Abstract
Background
The hyperglycemic condition in diabetes induces cellular senescence in vascular endothelial cells and causes cardiovascular complications. Alpha-mangostin is a xanthone found in Garcinia mangostana, and has shown protective effects in metabolic syndrome.
Objective
In this study, the anti-senescence effects of alpha-mangostin in the hyperglycemic condition are investigated.
Methods
HUVECs were incubated with high glucose for 6 days and co-treated by metformin or alpha-mangostin. After 6 days, cell viability, reactive oxygen species, the percentage of senescent cells, secretory interleukin-6, and the expression of SIRT1, AMPK, p53 and p21 were measured.
Results
High glucose (60 mM) significantly decreased cellular viability and increased reactive oxygen species and cellular senescence through the reduction of senescence-associated β-galactosidase activity. Moreover, high glucose increased the protein levels of p53, acetyl-p53 and p21. The protein levels of SIRT1 and total AMPK were decreased by high glucose. High glucose increased the secretion of IL-6. Alpha-mangostin (1.25 μM) and metformin (50 μM) reversed the toxic effects of high glucose in HUVECs.
Conclusion
These results show that alpha-mangostin, similar to metformin, has anti-senescence effects in high-glucose conditions, which is probably due to its antioxidant activity through the SIRT1 pathway. Alpha-mangostin has previously shown anti-inflammatory effects and metabolic status improvement in animal and clinical studies. Therefore, this natural agent can be considered as a supplement to prevent vascular complications caused by high glucose in patients with diabetes.
Graphical abstract
Keywords: Alpha-mangostin, High glucose, Diabetes, Senescence, SIRT1, HUVEC
Introduction
According to a report by the World Health Organization, diabetes will be the seventh most common cause of death by 2030. High blood glucose, insulin resistance, lipid profile disorders and obesity are different risk factors for inducing vascular dysfunction leading to complications of diabetes such as blindness, kidney failure, heart attack, stroke, and amputation of the lower extremities [1, 2]. HUVECs in high-glucose conditions undergo premature senescence. There are various reasons for the senescence of endothelial cells in a high-glucose condition including oxidative stress and advanced glycation end products (AGEs) induced by protein glycation [3, 4]. Excessive ROS) causes oxidative changes in cellular macromolecules, such as lipids, proteins, genomes, and ultimately induces cellular senescence [5, 6]. Senescence is a permanent cell cycle arrest to ensure that the cell with a damaged genome will not be proliferated. P53 and p21 are two proteins involved in this cell cycle arrest. These proteins are overexpressed in high genomic damage [7, 8]. Senescent cells secrete pro-inflammatory factors which are cleared by the immune system. However, the chronic secretion of pro-inflammatory factors causes tissue damage and diseases due to cellular death, ROS production, permanent activation of the immune system and induction of cellular senescence [3].
SIRT1 is a key regulator of cell longevity [9]. Donepezil via SIRT1 reduced the high-glucose-induced senescence of endothelial cells [3]. In microvascular endothelial cells of mice exposed to high glucose, the expressions of SIRT1 and BCl2 were reduced, whereas the levels of FOXO1 and p53 and the activity of β-galactosidase (cellular senescence marker) were increased. Treatment with metformin reversed these effects; thus, metformin protected endothelial cells from premature senescence [10]. Furthermore, SIRT1 can reduce cellular senescence and improve the viability of endothelial progenitor cells. Considering the key role of SIRT1, various SIRT1 activator compounds have been synthesized, and their effects on life span and health are being evaluated clinically [4].
Alpha-mangostin is one of the most abundant xanthones in G. mangostana (mangosteen) (Fig. 1).
Fig. 1.
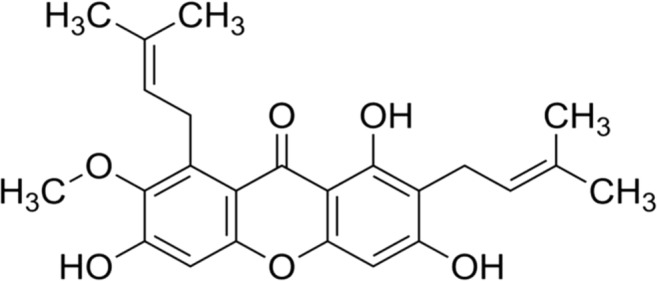
Chemical structure of alpha-mangostin
Alpha-mangostin has shown anti-inflammatory effects both in animal and clinical studies as evidenced by reduction in TNF-α and IL-6 levels [11]. Administration of alpha-mangostin decreased TNF-α and improved the lipid profile after 8 weeks in rats. Moreover, alpha-mangostin by its antihyperglycemic, antioxidant and anti-inflammatory effects, improved retinal blood flow and integrity [12]. In another study, it was found that alpha-mangostin, by increasing the expression of liver AMPK, SIRT1 and PPAR-gamma, regulates hepatic steatosis and obesity in obese mice receiving a fatty diet [13, 14]. The ethanolic extract of mangosteen pericarp showed glucose lowering and fat profile improvement effects in mice, and its hypoglycemic effect was probably due to the elevation in the population of pancreatic beta-cells [15].
Considering the antioxidant, anti-inflammatory and endothelial protective effects of alpha-mangostin, in this study for the first time, we investigated the effects of alpha-mangostin on high-glucose-induced senescence in HUVECs as well as its likely involved molecular mechanisms.
Materials and methods
Chemicals and materials
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; AtoCell, Budapest), alpha-mangostin (purity >90%, Trademax Pharmaceuticals & Chemicals Co, China), bovine serum albumin (BSA; Solarbio, China), dry skim milk (Quetlab, UK), ethylene glycol tetraacetic acid (EGTA; Sigma, USA), ethylenediaminetetraacetic acid (EDTA; Pars Tous Biotechnology, Mashhad, Iran), metformin (Sami Saz, Iran), NaF (Sigma, USA), sodium deoxycholate (Sigma, New Zealand), sodium orthovanadate (Na3VO4; Sigma, Madhya Pradesh, India) and others were purchased from Merck, Germany. Protease inhibitor cocktail was purchased from Thermo Fisher Scientific, USA. Protein assay kit (Bradford reagent) and polyvinylidene difluoride (PVDF) were bought from Bio-Rad, USA. Fetal bovine serum was purchased from Gibco, USA; Dulbecco’s modified Eagle's medium-F12 (DMEM-F12), trypsin and penicillin–streptomycin solution were brought from Bioidea, Iran. The senescence beta-galactosidase staining kit (#9860), rabbit polyclonal antibodies against SIRT1 (2310 s), AMPK (3632 s), p53 (9282 s), rabbit monoclonal antibody against p21 waf1/cip1 (2947 s), mouse monoclonal antibody against β-actin (3700 s), anti-rabbit IgG and anti-mouse IgG labeled with horseradish peroxidase were purchased from Cell Signaling. Rabbit monoclonal antibody against acetyl-p53 was purchased from Abcam.
Cell culture studies
We purchased the primary HUVECs from the Pasteur Institute, Tehran, Iran. Cells were cultured in DMEM F12 medium enriched by 10% v/v fetal bovine serum, 100 u/ml penicillin and 100 mg/ml streptomycin and maintained in an incubator at a temperature of 37 °C and atmosphere of 5% CO2.
Cellular senescence was induced by incubating HUVECs with 60 mM of D-glucose (HG) for 6 days. For investigating the protective effects of alpha-mangostin in HG-induced senescence, HG-incubated HUVECs were supplemented with alpha-mangostin or metformin as positive control. The media was changed every 24 h. HUVECs treated with normal concentration, 5 mM glucose (NG), for the entire 6 days served as the control group.
Cytotoxicity studies
Cells were seeded into 96-well culture plates at 1200 cells in 0.2 ml complete medium per well. HUVECs were incubated with alpha-mangostin (1.25–10 μM) for 6 days to determine its nontoxic concentrations. The concentration of metformin (50 μM) was selected based on previous studies and confirmed by MTT assay and western blotting [16]. For determining the toxic concentration of glucose, HUVECs were incubated with an increasing concentration of glucose (10–60 mM) for 6 days. Also, we investigated the impact of osmotic pressure on cell viability. Then, HUVECs were incubated with HG as described previously with alpha-mangostin (1.25 μM) or metformin (50 μM) or none of them during the entire period of incubation. After 6 days, cell viability was measured by MTT colorimetric assay. Briefly, after treatment, the culture media was withdrawn, and cells were incubated with 0.5 mg/mL MTT in fresh medium for 3 h at 37 °C. The MTT formazan crystals were dissolved in 150 μL of dimethyl sulfoxide (DMSO) for each well. The absorbance of dissolved formazan was measured at 570 and 630 nm using a microplate reader. A reference wavelength of 630 nm was used [17].
ROS assay
Cells were seeded into 96-well culture plates at 1200 cells in 0.2 ml enriched medium in each well. The cells were incubated as previously described. We used the dichlorofluorescin diacetate assay to investigate the cellular ROS [18]. H2DCF-DA, after entrance into the cell, is hydrolyzed by esterases to non-fluorescent H2DCF, and then H2DCF is oxidized by ROS to highly fluorescent DCF (2′,7′-dichlorofluorescin), with its intensity related to the degree of intracellular ROS presence. After incubation for 6 days, cells were washed two times with phosphate buffered saline (PBS) solution then were incubated with 10 μM of DCF-DA in PBS for 30 min at 37 °C in the dark. Afterward, cells were washed twice with PBS. The microplate reader measured fluorescence intensity of oxidized dichlorofluorescin at excitation/emission wavelengths of 485/527 nm [19].
Enzyme-linked immunosorbent assay (ELISA)
After incubation for 6 days as previously described, the cell culture supernatant was kept. The concentration of interleukin-6 (IL-6) was measured using the human IL-6 ELISA kit (IBL-International, Germany) based on the manufacturer’s instructions.
Senescence-associated beta-galactosidase activity assay
Senescence-associated β-galactosidase (SA-β-gal) activity is the indicator of cellular senescence, and it was evaluated using an SA-β-gal assay kit based on the manufacturer’s procedures. Briefly, HUVECs were seeded into 12-well culture plates at 2 × 104 cells in 1 ml complete medium per well. The cells were treated as previously described. After 6 days of incubation, cells were washed with PBS, fixed with 4% paraformaldehyde for 15 min and stained with SA-β-gal staining solution at pH 6.0, then incubated in a dry incubator for at least 24 h. The blue-stained cells were then observed and photographed under a bright-field microscope (Jenus, China). The number of SA-β-gal-stained cells was counted per field. The percentage of SA-β-gal-positive cells per 100 cells of each group was compared with the control.
Western blotting
The cells at 3 × 105 were seeded in t75 flasks. The cells were treated as previously described. At the end of treatment, each flask was washed with PBS at 37 °C followed by 3 ml trypsin (0.25%) then incubated in 5% CO2 at 37 °C up to 5 min. The trypsin effect was removed by adding 6 ml of complete medium at 37 °C, and detached cells were poured into a falcon. After centrifuging at 1100 rpm/5 min, the cell pellets were homogenized in ice-cold lysis buffer (50 mM Tris (pH 7.4), 150 mM NaCl, 1% TritonX-100, 2 mM EDTA, 2 mM EGTA, 10mMNaF, 1mMNa3NO4, 10mMβ-glycerol phosphate, 0.2% sodium deoxycholate, 10% 2-ME, 0.2% sodium dodecyl sulfate (SDS), 1 mM phenylmethyl-sulfonylfluoride (PMSF), 1% phosphatase inhibitor cocktail and 1% protease inhibitor cocktail). After centrifuging cell homogenate at 10,000 rpm for 20 min at 4 °C in a refrigerated centrifuge, the protein quantity of the supernatant was qualified using a Bradford protein assay kit. The supernatants were mixed with a sample loading buffer. One hundred micrograms of the homogenate protein was loaded to SDS-polyacrylamide gel (10%) and electrophoresed using a Bio-Rad Mini-PROTEAN Tetra system. The electrophoresed proteins were transferred to a PVDF membrane. Then the membrane was blocked in 5% nonfat milk for 2 h at room temperature. The membranes were probed using a range of primary antibodies against SIRT1, AMPK, p53, p21 waf1/cip1, β-actin and acetyl-p53 (1/1000 dilution) at room temperature with gentle shaking for 2 h. The membranes then were washed and incubated with anti-rabbit IgG and anti-mouse IgG (1:3000 dilutions) at room temperature with gentle shaking for 1.5 h. The antibodies were visualized using Pierce™ ECL Western Blotting Substrate (Thermo Fischer Scientific, USA) and Alliance 4.7 gel doc (UK), and quantified using UVIBand Map software (Uvitec, UK).
Statistical analysis
Statistical comparisons were carried out using GraphPad Prism (6.0) software. All results are expressed as mean ± standard deviation (SD) of the mean. One-way analysis of variance and Tukey–Kramer post hoc tests were performed to compare means. P values less than 0.05 were considered statistically significant.
Results
Cytotoxic effects of alpha-mangostin on HUVECs
At first, the cytotoxicity of alpha-mangostin was determined. HUVECs were incubated with increasing concentrations of alpha-mangostin (1.25, 2.5, 5 and 10 μM) for 6 days, and cell viability was determined by MTT colorimetric assay. Dose–response results showed 1.25 μM of alpha-mangostin increased cell viability, but the concentrations greater than 1.25 μM decreased cell viability that was significant at 5 μM. Therefore, we selected a non-cytotoxic concentration of alpha-mangostin (1.25 μM) as the protective dose for further experiments (Fig. 2).
Fig. 2.
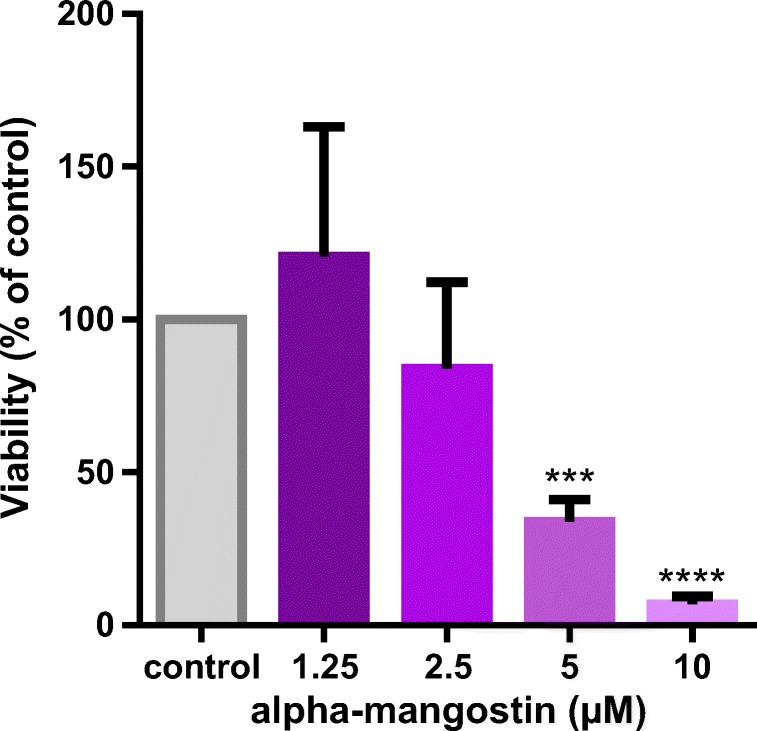
Cytotoxic effects of alpha-mangostin on HUVECs. HUVECs were incubated with increasing concentrations of alpha-mangostin for 6 days, and MTT assay was used to measure the cell viability in comparison with the control group. Values represent the mean ± SD of four independent experiments. Statistical significance was set at ***P < 0.001 and ****P < 0.0001 compared with control group
Cytotoxic effects of glucose on HUVECs
As we aimed to induce cellular senescence by high concentration of glucose, the cytotoxicity of glucose was determined. HUVECs were incubated with increasing concentrations of glucose (10, 20, 40, 50 and 60 μM) for 6 days, and cell viability was determined by MTT colorimetric assay. Dose–response results showed 10–30 mM of glucose slightly increased cell viability, but a higher concentration decreased cell viability in comparison with the control group, which was significant at 60 μM. Therefore, we selected 60 mM as the senescence induction dose for our experiments (Fig. 3).
Fig. 3.
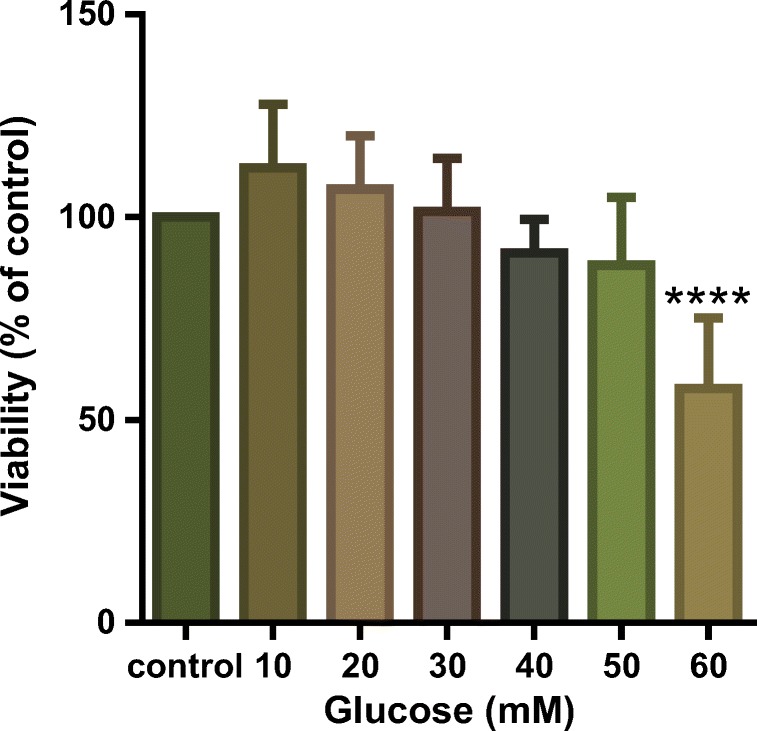
Cytotoxic effects of different concentrations of glucose on HUVECs. HUVECs were incubated with increasing concentrations of glucose for 6 days, and MTT assay was used to measure the cell viability in comparison with the control group. Values represent the mean ± SD of 4 independent experiments. Statistical significance was set ****P < 0.0001 compared with control group
Cytotoxic effects of osmotic pressure
Since we chose 60 mM concentration of glucose for our experiments and it is higher than a normal glucose condition, we investigated whether osmotic pressure affects cell viability. HUVECs were incubated with 55 mM mannitol as the osmotic pressure control (cell culture medium contains 5 mM glucose) for 6 days, and cell viability was determined by MTT colorimetric assay. The results showed mannitol had no effect on cell viability in comparison with the control group (Fig. 4).
Fig. 4.
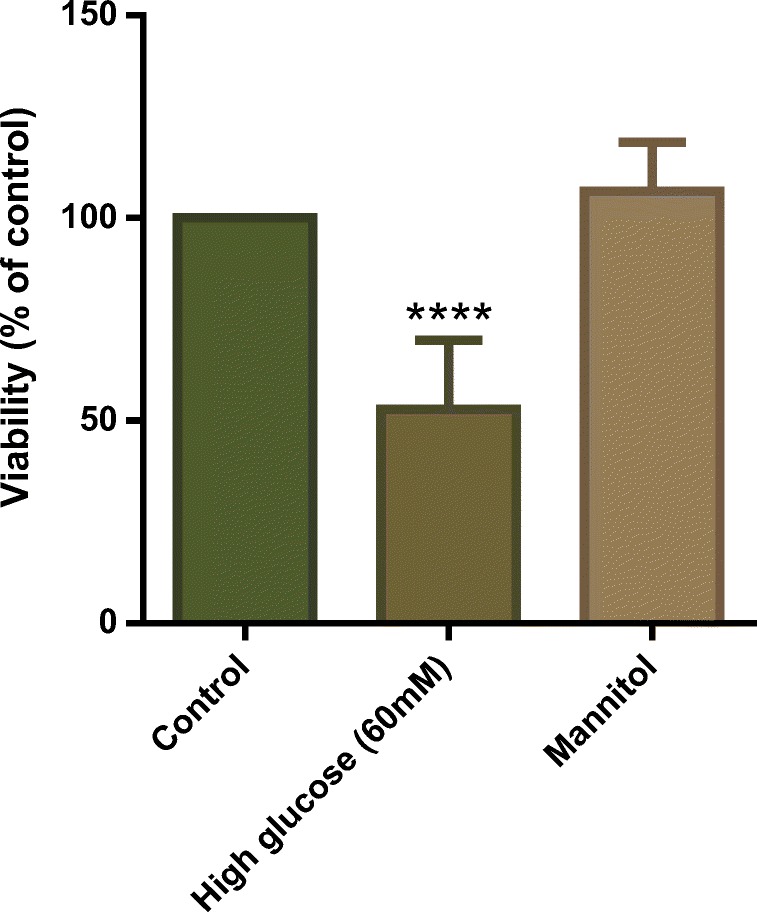
Cytotoxic effect of osmotic pressure on HUVECs. HUVECs were incubated with 55 mM mannitol as osmotic pressure control (cell culture medium containing 5 mM glucose) for 6 days, and cell viability was determined by MTT colorimetric assay. Values represent the mean ± SD of four independent experiments. Statistical significance was set at ****P < 0.0001 compared to control group
Protective effects of metformin on HUVECs
HUVECs were incubated with increasing concentrations of metformin (12.5, 25, 50, 100 and 200 μM) for 6 days, and cell viability was determined by MTT colorimetric assay. The concentrations used in this study were selected according to previous study [10]. Dose–response results showed that 12.5–200 μM of metformin had no cytotoxic effects on cell viability, but 400 μM significantly decreased cell viability (Fig. 5).
Fig. 5.
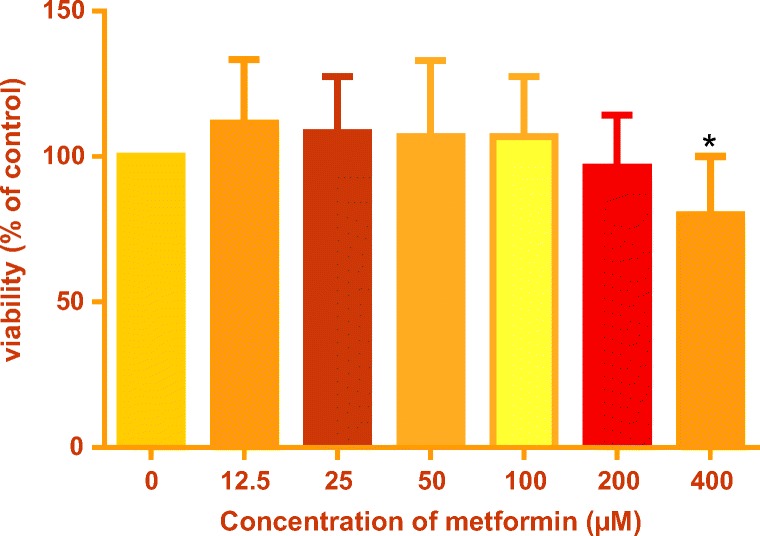
Cytotoxic effects of metformin on HUVECs. HUVECs were incubated with increasing concentrations of metformin for 6 days and MTT assay was used to measure the cell viability in comparison with the control group. Values represent the mean ± SD of 4 independent experiments. Statistical significance was set at *P < 0.05 compared with control group
The effects of different concentrations of metformin (12.5, 25 and 50 μM) on SIRT1 expression in HUVECs were also evaluated. Higher expression of SIRT1 was shown at 50 μM, although it was not significant compared with the normal glucose condition. Therefore, we selected 50 μM as the protective concentration for further experiments, in agreement with previous studies [10] (Fig. 6).
Fig. 6.
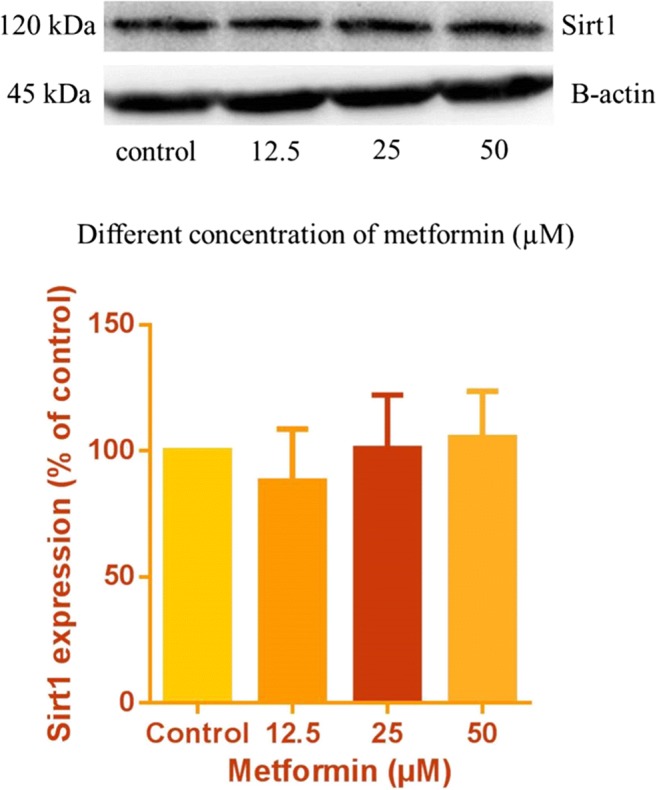
Western blots of SIRT1 protein in HUVECs incubated with metformin for 6 days. The internal standard was beta-actin, and 100 μg of sample protein was loaded in each well. Values represent the mean ± SD of four independent experiments
Alpha-mangostin protective effects in HUVECs
Next, we examined the protective effects of alpha-mangostin in HG-induced death in HUVECs. Treatment with alpha-mangostin (1.25 μM) similar to metformin (50 μM) significantly reversed the toxic effects of HG on HUVECs compared with the control (Fig. 7).
Fig. 7.
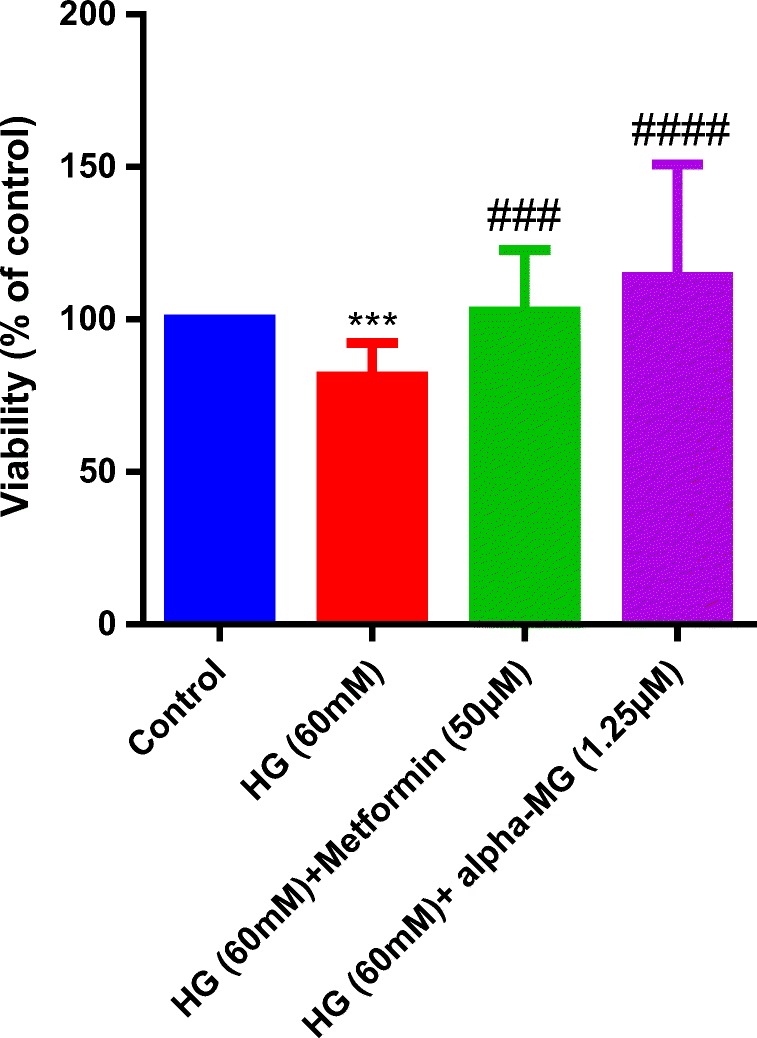
Alpha-mangostin protective effects against HG-induced death in HUVECs. Incubation of HUVECs with high glucose (60 mM) for 6 days significantly reduced viability vs. control group (normal glucose), alpha-mangostin and metformin significantly increased viability vs. control. The cellular viability was measured by MTT assay. Values represent the mean ± SD of four independent experiments. Statistical significance was set at ***P < 0.001 vs. control and ###p < 0.001, ####p < 0.0001 vs. HG group. HG = high glucose, alpha-MG = alpha-mangostin
Alpha-mangostin decreased ROS in HUVECs
Hyperglycemia-induced oxidative stress by mitochondria results in DNA damage and promotes endothelial senescence and angiopathy [3, 4]. Therefore, we examined the effects of alpha-mangostin and metformin, as positive control, on HG-induced ROS production. Treatment with alpha-mangostin and metformin significantly reversed the effects of HG on ROS production in HUVECs (Fig. 8).
Fig. 8.
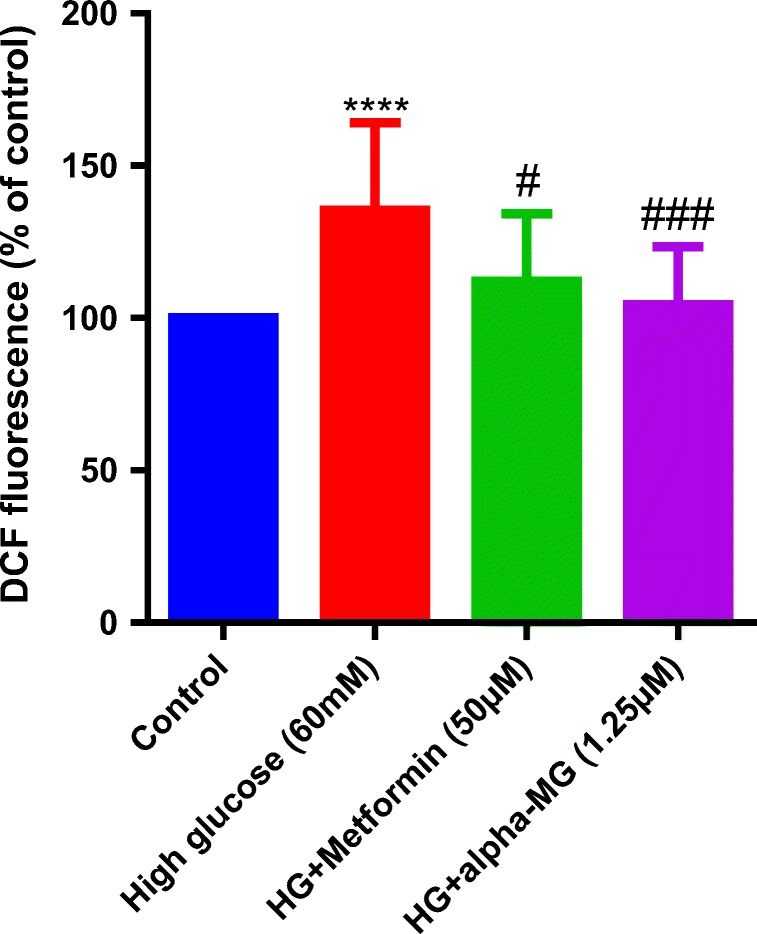
Alpha-mangostin decreased ROS induced by HG in HUVECs. HG induced ROS generation in HUVECs. Alpha-mangostin and metformin significantly reversed it. Values represent the mean ± SD of four independent experiments. Statistical significance was set at ****P < 0.0001 vs. control; #P < 0.05 and ###P < 0.001 vs. control. HG = high glucose, alpha-MG = alpha-mangostin
Alpha-mangostin decreased senescence in HUVECs
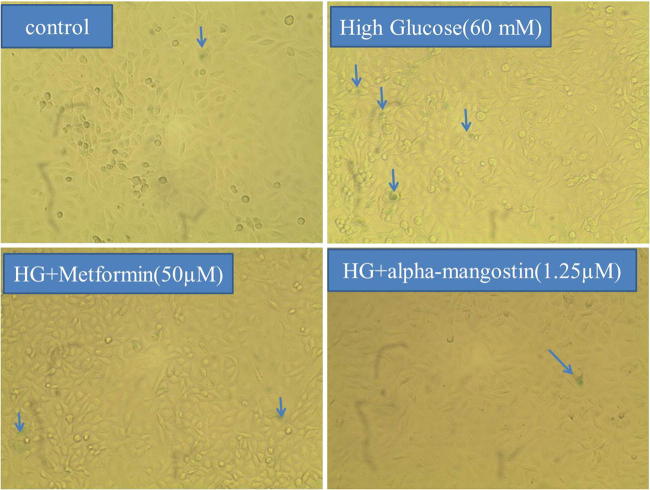
There was a significant increase in SA-β-gal-positive HUVECs after 6 days treatment by HG. SA-β-gal activity in HUVECs treated by HG was significantly higher than the control, whereas co-treatment with alpha-mangostin and metformin significantly reversed SA-β-gal activity (Figs. 9, 10).
Fig. 9.
Alpha-mangostin inhibited HG-induced senescence in HUVECs. After 6 days of incubation, cells were washed with PBS, fixed with 4% paraformaldehyde for 15 min and stained with SA-β-gal staining solution at pH 6.0, then incubated in a dry incubator for at least 24 h. Afterward, the blue-stained cells were observed and photographed under a bright-field microscope (×100 magnitude). The arrows show blue senescent cells
Fig. 10.
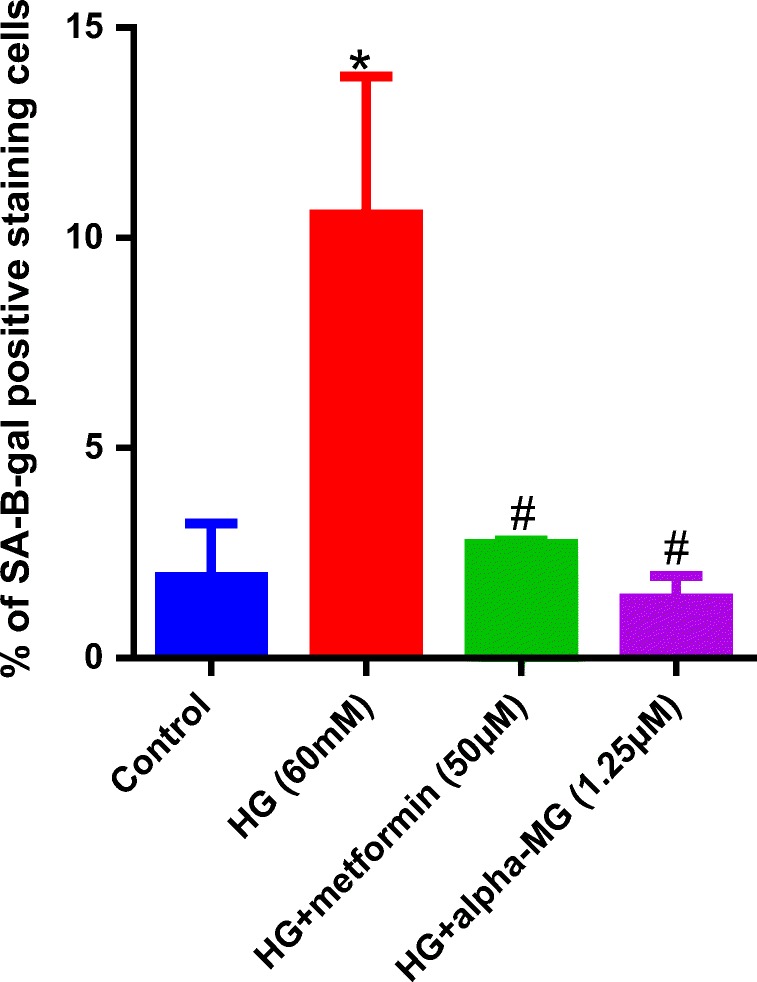
Alpha-mangostin inhibited HG-induced senescence in HUVECs. The percentage of SA-β-gal-positive cells per 100 cells of each group was compared with the control. The number of senescent cells was significantly decreased by alpha-mangostin and metformin. Values exhibit the mean ± SD of four independent experiments. Statistical significance was set at *P < 0.05 vs. control; #P < 0.05 vs. HG. HG = high glucose, alpha-MG = alpha-mangostin
Alpha-mangostin decreased secretory IL-6
The level of IL-6 induced by HG, as an inflammatory factor, was measured in the cell culture supernatant. The level of IL-6 was significantly higher in the HG group, and alpha-mangostin or metformin treatment significantly lowered secretory IL-6 (Fig. 11).
Fig. 11.
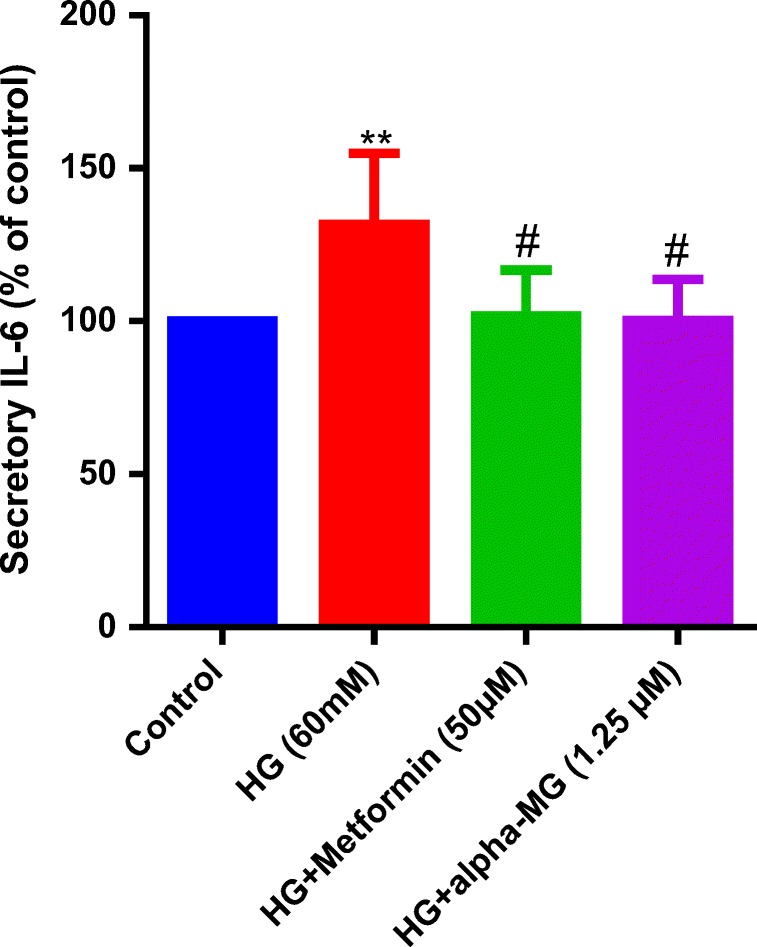
Alpha-mangostin decreased IL-6 secretion induced by HG in the cell culture. HG increased IL-6 secretion in cell culture. Alpha-mangostin and metformin significantly reversed IL-6 secretion. Values represent the mean ± SD of four independent experiments. Statistical significance was set at **P < 0.01 vs. control group; #P < 0.05 vs. HG. HG = high glucose, alpha-MG = alpha-mangostin
Alpha-mangostin decreased p53 protein and its downstream target, p21
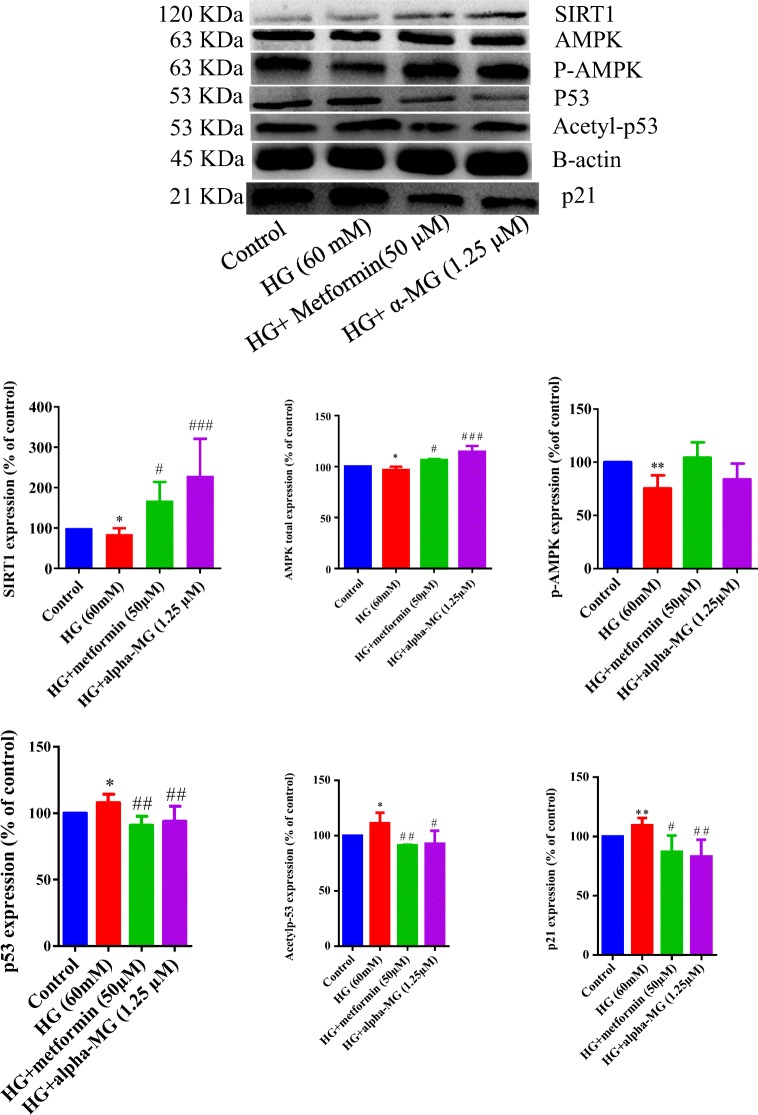
To determine whether alpha-mangostin regulates HUVEC senescence via altering the p53/p21 pathway, we examined the levels of p53, its active form (acetylated) and its downstream target, p21. Results from western blotting revealed that exposure of HUVECs to an HG condition for 6 days significantly increased total p53, acetylated p53 and p21 proteins. Alpha-mangostin or metformin significantly reversed these effects (Fig. 12).
Fig. 12.
Western blots of SIRT1, AMPK (total and phosphorylated), p53 (total and acetylated) and p21 proteins in HUVECs treated by high glucose (60 mM) for 6 days and the protective effects of α-mangostin and metformin. The internal standard was beta-actin, and 100 μg of sample protein was loaded in each well. Values represent the mean ± SD of four independent experiments. Statistical significance was set at *P < 0.05, **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. HG. HG = high glucose, alpha-MG = alpha-mangostin
Alpha-mangostin increased SIRT1 and AMPK in HUVECs
To determine whether alpha-mangostin regulates HUVEC senescence through the SIRT1 and AMPK, we investigated their expressions. Results from western blotting revealed that exposure of HUVECs to HG condition for 6 days significantly decreased SIRT1 and total AMPK. Co-treatment by alpha-mangostin or metformin significantly increased their expressions. Although HG decreased phosphorylated AMPK (active form), alpha-mangostin or metformin did not significantly reverse this effect (Fig. 12).
Discussion
In this study, we induced senescence in HUVECs by HG (60 mM) incubation for 6 days. HG reduced cell viability and increased oxidative stress and cellular senescence, which was characterized by an elevation in the positive cells for the SA-β-GAL marker. Also, the levels of SIRT1 and AMPK were decreased, and acetylated p53 (the active form of the p53 protein) and its downstream target, p21, were increased.
Metabolic diseases such as diabetes cause cardiovascular disease and complications such as blindness, kidney failure, heart attack, stroke, amputation of the lower limbs [20], and ultimately death. In this vascular injury, endothelial cellular senescence occurs due to ROS production by the mitochondrial respiratory chain in HG condition [21]. Due to various studies regarding the protective effects of metformin in endothelial cells, we considered it as positive control in this study. Luo and Lei in a study in 2017 showed that alpha-mangostin (15 μM), by inhibiting the activity of sphingomyelinase and ceramide, reduced HG-induced apoptosis (30 mM) after 24 h in HUVECs [22]. In another study, alpha-mangostin showed significant cytotoxicity at concentrations of 1 and 2 μM. The concentration of 100 nm, by inhibiting the activation of JNK and p38-MAPK phosphorylation, reduced oxidative stress and HG-induced apoptosis (25 mM) after 72 h in HUVECs [23]. In our study, alpha-mangostin showed cellular toxicity in concentrations greater than 1.25 μM, but at 1.25 μM, showed the protective effects against ROS production and cell survival. These differences between nontoxic concentrations in different studies may be due to different alpha-mangostin sources and its purity or cell culture conditions.
Due to DNA damage, proteins such as p53 and its downstream target, p21, halt the cell cycle to repair it. If a cell repairs the genome, the cell cycle will progress; otherwise, the cell becomes senescent [7, 8]. It has been shown that the expression and activity of SIRT1 in an aged vascular system are decreased and lead to oxidative stress, inflammation and senescence of the arteries and atherosclerosis. Activation of this molecule can be a suitable therapeutic target in treatment of metabolic-related vascular diseases [24]. Metformin is a common antidiabetic medicine that has shown endothelial protective effects independently of its glucose-lowering effect. One of its protective mechanisms is through SIRT1 activation [16]. Metformin (50 μM), by increasing the expression of SIRT1 and modifying FOXO1, reduced oxidative stress, apoptosis and senescence induced by HG (40 mM) after 3 days in endothelial cells [10]. Regarding the protective role of SIRT1 and AMPK in various studies related to the vascular system, these proteins were selected as possible targets of alpha-mangostin. In this study, alpha-mangostin, similar to the positive control, metformin, significantly increased SIRT1 and total AMPK. Results showed that although alpha-mangostin decreased oxidative stress and senescence as evidenced by downregulation of p53 and p21 proteins and reduced SA-β-GAL activity, the level of P-AMPK was not significantly increased by alpha-mangostin. Therefore, it can be concluded that other downstream targets of SIRT1 may be involved in these protective effects. The activation of FoxO3a/PGC-1α complex by SIRT1 has been shown to increase the expression of antioxidant genes such as catalase and manganese superoxide dismutase [25]. Moreover, SIRT1 not only increased PPAR-α and Nrf2 protein levels, but also diminished p66, so it can increase the antioxidant capacity of the cell. In addition, SIRT1 through the NF-κB pathway and p53 de-acetylation reduced the production of inflammatory factors such as IL-6, which led to vascular protective effects [26].Recent animal and clinical studies have shown that vascular aging and endothelial dysfunction are associated with oxidative stress and a pro-inflammatory phenotype. Through secretion of pro-inflammatory cytokines, chemokines, growth factors and proteases by senescent cells, these senescence-associated secretory phenotypes (SASP) can be created. For example, H2O2, by releasing free oxygen radicals, induced senescence in HUVECs. Coenzyme Q10, by increasing the activity of SIRT1 and AMPK, increased cell survival and reduced ROS, cellular senescence and SASP [27]. Furthermore, the reduced form of this agent, ubiquinol-10, decreased the activity of NF-κB and Il-6 secretion in senescent HUVECs incubated with LPS [28]. Alpha-mangostin has shown anti-inflammatory effects. Through inhibition of NF-κB in RAW 264.7 cells exposed to LPS, alpha-mangostin reduced IL-6 and TNF-α [29]. In our study, alpha-mangostin and metformin reduced the increased secretion of IL-6 into cell culture medium by HG.
Conclusion
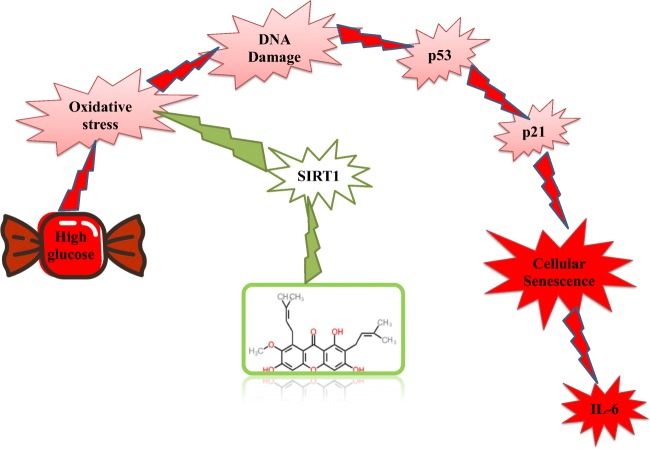
Our results show that alpha-mangostin, similar to metformin, exhibits anti-senescence and endothelial protective effects in hyperglycemic condition, which is due to its antioxidant activity, probably through SIRT1 (Fig. 13). Alpha-mangostin has shown anti-inflammatory effects and metabolic status improvement in previous in vivo and clinical studies. Thus, this natural agent can be considered as a supplement to prevent vascular complications caused by HG in patients with diabetes.
Fig. 13.
Schematic of α-mangostin protective effects in cellular senescence induced by high glucose
Acknowledgements
This study was financially supported by Mashhad University of Medical Sciences (Grant No. 327 941389).
Abbreviations
- AGEs
Advanced glycation end products
- Akt
Protein kinase B
- alpha-MG
Alpha-mangostin
- AMPK
5’-AMP-activated protein kinase
- eNOS
Endothelial nitric oxide synthase
- FOXO1
Forkhead box O1
- FoxO3a
Forkhead box O3
- HG
High glucose
- HO-1
Heme oxygenase 1
- HUVECs
Human umbilical vein endothelial cells
- IL-6
Interleukin-6
- JNK
c-Jun N-terminal kinases
- LPS
Lipopolysaccharides
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
Nitric oxide
- Nrf2
Nuclear factor erythroid 2-related factor 2
- p38-MAPK
P38 mitogen-activated protein kinases
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PPAR
Peroxisome proliferator-activated receptor
- ROS
Reactive oxygen species
- SASP
Senescence-associated secretory phenotype
- SA-β-GAL
Senescence-associated beta galactosidase
- SIRT1
Sirtuin 1
- TNF-α
Tumor necrosis factor alpha
Compliance with ethical standards
Conflict of interest
All authors state that there are no actual or potential conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hourieh Tousian, Email: houritsh@yahoo.com.
Bibi Marjan Razavi, Email: RazaviMr@mums.ac.ir.
Hossein Hosseinzadeh, Email: hosseinzadehh@mums.ac.ir.
References
- 1.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med. 2010;123(3 Suppl):S3–11. doi: 10.1016/j.amjmed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Burton DGA, Faragher RGA. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology. 2018;19(6):447–459. doi: 10.1007/s10522-018-9763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goligorsky MS. Vascular endothelium in diabetes. American journal of physiology Renal physiology. 2017;312(2):F266–Ff75. doi: 10.1152/ajprenal.00473.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43(4):477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Harman D. Aging: overview. Ann N Y Acad Sci. 2001;928(1):1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 7.Shin DH, Lee SJ, Kim JS, Ryu JH, Kim JS. Synergistic effect of immunoliposomal gemcitabine and bevacizumab in glioblastoma stem cell-targeted therapy. J Biomed Nanotechnol. 2015;11(11):1989–2002. doi: 10.1166/jbn.2015.2146. [DOI] [PubMed] [Google Scholar]
- 8.Signer RAJ, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12(2):152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science (New York, NY). 2004;303(5666):2011–5. 10.1126/science.1094637. [DOI] [PubMed]
- 10.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171(2):523–535. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bumrungpert A, Kalpravidh RW, Chitchumroonchokchai C, Chuang CC, West T, Kennedy A, et al. Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J Nutr. 2009;139(6):1185–1191. doi: 10.3945/jn.109.106617. [DOI] [PubMed] [Google Scholar]
- 12.Jariyapongskul A, Areebambud C, Suksamrarn S, Mekseepralard C. Alpha-mangostin attenuation of hyperglycemia-induced ocular hypoperfusion and blood retinal barrier leakage in the early stage of type 2 diabetes rats. Biomed Res Int. 2015;2015:785826. doi: 10.1155/2015/785826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi Y. al. e. α-Mangostin regulates hepatic Steatosis and obesity through SirT1-AMPK and PPARγ pathways in high-fat diet-induced obese mice. J Agric Food Chem. 2015;63:8399–8406. doi: 10.1021/acs.jafc.5b01637. [DOI] [PubMed] [Google Scholar]
- 14.Chae HS, Kim YM, Bae JK, Sorchhann S, Yim S, Han L, et al. Mangosteen extract attenuates the metabolic disorders of high-fat-fed mice by activating AMPK. J Med Food. 2016;19(2):148–154. doi: 10.1089/jmf.2015.3496. [DOI] [PubMed] [Google Scholar]
- 15.Taher M, Tg Zakaria TM, Susanti D, Zakaria ZA. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn in normoglycaemic and streptozotocin-induced diabetic rats. BMC complementary and alternative medicine 2016;16:135. 10.1186/s12906-016-1118-9. [DOI] [PMC free article] [PubMed]
- 16.Zhang E, Guo Q, Gao H, Xu R, Teng S, Wu Y. Metformin and resveratrol inhibited high glucose-induced metabolic memory of endothelial senescence through SIRT1/p300/p53/p21 pathway. PLoS One. 2015;10(12):e0143814. doi: 10.1371/journal.pone.0143814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rameshrad M, Imenshahidi M, Razavi BM, Iranshahi M, Hosseinzadeh H. Bisphenol a vascular toxicity: protective effect of Vitis vinifera (grape) seed extract and resveratrol. Phytotherapy research : PTR. 2018. 10.1002/ptr.6175. [DOI] [PubMed]
- 18.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27(5–6):612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 19.Tong YF, Liu Y, Hu ZX, Li ZC. A a. Protocatechuic aldehyde inhibits TNF-alpha-induced fibronectin expression in human umbilical vein endothelial cells via a c-Jun N-terminal kinase dependent pathway. Experimental and therapeutic medicine. 2016;11(1):277–282. doi: 10.3892/etm.2015.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tousian Shandiz H, Razavi BM, Hosseinzadeh H. Review of Garcinia mangostana and its xanthones in metabolic syndrome and related complications. Phytother Res. 2017;31(8):1173–1182. doi: 10.1002/ptr.5862. [DOI] [PubMed] [Google Scholar]
- 21.Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54(7):2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 22.Ding A-J, Zheng S-Q, Huang X-B, Xing T-K, Wu G-S, Sun H-Y, et al. Current perspective in the discovery of anti-aging agents from natural products. Natural products and bioprospecting. 2017;7(5):335–404. doi: 10.1007/s13659-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jittiporn K. Moongkarndi p, samer j, suvitayavat w. protective effect of α-Mangostin on high glucose induced endothelial cell apoptosis. Walailak J Sci & Tech. 2017;15(8):579–587. [Google Scholar]
- 24.Kitada M, Ogura Y, Koya D. The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging. 2016;8(10):2290–307. 10.18632/aging.101068. [DOI] [PMC free article] [PubMed]
- 25.Olmos Y, Sánchez-Gómez FJ, Wild B, García-Quintans N, Cabezudo S, Lamas S, et al. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid Redox Signal. 2013;19(13):1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. 2018;28(8):711–732. doi: 10.1089/ars.2017.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huo J, Xu Z, Hosoe K, Kubo H, Miyahara H, Dai J, et al. Coenzyme Q10 prevents senescence and dysfunction caused by oxidative stress in vascular endothelial cells. Oxidative Med Cell Longev. 2018;2018:3181759. doi: 10.1155/2018/3181759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivieri F, Lazzarini R, Babini L, Prattichizzo F, Rippo MR, Tiano L, et al. Anti-inflammatory effect of ubiquinol-10 on young and senescent endothelial cells via miR-146a modulation. Free Radic Biol Med. 2013;63:410–420. doi: 10.1016/j.freeradbiomed.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 29.Mohan S, Syam S, Abdelwahab SI, Thangavel N. An anti-inflammatory molecular mechanism of action of alpha-mangostin, the major xanthone from the pericarp of Garcinia mangostana: an in silico, in vitro and in vivo approach. Food Funct. 2018;9(7):3860–3871. doi: 10.1039/c8fo00439k. [DOI] [PubMed] [Google Scholar]