Highlights
-
•
Our results also indicated that the prevalence of diarrhoea, nausea/vomiting, and abdominal pain in COVID-19 patients was 9.1%, 5.2%, and 3.5%, respectively.
-
•
We found that 0.8-11% of COVID-19 patients had chronic liver comorbidities and 2.6-53% patients had abnormal levels of ALT, AST and TB, and 6-98% had abnormal levels of ALB during the COVID-19 progression.
-
•
Meanwhile, diarrhea and nausea/vomiting were not associated with the COVID-19 progression and patient prognosis.
Keywords: COVID-19, SARS-CoV-2, Liver injury, Diarrhoea, Gastrointestinal symptoms
Abstract
Backgrounds
Since December 2019, novel coronavirus (SARS-CoV-2)-infected pneumonia (COVID-19) occurred in Wuhan, and rapidly spread throughout China. Our study aimed to evaluate the association of liver injury and gastrointestinal symptoms (GIS) with the progression of COVID-19.
Methods
A comprehensive search was performed on the PubMed to identify eligible studies that summarized the liver injury and GIS in COVID-19.
Results
A total of 21 studies with 3024 patients were included. Up to 53% patients had liver dysfunctions and the degree of liver damage was associated the severity of the disease. The prevalence of diarrhoea, nausea/vomiting or abdominal pain in patients with COVID-19 were 9.1%, 5.2% and 3.5%, respectively. No significant was found in the prevalence of diarrhoea (OR, 1.24; 95%CI, 0.90 to 1.72; I2 = 0%, P = 0.19) and nausea/vomiting (OR, 1.24; 95%CI, 0.57 to 2.69; I2 = 61%, P = 0.58) between severe and non-severe patients. In addition, diarrhoea (OR, 1.22; 95%CI, 0.50 to 2.98; I2 = 0%, P = 0.66) and nausea/vomiting (OR, 1.09; 95%CI, 0.46 to 2.62; I2 = 0%, P = 0.84) were not associated with the prognosis of COVID-19 patients.
Conclusions
The incidences of GIS in patients with COVID-19 is relatively low and are not associated with the COVID-19 progression. Gastroenterologists should pay more attention to the liver injury induced by SARS-CoV-2 during the course of infection.
Introduction
Since December 2019, novel coronavirus (SARS-CoV-2)-infected pneumonia (COVID-19) occurred in Wuhan and spread rapidly across the world [1], [2]. The pathogen was confirmed to be a distinct clade from the β-coronaviruses, which was officially named SARS-CoV-2 with the disease termed COVID-19. Liver injury has been reported in several recent COVID-19 studies, but its incidence varies [3], [4]. In addition, Gu et al. and Xiao et al. concluded that gastrointestinal symptoms (GIS) (diarrhoea, vomiting or abdominal pain) should not be ignored during the outbreak of COVID-19 [5], [6]. Song et al. also reported a case with diarrhoea as the onset symptom, and emphasized that gastrointestinal system might be a potential route for SARS-CoV-2 infection [7]. However, the latest epidemiological study showed a relatively low prevalence of gastrointestinal symptoms induced by SARS-CoV-2 [3]. Therefore, we performed a systematic review and meta-analysis to determine the liver injury and the prevalence of GIS in COVID-19 patients.
Methods
Search strategy
This meta-analysis was performed and reported according to the PRISMA statement [8]. The study was approved by the Ethics Committee of the Zhongnan Hospital of Wuhan University (No. 2020011). A literature search was performed on the PubMed library from inception to 31 March 2020. The search strategy was a combination of (“COVID-19” OR “2019-ncov” OR “SARS-CoV-2”) AND (“clinical features” OR “clinical characteristics”). The relevant papers were also searched manually to identify additional studies that might have been missed in the above literature search.
Inclusion and exclusion criteria
A PICOS criteria was applied to strict the inclusion and exclusion criteria. Inclusion criteria were as follows:
-
•
studies about clinical characteristics of COVID-19;
-
•
number of samples > 10;
-
•
study contains indicators of liver dysfunction, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB)/globulin (GLB) and total bilirubin (TB);
-
•
specific gastrointestinal symptoms such as diarrhoea, nausea, vomiting, and abdominal pain are described.
Exclusion criteria were as follows:
-
•
case reports, letters or reviews;
-
•
non-English studies;
-
•
technical guideline;
-
•
unrelated research.
Data extraction
The data were extracted independently by two authors (Haizhou Wang and Fan Wang) from the included studies based on the following terms:
-
•
study ID;
-
•
date;
-
•
group size;
-
•
patient demographics;
-
•
group design;
-
•
liver dysfunction, including ALT/AST/ALB/GLB/TB;
-
•
GIS, including abdominal pain, diarrhoea and nausea or vomiting.
Discrepancies would be solved through discussion.
Statistical analysis
The Cochrane Review Manager (RevMan) program (RevMan 5.3, Denmark) and OpenMeta Analyst were used to pool and analyze the aforementioned outcomes extracted from the included studies. For the AEs (dichotomous data), odds ratios (OR) with 95% confidence intervals (CI) were selected to report the risk estimates following the Mantel-Haenszel method [9]. In addition, the heterogeneity among the studies we included was assessed by the Q and I 2 statistic [10]. When I 2 < 50%, the fixed effects model was applied to estimate risk using the DerSimonian and Laird method because of lower heterogeneity. In contrast, the random effects model was used when I 2 was > 50% [9]. A P value < 0.05 was considered to be statistically significant.
Results
Search results and liver injury in COVID-19
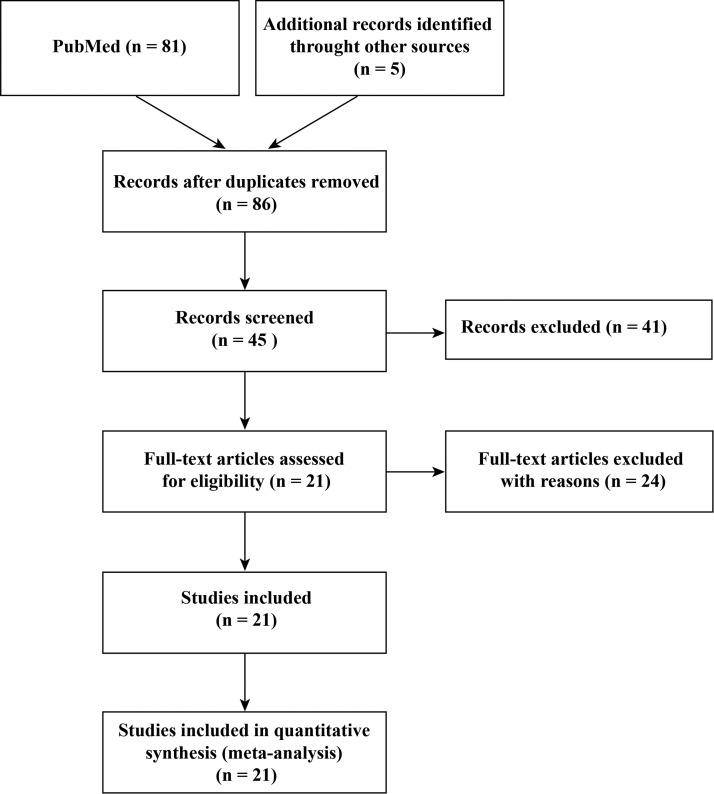
Fig. 1 showed the search flowchart and twenty-one studies were included with a total of 3024 COVID-19 patients [3], [5], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [2], [21], [22], [23], [24], [25], [26], [27]. The baseline characteristics of the included studies was presented in Table 1 . First, we evaluated how the liver is affected based on the included study. A total of 16 studies reported liver comorbidities or ALT/AST/ALB/GLB/TB abnormalities (Table 2 ). The data showed that 0.8-11% of COVID-19 patients had chronic liver comorbidities and 2.6-53% patients had abnormal levels of ALT, AST and TB, and 6-98% had abnormal levels of ALB during the COVID-19 progression. Moreover, patients with severe conditions or non-survival patients had higher rates of liver dysfunction. Thus, liver injury was more common in severe cases than mild cases in COVID-19.
Figure 1.
Flow chart of the whole procedures in this meta-analysis.
Table 1.
Baseline characteristics of the included studies.
| Study ID | Date | Group size | Gender (M/F) | Age (mean) | Gastrointestinal symptoms |
|---|---|---|---|---|---|
| Zhou 2020 |
12/29/2019- 1/31/2020 |
Non-survival (54) Survival (137) |
16/38 56/81 |
69 52 |
Nausea or vomiting and diarrhoea |
| Zhang 2020 |
16/1/2020- 3/2/2020 |
Non-severe (82) Severe (58) |
38/44 33/25 |
51.5 64 |
Nausea, abdominal pain and diarrhoea |
| Young 2020 |
23/1/2020- 3/2/2020 |
Non-severe (12) Severe (6) |
7/5 2/4 |
37 56 |
Diarrhoea |
| Yang 2020 | 12/24/2019- 1/26/2020 |
Non-survival (32) Survival (20) |
21/11 14/6 |
64.6 51.9 |
Vomiting |
| Yang 2020 | 17/1/2020- 10/2/2020 |
Overall (149) | 81/68 | 45.11 | Diarrhoea and nausea or vomiting |
| Xu 2020 |
10/1/2020- 26/1/2020 |
Overall (62) | 35/27 | 41 | Diarrhoea |
| Xu 2020 |
17/1/2020- 10/2/2020 |
Overall (90) | 39/51 | 50 | Diarrhoea, nausea and vomiting |
| Wang 2020 | 1/1/2020- 28//2020 |
Non-ICU (102) ICU (36) |
53/51 22/14 |
51 66 |
Diarrhoea, vomiting and abdominal pain |
| Shi 2020 |
12/20/2019- 1/23/2020 |
Overall (81) | 42/39 | 49.5 | Diarrhoea and vomiting |
| Huang 2020 |
12/2019- 1/2020 |
Overall (34) | 14/20 | 56 | Diarrhoea |
| Huang 2020 |
12/16/2019- 1/2/2020 |
Non-ICU (28) ICU (13) |
19/9 11/2 |
49 49 |
Diarrhoea |
| Guan 2020 |
12/11/2019- 1/29/2020 |
Non-survival (67) Survival (1032) Non-severe (926) Severe (173) |
45/22 592/437 537/386 100/73 |
63 46 45 52 |
Diarrhoea and nausea or vomiting |
| Chen 2020 |
1/1/2020- 1/20/2020 |
Overall (99) | 67/32 | 55.5 | Diarrhoea, nausea and vomiting |
| Chen 2020 |
1/20/2020- 2/6/2020 |
Overall (249) | 126/123 | 51 | Diarrhoea |
| Xiao 2020 |
2/1/2020- 2/14/2020 |
Overall (73) | 41/32 | 43 | Diarrhoea |
| Qin 2020 |
1/10/2020- 2/12/2020 |
Non-severe (166) Severe (286) |
80/86 155/131 |
53 61 |
Diarrhoea, nausea and vomiting, abdominal pain |
| Qian 2020 |
1/20/2020- 2/11/2020 |
Mild (82) Severe (9) |
NA | 49 66 |
Diarrhoea, nausea and vomiting |
| Mo 2020 |
1/1/2020- 2/5/2020 |
General (70) Refractory (85) |
33/37 55/30 |
46 61 |
Diarrhoea, nausea, vomiting and abdominal pain |
| Chang 2020 |
1/16/2020- 1/29/2020 |
Overall (13) | NA | 34 | Diarrhoea |
| Zhang 2020 |
1/17/2020- 2/8/2020 |
Normal CT (72) Abnormal CT (573) |
33/39 295/278 |
34 46 |
Diarrhoea, nausea and vomiting |
| Chung 2020 |
1/1/2020- 2/5/2020 |
Overall (21) | 13/8 | 51 | Nausea |
Abbreviation: ICU: intensive care unit; M: male; F: female; CT: computed tomography.
Table 2.
Comorbidity with liver injury in patients with SARS-CoV-2 infection.
| Study ID | Patients number | Patients with pre-existing liver conditions | Total patients with abnormal liver function | Notes |
|---|---|---|---|---|
| Chen et al. | 249 | 2 (0.8%) patients had chronic hepatitis B virus infection | NA | AST/ALT/ALB levels were within normal range. |
| Chen et al. |
99 | NA | ALT abnormal (28%) AST abnormal (35%) ALB abnormal (98%) TB abnormal (18%) |
43 (43.4%) patients had liver function abnormality, with ALT or AST above the normal range |
| Guan et al. |
1099 | 23 (2.1%) patients had chronic hepatitis B virus infection | ALT abnormal (21.3%) AST abnormal (22.2%) TB abnormal (10.5%) |
ALT abnormal: severe (19.8%) vs. non-severe (28.1%) survival (19.9%) vs. non-survival (40.8%) AST abnormal: severe (39.4%) vs. non-severe (18.2%) survival (20.1%) vs. non-survival (50.0%) TB abnormal: severe (13.3%) vs. non-severe (9.9%) survival (9.8%) vs. non-survival (20.8%) |
| Huang et al. |
41 | 1 (2%) patients had chronic liver disease | AST abnormal (37%) |
AST abnormal: ICU (62%) vs. non-ICU (25%) |
| Huang et al. |
34 | 1 (2.9%) patients had chronic liver disease | ALT abnormal (23.5%) AST abnormal (20.6%) ALB abnormal (73.5%) TB abnormal (8.8%) |
NA |
| Mo et al. |
155 | 7 (4.5%) patients had chronic liver disease | NA | The levels of ALT/AST all increased slightly, but all were within the normal range. The levels of ALB/GLB all decreased slightly, but all were within the normal range. |
| Qian et al. |
91 | NA | ALT abnormal (7.7%) AST abnormal (9.9%) ALB abnormal (47.3%) |
NA |
| Qin et al. |
452 | 6 (1.3%) patients had chronic liver disease | NA | NA |
| Shi et al. |
81 | 7 (9%) patients had chronic liver disease | AST abnormal (53%) |
The average level of ALT was 46.2U/L. |
| Wang et al. |
138 | 4 (2.9%) patients had chronic liver disease | NA | The levels of ALT/AST/TB all increased slightly in the ICU group, but all were within the normal range. |
| Xu et al. |
62 | 7 (11%) patients had chronic liver disease | AST abnormal (16.1%) | The average level of ALT was within the normal range. |
| Yang et al. |
149 | NA | ALT abnormal (12.1%) AST abnormal (18.1%) ALB abnormal (6.0%) TB abnormal (2.68%) |
NA |
| Yang et al. |
52 | NA | Liver dysfunction (29%) | NA |
| Zhang et al. |
645 | 24 (3.7%) patients had chronic liver disease | NA | The levels of ALT/AST/TB all increased in the abnormal CT imaging group, but all were within the normal range. The levels of ALB decreased in the abnormal CT imaging group, but all were within the normal range. |
| Zhang et al. |
140 | 8 (5.7%) patients had fatty liver and abnormal liver function | NA | NA |
| Zhou et al. |
191 | NA | ALT abnormal (31%) | The levels of ALB decreased below the normal range in the non-survival group. |
Abbreviation: AST: aspartate aminotransferase; ALB: albumin; GLB: globulin; ALT: alanine aminotransferase; TB: total bilirubin; ICU: intensive care unit; NA: not applicable.
The prevalence of GIS
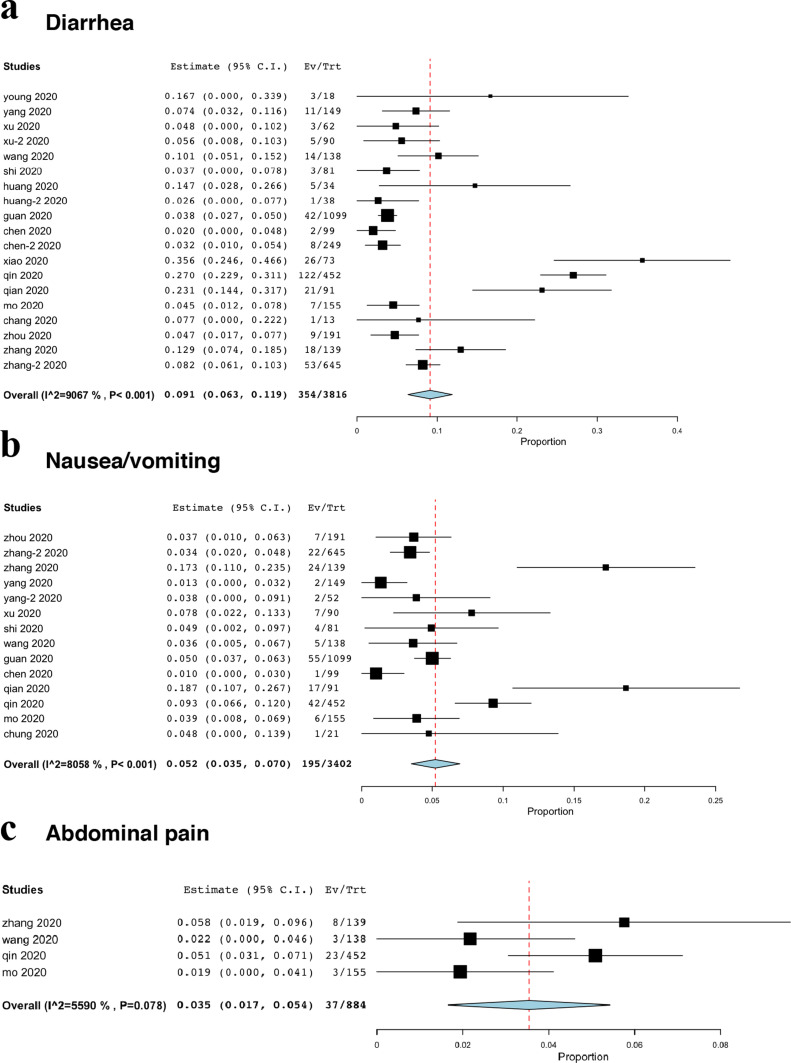
Diarrhoea was the most common gastrointestinal symptom (19 studies included symptoms of diarrhoea) and selected as the primary outcome, while its prevalence was only 9.1% (95%CI, 6.3% to 11.9%, Fig. 2 a). Fourteen studies reported symptoms of nausea or vomiting, with the prevalence of 5.2% (95%CI, 3.5% to 7.0%, Fig. 2b). In addition, only four studies reported abdominal pain, with the prevalence of 3.5% (95%CI, 1.7% to 5.4%, Fig. 2c).
Figure 2.
The prevalence of gastrointestinal symptoms. (a) diarrhoea; (b) nausea/vomiting; (c) abdominal pain.
Severe vs. non-severe
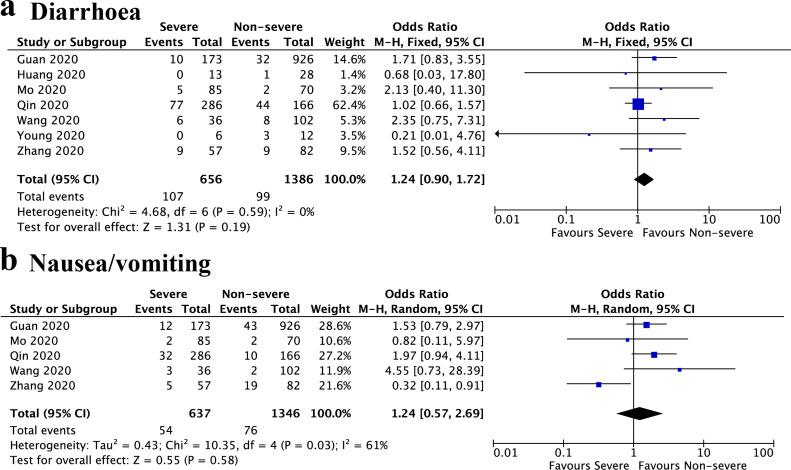
We then compared the prevalence of GIS between severe and non-severe patients. The definition of severe and non-severe also includes intensive care unit (ICU) vs. non-ICU and generally vs. refractory. Seven studies were included, and the results showed that no significant was found in the prevalence of diarrhoea between severe and non-severe patients (OR, 1.24; 95%CI, 0.90 to 1.72; I 2 = 0%, P = 0.19) (Fig. 3 a). In addition, the severe patients also had similar prevalence of nausea or vomiting with non-severe patients (OR, 1.24; 95%CI, 0.57 to 2.69; I 2 = 61%, P = 0.58) (Fig. 3b).
Figure 3.
Forest plot showed the odds ratio of diarrhoea (a) and nausea/vomiting (b) between severe and non-severe patients. M-H, Mantel–Haenszel; CI, confidence interval.
Survival vs. non-survival
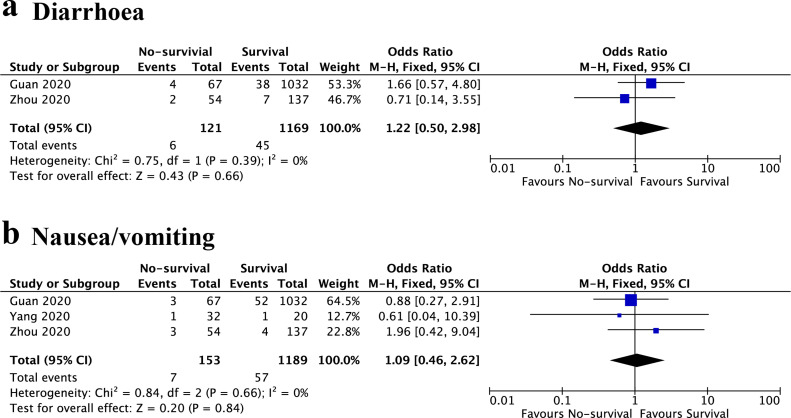
The relationship between GIS and prognosis of COVID-19 was also analyzed. Three studies were included, and the results also indicated that diarrhoea was not associated with the prognosis of COVID-19 patients (OR, 1.22; 95%CI, 0.50 to 2.98; I 2 = 0%, P = 0.66) (Fig. 4 a). The results also showed no significant difference in the prevalence of nausea/vomiting between non-survival and survival patients (OR, 1.09; 95%CI, 0.46 to 2.62; I 2 = 0%, P = 0.84) (Fig. 4b).
Figure 4.
Forest plot showed the odds ratio of diarrhoea (a) and nausea/vomiting (b) between survival and non-survival patients. M-H, Mantel–Haenszel; CI, confidence interval.
Discussion
In this study, we found that the prevalence of diarrhoea, nausea/vomiting, and abdominal pain in COVID-19 patients was 9.1%, 5.2%, and 3.5%, respectively. Meanwhile, they were not associated with the disease progression and patient prognosis. However, liver damage caused by SARS-CoV-2 infection was associated with disease progression.
Diarrhoea is the most common GIS in coronavirus infections, while nausea and vomiting are not specific. Moreover, nausea and vomiting are not necessarily caused by SARS-COV-2 infection and may be the result of various system dysfunctions. Therefore, diarrhea is the focus symptom of gastroenterologists on patients with coronavirus infection. It was reported that the prevalence of diarrhoea in patients with Middle East Respiratory Syndrome (MERS) and SARS was 30% and 10.6%, respectively [28]. One study indicated that MERS coronavirus could survive in simulated gastrointestinal fluids and has the ability to infect intestinal organoid models [28]. Hui et al. also proposed that SARS-CoV can be transmitted through the fecal-oral route [29]. Furthermore, Zhang et al. found that in the later stage of SARS-CoV-2 infection, the proportion of positive anal swabs was much higher than that of throat swabs [30]. The prolonged existence of SARS-CoV-2 viral RNA in faecal samples was also observed by Wu et al. [31]. They found that 55% of COVID-19 patients had anal swab positive results, and the average positive time of throat swab was 16.7 days, while the average positive time for anal swabs was 27.9 days. Importantly, positive staining of viral nucleocapsid protein (NP) and angiotensin-converting enzyme 2 (ACE2) could be visualized in the cytoplasm of stomach, duodenum and rectal gland epithelial cells through immunofluorescence [5]. These might provide the basis for virus mutation to obtain fecal-oral transmission capability. However, it is necessary to further identify whether diarrhoea is induced by antiviral drugs or antibiotics. For example, oseltamivir can cause drug-related diarrhoea [32]. Although there are some supportive findings, it is premature to determine whether SARS-CoV-2 can be transmitted through the fecal-oral route based on the current studies. Further well-designed studies are needed to identify the role of SARS-CoV-2 on the gastrointestinal tract.
In addition, up to 60% of SARS patients had liver impairment [33]. It was also reported that MERS patients had liver dysfunction [34]. Virus infection of liver cells may be the direct cause of liver damage, and recent study detected SARS-COV-2 RNA in blood samples, which provided a basis for viral exposure in the liver [35]. Several pathological studies further confirmed the presence of SARS-CoV in the liver tissue, although not detectable in the liver of MERS patients [33], [36]. Interestingly, Zou et al. found that liver showed lower ACE2 expression levels (< 1% ACE2 positive cells) through re-analyzing single cell RNA sequencing datasets [37], while some studies suggested that cholangiocytes were ACE2-enriched cells in the liver [38]. However, pathological findings found SARS-CoV-2 was not observed in the liver of a patient died from COVID-19 [39]. Therefore, the underlying mechanism of liver dysfunction induced by SARS-CoV-2 need further explore.
Based on the currently published data, it could be determined that liver injury in patients with mild COVID-19 could return to normal without special treatment, whereas liver injury in severe patients was more severe and required liver protection treatment. This may be due to the impaired immune function of patients with COVID-19. Our data showed 6-98% patients had abnormal ALB level and recent studies also demonstrated that lymphopenia, downregulation of CD4+ lymphocytes and cytokine storm were common in the severe or critical cases [14]. For patients with pre-existing chronic liver disease, such as chronic hepatitis B virus (HBV) infection, more evidence is needed to investigate the impact of co-infection (HBV and SARS-CoV-2) on the liver.
There are several limitations in our study. On the one hand, the patients in the results were the relatively focused patient population, mainly in China. During the current evolution into a global pandemic, we are eager to wait for more epidemiological studies in other countries. On the other hand, there are only 3 studies about the prognosis of COVID-19. More studies are needed to determine the relationship between liver dysfunction and disease prognosis.
In conclusion, liver injury caused by SARS-CoV-2 virus infection was associated with the severity of the disease. The prevalence of GIS was relatively low and was not associated with disease progression, with diarrhea of 9.1%, nausea/vomiting of 5.2% and 3.5% abdominal pain.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
HZW and QZ designed the study. HZW, FW and PSQ collected the data. HZW and FW drafted the manuscript. JL, HLW and QZ contributed to revise the manuscript. This study was supported by the Program of Excellent Doctoral (Postdoctoral) of Zhongnan Hospital of Wuhan University (Grant No. ZNYB2019003).
References
- 1.Hui D.S., E I.A., Madani T.A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu J., Han B., Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Y., Liu P., Shi X.L. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [W64] [DOI] [PubMed] [Google Scholar]
- 9.Yin P., Ji Q., Wang Y. Percutaneous kyphoplasty for osteoporotic vertebral compression fractures via unilateral versus bilateral approach: A meta-analysis. J Clin Neurosci. 2019;59:146–154. doi: 10.1016/j.jocn.2018.10.112. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemporary clinical trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 13.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W., Cao Q., Qin L. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.016. [S0163-4453(20)30099-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-4735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020:e201585. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30086-4. [S1473-3099(20)30086-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y., Tu M., Wang S. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel medicine and infectious disease. 2020:101606. doi: 10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J., Qi T., Liu L. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian G.Q., Yang N.B., Ding F. Epidemiologic and Clinical Characteristics of 91 Hospitalized Patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM. 2020 doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mo P., Xing Y., Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang D., Lin M., Wei L. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Cai H., Hu J. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung M., Bernheim A., Mei X. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui D.S.C., Zumla A. Severe Acute Respiratory Syndrome: Historical, Epidemiologic, and Clinical Features. Infect Dis Clin. 2019;33:869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W., Du R.H., Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y., Guo C., Tang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The Lancet Gastroenterology & Hepatology. 2020 doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beigel J.H., Bao Y., Beeler J. Oseltamivir, amantadine, and ribavirin combination antiviral therapy versus oseltamivir monotherapy for the treatment of influenza: a multicentre, double-blind, randomised phase 2 trial. Lancet Infect Dis. 2017;17:1255–1265. doi: 10.1016/S1473-3099(17)30476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chau T.N., Lee K.C., Yao H. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsaad K.O., Hajeer A.H., Al Balwi M. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? The Lancet Gastroenterology & Hepatology. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Y., He L., Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. The Journal of pathology. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Frontiers of medicine. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochemical and biophysical research communications. 2020 doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]