Abstract
Simultaneous transcriptome analyses of both host plants and pathogens, and functional validation of the identified differentially expressed genes (DEGs) allow us to better understand the mechanisms underlying their interactions. Here, we analyse the mixed transcriptome derived from Botrytis cinerea (the causal agent of grey mould) infected tomato leaves at 24 hr after inoculation, a critical time point at which the pathogen has penetrated and developed in the leaf epidermis, whereas necrotic symptoms have not yet appeared. Our analyses identified a complex network of genes involved in the tomato–B. cinerea interaction. The expression of fungal transcripts encoding candidate effectors, enzymes for secondary metabolite biosynthesis, hormone and reactive oxygen species (ROS) production, and autophagy‐related proteins was up‐regulated, suggesting that these genes may be involved in the initial infection processes. Specifically, tomato genes involved in phytoalexin production, stress responses, ATP‐binding cassette transporters, pathogenesis‐related proteins, and WRKY DNA‐binding transcription factors were up‐regulated. We functionally investigated several B. cinerea DEGs via gene replacement and pathogenicity assays, and demonstrated that BcCGF1 was a novel virulence‐associated factor that mediates fungal development and virulence via regulation of conidial germination, conidiation, infection structure formation, host penetration, and stress adaptation. The fungal infection‐related development was controlled by BcCGF‐mediated ROS production and exogenous cAMP restored the mutant infection‐related development. Our findings provide new insights into the elucidation of the simultaneous tactics of pathogen attack and host defence. Our systematic elucidation of BcCGF1 in mediating fungal pathogenesis may open up new targets for fungal disease control.
Keywords: BcCGF1, Botrytis cinerea, conidial germination, differentially expressed genes (DEGs), infection‐related development, mixed transcriptome, pathogenesis, virulence‐associated genes
Transcriptome profiling of both tomato and grey mould fungus at an early stage of infection and functional analysis reveal a novel pathogen factor, BcCgf1, that promotes fungal virulence by mediating infection‐related development.

1. INTRODUCTION
The necrotrophic fungal pathogen Botrytis cinerea causes grey mould on over 1,000 plant species (Fillinger and Elad, 2016). The pathogen infects almost all vegetable and fruit crops, including numerous economically important crops such as grapevine, strawberry, and raspberry, and annually causes enormous economic losses worldwide. The pathogen attacks many plant organs, including stems, fruits, leaves, and flowers, at both pre‐ and post‐harvest stages (Dean et al., 2012). In the field, the main infection source of this pathogen is conidia that form appressoria or appressorium‐like structures on host surfaces to facilitate infection (Gourgues et al., 2004; Choquer et al., 2007). In addition, B. cinerea is the most extensively studied necrotrophic fungal pathogen due to the availability of its genome sequence, its ease of study (e.g., easy to obtain gene knockout mutants or achieve gene silencing), as well as its economic relevance (Dean et al., 2012).
B. cinerea employs a range of toxic molecules to kill and decompose plant tissue prior to converting it into fungal biomass (Williamson et al., 2007). To initiate host infection, B. cinerea conidia on plant surfaces germinate and form appressoria for host penetration. Conidia of the pathogen hardly germinate in the presence of only water. Therefore, gluconeogenesis and glucose play a crucial role in the initiation of conidial germination; gluconeogenesis allows the pathogen to cope with the limitation of glucose and/or other carbon sources in the infection niches (Liu et al., 2018). Shortly after conidial germination, the germ tubes cease their polarized cell growth and start to form swollen melanized structures called appressoria. Besides appressoria, B. cinerea also forms highly melanized specialized hyphal networks or clumps of hyphal structures called infection cushions (Marschall and Tudzynski, 2016b). Infection structures (IFSs), including appressoria and infection cushions, are required for the pathogen to penetrate host cells. The fungal septin protein Sep4 is essential for pathogens to initiate IFS formation and host penetration (Feng et al., 2017). Plant cell walls are barriers that impair pathogen host colonization; however, they are also important reservoirs of energy‐rich sugars. To invade plant cells and to degrade or exploit cell wall components, B. cinerea secretes enzymes that disassemble cell wall polysaccharides during infection (Blanco‐Ulate et al., 2014). Interestingly, to disarm host resistance, the fungus also employs small RNAs as effectors that are delivered to the host plant cells where they can be loaded by the RNA silencing machinery and target plant defence genes (Weiberg et al., 2013). Although knowledge about B. cinerea pathogenesis has greatly expanded in the last two decades, mechanisms of pathogen factors that mediate the fungal pathogenesis and/or disarm host resistance remain obscure.
Once a pathogen is detected, the host plant activates signalling networks through the generation of small signalling molecules and coordination of hormonal signalling pathways to initiate defence mechanisms to the pathogen attack (AbuQamar et al., 2017). Plant responses to B. cinerea infection include host cell death, production of various secondary metabolites, antimicrobial peptides, and hormones, for example ethylene (ET), salicylic acid (SA), abscisic acid (ABA), and jasmonate (JA), as well as accumulation of reactive oxygen species (ROS), callose, and a variety of other cell wall modifications (Mengiste, 2012). ABA, ET, and auxin, are known to be actively involved in plant defence against B. cinerea (Navarro et al., 2006; El Oirdi et al., 2011; Windram et al., 2012; Sivakumaran et al., 2016). Other phytohormones, for example brassinosteroids, also regulate plant immunity by mainly interacting with transcription factors, or through camalexin biosynthesis and callose deposition. In addition, studies have notably provided evidence for cross‐talk among SA, JA, and ET or with other hormones in regulating plant defence responses to B. cinerea (for review, see AbuQamar et al., 2017).
Long non‐coding RNAs (lncRNAs) play important roles in gene expression and silencing pathways for several biological processes in eukaryotes. Host lncRNAs also play roles in plant defence. In response to pathogen infection, lncRNA‐dependent immune systems protect host plants via lncRNAs’ regulation of pathogen‐associated molecular patterns (PAMPs) and other effectors (Zaynab et al., 2018). Arabidopsis cells secrete exosome‐like extracellular vesicles to deliver small RNAs (sRNAs) into B. cinerea to silence fungal genes critical for pathogenicity, indicating that Arabidopsis has employed exosome‐mediated cross‐kingdom RNA interference as part of its immune responses during the evolutionary arms race with the pathogen (Cai et al., 2018). Despite some understanding of the antifungal mechanisms, more extensive studies on plant resistance to the pathogen are necessary, especially on host plants that are internally colonized by aggressive B. cinerea strains without displaying any signs of disease or stress (Shaw et al., 2016; Veloso and van Kan, 2018).
In B. cinerea pathosystems, host plant and/or pathogen transcriptomes have been investigated in Arabidopsis (Mulema and Denby, 2012), tomato (Blanco‐Ulate et al., 2013; Smith et al., 2014; Vega et al., 2015; Rezzonico et al., 2017), lettuce (De Cremer et al., 2013), and cucumber (Kong et al., 2015). These reports greatly increase our understanding of the mechanisms underlying B. cinerea interaction with its diverse hosts. However, plant or pathogen factors identified in the transcriptome analysis during their interactions remain to be functionally validated.
In this study, we investigated the simultaneous gene expression profiles of B. cinerea and tomato and identified a complex network of differentially expressed genes (DEGs) involved in the early stage of the B. cinerea–tomato interaction. We also functionally investigated several up‐regulated fungal genes and demonstrated that BcCGF1 is a novel B. cinerea factor that mediates the process of fungal development and infection via facilitating ROS production. Our work provides new insights into the molecular mechanisms underlying tomato–B. cinerea interaction at the early stage, which may be crucial to protect plants from damage caused by necrotrophic fungal pathogens.
2. RESULTS
2.1. DEGs in tomato and B. cinerea at the early interacting stage (24 hr post‐inoculation)
Transcriptomic analyses of B. cinerea‐infected tomato leaves as well as plant and pathogen controls (uninfected tomato plants and B. cinerea B05.10 cultured in ½ × potato dextrose broth, PDB) generated a total of 83,128,534 raw reads. After removing the adapter sequences and low‐quality reads, 70,186,664 clean reads remained (Table S1). From these, 96.2% and 84.4% of the clean reads in control and infected tomato, respectively, were mapped to the reference tomato genome. Likewise, 95.2% and 6.1% of the clean reads in B. cinerea at the noninfection and infection stages, respectively, were mapped to the B. cinerea genome (Tables S1 and S2). In tomato plants, RNA‐Seq yielded 1,319 DEGs (|log2FC [fold change]|> 1, p < .01); among them, 720 were up‐regulated and 599 were down‐regulated (Figure S1a, and Tables S3 and S4). In the fungus, 918 DEGs were detected and 621 and 297 DEGs were up‐regulated and down‐regulated, respectively (Figure S1b, and Tables S5 and S6). Our gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of tomato and B. cinerea DEGs revealed that the enriched GO terms (Figure S1c,d, and Tables S7 and S8) and KEGG pathways (Figure S2, and Tables S9 and S10) of the DEGs were similar to those previously reported (De Cremer et al., 2013; Kong et al., 2015; Rezzonico et al., 2017).
2.2. Characteristics of up‐regulated genes in B. cinerea‐infected tomato leaves
To understand early plant responses to B. cinerea infection, we selected some up‐regulated tomato genes for further functional annotation (Tables 1 and S3). Presumably, these transcripts play important roles in tomato plants against B. cinerea infection.
TABLE 1.
Highly up‐regulated (fold change > 50) tomato genes
| Gene ID | Protein symbol | Fold change | Annotation |
|---|---|---|---|
| Solyc03g020050.2 | CEVI57 | 1911.6 | Proteinase inhibitor type‐2 CEVI57 |
| Solyc06g066230.2 | LOC101248402 | 476.2 | Cytochrome P450 71D7 |
| Solyc02g078650.2 | LOC101263372 | 460.1 | Polyphenol oxidase, chloroplastic |
| Solyc12g010980.1 | LOC101260610 | 389.8 | Salutaridinol 7‐O‐acetyltransferase |
| Solyc04g072280.2 | LOC101261512 | 300.2 | Laccase‐14 |
| Solyc12g088800.1 | LOC101263295 | 273.1 | Hypothetical protein |
| Solyc01g101210.2 | TPS35 | 244.6 | Vetispiradiene synthase 1 |
| Solyc08g007090.1 | Solyc08g007090.1.1 | 226.2 | Expansin‐like B1 |
| Solyc05g021390.2 | LOC101251676 | 223.9 | Cytochrome P450 716B2 |
| Solyc01g101190.2 | TPS33 | 214.8 | Vetispiradiene synthase 1 |
| Solyc08g006750.2 | LOC101249664 | 178.5 | Histidine decarboxylase |
| Solyc00g247300.2 | LOC101247602 | 166.7 | Cytochrome P450 84A1 |
| Solyc01g095080.2 | ACS2 | 161.1 | 1‐aminocyclopropane‐1‐carboxylate synthase 2 |
| Solyc02g080840.1 | LOC101256060 | 157.3 | Probable F‐box protein At4g22030 |
| Solyc09g066400.1 | LOC101248046 | 156.6 | Premnaspirodiene oxygenase |
| Solyc02g070110.1 | LOC101263972 | 136.4 | Cannabidiolic acid synthase |
| Solyc04g016000.2 | LOC101266325 | 136.4 | Heat stress transcription factor B‐3 |
| Solyc01g101170.2 | TPS31 | 133.2 | Probable 5‐epi‐aristolochene synthase 4 |
| Solyc07g062370.1 | LOC101249107 | 122.5 | Hypothetical protein |
| Solyc02g067750.2 | CA3 | 115.5 | Carbonic anhydrase, chloroplastic |
| Solyc01g101180.2 | TPS32 | 109.5 | Viridiflorene synthase |
| Solyc03g119370.1 | LOC101266622 | 107.5 | Myb‐related protein 305 |
| Solyc08g036660.2 | LOC101253212 | 105.8 | Hypothetical protein |
| Solyc08g006740.2 | AADC2 | 100.0 | Histidine decarboxylase |
| Solyc02g070090.1 | LOC101255854 | 92.9 | Reticuline oxidase‐like protein |
| Solyc02g038740.2 | HMG2 | 88.0 | 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase 2 |
| Solyc01g087280.1 | LOC101263946 | 88.0 | Polygalacturonase |
| Solyc02g088160.2 | LOC101267864 | 87.3 | Casparian strip membrane protein |
| Solyc05g046340.1 | LOC101264724 | 84.0 | Phosphomannomutase |
| Solyc02g078930.1 | LOC101268840 | 82.6 | Hypothetical protein |
| Solyc09g075700.1 | LOC101255920 | 79.7 | Probable carboxylesterase 13 |
| Solyc04g083140.1 | LOC101251277 | 78.9 | Premnaspirodiene oxygenase |
| Solyc04g071070.2 | LOC544158 | 73.4 | Hypothetical protein |
| Solyc11g069560.1 | LOC101262484 | 71.4 | Hypothetical protein |
| Solyc07g064600.2 | CHRDi | 66.8 | RutC family protein C23G10.2 |
| Solyc06g065060.1 | LOC101254908 | 64.9 | Cannabidiolic acid synthase |
| Solyc09g013150.2 | LOC101254229 | 63.0 | Probable anion transporter 3, chloroplastic |
| Solyc11g067000.1 | ABCG51 | 62.7 | Pleiotropic drug resistance protein 2 |
| Solyc07g005380.2 | LOC101262431 | 61.7 | S‐norcoclaurine synthase |
| Solyc01g107080.2 | LOC101265373 | 61.5 | Uncharacterized acetyltransferase At3g50280 |
| Solyc09g011520.2 | LOC101267638 | 60.4 | Probable glutathione S‐transferase |
| Solyc01g005390.2 | LOC101248702 | 59.2 | Nudix hydrolase 18, mitochondrial |
| Solyc03g005500.1 | LOC101261887 | 58.7 | Ethylene‐responsive transcription factor ERF098 |
| Solyc02g093180.2 | LOC101266883 | 58.2 | Uncharacterized acetyltransferase At3g50280 |
| Solyc06g007180.2 | AS1 | 56.7 | Asparagine synthetase |
| Solyc05g047530.2 | LOC101262919 | 56.6 | Trans‐cinnamate 4‐monooxygenase |
| Solyc12g006530.1 | lTTS1 | 56.2 | β‐amyrin synthase |
| Solyc04g074770.2 | LOC101267111 | 54.6 | Unknown protein |
| Solyc10g084240.1 | LOC101249874 | 54.6 | Peroxidase 21 |
| Solyc07g054720.1 | LOC101256195 | 52.3 | Proteinase inhibitor type‐2 CEVI57 |
| Solyc05g056170.2 | PAL2 | 51.8 | Phenylalanine ammonia‐lyase |
2.2.1. Genes related to ROS and phytohormone production
In response to B. cinerea infection, several up‐regulated genes encoding ROS‐generating enzymes, including Solyc02g087070.2 (LOC543895), Solyc02g079500.2 (TMP1), Solyc02g078650.2 (LOC101263372), Solyc07g043590.2 (LOC101252005), and Solyc10g084240.1 (LOC101249874), were identified (Tables 1 and S3). The two type I 1‐amino‐cyclopropane‐1‐carboxylic acid synthase (ACS) isozymes ACS2 and ACS6 are responsible for B. cinerea‐induced ET production (Han et al., 2010). Many genes involved in ET biosynthesis were up‐regulated, including genes related to ET response factor (ERF) transcription factors (Solyc03g005500.1 [LOC101261887], Solyc05g051200.1 [LOC606712], Solyc06g053710.2 [ETR4], Solyc08g078180.1 [ERF‐A1], Solyc09g066360.1 [ERF‐C3], Solyc12g056590.1 [ERF‐D2], Solyc01g095080.2 [ACS2], Solyc03g080190.2 [DMR6‐1], and Solyc12g005940.1 [ACO2]) (Tables 1 and S3). Genes involved in biosynthetic pathways of ABA, for example Solyc04g071590.1 (ASR3) and Solyc04g071600.2 (LOC101247814), and auxin, for example Solyc03g120390.2 (IAA15) and Solyc03g121060.2 (IAA26), were also up‐regulated (Table S3).
2.2.2. Genes related to ATP‐binding cassette transporters
ATP‐binding cassette (ABC) transporters are involved in improving plant immunity (Mulema and Denby, 2012). Genes related to ABC transporters, including Solyc00g233480.1, Solyc02g087870.2 (MDR1), Solyc03g007530.2 (ABCC2), Solyc04g015970.2 (ABCA1), Solyc05g014380.2 (LOC101257768), Solyc05g014390.2, Solyc06g009290.2 (ABCB9), and Solyc06g074960.2 (LOC101266730), were up‐regulated in the infected leaves (Tables 1 and S3). Proteins encoded by these genes are classified in diverse ABC transporter families (Table S11). Our protein–protein interaction (PPI) network analysis suggested that the ABC transporters encoded by these genes interact with many other ABC transporters or related proteins (Figure 1a, Table S11) in response to B. cinerea infection.
FIGURE 1.

The protein–protein interaction (PPI) networks of tomato and Botrytis cinerea in the early stage of their interaction. The PPI networks of tomato ATP‐binding cassette (ABC) transporters (a) and B. cinerea autophagy‐related proteins (b) in the early stage of tomato–B. cinerea interaction. The proteins with green edges are encoded by the corresponding up‐regulated tomato ABC transporter genes (a) or B. cinerea autophagy‐related genes (b). The other interaction proteins in the networks are from STRING (https://string-db.org/), Uniprot (https://www.uniprot.org/), and NCBI (https://www.ncbi.nlm.nih.gov/). For the size of the circles, the bigger the circles, the more connecting strings/lines or interacting proteins the circles have. For the filled colours in the circles, the more connecting lines, the closer the colour to orange (ranging from yellow to orange). For the colours of the connecting lines, red indicates that proteins directly interact with the interested proteins (nodes with green edges) and black indicates other relationships of interactions. The bold connecting lines indicate that the combined score (0–1, the higher the score, the more reliable the interaction relationship) is greater than 0.95
2.2.3. Genes associated with autophagy machinery and WRKY DNA‐binding transcription factors
Autophagy is a mechanism of the cell that disassembles and recycles unnecessary or dysfunctional cellular components. The up‐regulation of an autophagy‐related (ATG) gene SlATG8 (Solyc08g078820.2) (Table S3) suggests that autophagy machinery plays a role in the tomato response to B. cinerea infection and may be conducive to host cell survival, in agreement with previous reports (Lai et al., 2011; Windram et al., 2012). Tomato WRKY DNA‐binding transcription factors mediate disease resistance against B. cinerea (Liu et al., 2014). Three tomato genes Solyc02g080890.2 (SlWRKY6), Solyc02g094270.1, and Solyc05g015850.2 (SlWRKY75) encoding WRKY DNA‐binding transcription factors were up‐regulated in response to B. cinerea attack (Table S3).
2.2.4. Genes related to PAMP receptors, PR proteins, and other factors involved in host defence
Fungal cell wall components such as oligosaccharides and chitin are fungal PAMPs that are recognized by host plant receptors and activate defence responses. Fungal polygalacturonases (PGs) are important components essential for virulence and detected by host plants (Windram et al., 2012). The tomato chitin elicitor receptor kinase gene Solyc04g072000.2 (LOC101256086) that encodes chitinase (Liu et al., 2012), and genes Solyc02g070910.1 (LOC101259509) and Solyc07g006770.2 (LOC101243723) encoding flagellin‐sensitive 2 (FLS2) that improve immune responses in Arabidopsis (Sun et al., 2013) were up‐regulated (Table S3). Arabidopsis Kazal‐type serine proteinase inhibitors (KPIs) are induced in response to B. cinerea infection and AtKPI‐1 displays a strong antifungal activity in inhibition of the pathogen conidial germination, demonstrating the roles of KPIs in defence against pathogens (Pariani et al., 2016). During B. cinerea infection, tomato genes related to KPI (LOC101256195), pathogenesis‐related (PR) proteins PR1b1 (Solyc00g174340.1), PR‐1a1 (Solyc01g106610.2), and Solyc01g097240.2 (PR‐P2), wounding Solyc01g097270.2 (LOC543758), peroxidases, for example Solyc10g084240.1 (Peroxidase 21), Solyc02g094180.2, Solyc01g105070.2, and Solyc01g007950.2 (Peroxidase 1), and respiratory burst Solyc01g099620.2 (LOC101251928) were up‐regulated (Table S3).
2.3. Characteristics of up‐regulated genes in B. cinerea at early stage of infection
To understand the possible roles of pathogen genes in host infection, we characterized some up‐regulated fungal DEGs, including BCIN_12g01020 encoding oxaloacetate acetylhydrolase (OAH) and BCIN_03g01540 encoding choline dehydrogenase (Tables 2 and S5). The pathogen may orchestrate these DEGs to facilitate host infection.
TABLE 2.
Highly up‐regulated (fold change ≥ 40) Botrytis cinerea genes
| Gene ID | Protein symbol | Fold change | Annotation | Secreted |
|---|---|---|---|---|
| BCIN_03g01540 | BCIN_03g01540 | 527.7 | Choline dehydrogenase | Yes |
| BCIN_12g01020 | Bcoah | 402.4 | Oxaloacetate acetylhydrolase (Bcoah1) | No |
| BCIN_15g05630 | BCIN_15g05630 | 349.4 | Putative NAD‐dependent epimerase dehydratase family protein | No |
| BCIN_15g01960 | BCIN_15g01960 | 211.3 | RNA methyltransferase, TrmH family, group 3 | Yes |
| BCIN_08g00280 | BCIN_08g00280 | 206.8 | Similar to carboxypeptidase S1 | Yes |
| BCIN_06g00650 | BCIN_06g00650 | 202.1 | Isotrichodermin C‐15 hydroxylase | No |
| BCIN_12g06300 | BCIN_12g06300 | 202.0 | Similar to metalloproteinase | No |
| BCIN_01g01260 | BCIN_01g01260 | 171.3 | DJ‐1/PfpI family protein DJ‐1 | No |
| BCIN_16g01820 | BCIN_16g01820 | 156.1 | Conidial germination protein/ Bccgf1 | Yes |
| BCIN_03g01560 | BCIN_03g01560 | 151.8 | Similar to aldo/keto reductase | No |
| BCIN_02g01260 | BCIN_02g01260 | 134.0 | Pisatin demethylase | No |
| BCIN_03g05820 | BCIN_03g05820 | 106.0 | Pectate lyase B precursor | Yes |
| BCIN_12g00380 | BCIN_12g00380 | 97.2 | Hypothetical protein | Yes |
| BCIN_15g02380 | Bcacp1 | 95.0 | Acidic protease | Yes |
| BCIN_03g01520 | BCIN_03g01520 | 78.0 | NADP‐dependent alcohol dehydrogenase | No |
| BCIN_12g02040 | Bcap8 | 76.3 | Polyporopepsin/Bcap8 | Yes |
| BCIN_02g08100 | BCIN_02g08100 | 76.0 | Hypothetical protein | No |
| BCIN_15g03320 | BCIN_15g03320 | 75.6 | Acid phosphatase precursor | Yes |
| BCIN_14g01070 | BCIN_14g01070 | 74.0 | Hypothetical protein similar to alcohol dehydrogenase | No |
| BCIN_05g04180 | BCIN_05g04180 | 73.8 | Hypothetical protein similar to betaine lipid synthase | No |
| BCIN_01g01550 | BCIN_01g01550 | 72.3 | Hypothetical protein | No |
| BCIN_06g00130 | BCIN_06g00130 | 70.0 | Oligopeptide transporter | No |
| BCIN_04g02930 | Bmr5 | 63.6 | Macrolide transporter ATP‐binding/permease protein | No |
| BCIN_02g04840 | BCIN_02g04840 | 62.9 | Inorganic phosphate transporter 1‐6/Pi cotransporter | No |
| BCIN_09g01150 | BCIN_09g01150 | 62.6 | Hypothetical protein | Yes |
| BCIN_03g03630 | BCIN_03g03630 | 61.1 | Endoglucanase A precursor | Yes |
| BCIN_11g02900 | BCIN_11g02900 | 60.0 | Trypsin | Yes |
| BCIN_06g05050 | BCIN_06g05050 | 59.9 | Hypothetical protein similar to glucoamylase | Yes |
| BCIN_02g08710 | BCIN_02g08710 | 58.4 | Hypothetical protein | No |
| BCIN_14g04260 | Bcgas2 | 57.6 | Protein of unknown function | Yes |
| BCIN_10g05620 | BCIN_10g05620 | 55.3 | Pectate lyase precursor | Yes |
| BCIN_05g01510 | BCIN_05g01510 | 51.7 | Hypothetical protein | Yes |
| BCIN_07g04510 | BCIN_07g04510 | 50.9 | Hypothetical protein | No |
| BCIN_09g02930 | Bcap1 | 49.5 | Aspergillopepsin A precursor (Bcap1) | No |
| BCIN_08g04910 | BCIN_08g04910 | 49.5 | Hypothetical protein | No |
| BCIN_04g05960 | BCIN_04g05960 | 49.2 | Hypothetical protein | Yes |
| BCIN_13g03660 | BCIN_13g03660 | 47.9 | Hypothetical protein | No |
| BCIN_14g05330 | BCIN_14g05330 | 44.9 | Similar to GPI anchored dioxygenase | Yes |
| BCIN_07g05430 | BCIN_07g05430 | 42.8 | Cytochrome P450 3A10 | No |
| BCIN_05g07630 | BCIN_05g07630 | 41.7 | Hypothetical protein | Yes |
| BCIN_06g00120 | BCIN_06g00120 | 41.3 | Oligopeptide transporter 7 | No |
| BCIN_10g01510 | BCIN_10g01510 | 40.9 | Hypothetical protein | Yes |
| BCIN_14g01770 | BCIN_14g01770 | 40.0 | Putative MFS multidrug protein | No |
| BCIN_16g02770 | Bcmp1 | 40.0 | Peptidase M35 domain of deuterolysins and related proteins | Yes |
2.3.1. Genes involved in secondary metabolite biosynthesis and toxin production
Oxalate secretion by fungal pathogens is usually associated with their pathogenesis (Han et al., 2007; Liang et al., 2015). The VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism (Bayram et al., 2008; Schumacher et al., 2015). During infection, fungal genes involved in secondary metabolites and toxin production were up‐regulated. For example, BCIN_12g01020 encoding BcOah1, BcVEL1 (BCIN_15g03390) and BcLAE1 (BCIN_05g01210) involved in secondary metabolism and virulence (Yang et al., 2013; Schumacher et al., 2015), and BCIN_03g01570 encoding polyketide synthases that regulates phytotoxin botcinic acid biosynthesis (Dalmais et al., 2011) were highly expressed (Tables 2 and S5).
2.3.2. Genes related to hormone and ROS production
Cytochrome P450 monooxygenases are essential for ABA biosynthesis in B. cinerea (Siewers et al., 2004). ABA decreases tomato resistance to B. cinerea via reduction of NO production, which also suppresses both ROS and ET production (Sivakumaran et al., 2016). Two P450 monooxygenase genes (BCIN_07g05430 and BCIN_03g06490) involved in ABA biosynthesis in B. cinerea were up‐regulated (Table S5). NADPH oxidases are required for ROS production and are involved in conidial germination, differentiation, and vegetative and pathogenicity development as well as virulence in B. cinerea (Marschall and Tudzynski, 2016a; Cao et al., 2018; Hou et al., 2020). NADPH oxidase genes BcNOXA (BCIN_05g00350) and BcNOXB (BCIN_02g04930) were also up‐regulated (Table S5).
2.3.3. Autophagy‐related genes
At the initiation of infection, the pathogen ATGs, including ATG1 (BCIN_07g00720), ATG2 (BCIN_14g01550), and ATG13 (BCIN_13g04910), were up‐regulated (Table S5). Our PPI network analysis suggests that the autophagy proteins encoded by these ATGs interact with many proteins, including Atg1, Atg3, Atg4, Atg7, and Atg8 (Figure 1b, Table S12).
2.3.4. Genes encoding carbohydrate‐active enzymes
Growing hyphae of B. cinerea secrete a large number of extracellular virulence components including cell wall degradation (CWD)‐associated enzymes that disassemble cell wall polysaccharides. B. cinerea polygalacturonases are essential for its virulence and can be detected by host plants (Blanco‐Ulate et al., 2014). Cutinases are extracellular degradative enzymes that hydrolyse cutin and facilitate fungal penetration through the cuticle (van der Vlugt‐Bergmans et al., 1997). During infection, a group of genes encoding enzymes involved in CWD were up‐regulated. These genes included two pectate lyase genes (BCIN_03g05820 and BCIN_10g05620), three endoglucanase genes (BCIN_10g06130, BCIN_12g06630, and BCIN_05g07690), a cutinase‐related gene (BCIN_03g04560), one endo‐1,4‐β‐xylanase gene (BCIN_12g00090), eight glucosidase‐related genes (e.g. BCIN_09g02640 and BCIN_09g05460), and three PG genes (BcPG3 [BCIN_04g04930], BcPG4 [BCIN_03g01680], and BcPG6 [BCIN_02g05860]) (Table S5).
2.3.5. Genes encoding candidate effectors
B. cinerea effectors play important roles in establishment of infection via suppression of plant innate immunity (Weiberg et al., 2013; Heard et al., 2015). Through prediction of secretory signal peptide and the cellular localization of protein encoded by each up‐regulated fungal transcript, we identified a larger number of transcripts (88/621) encoding putative secreted proteins that may be B. cinerea candidate effectors (Table S13). Among them, several candidate effector genes were highly up‐regulated, for example the expression levels of BCIN_16g01820 and BCIN_10g05620, encoding a hypothetic protein and a pectate lyase, respectively, were over 150‐ and 60‐fold of that of the control (Table 2).
2.4. Functional analysis of up‐regulated B. cinerea genes
Each DEG with unknown function may constitute a potential entry point for investigating the novel mechanism by which the pathogen secures a successful infection. To investigate gene function at early stage of infection, we representatively selected several up‐regulated genes (with known and unknown function) and performed quantitative reverse transcription PCR (RT‐qPCR) to confirm their expression levels (Figure 2a). We then generated gene knockout (KO) mutants as previously described (Feng et al., 2017) and conducted pathogenicity assays. Consistent with previous findings (Schumacher et al., 2015; Ren et al., 2017), the mutants lacking BcATG1, BcLAE1, or BcVEL1 lost their virulence (Figure S5). However, loss of individual β‐glucosidase genes BcBGL1 through BcBGL6 (BCIN_09g02640, BCIN_09g05460, BCIN_10g05590, BCIN_03g08710, BCIN_14g00650, and BCIN_10g02650) did not affect the virulence of the pathogen (Figure 2b), implying that those CWD‐associated factors may be functionally redundant in virulence and/or act on modulating fungal cell walls during growth and development of the grey mould fungus. Disruption of BCIN_03g01540, a gene encoding a glucose‐methanol‐choline (GMC) oxidoreductase, significantly reduced the fungal virulence (Figure 2c,d), suggesting that the factor is required for a successful infection.
FIGURE 2.
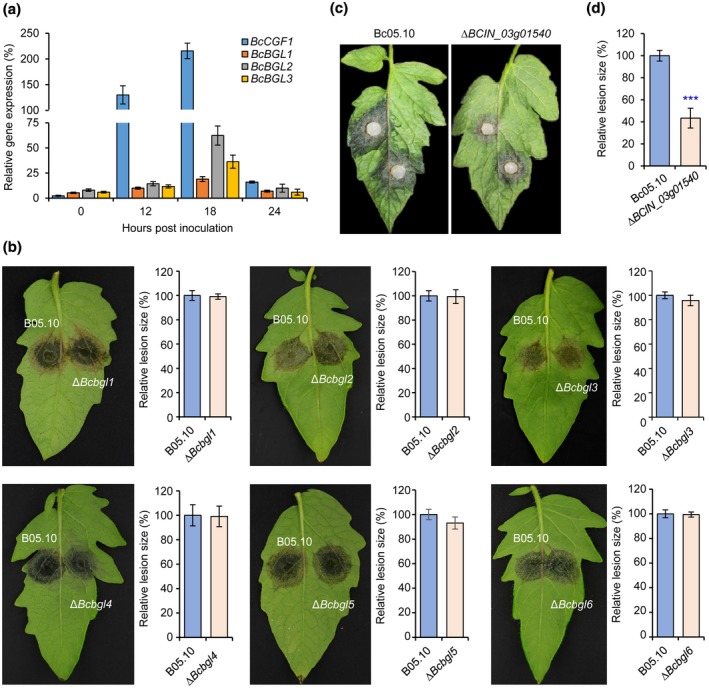
Functional analysis of the differentially expressed genes in Botrytis cinerea. (a) Quantitative reverse transcription PCR verifications of the up‐regulated genes in B. cinerea. BcCGF1, B. cinerea conidial germination factor 1. BcBGL, B. cinerea β‐glucosidase genes. (b) The up‐regulated genes encoding putative β‐glucosidases play a limited role in the virulence of B. cinerea. The indicated β‐glucosidase genes were separately deleted in B. cinerea and the resultant mutants, together with the wild‐type strain B05.10, were used for pathogenicity assays. (c) Loss of the up‐regulated gene BCIN_03g01540 encoding GMC oxidoreductase significantly reduced the fungal virulence. (d) Quantification of the lesion sizes caused by the indicated strains as shown in (c). Data represent means ± SD from at least four independent experiments. ***Significance at p < .001
2.5. BcCGF1 is a novel virulence‐associated gene required for virulence of B. cinerea
Among the fungal DEGs, we were particularly intrigued with these novel DEGs; BCIN_16g01820, encoding a hypothetical protein (Table 2), represented one such DEG because it had not been previously implicated as a factor that mediates fungal pathogenesis. We thus focused on the functional analysis of this DEG. Bioinformatics analysis indicated that the hypothetical protein contained 255 amino acid residues and shared 66% identity with the spore germination protein (PQE13889.1) of Rutstroemia sp. NJR‐2017a BBW (Figure S3); however, the protein has not been previously characterized. Our functional analysis demonstrated that BCIN_16g01820 was involved in B. cinerea conidial germination (see below), therefore BCIN_16g01820 was designated as B. cinerea conidial germination‐associated factor 1 (BcCGF1). BcCgf1 is conserved among some known fungi and putatively conserved domains have not been detected in the protein (Figure S3).
To analyse the roles of BcCGF1 in the fungal growth and pathogenesis, we generated B. cinerea CGF1 knockout (KO) mutant ΔBccgf1 and its complemented strain ΔBccgf1‐C using the illustrated strategies and performed functional analyses after confirmation of the absence of BcCGF1 in the mutants (Figure S4). Our data indicated that loss of BcCGF1 in B. cinerea did not affect mycelial growth (Figure 3a,b), but reduced conidial development (Figure 3c,d) on complete medium (CM) and disruption of BcCGF1 impaired virulence of the ΔBccgf1 mutants on both intact (Figure 4a–d) and wounded (Figure 4e–h) hosts. A similar result was observed when mycelial plugs of the tested strains were used to inoculate green bean leaves (Figure S6). Complementation of the mutants with the wild‐type (WT) CGF1 allele completely rescued the defects of the mutants (Figures 3 and 4). The findings demonstrate that BcCGF1 is a novel virulence‐associated factor required for conidial development, virulence, and in planta development of the pathogen.
FIGURE 3.
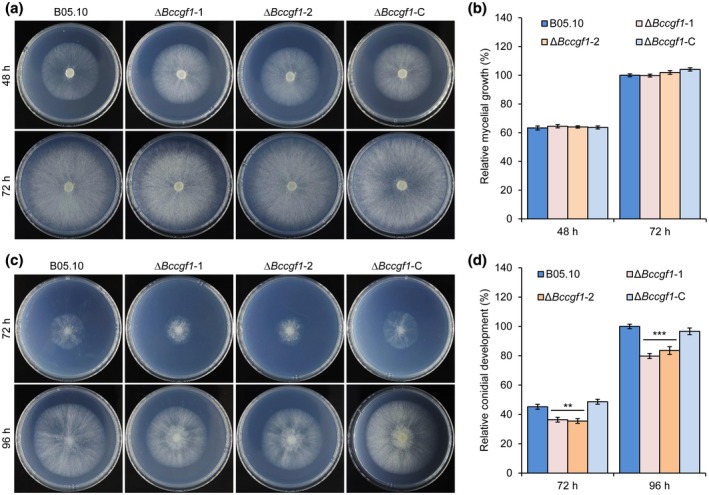
BcCGF1 is required for Botrytis cinerea conidial germination but not hyphal radial growth. Mycelium‐plug radial growth of the indicated wild type (B05.10), ΔBccgf1, and complemented (ΔBccgf1‐C) strains of B. cinerea on complete medium (CM) plates at 20 °C with a time course of 72 hr (a) and quantification of the mycelial growth (in diameter) at 72 hr post‐inoculation (hpi) (b). Conidial growth of the indicated strains on CM plates at 20 °C with a time course of 96 hr (c) and quantification of the growth at 96 hpi (d). Data represent means ± SD from at least four independent experiments. **, ***Significance at p < .01 and p < .001, respectively
FIGURE 4.

BcCGF1 is required for virulence of Botrytis cinerea. Host leaves and fruits were inoculated, incubated at room temperature in the dark, and at the indicated times after inoculation the inoculated materials were photographically documented and quantitatively analysed. Intact leaves were inoculated with the indicated strains via the conidial inoculation approach (105 conidia/ml, 8 μl) and the diseased tomato (a) or green bean (b) leaves at the indicated times after inoculation were photographically documented. Quantification of the relative lesion sizes on inoculated tomato (c) and green bean (d) leaves. Diseased apple fruits induced by conidia (105 conidia/ml, 5 μl) (e) or mycelial plugs (f) of the indicated strains at the indicated times after inoculation and quantification of the relative lesion sizes induced by conidia (g) or mycelial plugs (h) of the strains as shown in (e) and (f). Data represent means ± SD from at least four independent experiments. *, ***Significance at p < .05 and p < .001, respectively
2.6. BcCGF1 is required for B. cinerea IFS development and host penetration
To investigate the reduction in virulence of the ΔBccgf1 mutants, we evaluated the capability of the WT, ΔBccgf1, and ΔBcgf1‐C strains to form IFSs that play crucial roles in pathogen host penetration. We inoculated the tested strains on inductive surfaces (Feng et al., 2017) and determined their abilities to form appressoria and infection cushions. Our data demonstrated that loss of BcCGF1 reduced the pathogen formation of appressoria (Figure 5a,b) and infection cushions (Figure 5c,d). To further understand the attenuated pathogenicity of the ΔBccgf1 mutants, we performed onion epidermal cell infection assay to analyse host penetration of the strains and found that at 20 hr post‐inoculation/incubation (hpi), almost all the WT and ΔBccgf1‐C conidia penetrated the onion epidermis; host penetration by the ΔBccgf1 mutants was only 32% of that of the control (Figure 5e,f). These findings demonstrate that BcCGF1 is required for the pathogen IFS formation and host penetration.
FIGURE 5.

BcCGF1 mediates infection structure formation and host penetration. Disruption of BcCGF1 reduces appressorium (arrows) (a) and infection cushion (c) formation of the indicated strains on an inductive surface at 8 or 24 hr post‐inoculation (hpi) at 20 °C. Quantification of appressorium (b) and infection cushion (d) formation by the indicated strains at 8 and 24 hpi, respectively. (e) Loss of BcCGF1 impairs host penetration and invasive growth of the mutant strains. (f) Quantification of host penetration by the indicated strains at 20 hpi. Data represent means ± SD from three independent experiments with triplicate samples/slides examined for each strain in each experiment. ***Significance at p < .001
2.7. BcCGF1 mediates B. cinerea conidiation, conidial morphogenesis, and germination, but is dispensable for sclerotial formation and germination
To determine the roles of BcCGF1 in fungal development, we inoculated the WT, ∆Bccgf1, and ∆Bccgf1‐C strains on glass slides or CM plates and performed fungal development assays. Disruption of BcCGF1 reduced conidiation of the ∆Bccgf1 mutants (Figure 6a,b). The mutant conidia displayed an abnormal morphology (Figure 6a, lower panel). Quantitative analysis demonstrated that conidia produced by the mutants were smaller in both length and width than those produced by the WT and complemented strains (Figure 6c). The subsequent conidial germination assay indicated that disruption of BcCGF1 reduced the conidial germination rate and germ tube development (Figure 6d–f). Complementation of the mutants with the WT BcCGF1 rescued the defects (Figure 6). However, loss of BcCGF1 did not impair the production, morphology, or germination of the mutant sclerotia (Figure 6g–k). These findings indicate that BcCGF1 is required for conidiation, conidial morphogenesis and germination, and germ tube development, but dispensable for sclerotium formation and germination, of the pathogen.
FIGURE 6.
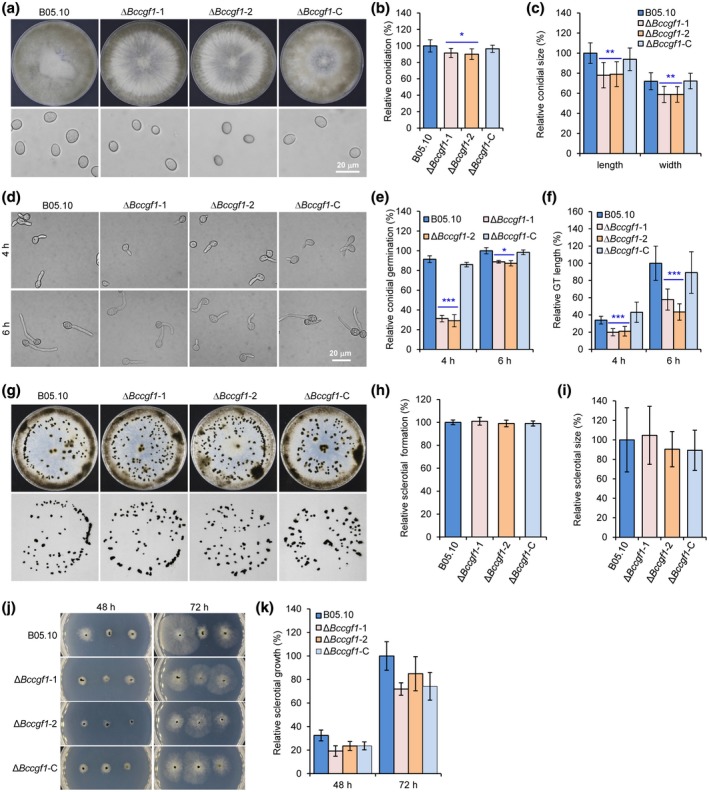
BcCGF1 is required for proper Botrytis cinerea conidiation, conidial morphogenesis, and germination but is dispensable for B. cinerea sclerotium production and morphogenesis. (a) Loss of BcCGF1 reduces B. cinerea conidiation (upper panel) and alters conidial morphology (lower panel). Quantification of conidiation (b) and conidial size (c) of the indicated strains. (d) Conidial germination of the indicated strains. Quantification of conidial germination (e) and germ tube (GT) length (f) at 4 and 6 hr post‐inoculation (hpi). Deletion of BcCGF1 in B. cinerea did not affect sclerotial production. The wild type (B05.10), ΔBccgf1, and ΔBccgf1‐C strains were inoculated on complete medium (CM) plates at 20 °C in darkness after 14 days. Sclerotial production by each strain was photographically documented (g) and quantitatively determined (h). (i) Quantification of the sizes of sclerotia produced by the indicated strains at 14 days. (j) Sclerotial germination of the indicated strain on CM plates at the indicated hpi. (k) Relative sclerotial growth of the indicated strains (nine sclerotia/plate). Data represent means ± SD from at least three independent experiments in which triplicate plates were analysed for each strain in each experiment. *, **, *** significance at p < .05, p < .01, and p < .001, respectively
2.8. BcCGF1 mediates B. cinerea osmotic and oxidative stress adaptation as well as cell wall integrity
To test whether BcCGF1 plays a role in B. cinerea adaptation to infection‐related stresses, we inoculated the WT, ΔBccgf1, and complemented strains on CM supplemented with the assorted stress‐mimicking agents, including osmotic stress agents NaCl and KCl, the oxidative stress agent H2O2, and the cell wall‐disturbing agents sodium dodecyl sulphate (SDS) and Congo Red (CR) (Feng et al., 2017), and compared the radial growth rates of these strains. The mutant strains displayed reductions in conidial germination and subsequent hyphal growth (Figure 7a,c) and reductions in radial growth of mycelia on CM containing the assorted stress agents (Figure 7b,d). Further analysis indicated that the relative mycelial radial growth inhibition (Cao et al., 2018; Hou et al., 2020) of the ΔBccgf1 strains significantly increased when cultured on CM containing the stress‐mimicking agents compared to that of the control and complemented strains (Figure 7e,f). These data suggest that BcCGF1 is required for pathogen osmotic and oxidative stress adaptation as well as cell wall integrity.
FIGURE 7.
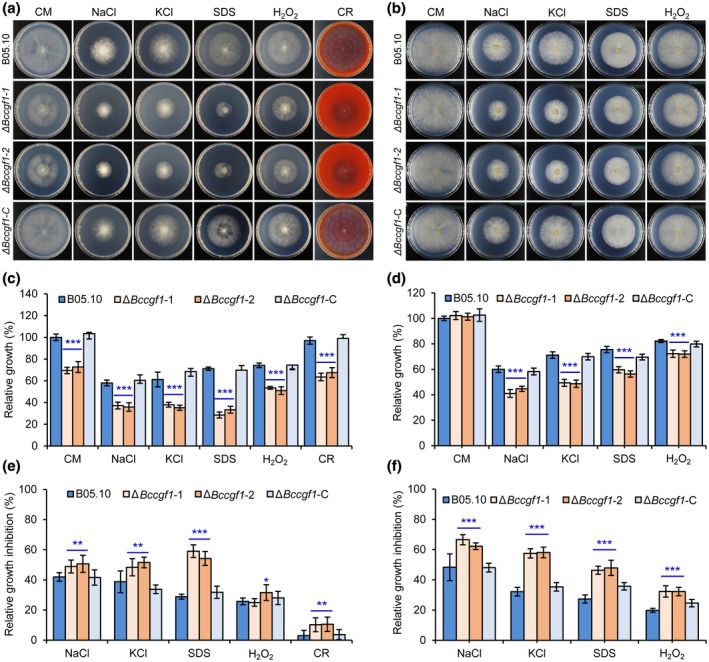
BcCGF1 mediates osmotic and oxidative stress adaptation as well as cell wall integrity of Botrytis cinerea. Conidial (5 × 105 conidia/ml, 5 μl) (a) and mycelial radial growth (mycelial plugs 5 mm in diameter) (b) of the wild type (B05.10), ΔBccgf1, and ΔBccgf1‐C strains of B. cinerea on complete medium (CM) supplemented with the osmotic stress agents NaCl (1 M) and KCl (1 M), the oxidative stress agent H2O2 (6 mM), the cell wall disturbing agents sodium dodecyl sulphate (SDS, 0.01%) or Congo Red (CR, 300 μg/ml). Quantification of the relative mycelial growth of the indicated strains growing from inoculated conidia (c) or mycelial plugs (d) on CM supplemented with the indicated stress‐mimetic agents as presented in (a) and (b). The relative mycelial growth inhibition of the indicated strains growing from inoculated conidia (e) or mycelial plugs (f) on CM supplemented with the indicated stress‐mimetic agents as presented in (a) and (b). Representative images are from one experiment (4 days post‐inoculation). Data represent means ± SD from three independent experiments in which triplicate plates were examined for each strain in each experiment. *, **, *** significance at p < .05, p < .01, and p < .001, respectively
2.9. Loss of BcCGF1 impairs endogenous ROS production and cAMP rescues the infection‐related development in the BcCGF1 deletion strains
Endogenous ROS and the cAMP signalling pathway play crucial roles in B. cinerea infection‐related development, including conidial germination and IFS formation (Marschall and Tudzynski, 2016b; Feng et al., 2017; Hou et al., 2020). Our data indicated that disruption of BcCGF1 impairs the pathogen conidial germination, IFS formation, host penetration, and virulence (Figures 3, 4, 5, 6). We thus wondered whether loss of BcCGF1 reduces endogenous ROS production and the cAMP signalling regulates these processes in BcCGF1‐deficient B. cinerea. To test these possibilities, we examined ROS production in the presence or absence of diphenylene iodonium (DPI), a NAD(P)H oxidase inhibitor, or antioxidant dithiothreitol (DTT), which prevents oxidation of 3,3′‐diaminobenzidine (DAB), in the tested strains using the DAB and nitroblue tetrazolium (NBT) staining approaches (Cao et al., 2018; Hou et al., 2020). DAB is converted to dark‐brown polymers in the presence of H2O2 and NBT forms a dark‐blue water‐insoluble formazan precipitate on reduction by superoxide radicals. We also determined the influence of cAMP signalling on conidial germination and/or IFS formation of the tested strains as previously described (Feng et al., 2017; Cao et al., 2018; Hou et al., 2020). Our data demonstrate that the ∆Bccgf1 mutants are difficult to be stained in the germ‐tubes and growing mycelia during conidial germination, appressorium, and infection structure formation (Figures 8a and S7); quantification analysis indicated that the relative ROS production by the mutants was significantly reduced (Figure 8b). These findings demonstrate that loss of BcCGF1 in B. cinerea impairs endogenous ROS production.
FIGURE 8.
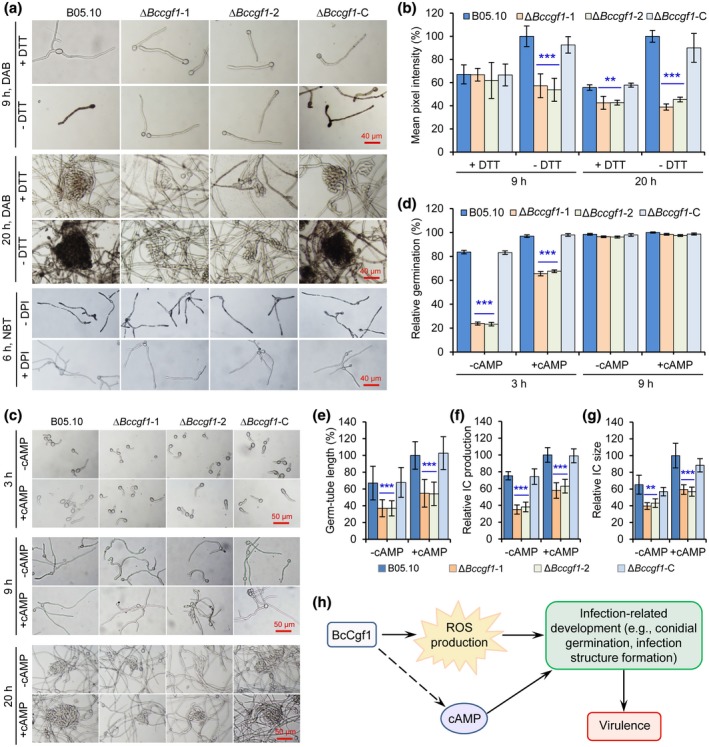
BcCGF1 regulates endogenous reactive oxygen species (ROS) production in Botrytis cinerea and cAMP rescues the infection‐related development in the BcCGF1 deletion strains. Conidia of the wild type (B05.10), ∆Bccgf1, and ∆Bccgf1‐C strains were cultivated on complete medium (CM) or CM supplemented with diphenylene iodonium (DPI) or dithiothreitol (DTT) at 20 °C for the indicated lengths of time, and then the germ tubes or hyphae/mycelia of the tested strains were stained with 3,3′‐diaminobenzidine (DAB) or nitroblue tetrazolium (NBT) solution as previously described (Cao et al., 2018; Hou et al., 2020). (a) Disruption of BcCGF1 reduced ROS production in the ∆Bccgf1 mutants during infection‐related development detected by DAB or NBT staining. (b) Quantification of relative ROS production by the mean pixel intensity in the tested strains shown in (a). (c) Exogenous cAMP rescued the defect of infection‐related development of the mutants. Quantification of conidial germination (d), germ tube length (e), infection cushion (IC) production (f), and infection cushion size (g) of the tested strains supplemented with or without exogenous cAMP. (h) A model describing BcCgf1 regulation of B. cinerea pathogenesis via mediating infection‐related development and virulence controlled by BcCgf1‐mediated ROS production. Data represent means ± SD from three independent experiments. **, *** significance at p < .01 and p < .001, respectively
The analysis of cAMP influence on the fungal infected‐related development indicated that exogenous cAMP (50 µM) significantly facilitates conidial germination, appressorium, and IFS formation of the mutants compared to that of the strains in the absence of cAMP (Figure 8c–g). Consistent with the previous reports (Feng et al., 2017; Hou et al., 2020), exogenous cAMP in the WT and ΔBccgf1‐C strains resulted in a promotion of conidial germination, and an earlier emergence and facilitation of IFS development (Figure 8c–g). These data suggest that cAMP signalling regulates the pathogen infection‐related development downstream of BcCgf1.
3. DISCUSSION
In this study, we performed simultaneous transcriptome analyses of tomato and B. cinerea during their early stage of interaction and identified 720 and 621 DEGs in tomato and B. cinerea, respectively. We used B. cinerea WT strain cultured in liquid medium, growing from spore suspension as a control. This culture condition can mimic the fungal infection environment inside host cells/tissues that may be hypoxic, and thus can minimize the interference of fungal genes that respond only to low‐oxygen conditions (Kawahara et al., 2012). After DEG enrichment analysis, we identified the tomato and B. cinerea genes and pathways that may play roles in their early stage of interaction and functionally investigated the gene functions of some fungal DEGs, including BcCGF1.
Similar to the previous findings (Blanco‐Ulate et al., 2013; Smith et al., 2014; Vega et al., 2015; Rezzonico et al., 2017), we identified a large number of up‐regulated tomato genes that may increase plant defence against B. cinerea. These genes are mainly associated with ROS and phytohormone production, stress responses, and PR proteins, and so on (Tables 1 and S3). The balance between two types of programmed cell death, autophagy and apoptosis, controls host cell life, and an induction of autophagic cell death is conducive to triggering host cell resistance to Botrytis infection (Veloso and van Kan, 2018). The up‐regulation of SlATG8 suggests that host autophagy machinery functions in tomato response to B. cinerea infection and cell survival.
Some new plant genes that may be involved in tomato plant resistance to B. cinerea attack were detected in our transcriptome analyses. Nine tomato ABC transporter‐related genes encoding protein members of diverse ABC transporter families were up‐regulated when infected by B. cinerea (Tables S3 and S11). These ABC transporters may interact with other ABC transporter‐related proteins to fulfil complicated biological processes involved in tomato defence against B. cinerea (Figure 1a and Table S11). The plant JA signalling pathway is activated mainly against necrotrophic pathogens (El Oirdi et al., 2011). The up‐regulation of Solyc12g009220.1 (JAZ2) (Table S3), encoding TIFY 10A in the JA pathway, may promote JA biosynthesis in the infected tomato. The calmodulin‐binding transcription activators (CAMTAs) are crucial in manipulating both biotic and abiotic stresses in plants by their efficiency to transduce calcium signals that mediate plant cell wall reinforcement, stomatal closure, and activation of defence‐related genes (Galon et al., 2010). Several tomato genes, for example Solyc01g105230.2 (LOC101055571), Solyc10g074570.1 (LOC101252935), Solyc02g083850.2 (LOC101260391), and Solyc06g073830.1 (LOC101265138) (Table S3), encoding CAMTAs or calcium‐related factors, were up‐regulated. The up‐regulation of these new tomato genes suggests that they may be required for tomato defence against pathogen attack or maintenance of survival of the infected cells.
Autophagy facilitates programmed cell developmental changes that occur during cellular remodelling, and thus serves as an adaptive mechanism for cellular nutrient starvation. Therefore, it is not surprising that some ATGs, including BcATG1, BcATG2, and BcATG13, were up‐regulated in the pathogen during initial infection. At early infection stage, B. cinerea may be in a condition of nutrient limitation and autophagy allows the pathogen to cope with nutrient starvation in the infection niches. The interactions of the autophagy proteins encoded by these ATGs with other autophagy‐related proteins in our PPI network analysis (Figure 1b, Table S12) suggest that the pathogen autophagy machinery is activated and functions during initial infection. The involvement of B. cinerea autophagy in securing a successful infection is likely via mediating its nutrition acquisition and development. These data also support the recent findings about the roles of autophagy in B. cinerea pathogenesis (Ren et al., 2017, 2018a, 2018b; Liu et al., 2019a).
Glucose metabolism is one of the basic characteristics of B. cinerea host infection. Glucose mediates pathogen development and pathogenesis via initiating conidial germination, mediating infection structure development, and host penetration (Liu et al., 2018). To invade plant cells and/or exploit the polysaccharides of plant cell walls, the pathogen secretes diverse enzymes to disassemble plant cell walls during infection. The expression of fungal glycoside hydrolases contributes to the degradation of host cell wall polysaccharides, and the soluble oligosaccharides produced by the digestion of glycoside hydrolases are transported to the inside of the fungal cell and metabolized (Blanco‐Ulate et al., 2014). Consistent with previous findings (Blanco‐Ulate et al., 2014), our data indicate that many transcripts encoding enzymes in glycosyl hydrolase family (GHF), including GHF 11 (BCIN_12g00090), GHF 28 (BCIN_10g06130), GHF 45 (BCIN_12g06630), and GHF 76 (BCIN_08g06110), polysaccharide lyase family 6 (BCIN_10g06130), and pectate lyases (BCIN_03g05820 and BCIN_10g05620) were highly up‐regulated in B. cinerea during initial infection (Table S5), suggesting that these enzymes may function in the pathogen CWD and break the plant physical barrier.
Unsurprisingly, a large number of genes encoding glucosidases were up‐regulated in B. cinerea during infection (Table S5). We generated the KO mutants of the six β‐glucosidase genes (BcBGL1 to BcBGL6) with up‐regulation levels ranging from 3‐ to 26‐fold (Table S5) and performed pathogenicity assays for these mutants; however, disruption of these genes did not impair virulence of the mutants (Figure 2b), suggesting that these individual β‐glucosidases may be dispensable for virulence of the pathogen. This may result from the functional redundancy of these enzymes in B. cinerea, thus loss of one or two of them did not impair virulence of the pathogen.
Our functional analysis suggested that fungal GMC oxidoreductase BCIN_03g01540 is involved in B. cinerea host infection. The GMC superfamily is a large and functionally diverse family of oxidoreductases that share a common structural fold. Fungal GMC enzymes of this superfamily that exhibit lignocellulose degradation include aryl‐alcohol oxidoreductase, alcohol oxidase, cellobiose dehydrogenase, glucose oxidase, glucose dehydrogenase, pyranose dehydrogenase, and pyranose oxidase (Sutzl et al., 2019). In polyporales, the members of the GMC oxidoreductase superfamily also play a central role in degradation of plant polymers because they generate extracellular H2O2, acting as the ultimate oxidizer in both white‐rot and brown‐rot decay (Ferreira et al., 2015). The roles of GMC oxidoreductases in B. cinerea host infection remain largely unknown; further investigation should be focused on the mechanisms of GMC oxidoreductases regulating the fungal pathogenesis.
The up‐regulation of many new fungal genes in the early stage of B. cinerea–tomato interaction implies that they may play roles in facilitating the pathogen host infection. However, more research is needed to clarify the functions of these newly identified genes in the pathogenesis of B. cinerea. In this work, we functionally validated the roles of novel virulence‐associated gene BcCGF1 in B. cinerea, identified by global mixed gene‐expression profiling. Bioinformatics analysis suggests that the deduced BcCgf1 may be a secreted protein containing a signal peptide, a transmembrane helix (TMhelix), and a noncytoplasmic domain (Figure S3a). Cgf1 proteins are evolutionarily conserved among pathogenic and nonpathogenic fungi (Figure S3b). However, the function of this protein has not been previously characterized. Disruption of BcCGF1 impairs conidiation, alters conidial morphogenesis, dramatically delays conidial germination, and reduces infection structure formation, host penetration, and invasive hyphal growth of the mutant strains, which raises an intriguing question about how BcCgf1 influences these processes. Further mechanism analysis indicates that the pathogen infection‐related development is controlled by BcCGF1‐mediated ROS production, and cAMP regulation of the developmental events is probably downstream of BcCgf1 (Figure 8h). However, the signal that stimulates the up‐regulation of BcCGF1 during infection remains unknown. Subcellular localization is an important functional characteristic of a protein, and subcellular localization of BcCgf1 needs to be determined, although bioinformatics analysis suggests that BcCgf1 may be a secreted protein (Figure S3a). The known functional domains of the deduced BcCgf1 are not detected (Figure S3a) and the functional characteristics of BcCgf1 also need to be further investigated. Further work is needed to address all the above‐mentioned issues to reveal the molecular mechanisms of BcCgf1 mediating the development and virulence of B. cinerea.
In summary, our global simultaneous transcriptome analyses of tomato and B. cinerea interaction at the early stage provides new insights into the mechanisms underlying tomato–B. cinerea interaction. We identified a novel fungal factor BcCGF1 through the analyses and demonstrated that BcCGF1 plays pleiotropic roles in B. cinerea conidial germination, asexual reproduction, infection structure formation, host‐penetration, stress adaptation, and virulence. BcCGF1 enhances the fungal virulence via promoting infection‐related development controlled by BcCGF1‐mediated endogenous ROS production (Figure 8h).
4. EXPERIMENTAL PROCEDURES
4.1. Fungal and plant materials
B. cinerea WT B05.10 and its derived mutant and complemented strains were used in this study (Table S14). All strains were maintained on potato dextrose agar (PDA) or CM as previously described (Liu et al., 2018). The plant materials used in this work included tomato, green bean, strawberry, and apple leaves or fruits.
4.2. Sample preparation for RNA‐Seq analysis
Tomato cultivar Moneymaker plants were grown in a growth chamber at 25 °C, 80% humidity, and a 14 hr:10 hr light/dark cycle. Seven‐week‐old plants (8–10 leaves/plant) were challenged with conidial suspension (106 conidia/ml in potato dextrose broth [PDB]) of B05.10 by the spray‐inoculation method. Plants inoculated with ½ × PDB served as a mock‐inoculation control. The inoculated plants were kept in containers sealed with a sheet of transparent plastic film on each top to maintain high‐humidity infection environments. At 24 hpi, inoculated leaves were detached from each inoculated plant (four plants per group), the leaf samples were quickly rinsed (in ddH2O) and frozen in liquid nitrogen and stored at −80 °C for RNA extraction. In each experiment, each treatment contained three biological replicates (three groups of plants). Two independent experiments were completed for RNA‐Seq analysis. Conidia (106 conidia/ml, 1 ml) of B. cinerea were inoculated into flasks containing 100 ml PDB and the flasks were incubated at room temperature with shaking (220 rpm). Homogenized mycelia were harvested at c.18 hpi and immediately snap‐frozen in liquid nitrogen and stored at –80 °C for RNA extraction.
4.3. RNA isolation and RNA‐Seq
Total RNAs were extracted using TRIzol reagent (Life Technologies, Shanghai, China). RNA quality was monitored on 1% agarose gels. RNA purity was determined by a NanoPhotometer spectrophotometer (Implen GmbH, München, Germany). RNA concentration was measured using a Qubit RNA Assay Kit in Qubit 2.0 Flurometer (Life Technologies). RNA integrity was assessed by the Bioanalyzer 2100 system (Agilent Technologies, Beijing, China). The extracted mRNA was used to construct the cDNA library and the library construction was sequenced on an Illumina Hiseq 2000 Platform (Novogene Bioinformatics Institute, Beijing, China). The preprocessed RNA‐Seq reads were mapped to the tomato or B. cinerea reference genome using the Bowtie, TopHat, and Cufflinks programs (Trapnell et al., 2009; 2010).
4.4. Transcriptome analysis
The DEGs were identified from RNA‐Seq data with the cut‐off of corrected p value < .01. Analyses of the biological information of DEGs were performed as previously described (Hou et al., 2020). The database used in the analyses included EggNOG (evolutionary genealogy of genes: Non‐supervised Orthologous Groups, http://eggnogdb.embl.de/#/app/home), a database of orthologous groups of genes, Gene Ontology Consortium, an international standard classification system for gene function, including biological processes, cellular components, and molecular function (http://www.geneontology.org/), KEGG (http://www.genome.jp/kegg/), Swiss‐Prot (http://web.expasy.org/docs/swiss-prot_guideline.html), NR (ftp://ftp.ncbi.nlm.nih.gov/blast/db/), and Pfam (http://pfam.xfam.org/).
4.5. RT‐qPCR
Total RNA for RT‐qPCR assay was extracted using RNAiso plus kid (TaKaRa), and cDNA was prepared using PrimerScript RT Reagent Kit with gDNA Eraser (TaKaRa) according to the manufacturer's instructions. RT‐qPCRs were carried out as previously described (Feng et al., 2017).
4.6. Bioinformatics analysis
The data of BcCGF1 DNA and protein sequence were obtained from NCBI (http://www.ncbi.nlm.nih.gov) and Ensembl Fungi (http://fungi.ensembl.org/Botrytis_cinerea) (B. cinerea B05.10) (Amselem et al., 2011; Van Kan et al., 2017). The deduced protein domains and functional sites of BcCgf1 were analysed via InterProScan (http://www.ebi.ac.uk/interpro/scan.html). Sequence alignments and phylogenetic trees were generated using GENEDOC software (http://www.softpedia.com/get/Science-CAD/GeneDoc.shtml) and MEGA 6 software (Tamura et al., 2013), respectively. The PPI networks were analysed using the SRTING software (v. 11.0, https://string-db.org/) and generated by the Cytoscape software (https://cytoscape.org/).
4.7. Generation of gene deletion mutants and complemented strains
Generation of gene KO mutants (including ΔBccgf1, ΔBclae1, ΔBcvel1, ΔBcatg1, ΔBCIN_03g01540, and ΔBcbgl1 to ΔBcbgl6) and the genetic complemented strain ΔBccgf1‐C was performed as previously described (Feng et al., 2017). Briefly, vector pXEH containing the HPH cassette was used for replacement of the targeted genes. The 5′ and 3′ homologous flanks of the targeted gene were amplified and cloned into pXEH upstream and downstream of HPH, respectively. The gene KO vector was transformed into Agrobacterium tumefaciens AGL‐1. The resultant deletion transformants were screened on PDA with 100 μg/ml hygromycin. Vector pSUL conferring resistance to chlorimuron‐ethyl was used for complementation of the ∆Bccgf1. A fragment containing BcCGF1 (1219 bp upstream and 686 bp downstream of the coding region of BcCGF1) was amplified by PCR and cloned into pSUL vector to generate the complementation vector. The complementary vector was transformed into A. tumefaciens AGL‐1 and the resultant transformants were screened on defined complex medium containing 100 μg/ml chlorimuron‐ethyl (Rolland et al., 2003). Diagnostic PCR was performed to verify the integration events of the selected transformants. The gene KO and complemented strains were further confirmed by RT‐qPCR (Weiberg et al., 2013). The primers used in the experiments are listed in Table S15.
4.8. Fungal growth and pathogenicity assays
For growth assays, conidia of B. cinerea WT, gene KO, and complemented strains were harvested with PDB, and conidial suspension was adjusted to approximately 105 conidia/ml. Conidial suspension (10 μl) was dropped onto glass slides to observe conidial morphology and germination, or onto CM plates to observe mycelial growth. Stress adaptation assays and the influence of cAMP on the development of the tested strains were performed as previously described (Feng et al., 2017; Hou et al., 2020). For pathogenicity assay, droplets of conidial suspension (5 × 105 conidia/ml in ½ × PDB, 5 μl) of the tested strains were dropped on host leaves/surfaces. When mycelial plugs were used in these assays, mycelial plugs (5 mm in diameter) taken from a 3‐day‐old culture of the tested strains were used, unless otherwise indicated. The inoculated slides or plant materials were incubated in the dark in a moistened box at 21 °C. Conidial germination, appressorium, and infection cushion formation were observed and counted under a microscope. At least 100 conidia were counted per replicate in each experiment. At least three independent experiments (triplicate samples examined for each treatment in each experiment) were performed. The assays were performed as previously described (Feng et al., 2017; Cao et al., 2018; Liu et al., 2018).
4.9. Cytological assay
Preparation of conidia and onion epidermal cells as well as sample lactophenol blue staining were performed as previously described (Liu et al., 2018, 2019b; Hou et al., 2020). The infected samples were microscopically observed, photographically documented, and analysed at 20 hpi.
4.10. Detection and quantification of ROS production
Detection and quantification of ROS production during conidial germination and infection structure formation were performed as previously described (Feng et al., 2017; Cao et al., 2018; Hou et al., 2020). The software ImageJ (http://rsbweb.nih.gov/ij/) was used to quantify ROS production.
4.11. Statistical analysis
The quantitative data in this study were derived from at least three independent experiments with triplicate treatments examined unless otherwise indicated. The data of controls, including conidial germination, mycelial growth, and lesion size, in each independent experiment were normalized as 1 or 100%. The significance between the data were assessed using the Student's t test and p < .05 was considered as a significant difference.
AUTHOR CONTRIBUTIONS
Q.M.Q. and M.Z.Z. conceived the experiments. M.Z.Z., C.H.S., Y.L., H.G.F., S.N.C., and J.H. performed the experiments. Q.M.Q., G.H.L., K.Z.S., H.W.C., C.C., and H.Z. analysed and interpreted the data. Q.M.Q. and G.H.L. provided reagents. Q.M.Q. supervised the work. Q.M.Q. and M.Z.Z. wrote the paper.
Supporting information
FIGURE S1 Differentially expressed genes in tomato and Botrytis cinerea at the early stage of their interaction
FIGURE S2 The 20 most enriched KEGG pathways in tomato (a) and Botrytis cinerea (b) during their early stage of interaction
FIGURE S3 Phylogenetic relationship of Cgf1 proteins from the indicated organisms
FIGURE S4 Strategies of generation of BcCGF1 deletion and complemented strains
FIGURE S5 Functional validation of the up‐regulated Botrytis cinerea differentially expressed genes BcATG1, BcLAE1, and BcVEL1
FIGURE S6 Loss of BcCGF1 reduces the virulence of Botrytis cinerea
FIGURE S7 BcCGF1 mediates endogenous reactive oxygen species production in Botrytis cinerea
TABLE S1 Mapping results of RNA‐Seq reads
TABLE S2 The percentages of the clean reads mapped to tomato and Botrytis cinerea genomes
TABLE S3 Up‐regulated tomato genes in response to Botrytis cinerea attack (24 hr post‐infection)
TABLE S4 Down‐regulated tomato genes in response to Botrytis cinerea attack (24 hr post‐infection)
TABLE S5 Up‐regulated Botrytis cinerea genes at the early stage of host infection (24 hr post‐infection)
TABLE S6 Down‐regulated Botrytis cinerea genes at the early stage of infection (24 hr post‐infection)
TABLE S7 GO enrichment of the differentially expressed genes in tomato infected by Botrytis cinerea at 24 hr post‐infection
TABLE S8 GO enrichment of the differentially expressed genes in Botrytis cinerea at 24 hr post‐infection
TABLE S9 KEGG enrichment of the differentially expressed genes in tomato attacked by Botrytis cinerea at 24 hr post‐infection
TABLE S10 KEGG enrichment of the differentially expressed genes in Botrytis cinerea at 24 hr post‐infection
TABLE S11 ABC transporters encoded by the up‐regulated tomato genes in response to Botrytis cinerea infection (early stage) and their interacting proteins
TABLE S12 Proteins encoded by the up‐regulated Botrytis cinerea autophagy genes at the early stage of infection and their interacting proteins
TABLE S13 Potential secreted Botryis cinerea proteins at the early stage of host infection
TABLE S14 Fungal strains used in this study
TABLE S15 Primers used in this study
ACKNOWLEDGEMENTS
We thank Professor Jan A. L. van Kan (Wageningen University, Netherlands) for sharing the predicted secreted proteins of B. cineare and Dr Chengguo Jia (Jilin University, China) for providing tomato seeds. This work was supported by the National Natural Science Foundation of China (grant nos. 31871913; 81371773), the Chinese Special Fund for Agro‐scientific Research in the Public Interest (201303025, subproject: Molecular basis for host–B. cinerea interaction), and the Overseas High‐level Talent Introduction Plan of Jilin University (4305050102) to Q.M.Q. The authors declare that no competing interests exist.
Zhang M‐Z, Sun C‐H, Liu Y, et al. Transcriptome analysis and functional validation reveal a novel gene, BcCGF1, that enhances fungal virulence by promoting infection‐related development and host penetration. Molecular Plant Pathology. 2020;21:834–853. 10.1111/mpp.12934
Funding information
This work was supported by the National Natural Science Foundation of China (grant nos. 31871913; 81371773), the Chinese Special Fund for Agro‐scientific Research in the Public Interest (201303025, subproject: Molecular basis for host–B. cinerea interaction), and the Overseas High‐level Talent Introduction Plan of Jilin University (4305050102) to Q.M.Q.
REFERENCES
- AbuQamar, S. , Moustafa, K. and Tran, L.S. (2017) Mechanisms and strategies of plant defense against Botrytis cinerea . Critical Reviews in Biotechnology, 37, 262–274. [DOI] [PubMed] [Google Scholar]
- Amselem, J. , Cuomo, C.A. , Van Kan, J.A. , Viaud, M. , Benito, E.P. , Couloux, A. et al (2011) Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea . PLoS Genetics, 7, e1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram, O. , Krappmann, S. , Ni, M. , Bok, J.W. , Helmstaedt, K. , Valerius, O. et al (2008) VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science, 320, 1504–1506. [DOI] [PubMed] [Google Scholar]
- Blanco‐Ulate, B. , Morales‐Cruz, A. , Amrine, K.C.H. , Labavitch, J.M. , Powell, A.L.T. and Cantu, D. (2014) Genome‐wide transcriptional profiling of Botrytis cinerea genes targeting plant cell walls during infections of different hosts. Frontiers in Plant Science, 5, 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco‐Ulate, B. , Vincenti, E. , Powell, A.L.T. and Cantu, D. (2013) Tomato transcriptome and mutant analyses suggest a role for plant stress hormones in the interaction between fruit and Botrytis cinerea . Frontiers in Plant Science, 4, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Q. , Qiao, L. , Wang, M. , He, B. , Lin, F.M. , Palmquist, J. et al (2018) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science, 360, 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, S.N. , Yuan, Y. , Qin, Y.H. , Zhang, M.Z. , de Figueiredo, P. , Li, G.H. et al (2018) The pre‐rRNA processing factor Nop53 regulates fungal development and pathogenesis via mediating production of reactive oxygen species. Environmental Microbiology, 20, 1531–1549. [DOI] [PubMed] [Google Scholar]
- Choquer, M. , Fournier, E. , Kunz, C. , Levis, C. , Pradier, J.M. , Simon, A. et al (2007) Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiology Letters, 277, 1–10. [DOI] [PubMed] [Google Scholar]
- Dalmais, B. , Schumacher, J. , Moraga, J. , Le Pecheur, P. , Tudzynski, B. , Collado, I.G. et al (2011) The Botrytis cinerea phytotoxin botcinic acid requires two polyketide synthases for production and has a redundant role in virulence with botrydial. Molecular Plant Pathology, 12, 564–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cremer, K. , Mathys, J. , Vos, C. , Froenicke, L. , Michelmore, R.W. , Cammue, B.P.A. et al (2013) RNAseq‐based transcriptome analysis of Lactuca sativa infected by the fungal necrotroph Botrytis cinerea . Plant, Cell and Environment, 36, 1992–2007. [DOI] [PubMed] [Google Scholar]
- Dean, R. , Van Kan, J.A. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di Pietro, A. , Spanu, P.D. et al (2012) The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oirdi, M. , Abd El Rahman, T. , Rigano, L. , El Hadrami, A. , Rodriguez, M.C. , Daayf, F. et al (2011) Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. The Plant Cell, 23, 2405–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, H.Q. , Li, G.H. , Du, S.W. , Yang, S. , Li, X.Q. , de Figueiredo, P. et al (2017) The septin protein Sep4 facilitates host infection by plant fungal pathogens via mediating initiation of infection structure formation. Environmental Microbiology, 19, 1730–1749. [DOI] [PubMed] [Google Scholar]
- Ferreira, P. , Carro, J. , Serrano, A. and Martinez, A.T. (2015) A survey of genes encoding H2O2‐producing GMC oxidoreductases in 10 polyporales genomes. Mycologia, 107, 1105–1119. [DOI] [PubMed] [Google Scholar]
- Fillinger, S. and Elad, Y. (2016) In: Fillinger S. and Elad Y. (Eds.). Botrytis – The Fungus, the Pathogen and Its Management in Agricultural Systems. Switzerland: Springer International Publishing. [Google Scholar]
- Galon, Y. , Finkler, A. and Fromm, H. (2010) Calcium‐regulated transcription in plants. Molecular Plant, 3, 653–669. [DOI] [PubMed] [Google Scholar]
- Gourgues, M. , Brunet‐Simon, A. , Lebrun, M.H. and Levis, C. (2004) The tetraspanin BcPls1 is required for appressorium‐mediated penetration of Botrytis cinerea into host plant leaves. Molecular Microbiology, 51, 619–629. [DOI] [PubMed] [Google Scholar]
- Han, L. , Li, G.J. , Yang, K.Y. , Mao, G. , Wang, R. , Liu, Y. et al (2010) Mitogen‐activated protein kinase 3 and 6 regulate Botrytis cinerea‐induced ethylene production in Arabidopsis. The Plant Journal, 64, 114–127. [DOI] [PubMed] [Google Scholar]
- Han, Y. , Joosten, H.J. , Niu, W. , Zhao, Z. , Mariano, P.S. , McCalman, M. et al (2007) Oxaloacetate hydrolase, the C–C bond lyase of oxalate secreting fungi. Journal of Biological Chemistry, 282, 9581–9590. [DOI] [PubMed] [Google Scholar]
- Heard, S. , Brown, N.A. and Hammond‐Kosack, K. (2015) An interspecies comparative analysis of the predicted secretomes of the necrotrophic plant pathogens Sclerotinia sclerotiorum and Botrytis cinerea . PLoS ONE, 10, e0130534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, J. , Feng, H.Q. , Chang, H.W. , Liu, Y. , Li, G.H. , Yang, S. et al (2020). The H3K4 demethylase Jar1 orchestrates ROS production and expression of pathogenesis‐related genes to facilitate Botrytis cinerea virulence. New Phytologist, 225, 930–947. [DOI] [PubMed] [Google Scholar]
- Kawahara, Y. , Oono, Y. , Kanamori, H. , Matsumoto, T. , Itoh, T. and Minami, E. (2012) Simultaneous RNA‐seq analysis of a mixed transcriptome of rice and blast fungus interaction. PLoS ONE, 7, e49423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, W. , Chen, N. , Liu, T. , Zhu, J. , Wang, J. , He, X. et al (2015) Large‐scale transcriptome analysis of cucumber and Botrytis cinerea during infection. PLoS ONE, 10, e0142221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, Z. , Wang, F. , Zheng, Z. , Fan, B. and Chen, Z. (2011) A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. The Plant Journal, 66, 953–968. [DOI] [PubMed] [Google Scholar]
- Liang, X. , Liberti, D. , Li, M. , Kim, Y.T. , Hutchens, A. , Wilson, R. et al (2015) Oxaloacetate acetylhydrolase gene mutants of Sclerotinia sclerotiorum do not accumulate oxalic acid, but do produce limited lesions on host plants. Molecular Plant Pathology, 16, 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. , Liu, Z. , Song, C. , Hu, Y. , Han, Z. , She, J. et al (2012) Chitin‐induced dimerization activates a plant immune receptor. Science, 336, 1160–1164. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Hong, Y.B. , Zhang, Y.F. , Li, X.H. , Huang, L. , Zhang, H.J. et al (2014) Tomato WRKY transcriptional factor SlDRW1 is required for disease resistance against Botrytis cinerea and tolerance to oxidative stress. Plant Science, 227, 145–156. [DOI] [PubMed] [Google Scholar]
- Liu, J.K. , Chang, H.W. , Liu, Y. , Qin, Y.H. , Ding, Y.H. , Wang, L. et al (2018) The key gluconeogenic gene PCK1 is crucial for virulence of Botrytis cinerea via initiating its conidial germination and host penetration. Environmental Microbiology, 20, 1794–1814. [DOI] [PubMed] [Google Scholar]
- Liu, N. , Ren, W. , Li, F. , Chen, C. and Ma, Z. (2019a) Involvement of the cysteine protease BcAtg4 in development and virulence of Botrytis cinerea . Current Genetics, 65, 293–300. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Liu, J.K. , Li, G.H. , Zhang, M.Z. , Zhang, Y.Y. , Wang, Y.Y. et al (2019b) A novel Botrytis cinerea‐specific gene BcHBF1 enhances virulence of the grey mould fungus via promoting host penetration and invasive hyphal development. Molecular Plant Pathology, 20, 731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall, R. and Tudzynski, P. (2016a) BcIqg1, a fungal IQGAP homolog, interacts with NADPH oxidase, MAP kinase and calcium signaling proteins and regulates virulence and development in Botrytis cinerea . Molecular Microbiology, 101, 281–298. [DOI] [PubMed] [Google Scholar]
- Marschall, R. and Tudzynski, P. (2016b) Reactive Oxygen Species in Development and Infection Processes. Paper presented at: Seminars in Cell and Developmental Biology (Elsevier: ). [DOI] [PubMed] [Google Scholar]
- Mengiste, T. (2012) Plant immunity to necrotrophs. Annual Review of Phytopathology, 50, 267–294. [DOI] [PubMed] [Google Scholar]
- Mulema, J.M.K. and Denby, K.J. (2012) Spatial and temporal transcriptomic analysis of the Arabidopsis thaliana–Botrytis cinerea interaction. Molecular Biology Reports, 39, 4039–4049. [DOI] [PubMed] [Google Scholar]
- Navarro, L. , Dunoyer, P. , Jay, F. , Arnold, B. , Dharmasiri, N. , Estelle, M. et al (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science, 312, 436–439. [DOI] [PubMed] [Google Scholar]
- Pariani, S. , Contreras, M. , Rossi, F.R. , Sander, V. , Corigliano, M.G. , Simon, F. et al (2016) Characterization of a novel Kazal‐type serine proteinase inhibitor of Arabidopsis thaliana . Biochimie, 123, 85–94. [DOI] [PubMed] [Google Scholar]
- Ren, W. , Liu, N. , Sang, C. , Shi, D. , Zhou, M. , Chen, C. et al (2018a) The autophagy gene BcATG8 regulates vegetative differentiation and plant infection of Botrytis cinerea . Applied and Environmental Microbiology, 84, e02455–e2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, W. , Sang, C. , Shi, D. , Song, X. , Zhou, M. and Chen, C. (2018b) Ubiquitin‐like activating enzymes BcAtg3 and BcAtg7 participate in development and pathogenesis of Botrytis cinerea . Current Genetics, 64, 919–930. [DOI] [PubMed] [Google Scholar]
- Ren, W. , Zhang, Z. , Shao, W. , Yang, Y. , Zhou, M. and Chen, C. (2017) The autophagy‐related gene BcATG1 is involved in fungal development and pathogenesis in Botrytis cinerea . Molecular Plant Pathology, 18, 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzonico, F. , Rupp, O. and Fahrentrapp, J. (2017) Pathogen recognition in compatible plant–microbe interactions. Scientific Reports, 7, 6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland, S. , Jobic, C. , Fevre, M. and Bruel, C. (2003) Agrobacterium‐mediated transformation of Botrytis cinerea, simple purification of monokaryotic transformants and rapid conidia‐based identification of the transfer‐DNA host genomic DNA flanking sequences. Current Genetics, 44, 164–171. [DOI] [PubMed] [Google Scholar]
- Schumacher, J. , Simon, A. , Cohrs, K.C. , Traeger, S. , Porquier, A. , Dalmais, B. et al (2015) The VELVET complex in the gray mold fungus Botrytis cinerea: impact of BcLAE1 on differentiation, secondary metabolism, and virulence. Molecular Plant‐Microbe Interactions, 28, 659–674. [DOI] [PubMed] [Google Scholar]
- Shaw, M.W. , Emmanuel, C.J. , Emilda, D. , Terhem, R.B. , Shafia, A. , Tsamaidi, D. et al (2016). Analysis of cryptic, systemic Botrytis infections in symptomless hosts. Frontiers in Plant Science, 7, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewers, V. , Smedsgaard, J. and Tudzynski, P. (2004) The p450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea . Applied and Environmental Microbiology, 70, 3868–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumaran, A. , Akinyemi, A. , Mandon, J. , Cristescu, S.M. , Hall, M.A. , Harren, F.J. et al (2016) ABA suppresses Botrytis cinerea elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Frontiers in Plant Science, 7, 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J.E. , Mengesha, B. , Tang, H. , Mengiste, T. and Bluhm, B.H. (2014) Resistance to Botrytis cinerea in Solanum lycopersicoides involves widespread transcriptional reprogramming. BMC Genomics, 15, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Li, L. , Macho, A.P. , Han, Z. , Hu, Z. , Zipfel, C. et al (2013) Structural basis for flg22‐induced activation of the Arabidopsis FLS2‐BAK1 immune complex. Science, 342, 624–628. [DOI] [PubMed] [Google Scholar]
- Sutzl, L. , Foley, G. , Gillam, E.M.J. , Boden, M. and Haltrich, D. (2019) The GMC superfamily of oxidoreductases revisited: analysis and evolution of fungal GMC oxidoreductases. Biotechnology for Biofuels, 12, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Stecher, G. , Peterson, D. , Filipski, A. and Kumar, S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell, C. , Pachter, L. and Salzberg, S.L. (2009) TopHat: discovering splice junctions with RNA‐Seq. Bioinformatics, 25, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell, C. , Williams, B.A. , Pertea, G. , Mortazavi, A. , Kwan, G. , van Baren, M.J. et al (2010) Transcript assembly and quantification by RNA‐Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology, 28, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kan, J.A. , Stassen, J.H. , Mosbach, A. , Van Der Lee, T.A. , Faino, L. , Farmer, A.D. et al (2017) A gapless genome sequence of the fungus Botrytis cinerea . Molecular Plant Pathology, 18, 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega, A. , Canessa, P. , Hoppe, G. , Retamal, I. , Moyano, T.C. , Canales, J. et al (2015) Transcriptome analysis reveals regulatory networks underlying differential susceptibility to Botrytis cinerea in response to nitrogen availability in Solanum lycopersicum . Frontiers in Plant Science, 6, 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso, J. and van Kan, J.A. (2018) Many shades of grey in Botrytis–host plant interactions. Trends in Plant Science, 23, 613–622. [DOI] [PubMed] [Google Scholar]
- van der Vlugt‐Bergmans, C. , Wagemakers, C. and van Kan, J. (1997) Cloning and expression of the cutinase A gene of Botrytis cinerea . Molecular Plant‐Microbe Interactions, 10, 21–29. [DOI] [PubMed] [Google Scholar]
- Weiberg, A. , Wang, M. , Lin, F.M. , Zhao, H. , Zhang, Z. , Kaloshian, I. et al (2013) Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science, 342, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, B. , Tudzynski, B. , Tudzynski, P. and van Kan, J.A. (2007) Botrytis cinerea: the cause of grey mould disease. Molecular Plant Pathology, 8, 561–580. [DOI] [PubMed] [Google Scholar]
- Windram, O. , Madhou, P. , McHattie, S. , Hill, C. , Hickman, R. , Cooke, E. et al (2012) Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high‐resolution temporal transcriptomic analysis. The Plant Cell, 24, 3530–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q.Q. , Chen, Y.F. and Ma, Z.H. (2013) Involvement of BcVeA and BcVelB in regulating conidiation, pigmentation and virulence in Botrytis cinerea . Fungal Genetics and Biology, 50, 63–71. [DOI] [PubMed] [Google Scholar]
- Zaynab, M. , Fatima, M. , Abbas, S. , Umair, M. , Sharif, Y. and Raza, M.A. (2018) Long non‐coding RNAs as molecular players in plant defense against pathogens. Microbial Pathogenesis, 121, 277–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Differentially expressed genes in tomato and Botrytis cinerea at the early stage of their interaction
FIGURE S2 The 20 most enriched KEGG pathways in tomato (a) and Botrytis cinerea (b) during their early stage of interaction
FIGURE S3 Phylogenetic relationship of Cgf1 proteins from the indicated organisms
FIGURE S4 Strategies of generation of BcCGF1 deletion and complemented strains
FIGURE S5 Functional validation of the up‐regulated Botrytis cinerea differentially expressed genes BcATG1, BcLAE1, and BcVEL1
FIGURE S6 Loss of BcCGF1 reduces the virulence of Botrytis cinerea
FIGURE S7 BcCGF1 mediates endogenous reactive oxygen species production in Botrytis cinerea
TABLE S1 Mapping results of RNA‐Seq reads
TABLE S2 The percentages of the clean reads mapped to tomato and Botrytis cinerea genomes
TABLE S3 Up‐regulated tomato genes in response to Botrytis cinerea attack (24 hr post‐infection)
TABLE S4 Down‐regulated tomato genes in response to Botrytis cinerea attack (24 hr post‐infection)
TABLE S5 Up‐regulated Botrytis cinerea genes at the early stage of host infection (24 hr post‐infection)
TABLE S6 Down‐regulated Botrytis cinerea genes at the early stage of infection (24 hr post‐infection)
TABLE S7 GO enrichment of the differentially expressed genes in tomato infected by Botrytis cinerea at 24 hr post‐infection
TABLE S8 GO enrichment of the differentially expressed genes in Botrytis cinerea at 24 hr post‐infection
TABLE S9 KEGG enrichment of the differentially expressed genes in tomato attacked by Botrytis cinerea at 24 hr post‐infection
TABLE S10 KEGG enrichment of the differentially expressed genes in Botrytis cinerea at 24 hr post‐infection
TABLE S11 ABC transporters encoded by the up‐regulated tomato genes in response to Botrytis cinerea infection (early stage) and their interacting proteins
TABLE S12 Proteins encoded by the up‐regulated Botrytis cinerea autophagy genes at the early stage of infection and their interacting proteins
TABLE S13 Potential secreted Botryis cinerea proteins at the early stage of host infection
TABLE S14 Fungal strains used in this study
TABLE S15 Primers used in this study


