Abstract
The gram‐positive bacterial species Clavibacter capsici causes necrosis and canker in pepper plants. Genomic and functional analyses of C. capsici type strain PF008 have shown that multiple virulence genes exist in its two plasmids. We aimed to identify the key determinants that control the virulence of C. capsici. Pepper leaves inoculated with 54 natural isolates exhibited significant variation in the necrosis. Six isolates showed very low virulence, but their population titres in plants were not significantly different from those of the highly virulent isolates. All six isolates lacked the pCM1Cc plasmid that carries chpG, which has been shown to be required for virulence and encodes a putative serine protease, but two of them, isolates 1,106 and 1,207, had the intact chpG elsewhere in the genome. Genomic analysis of these two isolates revealed that chpG was located in the pCM2Cc plasmid, and two highly homologous regions were present next to the chpG locus. The chpG expression in isolate 1,106 was not induced in plants. Introduction of chpG of the PF008 strain into the six low‐virulence isolates restored their virulence to that of PF008. Our findings indicate that there are at least three different variant groups of C. capsici and that the plasmid composition and the chpG gene are critical for determining the virulence level. Moreover, our findings also indicate that the virulence level of C. capsici does not directly correlate with bacterial titres in plants.
Keywords: Clavibacter capsici, pepper, plasmids, virulence factors, virulence mechanism
Natural isolates of the gram‐positive bacterium Clavibacter capsici show different levels of virulence in pepper due to different plasmid composition and the chpG gene, encoding a putative serine protease.

1. INTRODUCTION
Clavibacter species are gram‐positive plant pathogens that belong to phylum Actinobacteria (Eichenlaub and Gartemann, 2011). The genus Clavibacter includes six species: C. capsici causes necrosis, wilting, and bacterial canker in pepper; C. michiganensis causes wilting and bacterial canker in tomato; C. sepedonicus causes ring rot in potato; C. nebraskensis causes wilting and blight in maize; C. insidiosus causes wilting and stunting in alfalfa; and C. tessellarius causes bacterial mosaic in wheat (Holtsmark et al., 2008; Tambong, 2017; Li et al., 2018).
The genomic structures and plasmid composition of these Clavibacter species have been elucidated. C. michiganensis NCPPB382 carries one chromosome and two plasmids, pCM1Cm and pCM2Cm, and a 120‐kb pathogenicity island (PAI) in the chromosome has many important virulence genes required for effective colonization during infection in tomato plants (Gartemann et al., 2008). Similarly, C. capsici type strain PF008 carries one chromosome and two plasmids, pCMCc and pCM2Cc (Oh et al., 2016). However, C. capsici PF008 lacks PAI, but carries a part of the C. michiganensis PAI region in both plasmids (Hwang et al., 2018). At least five virulence genes in the PAI region of C. michiganensis have been found in pCM1Cc and pCM2Cc of C. capsici. pCMCc deletion by plasmid curing in C. capsici resulted in significantly reduced virulence compared with the virulence of the wild‐type strain (Hwang et al., 2018). The introduction of pCM1Cc‐borne chpG, encoding a putative serine protease, into the pCM1Cc‐cured strain of C. capsici PF008 restored its virulence in pepper, indicating that chpG is an important virulence gene in C. capsici for infection. In C. michiganensis, the celA gene, which encodes a cellulase and is located in pCM1Cm, is the most important virulence factor during infection in tomato plants (Jahr et al., 2000), and this gene ortholog is present in C. sepedonicus, but is missing in C. capsici and C. nebraskensis (Ahmad et al., 2015). The importance of celA for virulence was also confirmed by the overexpression of celA genes in C. capsici. C. capsici carrying celA of C. michiganensis or its ortholog of C. sepedonicus could cause wilting in tomato plants (Hwang et al., 2019).
Because the frequency of damage caused by Clavibacter species is reportedly high, the diversity of Clavibacter field isolates is being actively studied. C. michiganensis causes one of the most destructive tomato diseases in the world, including in the United States, therefore field isolates have been isolated and analysed from diverse geographic areas in the United States, Turkey, France, and Belgium (Jacques et al., 2012; Zaluga et al., 2013; Sen et al., 2018). Many studies have reported the natural variants of C. michiganensis in the United States, mainly in New York and California. Genomic profiles of the Californian isolates showed that celA in pCM1Cm and pelA, encoding a pectate lyase, in the chromosomes are essential for virulence, but there was one pathogenic isolate found in New York that lacked celA (Tancos et al., 2015; Thapa et al., 2017). Characterization and comparison of C. nebraskensis, including pathogenic and nonpathogenic isolates, isolated from maize fields in Iowa (United States) revealed that there was no direct correlation between sequence polymorphisms observed in putative virulence genes and the ability of disease development in maize (Ahmad et al., 2015). Natural isolates of C. capsici in Korea were reported in terms of morphological characteristics (Lee et al., 1999), but there is no evidence on the genetic diversity of C. capsici.
Plasmids in plant‐pathogenic bacteria are important virulence gene carriers that facilitate host infection. A tumour‐inducing plasmid in Agrobacterium tumefaciens, called the Ti plasmid, causes crown gall in many plants (Gordon and Christie, 2014). Pseudomonas syringae pv. phaseolicola possesses a plasmid‐borne PAI region for virulence in beans (Jackson et al., 1999). Clavibacter species are known to have virulence‐related plasmids (Eichenlaub and Gartemann, 2011). Artificial curing of virulence plasmid(s) in either C. michiganensis or C. capsici causes significant reduction of virulence (Jahr et al., 2000; Hwang et al., 2018). In C. michiganensis and C. capsici, the ability of a pathogenic isolate to be converted into a nonvirulent or low‐virulence variant depends on the presence/absence of plasmid(s), suggesting the evolutionary role of plasmid(s) in virulence in host plants (Vivian et al., 2001; Thapa et al., 2017).
In this study, we used 54 natural isolates of C. capsici from infected pepper plants and determined their virulence. Natural variations in the virulence of these isolates allowed us to find the major determinants required for their virulence. We found three variants of C. capsici natural isolates based on the composition of the two plasmids and the genomic position of chpG. We also found a direct correlation between the level of virulence and the composition of the two plasmids and the presence of chpG in C. capsici isolates.
2. RESULTS
2.1. Natural variation in the virulence of C. capsici isolates in pepper
Fifty‐four C. capsici natural isolates were isolated from pepper plants in Korea, and their identity was confirmed by PCR with a species‐specific primer set and DNA sequencing (Table S1) (Hwang et al., 2018). Their virulence was determined by checking their ability to cause necrosis in pepper leaves. C. capsici PF008, showing full virulence (severe necrosis) in the infected leaves, and its pCM1Cc‐lacking strain, PF008ΔpCM1Cc, showing reduced virulence (very mild necrosis), were the controls (Hwang et al., 2018). In this assay, the 54 isolates were categorized into three groups: a highly virulent group with PF008 and 12 isolates, a moderately virulent group with 36 isolates, and a low‐virulence group with 6 isolates and PF008ΔpCM1Cc strains (Figure 1a and Table S2). Six isolates categorized as low‐virulence isolates (1,065, 1,101, 1,106, 1,207, 1,905, and 2,093) showed less than 2.5 on the disease severity index, which was similar to that shown by PF008ΔpCM1Cc. These findings indicate that C. capsici isolates have natural variation in their ability to cause necrosis in pepper leaves.
FIGURE 1.
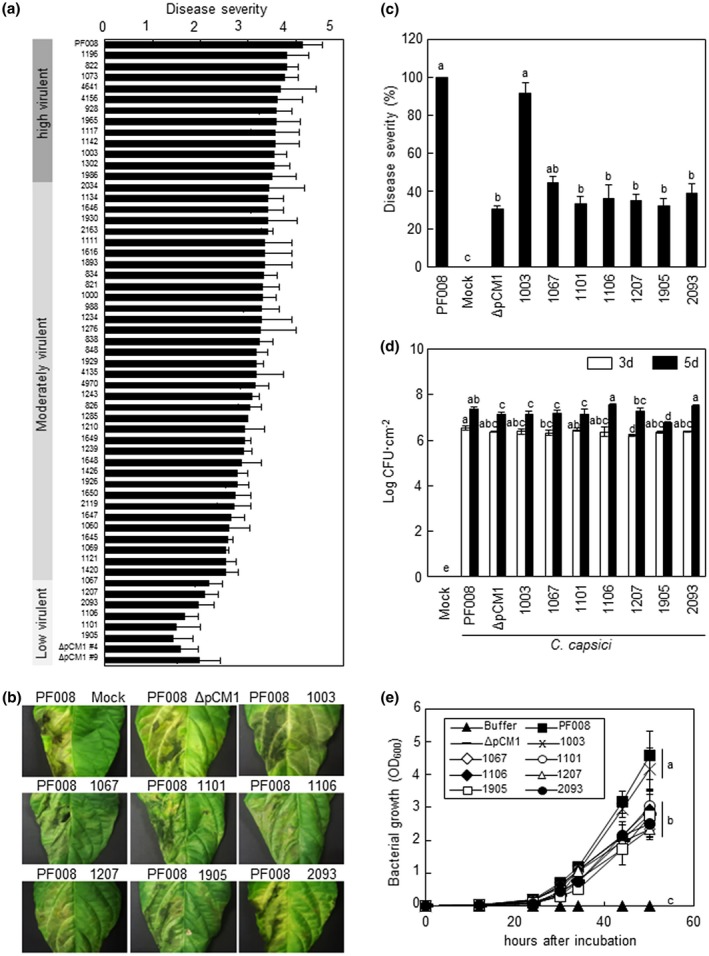
Virulence of the natural variants of Clavibacter capsici. (a) Disease severity of C. capsici isolates in comparison to the previously type strain PF008 and its pCM1Cc‐lacking derivative (ΔpCM1Cc). (b) Representatives of necrotic symptoms on the infected leaves with low‐virulence isolates. All inoculated leaves were photographed at 7 days after inoculation (dai). (c) Quantification of the disease severity in pepper leaves infected with low‐virulence isolates. Disease severity was expressed as a percentage compared to strain PF008‐infected leaves. C. capsici PF008 and natural isolate 1,003, and mock treatment with MgCl2 were used as positive and negative controls, respectively. Six leaves from three independent plants were used per treatment. Bars indicate SE. Different letters indicate statistically significant differences in disease severity, which was analysed by a nonparametric Kruskal–Wallis test with Dunnett's multiple comparisons (p < .05). (d) Bacterial growth of C. capsici isolates in pepper plants. The bacterial growth was measured at 3 and 5 dai. Bars indicate SD. All experiments were repeated three times and similar results were obtained. (e) Growth curves of low‐virulence C. capsici isolates and a pCM1Cc‐lacking strain (ΔpCM1Cc) on media. Optical density at 600 nm (OD600) was measured from 0 to 50 hr. The different letters in (d) and (e) indicate statistically significant differences as determined by Duncan's multiple range test (p < .05)
To further compare the virulence level of six low‐virulence isolates with that of C. capsici PF008, these isolates were inoculated next to each other in the same pepper leaves. In this assay, another highly virulent isolate, 1,003, was included. All six isolates showed less than 40% of the disease severity of the PF008 and 1,003 isolates, which means similar levels as in PF008ΔpCM1Cc (Figure 1b,c). When the pepper plant stems were inoculated with highly virulent PF008 and 1,003, typical bacterial canker developed at the inoculation sites. However, the cankers at the inoculation sites of the six low‐virulence isolates were significantly smaller than those of the highly virulent isolates (Figure S1). Regardless of the virulence level, all the isolates showed orange colonies (Figure S2). These findings indicate that low‐virulence isolates have poor ability to cause necrosis in leaves and cankers in the stems.
Consistent with the findings of previous studies (Hwang et al., 2018), pepper leaves inoculated with low‐virulence isolates contained similar titres and/or only slightly lower titres of bacteria than those inoculated with highly virulent isolates (Figure 1d). The bacterial growth of low‐virulence isolates in artificial media was examined. Interestingly, their growth was significantly delayed, and titres were lower than those of the highly virulent isolates, but their growth was similar to that of PF008ΔpCM1Cc (Figure 1e). These results imply that lower virulence is not strongly correlated with lower titre of bacterial cells in the pepper leaves.
2.2. Molecular characterization and geography of low‐virulence C. capsici
To examine whether the virulence level is correlated with the presence of two plasmids, pCM1Cc and pCM2Cc, all isolates were first confirmed by PCR using specific primer sets targeted to the plasmid‐backbone genes, rep and parA (Hwang et al., 2018). Out of the 54 isolates, 48 carried both plasmids, while the six low‐virulence isolates only carried pCM2Cc, like the PF008ΔpCM1Cc strain (Figure 2a and Table S2). Thus, there was a direct correlation between the presence of pCM1Cc and the level of virulence of C. capsici.
FIGURE 2.
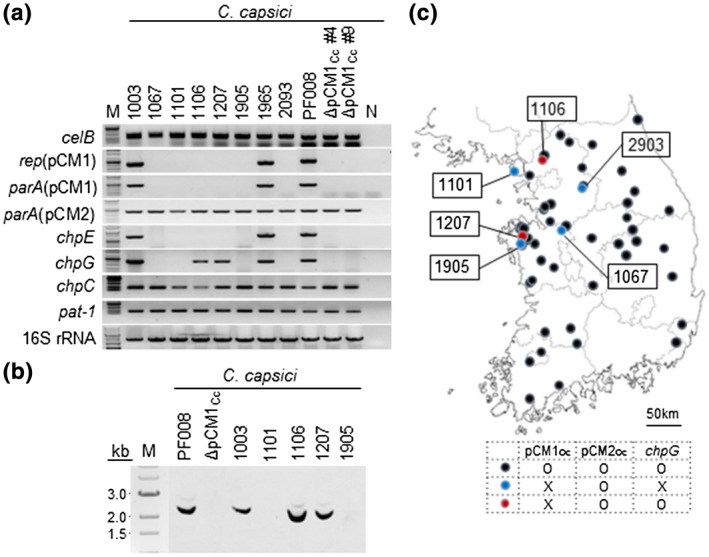
Determination of the absence of pCM1Cc and chpG in low‐virulence Clavibacter capsici variants. (a) PCR amplification of the plasmid‐backbone genes and putative virulence genes in two plasmids of low‐virulence C. capsici isolates. N, sterile water used as a negative control; M, 1 kb DNA size ladder. (b) Southern blot analysis of natural variants lacking pCM1Cc, but carrying chpG, with chpG as a probe in the digoxygenin detection system. Genomic DNAs extracted from each isolate were digested with the restriction enzyme XhoI. (c) Geographical location of C. capsici natural isolates collected in Korea. The map was obtained from the National Geographic Information Institute in Korea
The presence of the major virulence genes located in both plasmids was investigated. To this end, chpE and chpG as pCM1‐specific virulence genes, and chpC and pat‐1 as pCM2Cc‐specific virulence genes were selected. All four genes encode putative serine proteases. Additionally, a cellulase gene, celB, located in the chromosome was also investigated. All the isolates had celB, but lacked the other cellulase gene, celA, which was absent from C. capsici (Figure 2a, Table S2). chpC and pat‐ 1 in pCM2Cc were consistently detected in all the isolates, and chpE in pCM1 was only detected in isolates carrying pCM1Cc. However, chpG in pCM1Cc was detected not only in pCM1Cc‐carrying isolates PF008, 1,003, and 1985, but also in two out of the six pCM1Cc‐lacking isolates, 1,106 and 1,207 (Figure 2a and Table S2). The presence of chpG in the two pCM1Cc‐lacking isolates was confirmed again by Southern blot hybridization by using chpG from PF008 as a probe (Figure 2b). chpG from the two isolates 1,106 and 1,207 was cloned and sequenced. We found that chpG existed in these isolates in an intact form, and their open reading frames (ORFs) showed 100% identity at the amino acid level to ChpG of PF008 (Figure S3), although it is still unclear where chpG is located in these isolates. These results indicate that one of major virulence genes, chpG, is located in another genomic region in the two pCM1Cc‐lacking isolates and this relocation may affect the virulence.
The geographical location of the C. capsici isolates was mapped. This analysis showed that all the pCM1Cc‐lacking isolates were isolated from the north‐western regions of the Korean peninsula over 2 years and most of them originated from the same region where some isolates carrying both plasmids were isolated (Figure 2c and Table S2). Moreover, in one region, all three types were isolated. These results suggest the occurrence of more natural variants of C. capsici in certain regions of South Korea.
2.3. Genome analysis of C. capsici 1,101 and 1,106
To determine the overall genome structures and the location of chpG, we sequenced and analysed the whole genomes of two representative C. capsici isolates: 1,101 only carrying pCM2Cc and 1,106 with pCM2Cc and chpG. The genomic sequences of the two isolates were compared with those of C. capsici PF008 (GenBank accession nos. CP012573.1, CP012574.1, and CP012575.1), CFBP7576 (GenBank assembly ID GCA_002151115.1), and C. michiganensis LMG7333 (GenBank assembly ID GCA_002240575.1) as an outgroup. Genome sequences of 1,101 (GenBank accession nos. CP048049 and CP048050) and 1,106 (GenBank accession nos. CP048047 and CP048048) had comparable similarity to that of PF008, and 1,101 was found to be more similar to PF008 than 1,106 containing chpG (Figure 3a and Table S3). In the genome comparison of pCM2Cc between PF008 and newly sequenced isolates, the DNA sequence identity was 99.95% between pCM2Cc of PF008 and that of 1,101 with 100% of query coverage, whereas 1,106 had 99.89% DNA sequence identity with 99% of query coverage. Based on the comparison of the gene contents among these Clavibacter strains, 2,822 coding sequences (CDSs) were shared by PF008 and 1,101, and 64 and 6 CDSs were unique to PF008 and 1,101, respectively, while isolate 1,106 had 2,777 shared CDSs with 58 unique CDSs compared with PF008 (Figure 3b). Moreover, 2,771 CDSs were overlapping among three genomes of C. capsici, whereas 2,506 CDSs were overlapping with C. michiganensis type strain LMG7333 as the neighbouring strain, and 392 CDSs were specific to LMG7333. These analyses reveal that the number of shared and unique CDSs among the four strains reflects their phylogenetic relatedness.
FIGURE 3.
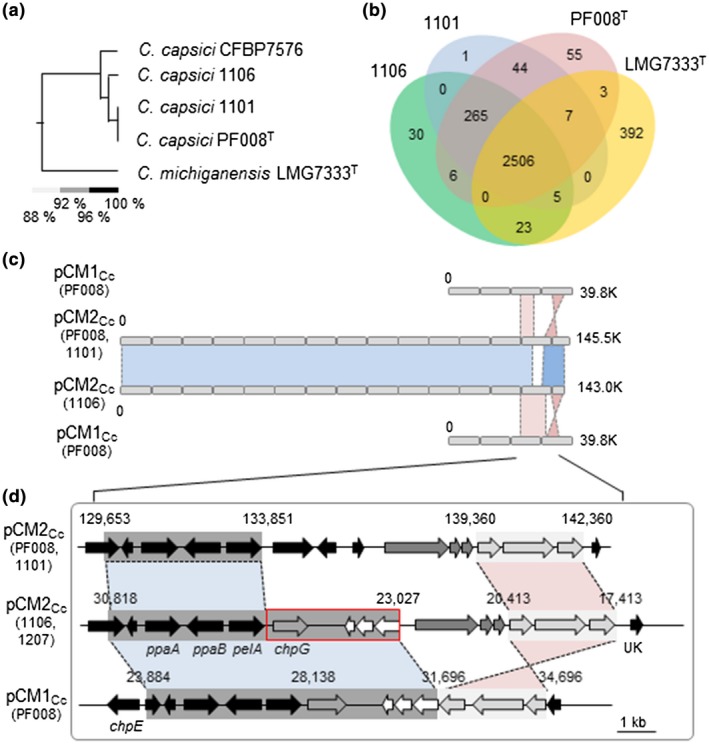
Genomic analysis of Clavibacter capsici isolates 1,106 and 1,101 and comparison with PF008. (a) Phylogenetic tree showing the relationship of four C. capsici isolates and one C. michiganensis isolate based on OrthoANI values. (b) Venn diagram representing the pan‐genomic landscape of three C. capsici isolates and one C. michiganensis isolate. The numbers in the Venn diagram indicate the number of strain‐specific coding sequences (CDSs) that were shared among the indicated genomes. (c) A comparison of the plasmids between C. capsici PF008 and natural isolates 1,106 and 1,101. Areas shaded in blue indicate highly conserved pCM2Cc regions between PF008 and 1,106. Areas shaded in red indicate highly conserved regions between pCM1Cc of PF008 and pCM2Cc of PF008 and 1,106. (d) Comparative studies of the virulence region containing chpG. Red boxes correspond to the conserved region between pCM1Cc of PF008 and pCM2Cc of 1,106. Dark and light grey boxes correspond to the conserved region between pCM1Cc of PF008 and pCM2Cc of natural isolates 1,106 and 1,207, defined as areas where the average identity is >99%. Detailed information for each open reading frame in this figure is given in Figure S4
We previously reported that in the pCM1Cc region carrying the five virulence genes of PF008, the 3.7‐kb region, including three putative virulence genes, was duplicated in pCM2Cc (Hwang et al., 2018). The pCM2Cc of 1,101 and 1,106 was almost identical to that of PF008, and there were two conserved regions in the pCM1Cc of PF008 with pCM2Cc of both 1,101 and 1,106. One was the 4.0‐kb region carrying the virulence genes ppaA, ppaB, and pelA in pCM2Cc (blue region in Figures 3c,d and S4), and the other was the 3.0‐kb region harbouring genes for putative recombinase, transposase, and ATP‐binding protein (pink region in Figure 3d). The pCM2Cc of 1,106 had a unique 3.8‐kb region that contained four ORFs and showed 99% identity with the conserved region in pCM1Cc of PF008 (Figures 3d and S4). One of them was chpG. Based on the DNA sequences of 1,106, the same 7.8‐kb region of 1,207 (GenBank accession nos. CP048045 and CP048046), which is another isolate carrying chpG (Figure 2), was cloned and sequenced. Our results show that this region of 1,207 is exactly matched with over 99.9% nucleotide identity to 1,106. chpG was also present in the same locus of pCM2Cc (Figure S5). These results confirm the presence of chpG in both 1,106 and 1,207 and indicate that its genomic location might play a significant role in virulence.
2.4. Expression profiles of the virulence genes in two plasmids of C. capsici
The expression of the virulence genes in pCM2Cc is affected by the lack of pCM1 (Hwang et al., 2018). Therefore, the expression of the virulence genes in pCM2Cc, which are chpC, pat‐1, ppaB2, and pelA1/A3, was investigated by quantitative reverse transcription PCR (RT‐qPCR) in pepper leaves after inoculation with the representatives of each group, PF008 carrying two plasmids, 1,101 carrying only pCM2Cc, 1,106 carrying only pCM2Cc and chpG, and PF008ΔpCM1Cc as a control. Moreover, the expression of two genes, chpE and chpG, which were shown to be located in the pCM1Cc of PF008, was also investigated. The expression of all the six genes was significantly increased after inoculation with PF008, and the expression of all the tested genes, except ppaB1/B2, peaked at 36 hr after inoculation (hai) and declined at 72 hai in the inoculated leaves (Figure 4). However, the expression of the genes in 1,101 and 1,106 was not increased as much as those of PF008 at either 36 or 72 hai, under the same conditions, like those in the PF008ΔpCM1Cc strain. Owing to the lack of chpE in both 1,101 and 1,106, its expression was not detected. In the case of chpG, no expression was detected in 1,101, but its expression was detected in 1,106, but this expression did not increase at any time point in the plants. When the expression of the six genes was investigated in rich medium at 48 hai, the level was almost the same as that in PF008 (Figure S6). These results suggest that the lack of pCM1Cc suppresses the ability to induce the expression of virulence genes in pCM2Cc, even chpG, in the pCM2Cc of 1,106, but not their basal expression levels.
FIGURE 4.
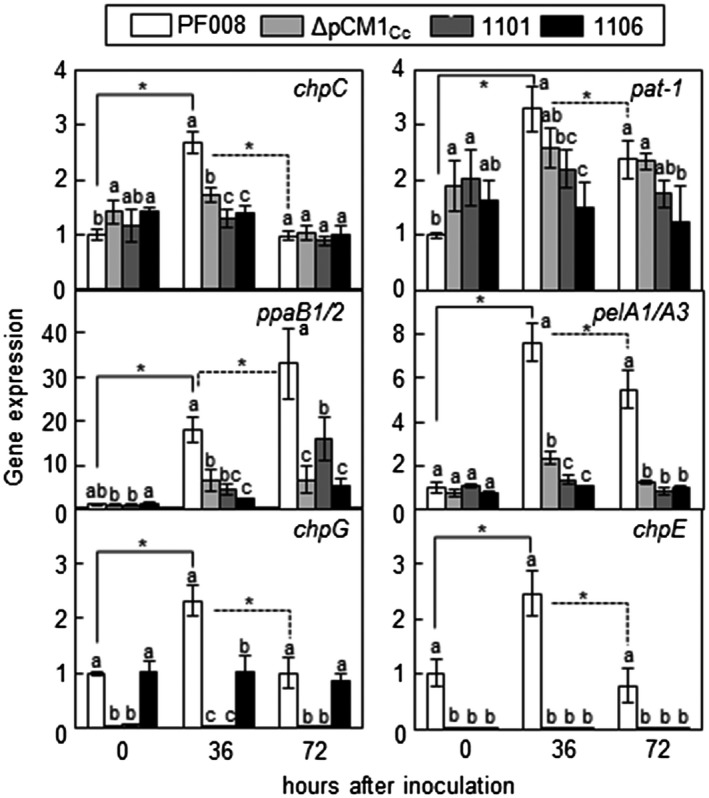
Expression profiles of the virulence genes in planta during infection with the representatives of three types of Clavibacter capsici isolates. Inoculated leaves were harvested at 0, 36, and 72 hr after inoculation (hai). Relative expression of the target gene was measured based on the expression of the housekeeping gene gyrA by quantitative reverse transcription PCR. Bars indicate SD. All experiments were repeated three times, and similar results were obtained. The different letters indicate statistically significant differences as determined by Duncan's multiple range test (p < .05). Asterisks in solid and dotted lines indicate statistically significant difference in gene expression between 0 and 36 hai and between 36 and 72 hai in each strain, respectively (p < .05)
2.5. Recovery of full virulence of low‐virulence C. capsici isolates by chpG overexpression
Previously, we showed that complementation of PF008ΔpCM1Cc strain with FLAG‐tagged chpG almost restored its full virulence (Hwang et al., 2018). Therefore, we transformed FLAG‐tagged chpG into the low‐virulence isolates and checked their ability to cause necrosis in pepper leaves. The virulence of all the six natural isolates was similar to that of PF008 during infection (Figure 5a,b). All six isolates that failed to cause severe canker in the stems were able to cause very strong cankers owing to chpG overexpression (Figure S7). The chpG‐overexpressing PF008ΔpCM1Cc, 1,101, and 1,106 strains grew similar to fully virulent PF008 (Figure S8). The chpG expression in PF008ΔpCM1Cc, 1,101, and 1,106 was determined by western blotting by using FLAG‐tag antibodies. The FLAG‐specific antibody reacted with a 29 kDa protein, and the protein level of all the strains was similar (Figure 5c). Moreover, the transcript levels of the endogenous and/or exogenous chpG gene were measured by RT‐qPCR in the inoculated pepper plants. The transcript levels of exogenous chpG were consistently similar to that of PF008 (Figure S9). These results further suggest that chpG is a critical virulence gene and its genomic location is critical for its expression and ability to contribute to the virulence of C. capsici in pepper.
FIGURE 5.
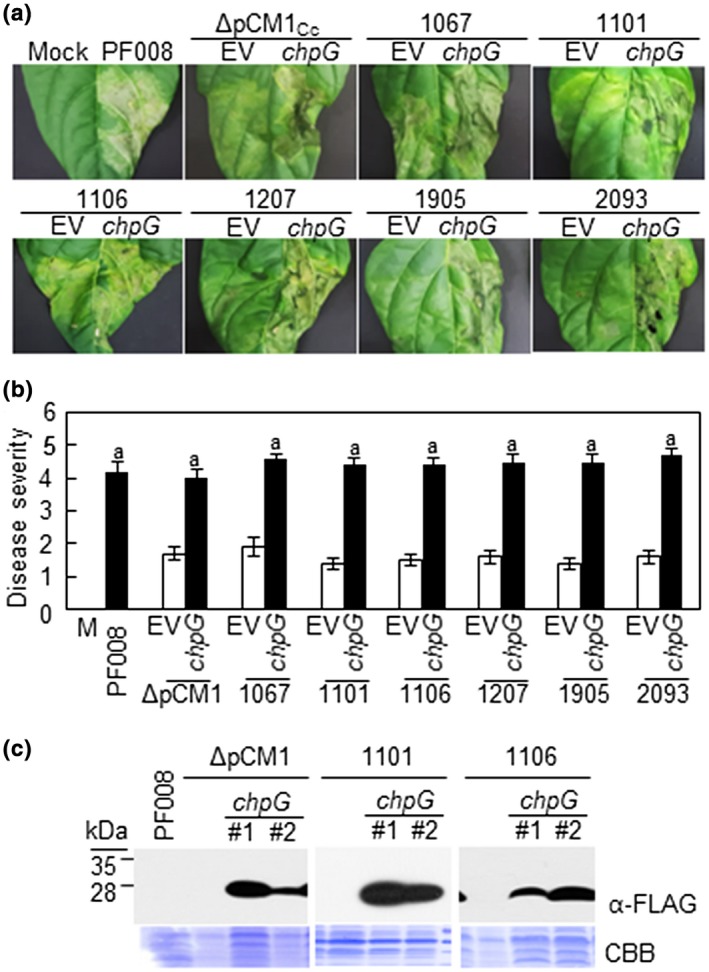
Effect of chpG expression in low‐virulence Clavibacter capsici isolates. (a) Observation of symptom development on the infected leaves with pCM1Cc‐lacking C. capsici isolates and strains complemented with chpG of PF008. All inoculated leaves were photographed at 7 days after inoculation. Mock, 10 mM MgCl2; EV, empty vector‐GFP. (b) Quantification of the disease severity in pepper leaves infected with each strain. EV, empty vector‐GFP. M, 10 mM MgCl2. The level of disease severity between PF008 and strains complemented with chpG of PF008 was analysed by a nonparametric Kruskal–Wallis test with Dunnett's multiple comparisons (p < .05), and the same letter indicates the similar level. (c). Western blot analysis of ChpG expression in ΔpCM1Cc and isolate 1,106, using FLAG‐tag antibody (α‐FLAG). CBB, Coomassie brilliant blue staining
2.6. Minor effects of chpE on the virulence of C. capsici 1,101 and 1,106
Another virulence gene, chpE, in the pCM1Cc of PF008, but absent from 1,101 and 1,106, was previously shown to contribute to the virulence of C. capsici (Hwang et al., 2018). We examined the effect of chpE on the virulence of 1,101 and 1,106 in pepper and compared its effects with that of chpG. Gene constructs carrying only chpE, only chpG, or both, from PF008 were generated and transformed into 1,101. Their virulence levels were evaluated at 7 days after inoculation (dai) in pepper leaves, and the wild‐type PF008 and PF008ΔpCM1Cc were used as positive and negative controls. The isolate 1,101 itself or that carrying green fluorescent protein (GFP) showed only 40% of the disease severity caused by the wild‐type PF008, which is similar to that of PF008ΔpCM1Cc (Figure 6a,b). Isolate 1,101 carrying chpG or chpE/chpG together rapidly caused necrosis, like the wild‐type PF008, while that carrying only chpE showed an increase of approximately 20% in the necrosis (Figure 6a,b). The bacterial titres of all the transformants were measured at 3 and 5 dai, and their titres were not much different from that of the wild‐type PF008 (Figure S10a), which was consistent with that reported previously (Hwang et al., 2018).
FIGURE 6.
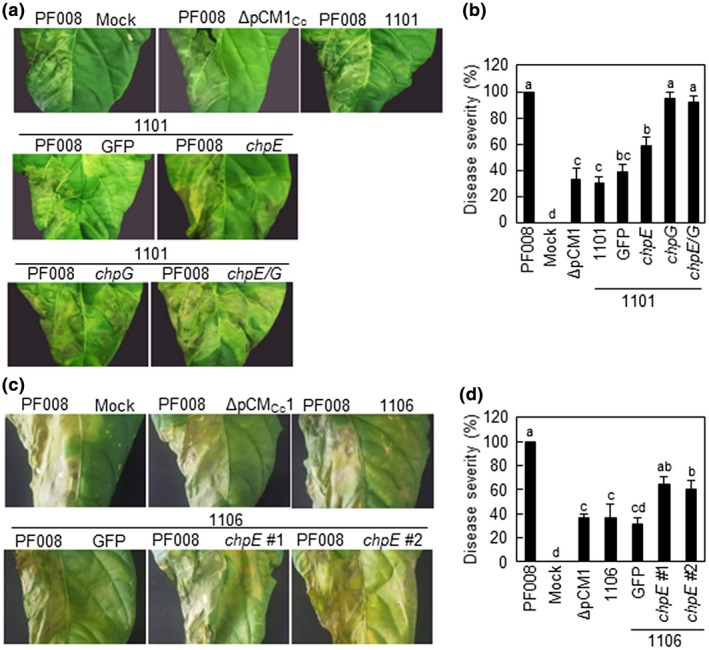
Virulence of pCM1Cc‐lacking Clavibacter capsici isolates 1,101, 1,106, and their complemented strains with chpE or chpG from PF008 on pepper leaves. (a) Necrotic disease symptoms on the infected leaves after infection with C. capsici isolate 1,101 and its complemented strains. All inoculated leaves were photographed at 7 days after inoculation (dai). (b) Disease severity at 7 dai. (c) Necrotic disease symptoms on the infected leaves after infection with C. capsici isolate 1,106 and its complemented strains. All inoculated leaves were photographed at 7 dai. (d) Disease severity at 7 dai. C. capsici type strain PF008 was used as a positive control, and mock treatment with 10 mM MgCl2 and green fluorescent protein (GFP) were used as negative controls. Bars indicate SE. Different letters in (b) and (d) indicate statistically significant differences in disease severity analysed by a nonparametric Kruskal–Wallis test with Dunnett's multiple comparisons (p < .05). All experiments were repeated three times and similar results were obtained
Next 1,106 carrying chpE of PF008 was generated, and its virulence was examined. The introduction of chpE increased approximately 20% of the disease severity (Figure 6c,d), like 1,101 carrying chpE. The bacterial titres of all the transformants were measured at 3 and 5 dai, and their titres were not much different from that of the wild‐type PF008 (Figure S10b), similar to the 1,101 transformants. Overall, these results indicate that chpE overexpression only partially contributes to the virulence of isolates 1,101 or 1,106.
3. DISCUSSION
We found natural variations in the virulence of 54 C. capsici isolates. Moreover, there was a direct correlation between the virulence level and the composition of the two plasmids along with the presence of chpG. Our previous reports showed that C. capsici type strain PF008 carries two plasmids, pCM1Cc and pCM2Cc, and both plasmids carry virulence genes, which are mostly present in the PAI region of C. michiganensis (Bae et al., 2015; Hwang et al., 2018). Moreover, the artificial curing of pCM1Cc dramatically reduced virulence. However, the artificial curing of another plasmid, pCM2Cc, failed. Based on these findings, we hypothesized that pCM1Cc is dispensable for bacterial survival although it contains at least five important virulence genes, including chpG. Our hypothesis was confirmed because six isolates naturally lacking pCM1Cc were found, but all isolates carried pCM2Cc. Similarly, natural variation in the plasmid composition was also found in C. michiganensis in two different studies (Tancos et al., 2015; Thapa et al., 2017). All C. michiganensis isolates in these cases carried pCM1‐like plasmids, while some isolates lacked pCM2Cc‐like plasmids. These findings imply that pCM1Cc may be essential, but pCM2Cc is dispensable in C. michiganensis, which is completely opposite to the findings in C. capsici shown in this study. Our study shows that essential genes for survival are located in different plasmids in both pathogens.
chpG encodes a putative serine protease, is present in the pCM1Cc of PF008, and is an important virulence gene in C. capsici (Hwang et al., 2018). Two natural isolates, 1,106 and 1,207, lacking pCM1Cc, carried the intact chpG, and genomic analysis revealed that chpG is located in pCM2Cc. Consistent with the previous finding that the expression of putative virulence genes in pCM2Cc was very low in the pCM1Cc‐cured strain (Hwang et al., 2018), the transcript level of chpG in 1,106 was not increased during infection. In addition to the lack of pCM1Cc‐carrying virulence genes, the lack of increase in the chpG expression in 1,106 can explain why this isolate showed very low virulence. Ectopic expression of chpG from PF008 as a plasmid form in all six low‐virulence isolates, including 1,106, restored their virulence to as much as that of isolates carrying both plasmids. These data also show that chpG expression is critical for complete virulence. The chpG homolog is present in the chromosomal PAI region of C. michiganensis, but a previous study showed that a chpG single mutant produced by transposon insertion in this bacterium still showed full virulence (Stork et al., 2008). These findings imply that the function of chpG in the virulence of Clavibacter species may be different. Chromosomal chpG of C. michiganensis and C. sepedonicus has previously been shown to induce a hypersensitive response (HR) in Nicotiana species (Gartemann et al., 2008; Lu et al., 2015). The above findings are yet to be elucidated in greater detail to investigate if ChpG has protease activity and, if so, whether this activity is important for virulence or HR induction in plants.
Comparative genome analysis revealed almost identical DNA regions next to chpG in the pCM1Cc of PF008 and pCM2Cc of 1,106. One of them is the 3.0‐kb region containing genes encoding an ATP‐binding protein, transposase, and recombinase family protein. The genome of PF008 contains putative insertion sequence (IS) elements and two intact transposases, which appear to be associated with extensive genetic rearrangements such as relocation and/or integration of gene(s) or a specific region. A transposase in the 3.0 kb region appears to be similar to that present in the IS1122 transposon discovered in the pCI1 plasmid of C. insidiosus strain R1‐1 (Lu et al., 2018). The presence of IS elements in the plasmid of C. capsici is probably related to the duplication or genetic rearrangement of certain DNA regions. Combined with this feature, the homologous recombination in the two identical DNA regions between pCM1Cc and pCM2Cc before the loss of pCM1Cc in 1,106 may serve as a mechanism for relocation of the chpG locus from pCM1Cc to pCM2Cc. This could be the reservoir of the key virulence gene, chpG, for C. capsici population in the field.
We showed that there are three variants of C. capsici natural isolates in terms of virulence, but not in terms of bacterial growth, and variation in the virulence was determined by the presence of pCM1Cc and the genomic position of chpG. However, all the variants grew similarly in the plants. There is no direct correlation between the virulence of Clavibacter and their ability to grow in the plants, in contrast with the general phenomenon that high virulence is associated with high bacterial titre (Thapa et al., 2017; Hwang et al., 2018). In addition, highly virulent isolates carrying both plasmids were present all over Korea, while the two types of low‐virulence isolates were present only in a limited area. Based on these findings, we propose the relationships among the three variants in terms of their virulence and lifestyles. The highly virulent strain of C. capsici may have evolved by the acquisition of the two plasmids. Owing to uncharacterized selection pressure, this strain may lose pCM1Cc to change its lifestyle for longer survival in host plants by not killing them, like endophytic bacteria. Simultaneously, to preserve the key virulence gene in the population, the genetic rearrangement through unknown mechanism(s) like homologous recombination may occur for relocating chpG into pCM2Cc, which is needed for bacterial growth and virulence, before the loss of pCM1Cc. Many studies have shown the beneficial effects of endophytic bacteria in their hosts, such as plant growth promotion or protection (Rosenblueth and Martinez‐Romero, 2006; Ryan et al., 2008). However, despite the biodiversity of the Clavibacter endophytes, there is little research on their positive effects, except that Clavibacter sp. strain Enf12 confers plant growth and chilling tolerance in Chorispora bungeana (Ding et al., 2011). The possible conversion of the lifestyles of plant‐pathogenic gram‐positive bacteria in nature by acquisition or loss of plasmids can help us to understand their virulence mechanisms.
4. EXPERIMENTAL PROCEDURES
4.1. Bacterial strains
The 54 C. capsici natural isolates were isolated from infected pepper fields in diverse regions of South Korea (Table S1). The C. capsici type strain PF008 was used as the control for virulence assays. For inoculation, all isolates were incubated in King's B (KB) medium (20 g protease peptone no. 3, 1.5 g K2HPO4, 6 ml of 1 M MgSO4, 16 ml of 50% glycerol per litre) at 26 °C for 24–48 hr.
4.2. Plant growth conditions
Pepper plants (C. annuum 'Nockwang') were grown in sterile commercial soil (Baroker, Seoul Bio Co., Ltd) in a growth chamber at 26 °C with a 14 hr:10 hr light:dark photoperiod at a light intensity of 80 ± 10 μmol⋅m−2⋅s−1 and a relative humidity of 70%.
4.3. PCR‐based confirmation of plasmids and virulence genes
The presence of plasmids and known virulence genes was confirmed in all the natural isolates by PCR with specific primer sets that have been previously described (Hwang et al., 2018). To verify the presence or absence of the two plasmids, plasmid‐backbone genes, including genes encoding replication protein or partitioning protein, were amplified from all the collected isolates. In addition, virulence genes celA, celB, chpE, chpG, chpC, and pat‐1, which are located in the chromosome or the two plasmids of C. capsici and C. michiganensis, were amplified by PCR.
4.4. Southern blot hybridization
Genomic DNA was isolated from the bacterial cells by using the HiGene Genomic DNA prep kit (BIOFACT), according to the manufacturer's instructions. Purified genomic DNA was digested with XhoI at 37 °C and size‐fractionated in 0.8% agarose gel. DNA was subsequently transferred to the nylon membrane by capillary blotting. As a probe, full‐length chpG gene was amplified by PCR and labelled with digoxygenin (DIG)‐dUTP. Hybridization with a biotin‐labelled probe and detection was performed according to the manufacturer's instructions for the DIG Luminescent Detection Kit (Roche).
4.5. Virulence assay in pepper
The natural isolates and mutant strains were grown in KB medium for 2 days. The plants were then inoculated with the bacterial suspension by either leaf infiltration or stem inoculation. For leaf infiltration, six‐ to eight‐leaf stage pepper plants were infiltrated using a syringe with 10 mM MgCl2 (mock) or the bacterial suspension (OD600 = 0.1). Pepper plants were infected according to previously reported methods (Hwang et al., 2018). In each assay, at least three leaves were used and the experiment was repeated four times. The disease severity of the inoculated pepper plants was recorded according to their visual appearance at 7 dai using an index ranging from 0 to 5: 0, no visible symptoms; 1, <5% leaf area affected; 2, 6%–25% leaf area affected; 3, 26%–50% leaf area affected; 4, 51%–75% leaf area affected; 5, >75% leaf area affected. If the average disease index was below 2.5, the tested C. capsici isolates were considered to be less virulent. For stem inoculation, 4‐week‐old pepper plants were infiltrated by stabbing a pipette tip containing 10 μl of bacterial suspension (OD600 = 0.1) into the stem and placed in a growth room at 26 °C.
To compare the virulence level, C. capsici PF008 was inoculated into the right half of the leaves, and each isolate was inoculated into the left half of the same leaves. At least eight leaves from three different plants were used. Symptom development was monitored visually at 7 dai by using the disease index and was recorded as a percentage of disease severity in left half‐leaves infected with natural isolates divided by those of the fully virulent strain PF008 inoculated into right half‐leaves.
4.6. C. capsici transformation
The gene constructs with chpE or chpG in a pK2‐22 vector, which was previously used for gene expression in C. michiganensis and C. capsici (Chalupowicz et al., 2012; Hwang et al., 2018), were transformed into the selected natural isolates. Briefly, the intact chpE and chpG with the native promoter and the terminator, flanked by SpeI/HindIII restriction enzyme sites, were amplified by PCR from C. capsici PF008 and cloned into a pK2‐22 vector via a conventional cloning method and directional cloning of DNA fragments using the EZ‐Fusion Cloning kit (Enzynomics), respectively. Transformation was performed as previously described (Meletzus and Eichenlaub, 1991). Fresh bacterial cells of the selected isolates of C. capsici were grown in KB broth at 26 °C for 24 hr and harvested by centrifugation, and the pelleted cells were resuspended in a protoplasting buffer containing 40 μg/ml of lysozyme. After incubation, the competent cells were prepared by centrifugation followed by serial washing. Electroporation was performed at a field strength of 2.5 kV/cm with up to 5 μg plasmid DNA. Transformed cells were cultured on KB medium with a neomycin (50 μg/ml) as a selectable marker.
4.7. Bacterial growth in vitro and in vivo
To measure the growth of C. capsici in vitro, each isolate was standardized to OD600 = 0.001. The growth of the cultures was measured over a time course in KB broth at 26 °C at 150 rpm. Growth was monitored by determining the OD600 using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc.).
To determine the bacterial growth inside pepper plants, leaf discs from the inoculated leaves of three different plants were collected at 3 and 5 dai, rinsed with 70% (vol/vol) ethanol for surface sterilization, and then washed with distilled water prior to homogenization. Homogenate samples were serially diluted and plated onto KB agar for measuring the bacterial titres. The numbers of colony‐forming units (cfu) were determined after incubation for 48 hr at 26 °C.
4.8. Whole‐genome sequencing
Whole‐genome sequencing of two C. capsici natural isolates, 1,101 and 1,106, was performed at ChunLab Inc. (Seoul, Korea) using the PacBio RSII method, and their sequencing depths of coverage were 125× and 296×, respectively. Sequencing data were assembled with PacBio SMRT Analysis v. 2.3.0 (Pacific Biosciences), and two contigs from each were generated. Gene prediction and functional annotation of the analysed genomes were performed using Prodigal v. 2.6.2 (Hyatt et al., 2010). To generate a phylogenetic tree and Venn diagram for the numbers of orthologous CDSs, comparative genome analysis was performed using the Comparative Genomics (CG) pipeline of BIOiPLUG Apps (https://www.bioiplug.com/apps, ChunLab Inc.). The OrthoANI (Orthologous Average Nucleotide Identity) algorithm was used to determine overall similarity values between natural isolates and reference genomes downloaded from the NCBI genome database (Lee et al., 2016). Based on OrthoANI analysis of C. capsici isolates and C. michiganensis LMG7333, the phylogenetic tree was constructed using the OrthoANI tool.
4.9. Gene expression measurement
To determine the expression level of the C. capsici virulence genes during pathogen infection, six‐ to eight‐leaf pepper plants were inoculated with the bacterial suspension (OD600 = 0.1). Samples were collected at 0, 36, and 72 hr post‐inoculation (hpi) and homogenized in near‐freezing conditions using liquid nitrogen. Total RNA of the samples was extracted using RNAiso Plus (Takara Bio Inc.), according to the manufacturer's instructions. The RNA samples were reverse transcribed using the ReverTra Ace RT‐qPCR Master Mix (Toyobo Co., Ltd), and then amplified using THUNDERBIRD SYBR qPCR Mix (Toyobo Co., Ltd). Gene‐specific primer sets for each virulence gene were used (Hwang et al., 2018). The expression levels of the target genes relative to the housekeeping gene gyrA were analysed by the comparative C t (2−ΔΔ C t) method. All experiments were repeated three times.
4.10. Western blotting
Total proteins were extracted from the bacterial strains containing C‐terminal FLAG‐tagged constructs. Bacteria were cultivated in half‐strength KB medium containing 0.4% carboxymethyl cellulose (Sigma‐Aldrich) at 26 °C for 2 days. The supernatant collected by centrifugation was precipitated with 10% wt/vol of chilled trichloroacetic acid/acetone at −20 °C for 2 hr. After centrifugation, protein precipitates were washed with ice‐cold acetone, and protein pellets were dried at room temperature. Proteins were resuspended in 0.1 ml of sodium dodecyl sulphate (SDS) sample buffer (100 ml of 1.5 M Tris [pH 6.8], 60 ml of 20% SDS, 300 ml glycerol, 150 ml β‐mercaptoethanol, 18 mg bromophenol blue per litre). Protein samples were fractionated on a 12% SDS‐polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (Millipore). The membrane was incubated with horseradish peroxidase‐conjugated α‐FLAG antibody (A8592; Sigma‐Aldrich) at a dilution of 1:2,000 for 1 hr at room temperature or overnight at 4 °C and then developed using the Enhanced Chemiluminescence Plus western blotting detection reagent (GE Healthcare).
4.11. Statistical analysis
Disease severity data were statistically analysed by the nonparametric Kruskal–Wallis test with Dunnett's multiple comparisons (p < .05). To analyse other results, Duncan's multiple range tests were performed for comparisons between independent groups (p < .05). All experiments were repeated at least three times.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by the grant from Cooperative Research Program for Agriculture Science and Technology Development (project no. PJ013285012019), Rural Development Administration, Republic of Korea and also the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019R1A2C2004568 and 2018R1A5A1023599, SRC).
Hwang IS, Lee HM, Oh E‐J, Lee S, Heu S, Oh C‐S. Plasmid composition and the chpG gene determine the virulence level of Clavibacter capsici natural isolates in pepper. Molecular Plant Pathology. 2020;21:808–819. 10.1111/mpp.12932
Funding information
This work was supported by the grant from Cooperative Research Program for Agriculture Science and Technology Development (project no. PJ013285012019), Rural Development Administration, Republic of Korea and also the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019R1A2C2004568 and 2018R1A5A1023599, SRC).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ahmad, A. , Mbofung, G.Y. , Acharya, J. , Schmidt, C.L. and Robertson, A.E. (2015) Characterization and comparison of Clavibacter michiganensis subsp. nebraskensis strains recovered from epiphytic and symptomatic infections of maize in Iowa. PLoS ONE, 10, e0143553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, C. , Oh, E.J. , Lee, H.B. , Kim, B.Y. and Oh, C.S. (2015) Complete genome sequence of the cellulase‐producing bacterium Clavibacter michiganensis PF008. Journal of Biotechnology, 214, 103–104. [DOI] [PubMed] [Google Scholar]
- Chalupowicz, L. , Zellermann, E.M. , Fluegel, M. , Dror, O. , Eichenlaub, R. , Gartemann, K.H. et al (2012) Colonization and movement of GFP‐labeled Clavibacter michiganensis subsp. michiganensis during tomato infection. Phytopathology, 102, 23–31. [DOI] [PubMed] [Google Scholar]
- Ding, S. , Huang, C.L. , Sheng, H.M. , Song, C.L. , Li, Y.B. and An, L.Z. (2011) Effect of inoculation with the endophyte Clavibacter sp. strain Enf12 on chilling tolerance in Chorispora bungeana . Physiologia Plantarum, 141, 141–151. [DOI] [PubMed] [Google Scholar]
- Eichenlaub, R. and Gartemann, K.H. (2011) The Clavibacter michiganensis subspecies: molecular investigation of gram‐positive bacterial plant pathogens. Annual Review of Phytopathology, 49, 445–464. [DOI] [PubMed] [Google Scholar]
- Gartemann, K.H. , Abt, B. , Bekel, T. , Burger, A. , Engemann, J. , Flugel, M. et al (2008) The genome sequence of the tomato‐pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382 reveals a large island involved in pathogenicity. Journal of Bacteriology, 190, 2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, J.E. and Christie, P.J. (2014) The agrobacterium Ti plasmids. Microbiology Spectrum, 2. doi: 10.1128/microbiolspec.PLAS-0010-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtsmark, I. , Takle, G.W. and Brurberg, M.B. (2008) Expression of putative virulence factors in the potato pathogen Clavibacter michiganensis subsp. sepedonicus during infection. Archives of Microbiology, 189, 131–139. [DOI] [PubMed] [Google Scholar]
- Hwang, I.S. , Oh, E.J. , Kim, D. and Oh, C.S. (2018) Multiple plasmid‐borne virulence genes of Clavibacter michiganensis ssp. capsici critical for disease development in pepper. New Phytologist, 217, 1177–1189. [DOI] [PubMed] [Google Scholar]
- Hwang, I.S. , Oh, E.J. , Lee, H.B. and Oh, C.S. (2019) Functional characterization of two cellulase genes in the gram‐positive pathogenic bacterium Clavibacter michiganensis for wilting in tomato. Molecular Plant‐Microbe Interactions, 32, 491–501. [DOI] [PubMed] [Google Scholar]
- Hyatt, D. , Chen, G.L. , Locascio, P.F. , Land, M.L. , Larimer, F.W. and Hauser, L.J. (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics, 11, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, R.W. , Athanassopoulos, E. , Tsiamis, G. , Mansfield, J.W. , Sesma, A. , Arnold, D.L. et al (1999) Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola . Proceedings of the National Academy of Sciences of the United States of America, 96, 10875–10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques, M.A. , Durand, K. , Orgeur, G. , Balidas, S. , Fricot, C. , Bonneau, S. et al (2012) Phylogenetic analysis and polyphasic characterization of Clavibacter michiganensis strains isolated from tomato seeds reveal that nonpathogenic strains are distinct from C. michiganensis subsp. michiganensis . Applied and Environmental Microbiology, 78, 8388–8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr, H. , Dreier, J. , Meletzus, D. , Bahro, R. and Eichenlaub, R. (2000) The endo‐β‐1,4‐glucanase CelA of Clavibacter michiganensis subsp. michiganensis is a pathogenicity determinant required for induction of bacterial wilt of tomato. Molecular Plant‐Microbe Interactions, 13, 703–714. [DOI] [PubMed] [Google Scholar]
- Lee, I. , Ouk Kim, Y. , Park, S.C. and Chun, J. (2016) OrthoANI: an improved algorithm and software for calculating average nucleotide identity. International Journal of Systematic and Evolutionary Microbiology, 66, 1100–1103. [DOI] [PubMed] [Google Scholar]
- Lee, S.‐D. , Yoon, C.‐M. , Lee, Y.‐K. , Choi, Y.‐H. and Cho, Y.‐S. (1999) Occurrence and distribution of bacterial canker of red pepper caused by Clavibacter michiganensis subsp. michignensis . Plant Disease and Agriculture, 5, 105–110. [Google Scholar]
- Li, X. , Tambong, J. , Yuan, K.X. , Chen, W. , Xu, H. , Levesque, C.A. et al (2018) Re‐classification of Clavibacter michiganensis subspecies on the basis of whole‐genome and multi‐locus sequence analyses. International Journal of Systematic and Evolutionary Microbiology, 68, 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Hatsugai, N. , Katagiri, F. , Ishimaru, C.A. and Glazebrook, J. (2015) Putative serine protease effectors of Clavibacter michiganensis induce a hypersensitive response in the apoplast of Nicotiana species. Molecular Plant‐Microbe Interactions, 28, 1216–1226. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Ishimaru, C.A. , Glazebrook, J. and Samac, D.A. (2018) Comparative genomic analyses of Clavibacter michiganensis subsp. insidiosus and pathogenicity on Medicago truncatula . Phytopathology, 108, 172–185. [DOI] [PubMed] [Google Scholar]
- Meletzus, D. and Eichenlaub, R. (1991) Transformation of the phytopathogenic bacterium Clavibacter michiganense subsp. michiganense by electroporation and development of a cloning vector. Journal of Bacteriology, 173, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, E.J. , Bae, C. , Lee, H.B. , Hwang, I.S. , Lee, H.I. , Yea, M.C. et al (2016) Clavibacter michiganensis subsp. capsici subsp. nov., causing bacterial canker disease in pepper. International Journal of Systematic and Evolutionary Microbiology, 66, 4065–4070. [DOI] [PubMed] [Google Scholar]
- Rosenblueth, M. and Martinez‐Romero, E. (2006) Bacterial endophytes and their interactions with hosts. Molecular Plant‐Microbe Interactions, 19, 827–837. [DOI] [PubMed] [Google Scholar]
- Ryan, R.P. , Germaine, K. , Franks, A. , Ryan, D.J. and Dowling, D.N. (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiology Letters, 278, 1–9. [DOI] [PubMed] [Google Scholar]
- Sen, Y. , Aysan, Y. , Mirik, M. , Ozdemir, D. , Meijer‐Dekens, F. , van der Wolf, J.M. et al (2018) Genetic characterization of Clavibacter michiganensis subsp. michiganensis population in Turkey. Plant Disease, 102, 300–308. [DOI] [PubMed] [Google Scholar]
- Stork, I. , Gartemann, K.H. , Burger, A. and Eichenlaub, R. (2008) A family of serine proteases of Clavibacter michiganensis subsp. michiganensis: chpC plays a role in colonization of the host plant tomato. Molecular Plant Pathology, 9, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambong, J.T. (2017) Comparative genomics of Clavibacter michiganensis subspecies, pathogens of important agricultural crops. PLoS ONE, 12, e0172295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancos, M.A. , Lange, H.W. and Smart, C.D. (2015) Characterizing the genetic diversity of the Clavibacter michiganensis subsp. michiganensis population in New York. Phytopathology, 105, 169–179. [DOI] [PubMed] [Google Scholar]
- Thapa, S.P. , Pattathil, S. , Hahn, M.G. , Jacques, M.A. , Gilbertson, R.L. and Coaker, G. (2017) Genomic analysis of Clavibacter michiganensis reveals insight into virulence strategies and genetic diversity of a gram‐positive bacterial pathogen. Molecular Plant‐Microbe Interactions, 30, 786–802. [DOI] [PubMed] [Google Scholar]
- Vivian, A. , Murillo, J. and Jackson, R.W. (2001) The roles of plasmids in phytopathogenic bacteria: mobile arsenals? Microbiology, 147, 763–780. [DOI] [PubMed] [Google Scholar]
- Zaluga, J. , Stragier, P. , Van Vaerenbergh, J. , Maes, M. and De Vos, P. (2013) Multilocus variable‐number‐tandem‐repeats analysis (MLVA) distinguishes a clonal complex of Clavibacter michiganensis subsp. michiganensis strains isolated from recent outbreaks of bacterial wilt and canker in Belgium. BMC Microbiology, 13, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


