Abstract
Previously, our group reported the establishment of a white callus cell line of Buddleja cordata Kunth that is a high producer of the secondary metabolite, verbascoside (VB, also named acteoside), under suspension culture conditions. Here, we present experimental evidence of the sustained ability of that cellular line to grow and produce high amounts of VB for 5 years of continuous culture. Cellular line profiles were determined at the early (at the beginning) and late stages (at the end of 5 years of continuous subculturing) by analyzing relevant parameters of culture growth, i.e., specific growth rate [µ], doubling time [dt], and growth index [GI], as well as VB production. Late-stage cultures exhibited a 61% faster growth rate than early-stage subcultures, and 25 and 3% lower doubling time and growth index. The extents of growth phases were found to be different. Similar amounts of biomass were found (9.5 g and 9.4 g L−1). Verbascoside production increased parallel to cell growth; maximal yield level occurred in the mid-exponential phase and lasted until the end of the stationary phase (i.e., from the 15th to the 25th day and from the 9th to the 21st day for the early and late stages, correspondingly). The content of VB was higher in the late-stage culture (1.43 ± 0945 g L−1) than in the early-stage culture (1.21 ± 0.0286 g L−1). Productivity values point out the potential use of B. cordata cell line in the biotechnological production of VB and for research focused on the biochemistry of secondary metabolism.
Keywords: Buddleja cordata, Long-term culture, Phenylpropanoid, Plant cell suspension cultures, Growth parameters, Verbascoside
Introduction
A major goal of biotechnology is the exploitation of plant metabolism to attain the large-scale production of secondary metabolites that hold potential as pharmaceutical drugs. One possible key to fulfill this goal is using in vitro culture systems to maximize the biosynthetic activity of cells, organs, or tissues grown in isolation from the whole plant. Among these systems, cell suspension cultures allow cells to grow in a tridimensional space supplied with constant aeration and defined nutrient conditions. Once initiated, suspension cultures are maintained by periodically subculturing cell samples in a fresh nutrient medium. By repeating subculture rounds, researchers may stimulate growth over extended periods while simultaneously selecting for fast cell division rates and clonality within cultures. Another important advantage of this culturing system is the possibility of exposing cell populations to chemicals in growth and metabolic response tests, and to experimentally evolve the adaptation to long-term growth as a preamble to the upscaling to bioreactor culturing (Bourgaud et al. 2001).
Notwithstanding the above advantages, cell suspension culture systems should be screened for the appearance of unexpected traits that may limit the biotechnological use of a cell line. Low tolerance to stress, decreased robustness against aging, and gradual loss of biosynthetic activities may result from the gene expression changes by which cells dedifferentiate and stabilize in the liquid environment (Hirasuna et al. 1991; Scragg 1995; Qu et al. 2005, 2011; Roberts 2007; Kolewe et al. 2008; Sánchez-Sampedro et al. 2009; Dubrovina and Kiselev 2012; Wilson and Roberts 2012; Kwiatkowska et al. 2014). Physiological and biochemical changes are also expected to happen as a by-product of continuous growth (i.e., long-term culturing and/or several numbers of subcultures) in a constant environment lacking cues for the maintenance of genome functionality resulting from molecular mechanisms (i.e., genetic and epigenetic factors) (Phillips et al. 1994; Kolewe et al. 2008). Genetic changes that take place during the plant cell culture are known as somaclonal variation (Jain 2001; Smýkal et al. 2007; Neelakandan and Wang 2012; Kiselev et al. 2013; Kwiatkowska et al. 2014; Dubrovina and Kiselev 2016; Yue et al. 2016).
Visual screening of cultures and growth-kinetics analyses are standard procedures which indicate cellular metabolic and growth variation under certain culture conditions. Its performance is necessary to minimize environmental cues leading to cell-line instability (Kwiatkowska et al. 2014; Isah et al. 2018). By comparing culture samples over extended periods, it can be determined whether the cell line maintains high growth efficiency and metabolic vigor (Bouque et al. 1998; Bourgaud et al. 2001). Biomass accumulation and secondary metabolite biosynthesis measurements are particularly significant as evidence of sustained metabolic ability. As a whole, these comparative analyses may be used as descriptions of trait trajectories to facilitate the selection and maintenance of a phenotypically robust and high-yielding cell line suitable for the upscaling of secondary metabolites production (Yue et al. 2016).
Buddleja cordata Kunth (Scrophulariaceae) is a plant species endemic to Mexico, where it is known as Tepozán and has widespread use in traditional medicine (Martínez 1989). Its therapeutic properties are believed to depend on major compounds of the phenylpropanoid biosynthetic pathway (Estrada-Zúñiga et al. 2009), among which verbascoside (VB) stands out for its anti-oxidant, anti-inflammatory, anti-parasitic, anti-proliferative, and anti-microbial activities (Alipieva et al. 2014; He et al. 2011; Sipahi et al. 2016, and references therein). VB has shown few side effects and thus is a target of research focused on developing sustainable methods for its large-scale production (Santoro et al. 2008; He et al. 2011; Santos-Cruz et al. 2012; Perucatti et al. 2018; Vazquez-Marquez et al. 2019).
Our group previously established a white callus cell line of B. cordata that biosynthesizes high amounts of VB (86.26 mg g−1 dry weight [DW]). Importantly, higher levels of VB (116 mg g−1 DW, equivalent to 1.44 g L−1) are observed after inducing the cell line to grow for a short time (10 subcultures) in liquid medium (Estrada-Zúñiga et al. 2009). In the present work, we evaluated the ability of that cell line to grow and biosynthesize VB in a long-term cell suspension culture that was maintained for 5 years.
The capability to accumulate high amounts of biomass and verbascoside prevailed over time. We propose that the cell line is well suited for its use in the biotechnological production of VB and the experimental determination of secondary metabolite biosynthesis.
Materials and methods
We used the B. cordata cell line previously established in a semisolid medium by Estrada-Zúñiga et al. (2009). Following the methodology of these authors, a cell suspension culture was initiated in 2014 and maintained by transferring callus cell aggregates from a semisolid culture into Erlenmeyer flasks containing liquid medium. We used half-strength MS liquid medium (Murashige and Skoog 1962) supplemented with 0.45 µM 2,4-dichlorophenoxyacetic acid (2,4-D), 2.32 µM kinetin (KIN), 3% (w/v) sucrose, 100 mg L−1 citric acid, and 150 mg L−1 ascorbic acid. Incubation was carried out at 26 ± 2 °C with constant shaking at 110 rpm and a photoperiod of 16 h light (30 µmol m−2 s−1).
Cell growth was renewed at 18-day intervals by subculturing inocula of weighed biomass. This long-term culture was maintained for approximately 100 subculture rounds (summing 5 years of continuous growth) and served as biomass source to prepare sample subcultures for experimentation. To determine the stability of the cell line, we performed growth kinetics to determine growth parameters (µ, dt, and GI) at the early (at the beginning) and late stages (at the end of the 5-year culturing period) (hereafter, we refer to these sample cultures as early- and late-stage cultures). Each stage consisted of three consecutive kinetic rounds. Fresh and dry weight measurements were made at 3-day intervals during 30 days of culture; sampling was carried out in triplicate by transferring 3 g of fresh biomass to 250-mL Erlenmeyer flasks containing 50 mL of liquid medium. Calculations were performed according to Godoy-Hernández and Vázquez-Flota (2012).
Growth index (GI) is a relative estimation of the growth capacity of a cellular line; it provides information about the ratio of the gained and the initial biomass. The gained biomass correlates the difference between the final and the initial masses:
where WF and W0 represent the final and the initial masses, respectively.
Specific growth rate (µ) is defined as the rate of growth of biomass of a cellular population per unit of biomass concentration. It is calculated during the exponential growth phase in which the growth in the cell population fits a straight line equation:
where x0 is the initial biomass and x is the biomass at time t.
Doubling time (dt) is defined as the time necessary for the accumulation of biomass in a cellular population to duplicate itself:
where µ represents the specific growth rate.
VB extraction from biomass samples, its detection and quantification, HPLC assays, and specific VB production rate calculations were performed as in Estrada-Zúñiga et al. (2009).
Specific production rate (qp) is defined as the rate of metabolite production in a cellular population per unit of product concentration. When metabolite production is associated with growth, qp is calculated during the growth phase as well as in the cellular growth case, and production in the cell population fits a straight line.
where p0 is the initial product and p is the product at time t.
The productivity of a culture (g product L−1 culture day−1) results from the sum of its growth rate, yield (product accumulation) and biomass accumulation (Scragg 1995).
OpenLAB software (version A.01.05 Agilent Technologies Inc., Santa Clara, CA, USA) was used to acquire and interpret chromatographic data. The NCSS software v.10 was used for statistical analyses of VB concentration and growth parameters. A p ≤ 0.05 was assumed for significant differences.
Results and discussion
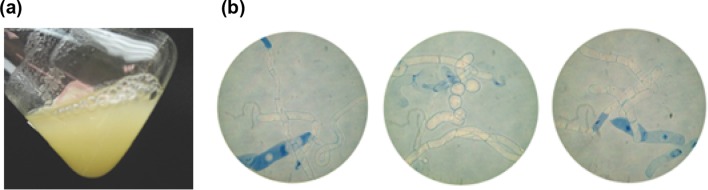
The B. cordata cell suspension culture system was easily initiated. Visual inspection of the first seven subculture rounds revealed no compact aggregates. Only a fast-proliferating, white, friable cell suspension was observed, similar to the appearance of cell suspension cultures reported by Estrada-Zúñiga et al. (2009). Also, cellular morphology was recognized by microscopic analysis as seen in Fig. 1. To determine whether the cell line was suitable enough for bioprocess engineering, we analyzed its growth behavior after 5 years of continuous culturing. Since culture age and cell density, among other environmental factors, are known to affect the growth behavior and biosynthetic activity of suspension cultures, we strictly controlled the timing, media composition, and the amount of biomass used as inoculum in each subculture round. An indication of methodological success was the invariable growth behavior shown by the cell line in successive subculture rounds at the early and late stages of the experiment. This stability was analytically ascertained by measuring the parameters of growth efficiency during three consecutive subculture rounds for each case (i.e., early- and late-stage culture) (Bourgaud et al. 2001). As shown in Table 1, no statistically significant differences were observed among the successive subcultures that made up the early stage, as well as among those for the late stage. These results indicate that the cell line holds the ability to become rapidly stabilized in the liquid environment and to grow robustly over long periods. However, the mean values of the measured parameters differed significantly between the early and late stages of the experiment, pointing to changes in growth behavior over the 5 years of continuous culturing.
Fig. 1.
Visual characteristics (a) and cellular morphology (b) of long-term cell suspension cultures of B. cordata . Cells were stained with Evans blue (dead cells were blue-stained). Cellular morphology was consistent during the five years of continuous culturing. Cells were observed at 100X
Table 1.
Growth-efficiency measurements in the early- and late-stages of the long-term cell suspension culture of B. cordata
| Subculture | Growth parameter | Value | |
|---|---|---|---|
| Early-stage | Late-stage | ||
| 1st | µ (day−1) | 0.102 | 0.153 |
| td (days) | 5.293 | 4.536 | |
| GI | 4.766 | 4.588 | |
| 2nd | µ (day−1) | 0.109 | 0.177 |
| td (days) | 5.129 | 3.918 | |
| GI | 4.787 | 4.706 | |
| 3rd | µ (day−1) | 0.099 | 0.168 |
| td (days) | 5.496 | 4.121 | |
| GI | 4.787 | 4.647 | |
| Mean | µ (day−1) | 0.103 ± 0.015 | 0.166 ± 0.012 |
| td (days) | 5.306 ± 0.184 | 4.192 ± 0.315 | |
| GI | 4.780 ± 0.012 | 4.647 ± 0.048 | |
Results are the means ± SD values of triplicate measurements
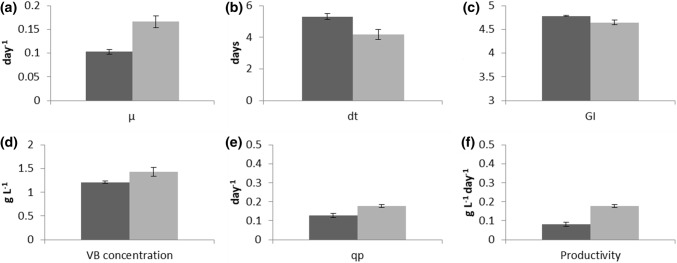
Figure 2 represents the statistically significant differences in the values obtained for the growth criteria tested between early- and late-stage cultures. Regarding specific growth rate (µ), (i.e., 0.103 ± 0.015 day−1 for early- and 0.166 ± 0.012 day−1 for late-stage cultures), the cell line showed a 61% higher rate of biomass accumulation over time. Differences also were observed in the ability of the cell line to accumulate enough biomass to undergo cell division. The doubling time (dt) of the early-stage culture (dt = 5.306 ± 0.184 days) was 25% higher than that of the late-stage culture (dt = 4.192 ± 0.315). As a whole, the increased µ and decreased dt values in late-stage culture suggest that the cell line increased its rate of biomass accumulation, but decreased its rate of cell division after 5 years of continuous growth. We used the growth index (GI) to estimate the ability of the cell line to accumulate biomass independently of the allocation of resources for cell division. The GI in the late-stage culture (4.647 ± 0.048) was 3% lower than that of the early-stage culture (4.780 ± 0.012).
Fig. 2.
A comparison of changes in growth parameters (a–c) and verbascoside production (d–f) between early- (black bars) and late-stage cultures (grey bars) of the long-term cell suspension culture of B. cordata. Results are the means ± SD of a specific growth rate (µ), b doubling time (dt), c growth index (GI), d VB concentration, e specific production rate (qp) and f productivity measurements made on biomass samples in triplicate
The growth behavior of the cell line was characterized further by plotting the growth kinetics of the early- and late-stage cultures (Fig. 3). Both cultures accumulated similar amounts of biomass (9.5 g DW L−1 and 9.4 g DW L−1, respectively), but they exhibited significant differences in the extent of each stage of their growth kinetics (see the summary of differences shown in Table 2). Unlike early-stage cultures, the late-stage cultures exhibited a faster exponential phase and attained maximum growth earlier; after the stationary phase, they lost viability over a period that was 1.8 times longer than that of the early-stage cultures. Furthermore, late-stage cultures did not exhibit a discernible lag phase at the time of renewing growth. These changes in growth behavior indicate that after 5 years of culture maintenance, the cell line was channeling a higher metabolic output into growth and viability.
Fig. 3.
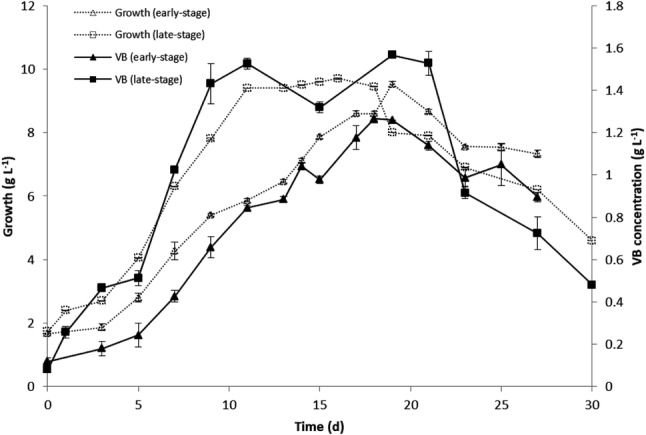
Growth kinetics and VB production profiles of the long-term cell suspension culture of B. cordata at the early- and late-stage of culture. Growth: (open triangles) at the early- and (open squares) at the late-stage. VB production: (filled triangles) at the early- and (filled squares) at the late-stage. Results are mean values ± SD of triplicate measurements
Table 2.
A comparison of growth phases extent in the early and late stages of the long-term cell suspension culture of B. cordata
| Phase of growth | Long-term culture | |
|---|---|---|
| Early-stage | Late-stage | |
| Extent (days) | ||
| Lag | 3 | 0 |
| Log | 14 | 11 |
| Stationary | 6 | 7 |
| Death | 6 | 12 |
An important concern in this work consisted of determining whether that metabolic ability persisted after 5 years of continuous subculturing, which was assessed by comparing the kinetics of VB production in the early- and late-stage cultures (Fig. 3). In both tested cultures, the highest level of VB occurred at the mid-exponential phase and lasted until the end of the stationary phase. However, following the growth-behavior differences described in the preceding paragraphs, the late-stage cultures exhibited maximum VB production from the 9th to the 21st day of growth, whereas the corresponding period for the early-stage cultures was from the 15th to the 25th day. Additionally, we confirmed that VB production increased parallel to cell growth, since specific production rates were similar to specific growth values in each stage, i.e., qp (0.127 ± 0.011 day−1) and µ (0.103 ± 0.015 day−1) for early-stage and qp (0.177 ± 0.007 day−1) and µ (0.166 ± 0.012 day−1) for late-stage cultures. VB production associated with growth had been observed previously in the original cell line (Estrada-Zúñiga et al. 2009).
The ability of the cell line to sustain high levels of VB production was determined by measuring the levels of VB by HPLC analysis (Fig. 4). VB content in the late-stage cultures (1.43 ± 0.0945 g L−1) was increased by 18% as compared to early-stage cultures (1.21 ± 0.0286 g L−1). This production level is similar to the previously reported value (1.44 g L−1) when the cell line was first tested by Estrada-Zúñiga et al. (2009) for its ability to grow and produce VB in suspension culture conditions. The calculation of the specific rate of VB production (qp) showed that VB was produced at a lower rate in early-stage cultures (qp = 0.127 ± 0.011 day−1) than in late stage-cultures (qp = 0.177 ± 0.007 day−1). Thus, by the end of the 5-year experiment, the cell line produced VB at a 39% faster rate. Moreover, as seen in Table 3, VB productivity rates also showed differences over time. In early-stage cultures, a combination of 9.5 g L−1 biomass and a yield of 12.73% corresponded to the productivity of 0.08 g L−1 day−1 with a 15-day run time. For late-stage cultures, 9.4 g L−1 biomass and a yield of 13.83% corresponded to the productivity of 0.14 g L−1 day−1 with a 9-day run time. In the case of the original line (established in 2009), a combination of 12.93 g L−1 biomass and a yield of 11.13% corresponded to the productivity of 0.1 g L−1 day−1 with 14-day run time. At the end of the 5 years of continuous culture, the B. cordata cell line increased 75% the productivity rate of VB.
Fig. 4.
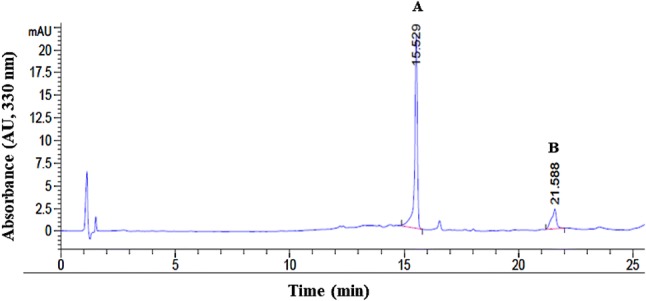
Chromatographic profile of an extract from long-term B. cordata cell suspension culture. Reprensented in the spectra are verbascoside (A) and internal standard (B)
Table 3.
Productivity in a long-term cell suspension culture of B. cordata in different stages of culture
| Stage of culture | Biomass (g L−1) | Time (days) | Yield (%) | Yield (g L−1) | Productivity (g L−1 day−1) |
|---|---|---|---|---|---|
| Early-stage | 9.5 | 15 | 12.73 | 1.21 ± 0.0286 | 0.08 |
| Late-stage | 9.4 | 9 | 13.83 | 1.43 ± 0.0945 | 0.14 |
| Line in 2009a | 12.93 | 14 | 11.13 | 1.44 | 0.1 |
aValues from ‘Line in 2009’ according to Estrada-Zúñiga et al. (2009)
The exploitation of high-value plant-derived chemicals by developing plant cell suspension cultures is often limited by the gradual loss of viability and metabolic abilities (i.e., low secondary metabolites production rates), mainly due to genetic changes that take place during the plant cell culture, particularly when growth is extended over long periods (Scragg 1995; Phillips et al. 1994; Kolewe et al. 2008; Neelakandan and Wang 2012; Wilson and Roberts 2012). Overcoming these limitations is feasible by improving culture conditions and monitoring cell population behavior, but the success of any methodological adjustment is mostly conditioned by inherent traits of the selected cell line. In this work, we assessed the long-term suitability of a cell line of B. cordata that previously showed the ability to continuously produce the secondary metabolite VB in coordination with cellular growth in a cell suspension culture system (Estrada-Zúñiga et al. 2009). Interestingly, this growth-dependent pattern of VB biosynthesis has been observed in other species such as Aphelandra spp., Cistanche deserticola, and Scrophularia striata (Nezbedová et al. 1999; Ouyang et al. 2005; Khanpour-Ardestani et al. 2015). However, none of these works determined whether the pattern may persist in a long-term cell suspension culture. In the present work, we determined that the association between growth and VB production persisted for 5 years of continuous culturing, indicating that the cell line of B. cordata holds inherent metabolic robustness suitable for its biotechnological application in VB production.
The long-term performance of the cell line may be partly associated with its low tendency to form aggregates, because aggregation may lead to the generation of metabolically heterogeneous cell subpopulations (Hulst et al. 1989; Naill and Roberts 2004, 2005; Kolewe et al. 2008; Wilson and Roberts 2012) by limiting the gas and nutrient diffusion to cells (Dixon 1985). Both the early- and late-stage cultures exhibited low growth variation upon subculturing (Table 1), indicating that population heterogeneity was successfully minimized (Fig. 1).
By the end of the 5 years of continuous growth, the cell line showed that its specific growth rate improved by 61%, whereas its doubling time was 25% reduced. Kinetic growth scrutiny showed, on the one hand, no discernible lag phase and, on the other, a substantially longer aging phase. Altogether, these results are suggestive of a high degree of trait plasticity regarding growth and viability. The cell line also exhibited 18% higher content of VB and a 39% increase in the rate of production of this secondary metabolite. Thus, changes in growth behavior were positively correlated with those in the biosynthetic activity. Our observations suggest that in some plant species, the underlying genetic circuitries controlling growth and VB production are partially overlapped. We posit that in the 5 years of careful subculturing, growth was renewed from an increasingly fit cell population that displayed plasticity for the allocation of resources to cell division and viability; this trait plasticity directly affected the biosynthesis of VB due to the genetic architecture of the cell line.
Diverse factors, other than those related to molecular changes (i.e., genetic variation), may contribute to phenotypic plasticity. For instance, it could be inherent to the biological specimen from which the cell line was originally derived, i.e., immature leave tissue. It has been noted elsewhere that the developmental phases of cultured plant cells resemble those of the leaves of herbaceous plants (Fountain et al. 2003; Kwiatkowska et al. 2014). Plant cells in culture can alter their molecular programs to endure and, ultimately, determine line fitness and adaptability to in vitro artificial conditions (i.e., temperature, agitation speed, and especially culture medium composition); that ability could be greatly influenced by plant growth regulators (Neelakandan and Wang 2012). In the particular case of our line, culture media content may exert a relevant role in maintaining the stability of the line, which could be predisposed to adjust its genetic and epigenetic programs in response to the plant growth regulators 2,4-D (a synthetic auxin) and KIN (a synthetic cytokinin) that were added to the culture medium, first, to induce callus development and, then, to maintain callus morphology and VB production. These two organic compounds are known regulators of the cell cycle and serve to induce cell dedifferentiation under in vitro growth conditions, but have shown also to be beneficial for the production of secondary metabolites such as alkaloids, terpenes, and phenolic compounds (Miller et al. 1955, 1956; Leguay and Guern 1975; Yang et al. 2002; Raghavan et al. 2006; Barciszewski et al. 2007; Perrot-Rechenmann 2010; Neelakandan and Wang 2012; Kieber and Schaller 2014; Jamwal et al. 2017; Isah et al. 2018). Also, plant growth regulators have been pointed out as a determining factor in the continuous production of secondary metabolites in long-term cultures. Sierra et al. (1992) reported that the stable alkaloid production and cellular growth in long-term cell suspension cultures of Tabernaemontana divaricata mainly depended on the type and concentration of plant growth regulators present in the culture medium. They noted that a given concentration of growth regulator grants a relatively constant production yield. However, changes in the medium (i.e., change in growth regulators) resulted in productivity loss, which then could be restored to the original level after some subcultures in the starting production medium. The latter led authors to conclude that no genetic change took place during the period the culture was maintained in suspension, or that, at least, the capacity for production of alkaloids in 'high' yields remained. To ascertain the adequate environmental conditions that confer and allow the development of cell lines is fundamental to achieve higher biomass and improve the productivity of bioactive compounds (Coppede et al. 2014; Jamwal et al. 2017; Isah et al. 2018).
Long-term culture is frequently considered disadvantageous for the industrial production of secondary metabolites as it results in decreased biomass production, in inconsistent patterns or gradual loss of metabolite production over time, as has been the case of long-term cultures for anthocyanins, alkaloids, taxanes, paclitaxel, and resveratrol production (Dubrovina and Kiselev 2016; Le et al. 2019). In contrast, successful examples of cell and organ cultures used for the stable and long-term production of bioactive compounds have been reported, like the production of alkaloids in cell suspension cultures of T. divaricata (Sierra et al. 1992), betaxanthins in callus cultures of Beta vulgaris (Trejo-Tapia et al. 2008), as well as the production of ginsenosides in long-term cultures of Panax ginseng (Le et al. 2019).
In this study, the long-term cell suspension cultures of B. cordata showed a reduction in growth index and biomass production over time, instead of variations in VB production during the culture period (i.e., long-term cultures produced more VB than short-term cultures). Total VB concentration in long-term cultures was comparable to the value reported when the line was originally generated (Estrada-Zúñiga et al. 2009). Le et al. (2019) reported in long-term cell suspension and adventitious root cultures of Panax ginseng that while biomass production decreased over time, ginsenoside production remained constant. On the other hand, Coppede et al. (2014) showed that in a 10-year-old cell suspension culture of Maytenus ilicifolia, the capacity to synthesize and accumulate quinone methide triterpenoids was higher than in 5-year-old cultures.
Concluding remarks
In this work, we provided experimental evidence that supports the ability of a B. cordata cell line to grow and produce verbascoside under long-term suspension culture conditions.
Although biomass decreased in long-term cultures, and VB production varied over time, total verbascoside production in long-term cultures was comparable to the production reported by Estrada-Zúñiga et al. in 2009 for the original cell line; however, productivity yield in long-term cultures increased.
Since the cell line retained the capacity to biosynthesize VB in high production rates after being subcultured for 5 continuous years, it represents a suitable in vitro system for biotechnological purposes. Further improvements of VB production could be attained by testing the cell line’s response to exogenous precursor and elicitor molecules. Due to its metabolic robustness, the cell line also holds potential as a model system for basic research focused on the biochemical and molecular basis of the biosynthesis of VB and other phenylpropanoids.
Acknowledgements
Hypatia Arano is grateful to the Consejo Nacional de Ciencia y Tecnología (CONACyT) for Grant 353160.
Author contributions
AVH, EZME, FFJ contributed to the design and implementation of the research and to the analyses of results. AVH and FFJ wrote the manuscript in consultation with EZME and CSF. CSF helped supervise the project. All authors reviewed the final manuscript. No other authors are involved in this work.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical standards
There is not any experiment inside it involving Human Participants and/or Animals.
Informed consent
Informed consent is not applicable for this work.
References
- Alipieva K, Korkina L, Orhan IE, Georgiev MI. Verbascoside—a review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol Adv. 2014;32:1065–1076. doi: 10.1016/j.biotechadv.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Barciszewski J, Massino F, Clark BF. Kinetin—a multiactive molecule. Int J Biol Macromol. 2007;40:182–192. doi: 10.1016/j.ijbiomac.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Bouque V, Bourgaud F, Nguyen C, Guckert A. Production of daidzein by callus cultures of Psoralea species and comparison with plants. Plant Cell Tissue Organ Cult. 1998;53:35–40. doi: 10.1023/A:1006057211490. [DOI] [Google Scholar]
- Bourgaud F, Gravot A, Milesi S, Gontier E. Production of plant secondary metabolites: a historical perspective. Plant Sci. 2001;161:839–851. doi: 10.1016/S0168-9452(01)00490-3. [DOI] [Google Scholar]
- Coppede JS, Pina ES, Paz TA, Fachin AL, Marins MA, Bertoni BW, França SC, Pereira AM. Cell cultures of Maytenus ilicifolia Mart. are richer sources of quinone-methide triterpenoids than plant roots in natura. Plant Cell Tissue Organ Cult. 2014;118:33–43. doi: 10.1007/s11240-014-0459-7. [DOI] [Google Scholar]
- Dixon RA. Plant cell culture: a practical approach. Oxford: IRL Press; 1985. [Google Scholar]
- Dubrovina AS, Kiselev KV. Effect of long-term cultivation on resveratrol accumulation in a high-producing cell culture of Vitis amurensis. Acta Physiol Plant. 2012;34:1101–1106. doi: 10.1007/s11738-011-0907-5. [DOI] [Google Scholar]
- Dubrovina AS, Kiselev KV. Age-associated alterations in the somatic mutation and DNA methylation levels in plants. Plant Biol. 2016;18:185–196. doi: 10.1111/plb.12375. [DOI] [PubMed] [Google Scholar]
- Estrada-Zúñiga ME, Cruz-Sosa F, Rodríguez-Monroy M, Verde-Calvo JR, Vernon-Carter EJ. Phenylpropanoid production in callus and cell suspension cultures of Buddleja cordata Kunth. Plant Cell Tissue Organ Cult. 2009;97:39–47. doi: 10.1007/s11240-009-9496-z. [DOI] [Google Scholar]
- Fountain MD, Valdes O, Fettig S, Beck E. Expression of cell cycle control factors in non-dividing and ageing photoautotrophic plant cells. Physiol Plant. 2003;119:30–39. doi: 10.1034/j.1399-3054.2003.00169.x. [DOI] [Google Scholar]
- Godoy-Hernández G, Vázquez-Flota FA (2012) Growth measurements: Estimation of cell division and cell expansion. In: Loyola-Vargas V, Ochoa-Alejo N (eds) Plant cell culture protocols. Methods in molecular biology (methods and protocols), vol 877. Humana Press, Totowa. 10.1007/978-1-61779-818-4_4 [DOI] [PubMed]
- He J, Hu XP, Zeng Y, Li Y, Wu HQ, Qiu RZ, Ma WJ, Li T, Li CY, He ZD. Advanced research on acteoside for chemistry and bioactivities. J Asian Nat Prod Res. 2011;13:449–464. doi: 10.1080/10286020.2011.568940. [DOI] [PubMed] [Google Scholar]
- Hirasuna TJ, Shuler ML, Lackney VK, Spanswick RM. Enhanced anthocyanin production in grape cell cultures. Plant Sci. 1991;78:107–120. doi: 10.1016/0168-9452(91)90167-7. [DOI] [Google Scholar]
- Hulst AC, Meyer MMT, Breteler H, Tramper J. Effect of aggregate size in cell cultures of Tagetes patula on thiophene production and cell growth. Appl Microbiol Biotechnol. 1989;30:18. doi: 10.1007/BF00255991. [DOI] [Google Scholar]
- Isah T, Umar S, Mujib A, Sharma MP, Rajasekharan PE, Zafar N, Frukh A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018;132:239–265. doi: 10.1007/s11240-017-1332-2. [DOI] [Google Scholar]
- Jain SM. Tissue culture-derived variation in crop improvement. Euphytica. 2001;118:153–166. doi: 10.1023/A:1004124519479. [DOI] [Google Scholar]
- Jamwal K, Bhattacharya S, Puri S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J Appl Res Med Aromat Plants. 2017;9:26–38. doi: 10.1016/j.jarmap.2017.12.003. [DOI] [Google Scholar]
- Khanpour-Ardestani N, Sharifi M, Behmanesh M. Establishment of callus and cell suspension culture of Scrophularia striata Boiss.: an in vitro approach for acteoside production. Cytotechnology. 2015;67:475–485. doi: 10.1007/s10616-014-9705-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev KV, Dubrovina AS, Shumakova OA (2013) DNA mutagenesis in 2- and 20-yr-old Panax ginseng cell cultures. In Vitro Cell Dev Biol Plant 49:175–182. 10.1007/s11627-012-9475-7
- Kolewe ME, Gaurav V, Roberts SC. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol Pharm. 2008;5:243–256. doi: 10.1021/mp7001494. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska A, Zebrowski J, Oklejewicz B, Czarnik J, Halibart-Puzio J, Wnuk M. The age-dependent epigenetic and physiological changes in an Arabidopsis T87 cell suspension culture during long-term cultivation. Biochem Biophys Res Commun. 2014;447:285–291. doi: 10.1016/j.bbrc.2014.03.141. [DOI] [PubMed] [Google Scholar]
- Le K-C, Jeong C-S, Lee H, Paek K-Y, Park S-Y. Ginsenoside accumulation profiles in long- and short-term cell suspension and adventitious root cultures in Panax ginseng. Horticult Environ Biotechnol. 2019;60(1):125–134. doi: 10.1007/s13580-018-0108-x. [DOI] [Google Scholar]
- Leguay JJ, Guern J. Quantitative effects of 2,4-dichlorophenoxyacetic acid on growth of suspension-cultured Acer pseudoplatanus cells. Plant Physiol. 1975;56:35–39. doi: 10.1104/pp.56.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez M (1989) Las plantas medicinales de México. Ediciones Botas, México
- Miller CO, Skoog F, Von Saltza MH, Strong F. Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc. 1955;77:1392. doi: 10.1021/ja01610a105. [DOI] [Google Scholar]
- Miller CO, Skoog F, Okomura FS, von Saltza MH, Strong FM. Isolation, structure and synthesis of kinetin, a substance promoting cell division. J Am Chem Soc. 1956;78:1345–1350. doi: 10.1021/ja01588a032. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Naill MC, Roberts SC. Preparation of single cells from aggregated Taxus suspension cultures for population analysis. Biotechnol Bioeng. 2004;86:817–826. doi: 10.1002/bit.20083. [DOI] [PubMed] [Google Scholar]
- Naill MC, Roberts SC. Flow cytometric analysis of protein content in Taxus protoplasts and single cells as compared to aggregated suspension cultures. Plant Cell Rep. 2005;23:528–533. doi: 10.1007/s00299-004-0875-y. [DOI] [PubMed] [Google Scholar]
- Neelakandan AK, Wang K. Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep. 2012;31:597–620. doi: 10.1007/s00299-011-1202-z. [DOI] [PubMed] [Google Scholar]
- Nezbedová L, Hesse M, Dušek J, Werner C. Chemical potential of Aphelandra sp. cell cultures. Plant Cell Tissue Organ Cult. 1999;58:133–140. doi: 10.1023/a:1006363612428. [DOI] [Google Scholar]
- Ouyang J, Wang X, Zhao B, Wang Y. Enhanced production of phenylethanoid glycosides by precursor feeding to cell culture of Cistanche deserticola. Process Biochem. 2005;40:3480–3484. doi: 10.1016/j.procbio.2005.02.025. [DOI] [Google Scholar]
- Perrot-Rechenmann C. Cellular responses to auxin: division versus expansion. Cold Spring Harb Perspect Biol. 2010;2:a001446. doi: 10.1101/cshperspect.a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucatti A, Genualdo V, Pauciullo A, Iorio C, Incarnato D, Rossetti C, Vizzarri F, Palazzo M, Casamassima D, Iannuzzi L, Iannuzzi A. Cytogenetic tests reveal no toxicity in lymphocytes of rabbit (Oryctolagus cuniculus, 2n=44) feed in presence of verbascoside and/or lycopene. Food Chem Toxicol. 2018;114:311–315. doi: 10.1016/j.fct.2018.02.053. [DOI] [PubMed] [Google Scholar]
- Phillips RL, Kaeppler SM, Olhoft P. Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA. 1994;91:5222–5226. doi: 10.1073/pnas.91.12.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu JG, Zhang W, Yu XJ, Jin MF. Instability of anthocyanin accumulation in Vitis vinifera L. var. Gamay Freaux suspension cultures. Biotechnol Bioprocess Eng. 2005;10:155–161. doi: 10.1007/BF02932586. [DOI] [Google Scholar]
- Qu JG, Zhang W, Yu XJ. Instability of anthocyanin composition under different subculture conditions during long-term suspension cultures of Vitis vinifera L. var. Gamay Fréaux. Chin J Biotechnol. 2011;27:1613–1622. [PubMed] [Google Scholar]
- Raghavan C, Ong EK, Dalling MJ, Stevenson TW. Regulation of genes associated with auxin, ethylene and ABA pathways by 2,4-dichlorophenoxyacetic acid in Arabidopsis. Funct Integr Genomics. 2006;6:60–70. doi: 10.1007/s10142-005-0012-1. [DOI] [PubMed] [Google Scholar]
- Roberts SC. Production and engineering of terpenoids in plant cell culture. Nat Chem Biol. 2007;3:387–395. doi: 10.1038/nchembio.2007.8. [DOI] [PubMed] [Google Scholar]
- Sánchez-Sampedro MA, Fernández-Tárrago J, Corchete P. Elicitation of silymarin in cell cultures of Silybum marianum: effect of subculture and repeated addition of methyl jasmonate. Biotechnol Lett. 2009;31:1633–1637. doi: 10.1007/s10529-009-0043-0. [DOI] [PubMed] [Google Scholar]
- Santoro A, Bianco G, Picerno P, Aquino RP, Autore G, Marzocco S, Gazzerro P, Lioi MB, Bifulco M. Verminoside- and verbascoside-induced genotoxicity on human lymphocytes: Involvement of PARP-1 and p53 proteins. Toxicol Lett. 2008;178:71–76. doi: 10.1016/j.toxlet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Santos-Cruz LF, Ávila-Acevedo JG, Ortega-Capitaine D, Ojeda-Duplancher JC, Perdigón-Moya JL, Hernández-Portilla LB, López-Dionicio H, Durán-Díaz A, Dueñas-García IE, Castañeda-Partida L, García-Bores AM, Heres-Pulido ME. Verbascoside is not genotoxic in the ST and HB crosses of the Drosophila wing spot test, and its constituent, caffeic acid, decreases the spontaneous mutation rate in the ST cross. Food Chem Toxicol. 2012;50:1082–1090. doi: 10.1016/j.fct.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Scragg AH. The problems associated with high biomass levels in plant cell suspensions. Plant Cell Tissue Organ Cult. 1995;43:163–170. doi: 10.1007/BF00052172. [DOI] [Google Scholar]
- Sierra MI, van der Heijden R, van der Leer T, Verpoorte R. Stability of alkaloid production in cell suspension cultures of Tabernaemontana divaricata during long-term subculture. Plant Cell Tissue Organ Cult. 1992;28:59–68. doi: 10.1007/BF00039916. [DOI] [Google Scholar]
- Sipahi H, Gostner JM, Becker K, Charehsaz M, Kirmizibekmez H, Schennach H, Aydin A, Fuchs D. Bioactivities of two common polyphenolic compounds: verbascoside and catechin. Pharm Biol. 2016;54:712–719. doi: 10.3109/13880209.2015.1072830. [DOI] [PubMed] [Google Scholar]
- Smýkal P, Valledor L, Rodríguez R, Griga M. Assessment of genetic and epigenetic stability in long-term in vitro shoot culture of pea (Pisum sativum L.) Plant Cell Rep. 2007;26:1985–1998. doi: 10.1007/s00299-007-0413-9. [DOI] [PubMed] [Google Scholar]
- Trejo-Tapia G, Balcazar-Aguilar JB, Martínez-Bonfil B, Salcedo-Morales G, Jaramillo-Flores M, Arenas-Ocampo ML, Jiménez-Aparicio A. Effect of screening and subculture on the production of betaxanthins in Beta vulgaris L. var. ‘Dark Detroit’ callus culture. Innov Food Sci Emerg Technol. 2008;9:32–36. doi: 10.1016/j.ifset.2007.04.009. [DOI] [Google Scholar]
- Vazquez-Marquez AM, Zepeda-Gómez C, Burrola-Aguilar C, Bernabe-Antonio A, Nieto-Trujillo A, Cruz-Sosa F, Rodriguez-Monroy M, Estrada-Zúñiga ME. Effect of stirring speed on the production of phenolic secondary metabolites and growth of Buddleja cordata cells cultured in mechanically agitated bioreactor. Plant Cell Tissue Organ Cult. 2019;39:155–166. doi: 10.1007/s11240-019-01673-9. [DOI] [Google Scholar]
- Wilson SA, Roberts SC. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J. 2012;10:249–268. doi: 10.1111/j.1467-7652.2011.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang J, Huang Z, Wang Z, Zhu Q, Liu L. Correlation of cytokinin levels in the endosperms and roots with cell number and cell division activity during endosperm development in rice. Ann Bot. 2002;90:369–377. doi: 10.1093/aob/mcf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Ming Q, Lin B, Rahman K, Zheng C-J, Han T, Qin L. Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol. 2016;36:215–232. doi: 10.3109/07388551.2014.923986. [DOI] [PubMed] [Google Scholar]