Abstract
Background
Weaning from mechanical ventilation (MV) is a cardiovascular stress test. Monitoring the regional oxygenation status has shown promising results in predicting the tolerance to spontaneously breathe in the process of weaning from MV. Our aim was to determine whether changes in skeletal muscle oxygen saturation (StO2) measured by near-infrared spectroscopy (NIRS) on the thenar eminence during a vascular occlusion test (VOT) can be used to predict extubation failure from mechanical ventilation.
Methods
We prospectively studied 206 adult patients with acute respiratory failure receiving MV for at least 48 h from a 30-bed mixed ICU, who were deemed ready to wean by their physicians. Patients underwent a 30-min spontaneous breathing trial (SBT), and were extubated according to the local protocol. Continuous StO2 was measured non-invasively on the thenar eminence. A VOT was performed prior to and at 30 min of the SBT (SBT30). The rate of StO2 deoxygenation (DeO2), StO2 reoxygenation (ReO2) rate and StO2 hyperemic response to ischemia (HAUC) were calculated.
Results
Thirty-six of the 206 patients (17%) failed their SBT. The remainder 170 patients (83%) were extubated. Twenty-three of these patients (13.5%) needed reinstitution of MV within 24 h. Reintubated patients displayed a lower HAUC at baseline, and higher relative changes in their StO2 deoxygenation rate between baseline and SBT30 (DeO2 Ratio). A logistic regression-derived StO2 score, combining baseline StO2, HAUC and DeO2 ratio, showed an AUC of 0.84 (95% CI 0.74–0.91) for prediction of extubation failure.
Conclusions
Extubation failure was associated to baseline and dynamic StO2 alterations during the SBT. Monitoring StO2-derived parameters might be useful in predicting extubation outcome.
Keywords: Near-infrared spectroscopy, Tissue oxygenation, Microcirculation, Regional blood flow, Mechanical ventilation, Weaning
Background
Weaning from ventilatory support is a challenge for critical care clinicians. Even in those patients who succeed a spontaneous breathing trial (SBT), failure of planned extubation occurs in up to 20% [1–5]. Importantly, failed extubation is associated with increased hospital mortality, prolonged ICU and hospital stays, and increased need for tracheostomy [6, 7]. Despite many respiratory and hemodynamic variables for predicting weaning success have been evaluated, few parameters have substantial predictive power [7, 8], and new predictive tools are needed.
The SBT is a cardiovascular stress test, and failure to wean from mechanical ventilation (MV) often reflects cardiovascular insufficiency to cope with the increased oxygen cost of breathing [9, 10]. Since the augmented oxygen cost of breathing is met by increases in respiratory muscle blood flow, diversion of flow away from “non-vital” tissues might occur, potentially causing hypoperfusion in areas such as the splanchnic and the peripheral circulation [11–15]. In fact, alterations in splanchnic and peripheral circulation, assessed by different technologies, have been associated with failure to tolerate an SBT [12–16]. In a preliminary study, our group demonstrated that changes in skeletal muscle oxygenation (StO2) measured non-invasively on the thenar eminence by near-infrared spectroscopy (NIRS) were associated with the outcome of a 30-min SBT [16].
In the present study, we hypothesized that regional oxygenation alterations within the SBT would be associated with extubation failure after a clinically successful SBT.
Methods
Setting
This prospective observational study was conducted in a 30-bed medical-surgical intensive care unit at a university hospital. The local Ethics Committee (CEIC 2008/554) approved the study. Informed consent was obtained from the patient or their next of kin prior to the study initiation. This study is presented according to the STROBE recommendations for reporting observational studies [17].
Patients and data collection
We included adult patients (≥ 18-year old) receiving invasive mechanical ventilatory support for > 48 h and considered ready to wean by their physicians, according to the local protocol, and who never experienced any previous attempt of separation from MV. Eligibility to perform a weaning trial included partial or complete recovery from the underlying cause of acute respiratory failure; adequate gas exchange, as indicated by a partial pressure of arterial oxygen (PaO2) > 60 mmHg to a fraction of inspired oxygen (FiO2) < 0.4, with a positive end-expiratory pressure (PEEP) < 5 cmH2O; adequate cough, with absence of excessive tracheobronchial secretion; core temperature < 38 °C; hemoglobin > 8 g/dL; stable cardiovascular status (heart rate < 120 beats min−1, systolic blood pressure 90–160 mmHg), with no need for vasoactive agents; adequate level of consciousness (awake, alert and aware of their surroundings), with no further need for sedative agents. Patients were also tested for adequate pulmonary function, defined as a rapid shallow breathing index < 100 breaths min−1 L−1, and a maximal inspiratory pressure (MIP) < − 20 cmH2O. Patients meeting these criteria were considered ready to wean, and were eligible for a subsequent spontaneous breathing trial.
Exclusion criteria included trauma in both upper limbs, and hematoma or skin lesions at the thenar eminence that could hinder placement of NIRS sensor probe. Patients with altered level of consciousness that could lead to central hypoventilation and/or impaired secretions’ management were excluded. Patients in whom their medical team decided to indicate preventive non-invasive ventilation following extubation were also excluded.
Study protocol
After inclusion, patients underwent a spontaneous breathing trial (SBT) for 30 min, defined as assisted spontaneous breathing with continuous positive airway pressure (CPAP) of 5 cm H2O, or a T-tube trial, as prescribed by their medical team. Patients were in a semi-recumbent position, and FiO2 was kept constant during the trial.
The evaluation criteria for SBT failure was defined as the presence of one or more of the following criteria during the trial: respiratory rate (RR) > 35 breaths/min for 5 min or longer; arterial pulse oximetry saturation (SpO2) < 90%, heart rate (HR) > 140 beats/min or sustained increase or decrease in HR > 20%; systolic blood pressure > 180 mmHg or < 90 mmHg; increased anxiety and diaphoresis. Decision to remove the endotracheal tube was made independently of the study investigators by the attending physicians, who did not have access to the StO2 data. Weaning success was defined as patient remaining free of MV for > 24 h after passing the SBT. Extubation failure was defined as the need for reinstitution of MV, either invasive or non-invasive, within 24 h following extubation.
Clinical parameters and outcomes
We collected demographic data: age, sex, diagnosis, and days on mechanical ventilation. Hemodynamic, respiratory and oxygenation variables were monitored continuously and recorded just before starting and at 30 min into the SBT. HR and mean arterial pressure (MAP) were recorded by routine bedside monitoring (Monitor Intellivue MP 70; Phillips Medizinsystems, Boeblingen, Germany). RR, tidal volume (Vt), minute ventilation (VE), FiO2 and SpO2 were recorded at start and 30 min into the SBT. Arterial and/or central venous blood gas analyses were made when an arterial line and/or a central venous line was in place, respectively (ABL 700 series; Radiometer Medical, Copenhagen, Denmark).
The primary outcome of interest was extubation failure.
NIRS measurements
Tissue oxygen saturation (StO2) was recorded continuously using the InSpectra 650 Tissue Spectrometer (Hutchinson Tech., Hutchinson, Minnessotta). This technology uses a wide gap 40-nm second derivative spectroscopic method, with measurements at four different wavelengths (680, 720, 760 and 800 nm), and has been previously validated for estimating local hemoglobin oxygen saturation in tissues [18]. The StO2 15-mm optical surface probe was placed on intact skin on the thenar eminence never placed adjacent to the site of radial artery cannulation. The InSpectra tissue spectrometer also measures relative hemoglobin concentration, presented as the tissue hemoglobin index (THI).
Vascular Occlusion Test (VOT) The VOT was performed as previously described [19, 20]. A blood pressure cuff (Portable Tourniquet System; Delfi Medical, Vancouver) was placed on the forearm, rapidly inflated 40 mmHg above systolic pressure, and kept inflated until StO2 decreased to 40%. Then, the cuff was rapidly deflated. The resulting deoxygenation (DeO2) and reoxygenation (ReO2) slopes are reported as change in saturation over time (Fig. 1). Hyperemic response following the reoxygenation is reported as an area under the curve (HAUC). We performed a VOT at the beginning of the SBT and again at 30 min into the SBT. NIRS-derived thenar muscle oxygen consumption (nirVO2) was calculated as described by Skarda [21]: nirVO2 = (DeO2 slope)−1/[(THIstart + THIend)/2]. Relative changes in StO2-derived parameters were calculated as the quotient between values at minute 30 and baseline (DeO2 ratio, nirVO2 ratio and ReO2 ratio). Absolute StO2- and VOT-derived variables were obtained using the InSpectra Research Software® v4.01 (Hutchinson Technology). Two researchers (JM and JM) separately analyzed all the registers, and a database was constructed. When discrepancies were observed, the two researchers performed a third joint analysis.
Fig. 1.
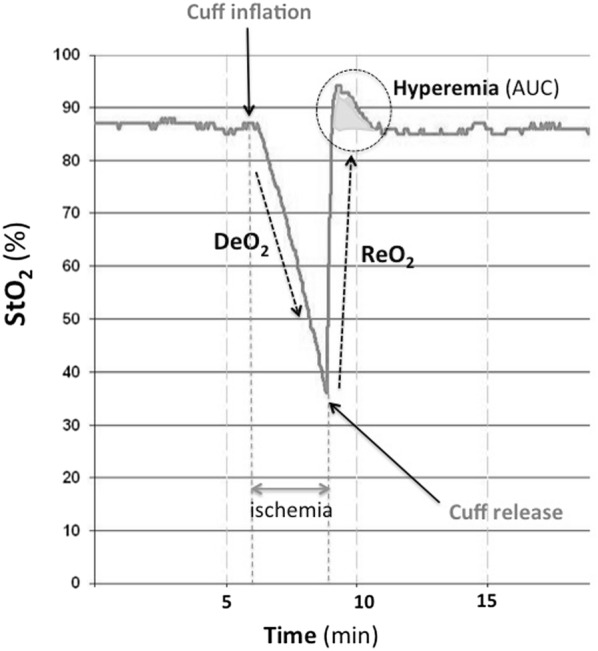
StO2 response to the Vascular Occlusion Test (VOT). The response to transient ischemia generates three main parameters in the continuous StO2 recording: the StO2 deoxygenation rate (DeO2), the StO2 reoxygenation rate (ReO2), and the hyperemic response to ischemia, which is computed as the area under the curve of the hyperemic response (HAUC) as referred to steady-state StO2
Sample size calculation
Accepting an alpha risk of 0.05 and a beta risk of 0.20 in a two-sided test, estimating an SBT failure rate of 20%, and an extubation failure rate of 15%, we calculated that 200 patients were necessary to detect a positive likelihood ratio (LR +) of the test equal to or greater than 5.
Statistical analysis
Statistical analysis was performed by means of IBM SPSS statistics 20.0 software (IBM Corporation). Normal distribution of the studied variables was confirmed using the Kolmogorov–Smirnov test. Accordingly, continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as absolute number and proportions (%). Chi square and Student’s t test were used to compare extubation success and failure groups. Student’s t test for paired measurements was used to analyze changes over time. Bivariate logistic regression analysis was used to obtain independent predictors of extubation success, and logistic regression models were used to create a predictive StO2-score. Statistical significance was defined as p < 0.05 (two-tailed test).
Results
Two hundred-and-six patients were studied. Thirty-six patients (17.5%) failed the SBT, and were excluded of the final analysis. One hundred-and-seventy patients passed the SBT and were extubated. Twenty-three (13.5%) of these patients failed extubation and required re-instauration of MV within 24 h. The suspected etiology of extubation failure was mainly increased respiratory muscle load (52%), and cardiac failure (35%) (Additional file 1: Table S1). No baseline differences in demographic, hemodynamic, and respiratory variables were observed when comparing extubation success and extubation failure groups (Table 1). Hemodynamic and respiratory changes within the SBT were similar in both groups (Table 1).
Table 1.
Characteristics of the patients who succeed the spontaneous breathing trial (SBT)
| Extubation success (n = 147) | Extubation failure (n = 23) | |||
|---|---|---|---|---|
| Age (years) | 66 ± 14 | 67 ± 12 | ||
| Days on MV (n) | 7 ± 5 | 8 ± 6 | ||
| Pre-existent comorbidities (%) | ||||
| Diabetes mellitus | 33 | 35 | ||
| Hypertension | 57 | 48 | ||
| Cardiac disease | 23 | 22 | ||
| COPD | 16 | 30 | ||
| Etiology of acute respiratory failure (%) | ||||
| Septic shock | 29 | 26 | ||
| Heart failure | 17 | 26 | ||
| Respiratory | 20 | 26 | ||
| Trauma | 6 | 0 | ||
| Other | 29 | 22 | ||
| Baseline | Minute 30 | Baseline | Minute 30 | |
|---|---|---|---|---|
| HR (beats/min) | 88 ± 16 | 91 ± 16† | 83 ± 17 | 87 ± 18† |
| MAP (mmHg) | 82 ± 13 | 85 ± 15† | 79 ± 14 | 80 ± 12 |
| RR (resp/min) | 19 ± 4 | 23 ± 5† | 18 ± 3 | 23 ± 4† |
| Vt (mL) | 454 ± 96 | 430 ± 123† | 438 ± 115 | 367 ± 132† |
| pH | 7.46 ± 0.05 | 7.44 ± 0.06† | 7.47 ± 0.04 | 7.44 ± 0.04† |
| pCO2 (mmHg) | 37 ± 6 | 38 ± 6† | 41 ± 9 | 42 ± 14 † |
| Hb (g/dL) | 9.9 ± 1.8 | 9.9 ± 1.8 | 10.1 ± 2 | 10.3 ± 2.1 |
| SaO2 (%) | 96 ± 8 | 97 ± 3 | 97 ± 3 | 96 ± 3 |
| ScvO2 (%) | 66 ± 9 | 66 ± 8 | 62 ± 9 | 61 ± 5* |
| StO2 (%) | 80 ± 6 | 80 ± 7 | 76 ± 11 | 77 ± 9 |
| DeO2 (%/min) | − 13.1 ± 8.4 | − 13.6 ± 10.7 | − 12.7 ± 7 | − 17.2 ± 10.3† |
| nirVO2 (U) | 139 ± 80 | 159 ± 135† | 129 ± 75 | 174 ± 97† |
| THI (U) | 10.7 ± 2.9 | 11.3 ± 3.1† | 10.0 ± 3.9 | 10.5 ± 4.0 |
| ReO2 (%/s) | 4.1 ± 1.6 | 4.2 ± 1.8† | 3.3 ± 1.9* | 3.7 ± 2.0 |
| Hyperemia AUC (U) | 12.2 ± 6.5 | 12.9 ± 5.7 | 7.1 ± 4.7** | 8.4 ± 4.1** |
| DeO2 ratio | 1.1 ± 0.3 | 1.5 ± 0.6** | ||
| nirVO2 ratio | 1.1 ± 0.4 | 1.5 ± 0.5* |
MV Mechanical ventilation, COPD Chronic obstructive pulmonary disease, HR heart rate, MAP mean arterial pressure, RR respiratory rate, Vt tidal volume, pCO2 arterial partial pressure of carbon dioxide, Hb Hemoglobin, SaO2 arterial oxygen saturation, ScvO2 central venous oxygen saturation, StO2 tissue oxygen saturation, DeO2 StO2 deoxygenation rate, nirVO2 NIRS-derived local oxygen consumption, THI tissue hemoglobin index, ReO2 StO2 reoxygenation rate
* p < 0.05; and ** p < 0.01, as compared to success group at the same time point; †p < 0.05, as compared to baseline values within the group
StO2 variables
Baseline ReO2 and HAUC values were significantly lower in the extubation failure group (Table 1). The weaning success group showed no significant changes in StO2, DeO2, and HAUC after 30 min of SBT, while nirVO2 and THI significantly increased. The extubation failure group showed a significant decrease in their DeO2 slope, as well as an increase in nirVO2 after 30 min of SBT, while no changes in StO2, HAUC and THI were observed. The DeO2 ratio (represented as the ratio of DeO2 at 30 min to baseline DeO2) was significantly higher in the failure group (1.5 ± 0.6 vs. 1.1 ± 0.3 in the success group, p < 0.01). The nirVO2 ratio was also higher in the failure group (1.5 ± 0.5 vs. 1.1 ± 0.4 in the success group, p < 0.05).
Extubation failure prediction
Univariate analysis showed that StO2, ReO2, and HAUC at baseline, StO2 and HAUC after 30 min of the SBT, DeO2 ratio, and nirVO2 ratio were associated with extubation failure. In a multivariate analysis, only baseline StO2, baseline HAUC and DeO2 ratio persisted significantly associated with extubation failure (Table 2).
Table 2.
Multivariate regression analysis
| B | ß | p | (95% CI) | |
|---|---|---|---|---|
| Baseline StO2 | − 0.075 | 0.93 | 0.03 | 0.87–0.99 |
| Baseline hyperemic AUC | − 0.192 | 0.83 | 0.001 | 0.74–0.92 |
| DeO2 ratio | 2.849 | 17.3 | < 0.001 | 4.1–72.8 |
The AUC for predicting extubation failure for baseline StO2, baseline HAUC, and DeO2 ratio are displayed in Table 3. A logistic regression-derived StO2 score, combining baseline StO2, baseline HAUC and DeO2 ratio, proved an AUC of 0.84 (95% CI 0.74–0.93, p < 0.001) for prediction of extubation failure. An StO2 score cut-off value of − 1.23 showed an LR + of 7 for predicting extubation failure (Additional file 2: Figure S1).
Table 3.
Prediction of extubation failure
| AUC | p | (95% CI) | |
|---|---|---|---|
| StO2 at baseline | 0.57 | ns | 0.43–0.72 |
| DeO2 ratio | 0.72 | < 0.01 | 0.58–0.85 |
| nirVO2 ratio | 0.69 | 0.02 | 0.56–0.81 |
| Hyperemia AUC0 | 0.73 | 0.01 | 0.58–0.88 |
| Hyperemia AUC30 | 0.74 | < 0.01 | 0.62–0.87 |
| StO2 score | 0.84 | < 0.001 | 0.74–0.93 |
| ScvO2 at min 30 | 0.72 | 0.04 | 0.58–0.85 |
| % change in ScvO2 | 0.59 | ns |
DeO2ratio quotient between StO2-deoxygenation rate at the end of SBT and at baseline, nirVO2ratio quotient between NIRS-derived local oxygen consumption at the end of SBT and at baseline, HAUC area under the curve of the hyperemic response, StO2 tissue oxygen saturation, ScvO2 central venous oxygen saturation, % change in ScvO2 refers to the % change in ScvO2 at min 30 of the SBT as compared to baseline ScvO2
Discussion
Our study demonstrates that alterations in StO2-derived parameters were predictive of extubation failure after a clinically successful SBT.
To our knowledge, this is the first study showing that regional oxygenation parameters are associated with extubation outcome. The peripheral circulation has been previously studied in the setting of a cardiovascular stress test such as the SBT. Several studies showed that changes in gastric mucosal pH within the SBT were predictive of the clinical outcome of such SBT [12–15]. Similar results were obtained when assessing the peripheral circulation by means of NIRS, non-invasively monitoring oxygenation of the skeletal muscle within the SBT [16, 22, 23]. However, they were all small studies, and never explored the ability to predict the outcome of the overall weaning process. In this prospective study, we analyzed the utility of StO2-derived parameters in the extubation process, after a clinically successful SBT. Our results add new evidence on the usefulness of regional non-invasive parameters as a complimentary tool in monitoring the cardiovascular performance of critically ill patients. Indeed, our data suggest that the inclusion of StO2 monitoring in the weaning process might help prevent failed extubations, and therefore, the potential negative consequences on the outcome of critically ill patients.
Transitioning from positive pressure ventilation to spontaneous ventilation determines an increase in the work of breathing, and thus, an increase in the oxygen demand of the respiratory muscles. Such increase in the metabolic demand causes a sympathetic activation, in an attempt to optimize cardiac output delivery to the metabolically active tissues [24], and to increase vasomotor tone, redistributing blood flow away from the periphery toward the respiratory muscles [10, 25]. The ability of the cardiovascular system to meet this increased demand might be closely related to the ability of the patient to tolerate spontaneously breathing. Therefore, when the cardiovascular performance is limited, and/or the cost of breathing excessive, extubation failure will be more likely to occur. In these situations, the increase in sympathetic tone might be more accentuated, as the cardiovascular system continues to attempt to match cardiac output to an increasing metabolic demand. Indeed, clinical criteria for determining the success of the SBT are essential symptoms of excessive sympathetic activity (i.e., tachycardia, hypertension, agitation). A subclinical manifestation of the sympathetic activation would be the degree of peripheral vasoconstriction and/or the increase in the metabolic rate, and this is the basis for regional oxygenation measurements with NIRS.
Increased local oxygen extraction rate
Our data confirmed that relative increases in DeO2 during a 30-min SBT are associated with extubation failure, independent of other respiratory and hemodynamic parameters. The observed increases in DeO2 within the SBT might be explained by two different, but potentially concurrent, pathophysiological mechanisms: (1) local supply–demand dependency in low or inadequate blood flow states, such as blood flow diversion from the periphery; and (2) increase in local metabolic rate.
The increase in oxygen demand of the respiratory muscles during an SBT may lead to blood flow redistribution via activation of the sympathetic–adrenal system. When accentuated, this compensatory mechanism might cause a stealing effect from the periphery [10]. Accordingly, one would expect decreases in blood content of the sensed area [19, 26], along with relative increases in the DeO2 rate (either due to the decreased Hb content or to the sympathetically driven increase in the metabolic rate). In our population, extubation failure was associated with significant increases in DeO2, but no change in THI. Conversely, the success group showed no change in DeO2, with significant increases in THI. Such observations suggest that cardiac output increased in those patients who succeeded the weaning process. In those patients who failed, since we did not measure stroke volume, the interpretation of the lack of changes in THI is more complex. In a model of simulated hypovolemia, Bartels et al. reported that THI measured on the thenar eminence detected slight decreases in stroke volume, but changes were minimal, as compared to measurements on the forearm [26]. Therefore, we cannot exclude some degree of stealing effect as results of a limited sensitivity of the technology. Finally, a balanced effect of increased cardiac output and peripheral vasoconstriction, resulting in no changes in THI, cannot be excluded.
NirVO2, an estimation of local oxygen consumption [20, 21], increased in both groups during the SBT, but the magnitude of this increase was significantly higher in the extubation failure group. NirVO2 differences might reflect dissimilar degrees in sympathetic activation in the setting of inadequate cardiovascular response. This hypothesis is supported by studies that showed significant increases in plasma catecholamines during the SBT, especially in patients who failed an SBT [27, 28]. Overall, our data suggest that the increase in DeO2 in the extubation failure group might be mainly related to an increase in local oxygen consumption, although some degree of blood flow diversion cannot be ruled out.
Local hyperemic response after transient ischemia
We also observed that baseline endothelial performance, as measured by the StO2 hyperemic response, was independently associated with extubation outcome. Our findings on the predictive value of the StO2 hyperemic response are not surprising. Activation of the sympathetic nervous system is a major component of the neurohumoral response to the increased oxygen cost of breathing. Catecholamine release mediates vasoconstriction of peripheral vascular beds, and also, via ß-adrenoceptors, promotes vasodilation of the coronary arteries, and improves contractility. The functional impairment of the endothelium has been associated not only with poor peripheral response, but also with diminished ß-adrenoceptor sensitivity, limiting the cardiac response [29]. Therefore, endothelial dysfunction might be a potential marker of the ability of the patient to respond to the hemodynamic changes produced by the discontinuation of ventilatory support. According to our observations, we might hypothesize that vascular endothelial integrity, evaluated in peripheral skeletal muscle, might be relevant when facing a cardiovascular stress test such as transitioning from MV to spontaneously breathing.
Study limitations
Our study has relevant limitations. First, it was carried out in a single center. Albeit we expect that similar patients should behave similarly, weaning approaches may vary across centers affecting the predictive value of these StO2-derived parameters. Thus, this study needs to be duplicated across other centers. Second, we did not determine the cause of weaning failure in patients who were considered to fail. We merely identified that they did fail. We can expect different behaviors of StO2 parameters in patients who fail because of limited cardiovascular reserve from patients who fail because of upper airway obstruction and/or impaired secretions’ management. This issue must be taken into account in future studies. Finally, we studied a heterogeneous ICU population. Since the observed predictive value of StO2 might vary across populations, further studies including selected homogeneous critically ill patients are required.
Conclusions
In a mixed ICU population, non-invasive StO2-derived parameters at baseline, and within a 30-min SBT, were predictive of extubation outcome. Therefore, StO2-derived parameters may be used as indicators of global VO2/DO2 balance when facing a cardiovascular stress test, such as liberation from mechanical ventilation.
Supplementary information
Additional file 1: Table S1. Suspected etiology of extubation failure.
Additional file 2: Figure S1. StO2-derived score according to extubation outcome. The cut-off value of − 1.23 is also represented.
Acknowledgements
The authors thank David Suarez for his statistical assistance. We also thank all the Critical Care Department staff of the Parc Taulí Hospital Universitari for their enthusiasm and teamwork.
Abbreviations
- DeO2
VOT-derived StO2 deoxygenation rate
- FiO2
Fraction of inspired oxygen
- HAUC
StO2 hyperemic response
- HR
Heart rate
- MAP
Mean arterial pressure
- MV
Mechanical ventilation
- NIRS
Near-infrared spectroscopy
- nirVO2
NIRS-derived thenar muscle oxygen consumption
- PaCO2
Arterial carbon dioxide tension
- PEEP
Positive end-expiratory pressure
- ReO2
VOT-derived StO2 reoxygenation rate
- RR
Respiratory rate
- SBT
Spontaneous breathing trial
- SaO2
Arterial oxygen saturation
- ScvO2
Central venous oxygen saturation
- SpO2
Pulse-oxymetry saturation
- StO2
Tissue oxygen saturation
- THI
Tissue hemoglobin index
- VO2
Global oxygen consumption
- VOT
Vascular occlusion test
- Vt
Tidal volume
Authors’ contributions
JM, GG, AV, HG, MP, FB and AA were involved in the conception and the design of the study. JM, GG, CE, JM and CS collected the data. JM and JM analyzed the StO2 records. JM performed the statistical analysis. JM, GG, AV, HG, MP, FB and AA discussed the results. All authors reviewed the manuscript and agreed with its final version. All authors read and approved the final manuscript.
Funding
The present study was supported by a Grant from the European Society of Intensive Care Medicine (ECCRN Clinical Research Award 2009), and a Grant from the Instituto de Salud Carlos III (FIS PS PI09/01000).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable demand.
Ethics approval and consent to participate
The local Ethics Committee (CEIC 2008/554) approved the study. Informed consent was obtained from the patient or their next of kin prior to the study initiation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13613-020-00670-y.
References
- 1.Thille AW, Cortés-Puch I, Esteban A. Weaning from the ventilator and extubation in ICU. Curr Opin Crit Care. 2013;19(1):57–64. doi: 10.1097/MCC.0b013e32835c5095. [DOI] [PubMed] [Google Scholar]
- 2.Esteban A, Alia I, Tobin M, Gil A, Gordo F, Vallverdu I, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1995;159:512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- 3.Esteban A, Frutos F, Tobin MJ, Alía I, Solsona JF, Valverdú I, et al. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332(6):345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 4.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39(12):2612–2618. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 5.Boles JM, Blon J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 6.Frutos-Vivar F, Esteban A, Apezteguia C, González M, Arabi Y, Restrepo MI, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;26(5):502–509. doi: 10.1016/j.jcrc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Funk GC, Anders S, Breyer MK, Burghuber OC, Edelmann G, Heindl W, et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur Respir J. 2010;35:88–94. doi: 10.1183/09031936.00056909. [DOI] [PubMed] [Google Scholar]
- 8.Meade M, Guyatt G, Cook D, Griffith L, Sinuff T, Kergl C, et al. Predicting success in weaning from mechanical ventilation. Chest. 2001;120(6 Suppl):400S–424S. doi: 10.1378/chest.120.6_suppl.400S. [DOI] [PubMed] [Google Scholar]
- 9.Field S, Kelly SM, Macklem PT. The oxygen cost of breathing in patients with cardiorespiratory disease. Am Rev Respir Dis. 1982;126(1):9–13. doi: 10.1164/arrd.1982.126.1.9. [DOI] [PubMed] [Google Scholar]
- 10.Vassilakopoulos T, Zakynthinos S, Roussos C. Respiratory muscles and weaning failure. Eur Respir J. 1996;9:2383–2400. doi: 10.1183/09031936.96.09112383. [DOI] [PubMed] [Google Scholar]
- 11.Peters J, Mack GW, Lister G. The importance of the peripheral circulation in critical illness. Intensive Care Med. 2001;27(9):1446–1458. doi: 10.1007/s001340101034. [DOI] [PubMed] [Google Scholar]
- 12.Mohsenifar Z, Hay A, Hay J, Lewis MI, Koerner SK. Gastric intramural pH as a predictor of success or failure in weaning patients from mechanical ventilation. Ann Intern Med. 1993;119(8):794–798. doi: 10.7326/0003-4819-119-8-199310150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Bouachour G, Guiraud MP, Gouello JP, Roy PM, Alquier P. Gastric intramucosal pH: an indicator of weaning outcome from mechanical ventilation in COPD patients. Eur Respir J. 1996;9(9):1868–1873. doi: 10.1183/09031936.96.09091868. [DOI] [PubMed] [Google Scholar]
- 14.Hurtado FJ, Berón M, Olivera W, Garrido R, Silva J, Caragna E, et al. Gastric intramucosal pH and intraluminal PCO2 during weaning from mechanical ventilation. Crit Care Med. 2001;29(1):70–76. doi: 10.1097/00003246-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Bocquillon N, Mathieu D, Neviere R, Lefebvre N, Marechal X, Wattel F. Gastric mucosal pH and blood flow during weaning from mechanical ventilation in patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1999;160:1555–1561. doi: 10.1164/ajrccm.160.5.9901018. [DOI] [PubMed] [Google Scholar]
- 16.Gruartmoner G, Mesquida J, Masip J, Martinez ML, Villagra A, Baigorri F, et al. Thenar oxygen saturation (StO2) during weaning from mechanical ventilation: an observational study. Eur Respir J. 2014;43:213–220. doi: 10.1183/09031936.00126312. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Myers DE, Anderson LD, Seifert RP, Ortner JP, Cooper CE, Beilman GJ, Mowlem JD. Noninvasive method for measuring local hemoglobin oxygen saturation in tissue using wide gap second derivative near-infrared spectroscopy. J Biomed Opt. 2005;10(3):034017. doi: 10.1117/1.1925250. [DOI] [PubMed] [Google Scholar]
- 19.Gómez H, Torres A, Polanco P, Kim HK, Zenker S, Puyana JC, et al. Use of noninvasive NIRS during a vascular occlusion test to assess dynamic tissue O2 saturation response. Intensive Care Med. 2008;34(9):1600–1607. doi: 10.1007/s00134-008-1145-1. [DOI] [PubMed] [Google Scholar]
- 20.Mesquida J, Gruartmoner G, Espinal C. Skeletal muscle oxygen saturation (StO2) measured by near-infrared spectroscopy in the critically ill patients. Biomed Res Int. 2013;2013:502194. doi: 10.1155/2013/502194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skarda DE, Mulier KE, Myers DE, Taylor JH, Beilman GJ. Dynamic near-infrared spectroscopy measurements in patients with severe sepsis. Shock. 2007;27(4):348–353. doi: 10.1097/01.shk.0000239779.25775.e4. [DOI] [PubMed] [Google Scholar]
- 22.Mergetis D, Maury E, Boelle PY, Alves M, Galbois A, Baudel JL, et al. Peripheral microcirculatory exploration during mechanical ventilation weaning. Minerva Anestesiol. 2014;80(11):1188–1197. [PubMed] [Google Scholar]
- 23.Poriazi M, Kontogiorgi M, Angelopoulos E, Vasileiadis I, Tripodaki ES, Nanou A, et al. Changes in thenar muscle tissue oxygen saturation assessed by near-infrared spectroscopy during weaning from mechanical ventilation. Minerva Anestesiol. 2014;80(6):666–675. [PubMed] [Google Scholar]
- 24.Jubran A, Mathru M, Dries D, Tobin MJ. Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med. 1998;158(6):1763–1769. doi: 10.1164/ajrccm.158.6.9804056. [DOI] [PubMed] [Google Scholar]
- 25.Kuwahira I, Gonzalez NC, Heisler N, Piiper J. Changes in regional blood distribution and oxygen supply during hypoxia in conscious rats. J Appl Physiol. 1993;74(1):211–214. doi: 10.1152/jappl.1993.74.1.211. [DOI] [PubMed] [Google Scholar]
- 26.Bartels SA, Bezemer R, de Vries FJ, Milstein DM, Lima A, Cherpanath TG, et al. Multi-site and multi-depth near-infrared spectroscopy in a model of simulated (central) hypovolemia: lower body negative pressure. Intensive Care Med. 2011;37(4):671–677. doi: 10.1007/s00134-010-2128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frazier SK, Stone KS, Moser D, Schlanger R, Carle C, Pender L, et al. Hemodynamic changes during discontinuation of mechanical ventilation in medical intensive care unit patients. Am J Crit Care. 2006;15(6):580–593. doi: 10.4037/ajcc2006.15.6.580. [DOI] [PubMed] [Google Scholar]
- 28.Oh TE, Bhatt S, Lin ES, Hutchinson RC, Low JM. Plasma catecholamines and oxygen consumption during weaning from mechanical ventilation. Intensive Care Med. 1991;17:199–203. doi: 10.1007/BF01709877. [DOI] [PubMed] [Google Scholar]
- 29.Larosa G, Forster C. Coronary beta-adrenoceptor function is modified by the endothelium in heart failure. J Vasc Res. 1996;33(1):62–70. doi: 10.1159/000159133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Suspected etiology of extubation failure.
Additional file 2: Figure S1. StO2-derived score according to extubation outcome. The cut-off value of − 1.23 is also represented.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable demand.


