Abstract
Background
Coronavirus disease 2019 (COVID-19) is characterized by risk of nosocomial transmission; however, the extent of environmental contamination and its potential contribution of environmental contamination to SARS-CoV-2 transmission are poorly understood. This study aimed to investigate whether environmental contamination may play a role in SARS-CoV-2 transmission.
Methods
Air samples were collected by natural precipitation, and environmental surface samples were collected by conventional surface swabbing. SARS-CoV-2 RNA detection was performed using reverse transcription polymerase chain reaction.
Results
Viral RNA was not detected in the 44 air samples. The positive rates in 200 environmental surface samples in medical areas (24.83%) was higher than that in living quarters (3.64%), with a significant difference (P < .05). The positive rates were 25.00% and 37.50% for the general isolation ward and intensive care unit, respectively, and no significant difference was observed between them (P = .238). The top 5 sampling sites with a positive rate in medical areas were beepers (50.00%), water machine buttons (50.00%), elevator buttons (42.86%), computer mouses (40.00%), and telephones (40.00%).
Conclusions
Most of the touchable surfaces in the designated hospital for COVID-19 were heavily contaminated, suggesting that the environment is a potential medium of disease transmission. These results emphasize the need for strict environmental surface hygiene practices and enhanced hand hygiene to prevent the spread of the virus.
Key Words: Air, Environmental surface, Disinfection, Hand hygiene, Hospital-associated infection
The coronavirus disease 2019 (COVID-19) is an emerging infectious disease caused by a novel coronavirus virus (SARS-CoV-2).1 , 2 The disease was first identified in Wuhan, China, in December 2019, which then spread rapidly from Wuhan to other areas of China. As of March 22, 2020, a total of 81,563 cases have been confirmed in China, and an outbreak occurred in several countries worldwide. The virus is highly contagious, and people are generally susceptible. Many healthcare workers have been infected during patient care.3 SARS-CoV-2 is mainly transmitted through infected respiratory droplets and close contact with the infected person. Further, there is risk for aerosol transmission when the virus is exposed to high concentrations of aerosol for a long time in a relatively closed environment.4 , 5 An environment contaminated with the feces and urine of infected patients may also cause transmission through aerosol or contact.6
To contain the spread of SARS-CoV-2, some designated hospitals have been appointed by the Chinese government as special treatment sites for the patients with COVID-19. Patients in the designated hospital were in high density, the SARS-CoV-2 could be excreted by respiratory droplets or aerosols, contaminating the surrounding environment. It has been reported that SARS-CoV-2 can remain viable and infectious in aerosols for hours and on surfaces up to days.7 If environmental disinfection is not thorough or effective, SARS-CoV-2 may spread widely and can even lead to nosocomial infection.
Researchers suggest that improving the environmental sanitation quality can reduce the spread of pathogens in hospitals and even prevent hospital infection outbreak.8 However, the extent of environmental contamination by SARS-CoV-2 and the potential contribution of this contamination to SARS-CoV-2 transmission are poorly understood. The present study was performed in a designated hospital to evaluate environmental contamination in the air and surfaces by SARS-CoV-2 RNA qualitative detection and to investigate whether environmental contamination may play a role in SARS-CoV-2 transmission.
Methods
Study setting
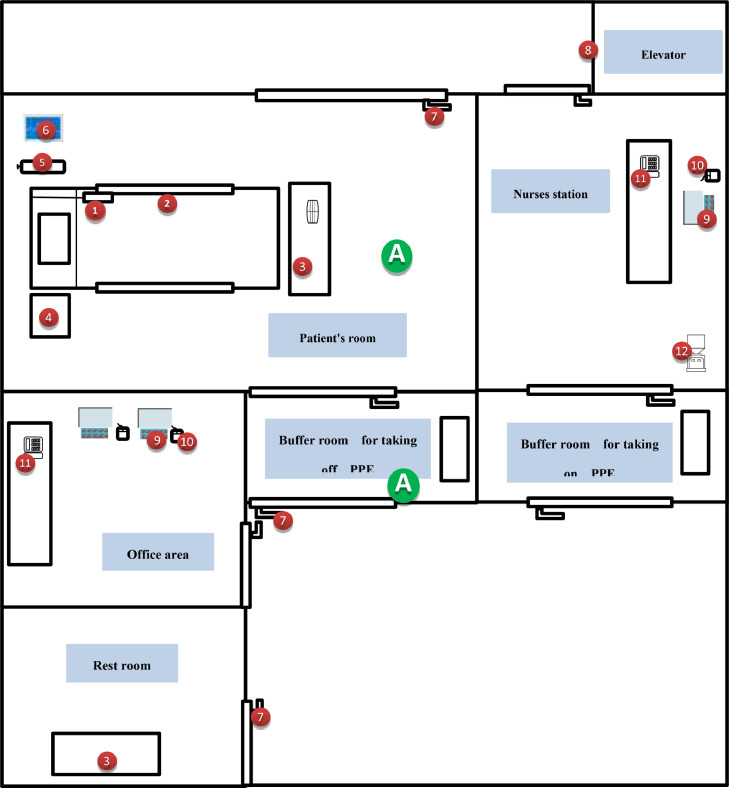
This study was conducted in Wuhan No. 7 Hospital, which was originally a comprehensive Grade 2A hospital and then became one of the first batch designated hospitals for COVID-19 in Hubei Province. The hospital started to treat patients with COVID-19 on January 22, 2020. It comprises general isolation wards, intensive care unit (ICU), fever clinic, clinical laboratory, office areas, and restrooms (Fig 1 ). All regions of the hospital were divided into 2 categories: (1) moderate- and high-risk regions, including the medical areas such as patient room, nurses’ station, buffer room for taking off personal protective equipment (PPE), and fever clinic, and (2) low-risk regions, including the living quarters such as the restrooms, office areas, and buffer room for taking on PPE.
Fig 1.
Room layout of the general isolation ward 1 and the living quarters showing environmental and air sampling sites. Numbered labels correspond to environmental sampling sites. ① beepers; ② bed rails; ③ desktops;④ bedside tables;⑤ oxygen cylinder valve;⑥ medical equipment such as ventilator, monitors, and X-ray devices, etc;⑦ door handles;⑧ elevator buttons;⑨ keyboards;⑩ computer mouses;⑪ telephones; ⑫ water machine buttons; A refers to air samples. The medical area with moderate and high risk contains patient's room, nurses station, buffer room for taking off PPE, and elevator; the living quarters with low risk contains the rest rooms, office area, and buffer room for taking on PPE.
Sample collection
Flocked swabs, premoistened with viral transport medium, were collected from environmental surfaces that were frequently touched by patients or healthcare workers. The surfaces included beeper, keyboard, computer mouse, telephone, door handle, desktop, medical equipment, bedrail, bedside table, oxygen cylinder valve, elevator button, and others such as refrigerator, IV port, and sample transfer box. Air samples from medical areas were collected through natural precipitation according to the Hygienic Standard for Disinfection in Hospitals.9 All samples were collected under emergency conditions around 8:00 AM before routine cleaning and disinfection and were delivered to the clinical laboratory immediately after collection.
Reverse transcription PCR and sequencing
The suspension was used for real-time reverse transcription polymerase chain reaction (RT-PCR) assay of SARS-CoV-2 RNA. The real-time RT-PCR assay was performed using a SARS-CoV-2 nucleic acid detection kit according to the manufacturer's protocol (Shanghai ZJ Bio-Tech Co., Ltd.). Two different targets on the SARS-CoV-2 genome, namely, the RNA-dependent RNA polymerase (RdRp) and nucleocapsid (N) genes, were employed, and a third target, the envelope (E) gene, was used for real-time quantitative PCR.10 A sample was considered positive when the qPCR Ct value was ≤43.
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 software (SPSS Inc.). The differences in the positive rates between the medical areas and the living quarters and the general isolation ward and ICU were compared by the χ2 test; the Fisher exact test was used when data were limited. A 2-sided α of less than 0.05 was considered to indicate statistical significance.
Results
Air samples
Of the 44 air samples collected in medical areas, none of all was positive for SARS-CoV-2 as assessed by RT- PCR (Table 1 ).
Table 1.
Positive rate of air samples from different departments in medical areas
| Areas | NO.of tests | NO.of Positive | Positive rate (%) |
|---|---|---|---|
| General isolation wards | 13 | 0 | 0.00 |
| Ward 1 | 3 | 0 | 0.00 |
| Ward 2 | 3 | 0 | 0.00 |
| Ward 3 | 1 | 0 | 0.00 |
| Ward 4 | 2 | 0 | 0.00 |
| Ward 5 | 3 | 0 | 0.00 |
| Ward 6 | 1 | 0 | 0.00 |
| Intensive care units | 13 | 0 | 0.00 |
| Fever clinic | 18 | 0 | 0.00 |
| Emergency room | 3 | 0 | 0.00 |
| Observation room | 1 | 0 | 0.00 |
| Treatment room | 1 | 0 | 0.00 |
| Infusion room | 1 | 0 | 0.00 |
| Diagnosis room 1 | 2 | 0 | 0.00 |
| Diagnosis room 2 | 2 | 0 | 0.00 |
| Throat swab sampling room | 2 | 0 | 0.00 |
| Public area | 6 | 0 | 0.00 |
| Total | 44 | 0 | 0.00 |
Environmental surface samples
The positive rates of samples from environmental surfaces in different areas are shown in Table 2 . Of the 200 swab samples taken from environmental surfaces, 38 were positive for SARS-CoV-2 RNA. Thirty-six (24.83%) of the 145 samples collected in medical areas and 2 (3.64%) of the 55 collected in living quarters were positive for the virus. The positive rate in medical areas was significantly higher than that in living quarters (P = .001). The positive rates were 25.00% and 37.50% for the general isolation ward and ICU, respectively, and no significant difference was observed between these regions (P = .238). Two positive samples were detected in the office area, one was detected on the surface of a keyboard, and another was on the surface of a telephone.
Table 2.
Positive rate of samples from environmental surface in different areas
| Areas | No. of tests | No. of positive | Positive rate (%) |
|---|---|---|---|
| Medical areas | 145 | 36 | 24.83 |
| General isolation ward | 72 | 18 | 25.00 |
| Ward 1 | 12 | 6 | 50.00 |
| Ward 2 | 12 | 0 | 0.00 |
| Ward 3 | 12 | 4 | 33.33 |
| Ward 4 | 12 | 3 | 25.00 |
| Ward 5 | 12 | 1 | 8.33 |
| Ward 6 | 12 | 4 | 33.33 |
| Intensive care units | 24 | 9 | 37.50 |
| Clinical laboratory | 7 | 0 | 0.00 |
| Fever clinic | 42 | 9 | 21.43 |
| Emergency room | 12 | 6 | 50.00 |
| Observation room | 4 | 1 | 25.00 |
| Treatment room | 4 | 0 | 0.00 |
| Infusion room | 4 | 0 | 0.00 |
| Diagnosis room 1 | 4 | 1 | 25.00 |
| Diagnosis room 2 | 4 | 0 | 0.00 |
| Throat swab sampling room | 8 | 0 | 0.00 |
| Public area | 2 | 1 | 50.00 |
| Living quarters | 55 | 2 | 3.64 |
| Office area | 22 | 2 | 9.09 |
| Rest room | 33 | 0 | 0.00 |
| Total | 200 | 38 | 19.00 |
The positive rates of samples from environmental surfaces of specific sites in medical areas are presented in Table 3 . In medical areas, the PCR-positive samples were obtained from the surface of keyboards, computer mice, beepers, bedside tables, bedrails, and medical equipment including ventilators and monitors. In living quarters, the PCR-positive samples were obtained from the surface of telephones and desktop. Samples taken from the surface of beepers (50.00%), water machine buttons (50.00%), elevator buttons (42.86%), computer mouses (40.00%), telephones (40.00%), and keyboards (33.33%) were positive for SARS-CoV-2 RNA.
Table 3.
Positive rate of samples from environmental surface of specific sites in medical areas
| Sampling site | No. of tests | No. of positivity | Positive rate (%) |
|---|---|---|---|
| Beepers | 6 | 3 | 50.00 |
| Water machine buttons | 8 | 4 | 50.00 |
| Elevator buttons | 7 | 3 | 42.86 |
| Computer mouses | 10 | 4 | 40.00 |
| Telephones | 10 | 4 | 40.00 |
| Keyboards | 15 | 5 | 33.33 |
| Medical equipment | 13 | 4 | 30.77 |
| Oxygen cylinder valve | 8 | 2 | 25.00 |
| Desktops | 18 | 3 | 16.67 |
| Bedrails | 7 | 1 | 14.29 |
| Bedside tables | 7 | 1 | 14.29 |
| Gloves | 7 | 1 | 14.29 |
| Door handles | 15 | 0 | 0.00 |
| Others | 14 | 1 | 7.14 |
| Total | 145 | 36 | 24.83 |
Discussion
Aerosol transmission of the virus could lead to the spread of an epidemic infectious disease. However, previous studies suggested that SARS-CoV-2 is transmitted within family and hospital-associated populations,3 , 11 , 12 indicating that this virus spreads mainly through close contact and infected respiratory droplets rather than through aerosols. In our study, the RNA of SARS-CoV-2 was not detected in any of the 44 air samples, indicating that the possibility of SARS-CoV-2 transmission by aerosols is yet to be confirmed. An air sampler that could force larger volumes of air should be required to detect low concentrations of the virus in the clinical environment in further studies.13 Conversely, in the designated hospitals for COVID-19, strict measures for air purification had been taken. The most important measure was to open windows to promote ventilation. If mobility could increase air exchange and reduce the virus concentration, then the probability of infection is greatly reduced.14 , 15 Previous studies have shown that ultraviolet light could kill the coronavirus effectively.16 For rooms with poor ventilation in the designated hospital, an ultraviolet air disinfection machine with 24 hours ultraviolet disinfection filtration was devoted to sterilize the air.
The positive rate of SARS-CoV-2 detection in the 200 surface samples was 19.00%. The positive rate in medical areas was higher than that in the living quarters, demonstrating the efficacy of the nosocomial infection prevention and control strategies. The wards were divided into different areas according to the risk level of infection, separating patients and healthcare workers using a physical barrier. Targeted measures for nosocomial infection prevention and control were carried out.
Individuals in the designated hospital were required to adhere to the regulations imposed in different areas, and crossing of those different areas was strictly prohibited. The results showed that application of physical barriers combined with behavioral management can effectively prevent the spread of SARS-CoV-2 in the designated hospital. Two positive samples taken from the living quarters indicated the importance of more careful and thorough environmental cleaning and disinfection in this area. The positive rates in areas such as general isolation ward 1, general isolation ward 3, general isolation ward 6, ICU, emergency room, and fever clinic in public area were, on average, higher. In our study, SARS-CoV-2 RNA was detected from the environmental surfaces frequently touched by patients and healthcare workers. The top 5 sites with a positive infection rate were beepers, water machine buttons, elevator buttons, computer mouses, telephones, and keyboards. Special emphasis should be placed on the cleaning and disinfection of the crucial parts of key departments, and intensive disinfection should be instituted and done by professional nurses.
Water machine buttons and elevator buttons that are frequently used by healthcare workers or patients are some of the neglected sites in the hospital, often not cleaned or disinfected. These sites can potentially become contaminated with the virus. Use of keyboard protection films was recommended for easier cleaning and disinfection.17 The positive rate in medical equipment surfaces such as ventilators, monitors, and X-ray devices was 30.77%, suggesting that the equipment surface can be contaminated with SARS-CoV-2 through infected respiratory secretions of infected patients; these contaminated surfaces caused the spread of the virus to healthcare workers during patient care. Fixed use of equipment for each patient was recommended to prevent cross contamination. Furthermore, all reusable medical equipment should be disinfected thoroughly.
One of 7 gloves was positive for SARS-CoV-2 RNA. This suggests that healthcare workers can come into contact with the contaminated surfaces mentioned above, and when hand hygiene or glove removal precautions fail, the virus and other microorganisms would spread to other surfaces and/or patients and/or healthcare workers, leading to hospital-associated infections. Hand hygiene is one of the most important measures to prevent the transmission of viruses like SARS-CoV-2 and has been recommended in numerous guidelines. Gloves are not a substitute for hand hygiene.18 , 19 Hand hygiene rules should be strictly observed.
Based on the results of our study, it can be concluded that the environment around patients with COVID-19 is widely contaminated. To protect healthcare workers, more thorough infection prevention and control guidelines are needed, as well as delivering methods to prevent contact transmission of COVID-19. Current guidelines suggest gloves, gowns, respirators, and eye protectors as PPE during COVID-19 patient care. There is also need for more careful and comprehensive procedures for putting on and removing PPE.20 In addition, the basic environmental hygiene and disinfection measures should be put in place.21 The environmental cleaning and disinfection regimes during the COVID-19 epidemic were similar in all areas: surface cleaning and disinfection using chlorine-based disinfectants were conducted twice per day. There were some limitations in the process of environmental disinfection in the designated hospital. First, environmental surface disinfection was implemented by spraying a chlorine-containing disinfectant. However, spraying cannot cover the surface evenly, such as the corners, and the effect of disinfection cannot be guaranteed without the wiping process. Moreover, spraying disinfectants may lead to harms to patients and health care workers, thus spraying disinfection should not be recommended. Second, the cleaning work in the designated hospital was mostly done by volunteer cleaners during this special period. They received the necessary training without professional experience. Third, all samples were collected under emergency conditions around 8:00 AM before routine cleaning and disinfection. The result may indicate that the usual frequency of disinfection did not meet the demand.
The data obtained in our study provided evidence of environmental contamination by SARS-CoV-2 and demonstrated the effectiveness of disinfection. Significant environmental contamination suggests that the environment is a potential medium of transmission.22 We proposed the following suggestions: (1) environmental surface disinfection should include wiping in an “S”-shaped motion and not repeating the area that has already been cleaned, according to regulations for hospital-associated infection control in the ward of healthcare facilities (WS/T510-2016); (2) the frequency of disinfection should be increased appropriately, at least 3 times per day: twice during the day and once at night (disinfection should be conducted at any time in case of obvious contamination); and (3) cleaners should be trained repeatedly to ensure that they are qualified for their job.
In conclusion, our study demonstrated that environmental surfaces in designated hospitals for patients with COVID-19 were widely contaminated by SARS-CoV-2, suggesting that the environment is a potential medium of transmission. Strict environmental surface hygiene practices should be implemented to prevent healthcare workers from coming into contact with contaminated environmental surfaces, and hand hygiene should be promoted to prevent the spread of virus.
Footnotes
Conflicts of interest: None to report.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [published correction appears in Euro Surveill. 2020 Feb;25(7):] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling Y., Xu S.B., Lin Y.X., et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H., Kang Z.J., Gong H.Y., et al. The digestive system is a potential route of 2019 nCoV infection: a bioinformatics analysis based on single-cell transcriptomes. Preprint. Posted online January 31, 2020. bioRxiv 927806. Available at: https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1. Accessed May 28, 2020.
- 7.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S.H., LIU Y.X., Mi Y.Q., et al. Epidemiological characteristics of nosocomial infection outbreaks in China in recent 13 years. Chin J Nosocomiol. 2018;28:2786–2788. +2792. [Google Scholar]
- 9.GB15982-2012. Hygienic Standard for Disinfection in Hospitals[S] Beijing, China, 2012. Available at:https://www.chinesestandard.net/PDF/English.aspx/GB15982-2012. Accessed May 28, 2020.
- 10.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu Y.Y., Wang S.Q., Wang X.L., et al. Epidemiological analysis on a family cluster of COVID-19. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:506–509. doi: 10.3760/cma.j.cn112338-20200221-00147. [DOI] [PubMed] [Google Scholar]
- 12.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso C., Raynor P.C., Goyal S., et al. Assessment of air sampling methods and size distribution of virus-laden aerosols in outbreaks in swine and poultry farms. J Vet Diagn Invest. 2017;29:298–304. doi: 10.1177/1040638717700221. [DOI] [PubMed] [Google Scholar]
- 14.Zuraimi M.S., Tham K.W., Chew F.T., Ooi P.L. The effect of ventilation strategies of child care centers on indoor air quality and respiratory health of children in Singapore. Indoor Air. 2007;17:317–327. doi: 10.1111/j.1600-0668.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Heating Refrigerating and Air-Conditioning Engineers . Handbook Heating, Ventilating, and Air-Conditioning Applications. ASHRAE; Atlanta, GA: 2013. Infection control; pp. 19–34. [Google Scholar]
- 16.Bedell K., Buchaklian A.H., Perlman S. Efficacy of an automated multiple emitter whole-room Ultraviolet-C disinfection system against coronaviruses MHV and MERS-CoV. Infect Control Hosp Epidemiol. 2016;37:598–599. doi: 10.1017/ice.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X.D., Hu B.J., Bao R., et al. Effect of keyboard covers on reduction of microbial contamination. Chin J Nosocomiol. 2014;24:2584–2585. +2588. [Google Scholar]
- 18.Storr J., Twyman A., Zingg W., et al. Core components for effective infection prevention and control programmes: new WHO evidence-based recommendations. Antimicrob Resist Infect Control. 2017;6:6. doi: 10.1186/s13756-016-0149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuruno N., Kasahara K., Mikasa K. Hand hygiene compliance in a universal gloving setting. Am J Infect Control. 2017;45:830–834. doi: 10.1016/j.ajic.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 20.General Office of National Health Commission. Prevention and control protocol for Novel Coronavirus Pneumonia (version 4) [EB/OL]. (2020-02-07)[2020-03-03]. Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202002/573340613ab243b3a7f61df260551dd4.shtml. Accessed May 28, 2020.
- 21.Krein S.L., Mayer J., Harrod M., et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: a qualitative study. JAMA Intern Med. 2018;178:1016–1057. doi: 10.1001/jamainternmed.2018.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]