Abstract
Background
Although hospital systems have largely halted elective surgical practices in preparing their response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, transplantation remains an essential and lifesaving surgical practice. To continue transplantation while protecting immunocompromised patients and health care workers, significant restructuring of normal patient care practice habits is required.
Methods
This is a nonrandomized, descriptive study of the abdominal transplant program at 1 academic center (University of California, San Francisco) and the programmatic changes undertaken to safely continue transplantations. Patient transfers, fellow use, and patient discharge education were identified as key areas requiring significant reorganization.
Results
The University of California, San Francisco abdominal transplant program took an early and aggressive approach to restructuring inpatient workflows and health care worker staffing. The authors formalized a coronavirus disease 2019 (COVID-19) transfer system to address patients in need of services at their institution while minimizing the risk of SARS-CoV-2 in their transplant ward and used technological approaches to provide virtual telehealth where possible. They also modified their transplant fellow staffing and responsibilities to develop an adequate backup system in case of potential exposures.
Conclusion
Every transplant program is unique, and an individualized plan to adapt and modify standard clinical practices will be required to continue providing essential transplantation services. The authors’ experience highlights areas of attention specific to transplant programs and may provide generalizable solutions to support continued transplantation in the COVID-19 era.
California was one of the early hot spots for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the United States, with 5 of the first 20 confirmed diagnoses [1]. The first coronavirus disease 2019 (COVID-19)-positive patient was admitted to the University of California, San Francisco (UCSF) Health System on February 3, 2020. The San Francisco Department of Public Health banned visitors to skilled nursing facilities on March 12, 2020, and to hospitals on March 14, 2020 [2]. A shelter-in-place order for San Francisco and 5 adjacent counties took effect on March 16, 2020; this injunction was extended to the entire state 3 days later [2]. As of May 20, 2020, there were 85,728 confirmed cases and 3485 deaths in the state, with 2185 cases and 37 deaths in San Francisco [3,4]. As of May 20, 2020, no documented nosocomial transmissions have been reported in the UCSF system.
Here, the authors describe their center’s approaches to 3 areas that may be of particular interest to the transplant community: management of patient transfers, modifications to fellow training, and patient education.
Methods
This is a descriptive analysis of a single US transplant center’s systemic responses to the COVID-19 pandemic. The UCSF transplant program performed 389 kidney transplants (34% living donor), 174 liver transplants (12% living donor), and 15 pancreas transplants in 2019. The authors’ transplant center actively follows approximately 7000 kidney transplant recipients, 3000 liver transplant recipients, and 300 pancreas transplant recipients. Between February 3, 2020, and April 15, 2020, they completed 49 deceased donor kidney transplants, 18 living donor kidney transplants, 18 laparoscopic donor nephrectomies, 29 deceased donor liver transplants, 4 living donor liver transplants. 4 donor hepatectomies, 3 simultaneous liver-kidney transplants, 1 kidney-pancreas transplant, 1 total pancreatectomy auto-islet cell transplant, and 2 kidney autotransplants. At the time of organ offers, patients were provided the risks and benefits of undergoing transplantation in the setting of the COVID-19 pandemic and were given the opportunity to defer transplantation per patient preference.
SARS-CoV-2 testing evolved during the period described. Through April 16, 2020, 4023 tests were completed at UCSF, with 144 (3.5%) positive results. Initially, the hospital had limited capacity to run in-house reverse transcription polymerase chain reaction assays, which required up to 24 hours for results. Overflow tests were sent to a nearby hospital with up to a 5-day turnaround time. This rendered it nearly impossible to obtain results in a timely fashion prior to a deceased donor transplant. During the course of the next several weeks, testing capacity and speed improved significantly. At the time of writing, reverse transcription polymerase chain reaction testing was performed on the ePlex system (GenMark Diagnostics, Carlsbad, Calif, United States) that required 2.5 to 3 hours per run. By mid-April, the authors were testing all potential recipients and donors and had developed a system to expedite processing pre-transplant. Their current policy is to screen all transplant recipients and living donors within 4 days of their operation. For recipients of deceased donor organs, the authors recommend early admission to provide additional time for COVID-19 testing while minimizing cold ischemia time.
This study was exempt from review by an institutional review board or ethics panel.
Results
Patient Transfers From Outside Hospital
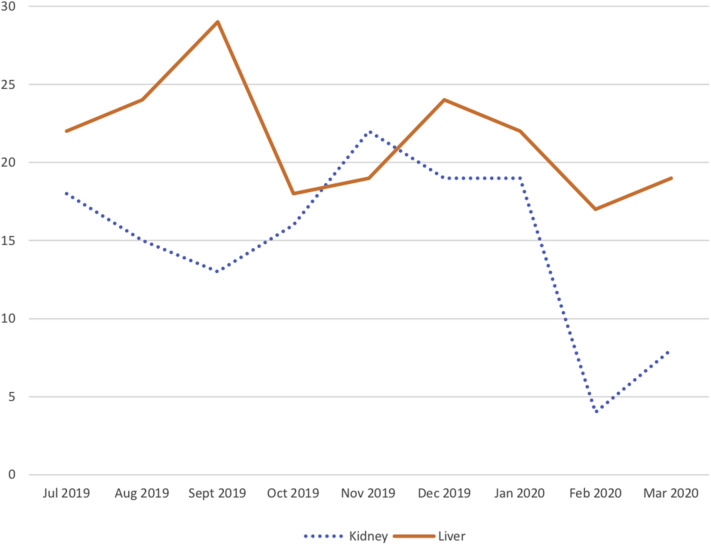
Prior to COVID-19, there were an average of 17 transfers a month to the kidney service and 22 transfers a month to the liver service from outside facilities (Fig 1 ). The authors’ customary practice is to ask most patients with postoperative complications requiring inpatient care to come to UCSF, either via presentation to the emergency department, direct admission, or transfer from an outside hospital. In addition, transfers to the liver service include patents with high Model for End-Stage Liver Disease scores who are referred for expedited evaluation for liver transplantation. Typically, transfers are approved by the on-service attending physician, then arranged through a transfer center that helps coordinate paperwork, bed management, and transportation. Patients are admitted to the multidisciplinary transplant service housed within a single hospital unit.
Fig 1.
Transfers to the UCSF Liver and Kidney Transplant Services.
UCSF began preparing for a surge in COVID admissions about the same time as the first COVID-19-positive patient was admitted on February 3, 2020. Shortly thereafter, UCSF established a transfer manager, whose authorization was required for any transfer from another facility. UCSF also designated a separate unit for patients with COVID-19. Transfers are restricted to patients who urgently needed specialized care. As a result, beginning in February 2020 the number of transplant patients being transferred has decreased notably on the kidney service (6 per month) but remained stable on the liver service (18 per month) (Fig 1). The exact reasons for this change are not clear, but the authors hypothesize that this difference is attributable to the fact that the postoperative kidney complications were more readily managed via telephone advice to outside facilities, whereas many postoperative liver complications required specialized interventional radiology or endoscopic interventions that could not be performed elsewhere. Moreover, the rate of transfers of patients with high Model for End-Stage Liver Disease scores for transplant evaluation is unchanged.
Post-transplant admissions are adjusted with the goal of minimizing the risk of SARS-CoV-2 transmission on the transplant unit. Post-transplant patients presenting with a confirmed COVID-19 diagnosis or symptoms highly concerning for COVID-19 are being admitted to an internal medicine service dedicated to initial evaluation for COVID-19, with the transplant team consulting. If COVID-19 is ruled out, they are transferred to the transplant service. Recipients who are deemed low-risk for COVID-19 but are admitted with fevers are admitted to the transplant service. The first such patient, a post-liver patient with reported temperatures up to 102°F at home and a history of recent biliary stent placement, was admitted March 8, 2020. He was met on arrival by nursing staff wearing personal protective equipment; a mask was placed on him, and he was brought to the transplant unit, where droplet precautions were instituted until his COVID-19 test result was negative.
Fellow Training and Use
The UCSF Abdominal Transplant Fellowship has 4 surgical fellows who alternate between liver transplant, kidney transplant, deceased donor organ recovery, and pediatric transplantation. The adult liver and kidney programs operate at the main campus; pediatric transplantation is based at UCSF Benioff Children’s Hospital (BCH), approximately 4 miles from the main campus. The authors participate in organ procurements in California, Nevada, New Mexico, and Arizona, with recent changes to organ allocation policy expanding their catchment to include Oregon and Idaho. They have a regional procurement center 40 miles from the main campus, and 26% of local donors are relocated to that site.
The geographic arrangement of the clinical assignments across multiple sites created a natural stratification of at-risk clinical exposures. The UCSF Mission Bay campus, which houses BCH, is a clinical site dedicated to pediatric, oncology, and obstetrics/gynecology specialty care. As such, the campus is without a primary medical inpatient service and adult emergency services, resulting in all patients suspicious for COVID-19 being routed to the main campus. In addition, the majority of admissions to the adult kidney and liver services were undergoing evaluation to rule out COVID-19, raising our suspicion that the fellows in the primary kidney and liver services would be at highest risk for nosocomial exposures.
The authors sequestered their donor and pediatric fellows off the main campus to leave 2 of the 4 fellows as relatively “unexposed” backups and to decrease the possibility of cross-campus and cross-team contamination. The pediatric transplant fellow only reported to BCH, where 4 pediatric patients were screened for COVID-19 and all tested negative. The donor fellow remained off campus under California’s “shelter-in-place” ordinance, with exception of participating in off-site organ procurements. They assisted in the main campus operating room as case volume required with section head approval for each case, and visits to the hospital were limited as much as possible.
The organ procurement practices were adjusted on a precautionary basis to limit fellow exposure risk to COVID-19. All donors within the organ procurement organization were screened for COVID-19. As an institution, the authors elected to generally defer on donor after cardiac death organ donation at this time. They believed that the rapid nature of such procurements, often with extubation in the operating room, posed a higher and perhaps unreasonable risk of exposure. Furthermore, they ruled out donors with respiratory symptoms or ground-glass opacities on chest computed tomography. Despite uniform donor testing, there is a false negative rate of 20% to 30% on COVID-19 assays [5], so the authors declined donors with any symptoms consistent with COVID-19 infection. Even with these precautions, travel to and operating in another hospital increases the exposure risk. Beginning in mid-March, they elected to use local organ recovery whenever possible, a practice that is now recommended by the United Network for Organ Sharing effective March 28, 2020 [6]. If local recovery was not available, they also used faculty without clinical responsibilities to minimize fellow exposures.
Similar to many institutions, the authors quickly identified their rounding process as a major risk for exposures to patients and staff. The rounding procedures across all campuses were transitioned to virtual rounds on March 16, 2020, in accordance with the social distancing ordinance for the San Francisco bay area. Traditionally, the authors conduct daily table and walk rounds as a multidisciplinary team, including surgeons, hepatologists and nephrologists, pharmacists, social workers, nutritionists, physical therapists, case managers, and trainees. This rounding model was inconsistent with the need for social distancing, and these multidisciplinary rounds were drastically restructured using Zoom videoconferencing (San Jose, Calif, United States). Rounding via telemedicine has been previously described as an adjunct to rounds, such as wound rounds in burn patients or allowing families to participate in intensive care unit rounds [7,8], but rarely as the primary mode of rounding [1,9]. Prior to rounds, patients are seen by the intern or nurse practitioner and the fellow responsible for their care. Multidisciplinary rounds then proceed via Zoom videoconference, with participants logging in from separate locations. All patient findings are discussed to form preliminary plans. Attendings only then see patients to confirm daily plans while minimizing exposure risk. In the authors’ experience, much of their usual rounding process is verbal exchange of data and chart review, which have naturally transitioned to the virtual setting and eliminated unnecessary congregation in the hallways. In theory, walk rounds could be conducted by a single individual, accompanied by a videoconferencing console to allow others to participate virtually.
The transplant section adopted a zero-tolerance policy mandating that any personnel with a single upper respiratory illness symptom, fever, or gastrointestinal distress be tested for COVID-19 prior to resuming work. To date, 2 of the fellows, 2 on-service interns, and 2 transplant nurse practitioners have been tested for COVID-19 for mild symptoms, and all were negative. They returned to clinical duties after 24 hours without symptoms. One attending developed COVID-19; his exposure was thought to be community-based.
Patient Education
Patient instruction surrounding transplant medication regimens is an essential part of the discharge process, one that typically takes place during several days during extended, face-to-face contacts between a pharmacist and a patient’s caretakers. Hospital restrictions on visitors since March 14, 2020, have drastically altered workflow, and patient education is now being completed entirely by videoconferencing with individual patients and their families, typically via cell phone group chat functions. This requires a pharmacist on 1 device, the patient on another, and the family on a third device. If the patient or family does not primarily speak English, a Health Insurance Portability and Accountability Act–compliant language line consultant is added to the group chat to translate.
A reliable Internet connection, video camera, and videoconferencing software are necessary to optimize this education strategy. As part of the authors’ hospital-wide discharge policy, family members have been completing patient education sessions on day-of-discharge from designated waiting areas on the hospital campus. This has presented issues with dropped calls in areas without strong cellular signal despite on-campus WiFi. Possible solutions include a designated on-campus site with reliable Internet connectivity or creation of an on-campus, contactless computer terminal with an established videoconferencing connection to the pharmacist and patient. Although these virtual education sessions can be accomplished while caretakers are logged on from their home Internet connection, variable home Internet services on a patient-to-patient basis pose similar connectivity issues. Efforts to decrease the number of connections per call, such as including an in-person translator on the pharmacist’s line, may help to further improve videoconferencing quality. Although each patient room has a computer workstation for the nursing care, many lack a video camera. When a patient does not have his or her own phone, the hospital has provided an iPad. Some family members have encountered difficulty with downloading the Zoom application.
Several workflow changes can be incorporated to streamline the education process. Patients and their caretakers can be provided reading materials and electronic resources in advance of videoconferencing education sessions. This has previously proven effective in transplant medication education as an adjunct to in-person teaching [10,11] and may offload some of the education burden from the pharmacist pool. Group education sessions covering general immunosuppression information could be conducted in a webinar to educate multiple caretakers simultaneously in advance of individualized sessions. A standard “read-back” policy can also help to ensure 2-way communication to demonstrate comprehension during teaching sessions.
Since implementing this patient education strategy, the authors have experienced 2 medication-related readmissions. Although medication-related readmissions do occur during normal practice, it is unclear if virtual education contributed to these readmissions. One readmitted patient had low health literacy and was highly reliant on caretakers to ensure medication compliance. It is important to consider that family members and caretakers may be minimizing contact with patients in the early post-transplant period to prevent infection. Although intended to limit potential COVID-19 exposures, this may impair the direct, in-person support network needed by some transplant recipients.
Conclusion
Transplantation remains an essential service in the COVID-19 era, with the American College of Surgeons recommending continuation of life-saving transplants without postponement [12]. However, significant modifications must be made to ensure the safety of transplant recipients and health care workers. With improved turnaround times for SARS-CoV-2 testing, the authors recommend screening for all donors and potential transplant recipients prior to surgery. Although the authors have described several workflow modifications, the transplant community will have to continually evaluate best practices on a center, regional, and national level as the course of the pandemic evolves.
Acknowledgments
The authors wish to thank John Hillman for providing data on patient transfers. Deborah Adey, Danielle Krieger, Claudia Praglin, and Giulia Worner also contributed background information relevant to the article.
Footnotes
Carrie Thiessen and Steven A. Wisel contributed equally to this work.
References
- 1.Centers for Disease Control Coronavirus disease 2019 (COVID-19): Cases in U.S. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html [accessed 23.03.20]
- 2.San Francisco Department of Public Health Coronavirus (COVID-19) health orders. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#investigation [accessed 23.03.20]
- 3.Los Angeles Times staff Tracking coronavirus in California. 2020. https://www.latimes.com/projects/california-coronavirus-cases-tracking-outbreak/ [accessed 19.05.20]
- 4.San Francisco Department of Public Health Coronavirus facts here. 2020. https://www.sfdph.org/dph/alerts/coronavirus.asp [accessed 19.05.20]
- 5.UCSF Infectious Disease Division. Daily COVID-19 digest [staff newsletter]. March 27, 2020.
- 6.United Network of Organ Sharing March 28 UNOS update on COVID-19: UNOS strongly encourages local recovery of organs. 2020. http://view.email.unos.org/?qs=0d8f6d229c18de8691bca8d95ce1f8122119b9b1af75caae2807a667568d48237114edd35f4ea0e13cda5bf5068aedbe9af65335711385b2b2a59a9a82e8574292ed14fbd0560bb8 [accessed 28.03.20]
- 7.Yenikomshian H.A., Lerew T.L., Tam M., Mandell S.P., Honari S.E., Pham T.N. Evaluation of burn rounds using telemedicine: perspectives from patients, families, and burn center staff. Telemed J E Health. 2019;25:25–30. doi: 10.1089/tmj.2017.0320. [DOI] [PubMed] [Google Scholar]
- 8.Stelson E.A., Carr B.G., Golden K.E. Perceptions of family participation in intensive care unit rounds and telemedicine: a qualitative assessment. Am J Crit Care. 2016;25:440–447. doi: 10.4037/ajcc2016465. [DOI] [PubMed] [Google Scholar]
- 9.Oh C.K., Kim K.H., Jeong W., Han W.K., Rha K.H., Ahn B. Research on patient satisfaction of robotic telerounding: a pilot study in a Korean population. Urology. 2019;130:205–208. doi: 10.1016/j.urology.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Harrison J.J., Badr S., Hamandi B., Kim S.J. Randomized controlled trial of a computer-based education program in the home for solid organ transplant recipients: impact on medication knowledge, satisfaction, and adherence. Transplantation. 2017;101:1336–1343. doi: 10.1097/TP.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 11.Tsapepas D.S., Salerno D., Jandovitz N. Using technology to enhance medication regimen education after solid organ transplantation. Am J Health Syst Pharm. 2018;75:1930–1937. doi: 10.2146/ajhp170799. [DOI] [PubMed] [Google Scholar]
- 12.American College of Surgeons COVID-19: Guidance for triage of non-emergent surgical procedures. 2020. https://www.facs.org/covid-19/clinical-guidance/triage [accessed 28.03.20]