Abstract
Purpose
Immune checkpoint inhibitors have recently been approved by the US FDA as first and/or second line therapy in a subset of cancer types. Recent evidence suggests that the quantity of tumor infiltrating lymphocytes (TILs) influences the likelihood of response to immune checkpoint inhibitors. Here, we set out to assess the density of CD8+ lymphocytes in a wide range of different cancer types and subtypes.
Methods
The density of CD8+ lymphocytes was compared across different cancer types using tissue microarrays (TMAs) composed of up to 50 tumor samples each from 84 different cancer types and subtypes. In total 2652 cancers and 608 normal tissues were successfully analyzed by CD8 immunohistochemistry followed by automated image analysis of digitized slides.
Results
We found that the median CD8+ lymphocyte counts ranged from 6 cells/mm2 in pleomorphic adenoma up to 1573 cells/mm2 in Hodgkin’s lymphoma. The CD8 counts were generally lower in normal tissues compared to cancer tissues. Blood vessels of the spleen were the only non-lymphatic tissue staining positive for CD8. Tumor types approved for checkpoint inhibitor therapy, including malignant melanoma (81), muscle invasive urothelial carcinoma (119), small cell lung cancer (120), clear cell renal cell cancer (153), squamous cell carcinoma (189) and adenocarcinoma of the lung (328) as well as Hodgkin’s lymphoma (1573) were all ranking among the upper half of our list. Comparably high CD8 densities (median cells/mm2) were also found in several rare and aggressive cancer types including Merkel cell carcinoma (70), angiosarcoma (95), anaplastic thyroid cancer (156) and embryonal carcinoma of the testis (186). In 73 of the 84 analyzed cancer types, the highly variable CD8 counts occasionally exceeded the average CD8 count of tumors for which checkpoint inhibitors have been approved.
Conclusion
These data support the concept that among most tumor types at least some individual cancers may benefit from treatment with immune checkpoint inhibitors.
Electronic supplementary material
The online version of this article (10.1007/s13402-020-00496-7) contains supplementary material, which is available to authorized users.
Keywords: Tissue microarray, Immune checkpoint, Lymphocytic infiltrate, Cytotoxic T cells
Introduction
Cancer drugs targeting the host immune reaction are increasingly employed in cancer therapy. Immune checkpoint inhibitors such as Pembrolizumab, Nivolumab and Atzolizumab, directed against programmed death-1 (PD-1) or its ligand 1 (PD-L1), have recently been approved by the US FDA as first and/or second line therapy in various cancer types including melanoma, non-small cell lung cancer, small cell lung cancer, renal cell carcinoma, urothelial carcinoma, cervical cancer and Hodgkin’s lymphoma [1–8]. Several additional drugs targeting the PD-1/PD-L1 system and other immune checkpoints or their ligands are currently being investigated in clinical trials. It is expected that the number of approved immune checkpoint inhibitors and the cancer types for which they are being utilized will increase markedly in the coming years [9].
Despite striking successes of these therapies in many patients, as yet the majority of treated individuals only shows a partial response [10]. Predictive tests that identify suited patients are urgently needed. An increasing number of publications has demonstrated that the number of tumor infiltrating lymphocytes (TILs) is one relevant factor determining the potential response of a cancer to checkpoint inhibitors [11]. As these medications combat cancer by stimulating cytotoxic lymphocytes, it is thought that the presence of more lymphocytes may increase the likelihood for a successful application of immune checkpoint inhibitors. Even in absence of specific therapy, tumors with high lymphocyte content such as medullary breast cancers, seminomas or colorectal carcinomas are often characterized by a particularly good prognosis [11–13]. Many studies describing (TILs) have shown associations with tumor phenotype, patient outcome or response to therapy [14–16]. Altogether, these data indicate that the number of lymphocytes in cancer tissues is of clinical importance.
Given the growing interest in the lymphocyte content of tumors, we utilized tissue microarrays (TMAs) composed of up to 50 tumors each from 84 different cancer types and subtypes to compare the density of CD8+ lymphocytes. The data provide a systematic overview with respect to the degree of cytotoxic T cell involvement in different cancers.
Material and methods
Patients and tissues
Formalin fixed paraffin embedded tissue samples from 3659 patients representing more than 80 different tumor types and subtypes and more than 70 different normal tissue types were retrieved from the archives of the Institute of Pathology at the University Medical Center Hamburg-Eppendorf. A pathologist identified representative cancerous and normal human tissue areas to assemble two different types of tissue microarrays (TMAs) from these samples: The first, a multitumor (TMA), contained 4–50 samples each from 84 different human tumor types and subtypes, as shown in Fig. 1. The samples of this first TMA were distributed among 8 different TMA blocks, each containing between 454 and 532 samples. The tissue cores were selected for a high tumor cell content on hematoxylin & eosin stained tissue sections of the donor tissue blocks, but not for particular features connected to the lymphocyte content such as the presence or density of infiltrating lymphocytes. The second TMA was composed of normal appearing tissues taken from organs removed for non-tumor reasons and encompassed 8 samples each of 76 different normal tissue types (608 samples on one slide), as shown in Supplementary Fig. 1. The exact composition of the study samples is shown in Table 1 and Supplementary Table 1. For both TMAs, a single 0.6 mm tissue punch was taken from each tissue sample. The TMA construction process was described previously [17]. The use of archived tissues has been approved by local laws (HmgKhG §12) and the local ethics committee (Ethics Commission Hamburg, WF-049/09). All studies have been carried out in compliance with the Helsinki Declaration.
Fig. 1.
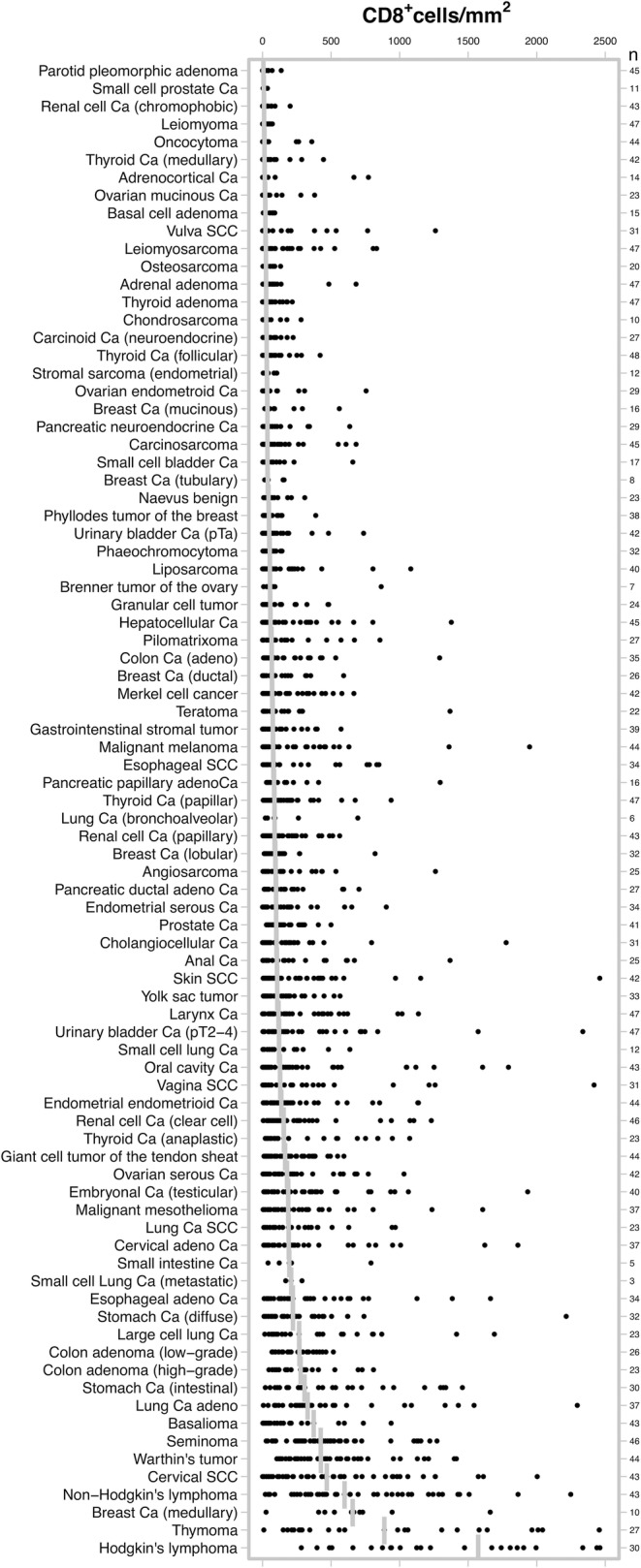
Distribution of CD8+ cell density (cell/mm2) across 84 different human tumor entities. In total 3339 tumor samples, represented by the black dots, were analyzed. The vertical bars indicate the median density per entity. Ca, carcinoma; SCC, squamous cell carcinoma
Table 1.
CD8+ cell densities (cells/mm2) in 84 different tumor types
| Tumor entity | N | Mean | SD | Median | Max |
|---|---|---|---|---|---|
| Parotid pleomorphic adenoma | 45 | 15 | 24 | 6 | 136 |
| Small cell prostate Ca | 11 | 14 | 10 | 9 | 35 |
| Renal cell Ca (chromophobic) | 43 | 23 | 35 | 10 | 201 |
| Leiomyoma | 47 | 19 | 18 | 11 | 71 |
| Oncocytoma | 44 | 33 | 72 | 13 | 359 |
| Thyroid Ca (medullary) | 42 | 43 | 83 | 14 | 445 |
| Adrenocortical Ca | 14 | 120 | 256 | 15 | 772 |
| Ovarian mucinous Ca | 23 | 53 | 95 | 18 | 380 |
| Basal cell adenoma | 15 | 31 | 28 | 20 | 89 |
| Vulva SCC | 31 | 141 | 279 | 21 | 1261 |
| Leiomyosarcoma | 47 | 113 | 193 | 24 | 832 |
| Osteosarcoma | 20 | 33 | 33 | 24 | 132 |
| Adrenal adenoma | 47 | 60 | 118 | 26 | 682 |
| Thyroid adenoma | 47 | 44 | 51 | 26 | 217 |
| Chondrosarcoma | 10 | 72 | 95 | 27 | 281 |
| Carcinoid Ca (neuroendocrine) | 27 | 53 | 55 | 30 | 223 |
| Thyroid Ca (follicular) | 48 | 58 | 80 | 30 | 420 |
| Stromal sarcoma (endometrial) | 12 | 43 | 35 | 31 | 104 |
| Ovarian endometroid Ca | 29 | 76 | 149 | 33 | 756 |
| Breast Ca (mucinous) | 16 | 98 | 146 | 35 | 560 |
| Pancreatic neuroendocrine Ca | 29 | 84 | 137 | 35 | 636 |
| Carcinosarcoma | 45 | 98 | 156 | 35 | 682 |
| Small cell bladder Ca | 17 | 94 | 159 | 36 | 658 |
| Breast Ca (tubulary) | 8 | 75 | 65 | 38 | 157 |
| Naevus benign | 23 | 69 | 72 | 43 | 308 |
| Basalioma | 42 | 142 | 212 | 46 | 938 |
| Phyllodes tumor of the breast | 38 | 62 | 67 | 46 | 388 |
| Urinary bladder Ca (pTa) | 42 | 89 | 138 | 48 | 737 |
| Phaeochromocytoma | 32 | 55 | 33 | 52 | 142 |
| Liposarcoma | 40 | 124 | 215 | 53 | 1081 |
| Brenner tumor of the ovary | 7 | 165 | 310 | 54 | 866 |
| Granular cell tumor | 24 | 118 | 138 | 55 | 483 |
| Hepatocellular Ca | 45 | 180 | 264 | 58 | 1377 |
| Pilomatrixoma | 27 | 157 | 227 | 65 | 856 |
| Colon Ca (adeno) | 35 | 169 | 243 | 67 | 1292 |
| Breast Ca (ductal) | 26 | 121 | 138 | 67 | 592 |
| Merkel cell cancer | 42 | 156 | 180 | 70 | 666 |
| Teratoma | 22 | 156 | 286 | 74 | 1368 |
| Gastrointenstinal stromal tumor | 39 | 115 | 137 | 75 | 573 |
| Esophageal SCC | 34 | 205 | 261 | 78 | 849 |
| Malignant melanoma | 43 | 231 | 371 | 81 | 1948 |
| Pancreatic papillary adenoCa | 16 | 199 | 313 | 83 | 1296 |
| Thyroid Ca (papillar) | 47 | 157 | 183 | 86 | 938 |
| Lung Ca (bronchoalveolar) | 6 | 198 | 258 | 87 | 695 |
| Renal cell Ca (papillary) | 43 | 141 | 149 | 88 | 563 |
| Breast Ca (lobular) | 32 | 119 | 143 | 92 | 821 |
| Angiosarcoma | 25 | 199 | 272 | 95 | 1262 |
| Pancreatic ductal adeno Ca | 27 | 158 | 188 | 98 | 703 |
| Endometrial serous Ca | 34 | 161 | 206 | 98 | 903 |
| Prostate Ca | 41 | 139 | 103 | 99 | 499 |
| Cholangiocellular Ca | 31 | 208 | 334 | 101 | 1777 |
| Anal Ca | 25 | 229 | 320 | 104 | 1368 |
| Skin SCC | 42 | 276 | 431 | 106 | 2460 |
| Yolk sac tumor | 33 | 145 | 154 | 107 | 566 |
| Larynx Ca | 47 | 230 | 281 | 115 | 1137 |
| Urinary bladder Ca (pT2–4) | 47 | 267 | 432 | 119 | 2336 |
| Small cell lung Ca | 12 | 192 | 200 | 120 | 637 |
| Oral cavity Ca | 43 | 304 | 452 | 127 | 1794 |
| Vagina SCC | 31 | 313 | 515 | 128 | 2418 |
| Endometrial endometrioid Ca | 44 | 237 | 283 | 137 | 1137 |
| Renal cell Ca (clear cell) | 46 | 306 | 479 | 153 | 2756 |
| Thyroid Ca (anaplastic) | 23 | 349 | 351 | 156 | 1073 |
| Giant cell tumor of the tendon sheat | 44 | 197 | 154 | 164 | 594 |
| Ovarian serous Ca | 42 | 235 | 248 | 178 | 1032 |
| Embryonal Ca (testicular) | 40 | 322 | 393 | 186 | 1934 |
| Malignant mesothelioma | 37 | 356 | 541 | 187 | 2844 |
| Lung Ca SCC | 23 | 274 | 273 | 189 | 969 |
| Cervical adeno Ca | 37 | 340 | 434 | 191 | 1863 |
| Small intestine Ca | 5 | 272 | 298 | 195 | 791 |
| Small cell Lung Ca (metastatic) | 3 | 222 | 61 | 208 | 288 |
| Esophageal adeno Ca | 34 | 378 | 386 | 220 | 1663 |
| Stomach Ca (diffuse) | 32 | 315 | 395 | 221 | 2215 |
| Large cell lung Ca | 23 | 440 | 446 | 266 | 1691 |
| Colon adenoma (low-grade) | 26 | 256 | 133 | 268 | 517 |
| Colon adenoma (high-grade) | 23 | 296 | 212 | 277 | 810 |
| Stomach Ca (intestinal) | 30 | 518 | 432 | 306 | 1458 |
| Lung Ca adeno | 37 | 488 | 488 | 328 | 2296 |
| Seminoma | 46 | 492 | 348 | 424 | 1272 |
| Warthin’s tumor | 44 | 501 | 356 | 425 | 1414 |
| Cervical SCC | 43 | 574 | 491 | 468 | 2004 |
| Non-Hodgkin’s lymphoma | 43 | 720 | 520 | 598 | 2248 |
| Breast Ca (medullary) | 10 | 677 | 424 | 657 | 1661 |
| Thymoma | 27 | 1017 | 833 | 889 | 2740 |
| Hodgkin’s lymphoma | 30 | 1649 | 954 | 1573 | 4100 |
Immunohistochemistry
Freshly cut TMA sections were all stained for CD8 in one run on the same day. The slides were deparaffinized, rehydrated, exposed to heat-induced antigen retrieval for 15 min at 98 °C in pH 9 DAKO target retrieval Solution (S2367) using a DAKO PT-LINK device, and then transferred to a DAKO Link 48 autostainer device. The autostainer protocol includes peroxidase blocking for 5 min (DAKO, Envision Flex-Kit 8002) and subsequent incubations with the primary antibody (Oncodianova, mouse monoclonal antibody, Clone TC8, dilution 1:200) for 20 min at room temperature, Flex HRP (DAKO EnVision Flex-Kit 8002) for 20 min, DAB-Chromogen (DAKO EnVision Flex-Kit 8002) for 10 min, and a final incubation with Hämatoxylin (DAKO K8008) for 5 min.
Definition of compartments and quantification of CD8 immunostaining
Digital images of stained slides were acquired using a Leica Aperio VERSA 8 automated microscope. TMA spots were automatically identified and analyzed using HALO™ (Indica Labs, US) in conjunction with the following procedure: Every TMA slide was scanned at 40 × magnification. Digital images were segmented using the HALO Tissue Microarray module to identify and annotate individual tissue spots. If necessary, the segmentation was corrected manually. The HALO Membrane IHC Quantification module was used to determine the number of CD8+ cells in each tissue spot and to measure the exact area of each tissue spot. The latter step was performed to compensate for uneven or incomplete tissue spots (average: 0.327 mm2). The number of stained cells and the area in square millimeters of each individual spot was used to calculate the density of stained cells/mm2 (number of cells per square mm). Examples of the image analysis procedure of tumor spots with a low, intermediate and high density of CD8+ lymphocytes are given in Supplementary Fig. 2.
Statistics
JMP Pro 12 software package (SAS Institute Inc., NC, US) and R version 3.5.1 (The R foundation) [18, 19] were used to plot the data, to calculate the median values and to perform analysis of variance (ANOVA).
Results
A total of 2652 (72%) of 3659 tumor samples and 608 (100%) of 608 normal tissue samples were interpretable in our TMA analysis. The remaining 1007 spots were excluded due to missing tissue or the absence of unequivocal cancer cells in the TMA spot. The number of interpretable spots for each tumor type ranged from 3 to 48 samples (mean: 32 ± 13). The vast majority, i.e., 2602 (98%) of the 2652 tissue spots, contained CD8+ lymphocytes, although there were 50 (2%) samples that completely lacked CD8+ T cells. These were from 31 different tumor entities. All data are summarized in Table 1 and Fig. 1. The median number of CD8+ lymphocytes was highly variable and ranged from 6 cells/mm2 in pleomorphic adenoma (Fig. 2b) up to 1572 cells/mm2 in Hodgkin’s lymphoma (Fig. 2f). The top positions in our ranking order were held by tumor entities known for their high lymphocyte density such as seminoma (median: 424), Warthin’s tumor (median: 425), medullary breast cancer (median: 657), and thymoma (median: 889). Also, cancers in tissues with particularly high lymphocyte densities such as stomach cancer (median: 306) or cervical squamous cell carcinoma (median: 468) exhibited high CD8 values. At the bottom end of our list were pleomorphic adenoma of the parotis (median: 6), small cell cancer of prostate (median: 9), leiomyoma (median: 10) and oncocytoma (median: 13).
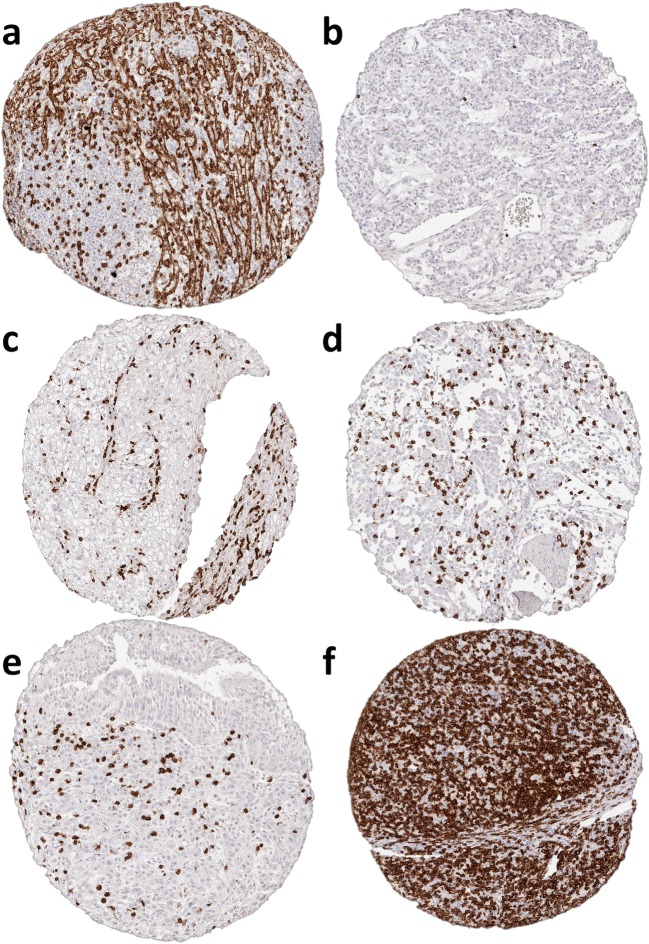
Fig. 2.
Representative pictures of CD8 immunohistochemistry in (a) healthy spleen, (b) pleomorphic adenoma, (c) clear cell renal cell cancer, (d) urinary bladder cancer, (e) squamous lung cancer and (f) Hodgkin’s lymphoma. Magnification 100×; TMA spot size 0.6 mm
580 (95%) of the 608 tissue spots representing 58 (76%) of the 76 analyzed healthy normal tissues contained CD8+ lymphocytes. The median number of CD8+ lymphocytes was highly variable ranging from 0 cells/mm2 in the white matter of cerebrum up to 1643 cells/mm2 in the spleen. Blood vessels of the spleen were the only non-lymphatic tissue staining positive for CD8. The data are summarized in Supplementary Table 1 and Supplementary Fig. 1.
Discussion
The data from this study provide a comprehensive overview of the density of tumor infiltrating CD8+ lymphocytes across a large number of different human tumor types. Numerous studies have recently quantified immune cell infiltrations in and around tumors. Most of these have used semi-quantitative methods to describe their data such as cell counts per high-power field or per predefined tissue area, positive pixel counts per area, semi-quantitative estimation of TIL numbers or the fraction of TILs in relation to the total intra-tumoral stroma [20–26]. Data on lymphocytic infiltration in cancers cannot always easily be compared between studies because objective metric data are often lacking. In this project, we described all data as “cells/mm2” in order to generate as comparable data as possible. An automated approach was developed that eliminates differences in tissue quantity occurring in TMA studies. Most spots are typically intact in TMA studies, but some are incomplete (Fig. 3). This may be due to specific properties of the arrayed tissue or may be caused by technical issues during embedding of tissue cores in a TMA or during sectioning. The validity of our approach is supported by our finding that tumor types among the top positions in our ranking order are morphologically characterized by high lymphocyte densities, such as seminoma, Warthin’s tumor, medullary breast cancer or thymoma. Other cancers with particularly high lymphocyte densities are those occurring in areas with a high density of mucosal lymphocytes, such as cervical or stomach cancer.
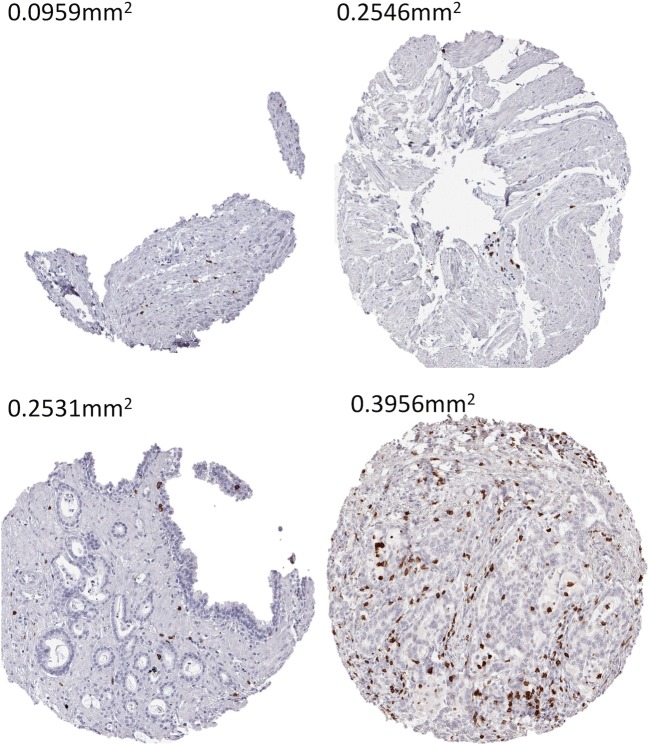
Fig. 3.
Area measurements of representative TMA cores that show an uneven or incomplete shape. Magnification 100×; TMA spot size 0.6 mm. Note that the tissue spots may have a larger area as theoretically expected from a 0.6 mm tissue punch (0.283 mm2). This is because the tissue expands when mounted on a slide
Several earlier CD8 density studies in cancers employing automated image analysis or manual cell counting to quantitate TILs in terms of “cells per mm2” yielded results that were comparable to those from our study. For example, an assessment of 220 gastric cancers by automated image analysis showed a median count of 436 CD8+ cells/mm2, which fits well with our results (mean: 413 CD8+ cells/mm2 in 62 intestinal and diffuse stomach cancers) [27]. Another study that counted T cells manually in 102 cervical squamous cell cancers reported a median of 441 CD8+ T cells/mm2, which is very similar to the median value of 468 CD8+ T cells/mm2 in our 43 patients [20]. One earlier study by Steele et al. [28] has used “cells/mm2” to compare the density of CD8+ cells in 8 different cancer types in a study analyzing the largest possible area per cancer on large sections. Several relevant observations were comparable between our and their study (Table 2), such as relatively low densities of CD8+ cells in kidney and prostate cancers and a high density in non-small cell lung cancer. The largest discrepancy was seen in pancreatic cancers, in which Steele at al. found 316 cells/mm2 and we found 93 cells/mm2 on average. It is a peculiarity of pancreatic cancer that it contains a high amount of tumor stroma and that the lymphocytes mostly reside in the stroma. It cannot be excluded that the selection process of tissues for TMA manufacturing has resulted in an overrepresentation of cancer cell-rich tissues with less stroma, as these are easier to target during the array manufacturing process.
Table 2.
Comparison of median CD8+ cell densities with findings by Steele et al.
| Current Study | Steele et al. | ||||
|---|---|---|---|---|---|
| Tumor entity | Subgroup | n | Density (cells/mm2) | n | Density (cells/mm2) |
| Bladder | Total | 89 | 76 | 50 | 124 |
| pTa | 42 | 48 | |||
| pT2–4 | 47 | 119 | |||
| Pancreatic | Total | 43 | 93 | 48 | 316 |
| Papillary | 16 | 83 | |||
| Ductal adenocarcinoma | 27 | 98 | |||
| Prostate | Total | 41 | 99 | 50 | 143 |
| RCC | Total | 89 | 112 | 49 | 104 |
| Papillary | 43 | 88 | |||
| Clear cell | 46 | 153 | |||
| Head and neck | Total | 90 | 118 | 50 | 220 |
| Larynx | 47 | 115 | |||
| Oral cavity | 43 | 127 | |||
| Lung squamous | Total | 23 | 189 | 41 | 346 |
| Gastroesophageal | Total | 153 | 221 | 50 | 207 |
| Esophageal SCC | 34 | 78 | |||
| Esophageal AdenoCa | 34 | 220 | |||
| Stomach Ca (diffus) | 32 | 221 | |||
| Stomach Ca (intestinal) | 30 | 306 | |||
| Lung non-squamous | Total | 60 | 328 | 42 | 238 |
| Large cell | 23 | 266 | |||
| Adenocarcinoma | 37 | 328 | |||
Cancer types for which treatment regimens with immune checkpoint inhibitors are already approved, such as malignant melanoma, muscle invasive urothelial carcinoma, clear cell renal cell cancer, squamous cell cancer and adenocarcinoma of the lung, small cell lung cancer and Hodgkin’s lymphoma, all ranked in the upper half of our list. This supports the concept that a high number of tumor infiltrating lymphocytes goes along with an increased likelihood for response to immune checkpoint inhibitors. The median content of CD8+ cells was 248 cells/mm2 for these tumor types. That 73 of our 84 analyzed tumor types had - at least occasionally - CD8+ cell counts that were higher than 248 cells/mm2 may be viewed as an argument for the applicability of immune checkpoint inhibitors - and probably other forms of immunotherapy - to a wide variety of different types of cancer. However, the CD8-positive cell density was found to be highly variable in most analyzed cancer types. All analyzed cancer types had at least one tumor with a CD8+ cell count below 282/mm2. This is in line with clinical observations that only a fraction of individual cancers from a certain tumor type will eventually respond favorably to treatment [29].
We identified several rare and aggressive tumor types that frequently exhibited a high density of CD8+ cells. These included anaplastic thyroid cancer, anal carcinoma, embryonal carcinoma of the testis, Merkel cell carcinoma, angiosarcoma and squamous cell carcinoma of the vagina. These cancers are candidates for a thorough assessment of the efficacy of immune checkpoint inhibitors. A clinically relevant effect of the lymphocyte content has already been suggested for some of these tumors, including reports on successful therapy attempts with checkpoint inhibitors. For Merkel cell carcinoma, a prognostic role of the associated immune cell count has been described in several studies [30, 31]. Furthermore, an objective response rate of more than 50% in a cohort of 26 Merkel cell carcinoma patients treated with anti-PD-1 therapy [32] has led to several ongoing phase I (NCT02488759, NCT02584829), II (NCT02196961) and III (NCT03271372) studies for this tumor entity. Also a patient with anaplastic thyroid cancer has recently reached complete radiographic and clinical remission after anti-PD-1 therapy [33]. Combination therapies can also be efficient in these tumor types as demonstrated by the success of combining monoclonal antibodies targeting PD-1 and CTLA-4 in angio- and other sarcoma types [34]. Moreover, a prognostic role of the CD8+ cell count was recently suggested in angiosarcoma [35].
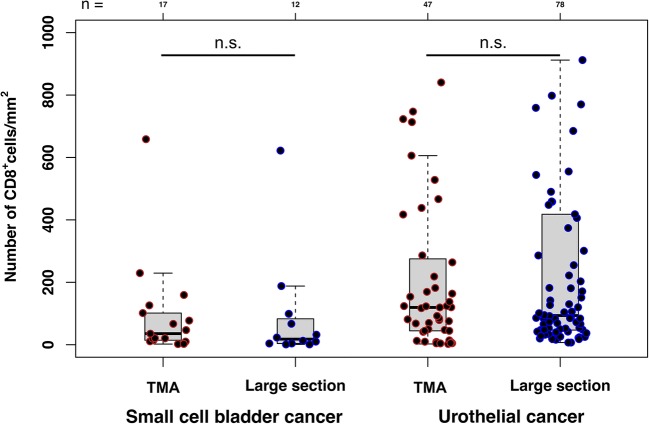
The premise that the number of lymphocytes in a cancer is linked to the likelihood of a positive response to immune checkpoint inhibitors is widely accepted [36]. Many studies have separately assessed lymphocyte counts in the tumor center and in its periphery [13, 37–39]. Some studies have recently suggested that the lymphocyte content at the tumor periphery may be more important clinically than the lymphocyte content in the tumor center. For example, in a study on 46 patients with metastatic melanoma, Tumeh et al. found that response to anti-PD-1 therapy was associated with higher CD8+ cell densities within the invasive margin [37]. In another study on metastatic colorectal cancer, the density of immune cells at the invasive margin predicted response to conventional chemotherapy [40]. However, quantification of TILs at the tumor margin is hampered by a “non-standardized” structure of the tumor periphery. For several tumor types such as bladder cancer, colorectal cancer and stomach cancer, different growth patterns have been described [41–43]. TIL quantification is easy in tumors with a solid growth and a clearly defined tumor margin, but is much more difficult in cancers showing insular or diffuse growth patterns with irregular borders. In contrast, lymphocyte quantification in the tumor center is independent of tumor growth patterns and thus simpler and perhaps more reproducible to measure than in the periphery. The high concordance between large sections and TMA CD8 counts in 17 small cell bladder carcinomas and in 47 urothelial cancers demonstrates that representative lymphocyte quantification is possible for tumor centers on a 0.6 mm tissue core (Fig. 4).
Fig. 4.
Comparison of CD8+ cell densities in large section and TMA formats. TMA, multi-tumor tissue microarray
Tissue microarrays with small 0.6 mm tissue cores were used in this study. When we introduced the TMA technology in 1998, several authors criticized the small diameter of the TMA spots. However, almost 20 years later, every known association between immune cell quantification and patient outcome [13, 44, 45] or between molecular markers and clinico-pathological features [46–49] have been successfully reproduced in TMA studies. In an earlier study on p53 staining, we even showed that the 0.6 mm TMA format provided better predictive power compared to the large section format technology [46]. Contrary to initial expectations, tissue heterogeneity does not negatively influence the ability of TMA spots to detect associations between molecular markers and tumor features. This is because of the high number of samples that are typically used in such analyses, as well as the high degree of standardization of the experimental conditions that cannot be achieved in standard slide-by-slide analyses [50–53].
Our analysis also included 76 different types of normal tissues, which apart from lymphatic tissues, exhibited much lower CD8+ lymphocyte counts than the tumors. Spleen blood vessels were the only non-lymphoid cell type staining unequivocally positive for CD8. CD8 expression in splenic vessels was first described by Buckley et al. in 1985 [54]. Further studies revealed that only the alpha chain of the CD8 molecule is expressed by the venous sinus-lining cells in the spleen’s red pulp (also known as littoral cells) and that this expression is unique to human spleen [55].
In summary, the data presented in this study provide a comprehensive overview of CD8 positivity across human normal tissues and cancers. Cancer types with high densities of CD8+ immune cells may be the best candidates for studies assessing the efficacy of immune checkpoint therapies.
Electronic supplementary material
(DOCX 136066 kb)
Funding Information
Open Access funding provided by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The usage of archived tissues has been approved by local laws (HmgKhG §12) and the local ethics committee (Ethics commission Hamburg, WF-049/09). All studies have been carried out in compliance with the Helsinki Declaration.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Niclas C. Blessin and Patrick Spriestersbach contributed equally to this work.
References
- 1.Hellmann MD, Ott PA, Zugazagoitia J, Ready NE, Hann CL, Braud FGD, Antonia SJ, Ascierto PA, Moreno V, Atmaca A, Salvagni S, Taylor MH, Amin A, Camidge DR, Horn L, Calvo E, Cai W, Fairchild JP, Callahan MK, Spigel DR. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032. J Clin Oncol. 2017;35:8503–8503. doi: 10.1200/JCO.2017.35.15_suppl.8503. [DOI] [Google Scholar]
- 2.Rihawi K, Gelsomino F, Sperandi F, Melotti B, Fiorentino M, Casolari L, Ardizzoni A. Pembrolizumab in the treatment of metastatic non-small cell lung cancer: A review of current evidence. Ther Adv Respir Dis. 2017;11:353–373. doi: 10.1177/1753465817725486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Chung HC, Schellens JHM, Delord J-P, Perets R, Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Wang J, Zeigenfuss S, Pruitt SK, Marabelle A. Pembrolizumab treatment of advanced cervical cancer: Updated results from the phase 2 KEYNOTE-158 study. J Clin Oncol. 2018;36:5522. doi: 10.1200/JCO.2018.36.15_suppl.5522. [DOI] [PubMed] [Google Scholar]
- 7.A. Ghahremanloo, A. Soltani, S.M.S. Modaresi, S.I. Hashemy, Recent advances in the clinical development of immune checkpoint blockade therapy. Cell Oncol 42, 609–626 (2019). 10.1007/s13402-019-00456-w [DOI] [PubMed]
- 8.H. Polioudaki, A. Chantziou, K. Kalyvianaki, P. Malamos, G. Notas, D. Mavroudis, M. Kampa, E. Castanas, P.A. Theodoropoulos, Nuclear localization of PD-L1: Artifact or reality? Cell Oncol 42, 237–242 (2019). 10.1007/s13402-018-00419-7 [DOI] [PubMed]
- 9.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 checkpoint signaling inhibition for Cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieci MV, Orvieto E, Dominici M, Conte P, Guarneri V. Rare breast cancer subtypes: Histological, molecular, and clinical peculiarities. Oncologist. 2014;19:805–813. doi: 10.1634/theoncologist.2014-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 14.Santoiemma PP, Powell DJ., Jr Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16:807–820. doi: 10.1080/15384047.2015.1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen R, Donia M, Westergaard MC, Pedersen M, Hansen M, Svane IM. Tumor infiltrating lymphocyte therapy for ovarian cancer and renal cell carcinoma. Hum Vaccin Immunother. 2015;11:2790–2795. doi: 10.1080/21645515.2015.1075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 17.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 18.Tippmann S. Programming tools: Adventures with R. Nature. 2015;517:109–110. doi: 10.1038/517109a. [DOI] [PubMed] [Google Scholar]
- 19.R-Core-Team, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. (2018)
- 20.Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K. Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer. 2007;97:1135–1138. doi: 10.1038/sj.bjc.6604001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korehisa S, Oki E, Iimori M, Nakaji Y, Shimokawa M, Saeki H, Okano S, Oda Y, Maehara Y. Clinical significance of programmed cell death-ligand 1 expression and the immune microenvironment at the invasive front of colorectal cancers with high microsatellite instability. Int J Cancer. 2018;142:822–832. doi: 10.1002/ijc.31107. [DOI] [PubMed] [Google Scholar]
- 22.Ingels A, Sanchez Salas RE, Ravery V, Fromont-Hankard G, Validire P, Patard JJ, Pignot G, Prapotnich D, Olivier F, Galiano M, Barret E, Rozet F, Weber N, Cathelineau X. T-helper 1 immunoreaction influences survival in muscle-invasive bladder cancer: Proof of concept. Ecancermedicalscience. 2014;8:486. doi: 10.3332/ecancer.2014.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KJ, Wen XY, Yang HK, Kim WH, Kang GH. Prognostic implication of M2 macrophages are determined by the proportional balance of tumor associated macrophages and tumor infiltrating lymphocytes in microsatellite-unstable gastric carcinoma. PLoS One. 2015;10:e0144192. doi: 10.1371/journal.pone.0144192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilic A, Landreneau RJ, Luketich JD, Pennathur A, Schuchert MJ. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res. 2011;167:207–210. doi: 10.1016/j.jss.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Jang N, Kwon HJ, Park MH, Kang SH, Bae YK. Prognostic value of tumor-infiltrating lymphocyte density assessed using a standardized method based on molecular subtypes and adjuvant chemotherapy in invasive breast Cancer. Ann Surg Oncol. 2018;25:937–946. doi: 10.1245/s10434-017-6332-2. [DOI] [PubMed] [Google Scholar]
- 26.Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- 27.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steele KE, Tan TH, Korn R, Dacosta K, Brown C, Kuziora M, Zimmermann J, Laffin B, Widmaier M, Rognoni L, Cardenes R, Schneider K, Boutrin A, Martin P, Zha J, Wiestler T. Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J Immunother Cancer. 2018;6:20. doi: 10.1186/s40425-018-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma P. Immune checkpoint therapy and the search for predictive biomarkers. Cancer J. 2016;22:68–72. doi: 10.1097/PPO.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behr DS, Peitsch WK, Hametner C, Lasitschka F, Houben R, Schonhaar K, Michel J, Dollt C, Goebeler M, Marx A, Goerdt S, Schmieder A. Prognostic value of immune cell infiltration, tertiary lymphoid structures and PD-L1 expression in Merkel cell carcinomas. Int J Clin Exp Pathol. 2014;7:7610–7621. [PMC free article] [PubMed] [Google Scholar]
- 31.T. Kervarrec, P. Gaboriaud, P. Berthon, J. Zaragoza, D. Schrama, R. Houben, Y. Le Corre, E. Hainaut-Wierzbicka, F. Aubin, G. Bens, J. Domenech, S. Guyetant, A. Touze, M. Samimi, Merkel Cell Carcinomas Infiltrated with CD33(+) Myeloid Cells and CD8(+) T Cells Are Associated with Improved Outcome. J Am Acad Dermatol 78, 973–982 e978 (2018). 10.1016/j.jaad.2017.12.029 [DOI] [PubMed]
- 32.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R. Kollipara, B. Schneider, M. Radovich, S. Babu, P.J. Kiel, Exceptional response with immunotherapy in a patient with anaplastic thyroid cancer. Oncologist 22, 1149–1151 (2017). 10.1634/theoncologist.2017-0096 [DOI] [PMC free article] [PubMed]
- 34.D'Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, Antonescu CR, Horvath E, Tap WD, Schwartz GK, Streicher H. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19:416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii H, Arakawa A, Utsumi D, Sumiyoshi S, Yamamoto Y, Kitoh A, Ono M, Matsumura Y, Kato M, Konishi K, Shiga T, Sano S, Sakaguchi S, Miyagawa-Hayashino A, Takahashi K, Uezato H, Miyachi Y, Tanioka M. CD8(+) tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer. 2014;134:2393–2402. doi: 10.1002/ijc.28581. [DOI] [PubMed] [Google Scholar]
- 36.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 37.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 39.Jakubowska K, Kisielewski W, Kanczuga-Koda L, Koda M, Famulski W. Stromal and intraepithelial tumor-infiltrating lymphocytes in colorectal carcinoma. Oncol Lett. 2017;14:6421–6432. doi: 10.3892/ol.2017.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, Feyen N, Martens UM, Beckhove P, Gnjatic S, Schirmacher P, Herpel E, Weitz J, Grabe N, Jaeger D. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 41.Fritsche HM, Novara G, Burger M, Gupta A, Matsumoto K, Kassouf W, Sircar K, Zattoni F, Walton T, Tritschler S, Baba S, Bastian PJ, Martinez-Salamanca JI, Seitz C, Otto W, Wieland WF, Karakiewicz PI, Ficarra V, Hartmann A, Shariat SF. Macroscopic sessile tumor architecture is a pathologic feature of biologically aggressive upper tract urothelial carcinoma. Urol Oncol. 2012;30:666–672. doi: 10.1016/j.urolonc.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1:1303–1306. doi: 10.1016/S0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- 43.T. Luebke, S.E. Baldus, G. Grass, E. Bollschweiler, J. Thiele, H.P. Dienes, A.H. Hoelscher, S.P. Moenig, Histological Grading in Gastric Cancer by Ming Classification: Correlation with Histopathological Subtypes, Metastasis, and Prognosis. World J Surg 29 (Discussion 1428), 1422–1427 (2005). 10.1007/s00268-005-7795-z [DOI] [PubMed]
- 44.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 45.Wirta EV, Seppala T, Friman M, Vayrynen J, Ahtiainen M, Kautiainen H, Kuopio T, Kellokumpu I, Mecklin JP, Bohm J. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J Pathol Clin Res. 2017;3:203–213. doi: 10.1002/cjp2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, Mross F, Dieterich H, Moch H, Mihatsch M, Kallioniemi OP, Sauter G. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol. 2001;159:2249–2256. doi: 10.1016/S0002-9440(10)63075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz C, Seibt S, Al Kuraya K, Siraj AK, Mirlacher M, Schraml P, Maurer R, Spichtin H, Torhorst J, Popovska S, Simon R, Sauter G. Tissue microarrays for comparing molecular features with proliferation activity in breast cancer. Int J Cancer. 2006;118:2190–2194. doi: 10.1002/ijc.21581. [DOI] [PubMed] [Google Scholar]
- 48.Moch H, Schraml P, Bubendorf L, Mirlacher M, Kononen J, Gasser T, Mihatsch MJ, Kallioniemi OP, Sauter G. High-throughput tissue microarray analysis to evaluate genes uncovered by cDNA microarray screening in renal cell carcinoma. Am J Pathol. 1999;154:981–986. doi: 10.1016/S0002-9440(10)65349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, Torhorst J, Haas P, Bucher C, Sauter G, Kallioniemi OP, Kallioniemi A. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst. 2000;92:1252–1259. doi: 10.1093/jnci/92.15.1252. [DOI] [PubMed] [Google Scholar]
- 50.Dancau AM, Simon R, Mirlacher M, Sauter G. Tissue microarrays. Methods Mol Biol. 2010;576:49–60. doi: 10.1007/978-1-59745-545-9_4. [DOI] [PubMed] [Google Scholar]
- 51.Simon R, Mirlacher M, Sauter G. Immunohistochemical analysis of tissue microarrays. Methods Mol Biol. 2010;664:113–126. doi: 10.1007/978-1-60761-806-5_12. [DOI] [PubMed] [Google Scholar]
- 52.Simon R. Applications of tissue microarray technology. Methods Mol Biol. 2010;664:1–16. doi: 10.1007/978-1-60761-806-5_1. [DOI] [PubMed] [Google Scholar]
- 53.Mirlacher M, Simon R. Recipient block TMA technique. Methods Mol Biol. 2010;664:37–44. doi: 10.1007/978-1-60761-806-5_4. [DOI] [PubMed] [Google Scholar]
- 54.Buckley PJ, Dickson SA, Walker WS. Human splenic sinusoidal lining cells express antigens associated with monocytes, macrophages, endothelial cells, and T lymphocytes. J Immunol. 1985;134:2310–2315. [PubMed] [Google Scholar]
- 55.Ogembo JG, Milner DA, Jr, Mansfield KG, Rodig SJ, Murphy GF, Kutok JL, Pinkus GS, Fingeroth JD. SIRPalpha/CD172a and FHOD1 are unique markers of littoral cells, a recently evolved major cell population of red pulp of human spleen. J Immunol. 2012;188:4496–4505. doi: 10.4049/jimmunol.1103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 136066 kb)