Abstract
We performed a retrospective cohort study in the Dutch patient population to identify active substances with a relatively high number of adverse drug reactions (ADRs) potentially related to drug switching. For this, we analyzed drug switches and reported ADRs related to switching between June 1, 2009, and December 31, 2016, for a selection of 20 active substances. We also compared pharmacovigilance analyses based on the absolute, switch‐corrected, and user‐corrected numbers of ADRs. In total, 1,348 reported ADRs and over 23.8 million drug switches were obtained from the National Health Care Institute in The Netherlands and from Lareb, which is The Netherlands Pharmacovigilance Centre. There was no correlation between the number of ADRs and the number of switches, but, on average, we found 5.7 reported ADRs per 100,000 switches. The number was relatively high for rivastigmine, levothyroxine, methylphenidate, and salbutamol, with 74.9, 50.9, 47.6, and 26.1 ADRs per 100,000 switches, respectively. When comparing analyses using the absolute number and the switch‐corrected number of ADRs, we demonstrate that different active substances would be identified as having a relatively high number of ADRs, and different time periods of increased numbers of ADRs would be observed. We also demonstrate similar results when using the user‐corrected number of ADRs instead of the switch‐corrected number of ADRs, allowing for a more feasible approach in pharmacovigilance practice. This study demonstrates that pharmacovigilance analyses of switch‐related ADRs leads to different results when the number of reported ADRs is corrected for the actual number of drug switches.
Generic drugs are highly similar to their brand‐name counterparts. Generic drugs comprise identical active substance(s), have the same pharmaceutical form, and have been shown to be bioequivalent to the brand‐name drug. Limits for bioequivalence are set to achieve similar clinical safety and efficacy, and if bioequivalence has been demonstrated, then branded and generic drugs are considered therapeutically equivalent.1 From a pharmacological perspective, it is, therefore, unexpected that a different clinical profile is observed for a generic drug as compared with a brand‐name drug, and that an adverse drug reaction (ADR) is experienced following a switch between bioequivalent drug products. However, cases of ADRs following drug switches are published;2, 3, 4 some studies on switching demonstrate increased health care utilization,5 and patients’ and prescribers’ perceptions of generics, and generic switches are not always positive.6, 7, 8, 9, 10 A need exists to systematically study the perceived problems and possibly elucidate points for improvement on the pharmacological and psychological assumptions of generic interchangeability.
Lareb, The Netherlands Pharmacovigilance Centre, is responsible for pharmacovigilance signal detection in The Netherlands and regularly receives ADR reports related to generic switching. In 2017, Lareb published a report on these ADRs for active substances with at least 25 reported switch‐related ADRs over a period of 10 years.11 Based on observed patterns for the absolute number of reported ADRs and a clinical review of each case, Lareb identified problems following switching: dysregulation of patients after switches of the thyroid hormone levothyroxine; breakthrough bleeding on oral contraceptives with ethinylestradiol and levonorgestrel; reduced efficacy with inhalation drugs for the treatment of asthma; salbutamol and fluticasone/salmeterol, skin reactions and curling of patches with rivastigmine; injection site pain and injection site reactions with methotrexate; and reduced efficacy with anti‐epileptics.
However, as acknowledged by the authors, and as is often the case for pharmacovigilance research, studying the absolute numbers of ADRs is a classic example of a “floating numerator”; that is, the number of cases is not related to the size of the population at risk. The relative risk may be low for drugs that are often switched, but could be high for drugs that are switched less often. Furthermore, we expect that there is a background rate of reported ADRs per number of drug switches. The background rate is difficult to discern without knowledge of the number of drug switches, specifically because we know from previous work that the number of switches fluctuates on a monthly basis (P.J. Glerum, M. Maliepaard, V. de Valk, D.M. Burger, C. Neef, unpublished data).
The primary aim of the current investigation is to calculate the relative number of reported ADRs per number of drug switches for the 20 active substances that were described in the Lareb report, in the time period between June 1, 2009, and December 31, 2016. (Temporary) increased numbers of reported ADRs (“peaks”) above the background level are of particular interest, as these could indicate a true increased incidence of clinical discomfort following a drug switch. Further investigation of such a relative peak should then be performed to determine whether a causal association between a specific drug switch and the experienced ADRs can be deduced.
In addition, we examine whether different peaks would be observed using the absolute number of ADRs or using the switch‐corrected number of ADRs. We also explore the feasibility of using the number of users, instead of drug switches, for correcting the absolute number of reported ADRs, because these data are more readily available.
METHODS
This is a retrospective cohort study of a selection of 20 active substances in the Dutch patient population, with qualitative and quantitative descriptive analyses. We related the number of reported ADRs obtained from the database of the Lareb to the drug switches from the National Health Care Institute (ZIN).
Active substance selection was based on a Lareb report from 2017, in which drug switch‐related ADRs were described for active substances with > 25 ADRs in a 10‐year period from 2006. The following active substances (20) are included: atorvastatin, enalapril, esomeprazole, ethinylestradiol/levonorgestrel, irbesartan, levothyroxine, losartan, metformin, methotrexate, methylphenidate, metoprolol, omeprazole, pantoprazole, paroxetine, perindopril, rivastigmine, salbutamol, salmeterol/fluticasone, simvastatin, and venlafaxine.
Patient switch data
We previously performed an extensive analysis of the drug switch data for the 20 drugs (P.J. Glerum, unpublished data). A switch is defined as the replacement of a patient’s prescribed drug with a similar drug dispensed within the preceding 150 days, for the same active substance, same strength, and same route of administration, but with the drug product coming from a different manufacturer. The drug products before and after the switch could be both generic or brand‐name drugs. In the ZIN data, analysis of consecutive dispenses is feasible from January 1, 2009, onward, thus we have reliable drug switch data from June 1, 2009, until December 31, 2016. Furthermore, we collected data on the total number of users of the 20 active substances from the same data source.
Reported ADR data
We obtained all spontaneously reported ADRs received by Lareb over the years 2009–2016, submitted by patients and healthcare professionals, both directly to Lareb and via marketing authorization holders. Only ADR reports that, according to the reporter, were highly related to drug switching or drug substitution were included. For this purpose, reports categorized under a specific subset of the Medical Dictionary for Regulatory Activities (MedDRA) Lowest‐Level Terms (LLTs) were included (Table S1 ). For each reported ADR, we obtained the unique anonymized identification number, date of onset of the ADR, report‐received date, MedDRA LLT, Anatomical Therapeutic Chemical (ATC) classification system code before switch, ATC code after switch, and sex and age of the patient.
The date of onset of the ADR was deemed most relevant to our study; however, this was not registered in 12.9% (375/2,901) of the reports. In these cases, the report‐received date was used, minus the median difference between the receive date and the date of onset of all reported ADRs (53 days). We excluded 5.3% (155/2,901) of the reports, whose date of onset of the ADR was not between June 1, 2009, and December 31, 2016. Furthermore, the interest of our study was drug switching between two products of the same active substance. We, therefore, excluded 5.2% (144/2,746) of the ADRs in which the registered ATC code before and after switch did not match. The final number of suitable ADRs was 2,602. We did not exclude reports in which the ATC code before the switch was not registered, which was the case in 33.7% (878/2,602) of the reports.
Data analysis
Aggregated data were exported from ZIN and Lareb databases as a Microsoft Excel workbook and imported into R software (version 3.5.1) using the package “xlsx.” Qualitative and quantitative descriptive analyses and data visualization were performed using base R and R Studio.
Descriptive analyses of reported ADR data were performed and presented per anatomic subgroup of the ATC classification system and for each of the 20 selected drugs. Both the patient switch data and the ADR data were aggregated on a quarterly (3‐month) basis, as this is a common time frame for analyses in pharmacovigilance. In the absence of switch data, ADR data before July 2009 were not included in the quarterly analyses.
The numbers of reported ADRs per number of switches were calculated by dividing the quarterly number of ADRs for each active substance by the quarterly number of switches for that active substance. Analogously, numbers of reported ADRs per number of users were calculated by dividing the quarterly number of ADRs by the number of users.
In this study, we define a peak in the number of ADRs as the value exceeding a threshold. This threshold was subjectively defined as the mean value of the number of ADRs in all quarters for all included active substances, plus one times its SD.
RESULTS
Number of reported ADRs
Our analyses included a total of 2,602 reported ADRs related to drug switching during the study period June 1, 2009, until December 31, 2016. Of the included ADRs, 63.9% were reported for female patients, and the mean age of the patients was 53.1 years (range 0–95 years). In Table S2 an overview is presented of the number of ADRs per ATC anatomic main group. Differences between anatomic groups are observed, with, for example, a large number of reported ADRs for the nervous system subgroup (n = 595) and a small number for dermatological drugs (n = 13). However, as the number of switches per active substance is unknown, no inferences can be made with regard to the relative risk per anatomic group.
In Figure 1 the data are illustrated graphically, with each shaded band indicating one specific active substance. From this figure, large differences can be observed regarding the relative contribution of active substances within each anatomic group, for example, a major contribution of levothyroxine in the subgroup “systemic hormonal preparations” (H) (95% (360/379) of the total number of switches in the ATC group) and of omeprazole (41.3% (124/300)) in the “alimentary tract and metabolism” subgroup (A). The 20 active substances included in our further analyses represent 51.8% (1,348/2,602) of all reported ADRs in our data set; they are marked in Figure 1. Table 1 provides an overview of both the number of reported ADRs and the number of drug switches of the included active substances.
Figure 1.
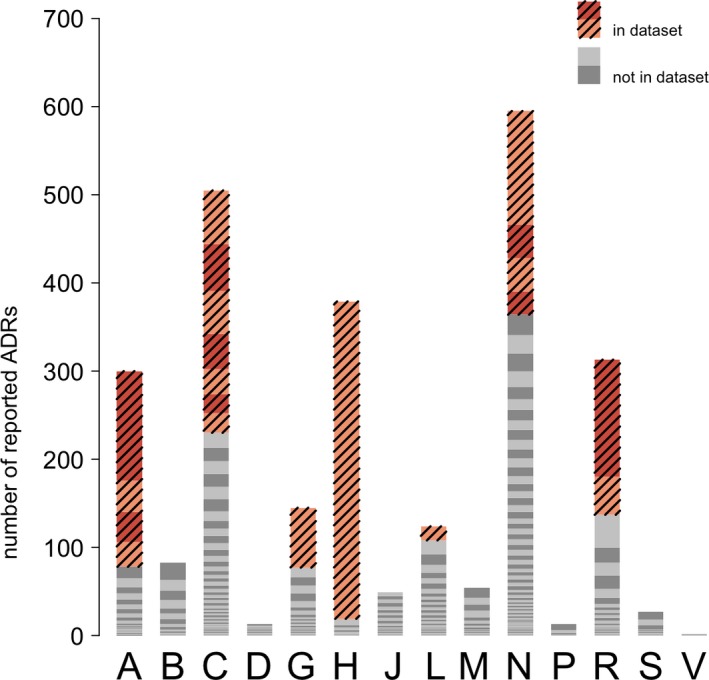
Overview of all spontaneously reported adverse drug reactions (ADRs) related to drug switching, for all active substances, from June 2009 to December 2016 in The Netherlands, as obtained from Lareb. Each bar is a sum of all reports in a specific anatomic group. Each individual color represents the total number of reported ADRs for one specific Anatomical Therapeutic Chemical code. Colored and striped parts indicate the 20 products selected for further study.
Table 1.
Overview of each specific active substance included in the analysis, including INN, European brand name, total number of reported ADRs related to drug‐switching, and total number of switches for these active substances, for the period June 2009 to December 2016 in The Netherlands
| INN | EU brand name | Total number of reported ADRs | Total number of switches |
|---|---|---|---|
| Levothyroxine | Thyrax Duotab | 360 | 507,396 |
| Salbutamol | Ventolin | 133 | 493,270 |
| Methylphenidate | Ritalin | 129 | 223,496 |
| Omeprazole | Losec | 124 | 2,742,659 |
| Ethinylestradiol/levonorgestrel | Microgynon | 68 | 483,996 |
| Atorvastatin | Lipitor | 61 | 2,007,886 |
| Metoprolol | Lopresor | 53 | 2,723,408 |
| Simvastatin | Zocor | 49 | 4,241,382 |
| Salmeterol/fluticasone | Seretide | 43 | 793,693 |
| Irbesartan | Aprovel | 40 | 446,312 |
| Paroxetine | Seroxat | 38 | 853,542 |
| Rivastigmine | Exelon | 38 | 30,722 |
| Pantoprazole | Pantozol | 36 | 2,192,881 |
| Metformin | Glucophage | 34 | 1,915,225 |
| Esomeprazole | Nexium | 28 | 716,320 |
| Losartan | Cozaar | 28 | 902,258 |
| Venlafaxine | Efexor | 26 | 422,532 |
| Enalapril | Renitec | 22 | 776,436 |
| Perindopril | Coversyl | 22 | 1,222,092 |
| Methotrexate | Metoject | 16 | 161,603 |
| Total | 1,348 | 23,857,109 |
ADRs, adverse drug reactions; INN, international nonproprietary name.
Relative number of reported ADRs
To examine the relation between the number of reported ADRs and the number of drug switches, the quarterly number of ADRs and quarterly number of switches for the 20 selected active substances are depicted in Figure 2. The mean number at which ADRs are reported for all active substances in our selection is 5.7 per 100,000 switches. However, no linear correlation between the number of ADRs and the number of switches was identified, and a high variability in the number of ADRs reported per quarter is observed.
Figure 2.
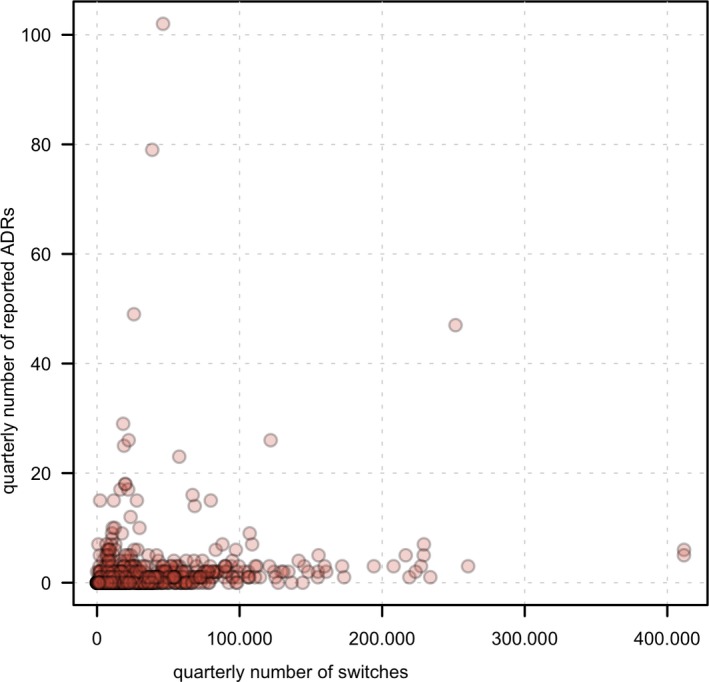
Plot of the absolute number of reported adverse drug reactions (ADRs) related to drug switching and number of switches in The Netherlands for all included active substances (n = 20) in the data set. Each data point is the number of reported ADRs and number of switches for one specific quarter year.
In Figure 3, the mean quarterly reported number of ADRs and mean quarterly number of switch‐corrected ADRs are compared for all 20 active substances in the data set. Figure 3 illustrates that there is a cluster of active substances with a similar, relatively low quarterly number of ADRs per 100,000 switches. In contrast, the following four active substances demonstrate notably higher numbers: rivastigmine (74.9 ± 188 per 100,000 switches, mean ± SD), levothyroxine (50.9 ± 64.2), methylphenidate (47.6 ± 37.1), and salbutamol (26.1 ± 45.2). The mean relative risk of reporting an ADR following a rivastigmine switch in our study period, therefore, seems 65‐fold higher, compared with a simvastatin product switch (i.e., 74.9 vs. 1.15 reported ADRs per 100,000 switches).
Figure 3.
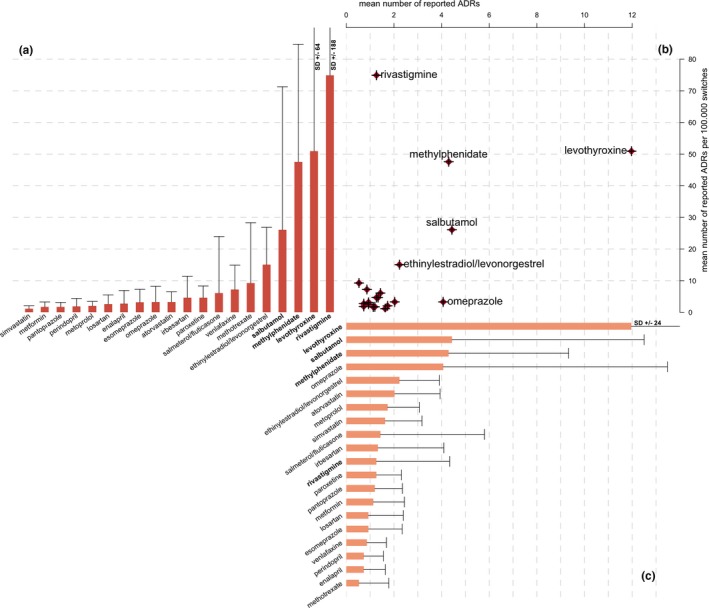
Comparison between mean number of quarterly reported adverse drug reactions (ADRs) and mean switch‐corrected number of quarterly ADRs, with bar chart (a) of mean switch‐corrected number of quarterly reported ADRs related to drug switching per 100,000 switches; plot (b) of each active substance, with the mean absolute number of quarterly reported ADRs on the x‐axis and the mean of switch‐corrected number of quarterly reported ADRs per 100,000 switches on the y‐axis; and bar chart (c) of mean absolute number of quarterly reported ADRs, error bars indicate quarterly SD.
Comparison between relative number and absolute number of reported ADRs
Figure 3 a,c depict the ranking of the active substances based on both mean number and switch‐corrected number of ADRs. The ranking order of these two measures does not fully overlap. More specifically, for rivastigmine, for example, the mean absolute number of ADRs (1.27 ± 3.07) is relatively low, whereas the mean number of switch‐corrected ADRs is relatively high (74.9 ± 188 per 100,000 switches). For omeprazole, it is the opposite: a relatively high mean number of ADRs (4.07 ± 9.41) and a relatively low mean number of switch‐corrected ADRs (3.28 ± 4.97 per 100,000 switches) exist. For levothyroxine, salbutamol, and methylphenidate, both the absolute number and switch‐corrected number of ADRs are relatively high.
Large SDs on the mean values are observed. This indicates that large differences can be observed between different quarters. Studying mean values ignores the fluctuation in the data, which are essential in the (real‐time) detection of temporal increases. Figure 4 presents an example of the study over time for the number of reported ADRs (absolute and switch‐corrected) related to esomeprazole. The largest absolute number of ADRs was observed in the first quarter of 2011 (six reported ADRs), and it coincides with the largest number of quarterly switches (83,254). As a result, the number of switch‐corrected ADRs in the first quarter of 2011 (7.21 per 100,000 switches) is lower than the number of switch‐corrected ADRs observed in the last quarter of both 2010 and 2012, the second quarter of 2015, and the first quarter of 2016 (with 8.68, 12.0, 11.96, and 12.65 reported ADRs per 100,000 switches, respectively), all representing quarters for which a lower absolute number of ADRs were reported (i.e., 2 or 3). This exemplifies that, when corrected for the number of switches, a peak in the absolute number of ADRs does not necessarily translate into the most relevant peak in ADRs. Similarly, a lower absolute number of ADRs could be more relevant if the number of switches in a quarter is relatively low.
Figure 4.
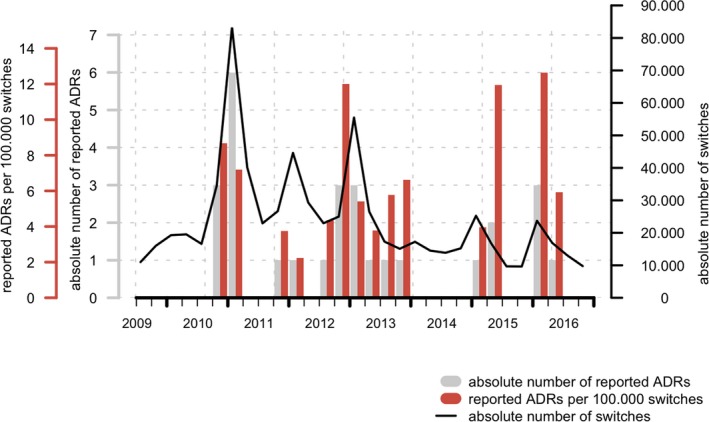
Number of quarterly switches (black line), absolute number of reported adverse drug reactions (ADRs; gray bar plot) and quarterly number of switch‐corrected reported ADRs (per 100,000 switches, color bar plot) for esomeprazole in The Netherlands.
To further examine the question as to whether different peaks would be observed using the absolute number of ADRs vs. the switch‐corrected number of ADRs, the absolute number of quarterly ADRs was plotted against the switch‐corrected number of ADRs for all 20 included active substances (Figure 5 a). This allows for a visual assessment of the difference between the two methods of “peak detection.” The figure illustrates a number of data points clustered at the lower values of both axes and a number of data points deviating from that cluster. To interpret the results, we arbitrarily defined a threshold above which a data point is considered to be a peak or a part thereof.
Figure 5.
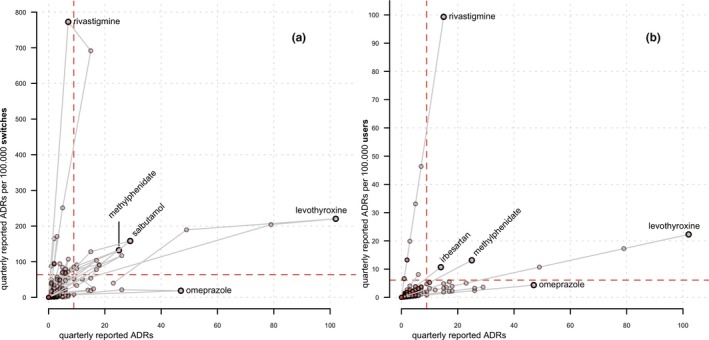
(a) Comparison between switch‐corrected, and user‐corrected quarterly reported adverse drug reactions (ADRs), with a plot (a) of the absolute number of quarterly reported ADRs per active substance on the x‐axis and number of switch‐corrected quarterly reported ADRs per 100,000 switches on the y‐axis; and a plot of (b) of absolute number of quarterly reported ADRs per active substance on the x‐axis and number of user‐corrected quarterly reported ADRs per 100,000 users on the y‐axis; gray lines connect values for quarters of a single active substance in chronological order, and dashed lines are the threshold values, defined as the mean number of the absolute, switch‐corrected, or user‐corrected reported ADRs + 1 SD. The total number of quarterly data points per active substance is 30.
Data are available for 30 quarter years for each individual active substance, amounting to 600 quarters. On the one hand, based on the absolute number of ADRs, 4.3% (26/600) of the quarters would be identified as a peak. On the other hand, based on the switch‐corrected ADRs, 5.7% (34/600) of the quarters would be identified as a peak. Furthermore, 2.5% (15/600) of the quarters would be identified by both methods, and 1.8% (11/600) of the peaks that were identified based on the absolute number of ADRs was not identified by the switch‐corrected ADR method. The latter 1.8% could be characterized as false positives of the absolute ADR method, or a “type 1” error, as these peaks would erroneously be identified as peaks when, in fact, they are explained by an increased number of switches. Similarly, 3.2% (19/600) of the ADR peaks would only be identified with the switch‐corrected ADR method and not by the absolute number of ADRs method. These could be defined as “type 2” errors or false negatives of the absolute number of ADRs method.
In Figure 5 a, the reported ADR peaks for the following five active substances visually stand out: rivastigmine, methylphenidate, levothyroxine, omeprazole, and salbutamol. For rivastigmine, a large switch‐corrected ADR peak is observed for 7 of 30 quarters, but only 1 of these 7 quarters (14.3%) is above the threshold for the absolute number of ADRs. Likewise, for methylphenidate, 26.7% (8/30) of the quarters is above the switch‐corrected ADR threshold but not above the threshold for the absolute number of ADRs. These data indicate false negatives in the absolute number of ADRs method.
For levothyroxine, 9 of 30 quarters are above the threshold for the absolute number of ADRs, of which 55.6% (5/9) is not identified by the switch‐corrected ADR method, thus indicating false positives in the absolute number of ADRs method. Likewise, for omeprazole, 10% (3/30) of the quarters is above the threshold for the absolute number of ADRs; however, none of these three were identified by the switch‐corrected ADR method, again indicating false positives for the absolute number of ADRs method. For salbutamol, no false positives or negatives are identified, as the same five quarters are identified by both methods.
User‐adjusted analysis
Data on the absolute number of drug switches are only available in The Netherlands in retrospect. This may hinder the use of the number of switches during real‐time pharmacovigilance analyses. A more practical approach to put the number of ADRs into perspective would, therefore, be to use the number of users instead of the number of switches.
The absolute number of quarterly ADRs and the user‐corrected number of quarterly ADRs are depicted in Figure 5 b. Comparing Figure 5 a,b, similar trends are observed for the values of rivastigmine, levothyroxine, methylphenidate, and omeprazole. Table 2 provides a detailed comparison of the number of ADRs above and below the threshold using either switch‐corrected or user‐corrected ADRs, as compared with the outcome for the absolute number of ADRs method, and it shows a similar trend using both methods.
Table 2.
Comparison between percentages of quarters above or below the threshold of either the switch‐corrected or user‐corrected reported ADR methods for the 20 selected active substances in this study
| Absolute number of reported ADRs below threshold | Absolute number of reported ADRs above threshold | |||
|---|---|---|---|---|
| Switch adjusted | User adjusted | Switch adjusted | User adjusted | |
| Reported ADR number above threshold | 3.17% (19/600) | 1.50% (9/600) | 2.50% (15/600) | 1% (6/600) |
| Reported ADR number below threshold | 92.5% (555/600) | 94.2% (565/600) | 1.83% (11/600) | 3.33% (20/600) |
ADRs, adverse drug reactions.
False negatives are marked in light gray, and false positives are marked dark gray.
Although numerical differences can be observed with respect to the number of false positives and false negatives between both methods, the overall impression is that both methods result in similar findings regarding the number of active substances with identified peaks and the percentage of quarters with values above or below the threshold. Similarity in the outcome of the switch‐corrected and user‐corrected ADR methods depends on the correlation between the quarterly number of users and the quarterly number of switches. Figure S1 is a depiction of the correlation and there is a linear correlation (R 2 = 0.72) with a significant slope (P = 2.48*10−6). On average, ~ 91 quarterly switches per 1,000 users are observed for the 20 active substances included in our analysis.
DISCUSSION
Pharmacovigilance is a useful tool in the identification of clinical consequences of (generic) drug switching. Given the importance for each individual patient, every reported ADR should always undergo thorough causal and clinical review.
We combined the number of drug switches and the number of related ADRs for a period of 7.5 years between June 1, 2009, and December 31, 2016. We demonstrated that, in this period, rivastigmine, levothyroxine, methylphenidate, and salbutamol have a relatively high number of switch‐related ADRs. Without switching data, the potential exists to draw false positive (noted, e.g., for omeprazole) or false negative (noted, e.g., for rivastigmine) conclusions. We also revealed that, without switching data, different active substances in our selection of 20 would be identified as having a high prevalence of switch‐related ADRs. The use of switch‐corrected ADR data should be considered in pharmacovigilance analyses to avoid misinterpretation of switch‐related ADR signals.
This is the first systematic, nationwide analysis of clinical discomfort from drug switches, using reported ADRs and the specific population at risk. Others have used indirect measures of discomfort, such as switchback rates.12 In a study on temporal trends in ADR reporting before and after generic introduction, the peak reporting numbers for five anti‐epileptic active substances were calculated as being in the range of 50–450 per 100,000 dispensed prescriptions.13 In that study, ADRs were not selected on specific LLTs and are expressed by number of prescriptions, which makes it difficult to compare with our study. In a recent analysis of a large switch in France between two levothyroxine drug products, an ADR reporting rate of 1.44% is mentioned.14 This is based on an estimated 2.1 million patients switching and 31,411 reported ADRs over a period of 13 months. The relative number of 1,440 of 100,000 switches in 13 months is higher than in our study, as we found a peak of 220 ADRs per 100,000 switches in 3 months for levothyroxine. The authors postulate a causal relation between the high number of reported ADRs and the proportion of subjects outside the bioequivalence limits, as found in a pharmacokinetic study. This relation is heavily debated.15, 16, 17, 18 In a similar situation for levothyroxine in 2007 and 2008 in New Zealand, the increased number of reported ADRs was attributed to other factors, such as inaccurate information and media attention.19 Others have postulated an analytical framework that is comparable to our approach, and this confirms the potential of combining spontaneously reported ADRs and health care claims data for the detection of generic issues.20
In our data set, we did not identify a correlation between the quarterly number of drug switches and the quarterly number of reported ADRs using data for all 20 active substances. This finding suggests that the increased number of ADRs is not merely a result of an increased number of switches. Thus, in many cases, the rate of reported ADRs indeed does change (temporarily), which may be an indication of an issue related to drug switches for those active substances. The reason for an increased rate of reported ADRs can be either an increase in the number of occasions on which a patient experiences a clinical consequence of a drug switch or a larger portion of the patients reporting their experienced ADRs. Furthermore, the absence of a correlation between the number of switches and the number of ARDs questions the idea that the same general “background ADR reporting rate” is present for all active substances for which ADRs related to drug switches are reported.
Further in our study, we demonstrated that correcting the number of ADRs by number of users, instead of number of switches, is a suitable alternative, because a comparable number of ADR peaks over time were identified by both methods. This is not an unexpected finding, as the number of switches is related to the number of users (see Figure S1 ). The number of users is, however, a mean value, and unlike the number of switches in The Netherlands, it does not demonstrate changes over a short period of time. Therefore, the user‐corrected ADR method cannot put into perspective the temporal increases in reported ADR peaks. This is not of great influence on our analysis, presumably because there is no strong correlation between peaks in the number of ADRs and peaks in the number of switches.
On average, in our data set of 20 active substances in The Netherlands, we found 364 switches per 1,000 users per year. In a study on the frequency of switching and adherence of inhalation medication, a yearly percentage switch of ~ 5% of total users was identified.21 This is low compared with our results, perhaps because multiple switches per patient and switches between generics were not accounted for in that publication. We are unaware of other literature references on drug switch frequencies.
Study limitations
This study is based on data from spontaneous ADR reporting, which is known to be subject to under‐reporting, varying by type of active substance and type of ADR.22 This limits the generalizability of our study, as we are not able to calculate an absolute risk of ADRs following drug switches, only a relative number of reported ADRs. Moreover, changes in the number of reported ADRs over time could simply be changes in the reporting rate and not true changes in the number of experienced ADRs. However, we believe that spontaneous reports are the most objective and systematically acquired data available to study clinical discomfort regarding drug switches. Furthermore, conclusions on the calculated number of reported ADRs can still be drawn relative to other active substances in the data set.
We present data for 20 active substances only. Nevertheless, these 20 active substances have the largest number of reported ADRs in The Netherlands and, therefore, provide an adequate number of ADRs on which to base calculations. As in our previous study (P.J. Glerum, unpublished data), we believe these 20 active substances represent a valuable sample, because active substances are included with both high and low numbers of switches, as well as high and low numbers of users. The active substance selection includes not only 5 of the 10 most prescribed active substances in The Netherlands in 2016 (between 688,000 and 1 million users), but also data for rivastigmine, which has, on average, only 15,000 users.
In theory, a more detailed level of analysis is possible using the Lareb and ZIN data sources, for instance, analysis of manufacturer‐specific switches or analysis of ADRs reported for a specific patient subgroup. Although interesting, more detailed analyses are limited because of the smaller number of ADRs that can be included in them. In addition, the data obtained for our study did not support manufacturer‐specific analysis, as this information was not registered by Lareb in the first part of the study period. Furthermore, subgroup analyses could not be performed, as the ZIN data were aggregated, and patient characteristics were not included.
We used all reported ADRs for which a certain LLT was registered. This limits the study, as allocation of LLTs to ADR reports is a subjective action. “Substitution” is particularly ambiguous terminology, as various interpretations are offered in literature. However, we gathered all ADRs from the same pharmacovigilance center and are, therefore, confident that the terminology and interpretation is consistent and similar within our study data.
The threshold for peak identification was arbitrarily chosen as the mean plus one SD. This is a common approach when analyzing data, such as those in this study; however, it is only a mathematical approach to peak identification. Conclusions drawn from these data can, therefore, differ if different threshold values are chosen, and the best threshold for actual pharmacovigilance analyses may be a subject for further research.
Future directions
In this study, we demonstrate the importance of incorporating the number of drug switches when analyzing switch‐related ADRs. Although we consider each reported ADR to be important, and we know that ADRs can have a significant impact on quality of life on an individual level,23 we believe that an ADR is less informative in analyzing the clinical discomfort of drug switching when a large number of switches underlies that specific ADR.
Our recommendation is to incorporate the number of drug switches in drug switch‐related ADR pharmacovigilance analyses, when feasible. If not, it would at least be desirable to incorporate the number of users, which is a reasonable alternative to the number of switches. Alternatively, it may be interesting to use an estimated number of drug switches by simulating the number of drug switches, based on the number of users, seasonality (known to occur in The Netherlands), and other factors that are unknown at this point. Further validation of either methodology is recommended.
CONCLUSION
We demonstrate that adjusting the absolute number of reported ADRs for the number of drug switches leads to identification of different ADR peaks between June 1, 2009, and December 31, 2016, at least in our selection of 20 active substances with a relatively large number of ADRs in The Netherlands. By using the switch‐corrected number of ADRs in pharmacovigilance analyses, the likelihood of identifying relevant switch‐related ADR peaks may be increased. Therefore, the use of switch‐corrected ADR data in routine pharmacovigilance analyses seems to be advisable. Although the number of switches is theoretically the best measure to put the reported ADRs related to drug switching in perspective, in the absence of such data, the mean number of users could be a useful alternative measure.
Funding
No external funding was received for this research project.
Conflicts of Interest
P.G. and M.M. are appointed to the Medicines Evaluation Board in The Netherlands. V.V. is appointed by the National Health Care Institute in The Netherlands. J.S., F.H. and E.P. are appointed by The Netherlands Pharmacovigilance Centre Lareb. All other authors declared no competing interests for this work.
Author Contributions
P.G. wrote the manuscript. P.G., M.M., F.H., E.P., D.B., and C.N. designed the research. P.G., V.V., and J.S. performed the research. P.G. analyzed the data.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The number of drug switches is currently not accounted for in the analysis of adverse drug reactions (ADRs) possibly resulting from those switches.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What are the relative number of reported ADRs per number of switches for the 20 active substances in our study and are those numbers different for ADR numbers not corrected for the number of switches?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ We now have a tool at hand that increased the likelihood of identifying relevant switch‐related ADR peaks and the use of switch‐corrected ADR data in routine pharmacovigilance analyses seems to be advisable.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ This systematic study of the perceived problems of drug switching can aid in further (pharmacovigilance) research and the discussion on the acceptability of drug switching and generic interchangeability.
Supporting information
Figure S1. Tif.
Table S1 and Table S2 . Docx.
Legends of Supplementary Figures and Tables S1 . Docx.
Acknowledgments
The authors thank all colleagues at the Department of Pharmacoepidemiology and Clinical Pharmacology at Utrecht University, The Netherlands, for all inspiring discussions on this research.
References
- 1. Guideline on the investigation of bioequivalence. Doc. Ref.: CPMP/EWP/QWP/1401/98 Rev. 1/ Corr** (2010).
- 2. Makus, K.G. & McCormick, J. Identification of adverse reactions that can occur on substitution of generic for branded lamotrigine in patients with epilepsy. Clin. Ther. 29, 334–341 (2007). [DOI] [PubMed] [Google Scholar]
- 3. Goldberg, J.F. A case of akathisia after switching from branded to generic high‐dose olanzapine. J. Clin. Psychiatry 73, 497 (2012). [DOI] [PubMed] [Google Scholar]
- 4. Gallelli, L. et al Recognizing severe adverse drug reactions: two case reports after switching therapies to the same generic company. Curr. Drug Saf. 11, 104–108 (2016). [DOI] [PubMed] [Google Scholar]
- 5. Kwan, P. & Palmini, A. Association between switching antiepileptic drug products and healthcare utilization: a systematic review. Epilepsy Behav. 73, 166–172 (2017). [DOI] [PubMed] [Google Scholar]
- 6. Colgan, S. , Faasse, K. , Martin, L.R. , Stephens, M.H. , Grey, A. & Petrie, K.J. Perceptions of generic medication in the general population, doctors and pharmacists: a systematic review. BMJ Open 5, e008915 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al Ameri, M.N. , Whittaker, C. , Tucker, A. , Yaqoob, M. & Johnston, A. A survey to determine the views of renal transplant patients on generic substitution in the UK. Trans. Int. 24, 770–779 (2011). [DOI] [PubMed] [Google Scholar]
- 8. Babar, Z.U. et al Evaluating pharmacists' views, knowledge, and perception regarding generic medicines in New Zealand. Res. Social Adm. Pharm. 7, 294–305 (2011). [DOI] [PubMed] [Google Scholar]
- 9. Dunne, S.S. What do users of generic medicines think of them? A systematic review of consumers' and patients' perceptions of, and experiences with, generic medicines. Patient 9, 499–510 (2016). [DOI] [PubMed] [Google Scholar]
- 10. O’Leary, A. et al Generic medicines and generic substitution: contrasting perspectives of stakeholders in Ireland. BMC Res. Notes 8, 790 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Netherlands Pharmacovigilance Centre Lareb . Overview on reports of adverse drug reactions related to drug substitution, April 2017.
- 12. Desai, R.J. et al Differences in rates of switchbacks after switching from branded to authorized generic and branded to generic drug products: cohort study. BMJ 361, k1180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bohn, J. , Kortepeter, C. , Munoz, M. , Simms, K. , Montenegro, S. & Dal Pan, G. Patterns in spontaneous adverse event reporting among branded and generic antiepileptic drugs. Clin. Pharmacol. Ther. 97, 508–517 (2015). [DOI] [PubMed] [Google Scholar]
- 14. Concordet, D. et al Levothyrox® new and old formulations: are they switchable for millions of patients? Clin. Pharmacokinet. 58, 827–833 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coste, J. , Bertagna, X. & Zureik, M. Comment on: “Levothyrox® new and old formulations: are they switchable for millions of patients?” Clin. Pharmacokinet. 58, 965–966 (2019). [DOI] [PubMed] [Google Scholar]
- 16. Munafo, A. , Krebs‐Brown, A. , Gaikwad, S. , Urgatz, B. & Castello‐Bridoux, C. Comment on "Levothyrox((R)) new and old formulations: are they switchable for millions of patients?". Clin. Pharmacokinet. 58, 969–971 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicolas, P. Comment on: "Levothyrox® new and old formulations: are they switchable for millions of patients?" Clin. Pharmacokinet. 58, 959–960 (2019). [DOI] [PubMed] [Google Scholar]
- 18. Tréchot, P. Comment on: Levothyrox® new and old formulations: are they switchable for millions of patients? Clin. Pharmacokinet. 58, 977–978 (2019). [DOI] [PubMed] [Google Scholar]
- 19. Faasse, K. , Cundy, T. & Petrie, K.J. Thyroxine: anatomy of a health scare. BMJ 339, 5613 (2009). [DOI] [PubMed] [Google Scholar]
- 20. Brown, J.D. , Henriksen, C. , Vozmediano, V. & Schmidt, S. Real‐world data approaches for early detection of potential safety and effectiveness signals for generic substitution: a metoprolol extended‐release case study. J. Clin. Pharmacol. 59, 1275–1284 (2019). [DOI] [PubMed] [Google Scholar]
- 21. Engelkes, M. et al Brand and generic use of inhalation medication and frequency of switching in children and adults: a population‐based cohort study. J. Asthma 55, 1086–1094 (2018). [DOI] [PubMed] [Google Scholar]
- 22. Hazell, L. & Shakir, S.A. Under‐reporting of adverse drug reactions: a systematic review. Drug Saf. 29, 385–396 (2006). [DOI] [PubMed] [Google Scholar]
- 23. Rolfes, L. , van Hunsel, F. , Taxis, K. & van Puijenbroek, E. The impact of experiencing adverse drug reactions on the patient's quality of life: a retrospective cross‐sectional study in the Netherlands. Drug Saf. 39, 769–776 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Tif.
Table S1 and Table S2 . Docx.
Legends of Supplementary Figures and Tables S1 . Docx.


