peripheral blood (PB), bone marrow (BM) and spleen (SP) from n = 4 mice/group are shown relative to the mean of the respective Vav− group. (b, right) AE9a expression in the hematopoietic compartment does not influence survival of mice. Kaplan–Meier plot illustrating that survival in AE9a mice (red line, mean survival 580 days, n = 25) is not altered compared to Vav− control mice (black line, mean survival 559 days, n = 9). ns, not significant; ***p < 0.001
We here report on the occurrence and prognostic impact of the AE9a splice variant in the so far largest set of adult t(8;21)-AML patients. Using a sensitive and robust quantitative RT-PCR assay, AE9a was detectable in all patients. In contrast to the previous studies, our study was performed in a large cohort of uniformly treated patients. Neither AE9a/AE, nor AE9a/ABL ratios correlate with any clinical feature and they do not impact on clinical outcome. These clinical observations are in line with data generated by our novel murine model which unequivocally demonstrates that expression of AE9a might contribute to leukemogenesis but is clearly not sufficient for the initiation of leukemia in mice.
We have recently investigated the molecular mechanisms of AE9a-dependent transformation in a viral transduction/transplantation model by analyzing its dual role in deregulation of the AML1 activating and the ETO repressing gene regulatory functions. In that system, the deregulation of both Notch and Aml1 target genes were required for the development of AE9a-driven leukemia [12] further supporting a necessary, but not sufficient role of AE9a for leukemia initiation. Thus, it is likely that a viral integration vector system for introducing AE9a in mice may cause leukemia through the activation of adjacent proto-oncogenes and therefore might not adequately recapitulate the human leukemogenesis.
In summary, in our large cohort of adult patients with t(8;21)-AML alternative splicing of the AML1-ETO fusion transcript represents a common feature. We could demonstrate that the allelic AE9a burden does not impact prognosis of this AML subtype therefore precluding its potential as a novel independent prognostic marker. Our clinical observation data were complemented by our recently established conditional AE9a knock-in mouse model showing that AE9a expression leads to enhanced proliferation and replating capacity but not to overt leukemia. Thus, AE9a rather acts as a precondition which requires a “second hit” for the development of AML. Alternative model systems like our tissue-specific knock-in mouse model may help to identify the critical “second hit” or additional environmental factors such as irradiation or chemotherapeutic agents.
Supplementary information
Acknowledgements
We are grateful to Drs. H. Jumaa, M. Bach, E. Surova and M. Stemmler for sharing reagents and knowledge. We want to thank E. Lopez and Dr. B. Kanzler from the Transgenic Mouse Unit of the Max Planck Institute in Freiburg (Germany) for the generation of the knock-in mice. This work was in part supported by the collaborative research grant TRR81 and BO 1639/9-1 by the DFG (German Research Foundation), the Max Planck Society and the EXC 294 in Freiburg and the Excellence Cluster for Cardio Pulmonary System (ECCPS) in Giessen to TB. BDG is supported by a Research Grant of the University Medical Center Giessen and Marburg (UKGM). This study was supported in part by the Excellence Initiative of the German Federal and State Governments (GSC-4, Spemann Graduate School). This study was additionally supported in part by the Deutsche Forschungsgemeinschaft (SFB 1074 project; subproject A3 and B3). MA was supported by the Else Kröner-Forschungskolleg and the Deutsche Forschungsgemeinschaft (DFG, AG252/1-1). We greatly acknowledge the members of the German-Austrian AML Study Group (AMLSG) for providing patient samples and clinical information.
Author contribution
MA, PS, TB, HG, LB, HD, KD, and FO designed the study and wrote the manuscript; BDG and IB generated the knock-in mouse model; MA, PS, AC, VIG, FGR, and NJ performed the experiments; MA, AC, DW, HD, PS, FO, and KD analyzed the results; DW performed statistical analyses; MA, AC, VIG, NJ, FGR, TS, TK, MW, KG, ML, HS, MR, EL, EK, FT, MH, AG, LB, PP, HD, and KD provided patient samples and clinical information.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mridul Agrawal, Peggy Schwarz
These authors jointly supervised this work: Konstanze Döhner, Franz Oswald
Supplementary information
The online version of this article (10.1038/s41375-019-0551-4) contains supplementary material, which is available to authorized users.
References
- 1.Higuchi M, O'Brien D, Kumaravelu P, Lenny N, Yeoh EJ, Downing JR. Expression of a conditional AML1-ETO oncogene bypasses embryonic lethality and establishes a murine model of human t(8;21) acute myeloid leukemia. Cancer Cell. 2002;1:63–74. doi: 10.1016/S1535-6108(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 2.Schessl C, Rawat VP, Cusan M, Deshpande A, Kohl TM, Rosten PM, et al. The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice. J Clin Invest. 2005;115:2159–68. doi: 10.1172/JCI24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolford JK, Prochazka M. Structure and expression of the human MTG8/ETO gene. Gene. 1998;212:103–9. doi: 10.1016/S0378-1119(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 4.Yan M, Kanbe E, Peterson LF, Boyapati A, Miao Y, Wang Y, et al. A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nat Med. 2006;12:945–9. doi: 10.1038/nm1443. [DOI] [PubMed] [Google Scholar]
- 5.Jiao B, Wu CF, Liang Y, Chen HM, Xiong SM, Chen B, et al. AML1-ETO9a is correlated with C-KIT overexpression/mutations and indicates poor disease outcome in t(8;21) acute myeloid leukemia-M2. Leukemia. 2009;23:1598–604. doi: 10.1038/leu.2009.104. [DOI] [PubMed] [Google Scholar]
- 6.Ommen HB, Ostergaard M, Yan M, Braendstrup K, Zhang DE, Hokland P. Persistent altered fusion transcript splicing identifies RUNX1-RUNX1T1 + AML patients likely to relapse. Eur J Haematol. 2010;84:128–32. doi: 10.1111/j.1600-0609.2009.01371.x. [DOI] [PubMed] [Google Scholar]
- 7.Faber ZJ, Chen X, Gedman AL, Boggs K, Cheng J, Ma J, et al. The genomic landscape of core-binding factor acute myeloid leukemias. Nat Genet. 2016;48:1551–6. doi: 10.1038/ng.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krönke J, Schlenk RF, Jensen KO, Tschurtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–16. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 9.Jahn N, Agrawal M, Bullinger L, Weber D, Corbacioglu A, Gaidzik VI, et al. Incidence and prognostic impact of ASXL2 mutations in adult acute myeloid leukemia patients with t(8;21)(q22;q22): a study of the German-Austrian AML Study Group. Leukemia. 2017;31:1012–5. doi: 10.1038/leu.2017.18. [DOI] [PubMed] [Google Scholar]
- 10.Paschka P, Du J, Schlenk RF, Gaidzik VI, Bullinger L, Corbacioglu A, et al. Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): a study of the German-Austrian AML Study Group (AMLSG) Blood. 2013;121:170–7. doi: 10.1182/blood-2012-05-431486. [DOI] [PubMed] [Google Scholar]
- 11.Schlenk RF, Döhner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 12.Thiel VN, Giaimo BD, Schwarz P, Soller K, Vas V, Bartkuhn M, et al. Heterodimerization of AML1/ETO with CBFbeta is required for leukemogenesis but not for myeloproliferation. Leukemia. 2017;31:2491–502. doi: 10.1038/leu.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan M, Ahn EY, Hiebert SW, Zhang DE. RUNX1/AML1 DNA-binding domain and ETO/MTG8 NHR2-dimerization domain are critical to AML1-ETO9a leukemogenesis. Blood. 2009;113:883–6. doi: 10.1182/blood-2008-04-153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.