Abstract
Microwave assisted synthesis of graft copolymer of polymeric blend of Fenugreek seed mucilage (FSM)-Polyvinyl alcohol (PVA) with acrylamide (AM) was done by free radical polymerization using ammonium per sulfate (APS) as initiator. Varying amount of AM and APS was used to optimize the best grade based on highest percentage grafting efficiency and investigated with intrinsic viscosity measurement, Fourier Transformation infrared spectroscopy (FTIR),13C NMR spectra, X-ray diffraction, elemental analysis, Thermogravimetric analysis, Scanning electron microscopy. The results of intrinsic viscosity indicate that the optimized sample GF4 has longer chain length than in comparison to the native mucilage and thus exhibits more swelling tendencies and thus can be used as very good controlled release matrix system. The thermal analysis and X-ray indicates that GF4 is more stable and possess more amorphous properties than the native FSM. The NMR and FT-IR studies reveal that in GF4 there is prominent presence of amide and the hydroxyl groups indicating that grafting mechanism has efficiently taken place. Histological studies & SEM image for optimized grade implanted on animals revealed sufficient tissue growth and exhibited biodegradability proving the material to be biocompatible and suitable to be used as tissue engineered scaffolds. The controlled release behavior of the optimized polymeric system GF4 was evidenced by 95% release of loaded drug Enalapril maleate for 16 h.
Graphical abstract
Electronic supplementary material
The online version of this article (10.1007/s40199-019-00237-8) contains supplementary material, which is available to authorized users.
Keywords: Graft copolymer, Metronidazole, Histopathology, Scaffold, Fenugreek seed mucilage
Introduction
The use of green chemistry design for polymer synthesis has eliminated the use of most toxic chemicals creating an environment free from chemical pollution [1]. Thus, chemical free radiation induced free radical initiated synthesis of various types of polymers like graft copolymer has brought a breakthrough in the field of modified polymers to be used in drug delivery and other biomedical applications [2]. The modification of a natural polysaccharide with another synthetic substance thereby retaining the chemical and physical properties of natural and synthetic polymers is mainly done by the process of graft polymerization [3, 4]. Also the modification of natural polymers by synthetic polymers improves their shelf life and stability, preventing from instant biodegradation and can be used in controlled release of potent drugs [5, 6]. In case of drug delivery, efficient controlled and extended release is mostly expected from any good formulations. The use of graft copolymers can serve the purpose of retarding the drug release by forming an efficient drug matrix which will slowly swell and erode to release the drugs in a controlled fashion preventing the peak and valley release pattern as expected in conventional delivery [7]. Besides the use of these modified polymers in drug delivery, other significant applications in tissue engineering are of importance. The biomedical nature of such modified polymers mainly depends on their abilities to interact with the living cells, cyto-compatibility and their macromolecular structure to form the three-dimensional (3D) structure to mimic the cells [8]. The use of natural polysaccharides is advantageous as they are nontoxic and biodegradable in nature [7]. Several polysaccharides are utilized for preparation of graft copolymer with vinyl or acryl monomers [4]. The acrylamide monomers have varied applications in the field of drug delivery, flocculation, stabilizing agents etc. [9]. Various plant sources like seeds, leaves, bark have plenty mucilage content as rich source of polysaccharides. Fenugreek seeds are one such source sufficient amount of mucilage and can be used for preparation of modified polymers [10]. Previously, there are several evidences of research on fenugreek seed mucilage grafted copolymers with acrylamide [10], but till date no where there are any evidences for the polymeric blend of fenugreek seed mucilage with Polyvinyl alcohol (PVA) grafted with acrylamide (AM) used as tissue scaffold. The current research is based on preparation of grafted polymeric blend of Fenugreek seed mucilage-Polyvinyl alcohol (FSM-PVA-g-PAM) with polyacrylamide for its use as polymeric tissue scaffold and its drug release properties.
Experimental
Fenugreek seed (Trigonella foenum-graecum) mucilage (FSM) was extracted by milling the soaked seeds in hot water to yield a thick mucilaginous solution which was filtered four times through very fine muslin cloth. For deproteinization, the mucilage solution was treated with solution of 0.3 N Ba(OH)2–5% aqueous ZnSO4.7H2O [10]. Acrylamide (AM), Ammonium Per sulfate (APS)(SD, Fine-Chem Ltd., India), Poly(vinyl alcohol)(CDH, New Delhi) were used as received.
Synthesis of FSM-PVA-g-PAM graft copolymer and its purification
The graft copolymer was prepared by using microwave assisted process by varying different concentration of APS and AM using APS as the redox initiator [11] as shown in composition Table 1. The time for the reaction was kept constant. Initially the required quantity of polymers (FSM with PVA) along with calculated amount of monomer AM were taken in a glass beaker and dissolved in milipore water (100 ml) stirred on a magnetic stirrer maintained at temperature 50 °C till all the polymers dissolve and then later APS is added and stirred for another 15 min. The mixture in the glass beaker was microwaved keeping the time constant at 40s till the polymer mixture thickens to gel consistency. The reaction vessel was immediately taken out and placed aside in ice bath for cooling. The unreacted PVA, monomer, APS and any other byproducts if formed are removed by using acetone as a solvent to obtain the purified graft copolymer [12, 13]. Reaction detail is outlined in Scheme 1.The optimization of the best grade of FSM-PVA-g-PAM is done using % Grafting Efficiency as shown in Eq. 1 [14–16]. The synthesized graft copolymer was dried in a hot air oven and ground to fine powder.
| 1 |
Table 1.
Composition of graft copolymer of FSM-PVA-g-AM
| Sl.No. | Grade Code | Weight in gm | % Yield | Microwave time (Sec) | % GE | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pure FSM | PVA | AM | APS | Yield | |||||
| 1 | GF1 | 0.5 | 0.5 | 10 | 0.2 | 8.752 | 78.142 | 30Sec | 77.52 |
| 2 | GF2 | 0.5 | 0.5 | 10 | 0.25 | 8.342 | 74.151 | 30Sec | 73.42 |
| 3 | GF3 | 0.5 | 0.5 | 10 | 0.15 | 7.166 | 64.269 | 30Sec | 61.66 |
| 4 | GF4 | 0.5 | 0.5 | 15 | 0.2 | 15.572 | 96.123 | 30Sec | 97.146 |
| 5 | GF5 | 0.5 | 0.5 | 20 | 0.2 | 18.934 | 89.311 | 30Sec | 89.67 |
| 6 | GF6 | 0.5 | 0.5 | 25 | 0.2 | 21.382 | 81.611 | 30Sec | 81.528 |
Scheme 1.
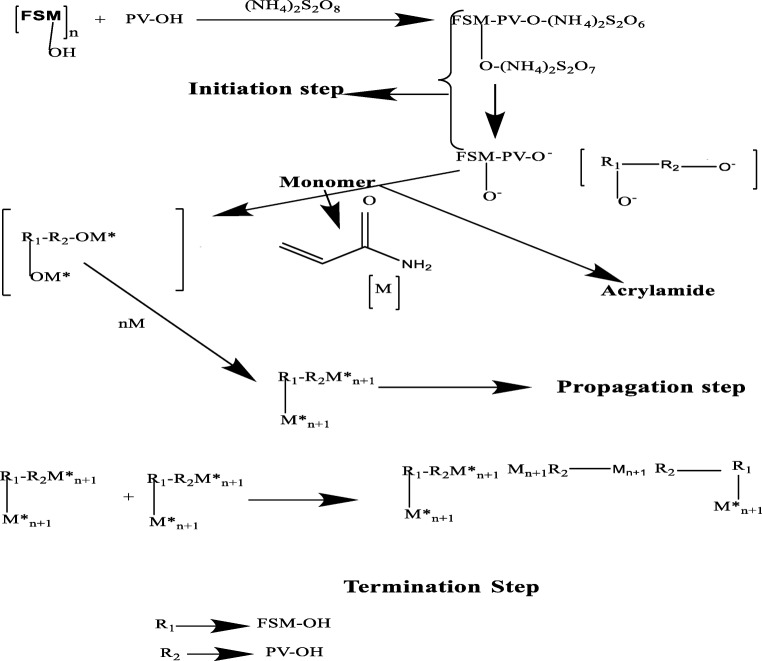
Reaction Mechanism of formation of graft-copolymer of Fenugreek seed mucilage (FSM)-Polyvinyl alcohol (PV-OH) with Acrylamide. Ammonium persulphate(NH4)2S2O8) is used as initiator in the initiation step leading to the formation of free radical on the Polymer blend of FSM with PV-OH and the monomer Acrylamide attaches to the free radical sites on to the Fenugreek seed mucilage-PVA and further propagates to form Polyacrylamide grafted copolymer in the Propagation step and later terminated by addition of acetone in the termination step
Characterization and analysis
The intrinsic viscosity measurement of all the prepared grades of graft copolymer were done using Ubbelodhe viscometer (capillary pore size 0.36 mm, measuring viscosity range from 0.3-1 mm2/s) at 25 °C. Other characterizations of graft copolymer along with the pure polymers and monomer was done by using Fourier Transformation Infrared Spectroscopy (FTIR) (Shimadzu, FTIR-8400S) using KBr pellets from 4000 to 400 cm−1 [16]. Thermo-gravimetric analysis (TGA) (Model: DTG-60; Shimadzu, Japan) was performed in an inert atmosphere (nitrogen) from 30 °C to 400 °C at the heating rate of 10 °C/min [17] .X-ray diffraction (XRD) was done with PW 1840 diffractometer and PW 1729 X-ray generator, which were used for the study to produce Cu-Ka radiation with the scattering angle (2Ɵ) varied from 10 to 800 [16]. 13C Nuclear magnetic resonance (NMR) was also done for the confirmation of grafting reaction in optimized graft copolymer which was compared with that pure FSM.
Intrinsic viscosity measurement
Intrinsic viscosity measurements of the aqueous solution of graft copolymer of different concentration (0.1, 0.05, 0.025 & 0.0125% w/v) were carried out with the help of Ubbelohde viscometer with a capillary diameter of 0.36 mm (constant 0.001) at 25 ± 0.1 °C in triplicate. The flow time was measured for polymeric solution. The intrinsic viscosity was calculated by plotting specific viscosity/concentration(ηsp/C) versus concentration (C) and then taking the intercept at C = 0 of the fitted straight line [6, 18].
FTIR spectroscopy
FTIR of optimized grade GF4 is done for the confirmation of structure. Sample was taken in 1:100 ratio by KBr and mix evenly in porcelain dish. About 10 mg of mixed sample was moved in sample holder and pressed evenly to make flat surface. The transmittance recorded between 4000 and 500 cm-1 on FTIR spectroscopy (Shimadzu, FTIR-8400S) [19].
Thermal analysis
The thermal stability of vacuum-dried samples of pure FSM, GF4, PVA and acrylamide were examined using thermal gravimetric analyzer (Shimadzu, Japan, DTG-60) and heated from room temperature to 700 °C in a nitrogen atmosphere at a heating rate of 10 °C/min [10].
XRD
The sample mainly pure FSM, PVA and GF4 were diffracted for 2θ value ranging from 20 to 80° at 2°/minute and chart speed of 2°/2 cm/2θ (Bruker AXC D8 Advance, Germany) [10].
13C NMR studies
Solid state 13C NMR spectroscopy was performed for native gum and GF-4. The analysis was performed by inserting about 300 mg of sample respectively in ceramic rotor on a JEOL ECX 400 (Peabody, MA, USA) spectrometer functioned at 75 MHz [20].
Surface morphology
The scanning electron microscopy of the GF4 was done in powdered form using SEM, Model Jeol JSM −6390 LV. The SEM photographs are used for characterizations of the surface morphology of the grafted polymer [9, 10].
Elemental analysis
The elemental analysis of FSM and GF4 was undertaken with an Elemental Analyzer (SEM, Jeol JSM -6390 LV). The estimation of three elements, i.e. carbon, nitrogen, oxygen was undertaken [21].
Invitro drug release studies
Preparation of tablets
The tablets of Enalapril Maleate using the optimized GF4 graft copolymer was prepared by hand powdering the graft polymer [22] and then weighing out a 225 mg of polymer along with 25 mg of drug which is 10% of total weight of the polymer and passing the mixture through a 20mesh sieve and to this mixture around 0.01 mL of water was added just to moisten the mixture and triturated. The dough was then hand molded to form a pellet like structure as shown in.
In-vitro release study
The in-vitro dissolution study of Enalapril Maleate from graft copolymer in the form of tablets as shown in Fig. 1, was carried out in a basket type 8-station dissolution test apparatus Dissolution apparatus(TDT -08 l), Electrolabs, TDT-08 L, Mumbai, India, with stirring speed of 50 rpm using phosphate buffer of pH 6.8 as dissolution medium under sink conditions at 37 °C ± 0.5 °C.Periodically, 10 ml of the solution was withdrawn from the dissolution medium and was filtered with a 0.45 μm (μ) membrane filter disc and analyzed by Double beam spectrophotometer (UV-1800), Shimadzu Corporation, Japan, at 205 nm for the drug [23]. Same volume of dissolution medium was replaced back after each sampling to maintain sink condition. The kinetic data obtained from the release profiles were also evaluated by fitting into different kinetic models.
Fig. 1.
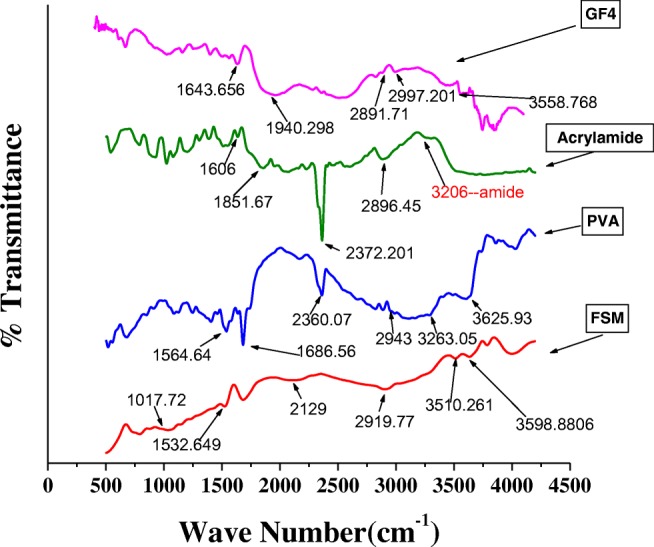
FTIR Spectra of FSM (Fenugreek seed mucilage); PVA (Polyvinyl alcohol); Acrylamide (AM); Graft copolymer (GF4)
Assessment of mice subcutaneous tissue response graft copolymer as tissue engineered scaffold
The experiment was conducted following the institutional animal use and care regulations of Birla Institute of Technology, Mesra, Ranchi (Institutional Animal ethical Committee of Birla Institute of Technology, Mesra, Ranchi, Approval number 1972/PH/BIT/25/17/IAEC). Male mice were housed under standard conditions with a controlled temperature of 25 °C and a light/dark cycle of 12/12 h. The animals were divided in two groups i.e. control & test each consisting of 6 animals [24]. Hairs on the skin were completely removed using hair removal cream. Then, the exposed skin of the animals was sterilized using povidone iodine. An incision was done and pure cotton ball as the control and cotton ball coated with solution of graft copolymer as the test material were placed in the skin pockets and stitched. After 15 days, each group was euthanized, and the test and control materials were taken out and freeze dried [25] and observed under SEM for the evidence of any cell growth on the scaffolds. Isolated liver, kidney & scaffold applied local tissue were preserved in formalin for histological procedures for determination of any toxicity. After collection, the carcasses were disposed by burial [24].
Histological procedure
Excised tissues from local site and liver were fixed in 10% formalin solution for 5 days. All tissues were processed by embedding in paraffin wax and then sectioned at 3 μm thicknesses, mounted on glass slides, deparaffinized and stained with Haematoxylin–Eosin (HE). Images were taken with an optical microscope (Leica, DME) [24].
Results and discussion
Synthesis of FSM-PVA-g-PAM
FSM-PVA-g-PAM has been synthesized using microwave irradiation. Initially for all grades of graft copolymer preparation, the quantity of polymers FSM, PVA and AM were kept constant. Only the ratio of APS was varied in the grades, to check at which concentration of APS, maximum % GE was achieved. At this stage, grade GF1 was having the highest %GE. Later, optimization of the monomer AM was quantified by keeping all the other contents constant and finally grade GF4 was selected as the optimum grade with maximum %GE and was further evaluated. The optimized graft copolymer was based on the % grafting efficiency. Generally, % grafting is used as the parameter for optimization of the best grade, but in the present process, the % grafting parameter did not provide expected results as there was continuous increase in grafting with the increase in monomer concentration. Thus, to get appropriate results, % grafting was utilized as the optimization parameter.
Characterizations
Intrinsic viscosity
The present optimized grade GF4 has higher intrinsic viscosity value when compared to other grades. The values of intrinsic viscosity of all the different grades of graft copolymer sample are given in Table 2. The graphs of intrinsic viscosities of all the grades are shown in Fig. S1. The intrinsic viscosity of a polymeric solution is a measure of the hydrodynamic volume of the polymer in solution which in turn depends on the polymer molecular weight, its structure, nature of the solvent and temperature of the medium. For the polymers which have a low intrinsic viscosity indicates that the hydrodynamic volume is less as it may be due to the smaller branched chains and for the polymers having large intrinsic viscosity, their hydrodynamic volume is also higher as this may be due to the reason for the long linear grafted chains on to the polymer backbone [19]. This may be an advantageous factor as more the hydrodynamic value, there may be more swelling and thus this can be an essential factor in controlling the release rate of drug from the polymeric matrix.
Table 2.
A comparative table depicting formula, intrinsic viscosities & % grafting efficiency of the graft copolymer grades
| Polymer grade | Amount of Fenugreek seed mucilage(gm) | Amount of PVA (gm) | Amount of Acrylamide(gm) | Time of irradiation (Sec) | Intrinsic viscosity (dl/g) | % GE |
|---|---|---|---|---|---|---|
| GF1 | 0.5 | 0.5 | 10 | 40s | 10.849 | 77.52 |
| GF2 | 0.5 | 0.5 | 10 | 40s | 3.9431 | 73.42 |
| GF3 | 0.5 | 0.5 | 10 | 40s | 7.619 | 61.66 |
| GF4 | 0.5 | 0.5 | 15 | 40s | 10.881 | 92.0 |
| GF5 | 0.5 | 0.5 | 20 | 40s | 5.6557 | 89.67 |
FTIR spectroscopy
The FTIR spectra of FSM shows characteristic peaks at 3598 cm−1 which corresponds to –OH [26] -CO stretching at 1017.72 [10, 11],-CH3 stretching at 2919.77 cm−1 as shown in Fig. 1 and Table S2. It is observed that the FTIR spectrum of GF4 is different from the FSM by the introduction of a small peak of secondary amide RCONHR at 1643 cm−1,broad and medium –NH stretching at 2997 and 3352 cm−1 [26]. The broadening in the spectra in GF4 after 3000 cm−1 may be due to the overlapping of –NH(amide) and –OH groups of mucilage.
Thermal analysis
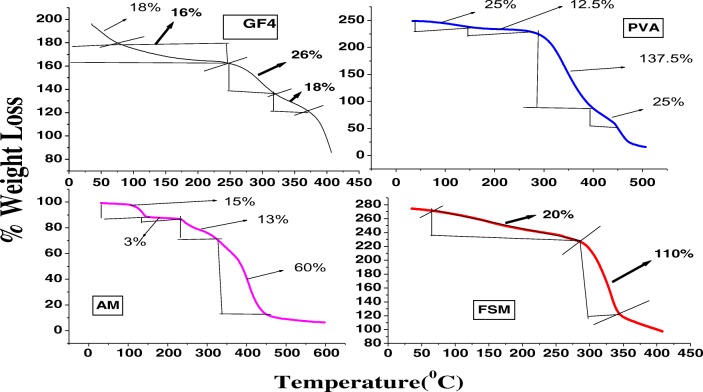
The TGA thermograms of FSM and FSM-PVA-g-PAM copolymer GF4, are shown in Fig. 2. In case of pure fenugreek seed mucilage FSM, there is only single stage degradation, where as in case of grafted copolymer GF4, there are multiple stages of degradation with very less amount of weight loss which is very high in case of pure mucilage. The percent weight loss in case of pure FSM and that of GF4 till 275 °C, was almost equal to about 18–20%, but after 275 °C, in case of FSM, there is a drastic weight loss whereas in case of GF4, there was very less amount of weight loss, which itself indicates that graft copolymer GF4, is more stable than that of pure polymer. Moreover, as in case of PVA, the percent weight loss after 275 °C is 137.55%, whereas in case of AM, the percent weight loss after 250 °C is only 13%. The percentage weight loss with an increase in temperature in both the cases is summarized in Table S3. The results showing less amount of % weight loss in case of GF4, when compared to pure FSM, PVA and AM, gives a clear indication that the polyacrylamide chains grafted with the polymer blend of pure FSM along with PVA contributed more stability towards temperature and thermal decomposition behavior [10].
Fig. 2.
Thermograms of FSM (Fenugreek seed mucilage); PVA (Polyvinyl alcohol); Acrylamide (AM); Graft copolymer (GF4)
X-ray diffraction
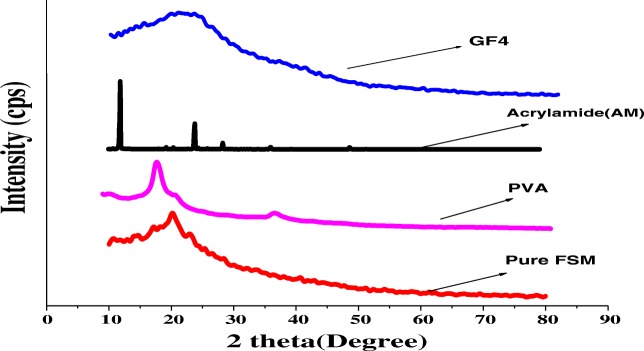
The XRD diffractograms of the pure native FSM shows broadened sharp peaks whereas in case of PVA and AM sharp peaks are evident in Fig. 3. But in case of GF4, the diffractogram does not have any sharp peaks and is almost bluntly broadened, which is different from the pure FSM, which indicates that GF4 will be easily soluble in aqueous phase [27]. The XRD diffractograms of the pure native FSM showing not so prominent sharp peaks indicates its partial crystalline nature [10], whereas in case of PVA and AM sharp peaks indicate their pure crystalline nature. In case of GF4, the presence of broad blunt diffractogram indicates the amorphous characteristics of the sample which will be easily soluble in aqueous phase. Also, this change in GF4 indicates that a solid phase was formed after grafting process and this led to the destruction of the crystalline nature of the polymer blend of mucilage and Polyvinyl alcohol [10].
Fig. 3.
XRD patterns of Pure native fenugreek seed mucilage (FSM), Polyvinyl Alcohol (PVA), Acrylamide (AM, Graft Copolymer (GF4)
NMR spectra
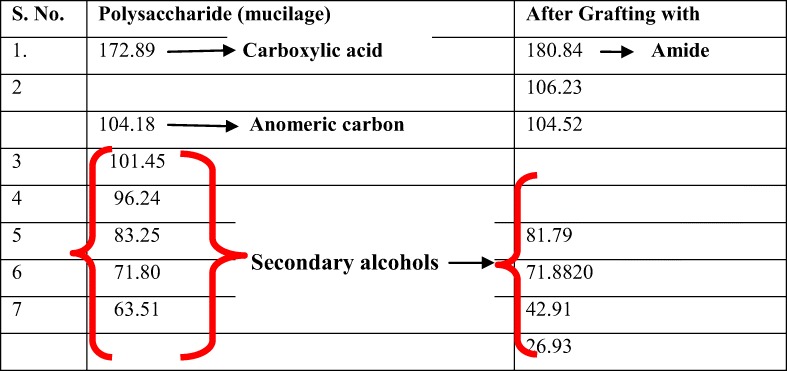
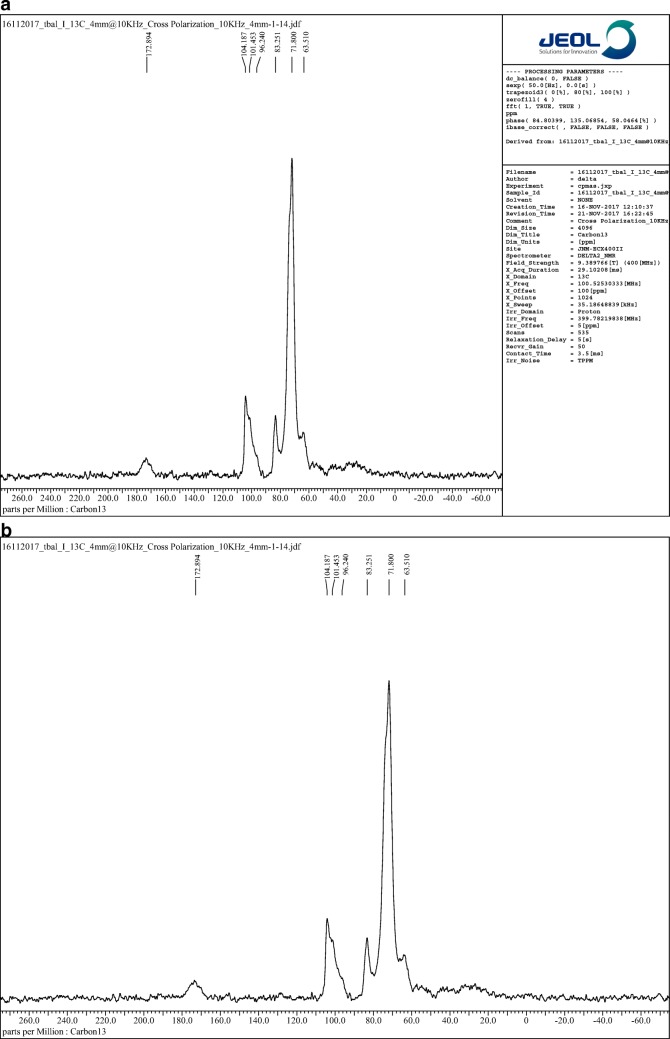
The 13C-NMR resonances of mucilage revealed the peaks as shown in Table 3. The NMR spectra of Pure Fenugreek seed mucilage (FSM) and that of GF4 are shown in Fig. 4a and b A small downfield shift of all the backbone carbons of the polysaccharide in the copolymer in comparison to those of the parent was observed, except for C-1 carbon showing upfield shift of 1.71 ppm in the copolymer as shown in Table 3. This may have been due to the introduction of the acrylamide moiety onto the polysaccharide, which is expected. The PVA also contributes to the upfield shift due to presence of secondary alcohol groups.
Table 3.
NMR peaks along with their interpretations
Fig. 4.
a spectra for Pure Fenugreek seed mucilage (FSM) b NMR spectra for optimized Graft copolymer GF4
Surface morphology
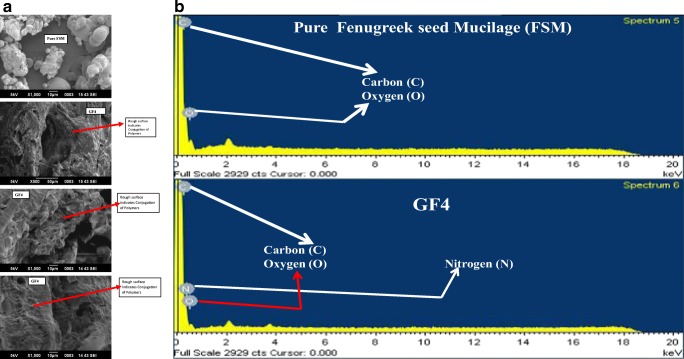
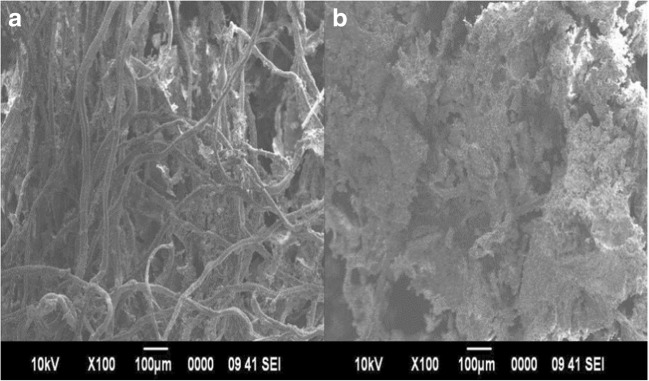
The scanning electron microscopic photographs of both the native FSM and that of GF4 are shown in Fig. 5a. The rough uneven surface of GF4 confirms the modification in the native fenugreek seed mucilage polymer by another polymer, thereby indicating the grafting of acrylamide (AM) over polymeric blend of FSM with PVA as shown in Fig. 8 [28].
Fig. 5.
a: Surface morphology Graft copolymer GF4 and Pure FSM b Elemental spectra of Pure FSM and GF4
Fig. 8.
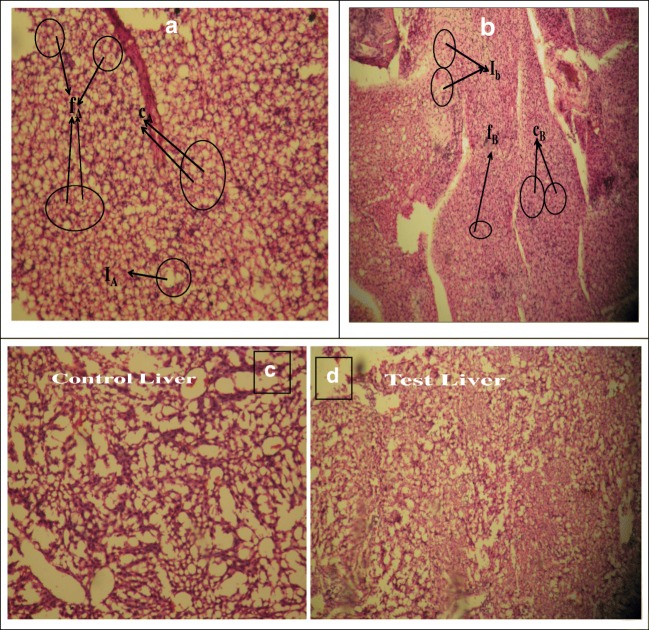
a Photomicrographs of histology of Control local tissue area stained with hematoxylin and eosin on day 15 after surgery captured by Leica Microscope at 40X respectively and f,c and I, represent spindle shaped fibroblast, collagen and Foreign body giant cells respectively b Photomicrographs of histology of Test local tissue area stained with hematoxylin and eosin on day 15 after surgery captured by Leica Microscope at 40X respectively and f,c and I, represent spindle shaped fibroblast, collagen and Foreign body giant cells respectively. c Photomicrographs of histology of liver of Test and control mice stained with hematoxylin and eosin on day 15 after surgery captured by Leica Microscope at 40X d: Photomicrographs of histology of kidney of control and test mice stained with hematoxylin and eosin on day 15 after surgery captured by Leica Microscope at 40X
Elemental analysis
The results of presence of different elements like oxygen, nitrogen, hydrogen and carbon are shown in Fig. 5b. This is also observed in practice from the elemental analysis result as summarized in Table S 4. The results as observed from Fig. 5b, confirms the presence of a considerable percent of nitrogen in GF4 compared to the pure FSM which also confirms that AM whose basic elemental content is nitrogen in the form of –CONH2, chains have been grafted on the backbone of polymeric blend of FSM and PVA [21].
In-vitro drug release studies
The drug release from graft copolymer grades GF1, GF2, GF3, GF4 and GF5 were done in presence of phosphate buffer of pH 6.8 and a comparative release profile was plotted for % Drug release versus time & drug release kinetics was calculated as depicted in Fig. 6, GF4 gives a prolonged release in a controlled fashion than in comparison to other grades. As soon as the graft copolymer tablet interacts with aqueous media, water diffuses into the tablet thereby causing the swelling of the matrix and this swelling continues and a monotonically hydrostatic pressure is developed within the tablets [29]. With the increase in swelling and decreased solubility of the grafted polymeric matrix, the polymeric matrix gets depleted slowly with slow diffusion of the drug from the matrix. The decreased solubility of the grafted copolymer GF4 shows decreased solubility due to increased grafting efficiency [30] and thus the grade GF4 shows controlled release properties by prolonging the release for more than 16 h uniformly as shown in Fig. S 5. The release kinetics of the optimized grade GF4 follows Higuchi model with R2 value almost nearing to 1 when compared to other drug release kinetic models as shown in Fig. 3, thereby showing that the polymeric matrix forms a permeable matrix [31] with diffusion type of release since value of n = 0.309 [32], as shown in Fig. S 5.
Fig. 6.
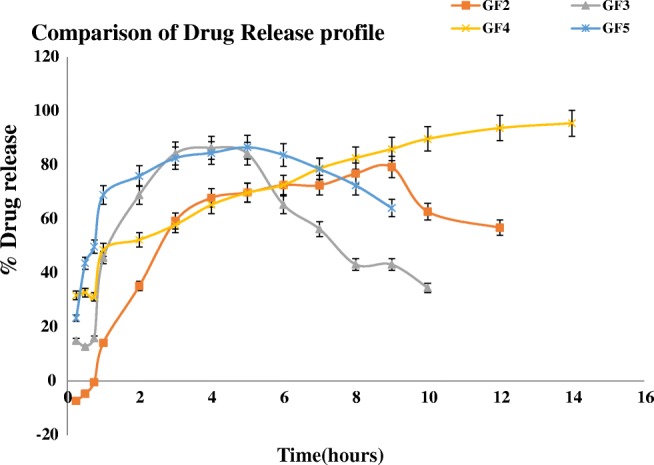
Comparative drug release profile
Assessment of response of graft copolymer scaffold towards mice tissue
All wounds experienced gradual healing without infection, after completion of 15 days, both the standard and test groups showed a complete loss of keratin layer and the epidermis was devoid of prickle cells. Efficient healing was confirmed by the existence of hair follicles and mature fibrous tissue. This was a clear indication towards the facility of moist environment at the site of the wound, which enabled the rapid epithelialization [33]. The scanning electron microscopy of the control cotton ball and the test cotton ball are shown in Fig. 7 to detect the growth of cells on to the polymer which can be used as tissue scaffold. The figure indicate that the cells have proliferated and differentiated onto the cotton ball as well as onto the cotton ball coated with GF4, [33] which also suggested that the inserted material are highly biodegradable in nature as shown in Fig. 7a and b.
Fig. 7.
a and b representing SEM images of the tissue growth on the control and Graft Copolymer GF4
Histological investigations
The histology slide of the local tissue, liver and kidney was investigated for both the control and test animals for detection of any obvious toxicity as shown in Fig. 8a-d. From the local tissue slides as shown in Fig. 8a and b, it is quite evident that there was denser growth of spindle shaped fibroblasts [34], collagen and granular cells as compared to that of control tissue [33]. The increased quantity of these cells in the local test tissue indicates that wound healing is rapid. Since collagen enhances the growth of extracellular matrix and thus its increase indicates rapid wound healing in the local test tissue. Foreign body giant cells observed in minor quantity in case of test shown in Fig. 8a and b, an inflammatory response are most common at the interface of both tissue and scaffold which is previously reported in several literature [35, 36]. These results ensure the nontoxic nature of the graft copolymer GF4 as well as its efficiency to be utilized as a template material for tissue growth. Also, the histological evaluation of the test liver and kidney when compared with control tissues as shown in Fig. 8c and d respectively suggests that the test material do not pose any toxicity in liver and kidney.
However, in this article, it can be visualized that our FSM-PVA-g-PAM is a versatile material having broader application. This novel material has potential to be applied in both tissue scaffold & drug delivery devices. The applicability of this material needs to be explored widely. In-depth toxicity study & applicability in some higher animal models like dog, monkey and human need to be explored. Full phase clinical trial need be observed to check the effect of material in the long run.
Conclusion
The graft copolymer of FSM-PVA-g-PAM was successfully prepared by microwave irradiation process using ammonium per sulfate as the redox initiator. The percentage grafting efficiency increased with increasing quantity of redox initiator but up to a certain limit and then again decreased, and whereas in presence of monomer the grafting efficiency continuously increased and thus the highest grafting efficiency value was taken as the best grade. The intrinsic viscosity of the optimized grade GF4 showed that the sample has longer polymeric chains leading to more swelling and delayed drug release. Moreover, the characterization of GF4 was done with FTIR, XRD, NMR, and TGA. The elemental analysis confirmed the presence of nitrogen along with carbon and oxygen in the sample GF4. The in-vitro tissue growth on the graft copolymer sample was successful and histopathological studies of the local tissues along with the liver and kidney showed that the sample is nontoxic. The in-vitro drug release from the optimized grade took place for more than 16 h and the release kinetics to followed Higuchi model with diffusion type of release from the matrix. This versatility makes the material suitable for tissue engineering scaffold & drug delivery device.
Electronic supplementary material
(DOCX 181 kb)
Acknowledgments
The authors acknowledge the instrumental support of Central Instrumentation Facility, Birla Institute of Technology, Mesra, for sophisticated instrument to carry out this experiment.
Author’s contributions
The experimental study was done by Corresponding author Trishna Bal (TB) as a part of her reserach work who did all the practical experiments and drafted the article. The other coauthor, Sabyasachi Swain (SS) did the insertion of the polymeric scaffolds in animals. All authors read and approved the final copy of the text.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dong H, Xu Q, Li Y, Mo S, Cai S, Liu L. The synthesis of biodegradable graft copolymer cellulose-graft-poly(l-lactide) and the study of its controlled drug release. Colloids Surf B: Biointerfaces. 2008;66:26–33. doi: 10.1016/j.colsurfb.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Mishra S, Mukul A, Sen G, Jha U. Microwave assisted synthesis of polyacrylamide grafted starch (St-g-PAM) and its applicability as flocculant for water treatment. Int J Biol Macromol. 2011;48:106–111. doi: 10.1016/j.ijbiomac.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Sen G, Singh RP, Pal S. Microwave-initiated synthesis of polyacrylamide grafted sodium alginate: synthesis and characterization. J Appl Polym Sci. 2010;115:63–71. doi: 10.1002/app.30596. [DOI] [Google Scholar]
- 4.Maiti S, Ranjit S, Sa B. Polysaccharide-based graft copolymers in controlled drug delivery. Int J PharmTech Res. 2010;2:1350–1358. [Google Scholar]
- 5.Srivastava A, Behari K. Synthesis and characterization of graft copolymer (guar gum–g–N-vinyl-2 pyrrolidone) and investigation of metal ion sorption and swelling behavior. J Appl Polym Sci. 2006;100:2480–2489. doi: 10.1002/app.23594. [DOI] [Google Scholar]
- 6.da Silva DA, de Paula RCM, Feitosa JPA. Graft copolymerisation of acrylamide onto cashew gum. Eur Polym J. 2007;43:2620–2629. doi: 10.1016/j.eurpolymj.2007.03.041. [DOI] [Google Scholar]
- 7.Sen G, Mishra S, Jha U, Pal S. Microwave initiated synthesis of polyacrylamide grafted guar gum (GG-g-PAM)—characterizations and application as matrix for controlled release of 5-amino salicylic acid. Int J Biol Macromol. 2010;47:164–170. doi: 10.1016/j.ijbiomac.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Khan F, Tanaka M. Designing smart biomaterials for tissue engineering. Int J Mol Sci. 2018;19:17. doi: 10.3390/ijms19010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabbagh F, Muhamad II. Acrylamide-based hydrogel drug delivery systems: release of acyclovir from MgO nanocomposite hydrogel. J Taiwan Inst Chem Eng. 2017;72:182–193. doi: 10.1016/j.jtice.2016.11.032. [DOI] [Google Scholar]
- 10.Mishra A, Yadav A, Pal S, Singh A. Biodegradable graft copolymers of fenugreek mucilage and polyacrylamide: a renewable reservoir to biomaterials. Carbohydr Polym. 2006;65:58–63. doi: 10.1016/j.carbpol.2005.12.015. [DOI] [Google Scholar]
- 11.Tanan W, Saengsuwan S. Microwave assisted synthesis of poly (acrylamide-co-2-hydroxyethyl methacrylate)/poly (vinyl alcohol) semi-IPN hydrogel. Energy Procedia. 2014;56:386–393. doi: 10.1016/j.egypro.2014.07.171. [DOI] [Google Scholar]
- 12.Kongparakul S, Prasassarakich S, Rempel LG. Effect of grafted methylmethacrylate on the catalytic hydrogenation of natural rubber. Eur Polym J. 2008;44:1915–1920. doi: 10.1016/j.eurpolymj.2007.09.021. [DOI] [Google Scholar]
- 13.Pal P, Pandey JP, Sen G. Synthesis, characterization and flocculation studies of a novel graft copolymer towards destabilization of carbon nano-tubes from effluent. Polymer. 2017;112:159–168. doi: 10.1016/j.polymer.2017.01.059. [DOI] [Google Scholar]
- 14.Pal S, Sen G, Ghosh S, Singh RP. High Performane polymeric flocculant based on modified polysaccharides-microwave assisted synthesis. Carbohydr Polym. 2012;87:336–342. doi: 10.1016/j.carbpol.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 15.Rahul R, Jha U, Sen G, Mishra S. A novel polymeric flocculant based on polyacrylamide grafted inulin: aquous microwave assisted synthesis. Carbohydr Polym. 2014;99:11–21. doi: 10.1016/j.carbpol.2013.07.082. [DOI] [PubMed] [Google Scholar]
- 16.Sharma RK, Lalita, Singh AP. Synthesis and characterization of chitosan based graft copolymers for drug release applications. J Chem Pharm Res. 2015;7(6):612–21.
- 17.Wesolowski M, Rojek B. Thermogravimetric detection of incompatibilities between atenolol and excipients using multivariate techniques. J Therm Anal Calorim. 2013;113:169–177. doi: 10.1007/s10973-013-3070-y. [DOI] [Google Scholar]
- 18.Schott H. Polymers. In: Martin A, Bustamante P, Chun AHC, editors. Physical pharmacy. 4. Maryland: B.I.Waverly Pvt. Ltd; 1994. pp. 561–563. [Google Scholar]
- 19.Azmeera V, Rastogi PK, Adhikary P. Synthesis, characterization and cyclic voltammetric study ofcopper(II) and nickel(II) polymer chelates. Carbohydr Polym. 2014;110:388–395. doi: 10.1016/j.carbpol.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Meena R, Prasad K, Mehta G, Siddhanta AK. Synthesis of the copolymer hydrogel k-carrageenan-graft-PAAm: evaluation of its absorbent and adhesive properties. J Appl Polym Sci. 2006;102:5144–5153. doi: 10.1002/app.24703. [DOI] [Google Scholar]
- 21.Sen G, Ghosha S, Jha U, Pal S. Hydrolyzed polyacrylamide grafted carboxymethyl starch (Hyd. CMS-g-PAM): an efficient flocculant for the treatment of textile industry wastewater. Chem Eng J. 2011;171:495–501. doi: 10.1016/j.cej.2011.04.016. [DOI] [Google Scholar]
- 22.Usha Rani G. Ananda Kumar Konreddy, Sumit Mishra∗, Gautam Sen. Synthesis and applications of polyacrylamide grafted agar as a matrix for controlled drug release of 5-ASA. Int J Biol Macromol. 2014;65:375–382. doi: 10.1016/j.ijbiomac.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Tosha SM, Chisty AASJ, Asaduzzaman M, Bhuiyan MA. Development and in vitro evaluation of pulsatile drug delivery system of Enalapril maleate. Bangladesh Pharmaceutical Journal. 2015;18(1):66–71. doi: 10.3329/bpj.v18i1.23519. [DOI] [Google Scholar]
- 24.Sumayya AS, Muraleedhara Kurup G. Biocompatibility of subcutaneously implanted marine macromolecules cross-linked bio-composite scaffold for cartilage tissue engineering applications. J Biomater Sci Polym Ed. 2018;29:257–276. doi: 10.1080/09205063.2017.1413759. [DOI] [PubMed] [Google Scholar]
- 25.Nishida E, Miyaji H, Kato A, HirokoTakita TI, TakehitoMomose KO, Murakami S, Sugaya T, Kawanami M. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int J Nanomedicine. 2016;11:2265–2277. doi: 10.2147/IJN.S104778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beauchamp. Infrared Tables (short summary of common absorption frequencies) https://www.cpp.edu/~psbeauchamp/pdf/spec_ir_nmr_spectra_tables.pdf. Accessed 5 Oct 2018.
- 27.Hancock BC, Parks M. What is true solubility advantage for amorphous pharmaceuticals? Pharm Res. 2000;17(4):397–404. doi: 10.1023/A:1007516718048. [DOI] [PubMed] [Google Scholar]
- 28.Songa Y, Wei D. Preparation and characterization of graft copolymers of silk Sericin and methyl methacrylate. Polym Polym Compos. 2006;14(2):169–174. [Google Scholar]
- 29.Muschert S, Siepmann F, Leclercq B, Carlin B, Siepmann J. Drug release mechanisms from ethylcellulose: PVA-PEG graft copolymer-coated pellets. Eur J Pharm Biopharm. 2009;72:130–137. doi: 10.1016/j.ejpb.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Sen G, Pal S. Microwave initiated synthesis of polyacrylamide grafted carboxymethyl starch (CMS-g-PAM): application as a novel matrix for sustained drug release. Int J Biol Macromol. 2009;45(1):48–55. doi: 10.1016/j.ijbiomac.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Paul DR. Elaborations on the Higuchi model for drug delivery. Int J Pharm. 2011;418(1):13–17. doi: 10.1016/j.ijpharm.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 32.YuanGao JZ, NadiaBou-Chacra T d JAP, Clas S-D, Walker RB, Löbenberg R. In vitro release kinetics of Antituberculosis drugs from nanoparticles assessed using a modified dissolution apparatus. Biomed Res Int. 2013;2013:1–9. doi: 10.1155/2013/136590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swain S, Bal T. Microwave irradiated carrageenan-guar gum micro-porous IPN: a novel material for isotropic tissue scaffolding. Int J Polym Mater Polym Biomater. 2018:1–9. 10.1080/00914037.2018.1506986.
- 34.Fatemeh Hajiaghaalipour MSK. Mahmood Ameen Abdulla, JunedahSanusi. The effect of Camellia sinensis on wound healing potential in an animal model. Evid Based Complement Alternat Med. 2013;2013:1–7. doi: 10.1155/2013/386734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wosgrau AC, Jeremias Tda S, Leonardi DF, Pereima MJ, Di Giunta G, Trentin AG. Comparative experimental study of wound healing in mice: Pelnac versus Integra. PLoS One. 2015;10(3):e0120322. doi: 10.1371/journal.pone.0120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Vries HJ, Mekkes JR, Middelkoop E, Hinrichs WL, Wildevuur CR, Westerhof W. Dermal substitutes for full-thickness wounds in a one-stage grafting model. Wound Repair Regen. 1993;1:244–252. doi: 10.1046/j.1524-475X.1993.10410.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 181 kb)