Abstract
Objective
In this review, we have summarized the pharmacokinetics, pharmacodynamics and adverse effects of imatinib, dasatinib, nilotinib, bosutinib, ponatinib and radotinib with focus on pharmacogenomic studies with clinical end points. We have discussed the key phase 3 trials of tyrosine kinase inhibitors (TKI) comparing with each other, treatment free remission (TFR) and selection of TKI. Upcoming concepts and related trials in the management of chronic myeloid leukemia (CML) along with future directions have been touched upon.
Evidence acquisition
PubMed, Embase, Google, Cochrane library and Medline were searched to identify relevant literature for the review. Clinicaltrial.gov was searched for upcoming data and trials.
Results
There are lot of gap in pharmacokinetics and pharmacodynamics of TKI. Imatinib appears to be the safest TKI. Newer TKI’s achieve better achievement of therapeutic milestones, deeper molecular response and less chances of progression of CML compared to imatinib. Newer TKI appears to be better choice for achieving TFR. When the objective is survival, imatinib is still the TKI of choice. Primary prophylaxis with antiplatelet drugs for TKI having cardiovascular and thromboembolic side effects should be considered.
Conclusion
Pharmacogenetic data of TKI is still immature to guide in therapeutic decision making in clinical practice. There is need for further research in pharmacology and pharmacogenomics of newer TKI’s. Randomized controlled trials are required to decide the optimum TKI for TFR. Safe and effective TKI for targeting T315I mutation, CML accelerated phase and blast crisis are an active area of research.
Keywords: Gene polymorphism and imatinib, Pharmacology of tyrosine kinase inhibitors, Selection of tyrosine kinase inhibitors , Tyrosine kinase inhibitors in chronic myeloid leukemia
Introduction
The landscape of chronic myeloid leukemia (CML) has transformed in the last few years due to the advent of novel tyrosine kinase inhibitors (TKI) and trials demonstrating the feasibility of treatment free remission (TFR). At present, we have imatinib as first generation TKI; nilotinib, dasatinib, bosutinib, radotinib as second generation TKI and ponatinib as third generation TKI. The pharmacokinetics, pharmacodynamics and pharmacogenetics of imatinib has been well studied followed by dasatinib and nilotinib, whereas there is lacunae in existing literature regarding ponatinib, bosutinib and radotinib. Here, we aim to review the existing literature regarding pharmacology of each TKI, key phase 3 trials comparing them, TFR, choice of TKI in different clinical settings and upcoming studies on TKI.
Chronic myeloid leukemia
World Health Organization classifies haematological malignancies into myeloid neoplasm, lymphoid neoplasm and, histiocytic/dendritic neoplasm. Myeloid neoplasms are further classified into major groups of myeloproliferative neoplasm, myelodysplastic syndromes, myelodysplastic/myeloproliferative neoplasm and acute myeloid leukemia. CML belongs to the group of myeloproliferative neoplasm [1]. CML is characterized by abnormal fusion due to reciprocal translocation between chromosome 9 and 22, t(9;22)(q34;q11), which gives rise to an abnormal chromosome 22 called Philadelphia chromosome. This chromosome codes for BCR-ABL1 fusion gene which codes for tyrosine kinase (TK). This constitutionally active TK activates downstream signalling pathways resulting in uncontrolled proliferation. Gradually, additional mutations are acquired and CML-chronic phase (CML-CP) evolves into accelerated phase (AP) and blast phase (BP) [2]. TKI are the cornerstone of treatment of CML and allogeneic hematopoietic stem cell transplant has a role to play in CML-AP and CML-BC.
Methods
PubMed, Embase, Google, Cochrane library and Medline were searched to identify relevant literature in English language for the review. Clinicaltrial.gov was searched for upcoming data and trials. The following search terms were used: “pharmacokinetics” or “pharmacodynamics” and “imatinib”; “nilotinib”, “dasatinib”, “bosutinib”, “ponatinib” and “radotinib”. Relevant published guidelines and citations of references were studied. We selected only those pharmacokinetic and pharmacogenomic studies where end points were clinical outcomes. For topics where there were a large number of studies, we preferred important studies and published meta-analysis to summarize the data. We did not include in vitro studies unless clinical, effect of TKI’s on other drugs, TKI’s currently in trial and omacetaxine mepesuccinate. For the section “choice of TKI”,we selected only phase 3 trials.
Results and discussion
Imatinib
Pharmacology
Imatinib is the first targeted therapy developed for CML. Imatinib competitively inhibits BCR-ABL TK by binding at the ATP-binding site [3]. It is highly specific for BCR-ABL and inhibits growth and apoptosis in hematopoietic cells expressing BCR-ABL but spares the normal cells [3].
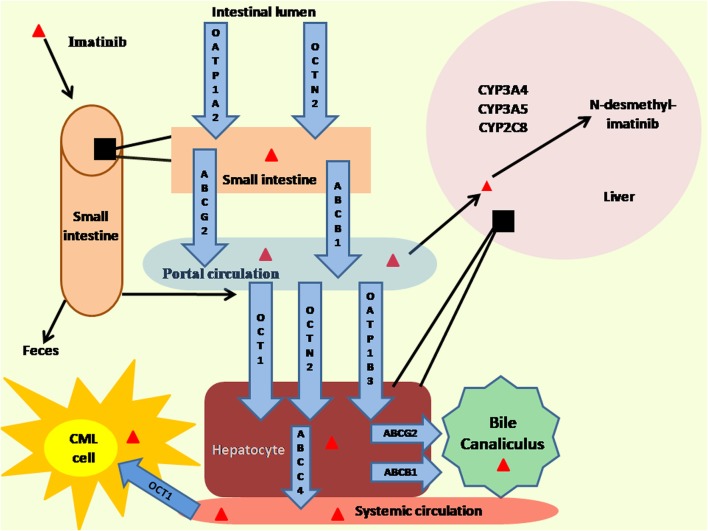
The metabolism of imatinib is summarized in Fig. 1. Imatinib is administered orally and is rapidly (t(max) 1–2 h) and completely absorbed through the gastrointestinal tract. It is not affected by food intake [4]. Absolute bioavailaibility of imatinib is more than 97% [5]. Elimination half life of imatinib is 18–20 h enabling once daily dosing [6]. Imatinib is actively transported in intestine and thought to be mediated by proteins, namely solute carrier organic anion transporter family member 1A2 (OATP1A2 (SLCO1A2)) and organic cation/carnitine transporter 2 (OCTN2(SLC22A5)) [7]. Imatinib is bound to α-1 acid glycoprotein (AGP) (95%) whereas the remaining is bound to albumin and erythrocytes. Organic anion-transporting polypeptide 1B3(OATP1B3),OCTN2, and organic cation-transporter 1(OCT1) are carriers which are speculated to transport imatinib from systemic circulation to hepatocytes [7]. In liver, ATP-binding cassette sub-family C member 4 (ABCC4) expressed on the basolateral membrane of hepatocytes could be a possible efflux transporter pumping imatinib from hepatocyte into systemic circulation [7]. The cellular efflux from hepatocyte to bile canaliculus is mediated by P-gp (ABCB) and ATP-binding cassette super-family G member 2 (ABCG2) [8]. Liver is the primary site of metabolism of imatinib by the cytochrome P450 complex. Majority of imatinib is metabolized in liver and a small fraction is excreted in bile and urine. Imatinib does not penetrate blood-brain barrier [7]. Imatinib influx into CML blast cells is mediated by OCT1(SLC22A1) [9].
Fig. 1.
Metabolism of imatinib. ABCB1: P-glycoprotein; ABCC4: ATP-binding cassette sub-family C member 4; ABCG2:ATP-binding cassette super-family G member 2; Cyp: Cytochrome; OATP: Solute carrier organic anion transporter family member; OCT1:Organic cation-transporter 1; OCTN2:Organic cation/carnitine transporter 2
Impact of OCT1 on clinical outcomes
Gene expression analysis in leukemia cell lines has shown that OCT1 expression is a composite surrogate for expression of transporters involved in intracellular uptake and retention of imatinib [10]. Patients with high pretreatment OCT1 had a significantly superior complete cytogenetic response (CCyR), progression free survival (PFS) and overall survival (OS); pretreament OCT1 emerged as the most important predictor of CCyR achievement at 6 months [11]. Data from patients enrolled in Trial of Imatinib with Dose Escalation in chronic myeloid Leukemia (TIDEL) and Tyrosine kinase inhibitor Optimization and Selectivity (TOPS) trial have shown that patients with high OCT1 activity achieved higher percentage of major molecular response (MMR) at 24 months, when compared with low OCT1 activity (85% vs 45%) [12, 13]. Patients with high OCT1 achieved MMR irrespective of the dose of imatinib used. Patients in low OCT1 group who received a higher dose of imatinib had significantly higher log reduction in MMR when compared with a patient who received lower dose of imatinib. This study demonstrated that low expression of OCT1 can be overcome by using higher dose of imatinib [14]. Updated data from TIDEL 1 showed that patients with high OCT-1 activity achieved significantly higher MMR at 60 months when compared to patients with low OCT-1 activity (89% vs 55%), higher OS (96% vs 87%) and higher event free survival (EFS) (74% vs 48%) and lower kinase domain mutation rate (4% vs 21%) [12]. Analysis of data from TOPS study showed that imatinib when administered at 400 mg/day, MMR was higher in patient with high OCT-1 activity, whereas at dose of 800 mg/day MMR was independent of OCT-1 activity and patients with low trough imatinib levels and low OCT-1 activity had lowest MMR and highest rate of imatinib failure [13]. Thus, OCT-1 activity appears to be a key determining factor for imatinib response, amenable to dose modification of imatinib and can be used to personalize imatinib dosing.
Polymorphism of OCT1 gene can influence intracellular uptake of imatinib in CML cells and can impact the outcome in CML. OCTN1 C allele (rs1050152) was significantly asscociated with deeper MMR [15]. The GG genotype at SLC22A1 (rs683369) was significantly associated with high rate of loss of response and treatment failure with Imatinib in Canadian population [16]. In a study of Malaysian patients G allele carriers of SLC22A1 C480G and A allele carriers of G1222A, were significantly associated with imatinib resistance [17]. Polymorphism of OATP1B3, which is encoded by SLCO1B3, can also influence therapeutic outcome of imatinib. In Indian patients SLCO1B3 334TT genotype was found to be significantly related with a higher risk of failure of cytogenetic response at 12 months though there was no significant difference in molecular response (MR) at 18 months [18]. In a study conducted in Brazil, SLCO1B3 699GG and 344TT genotypes were significantly associated with good imatinib response and carriers of 699GA/AA and 334TG/GG genotypes had significantly low chance of responding to standard dose of imatinib [19]. As these studies were done in limited ethnic subgroups and different polymorphisms were studied, further studies are required to understand the impact of OCT1gene polymorphism with clinical outcomes.
Effect of plasma protein binding of imatinib on clinical outcome
AGP is encoded by the ORM1gene. As AGP is the key molecule which binds to imatinib, polymorphism of ORM1 gene may affect the free drug availaibility and influence resistance to imatinib. In a study from France, patients with, higher level of AGP resulted in lower clearance of plasma imatinib and its main metabolite (CGP74588) [20]. Single nucleotide polymorphism (SNP) in AGP gene did not correlate with treatment failure of imatinib [16]. Unpublished data by Ankathil et al. [21] did not find any association of ORM1 520 G > A polymorphism with imatinib resistance, due to rarity of the genotype in Asian population. In populations, where the ORM1 520 G > A polymorphism is present at higher frequencies, it’s impact on imatinib resistance may be worth exploring.
Impact on clinical outcome of imatinib due to transport gene polymorphism
Genetic polymorphism of transporter genes can affect drug delivery and efficacy of imatinib. ABCB1gene which encodes Pgp has been studied with respect to imatinib pharmacogenetics in CML. Most frequent SNP polymorphism of ABCB1 gene are c.1236C > T, c.2677G > T/A and c.3435C > T [22]. A meta-analysis of 1826 patients analyzed the effect of these three SNP on imatinib response in CML patients [23]. On analysis, of ABCB1 c.1236C4 > T polymorphism, the 1236CC genotype was significantly associated with increased response to imatinib in Asian population. ABCB1 c.3435C > T polymorphism was significantly associated with better imatinib response and 3435 T allele was associated with worse response to imatinib [23]. Another meta-analysis of Asian patients found that ABCB1 C1236T polymorphism was associated with increased risk of imatinib resistance while, no association was found with ABCB1G2677T or C3435T polymorphism with imatinib resistance [24]. In contrast to the aforementioned meta-analysis, meta-analysis by Wang et al. [25] did not find any association of ABCB1 gene polymorphism with imatinib response. Another recent meta-analysis suggested that ABCB1 polymorphisms were not associated with MMR and complete molecular response (CMR) with use of imatinib [26]. These different results arise due to heterogenous studies in terms of study population, dose of drug, different inclusion and exclusion criteria and heterogenous endpoints of outcome.
In a meta-analysis of 771 patients, ABCG2 C421A polymorphism was significantly associated with higher MMR in patients receiving imatinib, specifically in those with Asian descent [26]. At present, data is heterogeneous and conflicting and requires further validation before being implemented in clinical practice.
Impact of cytochrome P450 on clinical outcomes
Imatinib is metabolized in the liver by CYP3A5, CYP3A4 and CYP2A8 to its predominant metabolite N-desmethyl-imatinib [27]. In Malaysian patients, heterozygous (AG) and homozygous variant (GG) genotype of CYP3A5*3 were significantly associated with low risk of imatinib resistance [28]. Similary, in a study from Egypt CYP3A5*3 polymorphism was associated with inferior outcome [29]. In a study from India, CYP1A1*2C wild type genotype (AA) was associated with poor cytogenetic response [30]. Apart from these, genetic polymorphism of glutathione-S-transferase, nuclear receptors and BIM gene have been studied in relation to therapeutic outcome of imatinib with variable results [21]. Though, polymorphism in imatinib metabolising genes have been identified, further studies are warranted for development of strategies which can modulate clinical outcome.
In contrast to above studies, a study of 112 CML patients, did not find any association of CYP3A5*3 (rs776746), CYP3A4*1 (rs2740574), CYP2C9*3 (rs1057910), SLC22A1 (rs683369), ABCB1(rs1045642, rs1128503), ABCG2 (rs2231142) and ABCC2 (rs717620) polymorphisms with imatinib plasma level and achieving an optimal clinical response was studied, did not find any association of the studied polymorphism with imatinib level and an optimal clinical response [31]. Unless well designed clinical trials explore the effects of transport molecules, gene polymorphism on clinical outcome and the appropriate dose of imatinb for such patients, it is not possible to tailor imatinib based on these variations.
Impact of therapeutic drug monitoring on clinical outcome
Imatinib plasma concentration has high interindividual variability with a coefficient of variation reported 50% and 45% for dose of 400 mg and 600 mg of imatinib respectively [32]. Imatinib trough plasma concentrations (Cmins) at day 29 were notably higher in patients who achieved CCyR and imatinib Cmin was predictive of higher CCyR independent of Sokal score [33]. In another study, Cmin in patients who achieved MMR was significantly higher than in subjects who didn’t achieve CMR and best sensitivity (77%) and best specificity (71%) for discriminating MMR was Cmin at 1002 ng/ml [34]. In a cohort of 254 Japanese patients with CML, Cmin > 1002 ng/ml had significantly higher probability of achieving MMR [35]. A review article has recommended Cmin of 1000 ng/ml as the pharamacokinetic target for CML and suggests increasing imatinib dose by 200 mg once daily stepwise [36]. Practically measuring imatinib trough concentration is difficult as the sample needs to be taken immediately before the administration of next dose and complex equipments are required, hence it is not performed routinely [37]. With the advent of newer TKI’s therapeutic drug monitoring of imatinib will have a limited role in future.
Adverse effects
Myelosuppression is a common in first 4–6 weeks after initiating imatinib. It is due to suppression of the leukemic clone and inhibition of normal haematopoiesis [38]. Myelosuppression is thought to be an expression of efficacy instead of drug toxicity [39]. In randomized trials grade 3/4 anemia has ranged from 5 to 7%, neutropenia 20–24% and thrombocytopenia 9–14% [40–42]. European LeukemiaNet (ELN) guidelines recommends to withhold drug at the first episode of grade 3/4 cytopenia till toxicity is less than grade 2 [39]. In case of recurrent episode imatinib can be restarted at lower dose and can be gradually escalated. Switching to alternate TKI is suggested in case of recurrent grade 3/4 cytopenia. G-CSF and erythropoetic agents can be administered to expedite recovery [39]. Grade 1 and 2 gastrointestinal side effects, nausea, diarrhoea, abdominal pain and vomiting are common whereas grade 3/4 toxicity is uncommon [39]. Skin side effects are also common ranging from 9.5% -69% [43]. They appear within the first 3–4 weeks of treatment [44]. They may occur in form of edema, maculopapular erythrematous rash, papulosquamous eruptions and pigmentary change. Musculoskeletal adverse effects such as myalgia and arthralgia are common, but serious events are rare [39]. Hepatotoxicity can occur with imatinib, grade 3/4 ranging from 3 to 6% which usually occur in first 2–8 weeks [45]. Imatinib causes hypophosphatemia in 25–49% of patients and decreased bone mineral density in approximately 50% of patients [40, 46, 47]. Thyroid abnormality was detected in 25% of patient treated with imatinib though majority were clinically asymptomatic [48]. Imatinib has does not male fertility. Females of reproductive age group are advised contraception as imatinib is teratogenic, though it is to be noted that normal pregnancies have also occurred when patients were on imatinib [49]. Effect of commonly prescribed drugs on imatinib have been summarized in Table 1.
Table 1.
Summary of tyrosine kinase inhibitors
| Characteristics | Imatinib | Dasatinib | Nilotinib | Bosutinib | Ponatinib | Radotinib |
|---|---|---|---|---|---|---|
| Tab strength | 100 mg,400 mg | 20 mg,50 mg, 70 mg, | 50 mg,150 mg,200 mg | 100 mg, | 15 mg, 45 mg | 100 mg, |
| 80 mg,100 mg, 140 mg | 500 mg | 200 mg | ||||
| Adult dose | ||||||
| CML-CP | 400 mg OD | 100 mg OD | 300 mg BD | 500 mg OD | 45 mg OD | 300 mg BD |
| CML-AP | 400 mg BD | 140 mg OD | 400 mg BD | 500 mg OD | Approved for T315I mutation | 400 mg BD |
| CML-BC | 400 mg BD | 140 mg OD | 400 mg BD | 500 mg OD | 400 mg BD | |
| Pediatric Dose | Not approved | Not approved | Not approved | |||
| CML-CP | 340 mg/sqm OD | 60 mg/sqm OD | 230 mg/sqm BD | |||
| CML-AP | 400 mg/sqmOD | 80 mg/sqm OD | 230 mg/sqm BD | |||
| CML-BC | 500 mg/sqmOD | 80 mg/sqm OD | 230 mg/sqm BD | |||
| Interaction with food | Taken with food | No interaction | Two hour before or one hour after food | Taken with food | No interaction | Not known |
| Common adverse effect | Fluid retention, Myalgia, arthralgia,GI-related, cytopenia | Pleural effusion, | Hyperlipidemia, hyperglycemia, myocardial infarction, stroke,peripheral artery occlusion, pancreatitis, QT prolongation | Diarrhea, elevated liver enzymes | Hypertension, | Myelosuppression, hepatotoxicity, hyperglycemia |
| Pulmonary artery hypertension,cytopenia Bleeding/platelet dysfunction | Abdominal pain, | |||||
| Arterial thrombosis.hepatotoxicity | ||||||
| Contraindication | None | None | Hypokalemia,hypomagnesemia, prolonged QT interval | Hypersensitivity | None | None |
| Black box warning for arterial thrombosis, hepatotoxicity | ||||||
| Drug interaction | Increased exposure: | Increased exposure: | Increased exposure: | Increased exposure: | Increased exposure: | Data not available |
| PPI,quinolones,azoles, CSA, valproic acid | PPI, Beta blocker,ACE inhibitor, macrolide,azole, CSA | Macrolides, valproic acid, cyclosporine,azoles | Azoles | Azole, protease inhibitor, macrolide, SRI | ||
| Decreased exposure: | Decreased exposure: | Decreased exposure: | Decreased exposure: | Decreaed exposure: | ||
| Dexamethosone, rifampicin, protease inhibitor, phenytoin carbamazepine, lamivudine,metformin | Dexamethasone, phenytoin, carbamazepine | Dexamethasone, phenytoin, carbamazepine, rifampicin | PPI, rifampicin | Rifampicin, carbamazepine, phenytoin | ||
| Drugs prolonging QT interval has additive effect | ||||||
| Female of childbearing age | Contraception advised | Contraception advised | Contraception advised | Contraception advised | Contraception advised | Data not available |
| Breast feeding | Contraindicated | Contraindicated | Contraindicated | Contraindicated | Contra-indicated | Data not available |
ACE angiotensin converting enzyme, AP accelerated phase, BC blast crisis, BD twice daily, CML chronic myeloid leukemia, CSA cyclosporine, GI gastrointestinal, OD once daily, PPI proton pump inhibitor, SRI serotononin reuptake inhibitor
Adverse effects specific to pediatric population
Growth deceleration occurs in prepubertal children. In a cohort of 108 patients 47.2% had growth impairment out of which 16.6% had grade 3 growth impairment [50]. In puberty catch-up growth occurs but its adequacy to attain the expected adult height is not known at present [51]. Imatinib dysregulates vitamin D, calcium and phosphate metabolism and has been reported to decrease bone mineral density in children [52, 53].
Dasatinib
Pharmacology
Dasatinib is a second generation TKI which inhibits c-KIT, BCR-ABL and SRC-family kinases, PDGFR-α and β and ephrin receptor kinase. Dasatinib binds to both active and inactive domain of BCR-ABL1 kinase protein [54]. Dasatinib is 325 times more potent than imatinib [55]. Dasatinib is administered orally, has rapid absorption with peak plasma concentration 0.5–3 h after administration. It is metabolized in liver by CYP3A4 and eliminated by fecal route. Dasatinib absorption is not influenced by food [54].
Imatinib uptake is predominantly mediated by OCT1 whereas dasatinib uptake is independent of OCT1 [56]. Dasatinib passively enters the cells [57]. In vivo studies suggest that ABCC4 (ATP Binding Cassette Subfamily C Member 4) mediates oral absorption of dasatinib from stomach [58]. Dasatinib efflux from the cell is mediated by ABCB1, ABCG2 and ABCC6 (ATP-binding cassette sub-family C member 6) [56, 57]. In vitro study on HEK293 and K562 cell lines have shown that ABCB1 variant (Asn400) is associated with lower intracellular dasatinib concentration due to increased efflux activity [59, 60]. In a retrospective study in Japanese population, natural killer group 2D receptor (NKG2D) HNK1/HNK1 (high-cytotoxic activity-related allele on NKG2D hb-1) haplotype was associated with quicker MMR [61, 62]. NKG2D gene polymorphisms need to be explored further biomarker for predictor of TFR. Data evaluating clinical outcomes of cytochrome polymorphism, efflux transporter protein encoding gene variants and therapeutic drug monitoring is limited.
Adverse effects
Dasatinib causes pleural effusions in 14–39% of patients. Median time of appearance is 5–11 months. It recurs in 70% of cases. They are managed by dose reduction or interruption, steroids and diuretics. Dasatinib can cause pulmonary hypertension in 0.45% of patients. Dasatinib has hypoglycaemic effect as it reduces fasting glucose [39]. Thyroid abnormalities were detected in 70% of patients though majority of them were subclinical and did not require therapy [48]. Dasatinib in frontline use caused grade 3/4 anemia, neutropenia and thrombocytopenia in 7%, 10% and 20% patients respectively [46]. In second and third line therapy dasatinib was associated with grade 3/4 anemia, neutropenia and thrombocytopenia in 19.2%, 45.8% and 47.2% patients respectively [39]. Management principles are same as imatinib. Dasatinib is associated with abortion and congenital abnormalities though uneventful pregnancies have also been documented. It has no clinically significant effect of male fertility [49]. In pediatric patients with CML, myalgia, arthralgia, bone growth impairment were reported, however pulmonary artery hypertension and effusions were not noted in children [63]. Drug interactions of dasatinib have been summarized in Table 1.
Nilotinib
Pharmacology
Nilotinib is 2nd generation TKI which competitively binds to the ATP binding site of the inactive conformation of BCR-ABL tyrosine kinase and it is 10–60 times more potent than imatinib. Nilotinib is administered orally and maximum mean concentration is achieved in median of 1.47 h with half life of 17 h therefore enabling daily dosing. Bioavailibility of nilotinib is 30% [60]. Food affects nilotinib absorption, hence nilotinib is advised to take 2 h before food or 1 h after food [64]. In vitro studies in cell line HEK293 and K562, suggests that ABCB1 variant (Asn 400) transports nilotinib from the cell, when compared to the wild variant [59, 60].
Nilotinib is 98% protein bound in in vitro studies. Nilotinib is predominantly metabolized by CYP3A4 [65, 66]. Patients with uridine diphosphate glucuronosyltransferase gene (UGT1A1) TA(7) polymorphism are susceptible to nilotinib induced hyperbilirubinemia [67, 68]. Clinical efficacy and toxicity of Nilotinib is not affected by OCT1 and ABCB1 polymorphism in contrast to imatinib [64].
In newly diagnosed CML-CP, Cmin of nilotinib was not associated with MMR [66]. In patients with imatinib resistant or intolerant CML, low Cmin of nilotinib was associated with longer time to achieve cytogenetic response and MMR [67, 68]. The potential role of therapeutic drug monitoring of nilotinib in optimizing nilotinib dose in imatinib resistant CML patients warrants further study.
Adverse effects
Nilotinib is associated with peripheral arterial occlusive disease, ischemic heart disease and cerebrovascular disease. Nilotinib is contraindicated in prolonged QT syndrome or if patients are on drugs causing prolonged QT syndrome. Hyperbilirubinemia is most frequent hepatic adverse effect [39]. In ENESTnd trial incidence of hyperglycemia was 6% and hypercholesterolemia 22% [40]. Hyperglycemia usually occurs in the first 2–3 week on initiation of nilotinib, and thus monitoring of blood gucose and lipid profile during treatment is advised [39]. Hypophospahtemia has been observed in 33% and thyroid abnormalities were seen in 55% patients, though they were rarely clinically significant [40, 48]. Grade 3/4 anemia, neutropenia and thrombocytopenia were observed in 3%, 10% and 12% patients respectively [40]. Common gastrointestinal adverse effects are nausea, diarrhea, abdominal pain and vomiting. Pancreatitis is rare ranging from 0.9 to 2% though lipase elevations have ranged from 29 to 47%. Nilotinib can cause fluid retention, skin rashes and myalgia [39]. Data on pregnancy outcome of nilotinib is limited. Though congenital anomalies have been reported with nilotinib, yet it appears that nilotinib is the safest TKI in pregnancy [49]. Effect of commonly prescribed drugs on nilotinib have been summarized in Table 1.
Bosutinib
Pharmacology
Bosutinib is a 2nd generation TKI which competitively inhibits Src and ABL tyrosine kinases [68]. Bosutinib is orally administered and attains peak plasma concentration in 3 to 6 h with bioavailability of 33.85% [69]. Half life ranges from 32.4 to 41.2 h enabling daily dose [69]. Bosutinib should be taken with food as food increases bosutinib solubility leading to enhanced absorption. Due to enhanced absorption, less bosutinib remains in the intestine leading to decrease gastrointestinal side effects [70]. Bosutinib is metabolized in liver by CYP3A4 [70]. In vitro and in vivo studies suggest that bosutinib efflux from the cell is mediated by P-gp, and its expression determines the intracellular concentration of bosutinib [71]. Drug transport molecues ABCG2 and SLC22A1 doesn’t appear to play role in bosutinib efflux [71, 72]. In Japanese patients, polymorphisms of ABCB1, ABCG2 and CYP3A4 did not influence bosutinib Cmin, however polymorphism of NR1I2 (Nuclear Receptor Subfamily 1 Group I member 2) influenced Cmin (notably, those with NR1I2 7635G/G and 8055 T/T genotype had lower Cmin) [73].The recommended dose of bosutinib is 400 mg once a day for CML-CP and 500 mg once daily for imatinib resistant and intolerant patients.
Adverse events
Diarrhea grade 3/4 is seen in 9–10% of patients. Hepatotoxicity with elevated aspartate transaminase/alanine transaminase is observed in 25–30% and 15–22% whereas grade 3/4 toxicity is seen in 11–9% and 6–9% patients respectively [46, 74, 75]. Grade 3/4 cardiac toxicity in the form of congestive cardiac failure and atrial fibrillation was seen 4% and 1% patients respectively [46]. Fatigue, rash, headache, edema and myalgia occur but grade 3/4 toxicity is rare [44, , ]. Grade 3/4 anemia, neutropenia and thrombocytopenia was seen in 3.5–11%, 6.7–16% and 16.4%–26% patients respectively [44, , ]. Effect of commonly prescribed drugs on bosutinib have been summarized in Table 1.
Ponatinib
Pharmacology
Ponatinib is 3rd generation TKI structurally designed to overcome the resistance of T315I mutation. Ponatinib is administered orally and peak concentration is achieved within 6 h [76]. It is metabolized in the liver by CYP3A4, CYP3A5, CYP2C8 and CYP2D6 to active metabolites [77]. Ponatinib is transported by P-gp and MRP1/2 whereas ABCB1, ABCG2 and OCT-1 are not involved in transport which are key in transport of imatinib [78, 79]. Ponatinib absorption is not affected by food intake [80]. Ponatinib Cmax correlates with the dose administered. A dose of 30 mg daily also appears to be sufficient to prevent resistance to ponatinib [81]. Ponatinib is administered at a dose of 45 mg once daily.
Adverse events
Rash, abdominal pain, headache, myalgia, arthralgia, fever, diarrhea and dry skin are common adverse effects but they are rarely grade ¾ [82, 83]. Grade 3/4 neutropenia and thrombocytopenia is seen in 4% and 12% respectively in newly diagnosed CML-CP patients. Lipase elevation grade 3/4 was seen in 14%. Hypertension of any grade was seen in 18% out of which 5% was grade 3/4 [82]. Serious adverse events cardiovascular(3%), cerebrovascular(2%) and peripheral vascular events (2%) were seen in newly diagnosed CML-CP patients [82]. In heavily pretreated patients grade 3/4 anemia(10%–32%), neutropenia (17%–37%) and thrombocytopenia (35%–44%) were seen [84]. Grade 3/4 hypertension was present in (8%–14%) and serious adverse effect cardiovascular (12%), cerebrovascular (10%), peripheral vascular(11%) and venous thromboembolism (5%) were seen in this heavily treated population [84]. Effect of commonly prescribed drugs on ponatinib have been summarized in Table 1.
Ponatinib is effective against patients who have received multiple TKI and achieves durable responses, with the drawback of serious thromboembolic events. Cardiovascular assessment and risk stratification should be done in patients who are considered to be candidates for ponatinib. Dose reduction of ponatinib is suggested as a strategy to reduce cardiovascular events in those at risk patient based on efficacy shown in trials and case series [81, 83, 85]. Patients at moderate risk of cardiovascular disease can be started at dose of 30 mg and reduced to 15 mg once patient achieves cytogenetic and molecular response. Patients who are already in molecular remission can be initiated at a dose of 15 mg [86]. Aspirin or clopidogrel as primary prophylaxis should be considered in all patients on ponatinib irrespective of dose because of very high risk of thromboembolic events, though at present data is lacking for the same [87].
Radotinib
Radotinib is second generation TKI with structure and resistance pattern similar to nilotinib [88]. Data for radotinib pharmacokinetics and pharmacodynamics is lacking. It is administered orally. Radotinib has been used in 300 mg twice daily and 400 mg twice daily; 300 mg twice daily was more effective and tolerable and is the approved dose [89].
Common toxicities were rash, pruritus, constipation, diarrhea, fatigue, myalgia and headache [89]. Grade 3/4 anemia (6%–10%), neutropenia (19%–24%) and thrombocytopenia (14%–16%) were seen in newly diagnosed CML-CP patients. Radotinib causes hepatotoxicity, grade 3/4 ALT elevation (20%–26%), AST elevation (5%–6%) and hyperbilirubinemia (27%–42%) were seen. Hyperglycemia was seen in 11% [89].
Which TKI to use?
With the advent of second generation and third generation TKI, each new TKI has been compared to imatinib in newly diagnosed CML-CP patients. Data from key phase 3 trials are summarized below.
Nilotinib vs Imatinib
Long term outcome of ENESTnd trial have been reported where imatinib has been compared to nilotinib 300 mg twice daily and nilotinib 400 mg twice daily in newly diagnosed CML-CP patients [90]. At the end of 5 years nilotinib significantly achieved higher MMR than imatinib. Number of progression to CML-AP/BC and deaths were significantly lower in nilotinib arm. Five year EFS, PFS and OS of nilotinib 400 mg twice daily was significantly higher than imatinib. Cardiovascular events were more with nilotinib 400 mg twice daily (all grade 13.4%, grade 3/4 8.7%), nilotinib 300 mg twice daily (all grade 7.5%, grade 3/4 4.7%) when compared to imatinib (all grade 2.1%, grade 3/4 1.8%). Hyperglycemia, increased lipase, liver enzymes and hyperbilirubinemia were also higher in nilotinib arms whereas hematological adverse events were higher in imatinib. On comparing the risk benefit ratio nilotinib 300 mg twice daily appears to be the appropriate dose [90]. In ENESTchina study patients on nilotinib 300 mg twice daily had significantly higher MMR at 12 and 24 months than patients on imatinib. Rates of CCyR at 24 months and the estimated rate of freedom from progression to CML-AP/BC were similar in both the arms [91].
Nilotinib is superior to imatinib in terms of depth of response and prevention of progression of CML-CP with a different toxicity profile. However, there was no notable difference in OS, EFS and PFS between the two drugs.
Dasatinib vs Imatinib
Dasatinib versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients (DASISION) a phase 3 randomized trial compared safety and efficacy of dasatinib with imatinib [46]. Cumulative 5 year MMR was significantly higher with dasatinib. Dasatinib achieved faster and deeper molecular responses, though OS and PFS were similar. Pleural effusion was seen in 28% vs 0.8%, pulmonary hypertension was seen in 5% vs 0.45% respectively with dasatinib and imatinib [46].
Dasatinib was similar to nilotinib and yielded better responses without benefit in OS. As OS rates are high, large sample size with longer follow up will be required to prove if nilotinib and dasatinib hav survival benefit over imatinib.
Bosutinib vs Imatinib
Bosutinib was compared with imatinib in, phase 3 Bosutinib Safety and Efficacy in Newly Diagnosed CML (BELA) randomized trial with primary end point of CCyR at 12 months [92]. MMR at 12 month and 24 month was significantly higher in bosutinib but there was no statistically significant difference in CCyR at 12 month and 24 month when compared with imatinib. There was no difference in EFS and OS at 2 years, though 2 years is a very short duration of follow up. Bosutinib had higher grade 3/4 diarrhea, increased ALT and vomiting. Imatinib had higher incidence of myalgia, muscle spasms, bone pain and peripheral edema though grade 3/4 toxicities were occasional. Bosutinib was not superior to imatinib in terms of primary end point CCyR but attained faster CCyR, superior MMR, and higher response rates at 3,6 and 9 months with a different toxicity profile [92]. In phase 3 randomized trial, Bosutinib Versus Imatinib for Newly Diagnosed CML (BFORE) trial MMR and CCyR at 12 month was significantly higher with bosutinib. Similar to BELA trial grade 3/4 gastrointestinal and hepatic toxicities were higher in bosutinib arm compared to imatinib arm [74].
Bosutinib appears to be a promising alternative of imatinib, but follow up duration of trial is short at present. Another phase 3 trial comparing with imatinib is undergoing (NCT02130557). There is a need to compare bosutinib with dasatinib or nilotinib in randomized controlled trials.
Ponatinib vs Imatinib
Ponatinib 45 mg once daily was compared with imatinib 400 mg once daily in phase 3 randomized trial to assess safety and efficacy wherein primary end point was MMR at 12 months [82]. The trial was terminated earlier due to vascular events with ponatinib reported in other trials. At 12 months only 13 patients on imatinib and 10 patients were available for evaluation of MMR out of which 5 and 8 patients respectively achieved MMR. At 3, 6 and 9 months percentage of patients achieving MMR and CCyR with ponatinib was significantly higher than imatinib. Out of 154 patients in the ponatinib arm five patients had cardiovascular, three cerebrovascular and three had peripheral vascular side effect. Grade 3/4 rash, abdominal pain hypertension, elevation in amylase and ALT were higher in ponatinib arm [82].
Multivariate analysis from pooled patients from trials of ponatinib has established that pancreatitis, cardiac failure, cardiovascular, cerebrovascular and atheroembolic events are significantly related to the dose of ponatinib [93]. The possibility of harnessing the unique activity of ponatinib and simultaneously by curtailing the serious adverse event with a dose of ponatinib is being explored in the OPTIC trial (NCT02467270) for upfront CML patients and OPUS trial (NCT02398825) in imatinib resistant CML-CP.
Radotinib vs Imatinib
Radotinib 300 mg twice daily and 400 mg twice daily was compared to imatinib 400 mg once daily in phase 3 randomized, Results of Safety and Efficacy of Radotinib Compared with Imatinib in Newly Diagnosed Chronic Phase CML (RERISE) trial [89]. At 12 months MMR rates were significantly higher in patients receiving radotinib 300 mg twice a day and radotinib 400 mg twice daily compared with imatinib 400 mg twice a day. The CCyR rate at 12 months with radotinib 300 mg twice daily was significantly higher when compared with imatinib. CCyR at 12 months with radotinib at a dose of 400 mg twice daily was not significantly different from imatinib. Radotinib had higher grade 3/4 elevated AST, elevated ALT, hyperbilirubinemia and elevated lipase compared to imatinib. Hematologic toxicities were less in radotinib 300 mg twice daily compared to radotinib 400 mg twice daily. Other toxicities were similar in all the three arms [89].
The follow up of RERISE trial is of short duration and done in Asian ethnicity with smaller body size. Tolerability of radotinib 300 mg twice daily was better than 400 mg twice daily, which can be attributed to small body size. There is evidence to suggest that radotinib 400 mg twice daily might be an appropriate dose for patients weighing more than 65 kg [94]. Further studies are required to determine the appropriate dose of radotinib, specially in other ethnicities.
Treatment free remission: Our current goal
Treatment free remission(TFR) is defined as a sustained deep molecular response after cessation of TKI in selected patients with predefined criteria [95]. Clinical trials have studied the stopping of imatinib and second generation TKI in CML. Discontinuation of imatinib has resulted in TFR outcome ranging from 39 to 61% [96–100]. All trials recruited patients with MR4(log reduction in BCR-ABL 4) for a year and with a median duration of therapy for more than 3 years [95–99]. Nilotinib when used in first line yielded a TFR of 48.9% [101]. Second generation TKI when used in second line resulted in TFR ranging from 48 to 63.3% [102, 103]. At present, patients older than 18 years, diagnosed in CML-CP with TKI treatment duration more than 5 years, MR4.5, deep molecular response duration of more than 2 years, and those who had an optimal response to TKI are recommended for TFR [104].
In EURO-SKI trial on the last day of TKI intake expression of ABCB1, OCT1 and ABCG2 were analyzed. Only high ABCG2 expression correlated with TFR, suggesting the role of ABCG2 as a predictive marker for TFR [105].
How and what to choose?
ELN guidelines recommend imatinib, nilotinib and dasatinib as first line options in CML-CP whereas NCCN guidelines recommend bosutinib in addition to these three [106, 107]. Ponatinib is currently approved only in patients with T315I-positive CML-CP/AP/BP or in situations where no other TKI can be used, hence it is the only TKI not used in first line [108]. Due to multiple TKI options and life of CML patients approaching that of normal population, choice of TKI depends on risk category, treatment goals, age, comorbidities, cost, ease of administration and availability of TKI [109, 110]. Sokal, Euro and European Treatment and Outcome Study (EUTOS) scores are used to predict outcome in CML-CP patients [107]. Nilotinib and dasatinib attained higher MMR and MR4.5 irrespective of risk category [46, 90] Nilotinib showed OS and PFS benefit for intermediate and high risk patients based on Sokal score, though they were not the primary objective [90]. High risk Sokal patients on bosutinib attained higher MMR compared to imatinib, though follow up was only 1 year [74]. Thus, second generation TKI should be preferred in intermediate and high risk category of CML patients [106].
Second and third generation TKI attain earlier, deeper response and fewer chances of progression to CML-AP/BC as compared to imatinib ue in newly diagnosed CML-CP patients. In all phase 3 trials primary end points of second and third generation have been met except with ponatinib which was terminated earlier. However, survival has not been the primary end point of these trials and there is no significant difference in survival when compared with imatinib [109]. In younger patients TFR is a desirable goal. For TFR a TKI with early, deep and sustained response is required, hence second generation TKI is the drug of choice. Elderly patients where survival is the goal imatinib will still be the preferred drug due to its safety profile [109].
Comorbidities play a further role in refining the choice of TKI. In patients with cardiovascular risk factors and diabetes mellitus, dasatinib or bosutinib is preferred over nilotinib. Nilotinib or bosutinib is preferred in patients with lung diseases, pulmonary hypertension and patients who are at risk of bleeding [106]. For other patients considering molecular response as end point nilotinib appears to be the best drug [111]. The drawback of nilotinib is twice daily administration and timing in relation to food. Bosutinib is not preferred in patient with pancreatitis, liver disease, inflammatory bowel disease and peptic ulcer disease. In patients with a history of pancreatitis dasatinib is the preferred drug [112]. Imatinib had the most favourable safety profile when it was compared with other TKI in a meta-analysis [111]. Imatinib can be used irrespective of any comorbidities. Generic imatinib is available and is comparatively cheaper. In many countries due to lower cost of imatinib and maximum clinical experience, it is still the first-line drug in CML-CP. Radotinib is currently approved only in Korea in the upfront setting for CML-CP [88].
Newer strategies in CML treatment: Upcoming trials
There is a need to explore the pharmacokinetics and pharmacogenetics of second and third generation TKI and how they impact clinical outcomes. At present, association of dasatinib, nilotinib, imatinib and bosutinib pharmacokinetic parameters with clinical outcome, toxicities and germline genetic variant are being studied (NCT03885830). Studies on the effect of genetic polymorphism of imatinib metabolizing enzymes and membrane transporter on molecular response of imatinib are going on (NCT03262974). The results of these studies will help in deciding the choice of TKI based on pharmacogenetic profile of the patient.
TKI tolerability and toxicity depend on dose. Dose reduction is a potential strategy to mitigate the adverse effects. Ponatinib dose reduction studies are under going (NCT02467270, NCT02398825) as mentioned above. Similarly, efficacy and safety of dasatinib 50 mg vs 100 mg daily is being evaluated in phase II trial (NCT03625388).
Newer TKI yield better responses than imatinib at the expense of cost and different toxicity profile. So switching TKI during maintenance is another possible option. A study where nilotinib is used to achieve cytogenetic response and subsequently imatinib will be used as maintenance is ongoing (NCT01316250).
ABL001(asciminib) is first allosteric inhibitor of BCR-ABL1 and is currently undergoing phase 3 trial (NCT03106779) with bosutinib as comparator arm in previously treated CML-CP with two or more TKI [113, 114]. In a three-arm trial, asciminib and imatinib combination is being compared with imatinib and nilotinib NCT03578367. K0706 a novel TKI, is currently under phase1/2 trial (NCT02629692) [115]. HQP1351 a multikinase inhibitor is undergoing phase 2 trial(NCT03883087) in CML-CP and in CML-AP(NCT03883100) with T315I mutations [116, 117]. PF-114 is a third generation TKI which is active against T315I and has completed phase I clinical trial [118]. Apart from ponatinib,T315I can be targeted in future, with these new molecules.
Drugs are being identified to administer in combination with TKI or sequentially after TKI administration to increase molecular response(NCT02767063). Ruxolitinib in combination with TKI (dasatinib or nilotinib) is being compared with single TKI(nilotinib or dasatinib) in randomized phase two trial (NCT03654768) [119].
Combination of TKI’s is another novel approach to enhance efficacy of TKI’s in CML. Nilotinib is being combined with interferon to attain deeper molecular response in phase III TIGER trial (NCT01657604) and another study (NCT02201459). Similarly,bosutinib is being combined with ropeginterferon (NCT03831776).
Attaining TFR is the new goal in CML, yet a major proportion of patient do not attain CMR. Patients who fail to achieve CMR on TKI are being investigated for the role of ruxolitinib (NCT01751425) and pembrolizumab (NCT03516279) to attain CMR.
CML-AP/BC has poor outcomes. DNA methylation of genes is a key event during progression of CML-AP/BC. Hence, a combination of hypomethylating agents with TKI are being investgated. Dastinib is being combined with decitabine in phase I/II trials for CML-AP/BC (NCT01498445).Ponatinib and 5-azacytidine combination are being studied in CML-AP/BC (NCT03895671). Combination of axitinib with bosutinib is also being evaluated in phase I/II trial (NCT02782403).
Future direction of TKI in CML
In this review we have summarized the key pharmacogenetic studies of imatinib and other TKI’s. However, the interpretation of these studies is limited due to lack of adequate statistical power and heterogeneous nature of the studies. There is an unmet need for multicentric, multiethnic well designed clinical trials with uniform and relevant end points to establish the role of pharmacogenetics in predicting TKI response and outcome. As CML progression on TKI still has poor outcomes, it is imperative to identify pharmacogenetic predictors of CML progression in patients treated with TKI.
There is a lack of studies delineating the detailed pharmacokinetics and pharmacodynamics of the newer TKI’, specially bosutinib, ponatinib and radotinib. Clinical trials studying newer TKI’s should also explore the molecules and their gene polymorphisms which are involved in transport and metabolism of these TKI.
TFR has emerged as new goal of CML treatment. Second generation TKI appears to be better than imatinib in attaining TFR than TKI, but it needs to be established in randomized control trial with TFR as an endpoint. Simultaneously, pharmacogenomics needs to be integrated in these trial so that pharmacogenetic predictor of TFR can also be identified. Sequential use of TKI’s needs to be explored to attain a deeper TFR than the conventional single agent TKI.
Nilotinib has cardiovascular side effects and ponatinib has life threatening thromboembolic events. We need data to define the optimal strategy for reduction of cardiovascular risk in patients on nilotinib and thromboembolic prophylaxis in patients on ponatinib. At present ponatinib is the only TKI effective against T315I. There is need for TKI which do not have life threatening side effect and are yet active against T315I mutation.
Pediatric CML is an understudied area. Optimal choice of TKI in pediatric patient and feasiblity of TFR in pediatric population needs to be studied. Pharmacokinetics and pharmacodynamics of TKI may not be the same in pediatric population as adult population and these need to be delineated.
Conclusion
We present here a comparative review of the pharmacokinetics, pharmacogenomics, clinical usage, efficacy and toxicity data of TKIs used for CML. Pharmacogenetic data for TKI at present are not mature enough to be implemented in clinical practice. Though, phase 3 trials, have been conducted for newer TKI’s there are lacunae in the pharmacology of newer TKI’s. Imatinib is still the most commonly used TKI worldwide. Where survival is the goal, imatinib appears to be a safe, cheap and reasonable option. When the goal is TFR second generation TKI appears to have greater potential, though trials are required to address this.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. Epstein FH, editor. N Engl J Med. 1999;341(3):164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and Safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 4.Gschwind H-P, Pfaar U, Waldmeier F, Zollinger M, Sayer C, Zbinden P, Hayes M, Pokorny R, Seiberling M, Ben-Am M, Peng B, Gross G. Metabolism and disposition of imatinib mesylate in healthy volunteers. Drug Metab Dispos Biol Fate Chem. 2005;33(10):1503–1512. doi: 10.1124/dmd.105.004283. [DOI] [PubMed] [Google Scholar]
- 5.Peng B, Dutreix C, Mehring G, Hayes MJ, Ben-Am M, Seiberling M, Pokorny R, Capdeville R, Lloyd P. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004;44(2):158–162. doi: 10.1177/0091270003262101. [DOI] [PubMed] [Google Scholar]
- 6.Peng B, Lloyd P, Schran H. Clinical Pharmacokinetics of Imatinib. Clin Pharmacokinet. 2005;44(9):879–894. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- 7.Eechoute K, Sparreboom A, Burger H, Franke RM, Schiavon G, Verweij J, et al. Drug transporters and Imatinib treatment: implications for clinical practice. Clin Cancer Res. 2011;17(3):406–415. doi: 10.1158/1078-0432.CCR-10-2250. [DOI] [PubMed] [Google Scholar]
- 8.Burger H, van Tol H, Brok M, Wiemer EAC, de Bruijn EA, Guetens G, de Boeck G, Sparreboom A, Verweij J, Nooter K. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005;4(7):747–752. doi: 10.4161/cbt.4.7.1826. [DOI] [PubMed] [Google Scholar]
- 9.Thomas J. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104(12):3739–3745. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, Burger H, Baker SD, Sparreboom A. Interaction of Imatinib with human organic ion carriers. Clin Cancer Res. 2008;14(10):3141–3148. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Giannoudis A, Lane S, Williamson P, Pirmohamed M, Clark R. Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to Imatinib in chronic myeloid leukemia. Clin Pharmacol Ther. 2008;83(2):258–264. doi: 10.1038/sj.clpt.6100268. [DOI] [PubMed] [Google Scholar]
- 12.White DL, Dang P, Engler J, Frede A, Zrim S, Osborn M, Saunders VA, Manley PW, Hughes TP. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with Imatinib. J Clin Oncol. 2010;28(16):2761–2767. doi: 10.1200/JCO.2009.26.5819. [DOI] [PubMed] [Google Scholar]
- 13.White DL, Radich J, Soverini S, Saunders VA, Frede AK, Dang P, Cilloni D, Lin P, Mongay L, Woodman R, Manley P, Slader C, Kim DW, Pane F, Martinelli G, Saglio G, Hughes TP. Chronic phase chronic myeloid leukemia patients with low OCT-1 activity randomized to high-dose imatinib achieve better responses and have lower failure rates than those randomized to standard-dose imatinib. Haematologica. 2012;97(6):907–914. doi: 10.3324/haematol.2011.056457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S, Zannettino A, Lynch K, Manley PW, Hughes T. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110(12):4064–4072. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- 15.Angelini S, Soverini S, Ravegnini G, Barnett M, Turrini E, Thornquist M, Pane F, Hughes TP, White DL, Radich J, Kim DW, Saglio G, Cilloni D, Iacobucci I, Perini G, Woodman R, Cantelli-Forti G, Baccarani M, Hrelia P, Martinelli G. Association between imatinib transporters and metabolizing enzymes genotype and response in newly diagnosed chronic myeloid leukemia patients receiving imatinib therapy. Haematologica. 2013;98(2):193–200. doi: 10.3324/haematol.2012.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DH, Sriharsha L, Xu W, Kamel-Reid S, Liu X, Siminovitch K, Messner HA, Lipton JH. Clinical relevance of a Pharmacogenetic approach using multiple candidate genes to predict response and resistance to Imatinib therapy in chronic myeloid leukemia. Clin Cancer Res. 2009;15(14):4750–4758. doi: 10.1158/1078-0432.CCR-09-0145. [DOI] [PubMed] [Google Scholar]
- 17.Makhtar SM, Husin A, Baba AA, Ankathil R. Genetic variations in influx transporter gene SLC22A1 are associated with clinical responses to imatinib mesylate among Malaysian chronic myeloid leukaemia patients. J Genet. 2018;97(4):835–842. [PubMed] [Google Scholar]
- 18.Nair D, Dhangar S, Shanmukhaiah C, Vundinti BR. Association of genetic polymorphisms of the ABCG2, ABCB1, SLCO1B3 genes and the response to Imatinib in chronic myeloid leukemia patients with chronic phase. Meta Gene. 2017;11:14–19. [Google Scholar]
- 19.de Lima LT, Bueno CT, Vivona D, Hirata RDC, Hirata MH, Hungria, VT de M, et al. Relationship between SLCO1B3 and ABCA3 polymorphisms and imatinib response in chronic myeloid leukemia patients. Hematology. 2015;20(3):137–142. [DOI] [PubMed]
- 20.Petain A, Kattygnarath D, Azard J, Chatelut E, Delbaldo C, Geoerger B, Barrois M, Séronie-Vivien S, LeCesne A, Vassal G, Innovative Therapies with Children with Cancer European consortium Population pharmacokinetics and Pharmacogenetics of Imatinib in children and adults. Clin Cancer Res. 2008;14(21):7102–7109. doi: 10.1158/1078-0432.CCR-08-0950. [DOI] [PubMed] [Google Scholar]
- 21.Ankathil R, Azlan H, Dzarr AA, Baba AA. Pharmacogenetics and the treatment of chronic myeloid leukemia: how relevant clinically? An update. Pharmacogenomics. 2018;19(5):475–393. doi: 10.2217/pgs-2017-0193. [DOI] [PubMed] [Google Scholar]
- 22.Wong M, Evans S, Rivory LP, Hoskins JM, Mann GJ, Farlow D, Clarke CL, Balleine RL, Gurney H. Hepatic technetium Tc 99m-labeled sestamibi elimination rate and ABCB1 (MDR1) genotype as indicators of ABCB1 (P-glycoprotein) activity in patients with cancer. Clin Pharmacol Ther. 2005;77(1):33–42. doi: 10.1016/j.clpt.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Q, Wu H, Yu Q, Kim DH, Lipton JH, Angelini S, Soverini S, Vivona D, Takahashi N, Cao J. ABCB1 polymorphisms predict imatinib response in chronic myeloid leukemia patients: a systematic review and meta-analysis. Pharmacogenomics J. 2015;15(2):127–134. doi: 10.1038/tpj.2014.54. [DOI] [PubMed] [Google Scholar]
- 24.Zu B, Li Y, Wang X, He D, Huang Z, Feng W. MDR1 gene polymorphisms and imatinib response in chronic myeloid leukemia: a meta-analysis. Pharmacogenomics. 2014;15(5):667–677. doi: 10.2217/pgs.13.222. [DOI] [PubMed] [Google Scholar]
- 25.Wang JL, Liu HJ, Li F, Yang WY, Wang JM, Tan SF, Wang Q. Multidrug resistance gene (MDR1) polymorphisms may not be directly associated with response to imatinib in chronic myeloid leukemia. Genet Mol Res. 2015;14(4):14967–14978. doi: 10.4238/2015.November.24.4. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Z-P, Zhao X-L, Takahashi N, Angelini S, Dubashi B, Sun L, Xu P. Trough concentration and ABCG2 polymorphism are better to predict imatinib response in chronic myeloid leukemia: a meta-analysis. Pharmacogenomics. 2017;18(1):35–56. doi: 10.2217/pgs-2016-0103. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Sutiman N, Chowbay B. Pharmacogenetics of drug transporters in modulating imatinib disposition and treatment outcomes in chronic myeloid leukemia & gastrointestinal stromal tumor patients. Pharmacogenomics. 2016;17(17):1941–1955. doi: 10.2217/pgs-2016-0124. [DOI] [PubMed] [Google Scholar]
- 28.Maddin N, Husin A, Gan SH, Aziz BA, Ankathil R. Impact of CYP3A4*18 and CYP3A5*3 polymorphisms on Imatinib Mesylate response among chronic myeloid leukemia patients in Malaysia. Oncol Ther. 2016;4(2):303–314. doi: 10.1007/s40487-016-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedewy AML, El-Maghraby SM. Do SLCO1B3 (T334G) and CYP3A5*3 polymorphisms affect response in Egyptian chronic myeloid leukemia patients receiving imatinib therapy? Hematol Amst Neth. 2013;18(4):211–216. doi: 10.1179/1607845412Y.0000000067. [DOI] [PubMed] [Google Scholar]
- 30.Lakkireddy S. Association of the Common CYP1A1*2C variant (Ile462Val polymorphism) with chronic myeloid leukemia (CML) in patients undergoing Imatinib therapy. Cell J. 2015;17(3):10. doi: 10.22074/cellj.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belohlavkova P, Vrbacky F, Voglova J, Racil Z, Zackova D, Hrochova K, Malakova J, Mayer J, Zak P. The significance of enzyme and transporter polymorphisms for imatinib plasma levels and achieving an optimal response in chronic myeloid leukemia patients. Arch Med Sci. 2018;14(6):1416–1423. doi: 10.5114/aoms.2018.73538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Titier K, Picard S, Ducint D, Teilhet E, Moore N, Berthaud P, Mahon FX, Molimard M. Quantification of imatinib in human plasma by high-performance liquid chromatography-tandem mass spectrometry. Ther Drug Monit. 2005;27(5):634–640. doi: 10.1097/01.ftd.0000175973.71140.91. [DOI] [PubMed] [Google Scholar]
- 33.Larson RA, Druker BJ, Guilhot F, O’Brien SG, Riviere GJ, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111(8):4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 34.Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard M-A, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109(8):3496–3499. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi N, Wakita H, Miura M, Scott SA, Nishii K, Masuko M, Sakai M, Maeda Y, Ishige K, Kashimura M, Fujikawa K, Fukazawa M, Katayama T, Monma F, Narita M, Urase F, Furukawa T, Miyazaki Y, Katayama N, Sawada K. Correlation between imatinib pharmacokinetics and clinical response in Japanese patients with chronic-phase chronic myeloid leukemia. Clin Pharmacol Ther. 2010;88(6):809–813. doi: 10.1038/clpt.2010.186. [DOI] [PubMed] [Google Scholar]
- 36.Yu H, Steeghs N, Nijenhuis CM, Schellens JHM, Beijnen JH, Huitema ADR. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53(4):305–325. doi: 10.1007/s40262-014-0137-2. [DOI] [PubMed] [Google Scholar]
- 37.Martins DH, Wagner SC, dos Santos TV, Lizot L d LF, Antunes MV, Capra M, et al. Monitoring imatinib plasma concentrations in chronic myeloid leukemia. Rev Bras Hematol Hemoter. 2011;33(4):302–306. doi: 10.5581/1516-8484.20110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sneed TB, Kantarjian HM, Talpaz M, O’Brien S, Rios MB, Bekele BN, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100(1):116–121. doi: 10.1002/cncr.11863. [DOI] [PubMed] [Google Scholar]
- 39.Steegmann JL, Baccarani M, Breccia M, Casado LF, García-Gutiérrez V, Hochhaus A, Kim DW, Kim TD, Khoury HJ, le Coutre P, Mayer J, Milojkovic D, Porkka K, Rea D, Rosti G, Saussele S, Hehlmann R, Clark RE. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30(8):1648–1671. doi: 10.1038/leu.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim D-W, Flinn IW, Kurokawa M, Moiraghi B, Yu R, Blakesley RE, Gallagher NJ, Saglio G, Kantarjian HM. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 41.Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, Wang J, Ipiña JJ, Kim DW, Ogura M, Pavlovsky C, Junghanss C, Milone JH, Nicolini FE, Robak T, van Droogenbroeck J, Vellenga E, Bradley-Garelik MB, Zhu C, Hochhaus A. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes JE, Kim D-W, Kantarjian HM, Brümmendorf TH, Dyagil I, Griskevicius L, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30(28):3486–3492. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5(3):228–231. [PubMed] [Google Scholar]
- 44.Brazzelli V, Grasso V, Borroni G. Imatinib, dasatinib and nilotinib: a review of adverse cutaneous reactions with emphasis on our clinical experience. J Eur Acad Dermatol Venereol. 2013;27(12):1471–1480. doi: 10.1111/jdv.12172. [DOI] [PubMed] [Google Scholar]
- 45.Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36(7):491–503. doi: 10.1007/s40264-013-0048-4. [DOI] [PubMed] [Google Scholar]
- 46.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: the Dasatinib versus Imatinib study in treatment-Naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;4(20):2333–2340. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berman E, Girotra M, Cheng C, Chanel S, Maki R, Shelat M, et al. Effect of long term imatinib on bone in adults with chronic myelogenous leukemia and gastrointestinal stromal tumors. Leuk Res. 2013;37(7):790–794. doi: 10.1016/j.leukres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Kim TD, Schwarz M, Nogai H, Grille P, Westermann J, Plöckinger U, Braun D, Schweizer U, Arnold R, Dörken B, le Coutre P. Thyroid dysfunction caused by second-generation tyrosine kinase inhibitors in Philadelphia chromosome-positive chronic myeloid leukemia. Thyroid. 2010;20(11):1209–1214. doi: 10.1089/thy.2010.0251. [DOI] [PubMed] [Google Scholar]
- 49.Abruzzese E, Trawinska MM, de Fabritiis P, Baccarani M. Management of pregnant chronic myeloid leukemia patients. Expert Rev Hematol. 2016;9(8):781–791. doi: 10.1080/17474086.2016.1205479. [DOI] [PubMed] [Google Scholar]
- 50.Suttorp M, Schulze P, Glauche I, Göhring G, von Neuhoff N, Metzler M, Sedlacek P, de Bont ESJM, Balduzzi A, Lausen B, Aleinikova O, Sufliarska S, Henze G, Strauss G, Eggert A, Kremens B, Groll AH, Berthold F, Klein C, Groß-Wieltsch U, Sykora KW, Borkhardt A, Kulozik AE, Schrappe M, Nowasz C, Krumbholz M, Tauer JT, Claviez A, Harbott J, Kreipe HH, Schlegelberger B, Thiede C. Front-line imatinib treatment in children and adolescents with chronic myeloid leukemia: results from a phase III trial. Leukemia. 2018;32(7):1657–1669. doi: 10.1038/s41375-018-0179-9. [DOI] [PubMed] [Google Scholar]
- 51.Samis J, Lee P, Zimmerman D, Arceci RJ, Suttorp M, Hijiya N. Recognizing Endocrinopathies associated with tyrosine kinase inhibitor therapy in children with chronic Myelogenous leukemia: TKI-associated Endocrinopathies in pediatric CML. Pediatr Blood Cancer. 2016;63(8):1332–1338. doi: 10.1002/pbc.26028. [DOI] [PubMed] [Google Scholar]
- 52.Giona F, Mariani S, Gnessi L, Moleti ML, Rea M, De Vellis A, et al. Bone metabolism, growth rate and pubertal development in children with chronic myeloid leukemia treated with imatinib during puberty. Haematologica. 2013;98(3):e25–e27. doi: 10.3324/haematol.2012.067447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaeger BAS, Tauer JT, Ulmer A, Kuhlisch E, Roth HJ, Suttorp M. Changes in bone metabolic parameters in children with chronic myeloid leukemia on imatinib treatment. Med Sci Monit. 2012;18(12):CR721–CR728. doi: 10.12659/MSM.883599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindauer M, Hochhaus A. Dasatinib. In: Martens UM, editor. Small molecules in oncology. Berlin: Springer Berlin Heidelberg; 2010. pp. 83–102. [Google Scholar]
- 55.O’Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 56.Giannoudis A, Davies A, Lucas CM, Harris RJ, Pirmohamed M, Clark RE. Effective dasatinib uptake may occur without human organic cation transporter 1 (hOCT1): implications for the treatment of imatinib-resistant chronic myeloid leukemia. Blood. 2008;112(8):3348–3354. doi: 10.1182/blood-2007-10-116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P, Eadie L, To LB, Melo J, Kumar S, Hughes TP, White DL. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14(12):3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 58.Furmanski BD, Hu S, Fujita K-I, Li L, Gibson AA, Janke LJ, et al. Contribution of ABCC4-mediated gastric transport to the absorption and efficacy of dasatinib. Clin Cancer Res Off J Am Assoc Cancer Res. 2013;19(16):4359–4370. doi: 10.1158/1078-0432.CCR-13-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dessilly G, Elens L, Panin N, Karmani L, Demoulin J-B, Haufroid V. ABCB1 1199G>a polymorphism (rs2229109) affects the transport of imatinib, nilotinib and dasatinib. Pharmacogenomics. 2016;17(8):883–890. doi: 10.2217/pgs-2016-0012. [DOI] [PubMed] [Google Scholar]
- 60.Garnock-Jones KP. Nilotinib: in the first-line treatment of newly diagnosed Philadelphia chromosome-positive chronic myeloid Leukaemia in chronic phase. Drugs. 2011;71(12):1579–1590. doi: 10.2165/11207770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Hara R, Onizuka M, Matsusita E, Kikkawa E, Nakamura Y, Matsushita H, Ohgiya D, Murayama H, Machida S, Ohmachi K, Shirasugi Y, Ogawa Y, Kawada H, Ando K. NKG2D gene polymorphisms are associated with disease control of chronic myeloid leukemia by dasatinib. Int J Hematol. 2017;106(5):666–674. doi: 10.1007/s12185-017-2294-1. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka C, Yin OQP, Sethuraman V, Smith T, Wang X, Grouss K, Kantarjian H, Giles F, Ottmann OG, Galitz L, Schran H. Clinical pharmacokinetics of the BCR–ABL tyrosine kinase inhibitor Nilotinib. Clin Pharmacol Ther. 2010;87(2):197–203. doi: 10.1038/clpt.2009.208. [DOI] [PubMed] [Google Scholar]
- 63.Gore L, Kearns PR, de Martino ML, Lee, De Souza CA, Bertrand Y, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330–1338. doi: 10.1200/JCO.2017.75.9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galimberti S, Bucelli C, Arrigoni E, Baratè C, Grassi S, Ricci F, et al. The hOCT1 and ABCB1 polymorphisms do not influence the pharmacodynamics of nilotinib in chronic myeloid leukemia. Oncotarget. 2017;20:8(50). doi: 10.18632/oncotarget.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nilotinib. FDA. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022068s004s005lbl.pdf. Accessed on 18 Mar 2019.
- 66.Larson RA, Yin OQP, Hochhaus A, Saglio G, Clark RE, Nakamae H, Gallagher NJ, Demirhan E, Hughes TP, Kantarjian HM, le Coutre PD. Population pharmacokinetic and exposure-response analysis of nilotinib in patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase. Eur J Clin Pharmacol. 2012;68(5):723–733. doi: 10.1007/s00228-011-1200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giles FJ, Yin OQP, Sallas WM, le Coutre PD, Woodman RC, Ottmann OG, Baccarani M, Kantarjian HM. Nilotinib population pharmacokinetics and exposure-response analysis in patients with imatinib-resistant or -intolerant chronic myeloid leukemia. Eur J Clin Pharmacol. 2013;69(4):813–823. doi: 10.1007/s00228-012-1385-4. [DOI] [PubMed] [Google Scholar]
- 68.Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, Frost P, Ye F, Boschelli DH, Boschelli F. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res. 2003;63(2):375–381. [PubMed] [Google Scholar]
- 69.Abbas R, Hug BA, Leister C, Gaaloul ME, Chalon S, Sonnichsen D. A phase I ascending single-dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI-606) in healthy adult subjects. Cancer Chemother Pharmacol. 2012;69(1):221–227. doi: 10.1007/s00280-011-1688-7. [DOI] [PubMed] [Google Scholar]
- 70.Abbas R, Hsyu P-H. Clinical pharmacokinetics and pharmacodynamics of Bosutinib. Clin Pharmacokinet. 2016;55(10):1191–1204. doi: 10.1007/s40262-016-0391-6. [DOI] [PubMed] [Google Scholar]
- 71.Redaelli S, Perini P, Ceccon M, Piazza R, Rigolio R, Mauri M, et al. In vitro and in vivo identification of ABCB1 as an efflux transporter of bosutinib. J Hematol Oncol. 2015;8:81. doi: 10.1186/s13045-015-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skoglund K, Boiso Moreno S, Jönsson J-I, Vikingsson S, Carlsson B, Gréen H. Single-nucleotide polymorphisms of ABCG2 increase the efficacy of tyrosine kinase inhibitors in the K562 chronic myeloid leukemia cell line. Pharmacogenet Genomics. 2014;24(1):52–61. doi: 10.1097/FPC.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 73.Abumiya M, Mita A, Takahashi S, Yoshioka T, Kameoka Y, Takahashi N, et al. Effects of polymorphisms in NR1I2, CYP3A4, and ABC transporters on the steady-state plasma trough concentrations of bosutinib in Japanese patient with chronic myeloid leukemia. Med Oncol. 2018;35(6):90. doi: 10.1007/s12032-018-1146-z. [DOI] [PubMed] [Google Scholar]
- 74.Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim D-W, Dyagil I, Glushko N, Milojkovic D, le Coutre P, Garcia-Gutierrez V, Reilly L, Jeynes-Ellis A, Leip E, Bardy-Bouxin N, Hochhaus A, Brümmendorf TH. Bosutinib versus Imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36(3):231–237. doi: 10.1200/JCO.2017.74.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gambacorti-Passerini C, Brümmendorf TH, Kim D-W, Turkina AG, Masszi T, Assouline S, Durrant S, Kantarjian HM, Khoury HJ, Zaritskey A, Shen ZX, Jin J, Vellenga E, Pasquini R, Mathews V, Cervantes F, Besson N, Turnbull K, Leip E, Kelly V, Cortes JE. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: minimum 24-month follow-up. Am J Hematol. 2014;89(7):732–742. doi: 10.1002/ajh.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poch Martell M, Sibai H, Deotare U, Lipton JH. Ponatinib in the therapy of chronic myeloid leukemia. Expert Rev Hematol. 2016;9(10):923–932. doi: 10.1080/17474086.2016.1232163. [DOI] [PubMed] [Google Scholar]
- 77.Ye YE, Woodward CN, Narasimhan NI. Absorption, metabolism, and excretion of [14C]ponatinib after a single oral dose in humans. Cancer Chemother Pharmacol. 2017;79(3):507–518. doi: 10.1007/s00280-017-3240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Klerk DJ, Honeywell RJ, Jansen G, Peters GJ. Transporter and Lysosomal mediated (multi)drug resistance to tyrosine kinase inhibitors and potential strategies to overcome resistance. Cancers. 2018;10(12). [DOI] [PMC free article] [PubMed]
- 79.Lu L, Saunders VA, Leclercq TM, Hughes TP, White DL. Ponatinib is not transported by ABCB1, ABCG2 or OCT-1 in CML cells. Leukemia. 2015;29(8):1792–1794. doi: 10.1038/leu.2015.35. [DOI] [PubMed] [Google Scholar]
- 80.Narasimhan NI, Dorer DJ, Niland K, Haluska F, Sonnichsen D. Effects of food on the pharmacokinetics of ponatinib in healthy subjects. J Clin Pharm Ther. 2013;38(6):440–444. doi: 10.1111/jcpt.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, O'Hare T, Hu S, Narasimhan NI, Rivera VM, Clackson T, Turner CD, Haluska FG, Druker BJ, Deininger MW, Talpaz M. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367(22):2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, Etienne G, Nicolini FE, le Coutre P, Clark RE, Stenke L, Andorsky D, Oehler V, Lustgarten S, Rivera VM, Clackson T, Haluska FG, Baccarani M, Cortes JE, Guilhot F, Hochhaus A, Hughes T, Kantarjian HM, Shah NP, Talpaz M, Deininger MW, EPIC investigators Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(5):612–621. doi: 10.1016/S1470-2045(16)00080-2. [DOI] [PubMed] [Google Scholar]
- 83.Cortes JE, Kim D-W, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DiPersio J, DeAngelo D, Abruzzese E, Rea D, Baccarani M, Müller MC, Gambacorti-Passerini C, Wong S, Lustgarten S, Rivera VM, Clackson T, Turner CD, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes T, Goldman JM, Shah NP, Kantarjian H, PACE Investigators A phase 2 trial of Ponatinib in Philadelphia chromosome–positive Leukemias. N Engl J Med. 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cortes JE, Kim D-W, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DeAngelo D, Abruzzese E, Rea D, Baccarani M, Müller MC, Gambacorti-Passerini C, Lustgarten S, Rivera VM, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes TP, Shah NP, Kantarjian HM. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393–404. doi: 10.1182/blood-2016-09-739086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iurlo A, Cattaneo D, Orofino N, Bucelli C, Molica M, Breccia M. Low-dose Ponatinib in intolerant chronic myeloid leukemia patients: a safe and effective option. Clin Drug Investig. 2018;38(5):475–476. doi: 10.1007/s40261-018-0623-7. [DOI] [PubMed] [Google Scholar]
- 86.Molica M, Scalzulli E, Colafigli G, Foà R, Breccia M. Insights into the optimal use of ponatinib in patients with chronic phase chronic myeloid leukaemia. Ther Adv Hematol. 2019;10:2040620719826444. doi: 10.1177/2040620719826444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breccia M, Efficace F, Iurlo A, Luciano L, Abruzzese E, Gozzini A, Pregno P, Tiribelli M, Rosti G, Minotti G. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: the possible role of ponatinib. Expert Opin Drug Saf. 2018;17(6):623–628. doi: 10.1080/14740338.2018.1480719. [DOI] [PubMed] [Google Scholar]
- 88.Eskazan AE, Keskin D. Radotinib and its clinical potential in chronic-phase chronic myeloid leukemia patients: an update. Ther Adv Hematol. 2017;8(9):237–243. doi: 10.1177/2040620717719851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwak J-Y, Kim S-H, Oh SJ, Zang DY, Kim H, Kim J-A, Do YR, Kim HJ, Park JS, Choi CW, Lee WS, Mun YC, Kong JH, Chung JS, Shin HJ, Kim DY, Park J, Jung CW, Bunworasate U, Comia NS, Jootar S, Reksodiputro AH, Caguioa PB, Lee SE, Kim DW. Phase III clinical trial (RERISE study) results of efficacy and Safety of Radotinib compared with Imatinib in newly diagnosed chronic phase chronic myeloid leukemia. Clin Cancer Res. 2017;23(23):7180–7188. doi: 10.1158/1078-0432.CCR-17-0957. [DOI] [PubMed] [Google Scholar]
- 90.Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim D-W, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, Shen Z-X, Saglio G, Jin J, Huang H, Hu Y, et al. Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase: ENESTchina. Blood. 2015;125(18):2771–2778. doi: 10.1182/blood-2014-09-601674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brümmendorf TH, Cortes JE, de Souza CA, Guilhot F, Duvillié L, Pavlov D, Gogat K, Countouriotis AM, Gambacorti-Passerini C. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. 2015;168(1):69–81. doi: 10.1111/bjh.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dorer DJ, Knickerbocker RK, Baccarani M, Cortes JE, Hochhaus A, Talpaz M, Haluska FG. Impact of dose intensity of ponatinib on selected adverse events: multivariate analyses from a pooled population of clinical trial patients. Leuk Res. 2016;48:84–91. doi: 10.1016/j.leukres.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Noh H, Park MS, Kim S-H, Oh SJ, Zang DY, Park HL, Cho DJ, Kim DW, Lee JI. Optimization of radotinib doses for the treatment of Asian patients with chronic myelogenous leukemia based on dose-response relationship analyses. Leuk Lymphoma. 2016;57(8):1856–1864. doi: 10.3109/10428194.2015.1113278. [DOI] [PubMed] [Google Scholar]
- 95.Caldemeyer L, Akard LP. Rationale and motivating factors for treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2016;57(12):2739–2751. doi: 10.1080/10428194.2016.1198959. [DOI] [PubMed] [Google Scholar]
- 96.Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, Guerci-Bresler A, Legros L, Varet B, Gardembas M, Dubruille V, Tulliez M, Noel MP, Ianotto JC, Villemagne B, Carré M, Guilhot F, Rousselot P, Mahon FX. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298–305. doi: 10.1200/JCO.2016.68.2914. [DOI] [PubMed] [Google Scholar]
- 97.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, Dang P, Goyne JM, Slader C, Filshie RJ, Mills AK, Melo JV, White DL, Grigg AP, Hughes TP. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 98.Lee S-E, Choi SY, Bang J-H, Kim S-H, Jang E-J, Byeun J-Y, Park JE, Jeon HR, Oh YJ, Kim HJ, Kim YK, Park JS, Jeong SH, Kim SH, Zang DY, Oh S, Koo DH, Kim H, Do YR, Kwak JY, Kim JA, Kim DY, Mun YC, Mauro MJ, Kim DW. Predictive factors for successful imatinib cessation in chronic myeloid leukemia patients treated with imatinib. Am J Hematol. 2013;88(6):449–454. doi: 10.1002/ajh.23427. [DOI] [PubMed] [Google Scholar]
- 99.Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424–430. doi: 10.1200/JCO.2012.48.5797. [DOI] [PubMed] [Google Scholar]
- 100.Mori S, Vagge E, le Coutre P, Abruzzese E, Martino B, Pungolino E, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study: ISAV study results: age and dPCR predict relapse. Am J Hematol. 2015;90(10):910–914. doi: 10.1002/ajh.24120. [DOI] [PubMed] [Google Scholar]
- 101.Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gómez Casares MT, Hellmann A, Stentoft J, Conneally E, García-Gutiérrez V, Gattermann N, Wiktor-Jedrzejczak W, le Coutre PD, Martino B, Saussele S, Menssen HD, Deng W, Krunic N, Bedoucha V, Saglio G. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525–1531. doi: 10.1038/leu.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rea D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846–854. doi: 10.1182/blood-2016-09-742205. [DOI] [PubMed] [Google Scholar]
- 103.Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K, Ishida Y, Kumagai T, Sato S, Ohashi K, Sakamaki H, Wakita H, Uoshima N, Nakagawa Y, Minami Y, Ogasawara M, Takeoka T, Akasaka H, Utsumi T, Uike N, Sato T, Ando S, Usuki K, Morita S, Sakamoto J, Kimura S, DADI Trial Group Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2(12):e528–e535. doi: 10.1016/S2352-3026(15)00196-9. [DOI] [PubMed] [Google Scholar]
- 104.Rea D, Ame S, Berger M, Cayuela J-M, Charbonnier A, Coiteux V, Cony-Makhoul P, Dubruille V, Dulucq S, Etienne G, Legros L, Nicolini F, Roche-Lestienne C, Escoffre-Barbe M, Gardembas M, Guerci-Bresler A, Johnson-Ansah H, Rigal-Huguet F, Rousselot P, Mahon FX, French Chronic Myeloid Leukemia Study Group Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: recommendations for clinical practice from the French chronic myeloid leukemia study group. Cancer. 2018;124(14):2956–2963. doi: 10.1002/cncr.31411. [DOI] [PubMed] [Google Scholar]
- 105.Rinaldetti S, Pfirrmann M, Manz K, Guilhot J, Dietz C, Panagiotidis P, et al. Effect of ABCG2, OCT1, and ABCB1 (MDR1) gene expression on treatment-free remission in a EURO-SKI subtrial. Clin Lymphoma Myeloma Leuk. 2018;18(4):266–271. doi: 10.1016/j.clml.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 106.Radich JP, Deininger M, Abboud CN, Altman JK, Berman E, Bhatia R, et al. Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. 2018;16(9):1108–1135. doi: 10.6004/jnccn.2018.0071. [DOI] [PubMed] [Google Scholar]
- 107.Hochhaus A, Saussele S, Rosti G, Mahon F-X, Janssen JJWM, Hjorth-Hansen H, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv41–iv51. doi: 10.1093/annonc/mdx219. [DOI] [PubMed] [Google Scholar]
- 108.ICLUSIG (ponatinib). FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203469lbl.pdf. Accessed 19 Mar 2019.
- 109.Rosti G, Castagnetti F, Gugliotta G, Baccarani M. Tyrosine kinase inhibitors in chronic myeloid leukaemia: which, when, for whom? Nat Rev Clin Oncol. 2016;14(3):141–154. doi: 10.1038/nrclinonc.2016.139. [DOI] [PubMed] [Google Scholar]
- 110.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM-L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–2857. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 111.Chen K-K, Du T-F, Wu K-S, Yang W. First-line treatment strategies for newly diagnosed chronic myeloid leukemia: a network meta-analysis. Cancer Manag Res. 2018;10:3891–3910. doi: 10.2147/CMAR.S177566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Isfort S, Brümmendorf TH. Bosutinib in chronic myeloid leukemia: patient selection and perspectives. J Blood Med. 2018;9:43–50. doi: 10.2147/JBM.S129821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Study of Efficacy of CML-CP Patients Treated With ABL001 Versus Bosutinib, Previously Treated With 2 or More TKIs - Full Text View - ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT03106779.
- 114.Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, Dodd S, Drueckes P, Fabbro D, Gabriel T, Groell JM, Grotzfeld RM, Hassan AQ, Henry C, Iyer V, Jones D, Lombardo F, Loo A, Manley PW, Pellé X, Rummel G, Salem B, Warmuth M, Wylie AA, Zoller T, Marzinzik AL, Furet P. Discovery of Asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem. 2018;61(18):8120–8135. doi: 10.1021/acs.jmedchem.8b01040. [DOI] [PubMed] [Google Scholar]
- 115.Safety, Tolerability, Pharmacokinetics and Activity of K0706 - Full Text View - ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT02629692.
- 116.A Pivotal Study of HQP1351 in Patients of Chronic Myeloid Leukemia in Chronic Phase With T315I Mutation - Full Text View - ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT03883087.
- 117.A Pivotal Study of HQP1351 in Patients of Chronic Myeloid Leukemia in Accelerated Phase With T315I Mutation - Full Text View - ClinicalTrials. Available from: https://clinicaltrials.gov/ct2/show/NCT03883100.
- 118.Turkina AG, Shukhov O, Chelysheva E, Nemchenko I, Petrova A, Bykova A, et al. PF-114 Mesylate, a novel third generation ATP-competitive BCR-ABL tyrosine kinase inhibitor: first Safety and efficacy data from a phase I study in patients with CML with failure of prior TKI therapy. Blood. 2017;130(Suppl 1):895. [Google Scholar]
- 119.Ruxolitinib Phosphate and Dasatinib or Nilotinib in Treating Patients With Chronic Myeloid Leukemia - Full Text View - ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT03654768.