Abstract
We report the synthesis and antimicrobial properties of a partially reduced dihydronathphthoquinone analogue of 2-methoxy, 5-acetoxy calamenene, extracted from Subergorgia reticulata. The growth of a pathogenic Vibrio harveyi strain was effectively controlled by the calamenene derivative 1 (Cala1) and its synthetic analog 2 (Cala2). Complete mortality of V. harveyi was observed with 2.5 and 0.5 µg mL−1 concentrations of Cala1 and Cala2, respectively. The metabolic assays demonstrated that Cala1 is a bacteriostatic agent while Cala2 showed bactericidal properties. It was confirmed that translocation of Cala2 into the cytoplasm does not induce any change to the integrity of the bacterial cell wall. The Cala2 induced damage to the genetic material of 70% of cells while genetic material of 91% of cells treated with Cala1 remained intact. The Cala2 is, therefore, proposed as a potential bactericidal compound against the aquaculture pathogen V. harveyi. The fact that the Cala2 exhibited minimal cytotoxicity to Artemia nauplii indicates its potential use as an antimicrobial agent for aquaculture operations.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02241-1) contains supplementary material, which is available to authorized users.
Keywords: Calamenene, Subergorgia reticulata, Synthetic analog, Antibacterial, Bactericidal, Bacteriostatic
Introduction
Massive mortalities of animals due to bacterial infections are common in aquaculture. Although probiotics, immunostimulants and water quality management have experimented widely as prophylactic strategies to control disease incidence, therapeutic measures are necessary when disease cases are reported. In the context on the ban of antibiotic applications in the aquaculture, several attempts have been made applying crude extracts of plants, animals and bacteria against disease-causing pathogens (Kanjana et al. 2011; Santhakumari et al. 2016; Ali et al. 2018). In general, Vibrio spp. are identified as native flora of marine environments, playing pivotal roles in biogeochemical cycles (Racault et al. 2019; Asplund et al. 2011). However, many of them are opportunistic pathogens causing mass mortalities at all stages of the life cycle of shrimps in both hatcheries and grow outs. Several V. harveyi isolates from aquaculture systems across Asia and Latin America have been reported to be resistant to multiple antibiotics (Vandenberghe et al. 1998). Nearly 60% of Vibrio isolated from aquaculture hatchery systems in India are resistant to erythromycin, nitrofurazone and oxytetracycline (Sahul Hameed and Balasubramanian 2000). There are also reports of the possible transfer of antibiotic-resistant genes from allochthonous to autochthonous microorganisms in the environment (Asok et al. 2012).
It is well known that marine organisms, especially corals and sponges, secrete secondary metabolites for rendering protection against various environmental stresses and pathogens (Thakur et al. 2005). Many of these compounds have unique structural features which exhibit promising biological properties, viz., target ion channels (Jones and Bulaj 2000), inhibit enzymes (Haefner 2003), interact with DNA (Burres et al. 1991), antioxidants (Reddy et al. 2011), immunostimulants (Heidarieh et al. 2012), antiproliferative (Essack et al. 2011) and antibacterial (Correa et al. 2011). Amongst marine coral reef diversity, gorgonian corals constitute an important group, secreting an array of secondary metabolites to protect themselves from fish and invertebrate predators (Fenical and Pawlik 1991; Harvell et al. 1988; Kelman et al. 2000), bacterial pathogens (Kelman et al. 1998) and surface fouling assemblages (Slattery et al. 1995; Raveendran et al. 2011). These secondary metabolites and their analogsf that selectively control the multiplication of pathogenic microorganisms on the highly nutritional mucus layer of these corals often find multiple applications in antimicrobial chemotherapy (Schug et al. 2013; Hunt et al. 2012).
The present study stems from an observation in one of our surveys in coral reef ecosystems of Lakshadweep islands of India that a bright orange colored gorgonian coral, Subergorgia reticulata, was visibly free from biofouling (Limna Mol et al. 2011). Later we attributed this to the presence of an antifouling agent calamenene, which is an aromatic bicyclic sesquiterpene having a cadinane skeleton, on the surface of S. reticulata (Limna Mol et al. 2011). Other studies also proposed the potential of gorgonian corals in secreting secondary metabolites to protect themselves from predators (Correa et al. 2011; Fenical and Pawlik 1991), bacterial pathogens (Gordaliza 2012) and surface fouling assemblages (Limna Mol et al. 2011; Haefner 2003). In the present study, we report the antibacterial properties of calamenene, extracted from S. reticulata (Cala1), and its synthetic analogue (Cala2). Also, its toxicity to aquaculture organisms was tested using brine shrimp as a model organism.
Materials and methods
Culture conditions
Vibrio harveyi strain was taken from the Marine Microbial Reference Facility (MMRF1107) maintained at CSIR-National Institute of Oceanography-Regional Centre (CSIR-NIO-RCK) Cochin India. V. harveyi was inoculated into Luria Bertani (LB) medium and grown overnight at 28 ± 2 °C on a shaker incubator at 120 rpm. Working cultures of V. harveyi were maintained on LB agar slants and subcultured every 2–3 weeks. The purity of the culture was confirmed occasionally by comparing the fatty acid profile with the data available in the library.
Synthesis and structural characterization of Calamenene-derivative
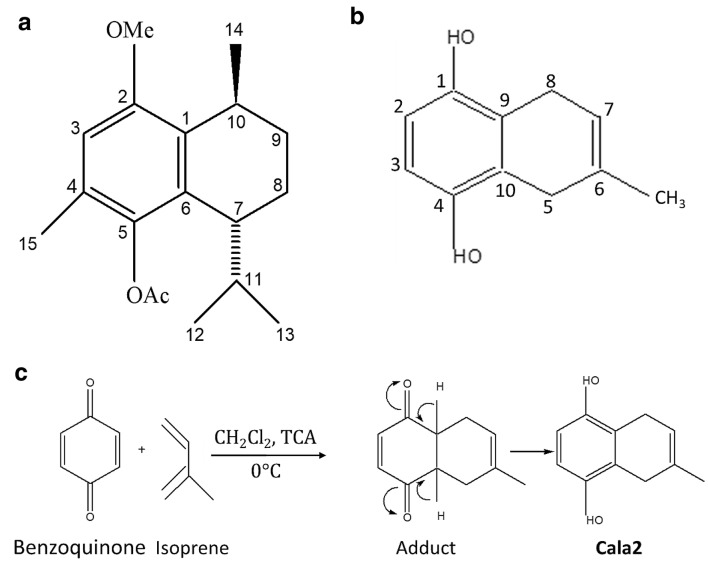
Three natural calamenenes were isolated from S. reticulata during our earlier studies (Limna Mol et al. 2011). Among these, the more potent, 2-methoxy, 5-acetoxy calamenene1 (Cala1) and its synthetic analog: 1,4-dihydroxy-5,8-dihydro-6-methyl naphthalene2 (Cala2) were selected for the present study (Fig. 1). Cala2 was synthesized simplistically through Diels Alder reaction using benzoquinone as the diene and isoprene as dienophile (Fig. 1). In a typical procedure, 108 mg of benzoquinone was dissolved in 10 ml of dichloromethane and to this 0.1 ml of isoprene was added, followed by 2 drops of trichloro acetic acid (TCA), and the reaction mixture was kept overnight with occasional stirring. The progress of the reaction was monitored using a thin layer chromatography. The crude adduct was then subjected to Sephadex column chromatography using CH2Cl2: MeOH (1:1) as eluant. Further purification on a silica gel column using acetone: hexane gradient elution yielded a white crystalline powder. The column was initially washed with 200 ml of pure hexane. The polarity of the column was increased with 2% acetone in hexane to remove the impurities. Different fractions were identified using TLC and collected separately. Finally, when all the impurities were removed, the desired product was eluted out from the column using 5% acetone in hexane. The structure of the compound was confirmed using FTIR, 1H NMR, 13C NMR, DEPT spectrum and MS spectral data.
Fig. 1.
Structure of 2-methoxy, 5-acetoxy calamenene1 (Cala1 (a) and its synthetic-analog, 1,4dihydroxy-5,8-dihydro-6-methyl naphthalene 2 (Cala2 (b) and schematic representation of the chemical synthesis of Cala2 (c)
The antibacterial property of compounds
The antibacterial property against V. harveyi was tested following standard death rate assay (Asok et al. 2012). Briefly, overnight grown V. harveyi cells (106 cells ml−1) were mixed with different concentrations of test compounds ranging from 0.1 to 5.0 µg ml−1 and incubated at room temperature for 1 h. Subsequently, 100 µl aliquots were plated in triplicate over the surface of a nutrient agar plate and incubated at 28 ± 2 °C for 24 h before enumerating the total number of colonies. Death rate ‘ε’ was calculated by using the following equation:
where N1 and N2 are the numbers of colonies grown on the control and experimental plates, respectively.
Also, the number of metabolically active cells in the experimental groups were counted under an upright microscope (Olympus, BX51) after staining with BacLight Redox sensor CTC viability kit (Molecular Probes, Invitrogen USA), following the protocols suggested in the product brochure.
Effect of Cala1 and Cala2 on cell wall integrity and genetic stability of V. harveyi
The effect of Cala1 and Cala2 on cell wall integrity and genetic stability of V. harveyi was investigated by SDS assay (Lok et al. 2006) and comet assay (Singh et al. 1988), respectively. For SDS assay, V. harveyi cells from an overnight culture were washed copiously with sterile phosphate-buffered saline (pH 7.5) and dispensed in the wells of a sterile microplate to a volume of 200 µl. After measuring the initial absorbance, the test solution was supplemented with test compounds and SDS (0.1%). The absorbance at 600 nm was recorded every 15 min for a period of 2.5 h. The decrease in absorbance compared to the initial reading was plotted against time. All the experiments were carried out three times in duplicate.
For comet assay, 106 bacterial cells treated with Cala1 and Cala2 were mixed with 100 µl of 0.5% low melting point agarose prepared in TAE buffer containing RNAse (5 g ml−1), SDS (0.25%) and lysozyme (0.5 mg ml−1). Bacterial cells impregnated in agarose solution were spread over a microscopic slide pre-coated with a thin layer of agarose (0.5%). The cells on the slides were lysed at 37 °C for 1 h, by immersing in a lysis solution, followed by incubation in an enzyme solution for 2 h at 37 °C. Subsequently, the slides were equilibrated with 300 mM sodium acetate and subjected for electrophoresis at 25 V for 1 h. Following electrophoresis, the slides were immersed in 1 M ammonium acetate in ethanol for 30 min, then in absolute ethanol for 1 h, air-dried at 25 °C, immersed in 70% ethanol for 30 min and air-dried. The slides were stained with SYBR green and comets were observed under a fluorescent microscope. The nucleic acids were classified based on comet length as intact (0–10 µm) and damaged (> 10 µm) groups and expressed as % of incidence in the histogram. The assay was repeated three times in duplicate.
Cytotoxicity assay using brine shrimp
The toxicity assay using nauplii of brine shrimp, Artemia sp., was conducted in sterile 6-well polystyrene multiwell plates. 10 competent nauplii (1-day-old) were introduced into each of the 6 replicates of 5 ml test solutions of the test compounds ranging from 0.1 to 5.0 µg ml−1 and incubated at room temperature for 24 h. The numbers of active, feeble and dead nauplii were recorded after 24 h of observation and expressed as a proportion of the total number of nauplii in the well.
Results and discussion
The synthesis of Cala2 was accomplished via Diels–Alder reaction, as described earlier (Limna Mol et al. 2011). The FTIR spectrum of the Cala2 had a broad band at 3260 cm−1, indicating the presence of –OH functional group. In addition to this, the absence of carbonyl absorption around 1700 cm−1 in the FTIR spectrum also confirmed enolisation of the intermediate ketone into the desired product Cala2. The observed m/z: 198.99 ([M + Na]+) in its mass spectrum indicated the molecular weight to be 176, corresponding to the formula C11H12O2, as expected. The 1H NMR spectrum displayed peaks at δ 1.85 (3H, s, CH3), δ4.45 (1H, s, OH), δ4.48 (1H, s, OH), δ6.56 (2H, s, Aromatic H), δ3.19 (2H, s, CH2) and δ3.285 (2H, s, CH2) are in agreement with the proposed structure. Further confirmation was provided by the presence of 11 carbon signals in the13C NMR, the multiplicities of which were deduced from DEPT 135 spectrum. These signals were at δ 23.35 (q), 25.04(t), 28.93(t), 112.14 (d), 112.23(d), 117.42(d), 122.48(s), 130.3 2(d), 146.69(s) and 146.86(s). The Cala2 has the same bicyclic skeleton as in the natural product Cala1. The methoxy and acetoxy groups of the latter have been replaced with phenolic hydroxyl groups in the former (Fig. 1). Probably due to the presence of free phenolic groups, Cala2 exhibited fivefold higher antimicrobial activity as compared to the Cala1 (Fig. 2).
Fig. 2.

Death rate of Vibrio harveyi with increasing concentrations of Cala 1 (blue line) and Cala 2 (red line). Results are expressed as death rate ± SD
The death rate of V. harveyi increases with the concentration of compounds in both cases. Complete inhibition of V. harveyi was observed with 2.5 and 0.5 µg ml−1 concentrations of Cala1 and Cala2 respectively (Fig. 2). A 7-hydroxycalamenene rich essential oil extracted from a shrub, Croton cajucara, was reported to inhibit methicillin-resistant Staphylococcus aureus (MIC 4.76 × 10–3 µg ml−1), Mycobacterium tuberculosis (MIC 4.88 µg ml−1) and M. smegmatis (MIC 39.06 µg ml−1) (Azevedo et al. 2013). Indeed, the possibility of differentiating bacteriostatic and bactericidal properties of a compound is limited with death rate assay. Also, V. harveyi are capable of entering into viable but non-culturable (VBNC) state to protect themselves from natural stresses (Oliver 2005; Griffitt et al. 2011). Under VBNC status, V. harveyi may be metabolically active but cannot grow on nutrient agar plates and therefore may not reflect in death rate assay. Although Cala1 expressed complete inhibition of V. harveyi at 2.5 µg ml−1, it was not bactericidal at this concentration. Nearly 70% of cells were metabolically active after 30 min of exposure to this concentration (Fig. 3). Interestingly the ratio of active: total V. harveyi was significantly lower in treatment groups exposed to 1 µg ml−1Cala2 for 30 min (15% metabolically active cells; Fig. 3). The inhibition of bacterial metabolism by test compounds was measured as the ability of electron transport chain of metabolically active cells to reduce 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) into red fluorescent formazan (supplementary Fig. 1). The results are expressed as ratio of total to active cells in a population after 30 min incubation.
Fig. 3.

Influence of test compounds, Cala1 and Cala 2 on the number of metabolically active cells of Vibrio harveyi
Further, we investigated the effect of Cala1 and Cala2 on the integrities of the cell membrane and genetic material of V. harveyi. The effect on cell wall integrity was measured as a function of its sensitivity towards detergent mediated cell lysis (Lok et al. 2006), following SDS assay. The cell wall integrity was evaluated from optical density recorded at every 15 min with or without compound. The optical density (A600) essentially remained intact, suggesting that the test compounds are not inducing any adverse effects to the cell wall of V. harveyi (Fig. 4). Bacteria with disintegrated cell membrane easily undergo SDS-mediated cell lysis and as a result, a sharp decrease in the optical density (A600) would be detected. Such cell lysis occurs because the detergent intensifies the damage to the membrane and causes the cell contents to leak out. In the current study, we suggest that the compounds enter the cytoplasm of V. harveyi passively without inducing any damage to the cell wall and interfere with the metabolism. A similar type of internalization without disturbing the cell membrane and targeting genetic material have been reported among the quinolone class of antibiotics (Kohanski et al. 2010). The calamenenes were reported as the active ingredient in essential oils extracted from plants, which work synergistically with antibiotics (Langeveld et al. 2014). Antimicrobial peptides and metal nanoparticles were also reported to interfere with the metabolism of bacteria, but their internalization induces disruption on the cell wall (Anas et al. 2013; Brogden 2005).
Fig. 4.

Changes in the Cell wall integrity of Vibrio harveyi on exposure to Cala1 and Cala2 for different time intervals. Results are expressed as cell wall integrity (%) ± SD
We confirmed the effect of Cala1 and Cala2 on the genetic stability of V. harveyi by comet assay. The extent of DNA damage is expressed as the length of comets formed by electrophoresis of V. harveyi treated with test compounds. The comets (500 numbers) formed as a result of electrophoresis were imaged using a fluorescence microscope and the nucleic acids were classified based on the length of the comets (Fig. 5). The fluorescence intensity in the tail of the comet is directly related to the frequency of the DNA breakage, which was assessed using densitometry followed by computer-aided analysis using Image-Pro Express software. The comets were ranked as intact (< 10 µm) and damaged (> 10 µm) ones based on the lengths of their tail. Comet assay is considered as a standard method for in vivo monitoring of the genotoxicity in both eukaryotic and prokaryotic cells. In comet assay, DNA fragments resulting from the single strand or double-strand breakage in cells embedded in the agarose gel migrate faster in the electric field than intact DNA. Since the bacterial DNA are not enclosed within a nucleus, they are easily accessible to nuclear damaging molecules. The internalized nanoparticles have been shown to induce severe DNA damage in marine Vibrio sp. (Jose et al. 2019). In the current work, the genetic material of over 91% of V. harveyi cells remained intact on treatment with Cala1. Interestingly, more than 70% of the cells treated with Cala2 suffered DNA damage. The results suggest that the Cala2 has high activity against bacterial DNA, however, molecular investigations are required for unraveling the mode of interaction. It is possible that planar compound Cala2 effectively intercalates to the minor/major groves. Also, the free phenolic OH groups may be responsible for the surge in activity compared to the parent compound. Aromatic hydroxyl groups have already been reported to be effective against ampicillin-resistant Enterobacter cloacae (Kim et al. 2012).
Fig. 5.
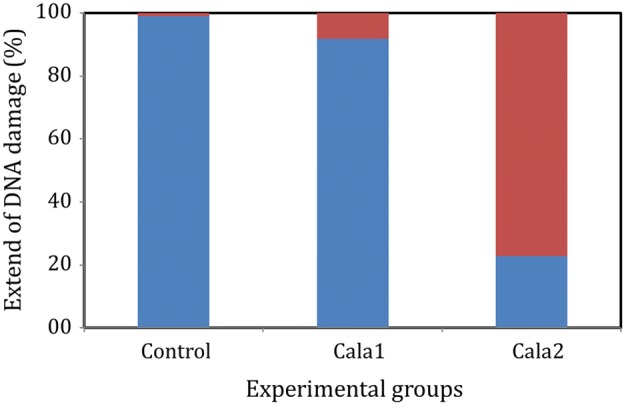
Comet assay histogram showing the integrity of DNA (intact-blue bars, damaged-red bars) in Vibrio harveyi before (control) and after treatment with Cala 1 and Cala 2. Comets are classified based on tail length into intact (<10 µm) and damaged (>10 µm) groups
The synthetic calamenene analog Cala2, with its high bactericidal activity against the pathogenic V. harveyi, could thus be a potential candidate for formulating alternative antimicrobial agents for bacterial pathogens in aquaculture. This aspect is further supported by the limited cytotoxicity exhibited by the Cala2. The cytotoxicity was tested in Artemia nauplii, which has been proven as a model organism for testing therapeutics agents for application in aquaculture (Overton and Bland 1981; Marques et al. 2004). The relative absence of cytotoxicity, thus, suggests the high potential of the molecule in aquaculture applications. When tested against the model organism, Artemia sp. nauplii at concentrations ranging from 0.1 to 5.0 µg ml−1, the Cala1 and Cala2 exhibited very little inhibitory activity. 73.33% and 83.33% of nauplii were active in wells treated with 5.0 µg ml−1 of Cala1 and Cala2, respectively (Fig. 6). The Artemia nauplii were 100% active when subjected to 0.5 µg ml−1 of the Cala2, i.e. the concentration at which it causes 100% death of V. harveyi. Higher concentrations of calamenenes and calamenene rich essential oils were found toxic to eukaryotic cells (Benoit-Vical et al. 1999; Limna Mol et al. 2011).
Fig. 6.
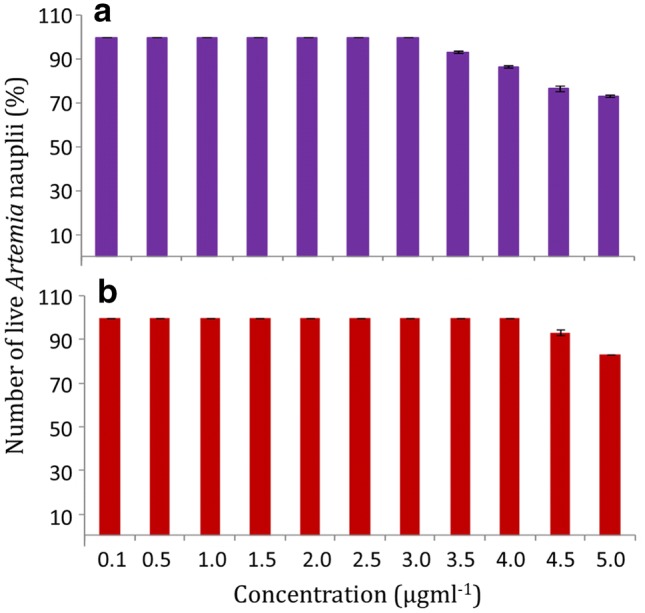
Toxicity of different concentrations of Cala 1 (1) and Cala 2 (2) to Artemia nauplii. Results are expressed as % inhibition ± SD
In conclusion, the present study proposes lower concentrations of Cala2 as a potential bactericidal compound against Gram-negative opportunistic pathogen, V. harveyi. Although natural Cala1 inhibited bacterial growth, we conclude it as a bacteriostatic effect because there was no significant difference in the metabolic activity and stability of cell membrane and genetic materials. Strikingly, the Cala2 showed enhanced bactericidal properties. It is hypothesized that the compound enters the cytoplasm without causing any damage to the cell wall. Inside the cytoplasm, the Cala2 interferes with bacterial metabolism and induces DNA damage leading to the death of the organism. We attribute the enhanced bactericidal properties to the additional hydroxyl groups on the 2nd and 5th carbon atom of the aromatic basal ring. Additionally, minimal cytotoxicity exhibited by the Cala2 enhances its potential for use as an alternative for antibiotics in aquaculture operations. However, further studies are intended to understand the mode of internalization of synthetic drug, and interaction with genetic material at the molecular level.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Directors of CSIR—National Institute of Oceanography, Goa and the Scientist-in-Charge, NIO Regional Centre, Kochi, for extending all required support in carrying out this work.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Ali MFZ, Yasin IA, Ohta T, Hashizume A, Ido A, Takahashi T, Miura C, Miura T. The silkrose of Bombyx mori effectively prevents vibriosis in penaeid prawns via the activation of innate immunity. Sci Rep. 2018;8(1):8836. doi: 10.1038/s41598-018-27241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anas A, Jiya J, Rameez MJ, Anand PB, Anantharaman MR, Nair S. Sequential interactions of silver–silica nanocomposite (Ag–SiO2NC) with cell wall, metabolism and genetic stability of Pseudomonas aeruginosa, a multiple antibiotic-resistant bacterium. Lett Appl Microbiol. 2013;56(1):57–62. doi: 10.1111/lam.12015. [DOI] [PubMed] [Google Scholar]
- Asok A, Arshad E, Jasmin C, Somnath Pai S, Bright Singh I, Mohandas A, Anas A. Reducing Vibrio load in Artemia nauplii using antimicrobial photodynamic therapy: a promising strategy to reduce antibiotic application in shrimp larviculture. Microb Biotechnol. 2012;5(1):59–68. doi: 10.1111/j.1751-7915.2011.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund ME, Rehnstam-Holm A-S, Atnur V, Raghunath P, Saeavanan V, Harnstrom K, Collin B, Karunasagar I, Godhe A. Water column dynamics of Vibrio in relation to phytoplankton community composition and environmental conditions in a tropical coastal area. Environ Microbiol. 2011;13(10):2738–2751. doi: 10.1111/j.1462-2920.2011.02545.x. [DOI] [PubMed] [Google Scholar]
- Azevedo MMB, Chavesm FCM, Almeida CA, Bizzo HR, Duarte RS, Campos-Takaki GM, Alviano CS, Alviano DS. Antioxidant and antimicrobial activities of 7-hydroxy-calamenene-rich essential oils from Croton cajucara Benth. Molecules. 2013;18:1128–1137. doi: 10.3390/molecules18011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Vical F, Valentin A, Mallíe M, Bastide J-M, Bessière J-M. In vitro antimalarial activity and cytotoxicity of Cochlospermum tinctorium and C. planchonii leaf extracts and essential oils. Planta Med. 1999;65(04):378–381. doi: 10.1055/s-2006-960794. [DOI] [PubMed] [Google Scholar]
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Burres NS, Barber DA, Gunasekera SP, Shen LL, Clement JJ. Antitumor activity and biochemical effects of topsentin. Biochem Pharmacol. 1991;42(4):745–751. doi: 10.1016/0006-2952(91)90031-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa H, Aristizabal F, Duque C, Kerr R. Cytotoxic and antimicrobial activity of pseudopterosins and seco-pseudopterosins isolated from the octocoral Pseudopterogorgia elisabethae of San Andres and Providencia Islands (Southwest Caribbean Sea) Mar Drugs. 2011;9(3):334–344. doi: 10.3390/md9030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essack M, Bajic VB, Archer JAC. Recently confirmed apoptosis-inducing lead compounds isolated from marine sponge of potential relevance in cancer treatment. Mar Drugs. 2011;9(9):1580–1606. doi: 10.3390/md9091580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenical W, Pawlik J. Defensive properties of secondary metabolites from the Caribbean gorgonian coral Erythropodium caribaeorum. Mar Ecol Prog Ser. 1991;75(1):1–8. doi: 10.3354/meps075001. [DOI] [Google Scholar]
- Gordaliza M. Synthetic strategies to terpene quinines/hydroquinones. Mar Drugs. 2012;10(2):358–402. doi: 10.3390/md10020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffitt KJ, III, Johnson CN, Jay Grimes D. Enumeration of Vibrio parahaemolyticus in the viable but nonculturable state using direct plate counts and recognition of individual gene fluorescence in situ hybridization. J Microbiol Methods. 2011;85:114–118. doi: 10.1016/j.mimet.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Haefner B. Drugs from the deep: marine natural products as drug candidates. Drug Discov Today. 2003;8(12):536–544. doi: 10.1016/S1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- Harvell C, Fenical W, Greene C. Chemical and structural defenses of Caribbean gorgonians (Pseudopterogorgia spp.). 1. Development of an in situ feeding assay. Mar Ecol Prog Ser. 1988;49(3):287–294. doi: 10.3354/meps049287. [DOI] [Google Scholar]
- Heidarieh M, Mirvaghefi AR, Akbari M, Farahmand H, Sheikhzadeh N, Shahbazfar AA, Behgar M. Effect of dietary Ergosan on growth performance, digestive enzymes, intestinal histology, hematological parameters and body composition of rainbow trout (Oncorhynchus mykiss) Fish Physiol Biochem. 2012;38:1169–1174. doi: 10.1007/s10695-012-9602-8. [DOI] [PubMed] [Google Scholar]
- Hunt LR, Smith SM, Downum KR, Mydlarz LD. Microbial regulation in gorgonian corals. Marine Drugs. 2012;10(6):1225–1243. doi: 10.3390/md10061225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Bulaj G. Conotoxins-new vistas for peptide therapeutics. Curr Pharm Des. 2000;6(12):1249–1285. doi: 10.2174/1381612003399653. [DOI] [PubMed] [Google Scholar]
- Jose J, Anas A, Jose B, Puthirath AB, Athiyanathil S, Jasmin C, Anantharaman MR, Nair S, Subrahmanyam C, Biju V. Extinction of antimicrobial resistant pathogens using silver embedded silica nanoparticles and an efflux pump blocker. ACS Appl Biomater. 2019;2(11):4681–4686. doi: 10.1021/acsabm.9b00614. [DOI] [PubMed] [Google Scholar]
- Kanjana K, Radtanatip T, Asuvapongpatana S, Withyachumnarnkul B, Wongprasert K. Solvent extracts of the red seaweed Gracilaria fisheri prevent Vibrio harveyi infections in the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 2011;30(1):389–396. doi: 10.1016/j.fsi.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Kelman D, Kushmaro A, Loya Y, Kashman Y, Benayahu Y. Antimicrobial activity of a Red Sea soft coral, Parerythropodium fulvum fulvum: reproductive and developmental considerations. Mar Ecol Prog Ser. 1998;169:87–95. doi: 10.3354/meps169087. [DOI] [Google Scholar]
- Kelman D, Benayahu Y, Kashman Y. Chemical defence of the soft coral Parerythropodium fulvum fulvum (Forskal) in the Red Sea. J Exp Mar Biol Ecol. 2000;243:309–312. doi: 10.1016/S0022-0981(99)00100-8. [DOI] [Google Scholar]
- Kim MK, Park J, Chong Y. Aromatic hydroxyl group plays a critical role in antibacterial activity of the curcumin analogues. Nat Prod Commun. 2012;7(1):57. [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8(6):423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld WT, Veldhuizen EJA, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol. 2014;40(1):76–94. doi: 10.3109/1040841X.2013.763219. [DOI] [PubMed] [Google Scholar]
- Limna Mol VP, Raveendran TV, Naik BG, Kunnath RJ, Parameswaran PS. Calamenenes—aromatic monocyclic sesquiterpenes from the Indian gorgonian Subergorgia reticulata. Nat Prod Res. 2011;25(2):169–174. doi: 10.1080/14786419.2010.495069. [DOI] [PubMed] [Google Scholar]
- Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PKH, Chiu JF, Che CM. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5(4):916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- Marques A, Dhont J, Sorgeloos P, Bossier P. Evaluation of different yeast cell wall mutants and microalgae strains as feed for gnotobiotically grown brine shrimp Artemia franciscana. J Exp Mar Biol Ecol. 2004;312(1):115–136. doi: 10.1016/j.jembe.2004.06.008. [DOI] [Google Scholar]
- Oliver JD. The viable but nonculturable state in bacteria. J Microbiol. 2005;43(S):93–100. [PubMed] [Google Scholar]
- Overton SV, Bland CE. Infection of Artemia salina by Haliphthoros milfordensis: a scanning and transmission electron microscope study. J Invertebr Pathol. 1981;37(3):249–257. doi: 10.1016/0022-2011(81)90083-5. [DOI] [Google Scholar]
- Racault M-F, Abdulaziz A, George G, Menon N, Punathil M, McConville K, Loveday B, Platt T, Sathyendranath S, Vijayan V. Environmental reservoirs of Vibrio cholerae: challenges and opportunities for ocean-color remote sensing. Remote Sens. 2019;11(23):2763. doi: 10.3390/rs11232763. [DOI] [Google Scholar]
- Raveendran TV, Limna Mol VP, Parameswaran PS. Natural product antifoulants from the Octocorals of Indian waters. Int Biodeterior Biodegrad. 2011;65(1):265–268. doi: 10.1016/j.ibiod.2010.11.013. [DOI] [Google Scholar]
- Reddy DRS, Audipudi AV, Reddy GD. Antioxidant, antiinflammatory and antifungal activity of marine sponge subergargoria suberosa-derived natural products. Int J PharmTech Res. 2011;3(1):342–348. [Google Scholar]
- Sahul Hameed A, Balasubramanian G. Antibiotic resistance in bacteria isolated from Artemia nauplii and efficacy of formaldehyde to control bacterial load. Aquaculture. 2000;183(3):195–205. doi: 10.1016/S0044-8486(99)00293-8. [DOI] [Google Scholar]
- Santhakumari S, Kannappan A, Pandian SK, Thajuddin N, Rajendran RB, Ravi AV. Inhibitory effect of marine cyanobacterial extract on biofilm formation and virulence factor production of bacterial pathogens causing vibriosis in aquaculture. J Appl Phycol. 2016;28(1):313–324. doi: 10.1007/s10811-015-0554-0. [DOI] [Google Scholar]
- Schug T, Abagyan R, Blumberg B, Collins T, Crews D, DeFur P, Dickerson S, Edwards T, Gore A, Guillette L. Designing endocrine disruption out of the next generation of chemicals. Green Chem. 2013;15(1):181–198. doi: 10.1039/C2GC35055F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Slattery M, McClintock JB, Heine JN. Chemical defenses in Antarctic soft corals: evidence for antifouling compounds. J Exp Mar Biol Ecol. 1995;190(1):61–77. doi: 10.1016/0022-0981(95)00032-M. [DOI] [Google Scholar]
- Thakur NL, Thakur AN, Muller WEG. Marine natural products in drug discovery. Nat Prod Radiance. 2005;4(6):471–477. [Google Scholar]
- Vandenberghe J, Li Y, Verdonck L, Li J, Sorgeloos P, Xu H, Swings J. Vibrios associated with Penaeus chinensis (Crustacea: Decapoda) larvae in Chinese shrimp hatcheries. Aquaculture. 1998;169(1):121–132. doi: 10.1016/S0044-8486(98)00319-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.