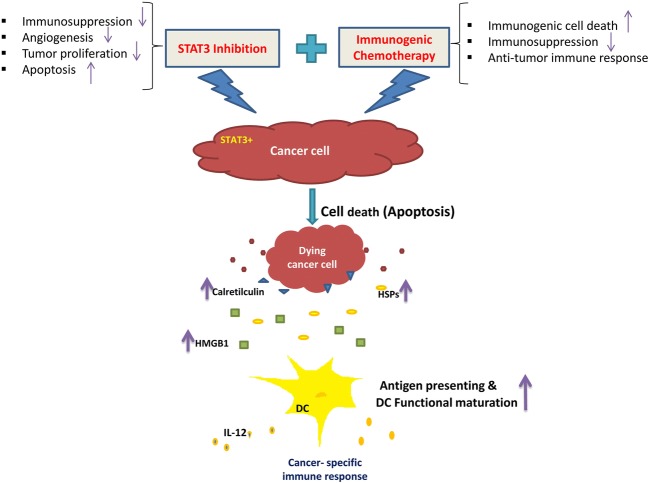
Abstract
Background
Induction of immunogenic cell death (ICD) is considered a promising strategy for cancer immunotherapy. Stattic is an inhibitor of STAT3, which is found constitutively active in many cancers and plays a major role in cancer progression.
Objectives
In the present study, we proposed to evaluate whether stattic can enhance the effects of chemotherapy in the induction of ICD in cancer cells harboring hyperactive STAT3.
Methods
The growth inhibitory effects of stattic and chemo agents including doxorubicin (DOX) and oxaliplatin (OXP) were evaluated using MTT assay in B16F10 and CT26 cell lines. Flow cytometry was applied to study cell apoptosis and calreticulin (CRT) surface exposure. The levels of high mobility group box 1 (HGMB1), heat shock protein70 (HSP70) and interleukin-12 (IL-12) were measured using ELISA.
Results
Treatment of B16F10 and CT26 cells with stattic in combination with DOX resulted in synergistic antitumor effects with combination index being 0.82 and 0.87, respectively. Interestingly, we found a higher level of ICD markers including CRT expression as well as HMGB1 and HSP70 secretion in the cells received combination therapy of stattic and DOX as compared with monotherapies. Moreover, exposure of dendritic cells (DCs) to conditioned media (CM) from cancer cells treated with stattic and/or DOX resulted in secretion of IL-12, which is an indicator of DCs maturation and induction of Th1 response. OXP and stattic monotherapy induced ICD in CT26 cells and stimulated IL-12 secretion by DCs; however, we did not observe a significant increase in the level of ICD in CT26 cells and IL-12 secretion by DCs when CT26 cells were treated with stattic and OXP combination as compared with monotherapy groups.
Conclusion
These findings indicate that STAT3 inhibitory stattic can increase ICD induced by DOX.
Graphical abstract
Keywords: Immunotherapy, Chemotherapy, Combination therapy, STAT3, Melanoma, Colon cancer
Introduction
Immunotherapy is one of the important strategies for cancer treatment. Despite advances in cancer immunotherapy over the last two decades, most of the developed therapeutic vaccines for cancer have not been moved from research laboratory into clinic due to poor therapeutic efficacy [1]. The poor clinical outcome of cancer vaccines, at least partly, has been attributed to immunosuppressive tumor microenvironment (TME), which suppresses the trafficking and function of activated immune cells [2]. Thus, modulation of immunosuppression in tumor microenvironment is considered a promising approach to enhance immunotherapy by cancer vaccines [3]. Previous studies have shown that the induction of immunogenic cell death (ICD) in cancer cells results in secretion of immunogenic signals which can activate dendritic cells (DCs) and change the immunosuppression in tumor [4, 5]. These studies suggest that killing cancer cells by anticancer agents with the ability of ICD induction can be a promising strategy for overcoming immunosuppression in TME [3].
ICD is a functionally distinct type of regulated cell death characterized by the secretion and exposure of immunogenic signals in dead tumor cells. The Immunogenic signals are known as damage-associated molecular patterns (DAMPs) [6, 7]. One of the main type of DAMPs includes surface-exposed calreticulin (CRT) produced during endoplasmic reticulum stress and pre-apoptotic death phase [5]. CRT, known as ‘eat-me’ signal, is a phagocytic signal, which promotes phagocytes to engulf dying tumor cells [4, 8]. Another immunogenic signal released in the autophagic stress by dying tumor cells, is adenosine triphosphate (ATP). ATP motivates purinergic receptors to attract macrophages and DCs into the tumor site [5, 9]. Non-histone chromatin binding protein high mobility group box 1 (HMGB1) (the late cell apoptosis signal) and heat shock proteins (HSP70 and HSP90) are other important types of DAMPs, which are released from dead tumor cells into the extracellular space. HMGB1 and HSP70 activate DCs by binding to toll-like receptor 4 (TLR4) and induce cancer specific T cell responses [10, 11]. The binding of HMGB1 and HSP70 to TLR4 stimulates Th1 type inflammatory responses, which in turn, can reverse tumor immunosuppressive microenvironment leading to eradication of cancer cells by tumor specific cytotoxic T cells [3, 12].
Previous studies have reported that radiotherapy and a number of the chemotherapeutic agents (such as doxorubicin and oxaliplatin) can induce ICD and stimulate immune responses against tumor cells in vitro and in vivo [13, 14]. Stattic is a nonpeptidic small molecule exhibited to selectively inhibit signal transducer and activator of transcription factor 3 (STAT3) through blocking the function of its SH2 domain [15]. STAT3 is an oncogenic protein, which is found constitutively active in many types of human malignancies including melanoma and colon cancer and plays a key role in their progression [16, 17]. Constitutively active STAT3 promotes cancer cells survival and proliferation, tumor angiogenesis, metastasis, and cancer-induced immunosuppression [18, 19]. Tumors harboring hyperactive STAT3 release a high level of immunosuppressive factors, which greatly contribute to the establishment of immunosuppression in TME. Besides, STAT3 as an oncogene is the negative regulator of Th1 immune responses and the main transcription factor involved in differentiation of Th17 cells which can be altered into regulatory T cells and promote tumor-associated immunosuppression [20]. Therefore, blocking STAT3 in tumor and tumor-associated immune cells has been suggested as a helpful strategy for effective immunotherapy of cancer [21, 22]. While some in vivo studies suggest that STAT3 inhibition can contribute to the induction of ICD [23], to our knowledge, there is no direct evidence on induction of ICD by STAT3 inhibitors in cancer cells, in vitro. The purpose of the present investigation was to examine whether suppression of STAT3 is able to enhance ICD induced by chemotherapy. B16F10 melanoma and CT26 colon cancer cell lines, which express constitutively active form of STAT3 [24, 25], were selected to assess the effects of STAT3 suppression on induction of ICD in this study.
Materials and methods
Reagents
Oxaliplatin (OXP) was purchased from Sigma-Aldrich (USA) and doxorubicin hydrochloride (DOX) was purchased from Ontario Chemicals Inc. (Ontario, Canada). Stattic (cat.no. ab120952) was obtained from Abcam (Cambridge, UK). AnnexinV-FITC apoptosis detection kit and Mouse IL-12 p70 Ready Set Go ELISA kit (cat.no. 88712122) were provided from Invitrogen eBioscience (San Diego, CA, USA). Mouse monoclonal antibodies to phosphorylated STAT3 (p-STAT3, Tyr705) (cat. no. 651002) and STAT3 (cat. no. 678002) were purchased from Biolegend (San Diego, CA, USA). B-Actin antibody (cat. no. sc-47,778) and horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody (cat. no. sc-516,102) were provided from Santa Cruz (USA). Super Signal molecular weight protein Ladder was obtained from Thermo Fisher Scientific (Waltham, USA). Protease inhibitor cocktail was provided from Roche Diagnostics. Enhanced chemiluminescence (ECL) western blotting substrate was obtained from Thermo scientific (Rockford, USA). Phycoerythrin (PE)–conjugated CRT monoclonal antibody (cat.no. ADI-SPA-601PE-D) was ordered from Enzo Life Sciences (Farmingdale, NY, USA). The HMGB1 (cat.no. E0523Mo) and HSP70 (cat.no. E1752Mo) ELISA Kits were purchased from bioassay technology laboratory (Shanghai, China). RPMI 1640, penicillin/streptomycin, fetal bovine serum (FBS) and L-glutamin were purchased from GIBCO laboratories (Grand Island, NY, USA). Animal-free recombinant murine granulocyte-monocyte colony-stimulating factor (GM-CSF) was provided from Peprotech (Rocky Hill, NJ). MTT reagents were provided from Sigma.
Cell lines and mice
B16F10 (melanoma) and CT26 (colon cancer) cell lines were purchased from Pasteur Institute of Iran (Tehran, Iran). The cell lines were maintained in RPMI 1640 supplemented with 10% FBS and 100 U/ml penicillin/streptomycin and incubated at 37°C in a humidified atmosphere with 5% CO2. C57Bl/6 mice were also purchased from Pasteur Institute of Iran. 8- 12 weeks old female mice were applied for primary DC culture preparation. Animal experiments were done in accordance with the Tabriz Medical University care and use of laboratory animal's guidelines. Ethical approval for the use of animals and performing the defined procedure in this study was obtained from the ethics committee at Tabriz University of Medical Science (IR.TBZMED.REC.1396.396 ethical code).
Cytotoxicity assay
The in vitro cytotoxicity of DOX, OXP and stattic was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. B16F10 and CT26 cells (0.3 × 104) were seeded into 96-well plates and grown overnight. Then, the cells were treated with each compound or combination of two compounds at different concentrations. Untreated and dimethyl sulfoxide (DMSO)-treated cells were considered as control cells. After 48 h incubation, the cells were added with MTT solution (0.5 mg/mL) and the cells were incubated for further 4 h at 37 °C. Finally, the medium was removed, DMSO was added and the absorbance was read at 570 nm by a microplate reader (BioTek Instruments, Inc. USA). Dose-response curve and IC50 value (the half-maximal inhibitory concentration) for either agent were calculated by Graphpad prism software. The combination index (CI) values for each dose were determined using data obtained from MTT assays and CompuSyn software. According to Chou-Talalay method, quantitative definition CI > 1.1, CI = 0.9–1.1 and CI < 0.9 have respectively antagonistic, additive and synergistic effect in drug combination [26]. The concentrations of drugs for combination therapy experiments were selected based on IC50 value of each compound in the selected cell lines. We choose to use the drugs at the concentration lower than their IC50 to avoid losing a major part of the cells due to the cytotoxicity of the compounds at higher concentrations. Besides, combination therapy with two drugs at the selected concentrations was found to result in an optimal anticancer effect indicated with CI.
Western blotting analysis
To evaluate pSTAT3 inhibition in the cells treated with stattic, western blotting analysis was conducted. B16F10 and CT26 cells (4 × 105) were seeded into 100 mm dishes for 24 h and then treated with DOX (7 and 50 nM), OXP (0.75 μM) alone and/or in combination with stattic (1.5 and 1.7 μM) according to the previously selected combinational treatment procedure. After incubation for 48 h, the cells were harvested and lysed in RIPA lysis buffer containing protease inhibitor cocktail and incubated at 4 °C for 30 min, and then the lysates were centrifuged at 100×g and 4 °C for 10 min. Bradford method was used for calculation of samples total protein concentration. The prepared protein samples were separated on 10% polyacrylamide gel electrophoresis, and then transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with Tris-buffered saline containing skim milk (5%) and supplemented with Tween-20 (0.05% v/v), then incubated with primary (anti-STAT3, anti-pSTAT3 and anti-B-Actin) and HRP-conjugated secondary antibodies. The protein bands were visualized with enhanced chemiluminescence (ECL) detection kit.
Apoptosis assay
To evaluate the induction of apoptosis, B16F10 and CT26 cells (1 × 105) were seeded into 60 mm dishes and incubated for an overnight. The cells were then treated with DOX and OXP alone or in combination with stattic for 48 h. The supernatants and the trypsinized cells were collected following treatment and centrifuged at 80×g for 5 min to harvest cell pellets. The harvested cell pellets were stained with Annexin V-FITC apoptosis kit according to the manufacturer’s protocol. Finally, the stained samples were immediately analyzed using FACS Calibur flow cytometer (Becton Dickinson, Franklin Lake, NJ, and USA).
Analysis of CRT exposure on the cell surface
Flow cytometry was utilized to examine the level of CRT on the cell surface of untreated and treated cells. B16F10 and CT26 cells (1 × 105) were seeded in 60 mm dishes. After overnight incubation, the cells were treated with each chemotherapeutic agent alone or in combination with stattic for 48 h. The cells were then stained with PE-conjugated anti-CRT antibody (1:100) and analyzed with FACS Calibur flow cytometer (Becton Dickinson, Franklin Lake, NJ, and USA).
HMGB1 and HSP70 release assays
B16F10 and CT26 cell (1 × 105) were plated into 60 mm dishes and incubated overnight. Then, the cells were treated with each compound alone or in combination with each other and incubated for 48 h. Supernatants of untreated and treated cells were collected and analyzed for the level of HMGB1 and HSP70 levels by ELISA kits according to the manufacturer’s instructions. The minimum detection levels of HMGB1 and HSP70 were 0.55 and 0.017 ng/mL, respectively.
Preparation of murine bone marrow derived dendritic cells (BMDC)
DC primary cultures were prepared by the previously established method of 7 days culture from bone marrow precursors of C57BL/6 mice in complete media (RPMI-1640 containing 10% heat inactivated-FBS (HI-FBS), 100 U/ml penicillin-streptomycin and L-glutamine) supplemented with 20 ng/mL of GM-CSF [27]. Briefly, femur was removed and cleared from surrounding tissues. The intact bone was disinfected with 70% ethanol, washed with phosphate buffered-saline (PBS) and then the both end of bone were cut with scissors. The bone marrow flushed with PBS using an insulin syringe. The obtained leukocytes were filtrate with 40 μm cell strainer to collect single cell suspension. After washing with PBS, about 2 × 107 leukocytes were achieved per femur.
To generate BMDC, at day 0, leukocytes were seeded at 2 × 106 cells in 100 mm dishes containing 10 mL of 1:1 mixture of DC complete media (RPMI with 10% HI-FBS, penicillin-streptomycin,-L-glutamine and 20 ng/mL GM-CSF) and collected conditioned media (CM) from B16F10 and CT26 cells. At day 3, another 10 mL of DC complete media was exceeded. At day 6, 10 mL of the culture supernatant was replaced with 10 mL of 1: 1 mixture of fresh DC complete media and CM. At the day of 7, the supernatant were collected for detection of interleukin-12 (IL-12) secretion [27].
One group of leukocytes grown in DC complete media without CM were used as a negative control and another one treated at day 7 with 100 ng/mL of lipopolysaccharide (LPS), the supernatant was collected after 24 h treatment and used as a positive control.
To make CM from CT26 and B16F10 tumor cells, the cells were grown and treated with the defined monotherapies and combination therapies in 60 mm dishes for 48 h and then the supernatants were collected, centrifuged, and filtrate. Then supplemented with 20 ng/mL of GM-CSF to be used in the experiments.
Assessment of BMDC functional maturation characteristic by ELISA
Matured DCs were obtained according to the procedure described in previous section and their supernatants were collected to be assessed for the level of IL-12 p70 by commercially available ELISA kit according to manufacturer’s instructions. The minimum detection level of IL-12 was 15 pg/mL. It should be noted that the absorbance for IL-12 at 7.8 pg/mL concentration (half of 15.6 pg/mL) was also used in building standard curve (ranging from 7.8 to 2000 pg/mL) with R2 being 0.998.
Statistical analysis
In the present study, each experiment was repeated in triplicate and all data were represented by the mean ± standard deviation (SD). Statistical analysis was conducted using one-way ANOVA analysis of variance followed by Tukey’s post hoc tests for multiple comparisons. p < 0.05 is indicated by *, p < 0.01 is indicated by **, p < 0.001 is indicated by *** and p < 0.0001 is indicated by **** in the figures.
Results
Growth inhibitory effects of stattic and chemotherapy combinational treatment in cancer cells harboring hyperactive STAT3
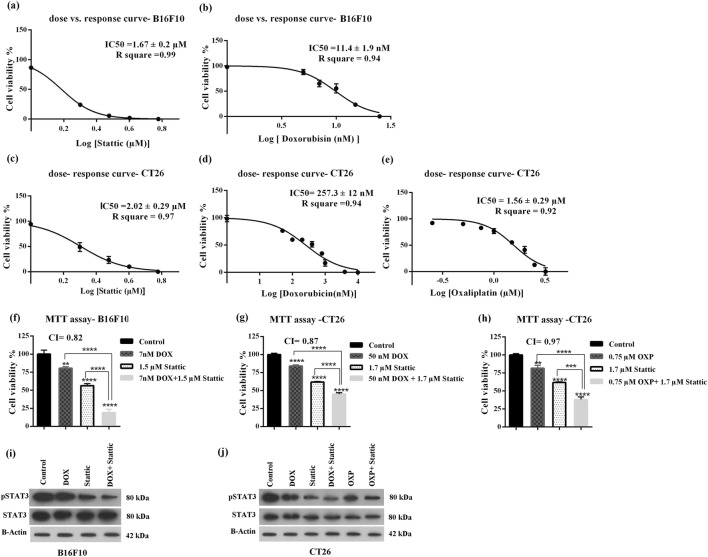
We first assessed the growth inhibitory effect of stattic and two chemotherapeutic agents (DOX and OXP) shown to induce ICD in cancer cells. Figure 1a, b show dose-response curves for the growth inhibitory effects of stattic and DOX in B16F10 melanoma cell, respectively. IC50 value for the growth inhibitory effects of stattic and DOX in B16F10 cells were found to be 1.67 ± 0.2 μM and 11.4 ± 1.9 nM, respectively. The dose-response curves for anticancer effects of stattic, DOX and OXP in CT26 cells are represented in Fig. 1c–e. OXP is the current standard frontline chemotherapeutic agent for treatment of colon cancer and CT26 is the model cancer cell line for this type of human malignancy. Stattic, DOX and OXP inhibited the growth of CT26 cells with IC50 being 2.02 ± 0.29 μM, 257.3 ± 12 μM and 1.56 ± 0.29 μM, respectively. Comparison of IC50 value of DOX in B16F10 cells with that of CT26 cells indicates that B16F10 cells are more sensitive to cytotoxic effect of DOX and consequently their viability can be further altered by slight changes of DOX concentration [28]. To study whether stattic can enhance the anticancer effects of the selected chemo agents (DOX, OXP) in B16F10 and CT26 cells, the cells were treated with stattic alone or in combination with either DOX or OXP. As depicted in Fig. 1f, treatment of B16F10 cells with stattic (1.5 μM) in combination with DOX (7 nM) for 48 h resulted in a significant reduction in the cell viability as compared with control and the cells treated with each agent alone. The CI value for stattic and DOX combinational therapy was 0.82 indicating synergistic anticancer effects of these two anticancer agents in B16F10 cells.
Fig. 1.
Dose-response curves of (a) stattic and (b) DOX in B16F10 cells, (c) stattic, (d) DOX and (e) OXP in CT26 cells. Dose-response curves were generated by GraphPad prism software to determine IC50 value of the drugs. Growth inhibitory effects of each monotherapies and combination therapies were obtained from MTT assay (f) DOX, stattic and DOX + stattic in B16F10 cell, (g) DOX, stattic and DOX + stattic and (h) OXP, stattic and OXP + stattic in CT26 cells. The data represent mean ± SD (n = 3). Representative western blotting analysis of p-STAT3, STAT3 and B-Actin in (i) B16F10 cells treated with DOX, stattic and DOX + stattic, (j) CT26 cells treated with DOX, stattic, OXP, DOX + stattic and OXP + Stattic. DOX, doxorubicin; OXP, oxaliplatin
In CT26 cell line, the CI value for stattic and DOX was 0.87 and the cell viability went down from 61.7 ± 0.7% in stattic treated cells and 83.96 ± 1.4% in DOX treated cells to 45 ± 1.6% in the cells treated with stattic (1.7 μM) in combination with DOX (50 nM) (Fig. 1g). As it was mentioned earlier, according to Chou-Talalay method, CI < 0.9 was defined as a synergetic inhibitory effect. The CI value for combination therapy with stattic (1.7 μM) and OXP (0.75 μM) was found to be CI = 0.97 (Fig. 1h), which demonstrates additive effect.
To confirm that stattic inhibits STAT3 activation, the level of active (phosphorylated) form of STAT3 (p-STAT3) were assessed by western blotting in both cell lines following 48 h treatment with mono- and combination therapies. As illustrated in Fig.1i,j, treatment of cancer cells with stattic alone or in combination with chemo agents, resulted in a remarkable decrease in the level of p-STAT3 in both cell lines as compared with control untreated group.
Induction of apoptosis in cancer cells treated with stattic and/or chemotherapeutic agents
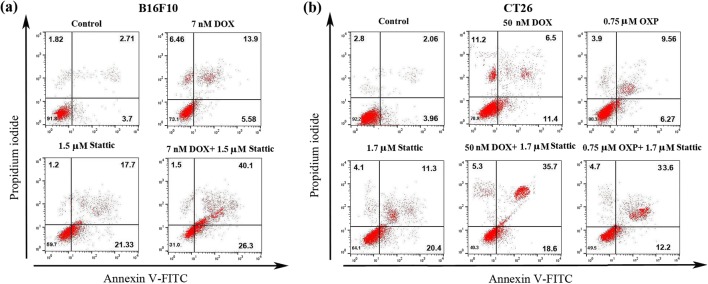
Apoptosis is generally considered to be non-immunogenic [11]. Nevertheless, it is well known that apoptosis can result in distinct biochemical subroutines under some circumstances and induce immune response. Indeed, ICD is considered to be a subtype of apoptosis [7, 12]. Therefore, we proposed to examine whether treatment of cancer cells harboring hyperactive STAT3, with stattic alone or in combination with chemotherapy can result in apoptosis. Figure 2 reveals that treatment of both cell lines with stattic alone or in combination with chemotherapy resulted in the induction of apoptosis in these cells. Of note, we found a small percentage of necrotic cells in B16F10 cells (6.4 ± 0.8%) and CT26 cells (11.2 ± 0.3%) treated with DOX alone (Fig. 2a). This observation is consistent with previous studies, indicating that DOX triggers necrotic death in tumor cells [28, 29].
Fig. 2.
Representative dot plot graph showing frequency of early and late apoptotic death after 48 h treatment of (a) B16F10 cells with DOX, stattic and DOX + stattic and (b) CT26 cells with DOX, OXP, stattic, DOX + stattic and OXP + stattic, as assessed by Annexin/PI staining and analyzed by flow cytometry. Each experiment was conducted in triplicate. DOX, doxorubicin; and OXP, oxaliplatin
Assessment of CRT cell surface expression in cancer cells treated with stattic and/or chemotherapeutic agents
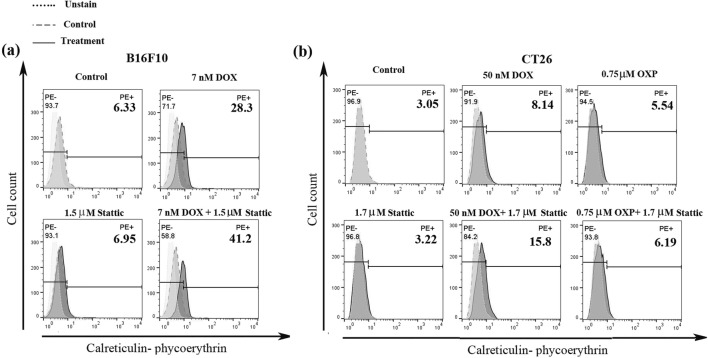
Exposure of CRT on the cell surface is one of the crucial hallmarks determining the immunogenicity of chemotherapeutics. Previous studies show that CRT is exposed on the cell surface during pre-apoptotic death phase and triggers tumor environment’s DCs to engulf dead tumor cells [4]. In the present study, to assess the potential of stattic to induce ICD or enhance the efficacy of chemotherapy in the induction of ICD, we determined cell surface expression of CRT. B16F10 and CT26 cells were treated with stattic and/or DOX for 48 h, then they were harvested and stained with PE-conjugated anti-CRT antibody. As it is shown in Fig. 3a, stattic alone (1.5 μM) caused a slight increase in the percentage of the cells expressing CRT (CRThigh cells). The percentage of CRThigh cells increased from 6.3% in control untreated cells to 28.3% in the cells treated with DOX alone. Interestingly, treatment of the cells with DOX (7 nM) in combination with stattic (1.5 μM) resulted in a remarkable increase in the percentage of CRThigh cells as compared with the cells treated with DOX alone (41.2% vs. 28.3%). In CT26 cells, the combinational therapy with stattic (1.7 μM) and DOX (50 nM) resulted in about 2 fold increase in CRT exposure compared to what observed in the cells treated with DOX alone (15.8% vs. 8.1%) (Fig. 3b).
Fig. 3.
Representative histogram of CRT surface exposure in (a) B16F10 cells treated with DOX, stattic and DOX + stattic, and (b) CT26 cell treated with DOX, OXP, stattic, DOX + stattic and OXP + stattic. Staining was assessed by PE-conjugated anti-CRT antibody and the experiments were analyzed by flow cytometry. The data represent one out of three independent experiments, which showed similar results. DOX, doxorubicin; OXP, oxaliplatin and PE, phycoerythrin
OXP is another chemo agent shown to induce ICD. Therefore, we proposed to examine the effects of OXP alone and in combination with stattic on induction of ICD in CT26 cells. CT26 cells were treated with stattic (1.7 μM) and/or OXP (0.75 μM) for 48 h and then they were evaluated for cell surface expression of CRT by flow cytometry. As shown in Fig. 3b, compared with control untreated cells, treatment of CT26 cells with either stattic or OXP resulted in a slight increase in the percentage of the cells expressing CRT (3.2% and 5.5%, respectively). The percentage of CRThigh cells increased to 6.1% in CT26 cells treated with OXP in combination with stattic.
Release of HGMB1 from cancer cells treated with stattic and/or chemotherapeutic agents
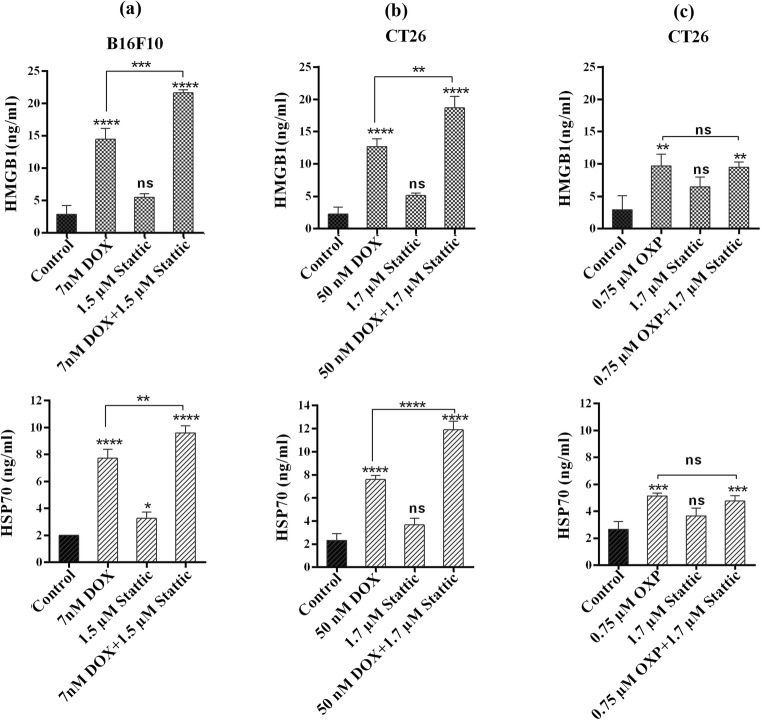
HMGB1 is a nuclear cytokine, which is shown to be released by the cells going through ICD. Previous studies have reported that HMGB1 activates T cell mediated immune responses [30]. Therefore, to examine the induction of ICD in cancer cells, we measured the level of HMGB1 in the supernatant of treated cells. As illustrated in Fig. 4a, the level of HMGB1 in the supernatant of B16F10 cells went up from 2.8 ± 1.08 ng/mL in control group to 14.4 ± 1.3 and 5.5 ± 0.9 ng/mL in DOX and stattic group, respectively. We observed a significantly higher level of HMGB1 in the supernatant of B16F10 cells treated with DOX in combination with stattic as compared with what we found in the cells treated with DOX alone (14.4 ± 1.3 vs. 21.6 ± 0.3 ng/mL) (p < 0.001). Likewise combinational treatment with DOX and stattic resulted in a significantly higher level of HMGB1 secretion in the supernatant of CT26 cell line as compared with DOX alone (12.7 ± 0.97 vs. 18.6 ± 1.4 ng/mL) (p < 0.01) (Fig. 4b). These findings indicate that stattic is able to enhance the efficacy of DOX in the induction of ICD in B16F10 and CT26 cells.
Fig. 4.
Assessment of HMGB1 and HSP70 release by ELISA following treatment with (a) DOX, stattic and DOX + stattic in B16F10, (b) DOX, stattic and DOX + stattic and (c) OXP, stattic and OXP + stattic in CT26 cells. The experiments were repeated in triplicate and data are indicated as the mean ± SD. DOX, doxorubicin; OXP; oxaliplatin, ns; not significant
In CT26 cell line, the release of HMGB1 significantly increased following the treatment of cells with OXP alone or in combination with stattic as compared with control group (Fig. 4c). However, we could not find a statistically significant difference in the level of HMGB1 between OXP treated cells and those treated with OXP in combination with stattic.
Release of HSP70 from cancer cells treated with stattic and/or chemotherapeutic agents
Prior studies show that HSP70 proteins are released into extracellular space in the cells undergoing ICD [10]. Therefore, we measured the level of HSP70 released from the cells treated with stattic and/or chemotherapeutic agents. As illustrated in Fig. 4a, b, similar to what we observed for the release of HMGB1, the level of HSP70 in the supernatant of the B16F10 and CT26 cells treated with stattic in combination with DOX, was significantly higher than what we found for the group treated with each drug alone. These results show that stattic can enhance the efficacy of DOX in the induction of ICD in B16F10 and CT26 cells. When we examined the level of HSP70 in CT26 cells following treatment with stattic and/or OXP, we found a significant increase in the level of HSP70 in the test groups treated with OXP alone and in combination with stattic as compared with untreated control group. However, the level of HSP70 in the cells treated with stattic and OXP was not significantly higher than that in the groups that received monotherapies (Fig. 4c).
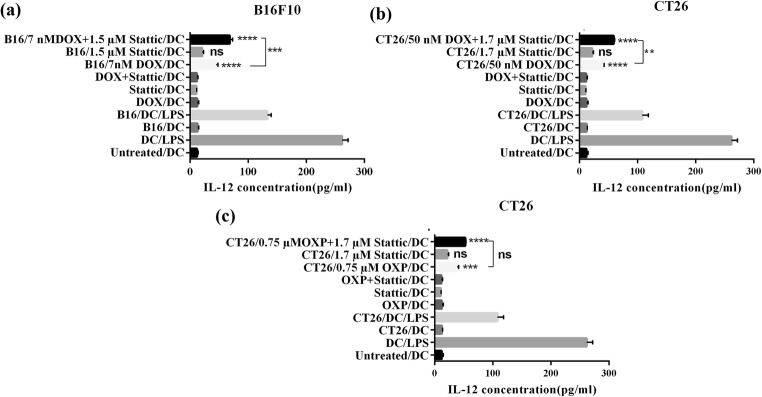
Secretion of IL-12 cytokine by BMDCs, which were exposed to the CM from cancer cells (B16F10 and CT26)
IL-12 cytokine is secreted by functionally mature DCs, which have encountered foreign antigen and danger signal. DCs producing IL-12 activate Th1 cells leading to induction of cell-mediated immune responses, which is the appropriate type of response against cancer. Indeed, secretion of IL-12 by DCs is an important indicator of their functional maturation as well as their ability to induce T cell-mediated immune responses [31, 32]. Therefore, in this research, in order to understand whether the enhanced level of ICD by stattic in cancer cells can potentially stimulate DC functional maturation and prime T cells response, the secretion of IL-12 by DCs was assessed. DCs were exposed to the collected CM from treated cancer cells and their supernatants were assessed after 7 days for secretion of IL-12 cytokine. As it was shown in Fig. 5a, b, the exposure of BMDCs to CM from cancer cells treated with DOX and/or stattic resulted in a significant increase in the level of IL-12 as compared to what observed in BMDCs exposed to CM of untreated cancer cells. Interestingly, in comparison to DCs exposed to CM of cancer cells received DOX and stattic monotherapy, DCs exposed to CM from cancer cells treated with DOX and stattic combination therapy, secreted significantly higher amount of IL-12. The level of IL-12 increased from 45 ± 2.4 pg/mL in BMDCs exposed to CM of B16F10 cells treated with DOX alone, to 68.4 ± 3.8 pg/mL in BMDCs exposed CM of B16F10 cells received DOX and stattic combination therapy (p < 0.001). Likewise in CT26 cells, the level of IL-12 secreted by BMDCs exposed to CM of the cells treated with stattic and DOX combination therapy, was significantly higher than that in BMDCs exposed to CM of the cells treated with DOX alone (59.3 ± 1.2 vs. 41.7 ± 0.8) (p < 0.01). With OXP and stattic treatment, the level of IL-12 significantly increased in BMDCs exposed to OXP and/or stattic as compared to control group exposed to CM from untreated CT26 cells. On the other hand, CM from CT26 cells treated with OXP and stattic combination therapy did not significantly increase the level of IL-12 in BMDCs as compared with monotherapy with each agent (Fig. 5c).
Fig. 5.
IL-12 secretion in DCs assessed by ELISA following the exposure to CM obtained from (a) B16F10 cells, (b) and (c) CT26 cells. Experiments were repeated in triplicate and data are indicated as the mean ± SD. DOX, doxorubicin; OXP, oxaliplatin; not significant, ns
These findings correlate well with what observed for the release of DAMPs in cancer cells treated with DOX and stattic combination therapy.
Discussion
Induction of ICD has attracted a great deal of attention as a promising strategy for reversing immunosuppression in TME, which is a major challenge for cancer immunotherapy [3]. In the present study, we proposed to examine whether an inhibitor of STAT3 (Stattic) can enhance the efficacy of chemo agents in the induction of ICD in cancer cells with hyperactive STAT3. A key finding of this research is that stattic significantly increases the induction of ICD by DOX, which is a widely used chemotherapeutic agent.
The reason behind the synergistic antitumor effect obtained by stattic in combination with DOX in B16F10 and CT26 cells, as compared with OXP in CT26 cells might be related to the difference in the mechanism of action of each chemo agent (DOX and OXP) alone and in combination with stattic in the selected cell lines. In line with our observations, previous studies have shown that STAT3 inhibitors (such as stattic) increase the direct cytotoxicity of anthracylines (DOX) in cancer cell lines, in vitro [33, 34].
Consistent with our findings, prior studies have shown that most of STAT3 inhibitory molecules including stattic have an ability to induce apoptosis in cancer cells [15, 35, 36]. We observed a small percentage of necrotic death in B16F10 and CT26 cells treated with DOX. This observation is similar to what reported in the previously published papers [28, 29]. Interestingly, the percentage of necrotic death in the DOX treated cells reduced when the cells were treated with DOX in combination with stattic. The ability of stattic in shifting the cell death from necrosis toward apoptosis is very beneficial in cancer immunotherapy settings such as ICD considered as a subtype of apoptosis, which can induce the right type of antitumor immune responses (cytotoxic T cell responses). In the support of this notion, previous studies found that necrotic cancer cells failed to induce effective cytotoxic T cell response against cancer [5, 12, 37].
Next, we found that STAT3 inhibitory stattic can improve the induction of ICD by DOX in cancer cells having constitutively active form of STAT3. DOX belongs to the first generation anthracyclines, which is well known for its immunogenic antitumor activity [38]. Consistent with previous reports, DOX significantly enhanced CRT cell surface expression and increased the release of HMGB1 and HSP70 in cancer cells [28, 39]. An important observation in this study was that stattic and DOX combinational treatment of B16F10 and CT26 cells resulted in a significant increase in CRT expression and the release of HMGB1 and HSP70 as compared with what we found in the cells treated with each agent alone. In line with our findings, Yang et al. have reported that targeted deletion of STAT3 improves therapeutic outcome of immunogenic chemotherapy (anthracyclines) by inducing the type-1 interferon production in immunocompetent mice [23]. The significance of anti-STAT3 and chemotherapy combinational treatment in the induction of ICD is marked by the previous studies showing that induction of ICD by anthracyclines significantly suppresses tumor growth in animal cancer models [23].
OXP is another chemotherapeutic agent which is found to induce ICD in several types of murine and human cell lines [5, 40]. In line with earlier reports, we observed that OXP induces CRT expression and the release of HMGB1 and HSP70 in a colon cancer cell line (CT26). However, addition of stattic to OXP treatment, did not significantly affect the level of CRT expression or HMGB1 and HSP70 release in CT26 cells compared to what we observed in DOX-treated cells. The better effects of stattic in enhancing the induction of ICD by DOX in cancer cells as compared with OXP, might be related to the difference in their potency for induction of ICD and the mechanisms by which each agent induces ICD. Our findings and several other reports show that DOX is more potent than OXP in induction ICD in cancer cells [6, 41, 42].
According to the previous studies, extracellular release of HMGB1 and HSP70 as a result of immunogenic chemotherapy induces DCs maturation through ligation of TLR4 leading to induction of Th1 type immune response [10]. Secretion of IL-12 by DCs is considered one of the main indicators of DC functional maturation and induction of cell mediated immunity [43]. In this research, we found that exposure of DCs to CM from cancer cells treated with stattic did not significantly induce concentration of IL-12 as compared to control group. On the other hand, the secretion of IL-12 was significantly increased in murine BMDC exposed to CM of cancer cells treated with DOX and stattic in comparison with what observed in DCs exposed to CM of the cells treated with DOX alone. These results suggest that DAMPs (such as CRT, HMGB1 and HSP70) released by cancer cells in response to combinational treatment are able to induce DCs functional maturation and have a potential to induce Th1 immune response. Correlating with what we observed for stattic and OXP combinational therapy in terms of the release of DAMPs by cancer cells, we did not observe significant changes in the level of IL-12 secreted by BMDCs exposed to the CM of cancer cells treated with stattic and OXP as compared with monotherapy and control groups.
Conclusion
In conclusion, we report that suppression of STAT3 activity significantly enhances the efficacy of DOX in induction of ICD in B16F10 and CT26 cancer cells. We also found that CM from these cancer cell lines treated with DOX and stattic combination therapy, induces DCs functional maturation and IL-12 secretion, which indicate the potential of such treatment for priming cell mediated immune responses. The significance of this observation is that DAMPs released from the cells undergoing ICD can modulate the immunosuppression in tumor, leading to induction of anticancer immune responses. Besides, due to the key role of STAT3 in cancer growth and tumor-induced immunosuppression, inhibition of STAT3 activity can greatly enhance the effects of chemotherapy in reversing the immunosuppression in tumor leading to the robust anticancer immune responses.
Acknowledgements
This study is part of a dissertation (No.128) by S.Jafari, submitted for Ph.D degree and supported financially by Biotechnology Research Center, Tabriz University of Medical Sciences.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Voena C, Chiarle R. Advances in cancer immunology and cancer immunotherapy. Discov Med. 2016;21(114):125–133. [PubMed] [Google Scholar]
- 2.Kalathil SG, Thanavala Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother. 2016;65(7):813–819. doi: 10.1007/s00262-016-1810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology. 2013;2(8):e25961. doi: 10.4161/onci.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini J-L, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 5.Radogna F, Diederich M. Stress-induced cellular responses in immunogenic cell death: implications for cancer immunotherapy. Biochem Pharmacol. 2018. [DOI] [PubMed]
- 6.Bezu L, Gomes-da-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, et al. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol. 2015;6:187. doi: 10.3389/fimmu.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20(5):504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15(9):1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 9.Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70(3):855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 10.Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y, et al. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell Mol Immunol. 2014;11(2):150–159. doi: 10.1038/cmi.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30(1):61–69. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 12.Montico B, Nigro A, Casolaro V, Dal CJ. Immunogenic apoptosis as a novel tool for anticancer vaccine development. Int J Mol Sci. 2018;19(2):594. doi: 10.3390/ijms19020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, et al. Trial watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4(4):e1008866. doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paolini A, Pasi F, Facoetti A, Mazzini G, Corbella F, Di Liberto R, et al. Cell death forms and HSP70 expression in U87 cells after ionizing radiation and/or chemotherapy. Anticancer Res. 2011;31(11):3727–3731. [PubMed] [Google Scholar]
- 15.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13(11):1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Yang CH, Fan M, Slominski AT, Yue J, Pfeffer LM. The role of constitutively activated STAT3 in B16 melanoma cells. Int J Interferon Cytokine Mediat Res. 2010;2010(2):1–7. doi: 10.2147/IJICMR.S6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7(6):545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Crowe PJ, Goldstein D, Yang J-L. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers. Int J Oncol. 2012;41(4):1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 19.Ilkovitch D, Lopez DM. Immune modulation by melanoma-derived factors. Exp Dermatol. 2008;17(12):977–985. doi: 10.1111/j.1600-0625.2008.00779.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Shen Y, Wang S, Shen Q, Zhou X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018;415:117–128. doi: 10.1016/j.canlet.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Kortylewski M, Pardoll D. Tumour immunology: crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 22.Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anti-Cancer Drugs. 2005;16(6):601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Yamazaki T, Pietrocola F, Zhou H, Zitvogel L, Ma Y et al. STAT3 inhibition enhances the therapeutic efficacy of immunogenic chemotherapy by stimulating type 1 interferon production by cancer cells. Cancer Res. 2015:canres. 1122.2015. [DOI] [PubMed]
- 24.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs. 2009;18(1):45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DS, Grandis JR, Johnson DE. STAT3 as a Major Contributor to Chemoresistance. Targeting Cell Survival Pathways to Enhance Response to Chemotherapy. Elsevier; 2019. p. 145–67.
- 26.Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. [DOI] [PubMed]
- 27.Garg SM, Vakili MR, Molavi O, Lavasanifar A. Self-associating poly (ethylene oxide)-block-poly (α-carboxyl-ε-caprolactone) drug conjugates for the delivery of STAT3 inhibitor JSI-124: potential application in Cancer immunotherapy. Mol Pharm. 2017;14(8):2570–2584. doi: 10.1021/acs.molpharmaceut.6b01119. [DOI] [PubMed] [Google Scholar]
- 28.K-j S, Ryung Choi K, Ryu C-K, Lee SJ, Kim HJ, Lee H. Induction of immunogenic cell death of tumors by newly synthesized heterocyclic quinone derivative. PLoS One. 2017;12(3):e0173121. doi: 10.1371/journal.pone.0173121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eom Y-W, Kim MA, Park SS, Goo MJ, Kwon HJ, Sohn S, et al. Two distinct modes of cell death induced by doxorubicin: apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene. 2005;24(30):4765–4777. doi: 10.1038/sj.onc.1208627. [DOI] [PubMed] [Google Scholar]
- 30.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 31.Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, Hiltbold EM. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol. 2008;181(12):8576–8584. doi: 10.4049/jimmunol.181.12.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karimi MH, Ebrahimnezhad S, Namayandeh M, Amirghofran Z. The effects of cichorium intybus extract on the maturation and activity of dendritic cells. DARU J Pharm Sci. 2014;22(1):28. doi: 10.1186/2008-2231-22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulesza DW, Carré T, Chouaib S, Kaminska B. Silencing of the transcription factor STAT3 sensitizes lung cancer cells to DNA damaging drugs, but not to TNFα-and NK cytotoxicity. Exp Cell Res. 2013;319(4):506–516. doi: 10.1016/j.yexcr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Ghorbani M, Sabzichi M, Ramezani F, Mohammadian J. Adjuvant therapy with stattic enriches the anti-proliferative effect of doxorubicin in human ZR-75-1 breast cancer cells via arresting cell cycle and inducing apoptosis. Biomed Pharmacother. 2019;109:1240–1248. doi: 10.1016/j.biopha.2018.10.183. [DOI] [PubMed] [Google Scholar]
- 35.Lin C-Y, Lee C-H, Huang C-C, Lee S-T, Guo H-R, Su S-B. Impact of high glucose on metastasis of colon cancer cells. World J Gastroenterol. 2015;21(7):2047–2057. doi: 10.3748/wjg.v21.i7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller AG, Sarker SD, Saleem IY, Hutcheon GA. Delivery of natural phenolic compounds for the potential treatment of lung cancer. DARU J Pharm Sci. 2019;1:433–449. doi: 10.1007/s40199-019-00267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. BBA-Rev Cancer. 2010;1805(1):53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011:canres. 0753.2011. [DOI] [PubMed]
- 39.Tongu M, Harashima N, Yamada T, Harada T, Harada M. Immunogenic chemotherapy with cyclophosphamide and doxorubicin against established murine carcinoma. Cancer Immunol Immunother. 2010;59(5):769–777. doi: 10.1007/s00262-009-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 41.Diederich M. Natural compound inducers of immunogenic cell death. Arch Pharm Res. 2019:1–17. [DOI] [PubMed]
- 42.Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019. [DOI] [PMC free article] [PubMed]
- 43.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3(4):3856–3893. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]