Abstract
Objectives
This study evaluated cardiac involvement in patients recovered from coronavirus disease-2019 (COVID-19) using cardiac magnetic resonance (CMR).
Background
Myocardial injury caused by COVID-19 was previously reported in hospitalized patients. It is unknown if there is sustained cardiac involvement after patients’ recovery from COVID-19.
Methods
Twenty-six patients recovered from COVID-19 who reported cardiac symptoms and underwent CMR examinations were retrospectively included. CMR protocols consisted of conventional sequences (cine, T2-weighted imaging, and late gadolinium enhancement [LGE]) and quantitative mapping sequences (T1, T2, and extracellular volume [ECV] mapping). Edema ratio and LGE were assessed in post–COVID-19 patients. Cardiac function, native T1/T2, and ECV were quantitatively evaluated and compared with controls.
Results
Fifteen patients (58%) had abnormal CMR findings on conventional CMR sequences: myocardial edema was found in 14 (54%) patients and LGE was found in 8 (31%) patients. Decreased right ventricle functional parameters including ejection fraction, cardiac index, and stroke volume/body surface area were found in patients with positive conventional CMR findings. Using quantitative mapping, global native T1, T2, and ECV were all found to be significantly elevated in patients with positive conventional CMR findings, compared with patients without positive findings and controls (median [interquartile range]: native T1 1,271 ms [1,243 to 1,298 ms] vs. 1,237 ms [1,216 to 1,262 ms] vs. 1,224 ms [1,217 to 1,245 ms]; mean ± SD: T2 42.7 ± 3.1 ms vs. 38.1 ms ± 2.4 vs. 39.1 ms ± 3.1; median [interquartile range]: 28.2% [24.8% to 36.2%] vs. 24.8% [23.1% to 25.4%] vs. 23.7% [22.2% to 25.2%]; p = 0.002; p < 0.001, and p = 0.002, respectively).
Conclusions
Cardiac involvement was found in a proportion of patients recovered from COVID-19. CMR manifestation included myocardial edema, fibrosis, and impaired right ventricle function. Attention should be paid to the possible myocardial involvement in patients recovered from COVID-19 with cardiac symptoms.
Key Words: cardiac involvement, cardiac magnetic resonance imaging, coronavirus disease-2019
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; AHA, American Heart Association; BSA, body surface area; CI, cardiac index; CO, cardiac output; CMR, cardiac magnetic resonance; COVID-19, coronavirus disease-2019; ECV, extracellular volume; EDV, end-diastolic volume; EF, ejection fraction; ER, edema ratio; ESV, end-systolic volume; FA, flip angle; FOV, field of view; hs-cTnI, high-sensitive cardiac troponin I; IQR, interquartile range; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; PSIR, phase-sensitive inversion-recovery; RT-PCR, reverse transcription and polymerase chain reaction; RV, right ventricle; RVEF, right ventricular ejection fraction; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; SI, signal intensity; STIR, short tau inversion recovery; SSFP, steady state free precession; SV, stroke volume; T2WI, T2-weighted imaging; TE, echo time; TR, repetition time
Central Illustration
Coronavirus disease-2019 (COVID-19) has been a global outbreak since March 2020 (1). To date, more than 2,725,000 patients have been confirmed with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection in more than 200 countries. The lung is the major organ involved in COVID-19, and angiotensin-converting enzyme 2 (ACE2) is the path for SARS-CoV-2 to attack pulmonary tissue (2). ACE2 is located not only in the lungs, but also in other organs, including the cardiovascular system (3). Previous studies (4,5) found that 12% to 15% of patients with COVID-19 had elevated high-sensitive cardiac troponin I (hs-cTnI) during hospital period, which indicated myocardial injury, and that cardiac involvement in severe-type patients was up to 31%. However, it is unknown if there is sustained cardiac involvement in patients after their recovery from COVID-19, especially those with moderate-type.
Cardiac involvement in myocarditis, including myocardial fibrosis, edema, and pericarditis (6), is associated with adverse events and poor prognosis; it is important to identify such involvement at an early stage for appropriate treatment. Cardiac magnetic resonance (CMR) is the current gold standard to evaluate cardiac morphology and function (7), and the recent CMR mapping techniques, including T1, T2, and extracellular volume (ECV), are unique tools to quantitatively assess myocardial diffuse fibrosis and edema (8,9). Although hs-cTnI is highly specific for myocardial injury, CMR has reported higher sensitivity for detecting occult cardiac involvement (10,11). The purpose of our study was to evaluate cardiac involvement in patients recovered from COVID-19 who reported cardiac symptoms, using cardiac CMR as a sensitive imaging tool.
Methods
Study design and participants
This single-center, retrospective, observational study was performed at Tongji Hospital, Tongji Medical College, Wuhan, China. Consecutive patients since March 2020 who were initially referred for cardiac CMR examination due to cardiac symptoms and who met the following inclusion criteria were retrospectively included: 1) patients were previously confirmed with SARS-CoV-2 infection using reverse transcription-polymerase chain reaction (RT-PCR) swab test (12); 2) patients were considered recovered by the discharging criteria (normal temperature lasting longer than 3 days, resolved respiratory symptoms, and substantially improved exudative lesions on chest CT images, and 2 consecutive negative RT-PCR test results separated by at least 24 h) and were isolated for 14 days (13); and 3) patients reported cardiac symptoms after being discharged, including chest pain, palpitation, and chest distress. Exclusion criteria were as follows: 1) a history of coronary artery disease or myocarditis; 2) contradictions to gadolinium contrast; and 3) CMR image quality that was not sufficient for analysis.
Healthy controls of similar age and gender distributions who previously underwent the same CMR examinations in our hospital were also included. The controls were selected from a database of healthy subjects without cardiovascular disease or systemic inflammation. This study was approved by the institutional review board of Tongji Hospital, Tongji Medical College (TJ-IRB20200417). The requirement for informed patient consent was waived by the ethics committee for this retrospective study.
CMR scanning protocol
All patients underwent CMR examination on a 3-T MR scanner (Skyra, Siemens, Healthineers, Erlangen, Germany). CMR scanning protocol included the following: 1) conventional sequences: short-axis and long-axis cine, T2-weighted imaging (T2WI), and late gadolinium enhancement (LGE); and 2) quantitative mapping sequences: native T1/T2 mapping and post-contrast T1 mapping. The stack of short-axis slices covered the left ventricle (LV) from apex to mitral annulus. Steady state free precession (SSFP) was used for cardiac cine imaging with the following parameters: echo time (TE) = 1.4 ms, repetition time (TR) = 37.7 ms, field of view (FOV) = 360 × 360 mm, matrix = 192 × 146, flip angle (FA) = 55°, slice thickness = 8 mm, and slice gap = 2 mm. T2WI, native T1/T2 mapping, LGE, and post-contrast T1 mapping had the same imaging plane as the short-axis cine.
T2WI, black blood T2-weight short tau inversion recovery (STIR) sequence was performed using TR = 2RR intervals, TE = 41 ms, slice thickness = 8 mm, and FOV = 360 mm × 360 mm. Native and post-contrast T1 mapping was acquired using an electrocardiograph-gated single-shot modified Look-Locker inversion recovery sequence with protocol 5(3)3 and 4(1)3(1)2, respectively. The acquisition parameters were TE = 1.2 ms, TR = 3.8 ms, FOV = 320 × 360 mm, matrix = 192 × 144, FA = 35°, and slice thickness = 8 mm. The T2 mapping technique involved a T2 preparation module to produce single-shot T2 prepared SSFP images (T2p-SSFP), with different T2 preparation times (0 ms, 24 ms, and 55 ms), TR = 208 ms, FA = 12°, and matrix = 206 × 256. LGE imaging was performed 10 to 15 min after intravenous administration of gadobenate dimeglumine (0.2 ml/kg of Multihance, Bracco Diagnostics, Shanghai, China) using a phase-sensitive inversion-recovery (PSIR) sequence (TR = 5.2 ms, TE = 1.2 ms, matrix = 224 × 156, and FA = 55°). Hematocrit level was measured within 3 days of CMR scanning for ECV calculation. All patients underwent laboratory hs-cTnI testing before cardiac CMR examination.
CMR images analysis
Two radiologists (LH with 10 years of CMR diagnosis experience and PZ with 4 years of CMR diagnosis experience) evaluated all CMR images using commercial software cvi 42, v.5.3 (Circle Cardiovascular Imaging, Calgary, Canada). Myocardial edema was evaluated on T2WI images (14) and divided into 16 American Heart Association (AHA) segments. Myocardial edema ratio (ER) was defined as the ratio between myocardial signal intensity (SI) to skeletal muscle SI (7). An ER >2.0 was considered as abnormal (15). The location (16 segments of AHA) and pattern (epicardial, mid-wall, or transmural) of LGE lesions on the LGE images were assessed by 2 observers who reviewed all PSIR images independently. A senior observer (LX, with 20 years of experience in CMR) adjudicated any discrepancies between the 2 observers. For each patient, the endo- and epicardial contours of LGE images were manually delineated, and LGE lesion was defined as SI >5 SDs above the mean SI of the remote reference myocardium (16). Ratios between the LGE volume and the total LV myocardium volume (LGE/myocardium) in the LGE-positive patients were calculated. Patients were further divided into 2 subgroups based on the presence or absence of positive conventional cardiac CMR findings, which were defined as increased myocardial edema ratio (>2.0) and/or LGE presence.
Global T1/T2 values were computed by manually delineating the whole LV myocardium region (including regions of LGE lesion) on the T1/T2 map. To assess the remote myocardium, T1/T2 values were also measured in the AHA myocardium segments free of apparent LGE lesion. Native T1 and post-contrast T1 of myocardium and blood pool were used to derive ECV as the described equation in a previous study (15).
LV and right ventricle (RV) function parameters were automatically calculated from endocardial and epicardial contours. Functional parameters included LV/RV end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), cardiac output (CO), LV mass, and ejection fraction (EF). All volumes and mass were normalized by body surface area (BSA).
Statistical analysis
All statistical analysis was performed using SPSS version 23.0 (IBM statistics, Armonk, New York) and GraphPad Prism version 8.1 (GraphPad Software Inc., La Jolla, California). Categorical variables were expressed as counts (percentage), and continuous variable as mean ± SD or median (interquartile range [IQR]). Normality of distribution was tested using Shapiro-Wilk test. Comparison between 2 groups were performed using unpaired Student’s t-test (for normal distribution) or Mann-Whitney U test (for non-normal distribution) with continuous variables, or chi-square test with categorical variable. Comparisons among 3 groups were performed using ordinary 1-way analyses of variance with Bonferroni corrected post hoc comparisons (for normal distribution) or Kruskal-Wallis tests with post hoc pairwise comparisons (for non-normal distribution), as appropriate. A value of p < 0.05 was considered statistically significant.
Results
Patient characteristics
Clinical characteristics and laboratory results of patients with COVID-19 are reported in Table 1 . A total of 26 patients (age 38 years; IQR: 32 to 45 years; 10 male) were enrolled in this study based on the inclusion and exclusion criteria. Twenty healthy controls of similar age and gender distributions (age 40 years; IQR: 29 to 50 years; 7 male) who previously underwent the same CMR examinations in our hospital were also included. All patients reported contact with patients who were diagnosed with COVID-19 pneumonia. Twenty-two patients of 26 (85%) were diagnosed as having moderate-type COVID-19 pneumonia and 4 (15%) as having severe-type, according to the Diagnosis and Treatment Protocol of Novel Coronavirus issued by the National Health Commission of the People’s Republic of China (13). The median age of the patients was 38 years (IQR: 32 to 45 years; range 25 to 60 years), and 10 (38%) were men. Two (8%) patients had a history of hypertension before COVID-19. During hospitalization due to COVID-19, all patients were administered antiviral and antibiotic therapy, and oxygen support was given to 21 (81%) patients. Antiviral drugs included Kaletra and Arbidol, and antibiotics included moxifloxacin and cefoperazone sulbactam. The median duration from onset of cardiac symptoms to CMR examination was 47 days (IQR: 36 to 58 days). Precordial chest pain, palpitation, and chest distress were reported in 3 (12%), 23 (88%), and 6 (23%) patients, respectively. At admission, 13 of 26 patients had hs-cTnI measurement during COVID-19 hospitalization, with median (IQR) peak value of 2.2 (IQR: 1.9 to 2.6) pg/ml. The hs-cTnI was in the normal range for all recovered patients at the time of CMR (2.0 [IQR: 1.9 to 2.2] pg/ml).
Table 1.
Clinical Characteristics and Laboratory Measurements of Patients Recovered From COVID-19
| Total (N = 26) | Conventional CMR Findings |
p Value∗ | ||
|---|---|---|---|---|
| Positive (n = 15) | Negative (n = 11) | |||
| Age (yrs) | 38 (32–45) | 39 (29–49) | 37 (34–39) | 0.61 |
| Male | 10 (38) | 4 (27) | 6 (55) | 0.23 |
| BMI (kg/m2) | 23.2 ± 3.6 | 22.3 ± 3.8 | 24.5 ± 3.1 | 0.12 |
| BSA (m2) | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.2 | 0.19 |
| HR (beats/min) | 77 ± 10 | 74 ± 8 | 81 ± 10 | 0.06 |
| Systolic pressure (mm Hg) | 121 ± 10 | 118 ± 10 | 124 ± 10 | 0.20 |
| Diastolic pressure (mm Hg) | 76 ± 8 | 77 ± 9 | 76 ± 7 | 0.82 |
| COVID-19 confirmed patient exposure | 26 (100) | 15 (100) | 11 (100) | NA |
| Duration between cardiac symptoms onset to CMR examination (days) | 47 (36–58) | 48 (35–56) | 50 (40–60) | 0.62 |
| Clinical types, moderate/severe/critical | 22/4/0 | 12/3/0 | 10/1/0 | 0.61 |
| Comorbidities | ||||
| Hypertension | 2 (8) | 1 (7) | 1 (9) | >0.99 |
| Diabetes mellites | 0 | 0 | 0 | NA |
| Coronary artery disease | 0 | 0 | 0 | NA |
| Chronic obstructive pulmonary diseases | 0 | 0 | 0 | NA |
| Cerebrovascular disease | 0 | 0 | 0 | NA |
| Chronic renal diseases | 0 | 0 | 0 | NA |
| Chronic liver diseases | 0 | 0 | 0 | NA |
| Cardiac symptoms | ||||
| Precordial chest pain | 3 (12) | 2 (13) | 1 (9) | >0.99 |
| Palpitation | 23 (88) | 12 (80) | 11 (100) | 0.24 |
| Chest distress | 6 (23) | 4 (27) | 2 (18) | 0.67 |
| Laboratory findings | ||||
| White blood cell count (×109/l) | 5.4 (4.5–6.8) | 4.7 (4.2–5.7) | 6.6 (5.8–6.9) | 0.06 |
| Lymphocyte count (×109/l) | 1.6 (1.3–1.9) | 1.5 (1.3–1.8) | 1.9 (1.6–2.0) | 0.38 |
| Hs-CRP (mg/l) | 1.4 (0.4–5.1) | 1.0 (0.4–7.5) | 2.3 (0.5–4.1) | 0.80 |
| DD (ug/ml FEU) | 0.28 (0.22–0.41) | 0.32 (0.24–0.44) | 0.22 (0.20–0.29) | 0.11 |
| IL6 (pg/ml) | 3.7 (2.2–14.4) | 4.2 (2.3–12.9) | 3.2 (2.2–11.6) | 0.80 |
| LDH (U/l) | 180 (158–193) | 185 (159–194) | 168 (154–192) | 0.43 |
| Hs-cTnI (pg/ml) | 2.0 (1.9–2.2) | 2.0 (1.9–2.1) | 2.0 (1.9–2.3) | 0.77 |
| NT-proBNP (pg/ml) | 28 (11–36) | 33 (20–57) | 14 (11–18) | 0.16 |
| Treatment before discharge | ||||
| Antiviral therapy | 26 (100) | 15 (100) | 11 (100) | NA |
| Antibiotic therapy | 26 (100) | 15 (100) | 11 (100) | NA |
| Use of corticosteroid | 13 (50) | 7 (47) | 6 (55) | >0.99 |
| Nasal cannula oxygen | 18 (69) | 11(73) | 7 (63) | 0.68 |
| Noninvasive ventilation or high-flow nasal cannula oxygen | 3 (12) | 2 (13) | 1 (9) | 0.62 |
Values are median (25th-75th percentiles), n (%), or mean ± SD.
BMI = body mass index; BSA = body surface area; CMR = cardiac magnetic resonance; COVID-19 = coronavirus disease-2019; DD = D-dimer; FEU = fibrinogen equivalent units; HR = heart rate; Hs-CRP = high-sensitivity C-reactive protein; IL6 = inerfertin-6; LDH = lactate dehydrogenase; Hs-cTnI = high-sensitivity cardiac troponin I; IQR = interquartile range; NA = not applicable; NT-proBNP = amino-terminal pro-brain natriuretic peptide.
The p value is for patients with positive conventional CMR findings vs. patients without positive conventional CMR findings.
Myocardial histological abnormalities using conventional T2WI and LGE sequences
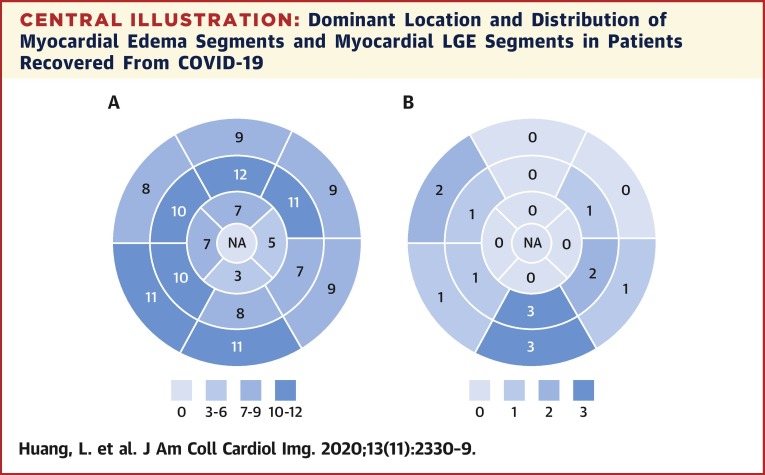
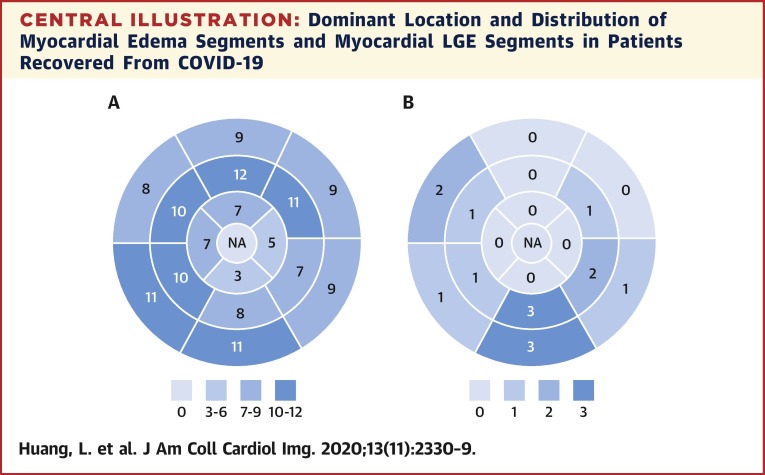
A total of 416 myocardial segments of 26 patients were analyzed. Fifteen patients of 26 (58%) were observed with increased T2 signal and/or positive LGE. Myocardial edema was found in 14 (14 of 26 [54%]) patients, involving 33% (137 of 416) of LV segments (Central Illustration, A ). Among them, 7 (7 of 14 [50%]) and 7 (7 of 14 [50%]) patients were observed with positive LGE and a small pericardial effusion, respectively. One patient (1 of 15 [4%]) was observed with positive LGE but without obvious myocardial edema. A total of 8 cases (8 of 26 [31%]) showed focal linear subepicardial and patchy mid-wall LGE, involving 15 (15 of 416 [4%]) myocardial segments (Central Illustration, B). The median of LGE/myocardium ratio was 7.2% (IQR: 6.2% to 8.4%; range 5.3% to 14.5%). Most LGE (9 of 15 [60%]) lesions were located at inferior and inferior-lateral segments at base and mid-chamber (Figure 1 ). Eleven patients (11 of 26 [42%]) had no positive cardiac CMR findings on the conventional T2WI and LGE sequences. Among 13 patients with admission cTnI data, 8 (62%) patients had no positive conventional CMR findings, 3 patients had myocardial edema without apparent LGE, 1 patient had LGE without myocardial edema, and 1 had both LGE and myocardial edema.
Central Illustration.
Dominant Location and Distribution of Myocardial Edema Segments and Myocardial LGE Segments in Patients Recovered From COVID-19
(A) Number of myocardial edemas distributed in the AHA 16 segments’ model in all 15 patients with positive conventional CMR findings. (B) Number of myocardial LGEs distributed in the AHA 16 segments’ model in all 15 patients with positive conventional CMR findings. AHA = American Heart Association; COVID-19 = coronavirus disease-2019; CMR = cardiac magnetic resonance; LGE = late gadolinium enhancement; NA = not applicable.
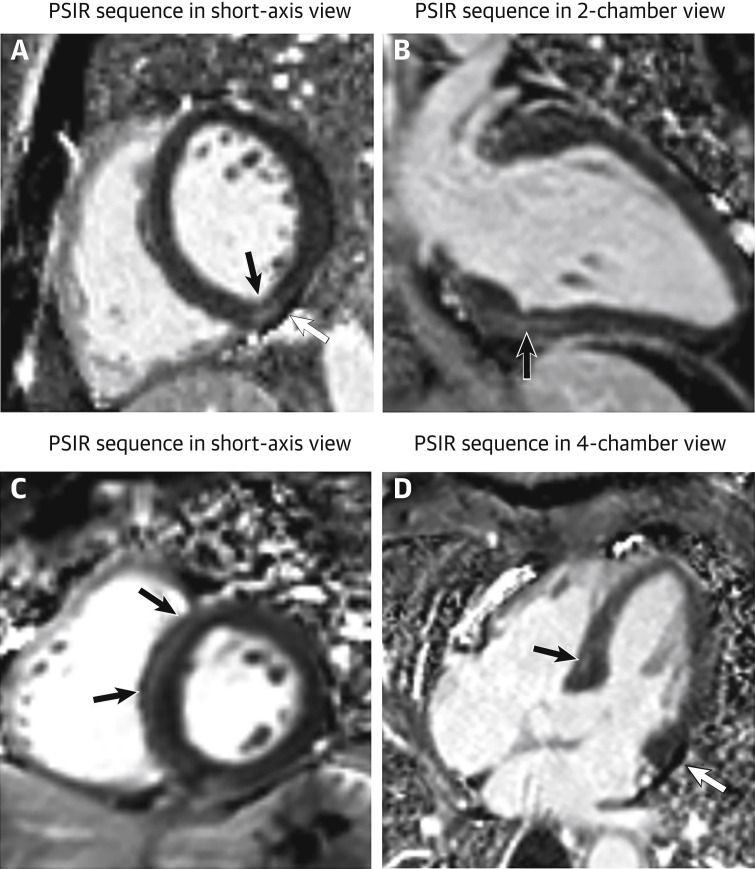
Figure 1.
Focal Myocardial Fibrosis in Patients Recovered From COVID-19
A 29-year-old male patient (first row) underwent cardiac CMR 1 month after the onset of palpitations. A 60-year-old male patient (second row) underwent cardiac CMR 2 months after the onset of palpitations. PSIR sequences in short-axis view (A, C) showed focal LGE (black arrows) in inferior and septal segments of left ventricle, respectively. Results were confirmed on the PSIR sequences in 2-chamber view (C) and 4-chamber view (D). Images A and D demonstrated a small pericardial effusion (white arrow) in both patients. COVID-19 = coronavirus disease-2019; CMR = cardiac magnetic resonance; LGE = late gadolinium enhancement; PSIR = phase-sensitive inversion recovery.
Elevated T1/T2/ECV values on quantitative CMR mapping sequences
Global native T1, T2, and ECV values all showed significantly elevated in recovered COVID-19 patients with positive conventional CMR findings, compared with patients without positive findings and healthy controls (native T1 1,271 ms [IQR: 1,243 to 1,298 ms] vs. 1,237 ms [IQR: 1,216 to 1,262 ms] vs. 1,224 ms [IQR: 1,217 to 1,245 ms]; T2 42.7 ± 3.1 ms vs. 38.1 ± 2.4 ms vs. 39.1 ± 3.1 ms; ECV 28.2% [IQR: 24.8% to 36.2%] vs. 24.8% [IQR: 23.1% to 25.4%] vs. 23.7% [IQR: 22.2% to 25.2%]; p = 0.002; p < 0.001, and p = 0.002, respectively). The T1, T2, and ECV values in the remote myocardium of the 8 LGE-positive patients were elevated compared with healthy controls (native T1 1,259 ms [IQR: 1,248 to 1,296 ms] vs. 1,224 ms [IQR: 1,217 to 1,245 ms]; T2 42.9 ± 3.1 ms vs. 39.1 ± 3.1 ms; ECV 28.7% ± 5.1%; vs. 23.8 ± 1.9%; p = 0.01; p = 0.03, and p = 0.03, respectively).
LV/RV function
LV and RV morphological and functional parameters are summarized in Table 2 . There was no significant difference of LV function among controls and patients with and without positive findings on conventional CMR sequences. Among patients with positive conventional CMR findings, only 1 (1 of 15 [7%]) patient showed impaired left ventricular ejection fraction (LVEF 45%), with obviously reduced contraction in the myocardial segments with edema. However, decreased RV function parameters including right ventricular ejection fraction (RVEF), CO, cardiac index (CI), SV, and SV/BSA were found in patients with positive conventional CMR findings, compared with healthy controls (p < 0.05). There was no significant difference of RV function parameters between patients with no positive CMR findings and healthy controls (p > 0.05).
Table 2.
Left and Right Ventricular Cardiac CMR Parameters of Patients Recovered From COVID-19 and Controls
| Conventional CMR Findings |
Controls (n = 20) | p Value∗ | |||||
|---|---|---|---|---|---|---|---|
| Positive (n = 15) | Negative (n = 11) | Adjusted p Value† | Adjusted p Value‡ | Adjusted p Value§ | |||
| Age (yrs) | 39 (29–49) | 37 (34–39) | 40 (29–50) | 0.83 | 0.99 | 0.69 | 0.78 |
| Male | 4 (27) | 6 (55) | 7 (35) | 0.30 | 0.50 | 0.88 | 0.34 |
| CMR parameters | |||||||
| Left ventricle | |||||||
| EF (%) | 60.7 ± 6.4 | 64.3 ± 5.8 | 63.0 ± 8.9 | 0.30 | 0.65 | 0.86 | 0.40 |
| EF<50% | 1 (7) | 0 (0) | 0 (0) | NA | NA | NA | NA |
| EDV (ml) | 71.6 (61.4–86.4) | 78.2 (64.0–92.1) | 86.1 (70.8–92.8) | 0.59 | 0.30 | 0.91 | 0.31 |
| ESV (ml) | 28.7 ± 8.6 | 28.2 ± 7.9 | 30.3 ± 10.3 | 0.98 | 0.89 | 0.81 | 0.80 |
| SV (ml) | 43.5 ± 8.0 | 49.9 ± 8.7 | 50.2 ± 12.1 | 0.16 | 0.13 | >0.99 | 0.10 |
| CO (l/min) | 3.0 (2.6–3.7) | 3.7 (3.5–4.5) | 3.5 (2.8–4.3) | 0.05 | 0.88 | 0.32 | 0.05 |
| Myo mass (g) | 57.1 ± 12.4 | 69.1 ± 17.2 | 63.9 ± 14.7 | 0.15 | 0.31 | 0.68 | 0.14 |
| EDV/BSA (ml/m2) | 43.9 ± 10.7 | 44.1 ± 6.7 | 47.3 ± 10.1 | >0.99 | >0.99 | 0.93 | 0.49 |
| ESV/BSA (ml/m2) | 17.5 ± 5.6 | 15.9 ± 4.1 | 18.0 ± 6.8 | 0.68 | 0.96 | 0.52 | 0.58 |
| SV/BSA (ml/m2) | 26.4 ± 6.2 | 28.2 ± 4.0 | 29.3 ± 5.5 | 0.64 | 0.34 | 0.81 | 0.29 |
| CI (l/min/m2) | 1.9 ± 0.5 | 2.3 ± 0.4 | 2.0 ± 0.5 | 0.15 | 0.84 | 0.30 | 0.19 |
| Myo mass/BSA (g/m2) | 34.3 ± 7.1 | 38.7 ± 6.6 | 37.4 ± 7.1 | 0.26 | 0.41 | 0.87 | 0.24 |
| Global T1 (ms) | 1,271 (1,243–1,298) | 1,237 (1,216–1,262) | 1,224 (1,217–1,245) | 0.03 | 0.002 | >0.99 | 0.002 |
| Global T2 (ms) | 42.7 ± 3.1 | 38.1 ± 2.4 | 39.1 ± 3.1 | <0.001 | 0.005 | 0.57 | <0.001 |
| Global ECV (%) | 28.2 (24.8–36.2) | 24.8 (23.1–25.4) | 23.7 (22.2–25.2) | 0.12 | 0.001 | 0.84 | 0.002 |
| Right ventricle | |||||||
| EF (%) | 36. 5 ± 6.1 | 41.1 ± 8.6 | 46.1 ± 12.0 | 0.31 | 0.01 | 0.38 | 0.01 |
| EDV (ml) | 73.0 ± 15.1 | 80.6 ± 19.9 | 81.2 ± 18.0 | 0.54 | 0.40 | >0.99 | 0.42 |
| ESV (ml) | 46.6 ± 11.5 | 47.8 ± 15.0 | 43.9 ± 14·8 | 0.97 | 0.82 | 0.76 | 0.72 |
| SV (ml) | 26.4 ± 6.1 | 32.8 ± 8.9 | 36.4 ± 11.3 | 0.13 | 0.01 | 0.61 | 0.01 |
| CO (l/min) | 1.9 ± 0.5 | 2.6 ± 0.8 | 2.6 ± 1.0 | 0.05 | 0.046 | 0.98 | 0.03 |
| EDV/BSA (ml/m2) | 44.1 ± 10.2 | 45.3 ± 8.8 | 47.2 ± 10.3 | 0.93 | 0.63 | 0.85 | 0.62 |
| ESV/BSA (ml/m2) | 28.1 ± 7.8 | 26.9 ± 7.4 | 26.0 ± 9.3 | 0.91 | 0.74 | 0.95 | 0.74 |
| SV/BSA (ml/m2) | 15.9 ± 3.6 | 18.4 ± 4.2 | 21.3 ± 5.7 | 0.26 | 0.01 | 0.28 | 0.01 |
| CI (l/min/m2) | 1.2 ± 0.3 | 1.5 ± 0.4 | 1.5 ± 0.4 | 0.09 | 0.03 | 0.98 | 0.03 |
Values are median (25th-75th percentiles), n (%), or mean ± SD. Bold indicates adjusted p < 0.05.
CI = cardiac index; CO = cardiac output; EF = ejection fraction; EDV = end-diastolic volume; ESV = end-systolic volume; Myo = myocardium; NA = not applicable; SV = stroke volume; other abbreviations as in Table 1.
p value is for patients with positive conventional CMR findings vs. patients with negative conventional CMR findings vs. controls.
Statistical difference between patients with positive and with negative conventional CMR findings.
Statistical difference between patients with positive conventional CMR findings and controls.
Statistical difference between patients with negative conventional CMR findings and controls.
Discussion
In this study, we present an CMR study of 26 patients who had recovered from COVID-19 but reported cardiac symptoms. None of the 26 patients selected for this retrospective analysis had known previous myocarditis or other heart diseases before COVID-19. However, 15 of 26 patients showed myocardial edema and/or foci LGE lesion. The presence of myocardial tissue abnormalities in otherwise healthy subjects suggests cardiac involvement as a lasting consequence of SARS-CoV-2 infection.
Myocardial edema and foci LGE lesion are the major image manifestations on conventional cardiac CMR sequences in our patient cohort. The majority of T2 signal hyperintensity was located in the interventricular septum, anterior, anterior-lateral, and inferior wall at the base and mid-chamber. The location of edema caused by SARS-CoV-2 appeared different from those caused by acute viral myocarditis, which commonly involves inferior and inferior-lateral wall (14,15). However, some recovered patients had subepicardial LGE lesions at inferior and inferior-lateral wall, similar to common types of viral myocarditis (inferior-lateral wall) (17). Pericardial involvement is a complication of myocardial damage, which was also found in a proportion of our cohort. Global T1, T2, and ECV values were significantly elevated in patients with COVID-19 with positive conventional cardiac CMR findings, compared with patients without positive findings and healthy controls (Figure 2 ). Also, elevated T1, T2, and ECV values were observed in the remote myocardium of LGE-positive patients, indicating diffuse involvement. Previous studies showed that elevated T2 suggested myocardial edema (17,18), whereas elevated native T1 and ECV suggested myocardial interstitial fibrosis (19). Therefore, the results suggest the existence of diffuse myocardial edema and fibrosis in patients with positive conventional CMR findings. We note that the range of ECV value in the healthy controls is lower than previously reported by Gottbrecht et al. (20), but close to that by Xu et al. (21) in a Chinese cohort.
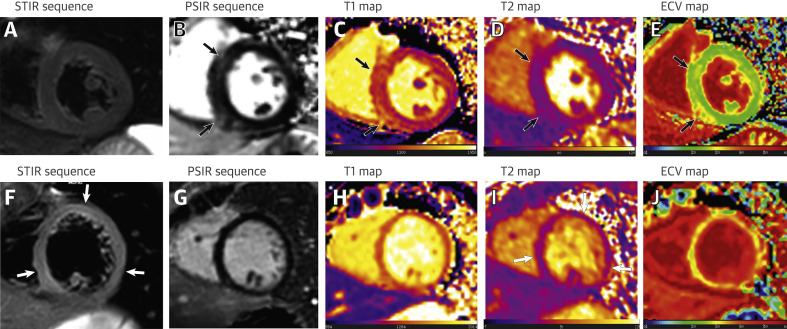
Figure 2.
Cardiac Involvement in Patients Recovered From COVID-19 Identified Using Quantitative Cardiac CMR
A 60-year-old male patient (first row) underwent cardiac CMR 2 months after the onset of palpitations. Short-axis STIR sequence (A) showed no evidence of myocardial edema. However, PSIR image (B) of the same slice showed focal LGE in the LV septal and inferior segments (black arrows). Increased native T1 (1,434 ± 43 ms), ECV (30 ± 2%), and normal T2 values (38 ± 2 ms) were shown in the corresponding location of focal LGE on the T1 (C), T2 (D), and ECV maps (E) (black arrows). A 29-year-old female patient (second row) underwent cardiac CMR 1 and a half months after the onset of palpitations. Short-axis STIR (F) and PSIR sequence (G) showed global myocardial signal hyperintensity but no apparent LGE, global T1, and ECV values were significantly increased on the T1 (H) and ECV maps (J). T2-mapping sequence (I) showed increased T2 values at inferior septal (41 ± 8 ms), anterior (41 ± 6 ms), and inferior lateral segments (43 ± 5 ms), which matched the location with significantly increased signal intensity on short-axis STIR sequence (F) (white arrows). ECV = extracellular volume; LV = left ventricle; STIR = short tau inversion recovery; other abbreviations as in Figure 1.
Eleven of 26 patients recovered from COVID-19 reported cardiac symptoms, but had no positive CMR findings either on conventional cardiac CMR sequences (cine, T2WI, and LGE) or quantitative mapping sequences (T1/T2/ECV mapping). There may be 2 reasons accounting for this phenomenon. First, it is possible that the chest symptoms were caused by the residual pulmonary disease, but further study is needed to confirm this. Second, because the median duration between clinical symptoms onset and CMR scan was as long as 50 days, the patients may have had acute myocarditis but were imaged at the sub-acute stage when edema already resolved. In either case, there is no sustained cardiac involvement in this patient subgroup.
It was previously reported that myocarditis and cardiac arrhythmias may be induced by COVID-19 associated with a high inflammatory burden (22). An autopsy study had reported infiltration of myocardial tissue by mononuclear inflammatory cells in a patient with COVID-19 postmortem (23). Our study also showed myocardial edema as the major image manifestation. Two pathological mechanisms may be involved in post-COVID-19 myocardial involvement (24). First, SARS-CoV-2 can directly cause myocardial inflammation because ACE2 receptor binding domain of spike protein coding S is similar in SARS-CoV-2 as in SARS-CoV, which was found to cause viral myocarditis after infection (25). Second, indirect injury may be caused by an inflammatory storm induced by the immune response (4).
Impaired RV function was found in the subgroup of post–COVID-19 patients who demonstrated cardiac involvement. Because RV mainly acts as a passive conduit in cardiac functioning, it is easily affected by a slight increase in pulmonary vascular resistance (26). Previous studies have reported RV failure in acute lung injury and acute respiratory distress syndrome (26,27). Because the lungs are the main target organ of SARS-CoV-2, RV may be more susceptible to impairment compared with LV.
In our cohort, LVEF was in the normal range for all patients except one. Previous studies suggested that myocardial tissue remodeling may precede functional remodeling in LV (28,29), and our results agree with the finding because abnormalities were identified mostly in myocardial tissue instead of LV function. This also indicates that the patients were in a relatively early stage of cardiac involvement, and they need to be followed up in a longer study. Quantitative cardiac CMR is a sensitive tool for early detection of cardiac involvement, and it can also be used to monitor further progress.
Study limitations
First, the sample size was small, limited by the current capacity of medical resources in the epidemic area. Second, most included patients had moderate COVID-19 previously, therefore, our report cannot reflect the full spectrum covering patients with severe and critical COVID-19. With both limitations, the reported proportion of cardiac involvement is limited to the present study and cannot be extrapolated to a larger population. Nevertheless, this study demonstrates the phenomenon of post–COVID-19 cardiac involvement, and the findings can be useful because cardiac involvement may be more easily overlooked in patients with mild SARS-CoV-2 infection. Last, we only had a 1–time point CMR examination, whereas longitudinal follow-ups will be valuable to confirm if the cardiac involvement will progress or regress.
Conclusions
There may be sustained cardiac involvement in patients recovered from COVID-19, as demonstrated by our cardiac CMR study. Major CMR manifestation included edema, fibrosis, and impaired RV contractile function. The cardiac status of patients with COVID-19 and survivors needs to be closely monitored; cardiac CMR can be a sensitive imaging tool in combination with laboratory tests for identifying cardiac involvement in patients with COVID-19.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: CMR is a sensitive and quantitative imaging tool to study early cardiac involvement. Our results showed that CMR was able to identify fibrosis and edema on the myocardium in a proportion of the patients recovered from COVID-19. Impaired RV function was also observed this patient subgroup.
TRANSLATIONAL OUTLOOK: Attention needs to be paid to the potential cardiac involvement and negative consequences in patients recovered from COVID-19. This is a relatively short-term small-cohort study; longitudinal follow-ups in a larger cohort are needed to confirm the prognosis value of cardiac CMR for patients recovered from COVID-19.
Author Relationship With Industry
This work was supported in part by the National Natural Science Foundation of China (81471637 and 81873889), the National Mega Project on Major Infectious Disease Prevention (2017ZX10103005-007), and the National Key Research and Development Program of China (2018YFE0204500). All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgements
The authors thank all colleagues who contributed to the present study. They are especially grateful to our frontline medical staff for their professionalism, dedication, and courage in the face of the COVID-19 outbreak.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Cardiovascular Imagingauthor instructions page.
References
- 1.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19 - 30 March 2020. World Health Organization. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---30-march-2020 Available at:
- 2.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;3099:1–10. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel V.B., Basu R., Oudit G.Y. ACE2/Ang 1-7 axis: a critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte. 2016;5:306–311. doi: 10.1080/21623945.2015.1131881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;6736:1–10. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1601–1609. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knockaert D.C. Cardiac involvement in systemic inflammatory diseases. Eur Heart J. 2007;28:1797–1804. doi: 10.1093/eurheartj/ehm193. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich M.G., Sechtem U., Schulz-Menger J. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira V.M., Schulz-Menger J., Holmvang G. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 9.Kammerlander A.A., Marzluf B.A., Zotter-Tufaro C. T1 mapping by CMR imaging: from histological validation to clinical implication. J Am Coll Cardiol Img. 2016;9:14–23. doi: 10.1016/j.jcmg.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Amano Y., Aita K., Yamada F., Kitamura M., Kumita S. Distribution and clinical significance of high signal intensity of the myocardium on T2-weighted images in 2 phenotypes of hypertrophic cardiomyopathy. J Comput Assist Tomogr. 2015;39:951–955. doi: 10.1097/RCT.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 11.Ho C.Y., Day S.M., Colan S.D. The burden of early phenotypes and the influence of wall thickness in hypertrophic cardiomyopathy mutation carriers. JAMA Cardiol. 2017;2:419. doi: 10.1001/jamacardio.2016.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao A., Zhenlu Y., Hongyan H. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 Cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health Commission of the People’s Republic of China Diagnosis and Treatment Protocol of Novel Coronavirus (trial version 7th). National Health Commission of the People’s Republic of China Website. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml Available at:
- 14.Luetkens J.A., Doerner J., Thomas D.K. Acute myocarditis: multiparametric cardiac MR imaging. Radiology. 2014;273:383–392. doi: 10.1148/radiol.14132540. [DOI] [PubMed] [Google Scholar]
- 15.Chaikriangkrai K., Abbasi M.A., Sarnari R. Prognostic value of myocardial extracellular volume fraction and T2-mapping in heart transplant patients. J Am Coll Cardiol Img. 2020;13:1521–1530. doi: 10.1016/j.jcmg.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohnen S., Radunski U.K., Lund G.K. Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging. 2015;8:e003073. doi: 10.1161/CIRCIMAGING.114.003073. [DOI] [PubMed] [Google Scholar]
- 17.Li H., Zhu H., Yang Z., Tang D., Huang L., Xia L. Tissue characterization by mapping and strain cardiac MRI to evaluate myocardial inflammation in fulminant myocarditis. J Magn Reson Imaging. 2020;52:930–938. doi: 10.1002/jmri.27094. [DOI] [PubMed] [Google Scholar]
- 18.Dolan R.S., Rahsepar A.A., Blaisdell J. Multiparametric cardiac magnetic resonance can detect acute cardiac allograft rejection after heart transplantation. J Am Coll Cardiol Img. 2019;12:1632–1641. doi: 10.1016/j.jcmg.2019.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinojar R., Varma N., Child N. T1 mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy: findings from the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.115.003285. [DOI] [PubMed] [Google Scholar]
- 20.Gottbrecht M., Kramer C.M., Salerno M. Native T1 and extracellular volume measurements by cardiac MRI in healthy adults: a meta-analysis. Radiology. 2019;290:317–326. doi: 10.1148/radiol.2018180226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J., Zhuang B., Sirajuddin A. MRI T1 mapping in hypertrophic cardiomyopathy: evaluation in patients without late gadolinium enhancement and hemodynamic obstruction. Radiology. 2020;294:275–286. doi: 10.1148/radiol.2019190651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–834. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z., Shi L., Wang Y. Case report pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y., Ma Y., Zhang J., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repessé X., Charron C., Vieillard-Baron A. Right ventricular failure in acute lung injury and acute respiratory distress syndrome. Minerva Anestesiol. 2012;78:941–948. [PubMed] [Google Scholar]
- 27.Osman D., Monnet X., Castelain V. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009;35:69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 28.Huang L., Ran L., Zhao P. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: tissue remodeling manifested prior to structure changes. Br J Radiol. 2019;92:20190634. doi: 10.1259/bjr.20190634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Florian A., Ludwig A., Rö Sch S., Yildiz H., Sechtem U., Yilmaz A. Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Img. 2014;15:1004–1012. doi: 10.1093/ehjci/jeu050. [DOI] [PubMed] [Google Scholar]