Abstract
Background/Aims
Teicoplanin can be used as an alternative to vancomycin when treating beta-lactam-resistant gram-positive bacterial infections. Both vancomycin and teicoplanin are associated with relatively high rates of adverse drug reactions (ADRs), including hypersensitivity reactions. There is limited data on teicoplanin-vancomycin cross-reactivity. This study examined the incidence of teicoplanin ADRs and risk factors for cross-reactivity between vancomycin and teicoplanin.
Methods
We analyzed the incidence of teicoplanin ADRs in a retrospective study of 304 newly teicoplanin-exposed, immunocompetent, hospitalized patients at a single Korean Medical Center between January 1, 2006 and December 31, 2015.
Results
Among 304 patients, 238 (78.3%) experienced vancomycin-associated ADRs prior to their teicoplanin exposure and 58 (19.1%) experienced teicoplanin- associated ADRs, which were mostly hypersensitivity reactions without acute kidney injury. The incidence of teicoplanin ADRs was higher in patients who previously experienced vancomycin-related ADRs (23.1% vs. 5.3%, p < 0.001). History of drug allergy was a statistically significant risk factor of teicoplanin ADRs. The incidence of teicoplanin ADRs significantly increased in patients with multiple organ involvement in vancomycin hypersensitivity reactions.
Conclusions
Teicoplanin should be administered with caution and clinicians must consider the risk factors of cross-reaction when prescribing teicoplanin to individuals with a history of vancomycin hypersensitivity.
Keywords: Cross reaction, Hypersensitivity, Teicoplanin, Vancomycin
INTRODUCTION
Antibiotic hypersensitivity is associated with high morbidity, mortality, and medical costs [1] and limits the use of appropriate antibacterial agents for bacterial infections. Vancomycin has reliable antibacterial activity and is recommended as the first-line treatment for infections caused by methicillin-resistant Staphylococcus aureus, methicillin-resistant coagulase-negative Staphylococcus, and ampicillin-resistant Enterococcus with extensive evidence for efficacy [2,3]. The use of vancomycin is increasing because of the increase in the development of resistant bacterial strains [4]. However, this antibiotic may elicit various adverse drug reactions (ADRs) including hypersensitivity reactions. The incidence of vancomycin ADRs is 13.4% to 47.0% [5-7]. Teicoplanin is also a glycopeptide antibiotic that can be used as an alternative in cases of vancomycin ADR or hypersensitivity [6]. It is widely used in Europe, Asia, and South America. The incidence of teicoplanin ADRs is reported to be 10.3% to 13.9% [6,8]. Several studies reported that the incidence of teicoplanin ADRs is lower than that of vancomycin ADRs [6,9]. However, these results do not guarantee that the use of teicoplanin is safe in cases of vancomycin ADRs. In fact, several reports show life-threatening cross-reactivity between the two drugs [10-15]. There are few reports regarding the incidence of teicoplanin hypersensitivity in patients with vancomycin hypersensitivity. In Korea, vancomycin is indicated as the first-line choice for resistant bacterial strains. Teicoplanin is a secondary choice, mainly used as an alternative to vancomycin. In this background, we analyzed the incidence of teicoplanin ADRs and cross-reactivity between vancomycin and teicoplanin.
METHODS
Study population
We analyzed the incidence of teicoplanin ADRs retrospectively using electronic medical records. All in-patients administered teicoplanin between January 1, 2006 and December 31, 2015 at the Seoul National University Bundang Hospital (SNUBH) were identified (n = 327). Exclusion criteria included patients previously administered teicoplanin (n = 21) and patients receiving immunosuppressive therapy within 30 days (n = 2). The primary aim of the study was to investigate the incidence of teicoplanin ADRs within 1 month. The secondary aim was to determine the rate of cross-reaction between teicoplanin and vancomycin. This study was approved by the Institutional Review Board of SNUBH with waiver of informed consent (IRB No: B-1703/385-106).
Definition of ADRs and hypersensitivity reactions
Demographic data, past medical history, concurrent medication, and history of hypersensitivity reaction to other drugs were retrieved from electronic medical records. Clinical information regarding antibiotic use including daily dosage and duration, infection focus, and suspected pathogen were also recorded. We reviewed all medical records including admission notes, progression notes, nursing notes, consultation papers, and laboratory results to identify the presence of hypersensitivity reactions related to teicoplanin administration. The time relationship between the onset of several reactions and antibiotic use was recorded and the improvement in a specific reaction after discontinuation of teicoplanin was assessed. A skin reaction was defined as severe pruritus, urticaria, and maculopapular rash persisting despite infusion rate modification. Fever was defined as body temperature ≥ 38℃. Acute kidney injury (AKI) was diagnosed using Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [16]. Neutropenia, thrombocytopenia, and eosinophilia were defined as follows: neutrophil < 1,500/mm3, platelet < 150,000/ mm3, and eosinophil count > 500/mm3 [17-19]. The relationship between a specific reaction and the drug was assessed by the World Health Organization-Uppsala Monitoring Center causality assessment system; cases of ‘certain’ or ‘probable’ causality was considered as ADRs [20]. When AKI was the only adverse reaction after vancomycin use, the case was not considered as a hypersensitivity reaction because vancomycin-induced AKI is not an immune-mediated response [21] and typically differs from other type of vancomycin associated ADRs that might be immune-mediated hypersensitivity reaction [22-28]. All assessments were cross-checked separately by two allergists in the drug allergy clinic.
Statistical analysis
To compare categorical variables, the chi-square test or Fisher’s exact test was used, and the independent t test was used for continuous variables. A p value below 0.05 was regarded as statistically significant. Multiple comparisons were corrected using Bonferroni correction. The odds ratio (OR) and 95% confidence interval (95% CI) were also calculated. All statistical analyses were performed using PASW software version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
A total of 304 patients were enrolled. Table 1 shows the characteristics of 304 patients who administered teicoplanin. The mean ± SD age of these patients was 52.7 ± 22.1 years, and 185 (60.9%) were male. A total of 51 (16.8%) patients experienced hypersensitivity to other drugs before admission. The top seven suspected sites of infection were wound infection (n = 67, 22.0%), respiratory infection (n = 51, 16.8%), osteomyelitis (n = 47, 15.5%), intra-abdominal infection (n = 26, 8.6%), soft tissue infection (n = 23, 7.6%), septic arthritis (n = 21, 6.9%), and catheter-related infection (n = 20, 6.6%). The most common pathogenic bacterium was methicillin-resistant Staphylococcus aureus (n = 167, 54.9%), while others included methicillin-resistant coagulase-negative Staphylococcus (n = 46, 15.1%), and ampicillin-resistant Enterococcus (n = 29, 9.5%).
Table 1.
Demographic and clinical characteristics of 304 patients
| Characteristic | Value |
|---|---|
| Age | 52.7 ± 22.1 |
| Sex, male:female | 185 (60.9):119 (39.1) |
| History of hypersensitivity to other drugs | |
| Yes | 51 (16.8) |
| No | 253 (83.2) |
| Infection focus | |
| Wound | 67 (22.0) |
| Respiratory | 51 (16.8) |
| Osteomyelitis | 47 (15.5) |
| Intra-abdominal | 26 (8.6) |
| Soft tissue | 23 (7.6) |
| Septic arthritis | 21 (6.9) |
| Catheter-related | 20 (6.6) |
| Cellulitis | 16 (5.3) |
| Infective endocarditis | 6 (2.0) |
| Central nervous system | 5 (1.6) |
| Others | 3 (1.0) |
| Unknown | 19 (6.3) |
| Pathogen | |
| Methicillin-resistant Staphylococcus aureus | 167 (54.9) |
| Methicillin-resistant coagulase-negative Staphylococcus | 46 (15.1) |
| Ampicillin-resistant Enterococcus | 29 (9.5) |
| Unknown or no growth | 62 (20.4) |
Values are presented as mean ± SD or number (%).
Details of teicoplanin use and teicoplanin hypersensitivity reactions
The mean ± SD duration and daily dose per weight of teicoplanin administration were 12.2 ± 10.6 days and 11.9 ± 3.98 mg/kg, respectively (Table 2). A total of 238 patients (78.3%) switched from vancomycin to teicoplanin because of vancomycin ADRs during the same hospitalization. No patients were re-administered vancomycin after the switch. There were two other reasons for which teicoplanin was used initially rather than vancomycin, preference for an antibiotic with once daily dosing (n = 22, 7.2%) and impaired renal function (n = 7, 2.3%). In the 238 patients who experienced vancomycin ADRs before teicoplanin use, the most common type of ADR was a non-infusion-related skin reaction (n = 84, 35.3%) followed by neutropenia (n = 74, 31.1%), drug fever (n = 63, 26.5%), AKI (n = 30, 12.6%), thrombocytopenia (n = 18, 7.6%), and eosinophilia (n = 7, 2.9%). Among the 304 patients administered teicoplanin, 58 (19.1%) experienced adverse reactions after teicoplanin administration. Among 55 patients who showed ADR after both two drugs, 40 patients (72.7%) could not continue to use teicoplanin and other kinds of antibiotics such as linezolid. Drug fever (n = 27, 8.9%) and skin reaction (n = 25, 8.2%) were the most common type of teicoplanin adverse reaction, followed by neutropenia (n = 10, 3.3%), eosinophilia (n = 4, 1.3%), and thrombocytopenia (n = 2, 0.7%). No patients experienced AKI after teicoplanin administration.
Table 2.
Details of teicoplanin treatment and adverse reactions
| Variable | Value |
|---|---|
| Teicoplanin use | |
| Duration, day | 12.2 ± 10.6 |
| Daily dose, mg/kg | 11.9 ± 3.98 |
| Reason for use of teicoplanin | |
| Adverse drug reaction to vancomycin | 238 (78.3) |
| Skin reaction | 84 (35.3) |
| Neutropenia | 74 (31.1) |
| Drug fever | 63 (26.5) |
| Acute kidney injury | 30 (12.6) |
| Thrombocytopenia | 18 (7.6) |
| Eosinophilia | 7 (2.9) |
| Others | 9 (3.8) |
| Once daily usage | 22 (7.2) |
| Impaired renal function | 7 (2.3) |
| Others | 37 (12.2) |
| Adverse reaction to teicoplanin | |
| No adverse reaction | 246 (80.9) |
| Any adverse reaction | 58 (19.1) |
| Skin reaction | 25 (8.2) |
| Neutropenia | 10 (3.3) |
| Drug fever | 27 (8.9) |
| Thrombocytopenia | 2 (0.7) |
| Eosinophilia | 4 (1.3) |
| Others | 4 (1.3) |
Values are presented as mean ± SD or number (%).
Incidence and risk factors of teicoplanin ADRs
Among 304 patients, histories of vancomycin ADR and of hypersensitivity reaction to any other drug were significant baseline variables predicting the occurrence of teicoplanin ADRs which were mostly hypersensitivity reactions ([p = 0.001; OR, 6.31; 95% CI, 1.91 to 20.89], [p = 0.015; OR, –2.30; 95% CI, 1.17 to 4.52], respectively). The rate of teicoplanin ADRs among patients with previous vancomycin hypersensitivity excluding AKI increased to 25.2% and was also significant (p < 0.001; OR, –6.01; 95% CI, 2.32 to 15.59). In patients without a history of vancomycin administration or vancomycin hypersensitivity reaction, the incidence of teicoplanin ADRs was 5.3%. When patients were divided into three groups, patients without a history of vancomycin administration, patients administered vancomycin but did not have vancomycin hypersensitivity, and patients who experienced vancomycin hypersensitive reactions excluding AKI, the incidences of teicoplanin ADRs were 3.7%, 6.0%, and 25.2%, respectively. These differences were significant between the first or second group and third group (p < 0.001 and p < 0.01, respectively) (Fig. 1). The latent period of teicoplanin ADRs was shorter if patients previously experienced vancomycin hypersensitivity reactions but it was not statistically significant (8.6 ± 8.0 days vs. 14.4 ± 8.0 days, p = 0.130).
Figure 1.
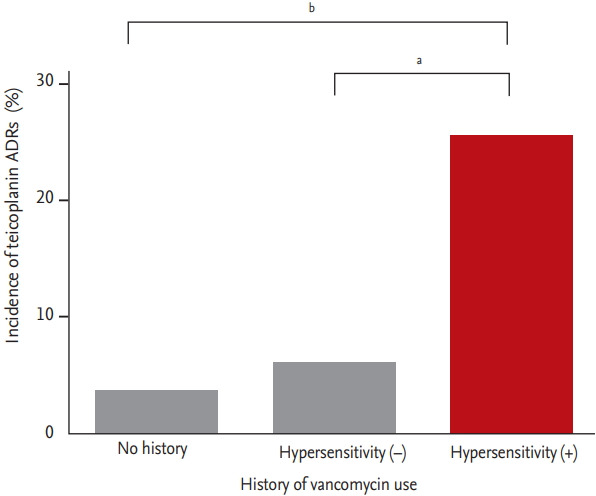
Incidence of teicoplanin adverse drug reactions (ADRs) according to history of vancomycin hypersensitivity. The incidences of teicoplanin ADRs were 3.7% (1/27) in patients with no history of vancomycin administration, 6.0% (4/67) in patients without hypersensitivity reaction after vancomycin administration, and 25.2% (53/210) in patients who previously experienced vancomycin hypersensitivity excluding acute kidney injury. a p < 0.01, b p < 0.001.
Predictors of teicoplanin ADRs among patients with history of vancomycin ADRs
Among the 238 patients who previously experienced vancomycin ADRs, risk factor of teicoplanin ADR was analyzed (Table 3). The duration of teicoplanin was shorter in patients with teicoplanin hypersensitivity and history of hypersensitivity reaction to other drugs was a risk factor of teicoplanin ADRs. Among the specific vancomycin reactions, drug fever was significantly associated with occurrence of teicoplanin ADRs (p = 0.025; OR, –2.07; 95% CI, –1.09 to 3.95). With analysis of patients with a history of vancomycin ADR, the group of patients with vancomycin AKI showed lower incidence of teicoplanin hypersensitivity (p = 0.022; OR, –0.21; 95% CI, 0.05 to 0.91). All the 28 patients who experienced only AKI as a vancomycin ADR showed no teicoplanin-associated ADR. Fig. 2 shows the incidence of teicoplanin hypersensitivity reaction according to the number of involved organs of vancomycin hypersensitivity reaction. The incidence of teicoplanin hypersensitivity increased with the number of involved organs of vancomycin hypersensitivity reaction (p for trend < 0.001). Classified by types of vancomycin hypersensitivity reaction, patients who experienced eosinophilia showed the highest incidence (42.9%) of teicoplanin hypersensitivity, followed by drug fever (33.3%), skin reaction (27.4%), and neutropenia (27.0%) (Fig. 3). Table 4 also shows the relationships of specific types of hypersensitivity reactions after vancomycin and teicoplanin use. Patients who previously had a vancomycin hypersensitivity reaction on the skin, drug fever, or neutropenia were most likely to show the same type of hypersensitivity after teicoplanin administration.
Table 3.
Risk factors of teicoplanin ADRs among patients who previously experienced vancomycin ADRs
| Characteristic | ADR to teicoplanin |
p value | |
|---|---|---|---|
| No (n = 183) | Yes (n = 55) | ||
| Age, yr | 60.1 ± 16.5 | 57.9 ± 17.6 | 0.388 |
| Sex | 0.262 | ||
| Male | 112 (61.2) | 29 (52.7) | |
| Female | 71 (38.8) | 26 (47.3) | |
| Daily dose of teicoplanin, mg/kg | 12.3 ± 3.8 | 12.6 ± 3.4 | 0.596 |
| Duration of teicoplanin use | 15.0 ± 11.7 | 9.5 ± 8.2 | 0.002 |
| Type of vancomycin hypersensitivity | |||
| Skin reaction (n = 84) | 61 (33.3) | 23 (41.8) | 0.248 |
| Neutropenia (n = 74) | 54 (29.5) | 20 (36.4) | 0.335 |
| Drug fever (n = 63) | 42 (23.0) | 21 (38.2) | 0.025 |
| Thrombocytopenia (n = 18) | 15 (8.2) | 3 (5.5) | 0.500 |
| Eosinophilia (n = 7) | 4 (2.2) | 3 (5.5) | 0.208 |
| Others (n = 9) | 8 (4.4) | 1 (1.8) | 0.384 |
| Acute kidney injury (n = 30) | 28 (15.3) | 2 (3.6) | 0.022 |
| History of hypersensitivity reaction to other drugs | 29 (15.8) | 16 (29.1) | 0.028 |
Values are presented as mean ± SD or number (%).
ADR, adverse drug reaction.
Figure 2.
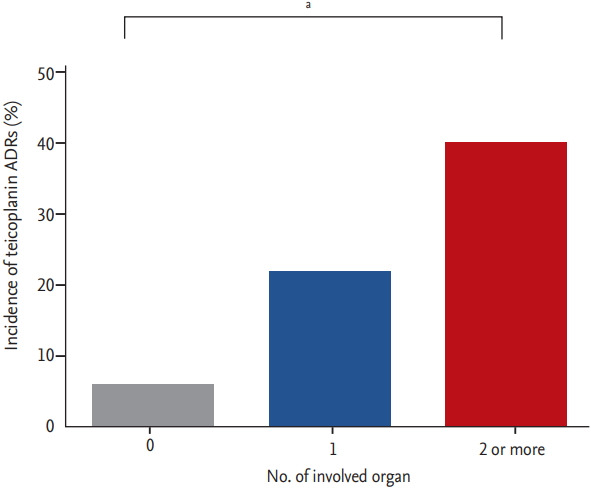
Incidence of teicoplanin adverse drug reactions (ADRs) according to the number of involved vancomycin hypersensitivity reactions. The incidences of teicoplanin ADRs were 6.0% (4/67) in patients without hypersensitivity reaction after vancomycin administration, 21.8% (37/170) in patients who experienced single type of vancomycin hypersensitivity reaction, and 40.0% (16/40) in patients who had multiple types of vancomycin hypersensitivity reaction. a p for trend < 0.001.
Figure 3.
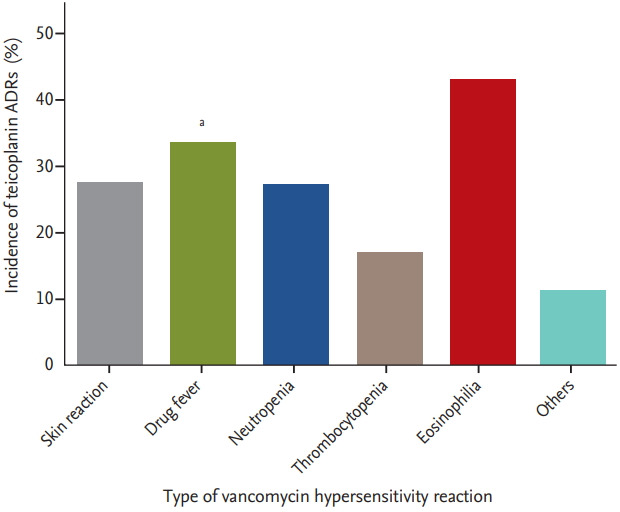
Incidence of teicoplanin adverse drug reactions (ADRs) according to type of vancomycin hypersensitivity reaction. The incidences were 27.4% (skin reaction), 33.3% (drug fever), 27.0% (neutropenia), 16.7% (thrombocytopenia), 42.9% (eosinophilia), and 11.1% (others). Drug fever associated with vancomycin was significantly associated with occurrence of teicoplanin ADR. a p = 0.025 compared with patients who did not experienced specific reaction.
Table 4.
Cross-reactivity between vancomycin and teicoplanin according to the type of hypersensitivity reaction after vancomycin use
| Previous vancomycin hypersensitivity reaction | Teicoplanin hypersensitivity |
||||||
|---|---|---|---|---|---|---|---|
| Any type of ADR | Skin reaction | Neutropenia | Drug fever | Thrombocytopenia | Eosinophilia | Others | |
| Any type of ADR (n = 238) | 53 (22.3)a | 22 (9.2) | 10 (4.2) | 22 (9.2) | 2 (0.8) | 2 (0.8) | 3 (1.3) |
| Skin reaction (n = 84) | 23 (27.4) | 14 (16.7)a | 1 (1.2) | 9 (10.7) | 0 | 1 (1.2) | 1 (1.2) |
| Neutropenia (n = 74) | 20 (27.0) | 5 (6.8) | 9 (12.2)a | 6 (8.1) | 2 (2.7) | 1 (1.4) | 1 (1.4) |
| Drug fever (n = 63) | 21 (33.3) | 11 (17.5) | 1 (1.6) | 13 (20.6)a | 0 | 1 (1.6) | 0 |
| Thrombocytopenia (n = 18) | 3 (16.7) | 0 | 3 (16.7) | 0 | 1 (5.6)a | 0 | 0 |
| Eosinophilia (n = 7) | 3 (42.9) | 1 (14.3) | 0 | 2 (28.6) | 0 | 2 (28.6)a | 0 |
| Others (n = 9) | 1 (11.1) | 0 | 0 | 0 | 0 | 0 | 1 (11.1) |
Values are presented as number (%). The numbers in table represent the incidence of specific types of teicoplanin hypersensitivity among patients with each type of vancomycin hypersensitivity.
ADR, adverse drug reaction.
The same type of reaction.
DISCUSSION
In a retrospective study of 304 patients who had been administered teicoplanin, we determined the incidence of teicoplanin ADRs and its risk factors. In patients without a history of vancomycin administration or vancomycin hypersensitivity reaction, the incidence of teicoplanin ADRs was 5.3%. This value was lower than previously reported rates of 10.3% to 13.9% [6,8], which did not consider history of hypersensitivity reaction to other drugs such as vancomycin. The incidence of teicoplanin ADRs in patients who previously experienced vancomycin hypersensitivity reaction excluding AKI was 25.2%, which was significantly higher than that in the other two groups (Fig. 1). After the first report of cross-reactivity between vancomycin and teicoplanin by McElrath et al. [29] in 1986, other case reports have been published regarding the cross-reactivity reactions of skin reactions or red-man syndrome [29,30], anaphylaxis [10], neutropenia [17], Stevens-Johnson syndrome [11], and drug hypersensitivity syndrome [12-15]. However, few studies have examined the exact incidence of cross-reactivity between vancomycin and teicoplanin hypersensitivity. Hung et al. [18] and Hsiao et al. [31] reported this value as 10.3% in 117 patients and 58.3% in 24 patients, respectively. Here, we examined the incidence and patterns of cross-reactivity between vancomycin and teicoplanin in 238 patients who previously experienced vancomycin ADRs.
The mechanism of cross-reactivity reaction between antibiotics is not well-known. Immunologic responses to similar antigenic structures may occur. For example, major and minor antigenic determinants for immunoglobulin E (IgE) binding in penicillin antibiotics have been identified and drug-specific T cells play an important role in delayed hypersensitivity reactions [1]. Some studies demonstrated that several immunologic mechanisms may be related to vancomycin and teicoplanin hypersensitivity and their cross-reactivity. Both drugs can induce anaphylaxis via IgE-mediated hypersensitivity [23,27]. Asero [22] and Azamgarhi et al. [23] revealed positive skin test results in patients with teicoplanin hypersensitivity. Knudsen and Pedersen [24] reported IgE-mediated histamine release in a patient who experienced hypersensitivity reactions to both vancomycin and teicoplanin. Additionally, vancomycin can cause not only immediate-type hypersensitivity reaction but also delayed type [26]. Mackett and Guay [25] demonstrated immune system sensitivity to vancomycin using the lymphocyte transformation test and Schwartz [28] isolated anti-granulocytic antibodies in the serum of a patient with vancomycin-induced neutropenia, suggesting that an immune-mediated mechanism such as delayed hypersensitivity involving T or B lymphocytes is involved rather than direct bone marrow destruction [17].
In this background, the probability of a cross-reaction can vary, depending on the type of response and we could clinically predict the occurrence of teicoplanin hypersensitivity according to the specific type of hypersensitivity reaction to vancomycin. Previous reports showed that patients who experienced vancomycin-induced neutropenia had a high possibility (33.3% to 50%) of experiencing a hypersensitivity reaction to teicoplanin [18,31]. We found that patients who experienced drug fever as vancomycin hypersensitivity reactions were at a significantly higher risk of cross-reactivity to teicoplanin (33.3%,) (Table 3 and Fig. 2). Eosinophilia, skin reaction, and neutropenia after vancomycin administration were also associated with a high risk of teicoplanin hypersensitivity (42.9%, 34.4%, and 27.0% respectively), although the results were not significantly different (Fig. 3). Additionally, patients who exhibited these four types of responses after vancomycin administration showed a high probability of same response to teicoplanin (Table 4).
As previously documented, hypersensitivity to vancomycin was the most important risk factor for teicoplanin ADR. In addition, a history of hypersensitivity to other drugs was also a risk factor. Interestingly, no patient experienced only AKI as the vancomycin ADR showed teicoplanin hypersensitivity. With respect to history of vancomycin ADR, vancomycin AKI was a predictive factor of lower incidence of teicoplanin ADRs which were mostly hypersensitivity reactions. This may be because of the different mechanisms of AKI and hypersensitivity reaction in vancomycin ADR. The hypersensitivity reaction refers to the response by an immunological mechanism among various ADRs [32]. Cross-reaction mediated by immunologic mechanisms is explained by a common antigenic determinant present in cross-reacting drugs [33]. However, AKI to vancomycin occurs via pro-inflammatory oxidation, mitochondrial dysfunction, and cellular apoptosis [21]. Therefore, vancomycin-induced AKI is not considered as hypersensitivity reaction and it might explain our result.
Our study also showed that the incidence of teicoplanin ADRs (hypersensitivity reactions) was higher in patients with multiple organ involvement in hypersensitivity reaction to vancomycin (Fig. 2). This result suggests that cross-reactivity between vancomycin and teicoplanin may depend on the immunological mechanism that causes hypersensitivity reactions. The duration of teicoplanin use was shorter in patients with teicoplanin hypersensitivity because administration was stopped after the occurrence of teicoplanin hypersensitivity.
This study has some limitations. First, we used a retrospective design and identified adverse reactions including hypersensitivity based on retrospective chart review of electronic medical records. This may resulted in insufficient information and observer bias. We attempted to overcome this bias by having two allergists analyze the data independently. Second, we identified hypersensitivity reactions based on the patients’ clinical presentation and did not perform in vivo or in vitro tests to confirm the immunologic mechanism. Non-immunologic reactions such as red-man syndrome may have occurred in the studied patients as a hypersensitivity reaction. However, most of red-man syndromes induced by vancomycin or teicoplanin are controlled by slow infusion of the drug or premedication with antihistamines in our hospital. All skin reactions in our study were not modified with an infusion rate control. Thus, we assumed that most of the hypersensitivity reactions other than AKI in our study were caused by an immunologic mechanism rather than a non-immunologic mechanism. Despite these limitations, this study enrolled a relatively large number of patients compared to previous studies and analyzed teicoplanin ADRs in terms of types of previous adverse reactions to vancomycin. Our study may improve the clinical prediction of the occurrence of teicoplanin ADRs in patients with vancomycin ADRs.
In conclusion, we analyzed the incidence and risk factors of teicoplanin ADRs and cross-reactivity between vancomycin and teicoplanin in 304 patients, including 238 patients who previously experienced vancomycin ADRs. We identified risk factors of teicoplanin ADRs and cross reaction between vancomycin and teicoplanin. This is the largest study regarding the cross-reactivity of vancomycin and teicoplanin hypersensitivity and first study to identify several risk factors of these reactions. Tolerability of teicoplanin mainly depends on the history of vancomycin hypersensitivity and its immunologic characteristics. In cases of vancomycin hypersensitivity, teicoplanin administration should be used with caution and clinicians must consider the risk of cross-reactivity according to risk factors and type of vancomycin ADRs.
KEY MESSAGE
1. The incidence of teicoplanin adverse drug reactions (ADRs) was higher in patients who previously experienced vancomycin-related ADRs and the rate of cross-reaction was 23.1%.
2. History of drug allergy and multiple organ involvement in vancomycin hypersensitivity reactions were significant risk factor of teicoplanin ADR.
3. In cases of vancomycin hypersensitivity, teicoplanin administration should be used with caution and clinicians must consider the risk of cross-reactivity according to risk factors and type of vancomycin ADRs.
Acknowledgments
This research was supported by a grant from Ministry of Food and Drug Safety to the regional pharmacovigilance center in 2018. We would like to express our gratitude to Ga-Young Oh for assisting with data processing.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Gruchalla RS, Pirmohamed M. Clinical practice. Antibiotic allergy. N Engl J Med. 2006;354:601–609. doi: 10.1056/NEJMcp043986. [DOI] [PubMed] [Google Scholar]
- 2.Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathwani D, Morgan M, Masterton RG, et al. Guidelines for UK practice for the diagnosis and management of methicillin-resistant Staphylococcus aureus (MRSA) infections presenting in the community. J Antimicrob Chemother. 2008;61:976–994. doi: 10.1093/jac/dkn096. [DOI] [PubMed] [Google Scholar]
- 4.Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care. 2011;17:254–265. doi: 10.1177/1078345811401363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimada K. Recent findings based on the results of the post-marketing surveillance of vancomycin hydrochloride for intravenous infusion. Jpn J Antibiot. 2003;56:259–271. [PubMed] [Google Scholar]
- 6.Wood MJ. The comparative efficacy and safety of teicoplanin and vancomycin. J Antimicrob Chemother. 1996;37:209–222. doi: 10.1093/jac/37.2.209. [DOI] [PubMed] [Google Scholar]
- 7.Wazny LD, Daghigh B. Desensitization protocols for vancomycin hypersensitivity. Ann Pharmacother. 2001;35:1458–1464. doi: 10.1345/aph.1A002. [DOI] [PubMed] [Google Scholar]
- 8.Davey PG, Williams AH. A review of the safety profile of teicoplanin. J Antimicrob Chemother. 1991;27 Suppl B:69–73. doi: 10.1093/jac/27.suppl_b.69. [DOI] [PubMed] [Google Scholar]
- 9.Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother. 2009;53:4069–4079. doi: 10.1128/AAC.00341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grek V, Andrien F, Collignon J, Fillet G. Allergic cross-reaction of teicoplanin and vancomycin. J Antimicrob Chemother. 1991;28:476–467. doi: 10.1093/jac/28.3.476. [DOI] [PubMed] [Google Scholar]
- 11.Yang LP, Zhang AL, Wang DD, Ke HX, Cheng Q, Wang C. Stevens-Johnson syndrome induced by the cross-reactivity between teicoplanin and vancomycin. J Clin Pharm Ther. 2014;39:442–445. doi: 10.1111/jcpt.12159. [DOI] [PubMed] [Google Scholar]
- 12.Kwon HS, Chang YS, Jeong YY, et al. A case of hypersensitivity syndrome to both vancomycin and teicoplanin. J Korean Med Sci. 2006;21:1108–1110. doi: 10.3346/jkms.2006.21.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao SH, Chen HH, Chou CH, Lin WL, Liu Yeh PY, Wu TJ. Teicoplanin-induced hypersensitivity syndrome with a preceding vancomycin-induced neutropenia: a case report and literature review. J Clin Pharm Ther. 2010;35:729–732. doi: 10.1111/j.1365-2710.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 14.Ebrahimpour S, Mohammadi M, Gholami K. Drug reaction with eosinophilia and systemic symptoms (DRESS) with teicoplanin: a case report. Drug Saf Case Rep. 2017;4:1. doi: 10.1007/s40800-016-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazu D, Kodama N, Yamashita D, et al. DRESS syndrome caused by cross-reactivity between vancomycin and subsequent teicoplanin administration: a case report. Am J Case Rep. 2016;17:625–631. doi: 10.12659/AJCR.899149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao SH, Chang CM, Tsai JC, et al. Glycopeptide-induced neutropenia: cross-reactivity between vancomycin and teicoplanin. Ann Pharmacother. 2007;41:891–894. doi: 10.1345/aph.1H633. [DOI] [PubMed] [Google Scholar]
- 18.Hung YP, Lee NY, Chang CM, et al. Tolerability of teicoplanin in 117 hospitalized adults with previous vancomycin-induced fever, rash, or neutropenia: a retrospective chart review. Clin Ther. 2009;31:1977–1986. doi: 10.1016/j.clinthera.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Tefferi A. Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment. Mayo Clin Proc. 2005;80:75–83. doi: 10.1016/S0025-6196(11)62962-5. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO)-Uppsala Monitoring Centre (UMC) Geneva (CH): WHO; c2019. The use of the WHO-UMC system for standardised case causality assessment [Internet] [cited 2019 Oct 23]. Available from: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf. [Google Scholar]
- 21.Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7:136–147. doi: 10.1177/2042018816638223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asero R. Teicoplanin-induced anaphylaxis. Allergy. 2006;61:1370. doi: 10.1111/j.1398-9995.2005.01021.x. [DOI] [PubMed] [Google Scholar]
- 23.Azamgarhi T, Shah A, Warren S. Teicoplanin anaphylaxis associated with surgical prophylaxis. Br J Clin Pharmacol. 2018;84:1038–1044. doi: 10.1111/bcp.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudsen JD, Pedersen M. IgE-mediated reaction to vancomycin and teicoplanin after treatment with vancomycin. Scand J Infect Dis. 1992;24:395–396. doi: 10.3109/00365549209061350. [DOI] [PubMed] [Google Scholar]
- 25.Mackett RL, Guay DR. Vancomycin-induced neutropenia. Can Med Assoc J. 1985;132:39–40. [PMC free article] [PubMed] [Google Scholar]
- 26.Minhas JS, Wickner PG, Long AA, Banerji A, Blumenthal KG. Immune-mediated reactions to vancomycin: a systematic case review and analysis. Ann Allergy Asthma Immunol. 2016;116:544–553. doi: 10.1016/j.anai.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otani IM, Kuhlen JL, Jr, Blumenthal KG, Guyer A, Banerji A. A role for vancomycin epicutaneous skin testing in the evaluation of perioperative anaphylaxis. J Allergy Clin Immunol Pract. 2015;3:984–985. doi: 10.1016/j.jaip.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz MD. Vancomycin-induced neutropenia in a patient positive for an antineutrophil antibody. Pharmacotherapy. 2002;22:783–788. doi: 10.1592/phco.22.9.783.34059. [DOI] [PubMed] [Google Scholar]
- 29.McElrath MJ, Goldberg D, Neu HC. Allergic cross-reactivity of teicoplanin and vancomycin. Lancet. 1986;1:47. doi: 10.1016/s0140-6736(86)91933-1. [DOI] [PubMed] [Google Scholar]
- 30.Khurana C, de Belder MA. Red-man syndrome after vancomycin: potential cross-reactivity with teicoplanin. Postgrad Med J. 1999;75:41–43. doi: 10.1136/pgmj.75.879.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiao SH, Chou CH, Lin WL, et al. High risk of cross-reactivity between vancomycin and sequential teicoplanin therapy. J Clin Pharm Ther. 2012;37:296–300. doi: 10.1111/j.1365-2710.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- 32.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 33.Romano A, Gueant-Rodriguez RM, Viola M, Gaeta F, Caruso C, Gueant JL. Cross-reactivity among drugs: clinical problems. Toxicology. 2005;209:169–179. doi: 10.1016/j.tox.2004.12.016. [DOI] [PubMed] [Google Scholar]


