Abstract
We reported a novel function of recombinant human thrombopoietin (TPO) in increasing hematopoietic stem and progenitor cell (HSPC) homing to the bone marrow (BM). Single doses of TPO treatment to the recipients immediately after BM transplantation showed significantly improved homing of HSPCs to the BM, which subsequently resulted in enhanced short‐ and long‐term engraftment of HSPCs in mice. We found that TPO could downregulate the expression and secretion of matrix metalloproteinase 9 in BM cells. As a result, SDF‐1α level was increased in the BM niche. Blocking the interaction of SDF‐1α and CXCR4 on HSPCs by using AMD3100 could significantly reverse the TPO‐enhanced HSPC homing effect. More importantly, a single dose of TPO remarkably promoted human HSPC homing and subsequent engraftment to the BM of nonobese diabetic/severe combined immunodeficiency mice. We then performed a clinical trial to evaluate the effect of TPO treatment in patients receiving haploidentical BM and mobilized peripheral blood transplantation. Surprisingly, single doses of TPO treatment to patients followed by hematopoietic stem cell transplantation significantly improved platelet engraftment in the cohort of patients with severe aplastic anemia (SAA). The mean volume of platelet and red blood cell transfusion was remarkably reduced in the cohort of patients with SAA or hematological malignancies receiving TPO treatment. Thus, our data provide a simple, feasible, and efficient approach to improve clinical outcomes in patients with allogenic hematopoietic stem cell transplantation. The clinical trial was registered in the Chinese Clinical Trial Registry website (http://www.chictr.org.cn) as ChiCTR‐OIN‐1701083.
Keywords: engraftment, hematopoietic stem and progenitor cells, homing, matrix metalloproteinase 9, thrombopoietin
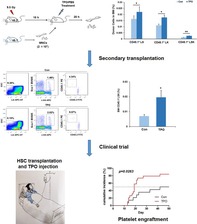
We reported a novel function of recombinant human thrombopoietin (TPO) in increasing hematopoietic stem and progenitor cell (HSPC) homing to the bone marrow (BM), which subsequently resulted in enhanced long‐term engraftment of HSPCs in mice. Notably, single doses of TPO treatment to patients followed by HSCT improved clinical outcomes, especially in patients with severe aplastic anemia.
Lessons learned.
• Thrombopoietin (TPO) administration on the day of transplantation was shown to be safe and beneficial for those patients in a haploidentical hematopoietic stem cell transplantation setting, especially for the patients with severe aplastic anemia.• TPO administration on the day of transplantation significantly reduced the number of platelet units and red blood cell units transfused for those patients with severe aplastic anemia or hematologic malignancies receiving hematopoietic stem cell transplantation.
Significance statement.
This article reports a novel function of recombinant human thrombopoietin (TPO) in increasing hematopoietic stem and progenitor cell (HSPC) homing to the bone marrow (BM), which subsequently resulted in enhanced long‐term engraftment of HSPCs in mice. Notably, TPO treatment to patients followed by hematopoietic stem cell transplantation improved platelet engraftment outcomes, especially in patients with severe aplastic anemia. To the best of authors' knowledge, the results of this study are innovative and might represent a valuable and rapid pathway for improving HSPC homing to the BM and the hematopoietic repopulation efficiency in patients.
1. INTRODUCTION
Successful repopulation of the hematopoietic and immune systems of patients after hematopoietic stem cell transplantation (HSCT) depends on the efficient homing of intravenous‐infused hematopoietic stem and progenitor cells (HSPCs) to the bone marrow (BM) and subsequent hematopoiesis in the niche. HSPC homing from peripheral blood to the BM is a rapid, critical, and multistep process in which stem and progenitor cells undergo rolling, firm adhesion to endothelial cells, transendothelial migration, lodging, and attachment to the BM microenvironment within a few hours after transplantation. However, not all infused HSPCs find their way to the BM home. The majority of HSPCs are tethered by various nonhematopoietic organs.1, 2 Therefore, the use of agents to ensure that as many HSPCs as possible seed to the BM is a favorable strategy for improving the subsequent engraftment and repopulation efficiency of transplanted HSPCs, particularly when infused umbilical cord blood (UCB) HSPC numbers are limited.3 Currently, various agents to enhance the homing efficiency of HSPCs have been reported,4, 5, 6 and several approaches for enhancing HSPC homing and engraftment are being evaluated in clinical trials.7, 8, 9 However, there is still an urgent need to discover a drug that is efficient in improving HSPC homing to the BM.
Thrombopoietin (TPO) is an important stimulator and clinical drug for promoting megakaryopoiesis and platelet production.8, 9, 10, 11, 12, 13, 14 Additionally, TPO has been proved to exert critical function on hematopoietic stem cells (HSCs), which can activate the c‐MPL receptor on HSCs and regulate hematopoiesis, HSC self‐renewal, and quiescence.12 Exogenous multiple‐day treatment with TPO in an irradiated mice model can promote hematopoietic recovery and HSPC engraftment, which is mainly ascribed to the role of TPO in regulating HSPC proliferation and survival.14 Given that homing of HSPCs to the BM is the first and critical step for subsequent HSPC engraftment, we are wondering whether TPO regulates the homing of HSPCs to the BM niche, a process that occurs within hours after transplantation. Considering the clinical use of recombinant human TPO in the treatment of thrombopenia,15, 16 further research on its role in affecting HSPC homing to the BM will represent a valuable and rapid pathway for identifying drugs that improve HSPC engraftment efficiency.
Here, we report that TPO exhibits a remarkable role in enhancing HSPC homing to the BM and, subsequently, leading to short‐ and long‐term hematopoietic engraftment in mice. The mechanism of TPO in enhancing HSPC homing to the BM involves the downregulation of matrix metalloproteinase 9 (MMP‐9) gene expression and subsequent perturbation of the SDF‐1α/CXCR4 axis. More importantly, we found that single doses of TPO treatment followed by HSCT demonstrated a therapeutic role in improving clinical outcomes. Our data provide insight into the early activation of TPO receptor signaling in the BM of patients, which might be an efficient strategy to enhance HSPC homing and engraftment after HSCT.
2. MATERIALS AND METHODS
2.1. Irradiation
Irradiation was administered using a 60Co irradiator (890‐1100 cGy/min) at 9.5 Gy (4.5 and 5 Gy at a 1‐hour interval) for the C57BL/6 mice and 3.5 Gy for the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.
2.2. Homing experiments
Eighteen hours after the CD45.2+ mice received the irradiation, BM mononuclear cells (MNCs) from donor CD45.1+ mice were harvested using lymphocyte separation medium (TBD Sciences, China) and transplanted into the irradiated CD45.2+ mice (tail vein injections, 2 × 107 each). Single doses of TPO (50 μg/kg) or phosphate‐buffered saline (PBS) were given to the recipient mice immediately after BM transplantation (BMT). After 20 hours, the chimeric CD45.2+ mice were sacrificed for homing assays. The homing efficiency of donor Lin−Sca1+ (LS), Lin−c‐Kit+ (LK), and Lin−Sca1+c‐Kit+ (LSK) cells was calculated according to the literature.17 Details of the calculation method are provided in the supplemental online Methods section.
2.3. Competitor transplantation experiments
BM MNCs (2 × 106), harvested from the 1° recipient mice at 20 hours after BMT with the different treatments, were mixed with 2 × 105 competitor CD45.2+ BM MNCs and cotransplanted into irradiated CD45.2+ mice (2° recipients). For the 3° transplantations, BM MNCs from 2° chimeric mice were transplanted into irradiated CD45.2+ mice (2 × 107 cells per mouse), and peripheral blood (PB) from the 3° recipient mice were harvested at 16 weeks after transplantation.
2.4. Human umbilical cord blood MNC homing in the NOD/SCID mice
MNCs were isolated from the fresh human umbilical cord blood (UCB) samples using a lymphocyte separation medium at 1.077 g/mL (TBD Sciences, China), and 1 × 107 MNCs (approximately 1 × 105 CD34+ cells) were then transplanted into the irradiated NOD/SCID mice. Single doses of TPO (50 μg/kg) or PBS were administered to the recipient mice immediately after cell transplantation. After 20 hours, two femurs and two tibias from each chimeric mouse were collected for flow cytometric assays.
2.5. Patient and transplantation characteristics
Patients diagnosed with severe aplastic anemia (SAA; n = 19) or hematologic malignancies (n = 38) in our department during 2014‐2016 who were suitable for receiving HSCT were subcutaneously injected with single doses of TPO (300 U/kg) on the day of transplantation. All the patients received both BM and mobilized PB HSCT and a total of two doses of TPO. Situation‐matched patients (14 patients with aplastic anemia, 43 patients with leukemia) between 2013 and 2014 were retrospectively analyzed as controls. The characteristics of patients, donors, and transplants are summarized in Table 1.
Table 1.
The characteristics of patients, donors, and transplants
| Characteristics | Severe aplastic anemia | Hematologic malignancies | ||||
|---|---|---|---|---|---|---|
| Control (n = 14) | TPO (n = 19) | Total (n = 33) | Control (n = 43) | TPO (n = 38) | Total (n = 81) | |
| Median age, years (range) | 16 (11‐26) | 20 (11‐32) | 18 (11‐32) | 24 (11‐49) | 28 (11‐59) | 26 (11‐50) |
| Sex | ||||||
| Male | 7 (50%) | 12 (63%) | 19 (58%) | 28 (65%) | 21 (55%) | 49 (60%) |
| Female | 7 (50%) | 7 (37%) | 14 (42%) | 15 (35%) | 17 (45%) | 32 (40%) |
| ABO mismatch | ||||||
| Major | 5 (36%) | 14 (74%) | 19 (58%) | 26 (60%) | 22 (58%) | 48 (60%) |
| Minor | 5 (36%) | 2 (11%) | 7 (21%) | 7 (16%) | 7 (18%) | 14 (20%) |
| No | 4 (29%) | 3 (16%) | 7 (21%) | 10 (23%) | 9 (24%) | 19 (20%) |
| Disease | ||||||
| ALL | 23 (53%) | 16 (42%) | 39 (48%) | |||
| AML | 10 (23%) | 15 (39%) | 25 (31%) | |||
| MDS | 7 (16%) | 5 (13%) | 12 (15%) | |||
| CML | 3 (7%) | 2 (5%) | 5 (6%) | |||
| Donor match (all partially matched) | ||||||
| 3/6 | 7 (50%) | 17 (89%) | 24 (73%) | 27 (63%) | 23 (61%) | 50 (62%) |
| 4/6 | 7 (50%) | 2 (11%) | 9 (27%) | 16 (37%) | 15 (39%) | 31 (38%) |
| Risk | ||||||
| H | 31 (72%) | 30 (79%) | 61 (75%) | |||
| L | 2 (5%) | 8 (21%) | 10 (14%) | |||
| M | 10 (23%) | 0 | 10 (14%) | |||
| Transplant characteristics | ||||||
| BM | ||||||
| Infused total nucleated cell dose (×108/kg, median, range) | 4.89 (1.20‐10.30) | 4.24 (0.68‐9.60) | 4.52 (0.68‐10.30 | 4.20 (0.90‐7.36) | 3.60 (1.48‐7.10) | 3.92 (0.90‐7.36) |
| Transplanted CD34+ cells (%, median, range) | 0.52 (0.19‐1.25) | 0.73 (0.14‐3.20) | 0.64 (0.14‐3.20) | 0.44 (0.20‐1.02) | 0.56 (0.22‐1.42) | 0.50 (0.20‐1.42) |
| PB | ||||||
| Infused total nucleated cell dose (×108/kg, median, range) | 5.5 (2.14‐11.50) | 6.4 (3.80‐12.30) | 6.02 (2.14‐12.30) | 4.57 (1.30‐7.96) | 6.45 (2.37‐9.90) | 5.45 (1.30‐9.90) |
| Infused CD34+ cells (%, median, range) | 0.42 (0.09‐0.76) | 0.61 (0.08‐1.42) | 0.53 (0.08‐1.42) | 0.55 (0.16‐1.21) | 0.57 (0.19‐1.54) | 0.56 (0.16‐1.54) |
| PB + BM | ||||||
| Infused total nucleated cell dose (×108/kg, median, range) | 10.39 (3.85‐17.19) | 10.64 (7.92‐16.35) | 10.53 (3.85‐17.19) | 8.76 (5.32‐13.73) | 10.06 (5.75‐16.30) | 9.37 (5.32‐16.30) |
| Infused CD34+ cells (×106/kg, median, range) | 4.19 (1.53‐12.49) | 6.22 (1.78‐19.35) | 5.36 (1.53‐19.35) | 4.34 (1.16‐8.76) | 5.31 (1.19‐17.90) | 4.80 (1.16‐17.90) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myelocytic leukemia; CML, chronic myelocytic leukemia; H, high risk; L, low risk; M, moderate risk; MDS, myelodysplastic syndromes.
2.6. Statistical analysis
The clinical data were analyzed using log‐rank tests to evaluate cumulative incidence for platelets and neutrophils. Unless otherwise noted, the P values were calculated using unpaired two‐tailed Student's t tests, and all error bars represent the means ± SDs. The variance similar between the groups has been statistically compared. Analyses were performed using SASS and GraphPad Prism, and P values <.05 were considered statistically significant.
3. RESULTS
3.1. TPO enhances homing of HSPCs to the BM and subsequently results in enhanced short‐ and long‐term engraftment of HSPCs in mice
We first tested whether the subcutaneous administration of TPO to the irradiated CD45.2 mice in single doses affected the in vivo homing of the donor CD45.1+ HSPCs to the recipient BM (Figure 1A). The results revealed that a single dose of the TPO treatment at 50 μg/kg caused a significant increase in the percentage of CD45.1+LS, CD45.1+LK, and CD45.1+LSK cells in the BM (Figure 1B). The homing efficiency of donor LK and LSK cells to the BM was significantly increased by TPO treatment (Figure 1C). We then used colony‐forming unit (CFU) assays to further assess the capacity of TPO to regulate the homing of HSPCs to the BM. Typical colonies, including BFU‐erythrocyte, CFU‐granulocyte/granulocyte and macrophage/macrophage, and CFU‐granulocyte, erythrocyte, monocyte/macrophage, and megakaryocyte (CFU‐GEMM), were scored based on morphologic criteria. Consistently, the homing efficiency of CFUs and different types of CFUs to the BM in TPO‐treated mice was much greater than those in the vehicle‐treated mice, with an approximately 3.39‐fold increase in total CFUs and a 3.46‐fold increase in CFU‐GEMMs (Figure 1D,E).
Figure 1.
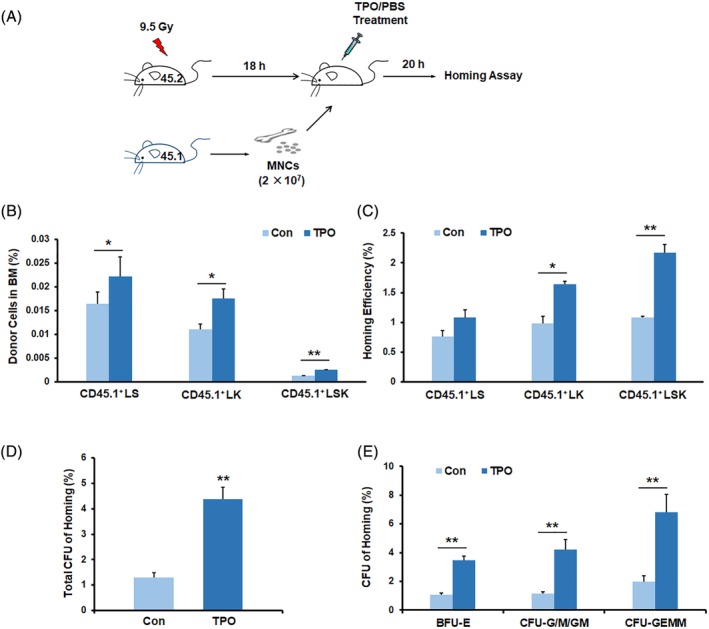
TPO enhances homing of HSPCs to the BM. A, Schematic representation of the homing experiments. Lethally irradiated CD45.2+ mice were transplanted with CD45.1+ BM MNCs and injected with single doses of TPO at 50 μg/kg or PBS as a control. BMs were harvested for homing assays 20 hours later. B,C, The percentage and homing efficiency of donor CD45.1+ HSPCs (LS, LK, and LSK cells) in the recipient BM (n = 3 each). D,E, The homing efficiency of CFU as calculated by comparing the homed CFU numbers with the initially injected CFU numbers. *P < .05, **P < .01, ***P < .001. TPO treatment group vs control group. BFU‐E, burst‐forming unit‐erythrocyte; CFU‐G/GM/M, colony‐forming unit‐granulocyte/granulocyte and macrophage/macrophage; CFU‐GEMM, colony‐forming unit‐granulocyte, erythrocyte, monocyte/macrophage, and megakaryocyte (n = 3 each). BM, bone marrow; HSPC, hematopoietic stem and progenitor cell; MNC, mononuclear cell; PBS, phosphate‐buffered saline; TPO, thrombopoietin
Enhanced HSPC homing could result in increased engraftment of transplanted HSPCs.18 To further assess the enhanced homing efficiency of HSPCs by TPO treatment, competitive repopulation models were then applied following the homing experiments (Figure 2A). BM MNCs (2 × 106), harvested from the 1° CD45.2+ recipient mice at 20 hours after CD45.1+ BMT with or without TPO treatments, were mixed with 2 × 105 competitor cells and cotransplanted into irradiated CD45.2+ mice (2° recipients). The increase in homed donor (CD45.1+) HSPC numbers in the mice that underwent a single dose of TPO administration was also reflected by increased engraftment of the primary donor CD45.1+ cells in the PB in the 2° recipients, particularly at 12 weeks after transplantation (Figure 2B). Long‐term donor CD45.1+ cell engraftment rates, particularly the CD45.1+LSK cell engraftment rates, in the BM were significantly increased in the mice that received chimeric BM cells from the TPO‐treated 1° recipient mice compared with those in the mice that received cells from the vehicle‐treated 1° recipients at 20 weeks after transplantation (Figure 2C‐E). BM cells from the 2° recipients were then transplanted into the 3° CD45.2 mice. The 3° recipients receiving transplants that incorporated the primary TPO‐enhancing homed BM cells exhibited increased engraftment of CD45.1+ blood lineage cells compared with the control group at 16 weeks after transplantation (Figure 2F). These results strongly indicate that the increased numbers of homed HSPCs by a single subcutaneous TPO injection to the primary recipients subsequently exhibited improved long‐term engraftment in the serial transplantation models.
Figure 2.
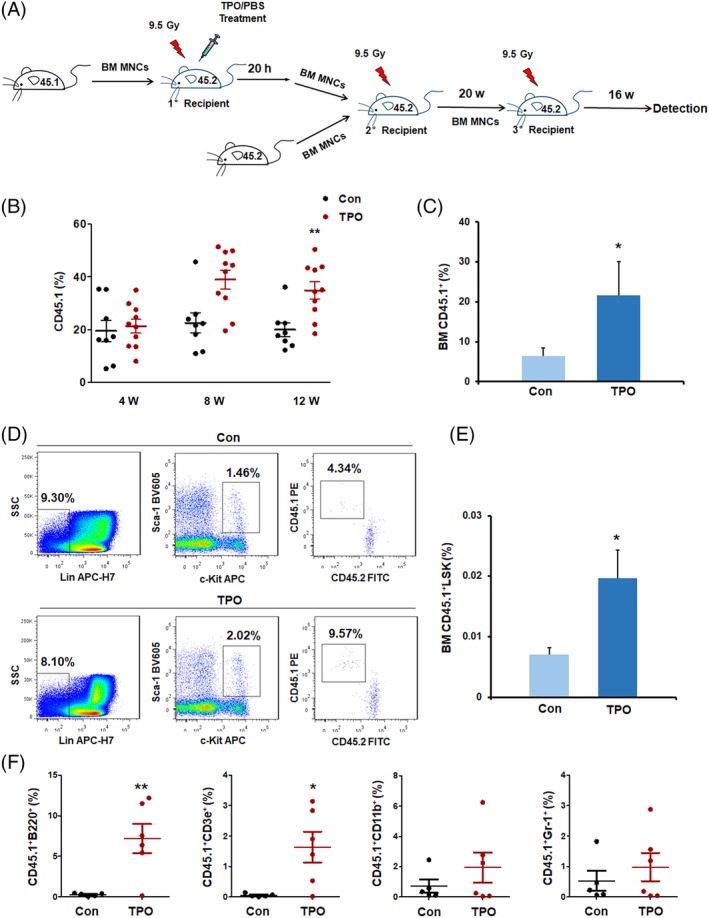
TPO enhances engraftment of HSPCs to the BM. A, Schematic representation of the homed CD45.1+ BM cell engraftment assays from the competitive and noncompetitive transplantation experiments. Lethally irradiated CD45.2+ mice (1° recipients) received CD45.1+ BM MNCs and single doses of TPO or PBS treatment (mean ± SD, n = 8 for the control group and n = 10 for the TPO treatment group). B, Flow cytometric analyses of the donor CD45.1+ cell percentages in the PB at 4, 8, and 12 weeks after 2° transplantation (mean ± SD, n = 8 for the control group and n = 10 for the TPO treatment group). C, The percentages of donor CD45.1+ cells in the 2° recipient BMs at 20 weeks after transplantation. D,E, Representative dot plots and percentages of donor LSK cells (CD45.1+CD45.2−LSK) in the 2° recipient BMs at 20 weeks after 2° transplantation. F, The donor CD45.1+ blood lineage cell percentages in the 3° recipients at 16 weeks after transplantation (mean ± SD, n = 5 for the control group and n = 6 for the TPO treatment group). BM, bone marrow; HSPC, hematopoietic stem and progenitor cell; MNC, mononuclear cell; PBS, phosphate‐buffered saline; TPO, thrombopoietin
Based on the effect of TPO in promoting HSPC homing to the BM and long‐term engraftment, we predicted that a single administration of TPO to recipients would enhance the short‐term engraftment of transplanted hematopoietic cells. Lethally irradiated wild‐type mice were transplanted with 1 × 106 BM cells from β‐actin‐luciferase transgenic mice and received one dose of the TPO or PBS treatment. The in vivo bioluminescence imaging results revealed that TPO administration significantly increased transplanted hematopoietic cell engraftment in the BM, particularly at 2‐3 weeks after transplantation (supplemental online Figure S1A,B). We also observed the survival rate of the recipients following low numbers of hematopoietic cell transplantation and TPO treatment. The results showed that the irradiated mice that received 2.5 × 105 BM cells and PBS treatment exhibited a low survival rate (50%). In contrast, 100% of the lethally irradiated mice that were given 2.5 × 105 BM cells and single injections of TPO survived (supplemental online Figure S1C). These results indicated that single administrations of TPO significantly improved homing of HSPCs to the BM and subsequently led to enhanced short‐ and long‐term engraftment of HSPCs in mice.
3.2. TPO enhances HSPC homing by downregulation of MMP‐9 expression and secretion
The process of HSPC homing to the BM involves several proteolytic enzymes and some important pathways.19, 20, 21, 22, 23, 24 A recent study suggested that MMP‐9 was affected by TPO in a brain ischemic model.25 Given that the proteolytic enzyme MMP‐9 was involved in the regulation of migration and adhesion‐related molecules and HSPC mobilization and homing in the BM,26, 27 we analyzed whether MMP‐9 played an important role in TPO‐stimulated homing of HSPC. Single doses of TPO (50 μg/kg) or PBS were given to the C57BL/6 mice receiving lethal irradiation without BMT. At 20 hours after TPO treatment, the mice were sacrificed for BM section and supernatant assays. The immunohistochemical assay results showed a notable decrease in MMP‐9 protein level in the BM extracellular stroma and BM cells of the TPO‐treated recipient mice (Figure 3A). Consistently, enzyme‐linked immunosorbent assay (ELISA) results showed lower MMP‐9 levels in the BM supernatants of the TPO‐treated mice compared with the control group (Figure 3B), which was indicative of the inhibitory role of TPO in MMP‐9 protein expression and secretion. We then used quantitative real‐time polymerase chain reaction (Q‐PCR) and Western blotting to evaluate the expression level of MMP‐9 in BM cells at 18 hours after TPO or PBS treatment. Similarly, the expression of MMP‐9 gene and protein was significantly reduced in the BM cells from lethally irradiated mice receiving TPO treatment compared with the control group (Figure 3C,D). Downregulated MMP‐9 gene expression was also detected in cultured BM cells at 12, 18, and 24 hours after the addition of TPO into the medium compared with the control group without TPO supplement (Figure 3E).
Figure 3.
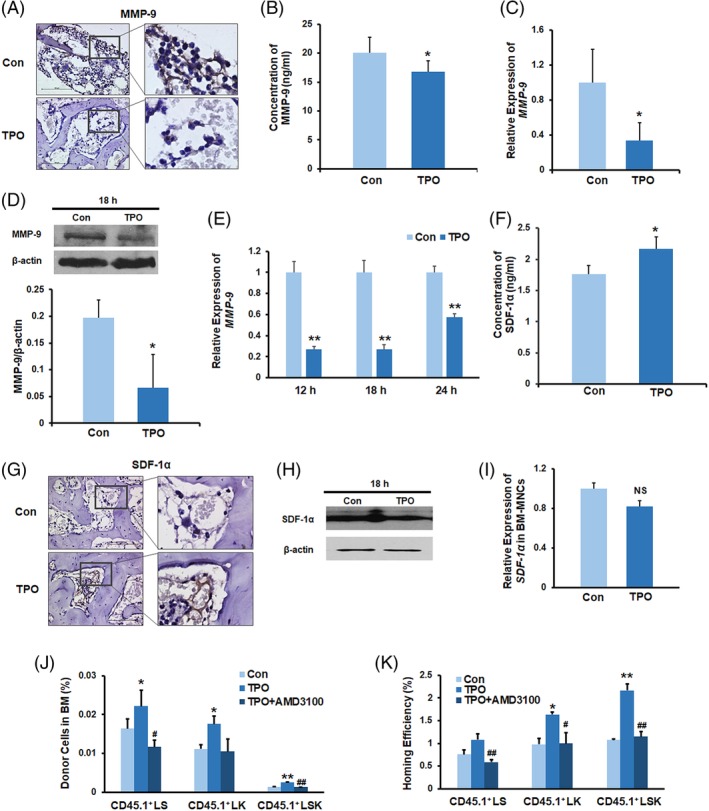
TPO enhances HSPC homing by the downregulation of MMP‐9 expression and secretion. A, Immunostaining of femur sections with anti‐MMP‐9 antibody (bar = 100 μm). B, BM MMP‐9 levels in the recipient mice without BMT. C, Q‐PCR for MMP‐9 gene expression in the recipient BM cells. D, Western blotting of the recipient BM cells without transplantation using an antibody against MMP‐9. E, Q‐PCR for MMP‐9 gene expression of in vitro cultured BM cells. F, BM SDF‐1α levels in the recipient mice at 20 hours following a single dose of TPO or PBS injection. The BM samples were collected from the supernatants of BM cell suspensions that were flushed from two femurs using 1 mL PBS and were assayed with a SDF‐1α ELISA kit. G, Immunostaining of femur sections with an anti‐SDF‐1α antibody (bar = 100 μm). BM sections were prepared at 20 hours after TPO or PBS treatment. H, Western blotting of the recipient BM cells without transplantation using an antibody against SDF‐1α. I, Q‐PCR for SDF‐1α gene expression in the recipient BM cells. J,K, The donor cell percentages and homing efficiency analyses indicating that treatment with AMD3100 blocked the TPO‐enhanced homing of donor LSK cells. n = 3 each. *P < .05, **P < .01, compared with the control group; # P < .05, ## P < .01, compared with the TPO group. All recipient mice were lethally irradiated and received single‐dose TPO or PBS injections without BMT. BM, bone marrow; BMT, bone marrow transplantation; HSPC, hematopoietic stem and progenitor cell; MNC, mononuclear cell; PBS, phosphate‐buffered saline; Q‐PCR, quantitative polymerase chain reaction; TPO, thrombopoietin
It has been reported that the chemokine SDF‐1α can be degraded by MMP‐928 and the alteration of SDF‐1α levels in the BM niche regulates HSPC homing.29, 30 Thus, we inferred that the reduction of MMP‐9 level in the BM from TPO‐treated mice would change the concentration of SDF‐1α in the BM niche. We next used ELISA to detect the SDF‐1α protein level in the BM supernatants from TPO‐ and PBS‐treated mice receiving lethal irradiation without BMT. We observed that a single dose of the TPO administration to the lethally irradiated mice caused an increase in the SDF‐1α levels in the BM supernatants (Figure 3F). Of note, stronger SDF‐1α staining in the BM extracellular stroma was observed at 20 hours after TPO treatment than that in the vehicle treatment group (Figure 3G). Interestingly, the levels of SDF‐1α protein and mRNA in the recipient BM cells exhibited no significant change at 18 hours following the TPO or vehicle injections (Figure 3H,I). in vitro BM cell culture experiments also suggested that the TPO treatment did not alter the expression of SDF‐1α (data not shown), indicating that the alteration in the SDF‐1α concentration in the BM niche caused by TPO was not correlated with the transcriptional regulation of SDF‐1α. These results indicated that TPO administration decreased MMP‐9 expression in the BM of lethally irradiated mice, which in turn reduced the degradation of SDF‐1α and increased the concentration of it in the BM.
It has been proved that the increased SDF‐1α levels in the recipient BM attract more CXCR4+ HSPCs to the BM.26 To further investigate the critical role of SDF‐1α/CXCR4 in TPO‐enhanced HSPC homing, we next used AMD3100, a CXCR4 antagonist, to block the interaction between SDF‐1α and CXCR4.31 The results revealed that blocking the SDF‐1α/CXCR4 pathway by AMD3100 remarkably inhibited the TPO‐enhanced homing of CD45.1+LSK cells to the BM (Figure 3J,K), indicating that TPO might promote the interaction of SDF‐1α/CXCR4 axis on HSPCs in BM niche and result in increased HSPC homing to the BM.
3.3. TPO enhances homing of human HSPCs to the BM of NOD/SCID mice
To further determine whether TPO accelerated the homing of human hematopoietic cells, we isolated UCB MNCs and injected these cells into NOD/SCID mice after the mice received 3.5 Gy of irradiation. Remarkably, single doses of TPO treatment increased the percentages and numbers of human CD45+CD34+ and CD45+CD34+CD38− cells that homed to the BM (Figure 4A‐C), which indicated a novel role of TPO in enhancing the homing of human HSPCs to the BM. Subsequently, 4 weeks after transplantation of human UCB MNCs, we harvested cells from the PB of these recipient mice and detected the incorporation of human cells. Of note, the percentage of human CD45+ cells in the PB of TPO‐treated mice showed a 2.4‐fold increase compared with that in the control group (Figure 4D,E), indicating that a single administration of TPO facilitated short‐term engraftment of human HSPCs in NOD/SCID mice.
Figure 4.
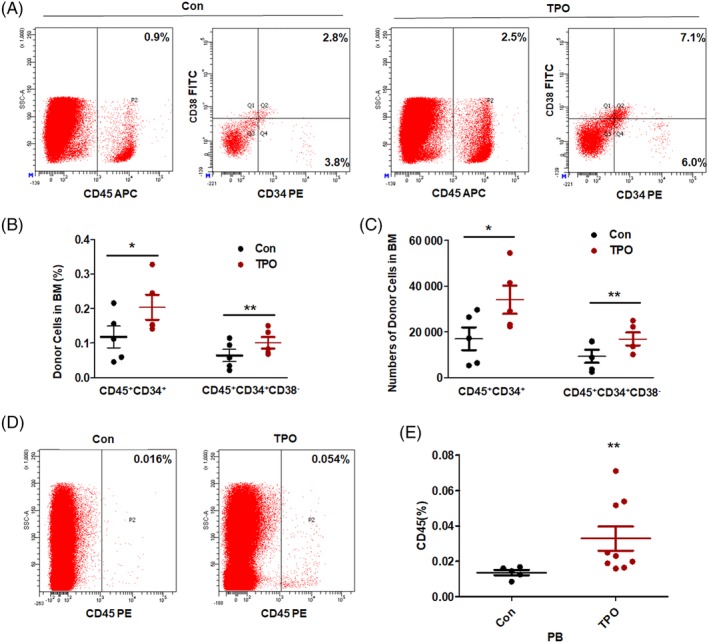
TPO enhances homing of human MNCs to the BM of NOD/SCID mice. A,B, Representative dot plots and percentages of donor human HSPCs (CD45+CD34+ and CD45+CD34+CD38−) in recipient BMs at 20 hours after cell transplantation. C, Numbers of human CD45+CD34+ and CD45+CD34+CD38− cells in the BMs of the mice. n = 5 each. D,E, Representative dot plots and percentages of the donor human CD45+ blood cells in the recipients at 4 weeks after transplantation. Mean ± SD, n = 5 for the control group and n = 9 for the TPO treatment group, *P < .05, **P < .01, ***P < .001. TPO treatment group vs control group. BM, bone marrow; HSPC, hematopoietic stem and progenitor cell; MNC, mononuclear cell; NOD/SCID, nonobese diabetic/severe combined immunodeficiency; TPO, thrombopoietin
3.4. Single administrations of TPO treatment on the day of transplantation improves the clinical outcomes in patients following HSCT
Increased HSPC seeding efficiency to the BM by TPO should lead to better engraftment of these transplanted cells in patients. We then conducted clinical trials to investigate the safety and therapeutic effect of TPO treatment in enhancing engraftment of human HSPCs. Nineteen patients with SAA at a median age of 20 years (range 11‐32 years) were enrolled and injected with TPO followed by haploidentical BM and PB HSCT (Table 1). Fourteen patients with SAA at a median age of 16 years (range 11‐26 years) who underwent haploidentical HSCT were retrospectively analyzed (Table 1). The cumulative incidence of neutrophil engraftment indicated that TPO treatment improved neutrophil recovery at 30 days after transplantation in patients with SAA. However, there was no statistical difference between the two groups (P = .0982; Figure 5A). Of note, the cumulative incidence of platelet engraftment at 50 days after transplantation was significantly different between the two groups (P = .0263; Figure 5A). Median time to platelet engraftment in patients with SAA who received the TPO treatment was 19 days, and for patients in the historical cohort, it was 46 days. The difference in the cumulative incidence of platelet recovery >50 000/μL at 50 days after transplantation between the two groups was statistically significant (P = .0257; Figure 5A). Seventy‐five percent of the patients with TPO treatment vs 40% of the patients in the control group achieved platelet recovery at 50 days after transplantation. We also conducted clinical trials in 38 patients with hematologic malignancies who received allogeneic HSCT and TPO treatment (Table 1). There was no statistically significant difference in the cumulative incidence of neutrophil and platelet engraftment between the TPO treatment cohort and the historical controls (Figure 5B). We found that the patients who received the TPO treatment showed a shorter median time in platelet engraftment than that in the historical control patients with hematologic malignancies at 50 days after transplantation (23 vs 33 days; Figure 5B). The cumulative incidence of platelet recovery (platelet number > 50 000/μL) in patients who received the TPO treatment was remarkably different from that of the patients in the historical cohort (P = .0063; Figure 5B). TPO administration significantly reduced the number of platelet units transfused before platelet engraftment for those patients with SAA or hematologic malignancies after HSPC transplantation (Figure 6A,B). The number of red blood cell (RBC) units transfused was also remarkably reduced within 60 days after transplantation in the cohort of patients with SAA or hematological malignancies receiving TPO treatment compared with the control group (Figure 6C,D). No severe adverse effects for TPO treatment were observed in any of the patients. These clinical data indicate that single injections of TPO followed by HSCT are simple, feasible, and useful ways to improve engraftment efficiency in a haploidentical HSCT setting, especially for those patients with SAA.
Figure 5.
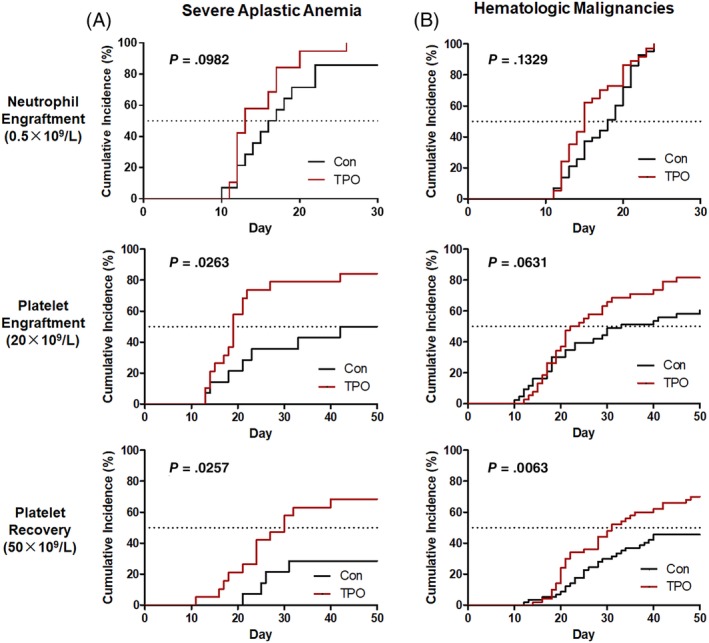
Clinical outcomes in patients receiving haploidentical HSCT with or without recombinant human TPO treatment. A, The neutrophil engraftment, platelet engraftment, and platelet recovery in patients with SAA receiving haploidentical HSCT with or without TPO administration. B, The neutrophil engraftment, platelet engraftment, and platelet recovery in patients with hematologic malignancies receiving haploidentical HSCT with or without TPO treatment. HSCT, hematopoietic stem cell transplantation; SSA, severe aplastic anemia; TPO, thrombopoietin
Figure 6.
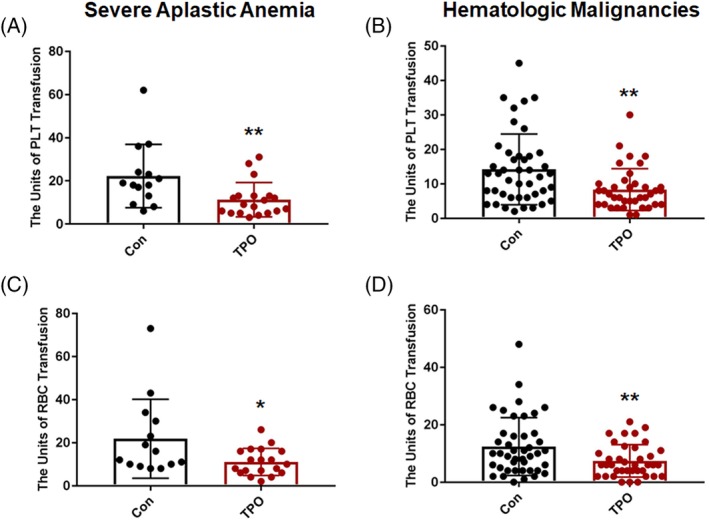
The platelet and red blood cell (RBC) transfusion for patients receiving haploidentical hematopoietic stem cell transplantation (HSCT) with or without single doses of recombinant human thrombopoietin (TPO) treatment. A, The number of platelet units transfused before platelet engraftment for patients with severe aplastic anemia (SAA) receiving haploidentical HSCT with or without TPO administration. B, The number of platelet units transfused before platelet engraftment for patients with hematologic malignancies receiving haploidentical HSCT with or without TPO treatment. C, The number of RBC units transfused within 60 days after transplantation in patients with SAA. D, The number of RBC units transfused within 60 days after transplantation in patients with hematologic malignancies. *P < .05, **P < .01
4. DISCUSSION
Homing of HSCs, which occurs within 24 hours after HSCT, is a first and critical step for BM seeding by HSPCs and seriously affects subsequent engraftment efficiency of transplanted HSPCs. However, only a small part of i.v.‐infused HSPCs seed into BM, which precedes proliferation and differentiation of these transplanted HSPCs in this environment. Most of the infused HSPCs are tethered by other nonhematopoietic tissue or organs.1, 2 The HSPC seeding efficiency to the BM varies between 1% and 10%, which indicates that homing to BM by HSPCs might be a random process.32 It was acceptable that low seeding efficiency of transplanted HSPCs can greatly influence subsequent hematopoiesis in BM. Given that the i.v.‐infused HSPC numbers from a single unit of UCB are much lower than adult BM and mobilized PB, the problem for low seeding efficiency of HSPCs from UCB is more prominent, which leads to an increase in the risk of graft failure and delays the engraftment time of neutrophils and platelets in patients. Therefore, improving homing efficiency of HSPCs from the beginning of HSCT is important to shorten the engraftment time of neutrophils and platelets in patients.
In our previous work, we found consecutive injections of TPO for 14 days after HSCT significantly increased the engraftment of HSPCs in mice.14 It was acceptable that two critical factors, homing efficiency to the BM and proliferation capacity in the BM of these i.v.‐infused HSPCs, can mainly affect the engraftment of HSPCs. Most reports showed that TPO can promote HSC proliferation and differentiation into megakaryocyte,8, 33, 34 which is one of the main ways to improve the engraftment efficiency of transplanted HSPCs. Given that the homing efficiency exerts great influence in subsequent engraftment results of HSPCs, we wondered whether TPO can affect the seeding efficiency of HSPCs to the BM. To discover the possibility of this cytokine in improving the homing of HSPCs to the BM, single doses of TPO, instead of 14 consecutive doses, were administered to the recipients immediately after HSCT. Homing efficiency of HSPCs was evaluated within 20 hours after transplantation. Surprisingly, we observed that single doses of TPO significantly increased the seeding efficiency of transplanted HSPCs to the BM of mice. We used three methods to determine the homing effect of HSPCs caused by TPO, which included CFU assays, surface marker analysis of these homed hematopoietic cells within 20 hours after transplantation, and serial transplantation experiments with homed hematopoietic cells. The results of these experiments provided the solid evidence for improving the homing effect of injected HSPCs to the BM by TPO. Although the homing process of HSPCs to the BM appears much shorter than subsequent hematopoiesis in the BM, it is the first step in increasing the seed numbers of HSPCs reaching to the BM for subsequent hematopoietic repopulation. Our experiments, in which single injections of TPO significantly improved the survival rate of irradiated mice transplanted with low hematopoietic cell numbers, further support this point. A competitive repopulation experiment has been considered a good surrogate assay to evaluate homing of long‐term HSCs. In our study, we showed that TPO treatment profoundly enhanced homing of long‐term multilineage repopulating HSCs, indicating that TPO signaling is critical for homing of primitive functionally HSCs into BM.
The proliferation effect on HSPCs by TPO could not be the reason for enhanced homing of these infused HSPCs to the BM. The homing process occurred within 24 hours and did not require cell division.20 Several reports have suggested that the proliferation of transplanted HSPC homing to the BM could not be detected even 48 hours after transplantation.35 Interestingly, Srour demonstrated that human BM and mobilized PB HSPCs were predominantly in the G0/G1 phases of the cell cycle up to 40 hours after transplantation.36 To investigate the mechanism of TPO in improving the homing of HSPCs to the BM, we focused on the influence of TPO on the chemotactic axis in the BM niche. Accumulated evidence indicates that a SDF‐1α chemotactic tug‐of‐war gradient between BM and PB explains mobilization and homing of HSPCs.37 By using an ELISA, we found that SDF‐1α levels showed an increase in the BM niche. However, a single TPO administration did not change the expression levels of SDF‐1α mRNA and protein in BM cells. To further find the clue, we analyzed the proteinase MMP‐9, which has been reported to regulate SDF‐1α levels by inactivating them. As our preclusion, MMP‐9 was decreased in expression and secretion from BM cells by TPO treatment. Consistently, in vivo TPO administration showed reduced MMP‐9 enzymatic activity in the BM supernatants of the lethally irradiated mice (supplemental online Figure S2A). in vitro BM cell culture experiments also suggested that TPO treatment inhibited the enzymatic activity of MMP‐9 (supplemental online Figure S2B). However, TPO administration showed no significant alteration on the gene expression of MMP inhibitors TIMP1‐4 (supplemental online Figure S3). The expression level of EGR‐1 and KLF6 gene was remarkably upregulated in the BM cells with TPO treatment (supplemental online Figure S4). It has been reported that EGR‐1 and KLF6 could inhibit MMP9 expression.38, 39, 40 The mechanism of MMP‐9 downregulation by TPO might be related with the activation of EGR‐1 and KLF6 expression. The enhanced SDF‐1α level by decreased MMP‐9 expression in BM by TPO caused much stronger attraction for infused HSPCs to the BM. Importantly, interruption of the strong connection of BM SDF‐1α and CXCR4 on HSPCs through AMD3100 administration remarkably blocked the homing effect of HSPCs to the BM by TPO. Our data provided the first evidence that TPO showed a new role in regulating the homing of HSPCs and discovered the novel mechanism that TPO regulated MMP‐9 gene expression in BM cells and subsequently affected the levels of SDF‐1α in the BM.
Recombinant human TPO has been used in the clinics in China for more than 10 years.15, 16 In recent years, the new generation of TPO receptor agonists, such as romiplostim and eltrombopag, have been proved to have clinical efficacy in increasing platelet counts in thrombocytopenia via modulating the TPO receptor.41, 42 Given that recombinant human TPO has been widely used in the hospital in China, we further conduct clinical trials to observe the role of TPO in improving engraftment of transplanted HSPCs in patients with SAA or hematologic malignancies. For a patient who needs to receive HSCT therapy and is lacking human leukocyte antigen‐identical donors, haploidentical HSCT is the treatment of choice.43, 44, 45 However, the engraftment outcome needs to be improved. In the clinical trial, one dose of TPO injection on the day of transplantation notably accelerated platelet engraftment and recovery in the patients with SAA receiving haploidentical HSCT, further indicating that TPO given once on the day of transplantation is a beneficial treatment approach for them. For patients with hematologic malignancies receiving HSCT, a single use of TPO on the day of transplantation also sped up platelet recovery and reduced the number of transfused platelet and RBC units. Given that we have proved that TPO has the function to enhance HSPC homing to the BM in animal experiments, the better engraftment effect of transplanted HSPCs in clinical trials caused by TPO administration together with HSCT might be ascribed to the homing‐promoting effect of this drug on HSPCs. The clinical data are encouraging and indicate that a simple and inexpensive strategy of using single doses of TPO can eventually improve clinical outcomes in patients following BM or mobilized blood transplantation. The new generation of TPO receptor agonists have similar biological function to recombinant TPO. Notably, eltrombopag is an orally available TPO receptor agonist and romiplostim is an injectable TPO peptide mimetic. Given that TPO receptor agonists can modulate HSPC function and stimulate megakaryopoiesis and have shown clinical efficacy in improving platelet levels in patients with thrombocytopenia,42, 46, 47 it is valuable to conduct new clinical trials to observe whether single doses of TPO receptor agonists along with HSPC transplantation can improve the engraftment efficiency of these transplanted HSPCs.
5. CONCLUSION
Our study first demonstrates that TPO has a novel role in improving the homing efficiency of mouse and human HSPCs to the BM and subsequently enhancing the short‐ and long‐term engraftment of transplanted HSPCs. TPO enhances the homing of HSPCs into the BM mainly by suppressing the expression and secretion of MMP‐9 and modulating the SDF‐1α/CXCR4 axis in the BM niche. More importantly, our clinical trial data indicate that subcutaneous single‐dose administration of TPO followed by HSCT is a powerful method to improve clinical outcomes in patients with SAA or hematological malignancies. This simple treatment might become an advantageous and economic approach to accelerate HSPC engraftment for patients receiving haploidentical adult HSCT or UCB transplantation.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
L.D., D.H., H.W.: collection and assembly of data, and provision of study material or patients; B.Z., Z.D., Y.H., S.W., S.Y., Z.F., J.Z., H.Y., L.H.: collection and assembly of data; W.Y.: data analysis and interpretation; Y. Li: data analysis and interpretation, conception and design, manuscript writing, and revising; X.P.: data analysis and interpretation, and financial support; Y. Liu: collection and assembly of data, and manuscript writing.
Supporting information
Supplemental Figure 1 TPO enhances short‐term engraftment of hematopoietic cells and increases survival of mice following BM transplantation. (A and B) The luciferase activities of donor hematopoietic cells in the recipients at 2 and 3 weeks after transplantation. (C) The survival rates of the lethally irradiated recipients receiving 2.5 × 105 donor BM MNCs and single doses of TPO or PBS (n = 10 each).
Supplemental Figure 2 TPO treatment reduces MMP‐9 proteolytic activity. (A) The MMP‐9 proteolytic activity in the BM supernatants of lethally irradiated mice. The BM supernatants were collected from the mice at 20 hours after single doses of TPO or PBS injection. Two femurs from each mouse were flushed with 500 μL PBS. The equal volume of the BM supernatants were loaded onto SDS‐polyacrylamide gel for zymographic analysis. (B) The MMP‐9 proteolytic activity in the culture medium of the mouse BM cells. The BM cells were isolated from normal mice and resuspended in serum‐free IMDM medium. After receiving 5 Gy irradiation, the cells were cultured with or without 50 ng/mL TPO for 20 hours. The cell‐conditioned medium at equal volume was used for zymographic analysis at 20 hours after in vitro treatment.
Supplemental Figure 3 Q‐PCR for TIMP1‐4 gene expression in the recipient BM cells at 18‐20 hours following single dose of TPO or PBS injection.
Supplemental Figure 4 Q‐PCR for EGR‐1 and KLF6 gene expression in cultured mouse BM cells with or without TPO treatment for 6 hours, 12 hours, 18 hours and 24 hours. BM cells were isolated from normal mice and cultured at the concentration of 5 × 106/ml in StemSpanTM SFEM medium with or without 50 ng/mL TPO for different time. *P < 0.05, **P < 0.01, compared to the control group.
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We thank Hongbo Luo and Weiping Yuan for helpful discussion and thank Huizuo Mao for drawing the patient picture in the graphical abstract. This work was supported by the National Key Research and Development Program of China (2017YFA0103100, 2017YFA0103103, 2017YFA0103104), National Natural Science Foundation of China (81872553, 81472908, 81800103), and Guangzhou Health Care and Cooperative Innovation Major Project (201704020218, 201803040005).
Liu Y, Ding L, Zhang B, et al. Thrombopoietin enhances hematopoietic stem and progenitor cell homing by impeding matrix metalloproteinase 9 expression. STEM CELLS Transl Med. 2020;9:661–673. 10.1002/sctm.19-0220
Yiming Liu and Li Ding contributed equally to this work.
Funding information National Key Research and Development Program of China, Grant/Award Numbers: 2017YFA0103100, 2017YFA0103103, 2017YFA0103104; National Natural Science Foundation of China, Grant/Award Numbers: 81872553, 81472908, 81800103; Guangzhou Health Care and Cooperative Innovation Major Project, Grant/Award Numbers: 201704020218, 201803040005
Contributor Information
Hengxiang Wang, Email: wanghengxiang123@aliyun.com.
Yanhua Li, Email: shirlylyh@126.com.
Xuetao Pei, Email: peixt@nic.bmi.ac.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Filip S, Mokry J, Vavrova J, et al. Splenectomy influences homing of transplanted stem cells in bone marrow‐ablated mice. Stem Cells Dev. 2012;21:702‐709. [DOI] [PubMed] [Google Scholar]
- 2. Shimizu T, Bannai M, Kawamura H, et al. Organ specificity of c‐kit+ lymphoid precursors in the liver, thymus, and bone marrow. Eur J Haematol. 2000;64:416‐425. [DOI] [PubMed] [Google Scholar]
- 3. Copelan EA. Hematopoietic stem‐cell transplantation. N Engl J Med. 2006;354:1813‐1826. [DOI] [PubMed] [Google Scholar]
- 4. Khurana S, Buckley S, Schouteden S, et al. A novel role of BMP4 in adult hematopoietic stem and progenitor cell homing via Smad independent regulation of integrin‐alpha4 expression. Blood. 2013;121:781‐790. [DOI] [PubMed] [Google Scholar]
- 5. Prabhash K, Khattry N, Bakshi A, et al. CD26 expression in donor stem cell harvest and its correlation with engraftment in human haematopoietic stem cell transplantation: potential predictor of early engraftment. Ann Oncol. 2010;21:582‐588. [DOI] [PubMed] [Google Scholar]
- 6. Aljitawi OS, Paul S, Ganguly A, et al. Erythropoietin modulation is associated with improved homing and engraftment after umbilical cord blood transplantation. Blood. 2016;128:3000‐3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cutler C, Multani P, Robbins D, et al. Prostaglandin‐modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074‐3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaushansky K, Lok S, Holly RD, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c‐Mpl ligand thrombopoietin. Nature. 1994;369:568‐571. [DOI] [PubMed] [Google Scholar]
- 9. Solar GP, Kerr WG, Zeigler FC, et al. Role of c‐mpl in early hematopoiesis. Blood. 1998;92:4‐10. [PubMed] [Google Scholar]
- 10. Fox N, Priestley G, Papayannopoulou T, Kaushansky K. Thrombopoietin expands hematopoietic stem cells after transplantation. J Clin Invest. 2002;110:389‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qian H, Buza‐Vidas N, Hyland CD, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671‐684. [DOI] [PubMed] [Google Scholar]
- 12. Rongvaux A, Willinger T, Takizawa H, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci USA. 2011;108:2378‐2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sitnicka E, Lin N, Priestley GV, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998‐5005. [PubMed] [Google Scholar]
- 14. Wang C, Zhang B, Wang S, et al. Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation. Sci Rep. 2015;5:12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu X, Bai X, Chen F, et al. Chinese guidelines for treatment of adult primary immune thrombocytopenia. Int J Hematol. 2018;107:615‐623. [DOI] [PubMed] [Google Scholar]
- 16. Wu Q, Ren J, Wu X, et al. Recombinant human thrombopoietin improves platelet counts and reduces platelet transfusion possibility among patients with severe sepsis and thrombocytopenia: a prospective study. J Crit Care. 2014;29:362‐366. [DOI] [PubMed] [Google Scholar]
- 17. Szilvassy SJ, Meyerrose TE, Ragland PL, Grimes B. Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood, and fetal liver. Blood. 2001;98:2108‐2115. [DOI] [PubMed] [Google Scholar]
- 18. Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444‐5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heazlewood SY, Oteiza A, Cao H, Nilsson SK. Analyzing hematopoietic stem cell homing, lodgment, and engraftment to better understand the bone marrow niche. Ann N Y Acad Sci. 2014;1310:119‐128. [DOI] [PubMed] [Google Scholar]
- 20. Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901‐1910. [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Li L. Understanding hematopoietic stem‐cell microenvironments. Trends Biochem Sci. 2006;31:589‐595. [DOI] [PubMed] [Google Scholar]
- 22. Levesque JP, Hendy J, Takamatsu Y, et al. Mobilization by either cyclophosphamide or granulocyte colony‐stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440‐449. [DOI] [PubMed] [Google Scholar]
- 23. Pruijt JF, Fibbe WE, Laterveer L, et al. Prevention of interleukin‐8‐induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP‐9). Proc Natl Acad Sci USA. 1999;96:10863‐10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP‐9 mediated release of kit‐ligand. Cell. 2002;109:625‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou J, Li J, Rosenbaum DM, Barone FC. Thrombopoietin protects the brain and improves sensorimotor functions: reduction of stroke‐induced MMP‐9 upregulation and blood‐brain barrier injury. J Cereb Blood Flow Metab. 2011;31:924‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim YH, Kwon HJ, Kim DS. Matrix metalloproteinase 9 (MMP‐9)‐dependent processing of betaig‐h3 protein regulates cell migration, invasion, and adhesion. J Biol Chem. 2012;287:38957‐38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoggatt J, Singh P, Tate TA, et al. Rapid mobilization reveals a highly engraftable hematopoietic stem cell. Cell. 2018;172:191‐204.e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jin F, Zhai Q, Qiu L, et al. Degradation of BM SDF‐1 by MMP‐9: the role in G‐CSF‐induced hematopoietic stem/progenitor cell mobilization. Bone Marrow Transplant. 2008;42:581‐588. [DOI] [PubMed] [Google Scholar]
- 29. Majka M, Ratajczak MZ. Biological role of the CXCR4‐SDF‐1 axis in normal human hematopoietic cells. Methods Mol Biol. 2006;332:103‐114. [DOI] [PubMed] [Google Scholar]
- 30. Peled A, Grabovsky V, Habler L, et al. The chemokine SDF‐1 stimulates integrin‐mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD‐3100, a novel antagonist of the CXCR‐4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Hennik PB, de Koning AE, Ploemacher RE. Seeding efficiency of primitive human hematopoietic cells in nonobese diabetic/severe combined immune deficiency mice: implications for stem cell frequency assessment. Blood. 1999;94:3055‐3061. [PubMed] [Google Scholar]
- 33. Ku H, Yonemura Y, Kaushansky K, Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996;87:4544‐4551. [PubMed] [Google Scholar]
- 34. Broudy VC, Lin NL, Kaushansky K. Thrombopoietin (c‐mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin‐11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85:1719‐1726. [PubMed] [Google Scholar]
- 35. Lanzkron SM, Collector MI, Sharkis SJ. Hematopoietic stem cell tracking in vivo: a comparison of short‐term and long‐term repopulating cells. Blood. 1999;93:1916‐1921. [PubMed] [Google Scholar]
- 36. Srour EF, Jetmore A, Wolber FM, et al. Homing, cell cycle kinetics and fate of transplanted hematopoietic stem cells. Leukemia. 2001;15:1681‐1684. [DOI] [PubMed] [Google Scholar]
- 37. Ratajczak MZ, Kim CH, Abdel‐Latif A, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF‐1 gradients. Leukemia. 2012;26:63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase‐9 (MMP‐9): the next decade. Crit Rev Biochem Mol Biol. 2013;48:222‐272. [DOI] [PubMed] [Google Scholar]
- 39. Bouchard F, Bélanger SD, Biron‐Pain K, St‐Pierre Y. EGR‐1 activation by EGF inhibits MMP‐9 expression and lymphoma growth. Blood. 2010;116:759‐766. [DOI] [PubMed] [Google Scholar]
- 40. Das A, Fernandez‐Zapico ME, Cao S, et al. Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor‐induced endothelial cell migration. J Biol Chem. 2006;281:39105‐39113. [DOI] [PubMed] [Google Scholar]
- 41. Levy B, Arnason JE, Bussel JB. The use of second‐generation thrombopoietic agents for chemotherapy‐induced thrombocytopenia. Curr Opin Oncol. 2008;20:690‐696. [DOI] [PubMed] [Google Scholar]
- 42. Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104:1112‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bachegowda LS, Saliba RM, Ramlal R, et al. Predictive model for survival in patients with AML/MDS receiving haploidentical stem cell transplantation. Blood. 2017;129:3031‐3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Passweg JR, Baldomero H, Bader P, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52:811‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Z, Zheng X, Yan H, li D, Wang H. Good outcome of haploidentical hematopoietic SCT as a salvage therapy in children and adolescents with acquired severe aplastic anemia. Bone Marrow Transplant. 2014;49:1481‐1485. [DOI] [PubMed] [Google Scholar]
- 46. Kao YR, Chen J, Narayanagari SR, et al. Thrombopoietin receptor‐independent stimulation of hematopoietic stem cells by eltrombopag. Sci Transl Med. 2018;10:eaas9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bal G, Fabian D, Maia D, Ringel F, Salama A. Effect of thrombopoietin receptor agonists on leukocyte and haematopoietic stem and progenitor cells in the peripheral blood of patients with immune thrombocytopenic purpura. Ann Hematol. 2017;96:2045‐2056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 TPO enhances short‐term engraftment of hematopoietic cells and increases survival of mice following BM transplantation. (A and B) The luciferase activities of donor hematopoietic cells in the recipients at 2 and 3 weeks after transplantation. (C) The survival rates of the lethally irradiated recipients receiving 2.5 × 105 donor BM MNCs and single doses of TPO or PBS (n = 10 each).
Supplemental Figure 2 TPO treatment reduces MMP‐9 proteolytic activity. (A) The MMP‐9 proteolytic activity in the BM supernatants of lethally irradiated mice. The BM supernatants were collected from the mice at 20 hours after single doses of TPO or PBS injection. Two femurs from each mouse were flushed with 500 μL PBS. The equal volume of the BM supernatants were loaded onto SDS‐polyacrylamide gel for zymographic analysis. (B) The MMP‐9 proteolytic activity in the culture medium of the mouse BM cells. The BM cells were isolated from normal mice and resuspended in serum‐free IMDM medium. After receiving 5 Gy irradiation, the cells were cultured with or without 50 ng/mL TPO for 20 hours. The cell‐conditioned medium at equal volume was used for zymographic analysis at 20 hours after in vitro treatment.
Supplemental Figure 3 Q‐PCR for TIMP1‐4 gene expression in the recipient BM cells at 18‐20 hours following single dose of TPO or PBS injection.
Supplemental Figure 4 Q‐PCR for EGR‐1 and KLF6 gene expression in cultured mouse BM cells with or without TPO treatment for 6 hours, 12 hours, 18 hours and 24 hours. BM cells were isolated from normal mice and cultured at the concentration of 5 × 106/ml in StemSpanTM SFEM medium with or without 50 ng/mL TPO for different time. *P < 0.05, **P < 0.01, compared to the control group.
Appendix S1: Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.


