Abstract
Background
The gut microbiota is closely associated with the bidirectional gut-brain axis that modulates neuropsychological functions of the central nervous system, thereby affecting mental disorders such as depression. Although it is known that probiotics affect brain functions, the impact of probiotics on the regulation of the prevalence and composition of gut microbiota, leading to anti-depressive effects has not been well understood.
Methods
Mice were randomly divided into four different groups (n = 10 for each group) as follows: Group G1 (normal group) as control and group G2 (stress group) were given sterile saline via oral route daily for 8 weeks without and with stress condition, respectively. Under the stress condition, group G3 (fluoxetine group) was administered with fluoxetine hydrochloride and group G4 (probiotic group) was orally given multi-strains of probiotics daily for 8 weeks. After treatment, all mice underwent behavioral testing. Furthermore, fecal samples were collected from randomly selected 5 mice of each group on day 60 and taxonomical analysis of intestinal microbial distribution was performed.
Results
Mice subjected to restraint stress showed depressive-like behaviors along with high corticosterone levels in serum. However, probiotic administration alleviated depressive-like behaviors and decreased corticosterone level. Moreover, fecal microbiota was distinctly altered in probiotic-treated mice of the stress group. The relative abundance of phylum and genus levels was significantly decreased in the stress group, but probiotic administration restored the composition of microbes restored.
Conclusion
Ingested probiotics alter the composition of gut microbiota, likely improving the symptoms of depression.
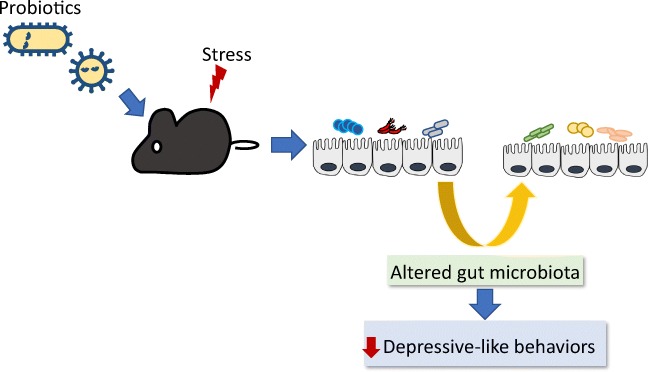
Graphical abstract.
Probiotic administration alters gut microbiota and reduces depressive-like behaviors
Electronic supplementary material
The online version of this article (10.1007/s40199-020-00329-w) contains supplementary material, which is available to authorized users.
Keywords: Depression, Stress, Probiotics, Gut microbiota, Anti-depressant
Introduction
Depression is known as major depressive disorder, which is accompanied by a feeling of guilt and hopelessness as well as a change in appetite and sleep and impairment of daily function [1]. Additionally, major depressive disorder often leads to disability and suicide [2]. Mortality risk associated with suicide was estimated to be more than 20-fold higher in patients with depressive disorder than in general population [3]. Furthermore, depressive symptoms are associated with a significantly higher risk of cardiovascular mortality [4]. Many studies have focused on the genetic, behavioral, and neurological characteristics for treating depressive symptoms; however, more attention has been focused toward the fact that environmental risk factors and immune dysregulation also contribute to the etiology of depression [5].
In the last decade, several studies have focused on variations in the gastrointestinal microbiome, and the consequent effects on various mental disorders [6]. Bidirectional communication network between the gastrointestinal tract and the central nervous system, referred to as the gut–brain axis, is associated with a complex crosstalk of the autonomic nervous, neuroendocrine, and immune system [7]. Gastrointestinal microbiome are known to produce and deliver neuroactive substances acting on the gut–brain axis [6, 8]. Although the pathways linking the gastrointestinal microbiome with the central nervous system are not clearly understood, changes in the gastrointestinal microbiome play an important role in the function of the central nervous system, and thereby affect mental disorders such as depression [5, 7].
Stress can lead to the loss of intestinal barrier function, resulting in increased intestinal permeability [9]. Increased intestinal permeability may lead to subsequent immune activation in the gut and also consequently affect the central nervous system; this is suggested to be a potential pathophysiological mechanism underlying major depression [9–11]. It has been elucidated that gut microbiota possibly plays a critical role in the maintenance of intestinal barrier function [12]. Several studies have suggested that probiotics positively influence the central nervous system by modulating critical neurotransmitters implicated in depression [13–15]. Although these findings imply that ingestion of probiotics may affect the gut–brain axis which participates in the alleviation of depression, it has not yet been well understood whether probiotic consumption regulates the prevalence and composition of gut microbiota, and in turn exert anti-depressive effects. Therefore, in this study, we aimed to determine the effect of administration with multi strains of probiotics including Lactobacillus, Bifidobacterium and Pediococcus on the regulation of gut microbiota and reduction of depressive symptoms in mice.
Materials and methods
Experimental animals
Six-week-old male ICR mice (20–26 g) were purchased from Orient Bio (Seongnam, Korea) and were allowed at least 1 week for quarantine and acclimatization. Animals were housed in polycarbonate cages under the standard conditions of constant temperature (22 ± 1 °C), relative humidity (55 ± 1%), and 12-h light/dark cycle and were given tap water and commercial rodent chow (Samyang Feed, Daejeon, Korea) ad libitum. This study was conducted in accordance with the National Institute of Health guidelines and approved by the Institutional Animal Care and Use Committee at the Dongguk University Ilsan Hospital (IACUC-2018-004). Forty mice were blindly randomized into four different treatment groups (n = 10/group). For 8 weeks, normal (G1) and stress (G2) groups received regular lab chow in the absence or presence of stress conditions, respectively. The mice in the stress group were immobilized by gentle insertion into a flexible triangle shaped vinyl screen that was closed and secured with a non-allergic adhesive tape for 2 h a day over a 8-week period. A probiotic formulation containing Lactobacillus plantarum LP3, L. rhamnosus LR5, Bifidobacterium lactis BL3, B. breve BR3, and Pediococcus pentosaceus PP1 provided by Cell Biotech Co. Ltd. (Gimpo, Korea) was dissolved in sterile saline and adjusted to an appropriate concentration before administration; probiotic formulation (500 μL; 2 × 108 CFU/mL) was subsequently administered to mice subjected to stress conditions over a 4-week period (G4). As a positive control, mice were orally administered with fluoxetine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA; 10 mg/kg/day) (G3) daily for 8 weeks. The mice in the normal group were orally administered with sterile saline (0.9% NaCl). All mice were orally administered with equal volume of samples. For taxonomical analysis of microbial distribution, fecal samples were collected from 5 mice of each group on day 60 after administration.
Forced swimming test (FST)
FST was performed as described previously [16]. Briefly, mice were individually placed into a Plexiglas cylinder (25 cm in height and 15 cm in diameter) filled with 10 cm of water (25 ± 1 °C). After performing 15 min pre-test for 3 days, mice were subjected to a 5-min FST after being exposed to the same experimental conditions as described above. The 5-min FST was used for analyzing behaviors including swimming, climbing, and immobility.
Tail suspension test (TST)
TST was performed as described previously with minor modifications [17]. Briefly, both acoustically and visually isolated mice were suspended 40 cm above the tabletop using an adhesive tape placed approximately 1 cm from the tip of the tail. The total time of immobility was measured during a 6-min test. When the mice hung passively and completely motionless, they were considered immobile owing to the absence of any limb or body movements.
Open field test (OFT)
To assess spontaneous locomotor activity of the mice, open-field test was performed. Mice were individually housed in a rectangular container (60 × 60 cm) with a height of 30 cm under dark or dim condition. Locomotor activities were indicated by the distance of movements and monitored by a computerized video-tracking system that employed the S-MART program (Pan Lab Co., Barcelona, Spain). The frequency of line crossing and distance traveled in the container were recorded for 5 min.
Elevated plus maze (EPM) test
For the EPM test, the apparatus consisted of two oppositely positioned open arms (50 × 10 cm) and two oppositely positioned closed arms (50 × 10 × 20 cm), which were connected with a central platform (10 × 10 cm). The maze was raised 50 cm above the floor and was made from black Plexiglas. Investigation of the open arms was conducted under indirect dim light. After testing the behavior of each mouse, the maze was cleaned with alcohol. At the beginning of each trial, mice were placed at the center of the maze, facing a closed arm. Anxiety reduction, as manifested by open arm exploration in EPM test, was defined as an increase in the number of entries made by mice into open arms compared with the total number of entries into both open and closed arms and an increase in the percentage of time spent by mice in open arms.
Assessment of corticosterone levels
Sera were obtained by centrifuging blood samples at 3000×g for 15 min at 4 °C and stored at −80 °C until use. The level of corticosterone was measured using a commercial enzyme-linked immunosorbent assay kit (Enzo Life Sciences, Farmingdale, NY, USA) according to the manufacturer’s instructions.
DNA extraction and 16S rRNA gene sequencing
The fecal samples of mice were collected after defecation on day 60 following administration with probiotics and were immediately frozen at −70 °C, and bacterial genomic DNA was extracted from 100 to 200 mg feces using FastDNA Spin Kit for Soil (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. The quality of the extracted genomic DNA was determined using Nano-drop, and fecal genomic DNA concentration was measured using the Qubit dsDNA BR assay kit (Thermo Fisher Scientific, Carlsbad, CA, USA). For 16S rRNA gene sequencing, the V4-V5 region of the bacterial 16S rRNA gene was amplified using forward primer in the v4 region (CCA GCM GCC GCG GTA ATW C) and a reverse primer in the v5 region (CC GTC AAT TYY TTT RAG TTT) by following the Illumina 16S Metagenomic Sequencing Library Preparation guide. Subsequently, the amplified sequencing libraries were purified with AMPure XP magnetic beads (Beckman Coulter, Brea, CA, USA), and the quality of libraries was checked using 2100 Bio-analyzer (Agilent, Santa Clara, CA, USA). Finally, sequencing libraries were mixed in equal molar concentrations to generate a 4-nM library pool and sequenced with 250-bp paired-end reads on the MiSeq systems using MiSeq v2 reagent kits (Illumina, San Diego, CA, USA).
Sequencing data analysis
Data quality control and analyses of raw sequences were performed with Quantitative Insights Into Microbial Ecology (QIIME, v1.9.1) pipeline by following the recommended guidelines [18]. Briefly, raw sequences were subjected to sequential pre-processing (demultiplexing and filtering), picking OTU at the 97% identity threshold using UCLUST, taxonomic assignment based on 97% identity clusters in Greengenes database (v13_8), and OTU table and taxa summary generation that provided proportional representation of taxonomic groups within each sample. For bacterial diversity analysis, the representative sequences of OTU were aligned with PyNAST, and a phylogenic tree was constructed with Fast Tree. Alpha diversity was calculated based on the parameters of observed species as well as Chao1, Shannon, and Simpson by employing rarefied OTU table. Beta diversity was calculated using unweighted Unifrac algorithm and represented using a principal coordinate analysis (PCoA) plot.
Data analysis
All statistical analyses were conducted with GrapPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS (ver. 19, Somers, NY, USA). Values are expressed as means ± SEM. All data were analyzed using Student’s t test. Statistical significance was accepted at a P value less than 0.05.
Results
Effect of probiotic administration on immobility time in FST and TST
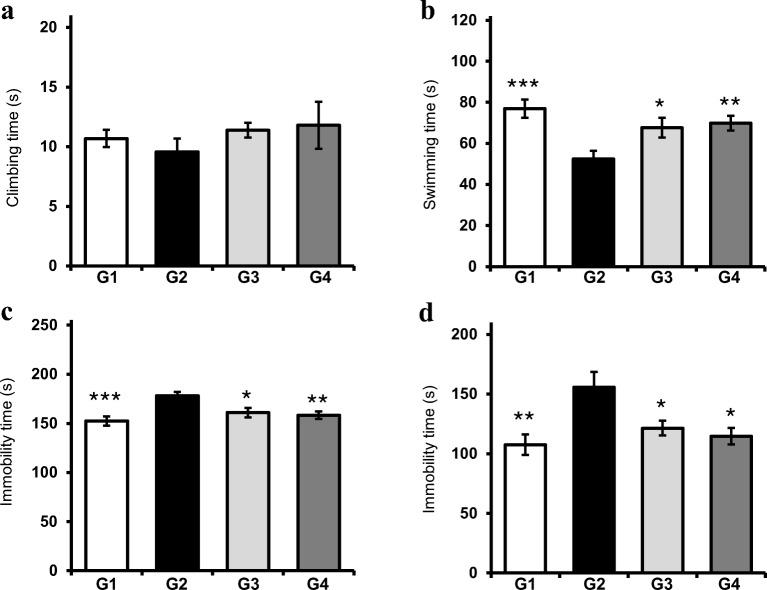
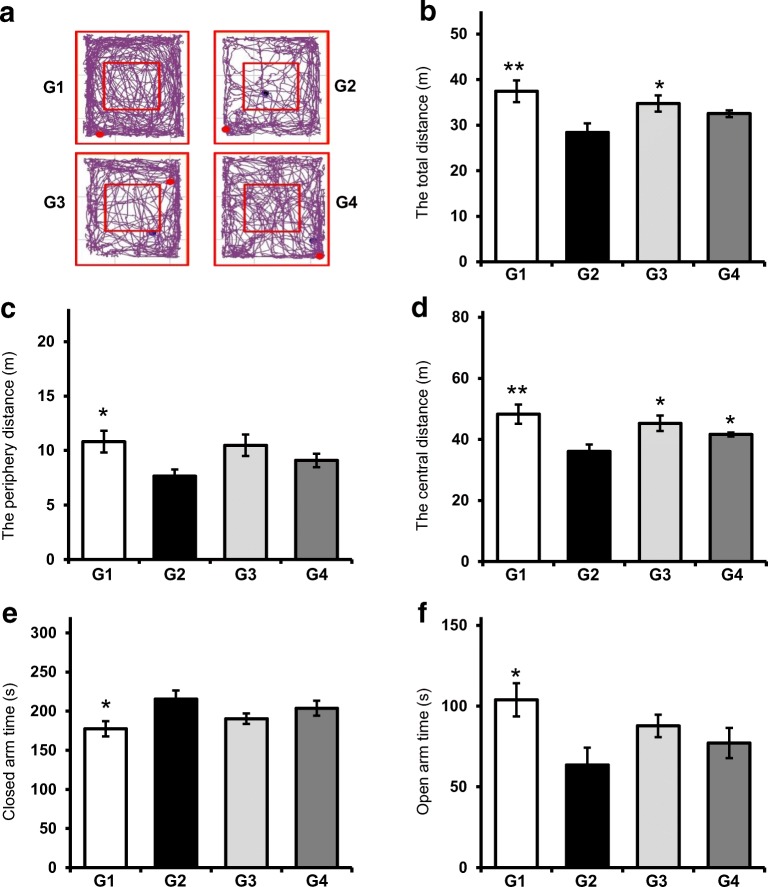
To examine whether probiotics attenuated depression in mice under stress conditions, mice were orally administered probiotics for 8 weeks, and FST and TST were performed. Figure 1a shows that probiotic-treated mice (G4) more frequently exhibited climbing behavior compared with stress mice (G2), although a statistically significant difference was not observed between groups. Swimming behavior was improved in mice administered with probiotics as well as those given fluoxetine (G3) (Fig. 1b). Immobility was considered as the total duration of floating, twitching, and kicking behaviors. Figure 1c also shows that immobility increased in stress mice, whereas probiotic administration significantly decreased immobility, causing the extent of movement in G4 mice to be similar to that in normal mice (G1). As a positive control, fluoxetine administration also effectively reduced immobility. Furthermore, administration with probiotics resulted in the reduction in immobility time in TST (Fig. 1d). In the open-field test, normal mice showed active movement in the overall area, including the peripheral and central areas, whereas mice exhibited conspicuously decreased movement in the peripheral and central areas of open field (Fig. 2a). More specifically, the total distance moved was significantly reduced in stress mice as opposed to the mice administered with fluoxetine, which exhibited increased movement (P < 0.05). Moreover, probiotic administration also increased the total distance of movement, although this increase was not statistically significant (Fig. 2b). In addition, the distance travelled by the stress mice (G2) in the mice peripheral areas was lesser than that travelled by both probiotic- (G4) and fluoxetine- administration (G3), but this difference was not statistically significant (Fig. 2c). Consistently, the distance travelled by the stress mice (G2) in the central area was significantly lesser than that travelled by mice administered with probiotics (G4) (P < 0.05) A similar increase in the distance travelled by fluoxetine-administered mice (G3) in the central area was also observed (Fig. 2d). In the elevated plus maze test, although the difference was not statistically significant, the probiotic-administered mice (G4) spent lesser time in the closed arms than the stress mice (G2) (Fig. 2e). Additionally, fluoxetine-administered mice (G3) also spent lesser time than the stress mice. Probiotic- and fluoxetine-administered mice spent more time in the open arms than the stress mice, but this difference was not statistically significant (Fig. 2f).
Fig. 1.
Effect of probiotic administration on depressive-like behaviors in FST and TST. Climbing behavior (a), swimming behavior (b), and immobile behavior (c) were measured during 6-min FST. d Immobile behavior was measured during 6-min TST. Results are presented as mean ± SEM values from 10 mice of each group. G1: normal mice, G2: stress mice, G3: stress + fluoxetine-treated mice, G4: stress + probiotic-treated mice. (*), (**), and (***) indicate P < 0.05, P < 0.01, and P < 0.005 compared with the control group, respectively
Fig. 2.
Effect of probiotic administration on depressive-like behaviors in OFT and EPM. a Representative track plots generated from OFT. Distance travelled in total (a) as well as in the peripheral (b), and central (c) areas. Each mouse was placed in the center of the open field and allowed to freely explore the apparatus during a 10-min test. Time spent in closed (e) and open (f) arms during EPM. Results are presented as mean ± SEM values from 10 mice of each group. G1: normal mice, G2: stress mice, G3: stress + fluoxetine-treated mice, G4: stress + probiotic-treated mice. (*) and (**) indicate P < 0.05 and P < 0.01 compared with the control group, respectively
Effect of probiotic administration on serum corticosterone level
Figure 3 displays the serum corticosterone level in stress mice (G2) and fluoxetine- (G3) and probiotic-administered mice (G4). The production of corticosterone in serum was significantly induced in stress mice (G2) than in the normal mice (G1) (P < 0.005), whereas probiotic administration caused a significant reduction in serum corticosterone level compared with the level in stress mice (P < 0.01). As expected, fluoxetine administration also considerably decreased serum corticosterone level (P < 0.05), suggesting that probiotics effectively reduced corticosterone production in mice serum.
Fig. 3.
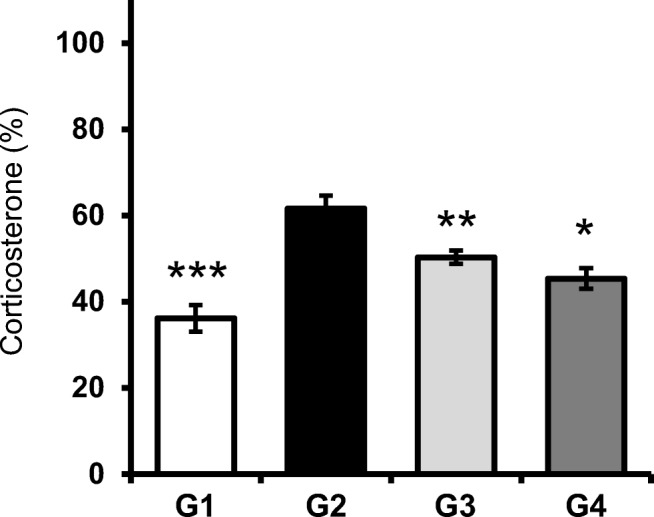
Effect of probiotic administration on the serum corticosterone level. Serum corticosterone level was measured after probiotic administration. Results are presented as mean ± SEM values from 10 mice of each group. (*), (**), and (***) indicate P < 0.05, P < 0.01, and P < 0.005 compared with the control group, respectively. G1: normal mice, G2: stress mice, G3: stress + fluoxetine-treated mice, G4: stress + probiotic-treated mice. (*) and (**) indicate P < 0.05 and P < 0.01 compared with the control group, respectively
Effect of probiotic administration on the diversity of gut microbiota
This experiment was individually repeated two times with four groups (n = 5), and the collected fecal samples were sequenced and analyzed using MiSeq and QIIME, respectively. Furthermore, 16S rRNA gene sequencing for each sample generated more than 120,000 reads, and more than 7700 OTUs were processed by QIIME. To evaluate the ecological features of bacterial communities among the four groups, we assessed diversity using various parameters. In alpha diversity, parameters of observed species and Chao1, which are species richness estimators, showed a slight increase only in the stress mice group (G2), and a similar tendency was also observed for parameters of Shannon and Simpson, species diversity estimators (Table 1). Beta diversity was calculated using unweighted Unifrac algorithm that determines microbial phylogenetic similarity showed an obvious difference in the microbial community between the normal (G1) and stress (G2) mice groups. The composition of microbial community in fluoxetine-administrated mice (G3) was similar to that in the normal mice group (G1) (Fig. 4). Although depressive-like behavior exhibited by both fluoxetine- and probiotic-administered mice (G3 and G4, respectively) was likely to improve and become similar to the behavior exhibited by normal mice, the probiotic-administered mice group showed a different microbiota community than the other groups, owing to the fact that the mice were given a direct delivery of probiotics containing lactobacilli and bifidobacteria. Thus, the alpha and beta diversity suggest that depression-induced stress causes remodeling of gut microbiota, and treatment with either fluoxetine or probiotics may remodulate gut microbiota.
Table 1.
Alpha diversity of fecal microbiota
| Group | Richness estimator | Diversity index | ||
|---|---|---|---|---|
| Observed_species | Chao 1 | Shannon | Simpson | |
| G1 (normal mice) | 7732.5 | 39,788.96 | 7.042 | 0.972 |
| G2 (stress mice) | 8809.0 | 41,881.26 | 7.586 | 0.980 |
| G3 (stress + fluoxetine-treated mice) | 8232.5 | 39,164.41 | 7.216 | 0.970 |
| G4 (stress + probiotic-treated mice) | 7251.5 | 34,310.12 | 7.072 | 0.973 |
The results are shown as the average value obtained from two individual experiments
Fig. 4.
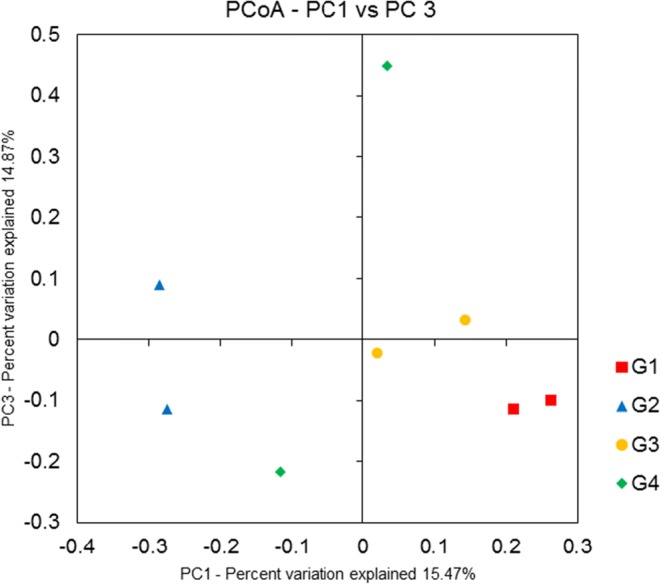
Principle coordinate analysis (PCoA) plots based on microbial OTUs of (G1) normal mice, (G2) stress mice, (G3) stress + fluoxetine-treated mice, and (G4) stress + probiotic-treated group. Unweighted Unifrac distance is presented by two-dimensional PCoA plots
Different microbial composition in the gut after probiotic administration
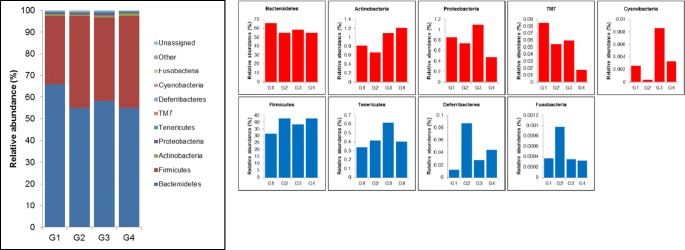
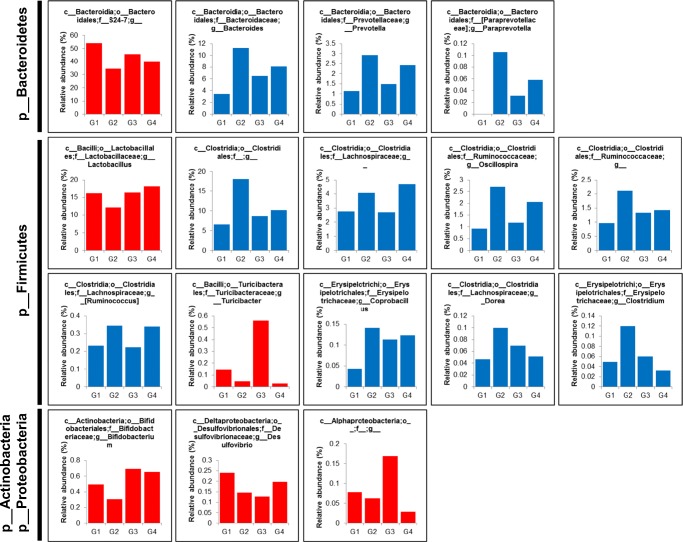
Taxonomical analysis of microbial distribution at the phylum level shows that microbiota composition is remodeled owing to stress (Fig. 5). The proportion of Bacteroides, Actinobacteria, Proteobacteria, Saccharibacteria (formerly known as TM7), and Cyanobacteria was decreased, whereas that of Firmicutes, Tenericutes, Deferribacteres, and Fusobacteria was increased in the gut of stress mice. Probiotic administration showed restoration of microbial compositions, such as Actinobacteria and Cyanobacteria, which had been altered owing to stress. At the genus level, 105 genera in the gut of stress mice showed the proportional tendency of either increase or decrease, and 85% (89 of 105 genera) of the gut microbiota was restored in both fluoxetine- or probiotic-administered mice (Supplementary Table 1). In particular, the relative abundance of proportionally dominant S24-7_unclassified, Lactobacillus, Turicibacter, Bifidobacterium, and Desulfovibrio decreased, whereas that of Bacteroides, Prevotella, Paraprevotella, Lachnospiraceae_unclassified, Ruminococcus, Dorea, Oscillospira, Ruminococcaceae_unclassified, Coprobacillus, and Clostridium increased in the gut of stress mice (Fig. 6). However, probiotic administration showed restoration of the gut microbiota at the genus level; in other words, the proportion of S24-7_unclassified, Lactobacillus, Bifidobacterium, and Desulfovibrio reduced owing to stress, suggesting that the delivered probiotics containing Lactobacillus and Bifidobacterium could alleviate depressive-like behaviors by modulating gut microbiota.
Fig. 5.
Stacked bar chart (left panel) and individual bar chart (right panel) showing relative abundance of the bacterial phylum of fecal microbiota in G1 (normal mice), G2 (stress mice), G3 (stress + fluoxetine-treated mice), or G4 (stress + probiotic-treated mice) groups
Fig. 6.
Individual bar chart showing relative abundance of bacterial genera of fecal microbiota in G1 (normal mice), G2 (stress mice), G3 (stress + fluoxetine-treated mice), or G4 (stress + probiotic-treated mice) groups
Discussion
Currently, the direct biochemical signaling between the gastrointestinal tract and the central nervous system, referred to gut-brain axis, has been revealed in the neurogastroenterology research [1]. Several reports have shown that gut microbiome can influence and modulate emotional behavior, suggesting that ingested probiotics could provide useful agent to alleviate depressive symptoms. The effects of probiotics on psychiatric symptoms are associated with the gut–brain axis, which are brought about by reduction in systemic inflammation and regulation of neurotransmission [19]. Although several animal models have shown a mimicry of depressive phenotypes by inducing neuronal changes similar to those observed in humans, the development of new treatments and biological mechanisms of depression are not easily applied to humans. Nevertheless, animal models, such as rodent model, are reliable to examine the molecular and cellular mechanisms of depressive symptoms in a controlled environment, providing that the animal model may provide the treatment strategies to apply to humans.
Several reports have revealed the effects of probiotic administration on psychiatric symptom attenuation. Administration with a single probiotic Bifidobacterium infantis reversed depression by regulating the hypothalamic–pituitary–adrenal axis in a rat model [20]. In another rat model, administration with B. infantis possibly increased tryptophan levels in plasma and reduced serotonin levels in the frontal cortex, thereby attenuating depressive symptoms [15]. In addition to B. infantis, L. rhamnosus JB-1 also reduced depression-related behavior by directly altering the receptor expression of gamma-aminobutyric acid [14]. In accordance with our observation, ingestion of the same strain, L. rhamnosus JB-1, caused a reduction in depressive-like behavior such as tail suspension in a mouse model [21]. A recent report also demonstrated that chronic mild stress-induced anxiety- and depressive-like behaviors in the sucrose preference test, elevated plus maze, and forced swim test were ameliorated by the administration of three probiotic strains, namely L. helveticus, L. plantarum, and B. longum [22]. Moreover, probiotics also exert anti-depressive and anxiolytic effects on humans. Significantly lesser psychological distress was observed in the treatment group administered with L. helveticus- and B. longum-containing probiotics [23]. Patients with chronic fatigue syndrome who received a probiotic formulation containing L. casei- showed significantly lesser anxiety symptoms than did the control group [24]. These positive findings, including our observations, suggest that probiotic treatment strategy is promising; in such a strategy, intestinal microbiota may be targeted for achieving therapeutic benefits in depressive disorders.
Although the role of probiotics in the amelioration of depressive disorders has not been precisely explored, our study showed that dysbiosis of intestinal microbiota was characterized by significant differences in taxonomy between the control and stress groups. At the phylum level, the proportion of Actinobacteria significantly decreased in the stress group, whereas probiotic treatment resulted in its increase. It is possible that probiotics containing Bifidobacterium could be directly delivered to the intestine, leading to an abundance of Actinobacteria in the probiotic-treated group. It has been reported that the composition of gut microbiota between major depressive disorder patients and healthy controls was characterized by a significant difference in the proportion of Actinobacteria and Firmicutes [5, 25]. However, in the present study, the proportion of Firmicutes was not restored after probiotic administration. In addition, inflammation in the gut may be involved in the pathogenesis of depression [26]. Although the results obtained from this study did not show the alleviation of inflammatory responses in the gut of mice administered with probiotics, it is possibly speculated that anti-inflammatory activity of probiotics in the gut may protect gut barrier function and modulate inflammatory mediators, consequently resulting in the reduced depression symptoms.
Conclusion
In conclusion, probiotic administration modulates stress-related behaviors in mice. Since the probiotics altered the distribution of gut microbiota, this study may support gut microbiome-based interventions for stress-related depression symptoms. Although further extensive studies need to explain the association of depression with ingested probiotics and incorporate metagenomics and metabolomics, based on our observations, we can elucidate a partial relationship between probiotic-induced gut microbiota alteration and depression.
Electronic supplementary material
(XLSX 28 kb)
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [2016R1D1A1B03932530 (B.S.K)].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Quan Feng Liu and Hong-Man Kim contributed equally to this work.
Contributor Information
Byung-Soo Koo, Email: koobs@dongguk.ac.kr.
Seok-Seong Kang, Email: sskang@dongguk.edu.
References
- 1.Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann General Psychiatry. 2017;16:18. doi: 10.1186/s12991-017-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry. 2000;157:1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- 4.Gump BB, Matthews KA, Eberly LE, Chang YF, Group MR Depressive symptoms and mortality in men: results from the multiple risk factor intervention trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 7.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13:239–244. doi: 10.9758/cpn.2015.13.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudzki L, Szulc A. “Immune gate” of psychopathology-the role of gut derived immune activation in major psychiatric disorders. Front Psychiatry. 2018;9:205. doi: 10.3389/fpsyt.2018.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008;29:117–124. [PubMed] [Google Scholar]
- 11.Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141:55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers:Tthe gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–95. [DOI] [PubMed]
- 14.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5. [DOI] [PMC free article] [PubMed]
- 15.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Jeon S, Lee CH, Liu QF, Kim GW, Koo BS, Pak SC. Alteration in brain-derived neurotrophic factor (BDNF) after treatment of mice with herbal mixture containing Euphoria Longana. Houttuynia cordata and Dioscorea japonica Daru. 2014;22:77. [DOI] [PMC free article] [PubMed]
- 17.Zeni AL, Zomkowski AD, Maraschin M, Rodrigues AL, Tasca CI. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: evidence for the involvement of the serotonergic system. Eur J Pharmacol. 2012;679:68–74. doi: 10.1016/j.ejphar.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. [DOI] [PMC free article] [PubMed]
- 19.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 21.McVey Neufeld KA, Kay S, Bienenstock J. Mouse strain affects behavioral and neuroendocrine stress responses following administration of probiotic Lactobacillus rhamnosus JB-1 or traditional antidepressant fluoxetine. Front Neurosci. 2018;12:294. doi: 10.3389/fnins.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, et al. Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Front Behav Neurosci. 2018;12:266. doi: 10.3389/fnbeh.2018.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 24.Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng P, Cheng K, Zeng L, Zhou CJ, Xie P. A new pathway for the gut microbiota to modulate the brain: activation of pattern-recognition receptors by microbial products. Mol Psychiatry. 2017;22:162–163. doi: 10.1038/mp.2016.210. [DOI] [PubMed] [Google Scholar]
- 26.Pfau ML, Menard C, Russo SJ. Inflammatory mediators in mood disorders: therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2018;58:411–428. doi: 10.1146/annurev-pharmtox-010617-052823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 28 kb)