Abstract
Objectives
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This paper aims to examine the CT imaging characteristics of COVID-19.
Methods
We evaluated CT images obtained between 10 January 2019 and 16 February 2020 at Taihe Hospital. Scans were conducted 2–6 times per patient and the re-testing interval was 2–7 days. Ninety-five patients with positive SARS-CoV-2 nucleic acid test results were included in this study and we retrospectively analysed their CT imaging characteristics.
Results
Ninety-five patients underwent 2–3 SARS-CoV-2 nucleic acid tests and received a definitive diagnosis of COVID-19. Fifty-three were male and 42 were female, and their mean age was 42 ± 12 years (range: 10 months to 81 years). Sixty-nine patients (72.6%) experienced fever, fatigue, and dry cough, while 15 (15.8%) had poor appetite and fatigue, and 11 (11.6%) had a dry cough and no fever. On CT imaging, early stage patients (n = 53, 55.8%) showed peripheral subpleural ground-glass opacities; these were mainly local patches (22/53, 41.5%), while some lesions were accompanied by interlobular septal thickening. Thirty-four (35.8%) patients were classified in the ‘progression stage’ based on CT imaging; these patients typically showed lesions in multiple lung segments and lobes (21/34,61.8%), and an uneven increase in ground-glass opacity density accompanied by consolidation and grid-like or cord-like shadows(30.5%). Two patients (2.1%) showed a severe presentation on CT. These showed diffuse bilateral lung lesions, mixed ground-glass opacities and consolidation with cord-like interstitial thickening and air bronchograms, entire lung involvement with a “white lung” presentation, and mild pleural effusion. Six patients in remission (6.3%), visible lesion absorption, fibrotic lesions. Based on clinical signs, 71 (74.7%), 22 (23.2%), and 2 (2.1%) patients had mild or moderate, severe, and critical disease, respectively. Within the follow-up period, 93 patients recovered and were discharged, including the 53 early stage patients and 34 progression stage patients. The length of hospitalisation was 7–28 days (mean: 10 ± 3.5 days). On discharge, lesions were significantly reduced in area and had in many cases completely disappeared, while slight pulmonary fibrosis was present in some patients. One severe stage patient was still hospitalised at the end of the follow-up period and the other severe stage patient died. The overall mortality rate was 1.05%.
Conclusions
Understanding the CT imaging characteristics of COVID-19 is important for early lesion detection, determining the nature of lesions, and assessing disease severity.
Highlights
-
•
The main clinical syndromes of COVID-19 were fever, weakness and dry cough, while upper respiratory symptoms such as nasal congestion and runny nose were rare.
-
•
Differentiation of COVID-19 with other viral pneumonitis requires the combination of epidemiological and laboratory tests, and there can be no pneumonia in the early stage.
-
•
Improving the early diagnosis accuracy so that timely quarantine and treatment may be carried out.
Keywords
SARS-CoV-2
Pneumonia
Viral infection
CT imaging characteristics
1. Introduction
Coronavirus disease 2019 (COVID-19) is an acute pneumonia caused by the β-coronavirus ‘severe acute respiratory syndrome coronavirus’ (SARS-CoV-2). This virus is enveloped, spherical or oval in shape, polymorphic, and has a diameter of 60–140 nm [1]. Genetically, it is significantly different from SARS-CoV and MERS-CoV. While COVID-19 patients are the main source of infection, asymptomatic patients can be similarly infectious. The main transmission routes are through respiratory droplets and close contact; transmission is also possible when individuals are exposed to high concentrations of aerosols in a sealed environment, while faecal-oral transmission remains to be confirmed [[2], [3], [4]]. The clinical signs of infection include lower respiratory tract disease accompanied by fever, dry cough, and dyspnoea. A positive test for the SARS-CoV-2 nucleic acid is required for definitive diagnosis [5]. However, while the specificity of nucleic acid testing is high, the sensitivity is relatively low and there is an appreciable false negative rate. Diagnosis with chest computed tomography (CT) is more intuitive and faster; chest CT is therefore the main method used for screening and diagnosis [6,7]. CT scans play an important role in the diagnosis and treatment of lung disease. Based on our experience, the imaging findings in COVID-19 infection are diverse and range from a normal appearance to diffuse pulmonary changes. In this study, we retrospectively analysed CT imaging data from 95 patients with confirmed COVID-19 in our hospital in order to examine the CT characteristics of infection.
2. Materials and methods
2.1. General information
In our hospital, 95 patients underwent CT scans and tested positive for SARS-CoV-2 nucleic acid. This study included patients who met one of the following three sets of criteria: 1. History of residence in the region of the epidemic within the last 2 weeks, apparent pulmonary lesions, and positive SARS-CoV-2 nucleic acid test results; 2. Patients with the above epidemiological history accompanied by fever, sore throat, diarrhoea, and other respiratory and gastrointestinal symptoms, abnormal pulmonary lesions, and positive SARS-CoV-2 nucleic acid test results; 3. Subjects who came into contact with patients with fever without personal protection, and who had a positive SARS-CoV-2 nucleic acid test result, positive first CT scan results, and lung abnormalities on re-examination. The following patients were excluded from analysis: 1. Patients with a medical history supportive of COVID-19 infection but no history of contact with people in the epidemic region or with patients with fever/respiratory symptoms; 2. Patients with respiratory diseases such as tuberculosis, tumours, and other types of pneumonia; 3. Patients with gastrointestinal symptoms due to a gastrointestinal disorder or lesions leading to loss of appetite; 4. Patients with complicated respiratory and gastrointestinal symptoms resulting from other causes such as trauma, bleeding, or malignancy.
This study included 53 males and 42 females aged from 10 months to 81 years (mean age: 42 ± 12 years). Sixty-nine patients (72.6%) experienced fever, fatigue, and dry cough, while 15 (15.8%) experienced poor appetite and fatigue, and 11 (11.6%) had a dry cough and no fever. Seventy-one (74.7%), 22 (23.2%), and 2 (2.1%) patients developed moderate, severe, and critical disease, respectively. At the time of the first CT scan, 19 (20%), 64 (67.4%), and 12 (12.6%) patients had experienced fever for 1–3, 4–7, and 7–10 days, respectively. Two to six CT scans were conducted; the interval between scans was 2–7 days.
2.2. Imaging methods
According to the “COVID-19 Imaging Examination and Diagnosis Quality Control Protocol (interim 3rd edition)”, patients with suspected COVID-19 wore N95 masks or surgical masks during imaging. Before entering the imaging room, their hands were disinfected. The radiology technician wore protective gear, ensured that no other people were present, and stayed within the isolation zone.
Patients adopted a supine position and held their breath. The GE HealthOptima660 and LightspeedCT machines were used for chest CT scans. The scan parameters were set as follows: tube voltage 120 kV, tube current 200 mA, slice interval 5 mm, acquisition slice thickness 0.625 mm, scanning duration <5 s, standard lung window level 530–430 HU, and window width 1400–1600 HU. The mediastinal window level was 35–40 HU and the window width was 300–350 HU. The scanning range was from the entrance of the thoracic cavity to the posterior costophrenic angle. After scanning, the image was evaluated to ensure that the examination was successful and the image quality was sufficient for diagnosis. Chest CT re-examinations were carried out every 5–7 days to assess lesion outcomes.
After imaging of suspected COVID-19 patients, personal protective equipment was removed and appropriate disinfection procedures were followed for equipment and personnel.
2.3. Image interpretation
Images were interpreted by two senior radiologists experienced in chest radiology. All imaging data were analysed in the absence of clinical or laboratory results. After independent evaluation, any discrepancies were resolved through discussion and negotiation.
2.4. CT typing
Patients with single or multiple local ground-glass opacities (GGOs) in the subpleural region of the lung periphery or along the bronchovascular bundle, as well as air bronchograms and mild septal thickening at adjacent lobes, were included in the early stage imaging presentation group [8]. The progression stage group comprised patients who developed new lesions or an increase in the original lesion area, multiple lobar involvement, an increased density and appearance of consolidations of varying size and degree, a grid-like shadow, “crazy-paving sign”, “halo sign”, or “reverse halo sign”. Patients with diffuse bilateral lung lesions, whole lung involvement and a “white lung” presentation, air bronchograms, pleural thickening, and mild pleural effusion were included in the severe stage group.
2.5. Patients were classified into the following three categories based on their clinical presentation
Moderate: fever and respiratory symptoms; signs of pneumonia on imaging.
Severe: respiratory distress; respiratory rate (RR) ≥30 breaths/min; finger oxygen saturation ≤ 93% at rest; arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mm Hg.
Critical: respiratory failure leading to the requirement for mechanical ventilation; shock; other organ failure necessitating treatment in the intensive care unit (ICU).
2.6. Discharge criteria
Patients were required to satisfy the following four criteria for discharge: 1. Normal temperature for 3 days or more; 2. Significant improvement in respiratory symptoms; 3. Significant absorption of exudative lesions observed on lung CT; 4. Two consecutive negative results for the SARS-CoV-2 nucleic acid on respiratory samples (with an interval of at least 1 day between tests).
2.7. Statistical methods
Quantitative data was expressed as mean ± standard deviation. SPSS software was used for statistical analysis (SPSS, Chicago, IL, USA).
3. Results
Ninety-five patients with suspected COVID-19 underwent two to three SARS-CoV-2 nucleic acid tests for definitive diagnosis. All patients showed signs of COVID-19 pneumonia on chest imaging during diagnosis. Fifty-three (55.8%) patients were classified as early stage (Fig. 1, Fig. 2 ). These cases frequently showed peripheral subpleural ground-glass opacities, which were observed as local patches in 22/53 patients (41.5%). Ground-glass opacities were seen with interlobular septal thickening in 4/53 patients (7.5%). Thirty-four (35.8%) patients were classified into the progression stage (Fig. 3, Fig. 4 ); these patients commonly exhibited lesions in multiple lung segments and lobes (21/34, 61.8%), with an uneven increase in ground-glass opacity density accompanied by consolidation and grid-like or cord-like shadows (30%).There were two (2.1%) patients in the severe stage group (Fig. 5 ), these presented with diffuse bilateral lung lesions, mixed ground-glass opacities and consolidation with cord-like interstitial thickening or air bronchograms, entire lung involvement with a “white lung” presentation, and a slight pleural effusion. Six patients in remission (6.3%) (Table 1 ). The distribution and severity of lesions on imaging progressed during the course of the disease, and one or multiple imaging presentations could be identified at each stage (Table 2 ). Seventy-one (74.7%), 22 (23.2%), and two (2.1%) patients had mild or moderate (Fig. 6 ), severe (Fig. 7 ), and critical clinical disease, respectively (Table 3 ).
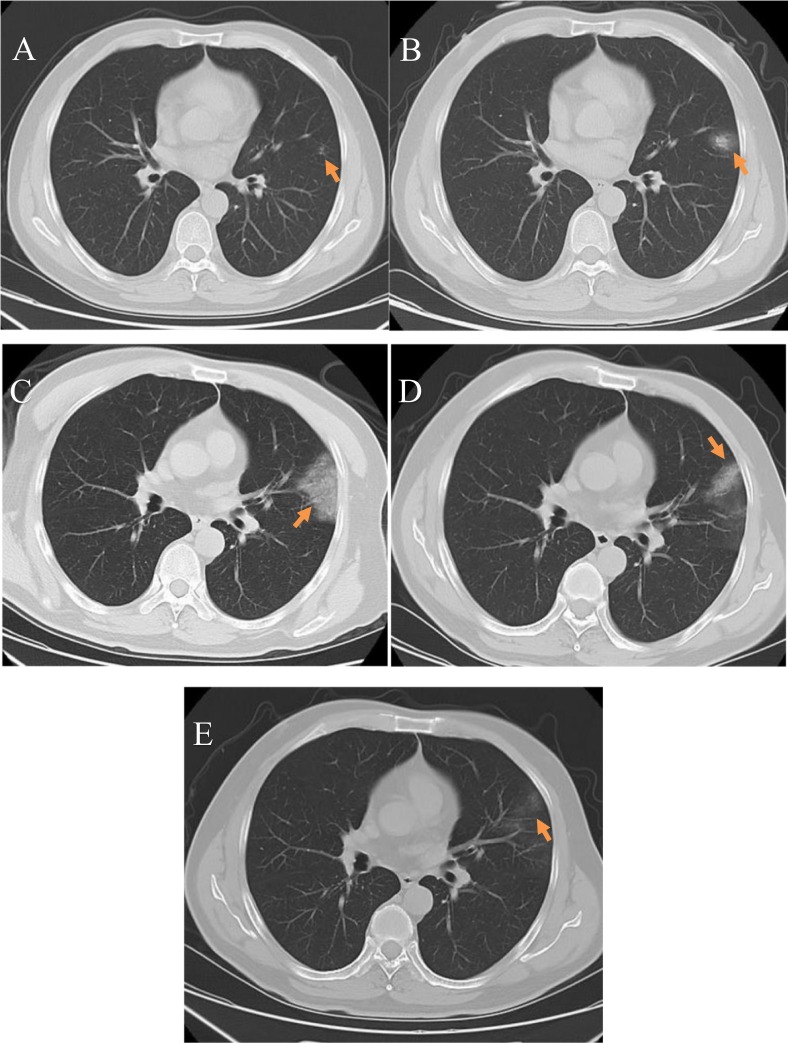
Fig. 1.
Early changes: A 41-year-old male COVID-19 patient with a history of occupational exposure to the disease who had experienced fever for 2 days. A) An initial plain axial chest CT image showed small patchy ground-glass opacities (arrow) located along vascular bundles in the subpleural region of the left upper lobe lingular segment; B) A plain axial CT image taken 2 days later showed that the ground-glass opacities had increased in area and density; C) A plain axial CT image taken after 9 days showed that the lesion area had significantly increased with an uneven increase in density, and an air bronchogram and vascular shadows were observed; D) A plain axial CT image taken after 15 days showed that the density of ground-glass opacities was decreased and partial absorption was observed; E) A plain axial CT image taken after 20 days showed that the lesions in the left upper lobe lingular segment were practically absorbed; a SARS-CoV-2 nucleic acid test taken at this time was negative.
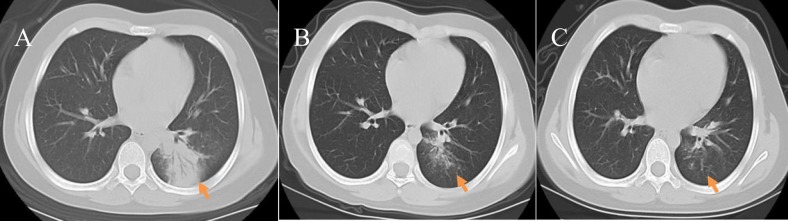
Fig. 2.
Early changes: A 7-year-old female COVID-19 patient who had experienced a fever and fatigue for 5 days; she had a history of contact with relatives who returned from Wuhan. A) A plain axial CT image showed cord-like consolidation in the left lower lung lobe with even density and blurred boundaries, and an air bronchogram was observed(arrow), as is seen in lobar pneumonia; B) A plain axial CT image taken 6 days later showed that the consolidation had been partially absorbed compared with the previous image and the lesion boundaries were slightly blurred; C) A plain axial CT image taken after 16 days showed that left lower lung lobe lesions had been virtually absorbed; a SARS-CoV-2 nucleic acid test taken at this time was negative.
Fig. 3.
Progressive changes: A 45-year-old female COVID-19 patient who had experienced a fever for 7 days and had a sore throat, dyspnoea, and a history of a short residence in Wuhan. A, B) Plain axial CT images showed multiple bilateral subpleural ground-glass opacities(arrow); the long axis of the lesions was vertical to the thoracic wall; C, D) Plain axial CT images taken 7 days later showed diffuse bilateral subpleural and central patchy ground-glass opacities involving multiple lung lobes.
Fig. 4.
Progressive changes: A 69-year-old female COVID-19 patient with a history of residence in Wuhan who reported a productive cough accompanied by systemic muscle soreness for 14 days. A–D) Plain axial CT images showed patchy ground-glass opacities and grid-like changes bilaterally in the subpleural areas of the lung periphery, with a “crazy-paving sign”(arrow); E–H) Plain axial CT images taken 3 days later showed an increased lesion area and an uneven increase in ground-glass opacities bilaterally in the lung periphery accompanied by consolidation. Ground-glass opacities and grid-like shadows were also present inside the lungs, accompanied by interlobular septal thickening.
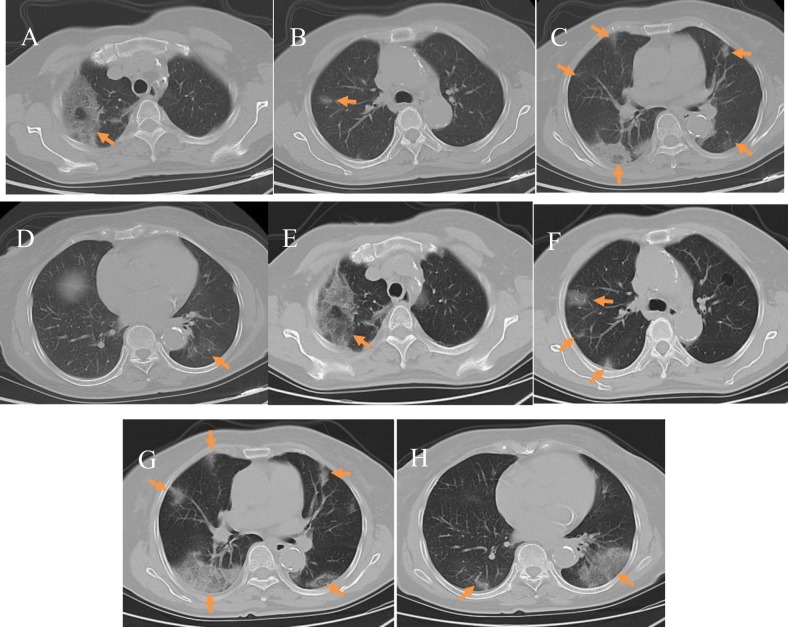
Fig. 5.
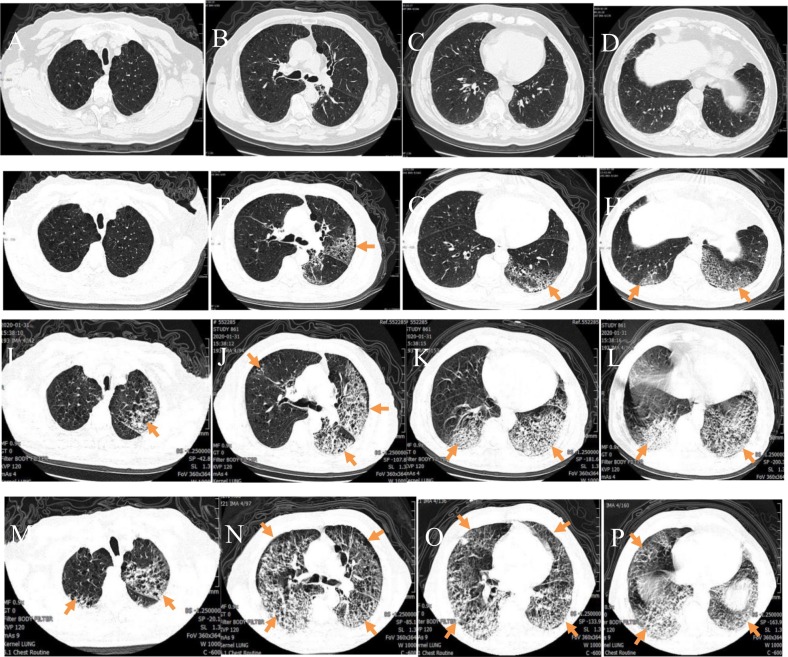
Early to Severe changes: A 67-year-old male COVID-19 patient with a history of residence in Wuhan who reported fever, fatigue, and poor appetite for 6 days. A–D) Initial plain axial chest CT images showed bilateral bronchial wall thickening and mild dilation, bilateral centrilobular emphysema, and bilateral lower lung interstitial thickening; E–H) Plain axial CT images taken 4 days later showed diffuse patchy ground-glass opacities in both lungs (arrow); in the left lung the lesions have a fan-like distribution and grid-like shadows, and vascular thickening is also seen; I–L) Plain axial CT images taken after 7 days showed a significant expansion of the lesion area with multiple patchy and grid-like shadows and areas of consolidation in both lungs; M–P) Plain axial CT images taken after 10 days showed diffuse bilateral lung lesions, with a “white lung” presentation.
Table 1.
Staging of coronavirus disease 2019 based on CT imaging
| Imaging presentation | Imaging stage |
||||
|---|---|---|---|---|---|
| Early stage | Progression stage | Severe stage | Remission stage | Proportion (%) | |
| Lesion distribution | |||||
| Subpleural subsegmental or segmental distribution | 19 | 35.8% | |||
| Involves >3 lung segments or 2 lung lobes | 21 | 61.8% | |||
| Diffuse bilateral lung distribution accompanied by air bronchogram | 1 | 50% | |||
| Lesion characteristic | |||||
| Normal or few abnormal dense shadows | 5 | 9.4% | |||
| Absence of specific ground-glass opacities or unilateral lung segment patchy shadows | 3 | 5.7% | |||
| Local patchy ground-glass opacity | 22 | 41.5% | |||
| Ground-glass opacity or interlobular septal thickening | 4 | 7.5% | |||
| Ground-glass opacity, non-uniform consolidation, and grid-like or cord-like shadows | 6 | 17.6% | |||
| Mixed consolidation shadow and cord-like interstitial thickening | 1 | 50% | |||
| Ground-glass opacity and diffuse fibrous cord-like shadow | 4 | 66.7% | |||
| Lesion progression | |||||
| Lesion expansion by >50% | 7 | 20.6% | |||
| Lesion outcome | |||||
| Absorption of exudative lesions | 2 | 33.3% | |||
| Total (n) | 53 | 34 | 2 | 6 | 100% |
Table 2.
CT imaging presentation of coronavirus disease 2019
| Imaging characteristic | Number of patients (n = 95) | Proportion (%) |
|---|---|---|
| Lesion distribution | ||
| Unilateral lung lobe | 39 | 41.2 |
| Bilateral lung lobes | 56 | 58.9 |
| Lesion site | ||
| Lung periphery, subpleural distribution | 45 | 47.4 |
| Central distribution | 13 | 13.7 |
| Simultaneous peripheral and central distribution | 37 | 38.9 |
| Number of lesions | n = 79 | 83.2% |
| Single | 22 | 23.2 |
| Multiple | 57 | 60 |
| Ground-glass opacity | n = 79 | 83.2% |
| Ground-glass opacity | 41 | 43.2 |
| Ground-glass opacity with consolidation and grid-like or cord-like shadows | 29 | 30.5 |
| Mixed type | 9 | 9.5 |
| Other imaging signs | n = 21 | 22.1% |
| Fibrous cord-like shadow | 13 | 13.7 |
| Pleural hypertrophy | 5 | 5.3 |
| Pleural effusion | 2 | 2.1 |
| Mediastinal lymph node enlargement | 1 | 1.1 |
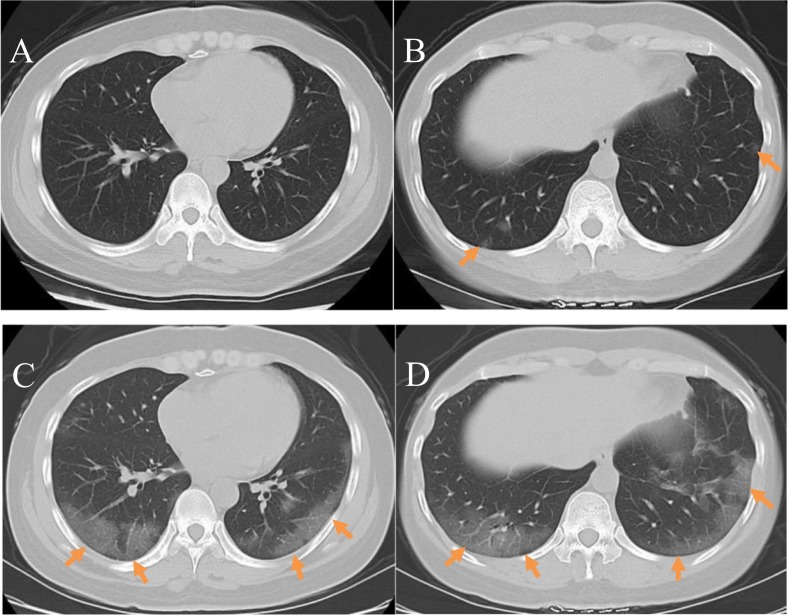
Fig. 6.
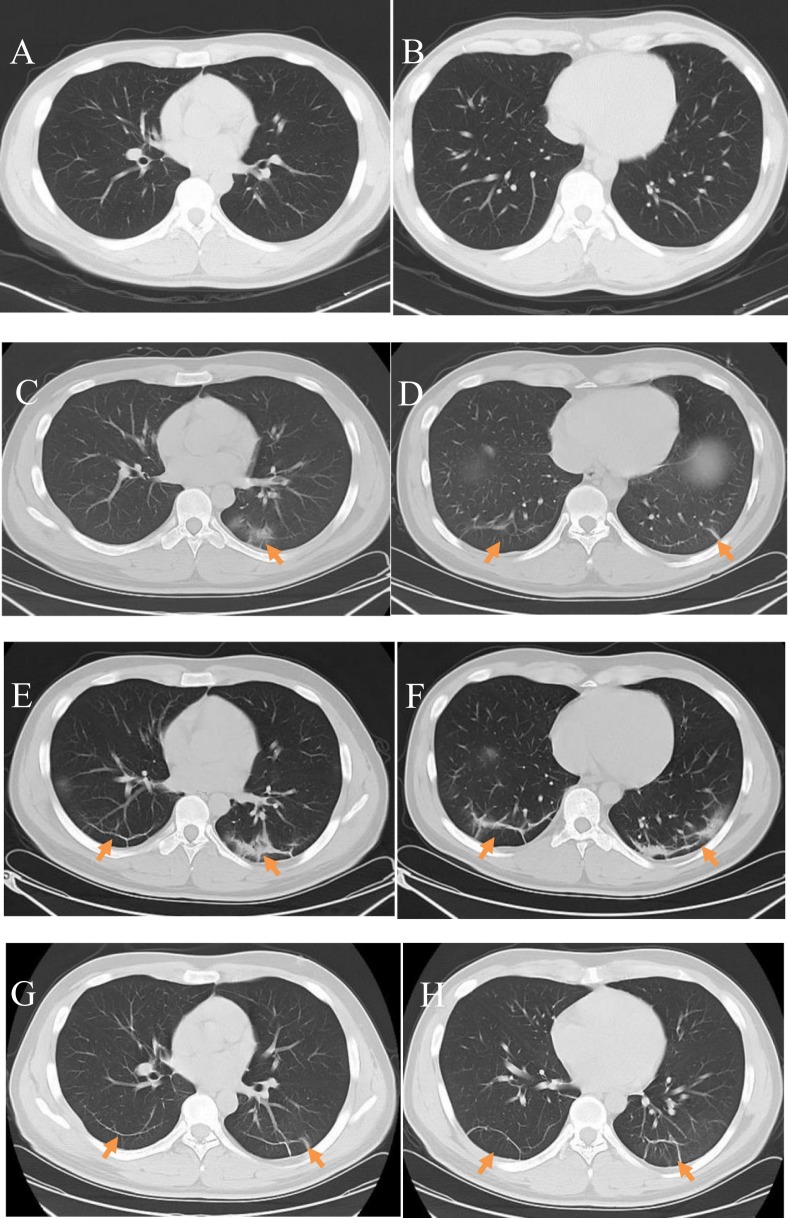
Mild or moderate changes: A 28-year-old male COVID-19 patient who had experienced fever for 1 day, but reported no history of contact with people from the epidemic region or confirmed COVID-19 patients. Clinical type: mild or moderate. A,B) Initial plain axial chest CT images did not show any apparent abnormalities; C,D) Plain axial CT images taken after 3 days showed patchy ground-glass opacities and cord-like shadows bilaterally in the subpleural regions(arrow); these are more significant in the left lung; E, F) Plain axial CT images taken after 6 days showed increased areas of subpleural ground-glass opacity bilaterally accompanied by consolidation and cord-like shadows, as well as interlobular septal thickening; G, H) Plain axial CT images taken after10 days showed that the lesions were mostly absorbed and there were fibrous cord-like shadows with clear boundaries in the subpleural area; a SARS-CoV-2 nucleic acid test taken at this time was negative.
Fig. 7.
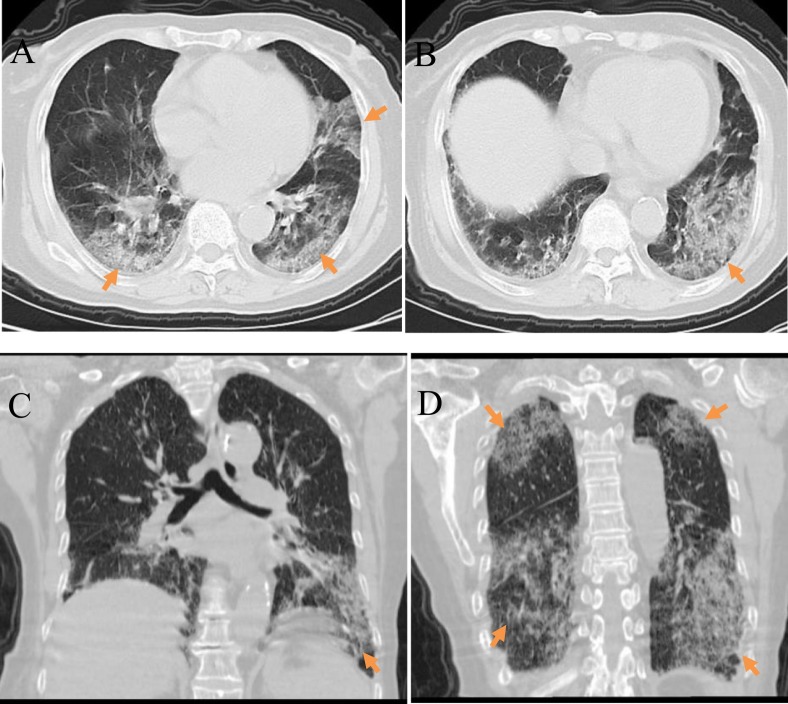
Severe changes: A 78-year-old female COVID-19 patient who reported an intermittent fever and cough for 1 week, and had a history of contact with family members from the epidemic region. Clinical type: severe. A,B) Plain axial chest CT images; C, D) Plain coronal chest CT images. All images showed extensive ground-glass opacities and areas of consolidation bilaterally in the lung periphery and subpleural areas (arrow); these are more significant in the lower lungs. Air bronchograms and vascular thickening shadows were also observed.
Table 3.
Clinical typing of coronavirus disease 2019 in relation to CT imaging presentation
| Imaging presentation | Clinical type (n (%)) |
||
|---|---|---|---|
| Mild or moderate | Severe | Critical | |
| Lesion distribution | |||
| Subsegmental or segmental distribution | 15 (21.1%) | ||
| Unilateral or bilateral lung periphery, subpleural distribution | 56 (78.9%) | 5 (22.7%) | |
| Involves 2 or more lung lobes | 9 (40.9%) | ||
| Diffuse bilateral lung distribution | 8 (36.4%) | 2 (100%) | |
| Ground-glass opacity characteristics | |||
| Single | 20 (28.2%) | 2 (9.1%) | |
| Multiple | 45 (63.4%) | 12 (63.4%) | |
| Vascular thickening and air bronchogram | 27 (38%) | 13 (59.1%) | 1 (50%) |
| Consolidation and grid-like or cord-like shadows | 23 (32.4%) | 5 (22.7%) | 1 (50%) |
| Mixed type | 1 (1.4%) | 8 (36.4%) | |
| Other imaging signs | |||
| Interlobar or bilateral pleural thickening | 1 (1.4%) | 4 (18.2%) | |
| Free or local encapsulated effusion | 1 (4.5%) | 1 (50%) | |
| Total (n) | 71 (5 + 66) | 22 | 2 |
Within the follow-up period, 93 patients recovered and were discharged, including the 53 early stage patients and 34 progression stage patients. The length of hospitalisation was 7 to 28 days and the mean length of hospitalisation was 10 ± 3.5 days. On discharge, lesions had significantly reduced in area or had completely disappeared, though slight pulmonary fibrosis was present in some patients. One severe stage patient was still hospitalised at the end of the follow-up period and one severe stage patient died. The overall mortality rate was 1.05%.
4. Discussion
The typical clinical presentation of COVID-19 is fever, fatigue, and dry cough; this presentation is consistent with lower respiratory tract infection. Nasal congestion, runny nose, and other upper respiratory tract symptoms are rare. Dyspnoea may occur 1 week after disease onset, and severely affected patients rapidly progress to acute respiratory distress syndrome, septic shock, refractory metabolic acidosis, coagulation disorders, and multiorgan failure. Most patients have a good prognosis [9,10]. At the early stage of COVID-19 infection, the peripheral white blood cell count is normal or decreased, the lymphocyte count is reduced, and the neutrophil percentage is not elevated. Most patients have elevated serum amyloid A (SAA), C-reactive protein (CRP), and erythrocyte sedimentation rate; these signs are more significant in severe pneumonia cases. Creatine kinase (CK), lactate dehydrogenase (LDH), and aspartate transaminase (AST) are elevated in severe disease. Some patients have elevated myoglobin and normal procalcitonin levels. A positive SARS-CoV-2 nucleic acid test result is required for a definitive diagnosis of COVID-19, but a negative test result does not necessarily preclude infection [2,5].
Among the 95 COVID-19 patients in this study, ground-glass opacities (43.1%) were mostly seen in the early stage imaging presentation and lesions were mainly located at the subpleural region in the lung periphery; this distribution may be due to the fact that virus particles fuse with the alveolar epithelium when they reach the cortical lobules in the lower lungs. This causes injury to the alveolar walls, vascular congestion, the accumulation of exudate in the alveoli, swelling around the lobule, and the simultaneous involvement of multiple adjacent lobules. In the early stage of disease, this mainly presents as interstitial ground-glass opacities. At later stages of disease, the lobules fuse, resulting in an irregular morphology and distribution of lesions that does not follow lung segments, and a fan-like distribution of lesions in subpleural areas [11,12]. In the progression stage, expansion of the lesion area is typically seen, with frequent involvement of multiple lung segments and lobes (21/34, 61.8%). Some patients may present with ground-glass opacities in combination with consolidation and grid-like or cord-like shadows (30.5%). This is mainly due to the collapse of alveolar walls, and the replacement of air in the alveoli with inflammatory exudate, cells, or tissue. The loss of air contrast causes the walls of pulmonary blood vessels and the trachea to become blurry; this often results from interstitial inflammatory thickening [13]. During the progression stage, air bronchograms or vascular thickening can be seen in consolidated areas. The increase in permeability that underlies these changes may be due to pathogen invasion of the bronchiolar and alveolar epithelium, which results in inflammatory cell infiltration and thickening and swelling of the tracheal walls; however, this does not lead to bronchiolar obstruction. Pathogen invasion of the epithelial cells in the lesion can also result in local vascular congestion and swelling. In the latter stages of disease, lesions may fuse or become partially absorbed [14]. In the severe stage, disease progression is rapid. Ground-glass opacities can be seen with cord-like interstitial thickening, which is considered to be due to the accumulation of cells or exudate; furthermore, the entire lung can be involved with a ‘white lung sign”.
In this study, a few ground-glass opacities were located at the periphery of bronchovascular bundles in some early stage and progression stage patients. In early stage patients, the long axis of these lesions was typically vertical to the thoracic wall and parallel to the bronchovascular bundle (3/53, 5.7%) while some patients in the progression stage showed interstitial thickening (6/34, 17.6%). Adjacent tracheal and bronchial walls did not show significant thickening in early stage patients, while adjacent pleural oedema sometimes developed. At the progression stage, local interstitial thickening gradually became apparent. Some of the patients in this study had pleural thickening (5.3%) while both of the severe stage patients showed mild pleural effusion; this may have been due to pleural inflammation resulting in inflammatory exudates. Only one patient (1.1%) had mediastinal lymph node enlargement due to other comorbidities.
In this study, in 5.3% of mild cases, the first CT scan was negative and re-examination 2–7 days later showed abnormalities. The initial scans can be negative because the virus is located in the upper respiratory tract during the early stage and does not cause pulmonary infiltration and exudative lesions [14]. Some mild cases progress to moderate disease; imaging in these cases typically reveals multiple lesions in the lung periphery or subpleural areas (56/71, 78.9%). Lesions are mostly seen in the lower lungs and multiple lesions are often seen bilaterally (45/71, 63.4%). These cases typically have a similar ground-glass opacity presentation to cases in the progression stage, but the lesions do not usually involve the entire lung segment. Patients with a severe clinical presentation (23.2%) presented with diffuse bilateral lung consolidation, air bronchograms and bronchiectasis, and patchy ground-glass opacities visible in non-consolidated regions. In critical patients (2.1%), the “white lung” presentation was seen on imaging and the lesion area was extensive. By combining the imaging and clinical data in our study, we found that the progression of clinical symptoms in COVID-19 patients often does not closely correspond to the disease stage seen on imaging; this may partially be due to differences in patients' immune status that affect their response at different stages of the disease. Another reason for this discrepancy could be that the history provided by patients regarding the onset of symptoms is not always accurate [[15], [16], [17], [18], [19], [20]].
COVID-19 disease can present with a diverse range of imaging findings; a combination of epidemiological, clinical, and laboratory data, as well as SARS-CoV-2 nucleic acid test results, are required to differentiate it from other viral pneumonias. In children, uncharacteristic or unique imaging presentations may be seen in this disease, so further studies of imaging findings in children are required [[21], [22], [23], [24]]. As COVID-19 patients are infectious during the incubation period and after disease onset, early diagnosis by imaging is vital.
Our study shows that certain findings on CT imaging are characteristic of COVID-19 infection and progression. Understanding the imaging characteristics of COVID-19 is important to support early diagnosis, control, and the evaluation of treatment results.
Table 1. Staging of coronavirus disease 2019 based on CT imaging.
Acknowledgments
Acknowledgments
Not applicable.
Funding
Not applicable.
References
- 1.Hui D.S., Azhar E.I., Madani T.A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health Commission, State Administration of Traditional Chinese Medicine Coronavirus disease 2019 diagnosis and treatment protocol (interim 7th edition) [EB/OL] http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf
- 3.Chan J.F.W., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New England Journal of Medicine. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C., Horby P.W., Hayden F.G. A novel coronavirus outbreak of global health concern. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Clinical Management of Severe Acute Respiratory Infection when novel CoronaVirus (nCoV) Infection is Suspected: Interim Guidance. 2020-01-13. HYPERLINK "https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected" https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 8.Coronavirus disease 2019 imaging diagnostic guidelines 2020 first edition. Chinese Journal of Radiology. 2020;54:E001. doi: 10.3760/cma.j.issn.1005-1201.2020.0001. [DOI] [Google Scholar]
- 9.World Health Organization Infection Prevention and Control During Health Care When Novel CoronaVirus (nCoV) Infection is Suspected: Interim Guidance [EB/OL] 2020-01-15. https://www.who.int/internal-publications-detail/surveillance-case-definitions-for-human-infection-withnovel-coronavirus-(ncov)
- 10.Pan Y., Guan H. Imaging changes in patients with 2019-nCov. Eur Radiol. 2020:1–2. doi: 10.1007/s00330-020-06713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Ren M.J., Zhang Y. Lung CT findings of 1 confirmed coronavirus disease 2019 (2019-nCoV) patient (attached with SARS pathology and differential diagnosis) New Medicine. 2020;30:4–6. doi: 10.12173/j.issn.1004-5511.2020.01.001. [DOI] [Google Scholar]
- 12.Tian S., Hu W., Niu L. 2020. Pulmonary pathology of early phase SARS-COV-2 pneumonia. Preprints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J., Zheng X., Tong Q. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W., Ni Z., Hu Y. 2020. Clinical characteristics of 2019 novel coronavirus infection in China. Med Rxiv. [Google Scholar]
- 16.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New England Journal of Medicine. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang B., Wang X., Li Q. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J Clin Med. 2020;9:462. doi: 10.3390/jcm9020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diao K., Han P., Pang T. HRCT imaging features in representative imported cases of 2019 novel coronavirus pneumonia. Precision Clinical Medicine. 2020;3:9–13. doi: 10.1093/pcmedi/pbaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y., Guan H., Zhou S. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020:1–4. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang P., Liu T., Huang L. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295:22–23. doi: 10.1148/radiol.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCov) in suspected human cases [EB/OL]. HYPERLINK "https://www.who.int/internal-publications-detail/surveillance-case-definitions-for-human-infection-withnovel-coronavirus-(ncov)-infection-is-suspected" https://www.who.int/internal-publications-detail/surveillance-case-definitions-for-human-infection-withnovel-coronavirus-(ncov)-infection-is-suspected.
- 23.Chen Z.-M., Fu J.-F., Shu Q. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020 doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen K., Yang Y., Wang T. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J Pediatr. 2020 doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]