Abstract
Background
Imatinib is a platelet-derived growth factor receptor (PDGFR) inhibitor with very low water solubility. Previous studies in atherosclerosis have shown that PDGFR activity has an egregious effect on vascular disease and progression of atherosclerosis. Specific ligands of atherosclerotic plaques can be used for targeting of nanoparticles. Studies in atherosclerosis proved that stabilin-2 is a glycoprotein which exists abundantly in atherosclerotic plaques and it is produced from both macrophages and endothelial cells.
Objectives
The objective of this study is the targeting drug delivery to atherosclerotic plaques by using imatinib-loaded nanoparticles modified by S2P peptide.
Methods
The imatinib-loaded nanoparticles were fabricated through a modified emulsion/solvent evaporation technique. After fabricating PLGA nanoparticles, maleimide PEG was used as linker between PLGA nanoparticles and S2P peptide. Because of presence cysteine in both side of S2P peptide, maleimide formed a thiolether linkage by thiol group of cysteine. Then the physicochemical analysis like H-NMR, FT-IR, DSC, SEM, particle size, zeta potential, and drug release were studied.
Results
Stabilin-2 peptide with sequence of CRTLTVRKC is a specific ligand to stabilin-2, so it was synthesized for using as the targeting agent for atherosclerosis. S2P peptide conjugation to the surface of nanoparticles was proved by H-NMR and FT-IR, and the percentage of S2P peptide in nanoparticles was 1.3%. The final nanoparticles were spherical and their size were 183 nm. The loading capacity of the imatinib-loaded nanoparticles was 5.05%. The sustained release profile was observed for peptide targeted nanoparticles.
Conclusion
The chosen method was simple, reproducible, and specific in peptide conjugation of nanoparticles for targeting delivery to atherosclerotic regions.
Graphical abstract.

.
Keywords: Nanomedicine, Surface conjugation, S2P peptide, Imatinib, Atherosclerosis
Introduction
About 30% of deaths in United States are established by atherosclerosis and complications thereof [1]. Atherosclerotic disease of the carotid veins is existed in the ageing population [2].
Angiography, angioscopy X-ray, and intravascular ultrasound are the standard methods to diagnose atherosclerosis. Although, these imaging methods do not efficiently indicate functional changes in vein walls, but specific targeting wards for atherosclerosis have been developed. Such molecules should be efficiently and specifically expressed in atherosclerotic plaques, which are recognized by proteolytic activity, inflammation, apoptosis, and angiogenesis [3]. Because of that, researches have focused on ligands such as antibodies and peptides that selectively joint to the activated endothelium, macrophages, cells involved in angiogenesis, and apoptotic cells. All of these cells or biological features are sign of different phases of atherosclerotic plaque progress [1]. Therefore necessary to focus on inhibition of platelet adhesion, activation and aggregation as one of efficient therapeutic approaches [4].
Restenosis may promote by enhancement of vascular smooth muscle cells (VSMC) proliferation via release of platelet-derived growth factor. Various methods to inhibit the PDGFR and its signaling cascades have been suggested. Results with PDGFR kinase inhibitors were acceptable. Offering a selective and bioavailable drug might cause an effective therapeutic rate for reduction of restenosis [5].
Imatinib is an inhibitor for PDGFR tyrosine kinase and is confirmed for the treatment of chronic myeloid leukemia. Imatinib with IC50 < 10 nM was reported to be a specifically more effective inhibitor of VSMC proliferation than other inhibitors while has little influence on the vascular endothelial cell growth factor receptor tyrosine kinase. This information approves a rational for the use of imatinib as a VSMC-specific molecular-targeting drug in the prevention of in-stent restenosis [6].
Peptide conjugated nanoparticles have emerged as powerful tools for drug delivery [7, 8]. Stabilin-2/FEEL-2/HARE is a type of multi-functional protein that reportedly identifies [9], as a scavenger receptor that joints to bacteria. It endocytoses changed the end-products of glycation and low-density lipoprotein [10, 11]. It has been reported that stabilin-2 acts as a membrane phosphatidylserine receptor among apoptotic cells [11]. Stabilin-2 was highly expressed on endothelial cells, smooth muscle, macrophages, atherosclerotic plaques [12, 13], and a peptide with CRTLTVRKC sequence (S2P) that selectively bind to stabilin-2 has been identified [1], for this reason localization of nanoparticles containing S2P peptide as a targeting agent for atherosclerosis has been bring up [14].
Materials and methods
Materials
Imatinib was obtained from Persian Pharmaceutical Company (Tehran, Iran). Polyvinyl alcohol (PVA), 90% hydrolyzed, with molecular weight of 30,000–70,000 Kda was obtained from Sigma-Aldrich (Carlsbad, USA). PLGA 502 H was obtained from Evonik (Essen, Germany). Maleimide-PEG-Amine (MW 2000) was purchased from Jenkem (Tianjin, China). Sodium chloride was purchased from Samchun (Pyeongtaek, South Korea). Di-sodium, hydrogen phosphate (Na2HPO4.12H2O), potassium chloride, Potassium Di-hydrogen phosphate (KH2PO4), Tween® 80, dichloromethane (DCM), acetone, and acetonitrile were purchased from Merck (Darmstadt, Germany). Methanol was purchased from Duksan (Ansan, South Korea). N-HydroxySulfoSuccinimide sodium (Sulfo-NHS) and N-(3-Di methyl amino propyl)-N′-ethyl carbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich (Carlsbad, USA). Dialysis bag with molecular weight of 12,000 Kda was obtained from Spectrum (New Jersey, USA).
Peptide synthesis
For synthesizing S2P peptide (H-Cys1-Arg2-Thr3-Leu4-Thr5-Val6-Arg7-Lys8-Cys9-OH), method of “synthesize of peptide on solid bed” was chosen. All the amino acids were protected by fluorenylmethyloxycarbonyl chloride (Fmoc) group. 2-chlorotrityl chloride resin was used for peptide synthesis and TBTU was employed as a coupling reagent in solution of dimethylflormamide activated by diisopropylethylamine. Conjugation of thiol groups in Cys1 and Cys9 to each other are probable during deprotection phase so the thiol groups of both Cys were protected by acetamidomethyl (Acm). After completion of peptide synthesis, trifluoroacetic acid was used for separating of protected peptide from resin surface. Finally purification by high performance liquid chromatography (HPLC) was been administrated and LC- mass (Agilent 6410 QQQ, USA) spectrometry was employed to confirm the sequence [15].
Preparation of imatinib-PLGA nanoparticles
The imatinib-loaded nanoparticles were fabricated through a modified emulsion/solvent evaporation technique [15]. Briefly, 450 mg of PLGA and 45 mg of imatinib were dissolved in 15 ml of DCM/acetone with ratio of 2:1 v/v as internal phase. It was slowly dispersed in 45 ml of PVA 0.5% w/v under an ultrasonic probe (Misonix, USA) at 30 amplitudes for 5 min. Next, the organic phase was evaporated using mechanical stirrer for 3 h. Nanoparticles were twice washed and isolated by centrifuge (Sigma 3 K30, Germany) for 25 min at 20,000 rpm. The collected nanoparticles were lyophilized for 48 h at −50 °C (Christ, Alpha 2–4 LD, Germany) to obtain a fine nanoparticles powder.
Pegylation of PLGA-imatinib nanoparticles
Pegylation of nanoparticles was performed via conjugation of maleimide-PEG-NH2 to carboxylic group of PLGA. This reaction was accomplished in following steps: first; 180 mg of nanoparticles, 75 mg sulfo-NHS, and 75 mg EDC were suspended in 5 ml phosphate buffer (pH 6.5) and stirred for 1 h at room temperature to obtain an ester. Sulfo-NHS was used to prepare amine-reactive esters of carboxylic groups. Then 60 mg maleimide-PEG-amine was dissolved in 1.5 ml phosphate buffer (pH 6.5) and finally the maleimide-PEG-amine mixture was added to first solution and incubated under gentle stirring for 18 h at room temperature [14]. At the end, nanoparticles were twice washed and centrifuged for 25 min at 20,000 rpm.
Conjugation of S2P to maleimide-PEG-PLGA-imatinib nanoparticles
Sulfhydryl group of S2P peptide was conjugated to the maleimide function of PEG surrounding the nanoparticles surface. The thiol of S2P peptide was reacted with maleimide of PEG-PLGA nanoparticle at 1:1 M ratio in minimal volume of phosphate buffer (pH 7.0), stirring at 100 rpm in room temperature for 20 h. Thiol as a strong nucleophilic group spontaneously react and couple with maleimide (the π-bond) preferentially under pH 6.5–7.5 [16]. Afterwards the final nanoparticles were twice washed and centrifuged for 25 min at 20,000 rpm. The obtained product was lyophilized for 48 h at −50 °C to leave a fine nanoparticles powder.
Particle size
For determining particle size, Zetasizer (Malvern ZS, UK) was employed. The particle size measurement was done by using method of “dynamic light scattering (DLS)” based on the dynamic brownian motion of the suspended particle in water medium [17]. A sample at concentration of nearly 1 mg nanoparticles/ml water was prepared. All measurements were carried out at a scattering angle of 90° at 25 °C.
Scanning electron microscopy
The samples were examined to determine surface morphology of the final nanoparticles by using scanning electron microscopy (SEM, Philips XL30 scanning microscope, Netherlands) [18]. A suspension of nanoparticles in water was made and a drop was trickled on a lamella, the lamella was taken for SEM after drying. Nanoparticles were coated by gold before SEM observation.
H-NMR, FT-IR and DSC
H-NMR (Bruker 500 Ultrashield, Germany), FT-IR (Nicolet Magna-IR 550 spectometer, USA) and DSC (DSC 823 MettlerTolledo, Switzerland) were used for chemical and thermal characterization of samples.
Loading efficiency measurements
For determining of drug loading, 10 mg of nanoparticles was added to 10 ml of acetonitrile and then 20 ml of methanol was added. The insoluble polymers were collected by centrifuge (for 25 min at 20,000 rpm. Afterward concentration of imatinib in mentioned solution was measured by reading of UV absorption at λ = 268.5 nm (Scinco S-3100, Korea). The drug loading and encapsulation efficiency were calculated using the following equations:
Drug loading = (weight of imatinib in nanoparticles/total weight of nanoparticles) × 100%.
Encapsulation efficiency = (actual/theoretical imatinib loading) × 100%.
In vitro drug release
An appropriate amount of the freeze-dried nanoparticles (50 mg) was dispersed in 5 ml isotonic phosphate buffer saline (pH 7.4), then the obtained suspension was poured in a dialysis bag [19]. Being selective permeability is one of the advantages of using dialysis bag so only imatinib molecules can pass via its pores. Both side of the bag were tied tightly, and then it was plunged in 45 ml phosphate buffer saline inside of a closed beaker. The beaker was placed in a shaker (Heidolph Incubator 1000-Unimax10F0, Germany) maintained at 37 ± 0.5 °C and shaken at 90 rpm. At specified time intervals, 45 ml fresh phosphate buffer saline was replaced instead of previous medium. The concentration of released imatinib was measured by reading amount of UV absorption at λ = 268.5 nm (Scinco S-3100, Korea).
Results and discussion
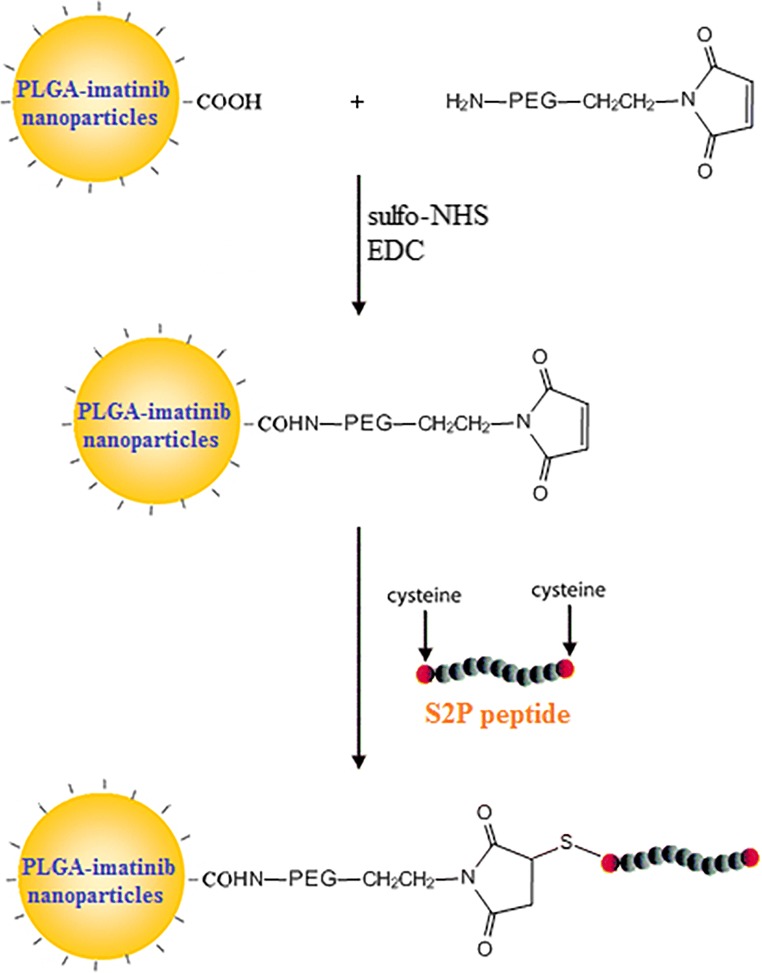
Imatinib was encapsulated in the PLGA nanoparticles by emulsion/solvent evaporation technique. After fabricating nanoparticles, maleimide PEG was used as linker between PLGA nanoparticles and S2P peptide. Because of the presence cysteine in both side of S2P peptide, maleimide form a thiolether linkage by thiol group of cysteine. The schema of synthesis is shown in Fig. 1.
Fig. 1.
Synthesis schema of peptide conjugation to PLGA nanoparticles
The average particle size and polydispersity data of nanoparticles are presented in Table 1. In emulsion/solvent evaporation technique, the organic phase was PLGA/imatinib dissolved in DCM/acetone. Acetone was used for reducing of particle size and DCM was used for improving of drug loading. DCM has a low miscibility with aqueous phase and maintain more imatinib molecule in organic droplets. The particle size is a key for the biological fate of nanoparticles and it has a great impact on their biodistribution [20].
Table 1.
Mean particle size and polydispersity index (PdI) of nanoparticles containing imatinib (mean ± SD)
| Mean particle size (d.nm) | PdI | |
|---|---|---|
| PLGA nanoparticles | 171.5 ± 1.2 | 0.075 ± 0.009 |
| S2P Peptide-PLGA-Maleimide-PEG nanoparticles | 183.3 ± 1.4 | 0.195 ± 0.008 |
The evaluation of particle size revealed that the mean particle size of NPs was 171.5 ± 1.2 nm and when it was conjugated with S2P peptide it increased to 183.3 ± 1.4 nm. S2P peptide targeted and non-targeted nanoparticles showed no statistically significant difference in the particle size, suggesting that conjugation process didn’t influence the particle size. The polydispersity index (PDI) was low for both nanoparticles, indicating that an uniform dispersion was obtained.
SEM microscopy was used to investigate the surface morphology. As showed in the Fig. 2, nanoparticles display almost spherical shape with uniform size distribution.
Fig. 2.

Scanning electron microscopic photograph of the peptide-PLGA-PEG nanoparticles containing imatinib
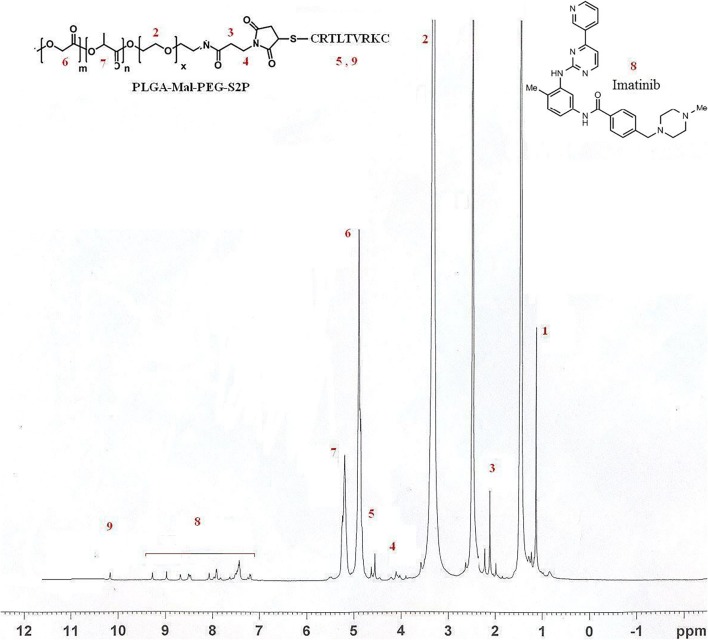
H-NMR was used to prove presence of imatinib, PLGA, maleimide-PEG, and S2P peptide in the final nanoparticles. Peaks of each active groups are marked in Fig. 3. The amidation reaction (between PEG and PLGA) was proved by presence of 3.4 (–CH2 in PEG), 4.8 (–CH2 in PLGA) and 5.2 (–CH in PLGA) peaks at H-NMR. Based on peak area of S2P peptide to total peak area, the percentage of S2P peptide in nanoparticles was 1.3%.
Fig. 3.
H-NMR spectrum of the peptide-PLGA-PEG nanoparticles containing imatinib
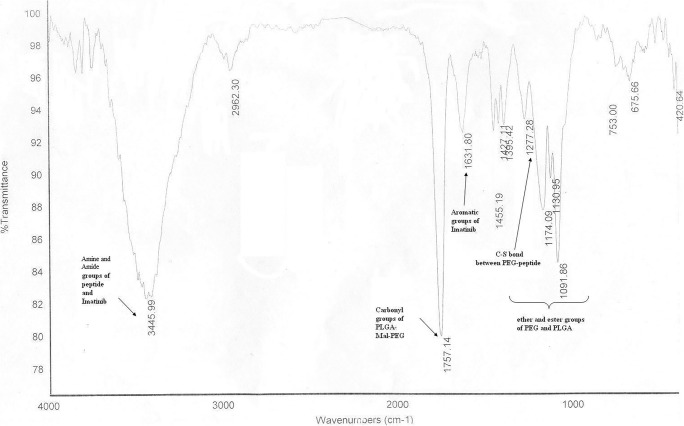
The chemical structure of final nanoparticles was analyzed by FT-IR spectra (Fig. 4). The strong peaks at 1091 cm−1 to 1174 cm−1 correlated to the ether and the ester groups of PEG and PLGA. The peak at 1277 cm−1 related to the C-S bond between PEG and peptide. The peak at 1631 cm−1 correlated to the aromatic groups of imatinib. The strong peak at 1757 cm−1 correlated to the carbonyl groups of PLGA-Mal-PEG. The strong and wide peak at 3445 cm−1 correlated to the amine and the amide groups of peptide and imatinib.
Fig. 4.
FT-IR spectrum of the peptide-PLGA-PEG nanoparticles containing imatinib
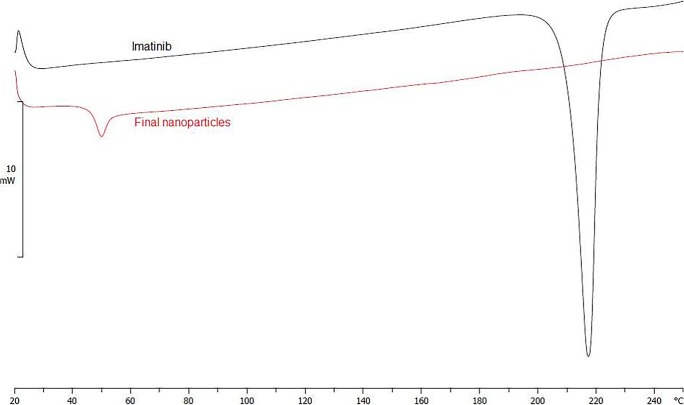
DSC analysis provided data on the thermal transitions and the state of imatinib after encapsulation in nanoparticles. Figure 5 shows DSC thermograms of pure imatinib and nanoparticles containing imatinib. The pure imatinib shows an endothermic peak at 218 °C that corresponds to melting point, indicating its crystalline state. The thermograms confirm the amorphous state of PLGA as it only shows the glass transition peak at 50 °C. The absence of imatinib melting point peak in the thermogram of nanoparticles suggested that imatinib in the nanoparticles was converted to amorphous state [21].
Fig. 5.
DSC thermogram of (a) pure imatinib and (b) final nanoparticles
The encapsulation efficacy of imatinib in PLGA nanoparticles was 68%. The drug loading of imatinib in the targeted peptide nanoparticles was 5.05%. Different factors influence the encapsulation efficacy and the drug loading in nanoparticles. The critical parameters for current method are the type and molecular weight of polymer, the type and amount of organic phase, and drug/polymer ratio [22].
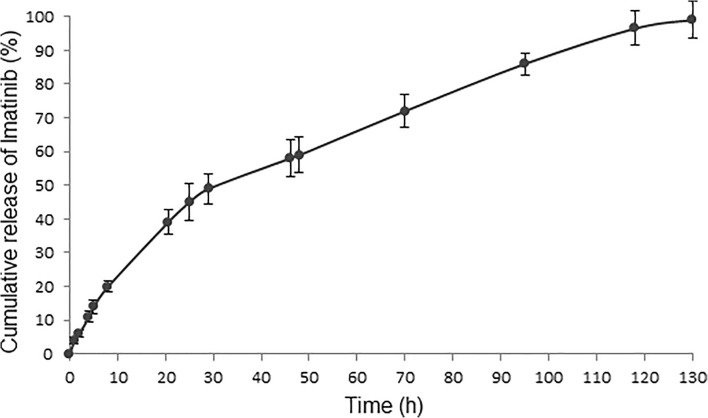
The release kinetic of final nanoparticles was studied by the dialysis bag diffusion technique. The release profile of imatinib from nanoparticles in phosphate buffer saline with 0.03% tween 80 is summarized in the cumulative percentage release (Fig. 6). The release kinetic was similar to the zero-order model (R2 = 0.95). Nanoparticles had an initial burst release during the first 8 h and about 21% of total amount of imatinib. Then remaining imatinib was released up to 100% after 130 h. The initial burst release could be attributed to the surface encapsulated imatinib. The drug release gradually decreased and remained constant even after 130 h. It might be due to the diffusion release of imatinib encapsulated near the surface and in the outer portion of the nanoparticles [23]. Afterwards, the polymer matrix was eroded in the medium, and then the release mechanisms of swelling, diffusion, and erosion might be the main cause of the release behavior [24]. Hydrolysis of polymer can result in erosion and pore formation, which may increase the drug release [25]. Imatinib-encapsulated nanoparticles with controlled release properties could be used to minimize off-target toxicity of imatinib [26].
Fig. 6.
In vitro cumulative drug release from the peptide-PLGA-PEG nanoparticles
Conclusions
Because of significant role of atherosclerosis in cardiovascular disease, targeting delivery to atherosclerotic regions is an area of important research interest. The present work proposed a novel formulation of imatinib-loaded nanoparticles targeted by S2P peptide. The biodegradable nanoparticles from maleimide PEG-PLGA were developed and the S2P peptide was conjugated to the surface of nanoparticles. The maleimide PEG was used as linker for the conjugation of S2P peptide to PLGA NPs. Results demonstrated that by using S2P peptide as targeting agent, maleimide PEG as linker and emulsion/solvent evaporation technique, the targeted nanoparticles would be obtained with desired particle size, morphology, and drug loading properties. The chosen method was simple, reproducible, and specific in the peptide conjugation. This study will be useful for the future studies aiming at the development of targeting drug delivery system for atherosclerotic regions and investigating in vivo efficiency.
Acknowledgements
The authors are grateful to Nanotechnology Research Center of Tehran University of Medical Sciences (Tehran, Iran) for financial support.
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest in this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee GY, Kim J-H, Oh GT, Lee B-H, Kwon IC, Kim I-S. Molecular targeting of atherosclerotic plaques by a stabilin-2-specific peptide ligand. J Control Release. 2011;155(2):211–217. doi: 10.1016/j.jconrel.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36(3):443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 3.Douma K, Prinzen L, Slaaf DW, Reutelingsperger CP, Biessen EA, Hackeng TM, et al. Nanoparticles for optical molecular imaging of atherosclerosis. Small. 2009;5(5):544–557. doi: 10.1002/smll.200801079. [DOI] [PubMed] [Google Scholar]
- 4.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103(6):2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 5.Jandt E, Mutschke O, Mahboobi S, Uecker A, Platz R, Berndt A, et al. Stent-based release of a selective PDGF-receptor blocker from the bis-indolylmethanon class inhibits restenosis in the rabbit animal model. Vasc Pharmacol. 2010;52(1):55–62. doi: 10.1016/j.vph.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Masuda S, Nakano K, Funakoshi K, Zhao G, Meng W, Kimura S, et al. Imatinib mesylate-incorporated nanoparticle-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries. J Atheroscler Thromb. 2011;18(12):1043–1053. doi: 10.5551/jat.8730. [DOI] [PubMed] [Google Scholar]
- 7.Mahdaviani P, Bahadorikhalili S, Navaei-Nigjeh M, Vafaei SY, Esfandyari-Manesh M, Abdolghaffari AH, et al. Peptide functionalized poly ethylene glycol-poly caprolactone nanomicelles for specific cabazitaxel delivery to metastatic breast cancer cells. Mater Sci Eng C. 2017;80:301–312. doi: 10.1016/j.msec.2017.05.126. [DOI] [PubMed] [Google Scholar]
- 8.Esfandyari-Manesh M, Mohammadi A, Atyabi F, Nabavi SM, Ebrahimi SM, Shahmoradi E, et al. Specific targeting delivery to MUC1 overexpressing tumors by albumin-chitosan nanoparticles conjugated to DNA aptamer. Int J Pharm. 2016;515(1–2):607–615. doi: 10.1016/j.ijpharm.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 9.McCourt PA, Smedsrød BH, Melkko J, Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology. 1999;30(5):1276–1286. doi: 10.1002/hep.510300521. [DOI] [PubMed] [Google Scholar]
- 10.Tamura Y, Adachi H, Osuga J-i, Ohashi K, Yahagi N, Sekiya M, et al. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J Biol Chem. 2003;278(15):12613–12617. doi: 10.1074/jbc.M210211200. [DOI] [PubMed] [Google Scholar]
- 11.Park S-Y, Jung M-Y, Kim I-S. Stabilin-2 mediates homophilic cell–cell interactions via its FAS1 domains. FEBS Lett. 2009;583(8):1375–1380. doi: 10.1016/j.febslet.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE) J Biol Chem. 2000;275(48):37733–37741. doi: 10.1074/jbc.M003030200. [DOI] [PubMed] [Google Scholar]
- 13.Park S, Jung M, Kim H, Lee S, Kim S, Lee B, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15(1):192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 14.Luo G, Yu X, Jin C, Yang F, Fu D, Long J, et al. LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors. Int J Pharm. 2010;385(1):150–156. doi: 10.1016/j.ijpharm.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Maghari S, Ramezanpour S, Balalaie S, Darvish F, Rominger F, Bijanzadeh HR. Synthesis of functionalized Pseudopeptides through five-component sequential Ugi/Nucleophilic reaction of N-substituted 2-Alkynamides with Hydrazides. J Org Chem. 2013;78(13):6450–6456. doi: 10.1021/jo4003294. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Jothar L, Doulkeridou S, Schiffelers RM, Torano JS, Oliveira S, van Nostrum CF, et al. Insights into maleimide-thiol conjugation chemistry: conditions for efficient surface functionalization of nanoparticles for receptor targeting. J Control Release. 2018;282:101–109. doi: 10.1016/j.jconrel.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Goldburg W. Dynamic light scattering. Am J Phys. 1999;67(12):1152–1160. doi: 10.1119/1.19101. [DOI] [Google Scholar]
- 18.Mohanraj V, Chen Y. Review on nanoparticles. Trop J Pharm Res. 2006;5:561–573. [Google Scholar]
- 19.Esfandyari-Manesh M, Darvishi B, Ishkuh FA, Shahmoradi E, Mohammadi A, Javanbakht M, et al. Paclitaxel molecularly imprinted polymer-PEG-folate nanoparticles for targeting anticancer delivery: characterization and cellular cytotoxicity. Mater Sci Eng C. 2016;62:626–633. doi: 10.1016/j.msec.2016.01.059. [DOI] [PubMed] [Google Scholar]
- 20.Calvo P, Gouritin B, Chacun H, Desmaële D, D'Angelo J, Noel J-P, et al. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res. 2001;18(8):1157–1166. doi: 10.1023/A:1010931127745. [DOI] [PubMed] [Google Scholar]
- 21.Esfandyari-Manesh M, Mostafavi SH, Majidi RF, Koopaei MN, Ravari NS, Amini M, et al. Improved anticancer delivery of paclitaxel by albumin surface modification of PLGA nanoparticles. Daru. 2015;23(1):1. doi: 10.1186/s40199-015-0107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esfandyari-Manesh M, Ghaedi Z, Asemi M, Khanavi M, Manayi A, Jamalifar H, et al. Study of antimicrobial activity of anethole and carvone loaded PLGA nanoparticles. J Pharm Res. 2013;7(4):290–295. [Google Scholar]
- 23.Ghasemi Z, Dinarvand R, Mottaghitalab F, Esfandyari-Manesh M, Sayari E, Atyabi F. Aptamer decorated hyaluronan/chitosan nanoparticles for targeted delivery of 5-fluorouracil to MUC1 overexpressing adenocarcinomas. Carbohydr Polym. 2015;121:190–198. doi: 10.1016/j.carbpol.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Malek SJ, Khoshchehreh R, Goodarzi N, Khoshayand MR, Amini M, Atyabi F, et al. Cis-Dichlorodiamminoplatinum (II) glyconanoparticles by drug-induced ionic gelation technique targeted to prostate cancer: preparation, optimization and in vitro characterization. Colloids Surf B: Biointerfaces. 2014;122:350–358. doi: 10.1016/j.colsurfb.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Kim CS, Saylor DM, Koo D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: a review of experiments and theories. J Biomed Mater Res B Appl Biomater. 2017;105(6):1692–1716. doi: 10.1002/jbm.b.33648. [DOI] [PubMed] [Google Scholar]
- 26.Marslin G, Revina AM, Khandelwal VKM, Balakumar K, Prakash J, Franklin G, et al. Delivery as nanoparticles reduces imatinib mesylate-induced cardiotoxicity and improves anticancer activity. Int J Nanomedicine. 2015;10:3163. doi: 10.2147/IJN.S75962. [DOI] [PMC free article] [PubMed] [Google Scholar]