Abstract
Aims
Although immune-mediated inflammatory diseases (IMID) are associated with multiple mental health conditions, there is a paucity of literature assessing personality disorders (PDs) in these populations. We aimed to estimate and compare the incidence of any PD in IMID and matched cohorts over time, and identify sociodemographic characteristics associated with the incidence of PD.
Methods
We used population-based administrative data from Manitoba, Canada to identify persons with incident inflammatory bowel disease (IBD), multiple sclerosis (MS) and rheumatoid arthritis (RA) using validated case definitions. Unaffected controls were matched 5:1 on sex, age and region of residence. PDs were identified using hospitalisation or physician claims. We used unadjusted and covariate-adjusted negative binomial regression to compare the incidence of PDs between the IMID and matched cohorts.
Results
We identified 19 572 incident cases of IMID (IBD n = 6,119, MS n = 3,514, RA n = 10 206) and 97 727 matches overall. After covariate adjustment, the IMID cohort had an increased incidence of PDs (incidence rate ratio [IRR] 1.72; 95%CI: 1.47–2.01) as compared to the matched cohort, which remained consistent over time. The incidence of PDs was similarly elevated in IBD (IRR 2.19; 95%CI: 1.69–2.84), MS (IRR 1.79; 95%CI: 1.29–2.50) and RA (IRR 1.61; 95%CI: 1.29–1.99). Lower socioeconomic status and urban residence were associated with an increased incidence of PDs, whereas mid to older adulthood (age 45–64) was associated with overall decreased incidence. In a restricted sample with 5 years of data before and after IMID diagnosis, the incidence of PDs was also elevated before IMID diagnosis among all IMID groups relative to matched controls.
Conclusions
IMID are associated with an increased incidence of PDs both before and after an IMID diagnosis. These results support the relevance of shared risk factors in the co-occurrence of PDs and IMID conditions.
Key words: Chronic conditions, epidemiology, mental health, risk factors
Introduction
Immune-mediated inflammatory diseases (IMID) are characterised by systemic inflammation and immune dysregulation, with three of the most common and functionally severe IMID in Canada being inflammatory bowel disease (IBD), multiple sclerosis (MS) and rheumatoid arthritis (RA; Wong et al., 2010; Coward et al., 2018; Gilmour et al., 2018). Mental health disorders, particularly anxiety and depressive disorders are more prevalent among individuals with IMID relative to the general population (Matcham et al., 2013, 2015; Tribbick et al., 2015; Marrie et al., 2017) and those experiencing comorbid mental health difficulties exhibit poorer functional outcomes, including lower employment rates (Gilworth et al., 2003; De Boer et al., 2016), greater disability (Bombardier et al., 2011; Chan et al., 2017) and lower quality of life (Matcham et al., 2016; Kochar et al., 2017; Amtmann et al., 2018).
The high co-occurrence of mental health disorders in IMID populations is likely multifactorial in aetiology. The onset and experience of disease is a stressor, capable of producing or exacerbating mental health concerns (Sokal et al., 2004). Conversely, symptomatology associated with poor mental health (e.g., chronic sleep difficulties) may also trigger physical health problems (Leng et al., 2016). Some evidence suggests mutual points of origin between IMID and mental health disorders such as depression (Krishnadas and Cavanagh, 2012), including hypothalamic-pituitary-adrenal axis overactivity (McEwen, 2004), upregulation of cytokine-induced inflammatory processes (Kendall-Tackett, 2009) and genetic mutations (Euesden et al., 2017).
Although personality disorders (PDs) are elevated among those with various chronic physical health conditions (Quirk et al., 2015) and potentially complicate the management of an IMID, they have been largely overlooked in the IMID literature. In part, this is due to taxonomic concerns with the diagnosis of a PD. There are shared characteristics, albeit varying in salience to the presentation, among PDs (e.g., hostility, evident in paranoid, narcissistic and antisocial PD; Westen et al., 2012). Further, given personality pathology is an extension of normative personality functioning, distinction is open to bias (Bakker, 2019). However, recent emphasis has been placed on improving empirically based delineation of personality dysfunction, allowing for new avenues of investigation.
In the existing literature, neuroticism has been reported in persons with IBD and RA (Hyphantis et al., 2006; Tosic-Golubovic et al., 2010), obsessive-compulsive personality characteristics have been reported in persons with RA and MS (Marcenaro et al., 1999; Mohamadi et al., 2016), and indications of Cluster B personality styles (i.e., narcissistic, borderline, histrionic) have been described in persons with MS and RA (Marcenaro et al., 1999; Incerti et al., 2015). However, much of this research has relied on personality inventories rather than clinician-based diagnoses, given the diagnostic issues, and population-based estimates have been particularly limited (Hyphantis et al., 2006; Vidal et al., 2008; Incerti et al., 2015). As such, differences in study design have limited comparability across diseases, which may drive apparent discrepancies between IMID conditions. For example, Harel et al. (2007) reported 3% of their MS sample presented with personality dysfunction, whereas Robertson et al. (1989) identified 60% of their IBD sample as non-normative in terms of a personality profile.
We aimed to examine the association between any PD and three IMID concurrently (IBD, MS and RA) in a large, population-based sample using physician-based administrative clinical data. Specifically, we compared the incidence over time of any PD in IMID cohorts and matched controls, and examined sociodemographic factors associated with PDs. We also assessed whether the incidence of any PD is increased in the 5 years before the diagnosis of IMID, as compared to a matched population and compared to the 5-years post-diagnosis.
Methods
Setting
This retrospective cohort study was conducted in Manitoba, Canada, a province with a population of approximately 1.3 million. Health care in Manitoba is universal and publically funded, and the province maintains administrative databases of all health services delivered; these data are collected at the time of service delivery. We accessed these databases through the Manitoba Population Research Data Repository at the Manitoba Centre for Health Policy. This study was approved by the University of Manitoba Health Research Ethics Board and data access was approved by the Manitoba Health Information Privacy Committee.
Data sources
We used administrative databases for the period from April 1, 1984 (the earliest date available) to March 31, 2013 (latest date available at the time of study approval). The population registry includes sociodemographic data, dates of health care coverage, as well as residence location by postal code for each provincial resident eligible to receive health services. Since 1984, every Manitoba resident has been assigned a unique personal health identification number (PHIN). All physician claims and hospital records include the individual's unique PHIN. Physician claims data provide the date of service and one physician-assigned diagnosis per visit, using the International Classification of Diseases (ICD), 9th revision, Clinical Modification (ICD-9-CM). Hospital records data provide hospital admission and separation dates and information regarding hospital admissions, including up to 25 diagnoses using ICD-9-CM (and ICD-10-CA codes after 2004). We also identified outpatient prescription dispensations, including date, drug name and drug identification number (DIN) using the Drug Program Information Network (DPIN; beginning in 1995), which uses the World Health Organization's Anatomical Therapeutic Chemical (ATC) Classification System. To maintain confidentiality, databases were linked using anonymised unique identifiers at the individual level.
Study populations
First, using validated case definitions we created cohorts of all Manitobans with IBD (Bernstein et al., 1999), MS (Al-Sakran et al., 2018) and RA (Hitchon et al., 2019). The date of diagnosis, or index date, was defined as the date of the first health claim for the IMID condition during the study period. To identify true index dates and thereby incident cases of IMID, individuals with relevant health claims (that is, claims for IBD, MS or RA) 5 years before the date of their IMID diagnosis were excluded. Given the need to look back 5 years and availability of administrative data beginning in 1984, the earliest index dates occurred in 1989 and the last in 2011. We established cohorts matched 5:1 to each IMID participant on sex, year of birth within a range of ±5 years and region of residence as determined by the first three digits of the postal code. These matched cohorts excluded individuals with ICD-9-CM/ICD-10-CA diagnosis codes for IBD, demyelinating disease, RA and related disorders, and use of MS-specific disease-modifying therapies (which were part of the MS case definition). Each control was assigned the index date of its matched case.
For analyses involving pre- and post-IMID index comparisons in the incidence of any PD, we further restricted the study population. Specifically, we required the incident disease cases and their matched controls to have ⩾5.5 continuous years of data available before and after the index date; this allowed for a 5-year pre-index and 5-year post-index window, with the 6-month intervals before and after the index date comprising the index year. Thus index dates ranged from 1989 to 2008.
Personality disorders
PDs were identified based on the presence of ⩾1 hospitalisation or physician claim with ICD-9-CM/10-CA codes 301, F21, F60, F61 and F69 (Chartier et al., 2018; Beaulieu et al., 2019). The case definition selection is intended to exclude those with personality changes secondary to medical conditions. To identify any incident PD, the first claim for the PD had to be preceded by 5 years with no PD claims as defined above (301, F21, F60, F61 and F69). Therefore incidence is reported from April 1, 1989 through March 31, 2012. To estimate lifetime (period) prevalence, once a person met the case definition for a PD, he or she was considered affected in all subsequent years if living in Manitoba. However, some individuals with a PD may experience periods of remission (Gunderson et al., 2011). Therefore, we estimated the annual period prevalence of PDs through those requiring ongoing care each year; a person was only counted as an annual prevalent case if there was ⩾1 hospital or physician claims for the disorder in that year, otherwise, they were considered unaffected.
Covariates
Covariates included sex (male as reference group), age (18–24, 25–44, 45–64, ⩾65; 18–24 years as reference group), socioeconomic status (SES, in quintiles; the highest quintile as reference group), region (urban, rural; rural as reference group) and annual number of visits to a physician unrelated to a psychiatric disorder. SES was determined through linking postal codes to dissemination-area level census data from Statistics Canada to derive the Socioeconomic Factor Index version 2 (SEFI-2), an indicator based on average household income, per cent of single parents households, unemployment rate and high school education rate, where higher scores indicate lower SES (Chateau, Metge, Prior, and Soodeen, 2012). Urban regions encompassed Winnipeg (population >600 000) and Brandon (population >47 000). Models of incidence pre and post-IMID index also included a variable for index year (1999–2007 v. 1989–1998).
Analyses
We summarised the sociodemographic characteristics of the study cohorts using descriptive statistics. We estimated the crude annual incidence, lifetime and annual period prevalence, and 95% confidence intervals (CI) of any PD for the disease cohorts (i.e., combined IMID, IBD, MS, RA) and their matched control cohorts. Estimates were age- and sex-standardised to the 2010 Canadian population. We then tested for differences in incidence rates of any PD between the disease cohorts and the matched cohorts using unadjusted and covariate-adjusted negative binomial regression models. These models included the natural logarithm of the number of person-years as an offset to account for variable follow-up, and the covariates defined above. Additional covariate-adjusted models included the interaction of cohort × year to assess if there was a significant difference in the temporal trend for the disease and matched cohorts. We report incidence rate ratios (IRR) and 95%CI for these models.
In the subgroup with 5 years of data before and after the IMID index date, we estimated the annual incidence of any PD in each year of the pre-index, index and post-index periods. We tested whether the temporal trends in the incidence of any PD changed within the pre- and post-index periods, and whether these trends differed between the pre- and post-index periods. We also compared whether the findings differed between the IMID and matched cohorts. Therefore we created multivariable negative binomial regression models that incorporated three main effects of cohort (IMID v. matched [reference]), period (pre-diagnosis [reference], diagnosis, post-diagnosis) and year (continuous variable from 1 to 5 in the pre-diagnosis and post-diagnosis periods, 0 for the year of diagnosis) as well as two-way interactions between cohort and period, and year and period, and a three-way interaction between cohort, period and year. These models also included covariates as described above. We conducted separate models for each IMID cohort.
Statistical analyses were performed using SAS V9.4 (SAS Institute Inc., Cary, NC.)
Results
Study population
We identified 19 572 incident cases of IMID, including 6119 incident cases of IBD, 3514 incident cases of MS, and 10 206 incident cases of RA (Marrie et al., 2017). The matched cohort comprised 97 727 persons, with 30 573 persons matched to the IBD cohort, 17 526 persons matched to the MS cohort, and 50 960 persons matched to the RA cohort. A majority of the sample was female (66.7%), with a mean age at diagnosis ranging from 40.8 (12.5) years for MS to 53.7 (16.0) years for RA (Table 1).
Table 1.
Characteristics of the study cohorts and subgroups
| Characteristics | Combined IMID | Combined IMID matches | IBD | IBD matches | MS | MS matches | RA | RA matches |
|---|---|---|---|---|---|---|---|---|
| Whole population (index dates: 1989–2013) | ||||||||
| n | 19 572 | 97 727 | 6119 | 30 573 | 3514 | 17 526 | 10 206 | 50 960 |
| Female: n (%) | 13 053 (66.7) | 65 185 (66.7) | 3330 (54.4) | 16 642 (54.4) | 2544 (72.4) | 12 697 (72.4) | 7369 (72.2) | 36 793 (72.2) |
| Age at diagnosis (yrs): mean (s.d.) | 47.7 (17.0) | 47.7 (16.9) | 41.9 (17.0) | 41.9 (17.0) | 40.8 (12.5) | 40.8 (12.5) | 53.7 (16.0) | 53.7 (16.0) |
| Duration (yrs) of follow-up from index date: median (IQR) | 9.67 (4.66, 15.5) | 9.51 (4.45, 15.5) | 9.98 (4.64, 16.0) | 9.69 (4.34, 15.9) | 10.3 (4.90, 16.1) | 10.5 (5.0, 16.3) | 9.19 (4.58, 14.8) | 9.05 (4.33, 14.9) |
| Urban residence: n (%) | 12 244 (62.6) | 61 138 (62.6) | 4095 (66.9) | 20 460 (66.9) | 2344 (66.7) | 11 685 (66.7) | 5981 (58.6) | 29 870 (58.6) |
| Socioeconomic status | −0.05 (0.95) | −0.09 (0.99) | −0.26 (0.91) | −0.20 (0.88) | −0.21 (0.89) | −0.18 (0.87) | 0.05 (1.03) | 0.08 (1.00) |
| Restricted subgroup (index dates: 1989–2008) | ||||||||
| n | 12 141 | 65 424 | 3766 | 20 355 | 2190 | 12 315 | 6350 | 33 584 |
| Female: n (%) | 8220 (67.7) | 44 427 (67.9) | 2062 (54.7) | 11 230 (55.2) | 1621 (74.0) | 9108 (74.0) | 4658 (77.2) | 24 692 (73.5) |
| Age at diagnosis (yrs): mean (s.d.) | 47.0 (16.2) | 46.6 (16.0) | 41.5 (16.4) | 41.0 (15.9) | 40.2 (11.5) | 40.5 (11.7) | 52.7 (15.4) | 52.3 (15.3) |
| Duration (yrs) of follow-up from index date: median (IQR) | 12.8 (9.0, 17.4) | 12.8 (9.0, 17.4) | 13.4 (9.3, 18.1) | 13.3 (9.2, 17.9) | 13.4 (9.4, 18.0) | 13.2 (9.3, 18.0) | 12.4 (8.8, 16.7) | 12.4 (8.8, 16.7) |
| Urban residence: n (%) | 7495 (61.7) | 40 809 (62.4) | 2491 (66.1) | 13 545 (66.5) | 1435 (65.5) | 8172 (66.3) | 3681 (58.0) | 19 637 (58.5) |
| Socioeconomic status | −0.11 (0.99) | −0.06 (0.96) | −0.28 (0.92) | −0.20 (0.89) | −0.27 (0.90) | −0.20 (0.87) | 0.04 (1.00) | 0.08 (1.00) |
IMID, immune-mediated inflammatory diseases; IBD, inflammatory bowel disease; MS, multiple sclerosis; RA, rheumatoid arthritis.
Note. Adapted from Marrie et al. (2017).
Prevalence and incidence of personality disorders
In 2011, the crude lifetime prevalence of any PD per 100 persons was higher in the combined IMID cohort (4.72; 95% CI: 4.38–5.09) than in the matched cohort (3.10; 95% CI: 2.98–3.24). After age and sex-standardisation, the lifetime prevalence of any PD remained 50% higher in the combined IMID than the matched cohort (prevalence ratio 1.51; 95% CI: 1.34–1.70). Similarly, the lifetime prevalence of any PD was elevated in the IBD, MS and RA cohorts as compared to their matched controls. In 2011, the standardised annual prevalence of any PD per 100 persons was almost two-fold higher in the combined IMID cohort (0.63; 95% CI: 0.42–0.94) than in the matched cohort (0.33; 95% CI: 0.26–0.41). The annual prevalence of any PD was also higher in the individual disease cohorts as compared to their matches (IBD: 0.64% v. 0.32%; MS: 0.69% v. 0.28%; RA: 0.46% v. 0.28%).
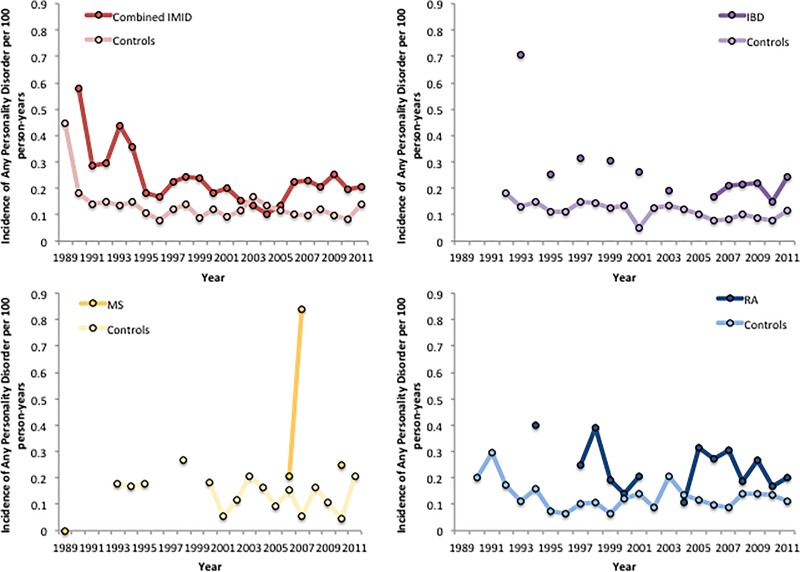
In 2011, the crude incidence of any PD per 100 person-years was higher in the combined IMID cohort (0.21; 95% CI: 0.14–0.30) than in the matched cohort (0.13; 95% CI: 0.11–0.16). After age and sex-standardisation the incidence of any PD remained non-significantly higher in the combined IMID cohort (IRR 1.48; 95% CI: 0.94–2.33). The incidence of any PD was also higher in the individual IMID cohorts (see Fig. 1).
Fig. 1.
Incidence of any personality disorder per 100 person-years, age and sex-standardized to the 2010 Canadian population, in disease cohorts and matched controls across study period. Absent lines are due to suppressed cells.
Sociodemographic factors associated with personality disorders
After adjusting for age, sex, year, SES, region and number of physician visits, the combined IMID cohort had a higher incidence of any PD than the matched cohort (Table 2). Compared to the highest SES quintile, all lower SES quintiles were associated with an increased incidence of any PD. Compared to those living in a rural region, individuals living in an urban region had an increased incidence of any PD. Individuals aged 25–64 years had a lower incidence of any PD compared to those aged 18–24 years. The incidence of any PD declined slightly over time but there was no observed interaction between cohort and time. The findings were similar for each individual IMID (Table 2).
Table 2.
Adjusteda incidence rate ratios and 95% confidence intervals for the association between sociodemographic characteristics and any personality disorder among study cohorts
| Combined IMID | IBD | MS | RA | |
|---|---|---|---|---|
| Variable | ||||
| Cohort | ||||
| Matches | 1.00 | 1.00 | 1.00 | 1.00 |
| IMID | 1.72 (1.47–2.01)* | 2.19 (1.69–2.84)* | 1.79 (1.29–2.50)* | 1.61 (1.29–1.99)* |
| Sex | ||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 1.10 (0.95–1.28) | 1.22 (0.99–1.51) | 0.79 (0.57–1.11) | 1.06 (0.85–1.32) |
| Age (years) | ||||
| 18–24 | 1.00 | 1.00 | 1.00 | 1.00 |
| 25–44 | 0.83 (0.67–1.02) | 0.85 (0.63–1.14) | 0.71 (0.44–1.13) | 0.69 (0.48–1.00) |
| 45–64 | 0.54 (0.43–0.68)* | 0.56 (0.41–0.77)* | 0.51 (0.32–0.82)* | 0.42 (0.28–0.64)* |
| ⩾65 | 0.98 (0.80–1.21) | 0.92 (0.66–1.29) | 1.03 (0.53–2.01) | 0.84 (0.57–1.24) |
| Socioeconomic status | ||||
| Quintile 1 (lowest) | 2.39 (1.98–2.87)* | 2.45 (1.78–3.38)* | 2.90 (1.88–4.46)* | 2.26 (1.74–2.93)* |
| Quintile 2 | 1.94 (1.60–2.35)* | 2.09 (1.53–2.87)* | 1.57 (0.99–2.49) | 2.00 (1.53–2.62)* |
| Quintile 3 | 1.49 (1.22–1.82)* | 1.45 (1.04–2.03)* | 1.49 (0.94–2.35) | 1.56 (1.18–2.07)* |
| Quintile 4 | 1.56 (1.29–1.89)* | 1.58 (1.15–2.16)* | 1.74 (1.15–2.63)* | 1.48 (1.12–1.95)* |
| Quintile 5 (highest) | 1.00 | 1.00 | 1.00 | 1.00 |
| Region | ||||
| Rural | 1.00 | 1.00 | 1.00 | 1.00 |
| Urban | 1.86 (1.64–2.11)* | 1.56 (1.24–1.97)* | 1.78 (1.32–2.41)* | 2.02 (1.71–2.38)* |
| Year | 0.99 (0.98–1.00) | 0.97 (0.95–1.00)* | 0.97 (0.95–1.00)* | 1.00 (0.98–1.02) |
| Number of physician visits | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
Adjusted for age, sex, year, socioeconomic status, region and number of physician visits.
*p < 0.05.
Bold indicates statistical significance.
Personality disorders before and after IMID diagnosis
After we restricted the study population to individuals with 5 years of data before and after the IMID-index year, we were able to include 12 141 incident cases with IMID, 3766 with IBD, 2190 with MS and 6350 with RA, as well as a matched subgroup of 65 424 persons (Table 1). The characteristics of the IMID and matched subgroups were similar to those of the larger study population from which they were drawn (Table 1).
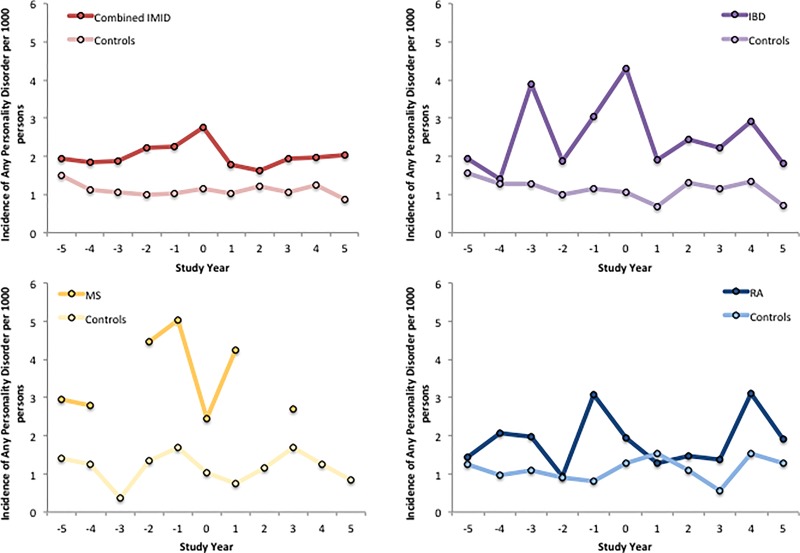
After age and sex-standardisation, incidence of any PD was higher in all IMID subgroups compared to their matched subgroups in the index year, although this did not reach statistical significance for the MS or RA subgroups (Table 3, Fig. 2). The incidence of any PD was also consistently higher in the combined IMID subgroup than the matched subgroup during the pre-index and post-index periods (Table 3, Fig. 2).
Table 3.
Standardised incidence rate ratios for any personality disorder in disease cohorts (v. matched controls), presented 5 years pre-index to 5 years post-index
| Incidence rate ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Combined IMID | IBD | MS | RA | |
| 5 years pre- | 1.29 (0.81–2.06) | 1.25 (0.54–2.86) | 2.09 (0.88–4.99) | 1.15 (0.52–2.55) |
| 4 tears pre- | 1.61 (1.00–2.61) | 1.08 (0.43–2.74) | 2.24 (0.77–6.51) | 2.14 (0.99–4.61) |
| 3 years pre- | 1.81 (1.10–2.95)* | 3.04 (1.50–6.19)* | – | 1.80 (0.77–4.22) |
| 2 years pre- | 2.24 (1.36–3.70)* | 1.87 (0.81–4.31) | 3.34 (1.19–9.37)* | 1.03 (0.35–3.01) |
| 1 year pre- | 2.22 (1.37–3.60)* | 2.62 (1.32–5.19)* | 2.97 (0.71–12.50) | 3.86 (1.10–13.55)* |
| Year 0a | 2.38 (1.52–3.72)* | 4.06 (2.08–7.94)* | 2.39 (0.84–6.83) | 1.54 (0.66–3.63) |
| 1 year post- | 1.70 (1.02–2.84)* | 2.77 (1.16–6.64)* | 5.74 (1.65–20.01)* | 0.83 (0.34–2.02) |
| 2 years post- | 1.34 (0.79–2.26) | 1.85 (0.86–3.99) | – | 1.36 (0.51–3.60) |
| 3 years post- | 1.84 (1.10–3.10)* | 1.95 (0.87–4.37) | 1.60 (0.56–4.62) | 2.44 (1.00–5.95)* |
| 4 years post- | 1.57 (0.96–2.56) | 2.17 (1.05–4.49)* | – | 2.02 (0.55–7.49) |
| 5 years post- | 2.31 (1.38–3.85)* | 2.51 (1.01–6.24)* | – | 1.48 (0.62–3.55) |
IMID, immune-mediated inflammatory disease, IBD, inflammatory bowel disease, MS, multiple sclerosis, RA, rheumatoid arthritis.
Index year is represented by 0. Matched controls as reference group, *p < 0.05.
Bold indicates statistical significance.
Fig. 2.
Incidence of any personality disorder per 1,000 person-years, age and sex-standardized to the 2010 Canadian population, in disease cohorts and matched controls, across the 5 years before and 5 years after the index date. Index year is represented by 0. Absent lines are due to suppression.
After adjusting for age, sex, index year, SES, region of residence and number of physician visits, there was no linear change in any PD incidence during the pre-index or post-index periods in the IMID subgroups. No difference in rates of change was observed between the pre-index and post-index periods (Table 4).
Table 4.
Incidence rate ratios (95% confidence intervals) showing association between personality disorders pre- and post-index date and immune-mediated inflammatory disease
| Cohorts | Model 1 | Model 2 |
|---|---|---|
| Combined IMID | ||
| Year effect: cases pre-index | 1.01 (0.89–1.15) | 1.01 (0.89–1.15) |
| Year effect: cases post-index | 0.98 (0.89–1.08) | 0.98 (0.89–1.08) |
| Post-pre ratio: cases | 0.97 (0.82–1.14) | 0.97 (0.83–1.13) |
| Year effect: controls pre-index | 0.91 (0.84–0.98)* | 0.96 (0.92–1.01) |
| Year effect: controls post-index | 0.97 (0.91–1.03) | 1.00 (0.95–1.05) |
| Post-pre ratio: controls | 1.07 (0.97–1.18) | 1.04 (0.95–1.13) |
| Case ratio/control ratio | 0.91 (0.75–1.10) | 0.93 (0.78–1.12) |
| Inflammatory bowel disease | ||
| Year effect: cases pre-index | 1.18 (0.96–1.46) | 1.19 (0.97–1.46) |
| Year effect: cases post-index | 0.93 (0.80–1.08) | 0.93 (0.80–1.08) |
| Post-pre ratio: cases | 0.79 (0.61–1.02) | 0.78 (0.61–1.01) |
| Year effect: controls pre-index | 0.89 (0.79–1.01) | 0.92 (0.85–1.00) |
| Year effect: controls post-index | 0.99 (0.89–1.09) | 1.00 (0.92–1.10) |
| Post-pre ratio: controls | 1.10 (0.94–1.29) | 1.09 (0.94–1.27) |
| Case ratio/control ratio | 0.71 (0.53–0.97)* | 0.72 (0.53–0.97)* |
| Multiple sclerosis | ||
| Year effect: cases pre-index | 0.96 (0.77–1.21) | 0.97 (0.77–1.22) |
| Year effect: cases post-index | 0.86 (0.69–1.07) | 0.85 (0.69–1.06) |
| Post-pre ratio: cases | 0.89 (0.65–1.23) | 0.88 (0.64–1.21) |
| Year effect: controls pre-index | 0.86 (0.73–1.01) | 0.94 (0.85–1.05) |
| Year effect: controls post-index | 0.96 (0.84–1.08) | 1.00 (0.90–1.12) |
| Post-pre ratio: controls | 1.11 (0.91–1.36) | 1.06 (0.88–1.29) |
| Case ratio/control ratio | 0.80 (0.55–1.17) | 0.83 (0.58–1.20) |
| Rheumatoid arthritis | ||
| Year effect: cases pre-index | 0.88 (0.71–1.09) | 0.88 (0.72–1.08) |
| Year effect: cases post-index | 1.11 (0.95–1.30) | 1.11 (0.95–1.3) |
| Post-pre ratio: cases | 1.26 (0.97–1.64) | 1.27 (0.98–1.64) |
| Year effect: controls pre-index | 0.92 (0.82–1.03) | 0.99 (0.93–1.06) |
| Year effect: controls post-index | 0.95 (0.87–1.03) | 0.98 (0.92–1.06) |
| Post-pre ratio: controls | 1.03 (0.89–1.18) | 0.99 (0.87–1.12) |
| Case ratio/control ratio | 1.23 (0.91–1.65) | 1.28 (0.96–1.70) |
The year variable assesses whether there is an annual linear increase in incidence in the cohort (cases or controls) and period (pre-index or post-index) of interest. Post-pre ratio compares the year effect in the post-index v. pre-index periods. A ratio <1 indicates the rise in incidence was greater in the pre-index period than the post-index period whereas a ratio indicates the yearly rise in incidence is greater in the post-index than the pre-index period. The case ratio/control ratio variable assesses whether the pre-post ratios differ in the cases and controls. Model 1, unadjusted; Model 2, adjusted for sex, age, index year, urban/rural, SEF12 quintiles; Model 3, adjusted for sex, age, index year, urban/rural, SEF12 quintiles, non-psychiatric physician visits, *p < 0.05.
Bold indicates statistical significance.
Discussion
Using population-based administrative data for the full study population, we found that the incidence of any PD was elevated in the IMID population relative to matched controls, regardless of the specific IMID condition; the same was true for prevalence. The elevated incidence of any PD was consistent over study years. Younger age, lower SES and urban residence were associated with an increased incidence of any PD. In the restricted subgroup, trends in the incidence of any PD did not differ before and after the IMID diagnosis.
Several possible explanations exist for the increased incidence of PDs in IMID. The increased incidence before IMID diagnosis, even after adjusting for number of physician visits, suggests that the findings do not reflect surveillance bias (i.e., increased probability of detection due to increased observation). A prodromal syndrome characterised by pathological personality patterns is possible, but the stability of our incidence rates argues against this explanation. The most compelling explanation for our results is shared risk factors. Poor psychosocial health, for example, encompassing variables such as past sexual abuse and violence, has been associated with personality dysfunction (Battle et al., 2004), as well as IBD (Caplan et al., 2014), MS (Spitzer et al., 2012) and arthritic diseases (Brennan-Olsen et al., 2019). A pro-inflammatory state secondary to chronic stress exposure (Miller et al., 2002) could also play a role (Cătană et al., 2015; Li et al., 2015; Oglodek et al., 2015; Bartlett et al., 2016). Shared genetic markers (Kendler et al., 2008; Liu et al., 2015; Yarwood et al., 2015; Parnell and Booth, 2017) may also contribute to the development of PDs and IMID. In such situations, we would expect increases in incidence to remain stable over time given inter-individual variance in the timing of the mental and physical health presentations. This phenomenon, previously supported in the context of comorbid anxiety disorders and chronic pain (Asmundson et al., 2002), appears to be demonstrated with our results. The search for common risk factors is an important area for future research on psychiatric comorbidity in IMID populations, as it provides a means of understanding potential mechanisms for the occurrence and maintenance of comorbidity.
There may also be a form of mutual maintenance at play for all IMID groups, similar to that first discussed between chronic health conditions and post-traumatic stress disorder (Sharp and Harvey, 2001). Any of the disease experiences discussed above may amplify hyper-controlled or emotionally dysregulated personality structures, such as those corresponding with obsessive-compulsive PD (OCPD) and borderline PD (BPD). Personality-pathology related outcomes, such as interpersonal difficulties, impede healthy adjustment to illness (Stanton et al., 2007), interact adversely with self-concept in the context of chronic disease (Juth et al., 2008) and worsen disability (Evers et al., 2003). Therefore, future studies should explore the specific nature of personality pathology in IMID populations, the relevance of IMID-specific disease experiences and the role of mutual maintenance in this comorbidity.
Lower SES and urban living have been consistently associated with poorer mental health (Miech et al., 1999; Judd et al., 2002). The former may reflect increased stressors (e.g., lower finances, less social supports) or poorer health behaviours such as smoking or unhealthy diet (Wadsworth, 2015). The latter may reflect relocation into urban settings upon personal need for greater access to mental health supports (Brems et al., 2006) as well as mental health stigma interfering with the access of supports in rural regions (Rost et al., 1993). While individuals between the ages of 45 and 64 years were at a reduced risk of a PD diagnosis, this may be explained by the fact that some PD symptomatology, such as that associated with BPD, presents less explicitly with age, therefore becoming harder to detect (Van Alphen et al., 2012). Relatedly, assessment of personality dysfunction across the lifetime can be affected by clinicians' relatively limited exposure to, and understanding, of healthy aging, thereby confounding norm comparisons (Zweig, 2008). Alternatively, this finding may reflect a true effect, given evidence that mental health symptoms improve with age (Reynolds et al., 2015). Significant findings for the oldest age group may simply be undetected due to a lack of statistical power. Sex was not a predictor of PDs in our study, but this may have been because we combined all PDs and personality pathology demonstrates condition-specific gender differences. For example, schizoid PD and OCPD are more common in males, whereas dependent PD and BPD are more commonly diagnosed in females (Paris, 2004).
Clinicians' recognition of the elevated rates of PD diagnoses across IMID groups is important due to the inherent interpersonal difficulties associated with personality pathology (APA, 2013). Given the need for medical supports among those with chronic physical conditions, this comorbid population is likely to have greater difficulties navigating the health care system (Van Alphen et al., 2012). Our findings highlight the need for increased supports for these individuals. Examples of these supports might include interpersonal effectiveness training (e.g., assertiveness skills) and distress tolerance (e.g., radical acceptance), hallmarks of dialectical behaviour therapy (DBT; Linehan, 2018). In further support of this approach, DBT has previously been postulated as an intervention that may be appropriate for difficult patients reliant on the medical system (Huffman et al., 2003). Future research directions should explore assessment considerations and treatment options in this vulnerable population.
Although our study strengths include large sample size, population-based design and long study period, there are limitations. PDs were identified using hospital and physician claims, therefore diagnoses by non-physician providers may not have been captured. Nonetheless, we expect this potential bias to be non-differential between groups. Nuances in the presentation of personality pathology in the context of physical health conditions may further complicate assessment; for example, BPD in medical settings has been shown to present less characteristically (i.e., graphic self-harm, labile mood) and more somatically (i.e., pain sensitivity, somatic preoccupation; Sansone and Sansone, 2015). Relatedly, given an association between BPD and pain catastrophising (Sansone et al., 2013), the possibility of a reporting bias to physicians cannot be dismissed; yet notably, this association appears to be more related to depressive experience as opposed to personality structure per se (Mun et al., 2016). We focused on PDs, yet less severe pathological personality presentations would not have been captured. We were unable to differentiate between specific PDs. Finally, caution should be applied to interpretation regarding the individual IMID groups, given some small cell sizes.
In summary, persons with IMIDs are at an increased risk of a PD, regardless of the specific IMID. Elevated comorbidity rates may relate to shared risk factors between IMID and PDs but this requires further investigation. PDs warrant greater attention in IMID research and in the care of IMID patients, due to the potential for improving our understanding of the aetiology and treatment of these conditions.
Acknowledgement
The sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript. The authors acknowledge the Manitoba Centre for Health Policy for use of the Manitoba Population Research Data Repository under project #2014-030 (HIPC #2015/2015-19A). The results and conclusions presented are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. Members of the CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease are: Ruth Ann Marrie, James M Bolton, Jitender Sareen, Scott B Patten, Alexander Singer, Lisa M. Lix, Carol A Hitchon, Renée El-Gabalawy, PhD, Alan Katz, John D Fisk, Charles N Bernstein, Lesley Graff, Lindsay Berrigan, Ryan Zarychanski, Christine Peschken, James Marriott
Availability of Data and Materials
The authors received permission to access the data used in this study, however, they are unable to share the data as they are not the data custodians.
Financial support
This study was funded by the Canadian Institutes of Health Research (THC-135234), Crohn's and Colitis Canada, and the Waugh Family Chair in Multiple Sclerosis (to RAM). Dr Bernstein is supported in part by the Bingham Chair in Gastroenterology. Dr Sareen is supported by CIHR #333252. Dr Lix is supported by a Tier I Canada Research Chair. This study and Dr El-Gabalawy, Caitlin Blaney and Jordana Sommer were also supported by University of Manitoba Max Rady College of Medicine Start-Up Funding (to REG).
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of interest
Charles Bernstein has served on Advisory Boards for AbbVie Canada, Ferring Canada, Janssen Canada, Shire Canada, Takeda Canada and Pfizer Canada; Consultant for Mylan Pharmaceuticals; Educational grants from Abbvie Canada, Pfizeer Canada, Shire Canada, Takeda Canada and Janssen Canada. Speaker's panel for Abbvie Canada, Ferring Canada, Medtronic Canada and Shire Canada. Received research funding from Abbvie Canada. Alex Singer holds a grant administered by the Canadian Institute for Military and Veterans Health Research that has funding and in-kind support from IBM and Calian.
References
- Al-Sakran LH, Marrie RA, Blackburn DF, Knox KB and Evan CD (2018) Establishing the incidence and prevalance of multiple sclerosis in Saskatchewan. The Canadian Journal of Neurological Sciences 45, 295–303. [DOI] [PubMed] [Google Scholar]
- Amtmann D, Bamer AM, Kim J, Chung H and Salem R (2018) People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disability and Health Journal 11, 99–107. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Asmundson GJG, Coons MJ, Taylor S and Katz J (2002) PTSD And the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. The Canadian Journal of Psychiatry 47, 930–937. [DOI] [PubMed] [Google Scholar]
- Bakker GM (2019) A new conception and subsequent taxonomy of clinical psychological problems. BMC Psychology 7, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DB, Connelly MA, AbouAssi H, Bateman LA, Tune KN, Huebner JL, Kraus VB, Winegar DA, Otvos JD, Kraus WE and Huffman KM (2016) A novel inflammatory biomarker, GlycA, associates with disease activity in rheumatoid arthritis and cardio-metabolic risk in BMI-matched controls. Arthritis Research and Therapy 18, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle CL, Shea MT, Johnson DM, Yen S, Zlotnick C, Zanarini MC, Sanislow CA, Skodal AE, Gunderson JG, Grilo CM, McGlashan TH and Morey LC (2004) Childhood maltreatment associated with adult personality disorders: findings from the collaborative longitudinal personality disorders study. Journal of Personality Disorders 18, 193–211. [DOI] [PubMed] [Google Scholar]
- Beaulieu T, Krishnamoorthy A, Lima V, Li T, Wu A, Montaner J, Barrios R, Ti L and Stop HIV/AIDS in BC Study Group (2019) Impact of personality disorders on leaving hospital against medical advice among people with HIV in British Columbia, Canada. Social Psychiatry and Psychiatric Epidemiology, 1–7. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Blanchard JF, Rawsthorne P and Wajda A (1999) Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. American Journal of Epidemiology 149, 916–924. [DOI] [PubMed] [Google Scholar]
- Bombardier C, Hawker G and Mosher D (2011) The impact of arthritis in Canada: today and over the next 30 years. Arthritis Alliance of Canada. Available at http://www.arthritisalliance.ca/images/PDF/eng/Initiatives/20111022_2200_impact_of_arthritis.pdf [Google Scholar]
- Brems C, Johnson ME, Warner TD and Roberts LW (2006) Barriers to healthcare as reported by rural and urban interprofessional providers. Journal of Interprofessional Care 20, 105–118. [DOI] [PubMed] [Google Scholar]
- Brennan-Olsen SL, Tailieu TL, Turner S, Bolton J, Quirk SE, Gomez F, Duckham RL, Hosking SM, Duque G, Green D and Afifi T (2019) Arthritis in adults, socioeconomic factors, and the moderating role of childhood maltreatment: cross- sectional data from the national epidemiological survey on alcohol and related conditions. Osteoporosis International 30, 363–373. [DOI] [PubMed] [Google Scholar]
- Caplan RA, Maunder RG, Stempak JM, Silverberg MS and Hart TL (2014) Attachment, childhood abuse, and IBD-related quality of life and disease activity outcomes. Inflammatory Bowel Diseases 20, 909–915. [DOI] [PubMed] [Google Scholar]
- Cătană CS, Neagoe IB, Cozma V, Magdaș C, Tăbăran F and Dumitrașcu DL (2015) Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World Journal of Gastroenterology 21, 5823–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W, Shim HH, Lim MS, Sawadjaan FLB, Isaac SP, Chuah SW, Leong R and Kong C (2017) Symptoms of anxiety and depression are independently associated with inflammatory bowel disease-related disability. Digestive and Liver Disease 49, 1314–1319. [DOI] [PubMed] [Google Scholar]
- Chartier M, Bolton J, Mota N, MacWilliam L, Ekuma O, Nie Y, McDougall C, Srisakuldee W and McCulloch S (2018) Mental Illness among Adult Manitobans. Winnipeg, MB: Available at http://mchp-appserv.cpe.umanitoba.ca/reference/mh2015_Report_web.pdf [Google Scholar]
- Chateau D, Metge C, Prior H and Soodeen RA (2012) Learning from the census: the Socio-Economic Factor Index (SEFI) and health outcomes in Manitoba. Canadian Journal of Public Health 103, S23–S27. Available at http://www.jstor.org/stable/41995685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward S, Clement F, Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Hazlewood G, Jelinski S, Jones J, Kuenzig E, Leddin D, McBrien K, Murthy S, Nguyen GC, Otley A, Rezaie A, Pena-Sanchez J, Singh H, Targownik L and Kaplan GG (2018) The rising prevalence of inflammatory bowel disease in Canada: analyzing the past to predict the future. Journal of the Canadian Association of Gastroenterology 1, 47–48. [Google Scholar]
- De Boer AGEM, Evertsz FB, Stokkers PC, Bockting CL, Sanderman R, Hommes DW, Sprangers MAG and Frings-Dresen MHW (2016) Employment status, difficulties at work and quality of life in inflammatory bowel disease patients. Euopean Journal of Gastroenterology & Hepatology 28, 1130–1136. [DOI] [PubMed] [Google Scholar]
- Euesden J, Danese A, Lewis CM and Maughan B (2017) A bidirectional relationship between depression and the autoimmune disorders – New perspectives from the National Child Development Study. PLoS ONE 12, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers AWM, Kraaimaat FW, Geenen R, Jacobs JWG and Bijlsma JWJ (2003) Pain coping and social support as predictors of long-term functional disability and pain in early rheumatoid arthritis. Behaviour Research and Therapy 41, 1295–1310. [DOI] [PubMed] [Google Scholar]
- Gilmour H, Ramage-Morin PL and Wong SL (2018) Multiple sclerosis: prevalence and impact. Statistics Canada Health Reports 29, 3–8. Available at https://www150.statcan.gc.ca/n1/pub/82-003-x/2018001/article/54902-eng.pdf [PubMed] [Google Scholar]
- Gilworth G, Chamberlain MA, Harvey A, Woodhouse A, Smith J, Smyth MG and Tennant A (2003) Development of a work instability scale for rheumatoid arthritis. Arthritis & Rheumatism 49, 349–354. [DOI] [PubMed] [Google Scholar]
- Gunderson JG, Stout RL, McGlashan TH, Shea MT, Morey LC, Grilo CM, Zanarini MC, Yen S, Markowitz JC, Sanislow C, Ansell E, Pinto A and Skodol AE (2011) Ten-year course of borderline personality disorder: psychopathology and function from the collaborative longitudinal personality disorders study. Archives of General Psychiatry 68, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel Y, Barak Y and Achiron A (2007) Dysregulation of affect in multiple sclerosis: new phenomenological approach. Psychiatry and Clinical Neurosciences 61, 94–98. [DOI] [PubMed] [Google Scholar]
- Hitchon CA, Khan S, Elias B, Lix LM and Peschken CA (2019) Prevalence and incidence of rheumatoid arthritis in Canadian first nations and non-first nations people: a population-based study. Journal of Clinical Rheumatology. doi: 10.1097/RHU.0000000000001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Stern TA, Harley RM and Lundy NA (2003) The use of DBT skills in the treatment of difficult patients in the general hospital. Psychosomatics 44, 421–429. [DOI] [PubMed] [Google Scholar]
- Hyphantis TN, Bai M, Siafaka V, Georgiadis AN, Voulgari PV, Mavreas V and Drosos AA (2006) Psychological distress and personality traits in early rheumatoid arthritis: a preliminary survey. Rheumatology International 26, 828–836. [DOI] [PubMed] [Google Scholar]
- Incerti CC, Argento O, Pisani V, Mannu R, Magistrale G, Di Battista G, Caltagirone C and Nocentini U (2015) A preliminary investigation of abnormal personality traits in MS using the MCMI-III. Applied Neuropsychology: Adult 22, 452–458. [DOI] [PubMed] [Google Scholar]
- Judd FK, Jackson HJ, Komiti A, Murray G, Hodgins G and Fraser C (2002) High prevalence disorders in urban and rural communities. Australian & New Zealand Journal of Psychiatry 36, 104–113. [DOI] [PubMed] [Google Scholar]
- Juth V, Smyth JM and Santuzzi AM (2008) How do you feel? Self-esteem predicts affect, stress, social interaction, and symptom severity during daily life in patients with chronic illness. Journal of Health Psychology 13, 884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett K (2009) Psychological trauma and physical health: a psychoneuroimmunology approach to etiology of negative health effects and possible interventions. Psychological Trauma: Theory, Research, Practice, and Policy 1, 35–48. [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Røysamb E, Tambs K, Torgensen S, Neale MC and Reichborn-Kjennerud T (2008) The structure of genetic and environmental risk factors for DSM-IV personality disorders: a multivariate twin study. Archives General Psychiatry 65, 1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochar B, Barnes EL, Long MD, Cushing KC, Galanko J, Martin CF, Raffals LE and Sandler RS (2017) Depression is associated with more aggressive inflammatory bowel disease. The American Journal of Gastroenterology 113, 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R and Cavanagh J (2012) Depression: an inflammatory illness? Journal of Neurology, Neurosurgery, and Psychiatry 83, 495–502. [DOI] [PubMed] [Google Scholar]
- Leng Y, Wainwright NWJ, Cappuccio FP, Surtees PG, Hayat S, Luben R, Brayne C and Khaw K (2016) Daytime napping and increased risk of incident respiratory diseases: symptom, marker, or risk factor? Sleep Medicine 23, 12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, Moore CS, Michel L, Althekair F, Rajasekharan S, Gommerman JL, Prat A, Fillatreau S and Bar-Or A (2015) Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Science Translational Medicine 7, 1–10. [DOI] [PubMed] [Google Scholar]
- Linehan MM (2018) Cognitive-behavioral Treatment of Borderline Personality Disorder. New York City, New York: Guilford Publications. [Google Scholar]
- Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shan T, Abedian S, Cheon JH, Cho J, Daryani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JJY, Malekzadeh R, Westra H, Yamazaki K, Yang S, International Multiple Sclerosis Genetics Consortium, International IBD Genetics Consortium, Barrett JC, Franke A, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Anderderson CA and Weersma RK (2015) Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nature Genetics 47, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcenaro M, Prete C, Badini A, Sulli A, Magi E and Cutolo M (1999) Rheumatoid arthritis, personality, stress response style, and coping with illness: a preliminary study. Annals of the New York Academy of Sciences 876, 419–425. [DOI] [PubMed] [Google Scholar]
- Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, Cutter G and Reider N (2015) The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Multiple Sclerosis Journal 21, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, Singer A, Lix LM, Hitchon CA, El-Gabalawy R, Katz A, Fisk JD and Bernstein CN (2017) Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. Journal of Psychosomatic Research 101, 17–23. [DOI] [PubMed] [Google Scholar]
- Matcham F, Rayner L, Steer S and Hotopf M (2013) The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology 52, 2136–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcham F, Norton S, Scott DL, Steer S and Hotopf M (2016) Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology 55, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2004) Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences 1032, 1–7. [DOI] [PubMed] [Google Scholar]
- Miech RA, Caspi A, Moffitt TE, Wright BRE and Silva PA (1999) Low socioeconomic status and mental disorders: a longitudinal study of selection and causation during young adulthood. American Journal of Sociology 104, 1096–1131. [Google Scholar]
- Miller GE, Cohen S and Ritchey AK (2002) Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychology 2, 531–541. [DOI] [PubMed] [Google Scholar]
- Mohamadi A, Davoodi-Makinejad M, Azimi A and Nafissi S (2016) Personality characteristics in MS patients: the role of avoidant personality. Clinical Neurology and Neurosurgery 144, 23–27. [DOI] [PubMed] [Google Scholar]
- Mun CJ, Karoly P, Ruehlman L and Kim H (2016) Borderline personality features and pain severity: exploring the meditational role of depression and catastrophizing. Journal of Social and Clinical Psychology 35, 386–400. Available at doi:https://www.researchgate.net/profile/Chung_Mun/publication/301791858_Borderline_Personality_Features_and_Pain_Severity_Exploring_the_Mediational_Role_of_Depression_and_Catastrophizing/links/5cc4feb6299bf1209784d0a4/Borderline-Personality-Features-and-Pain-Severity-Exploring-the-Mediational-Role-of-Depression-and-Catastrophizing.pdf [Google Scholar]
- Oglodek EA, Szota AM, Just MJ, Moś DM and Araszkiewicz A (2015) The MCP- 1, CCL-5 and SDF-1 chemokines as pro-inflammatory markers in generalized anxiety disorder and personality disorders. Pharmacological Reports 67, 85–89. [DOI] [PubMed] [Google Scholar]
- Paris J (2004) Gender differences in personality traits and disorders. Current Psychiatry Reports 6, 71–74. [DOI] [PubMed] [Google Scholar]
- Parnell GP and Booth DR (2017) The multiple sclerosis (MS) genetic risk factors indicate both acquired and innate immune cell subsets contribute to MS pathogenesis and identify novel therapeutic opportunities. Frontiers in Immunology 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk SE, El-Gabalawy R, Brennan SL, Bolton JM, Sareen J, Berk M, Chanen AM, Pasco JA and Williams LJ (2015) Personality disorders and physical comorbidities in adults from the United States: data from the national epidemiologic survey on alcohol and related conditions. Social Psychiatry and Psychiatric Epidemiology 50, 807–820. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Pietrzak RH, El-Gabalawy R, Mackenzie CS and Sareen J (2015) Prevalence of psychiatric disorders in U.S. Older adults: findings from a nationally representative survey. World Psychiatry 14, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DAF, Ray J, Diamond I and Edwards GJ (1989) Personality profile and affective state of patients with inflammatory bowel disease. Gut 30, 623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost K, Smith GR and Taylor JL (1993) Rural-urban differences in stigma and the use of care for depressive disorders. The Journal of Rural Health 9, 57–62. [DOI] [PubMed] [Google Scholar]
- Sansone RA and Sansone LA (2015) Borderline personality in the medical setting. The Primary Care Companion for CNS Disorders 17, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone RA, Watts DA and Wiederman MW (2013) Pain and pain catastrophizing among internal medicine outpatients with borderline personality symptomatology: a cross-sectional self-report survey. The Primary Care Companion for CNS Disorders 15, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp TJ and Harvey AG (2001) Chronic pain and posttraumatic stress disorder: mutual maintenance? Clinical Psychology Review 21, 857–877. [DOI] [PubMed] [Google Scholar]
- Sokal J, Messias E, Dickerson FB, Kreyenbuhl J, Brown CH, Goldberg RW and Dixon LB (2004) Comorbidity of medical illnesses among adults with serious mental illness who are receiving community psychiatric services. Journal of Nervous and Mental Disease 192, 421–427. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Bouchain M, Winkler LY, Wingenfeld K, Gold SM, Grabe HJ, Barnow S, Otte C and Heesen C (2012) Childhood trauma in multiple sclerosis: a case-control study. Psychosomatic Medicine 74, 312–318. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Revenson TA and Tennen H (2007) Health psychology: psychological adjustment to chronic disease. The Annual Review of Psychology 58, 565–592. [DOI] [PubMed] [Google Scholar]
- Tosic-Golubovic S, Miljkovic S, Nagorni A, Lazarevic D and Nikolic G (2010) Irritable bowel syndrome, anxiety, depression and personality characteristics. Psychiatria Danubina 22, 418–424. Available at http://search.proquest.com/docview/755164944/ [PubMed] [Google Scholar]
- Tribbick D, Salzberg M, Ftanou M, Connell WR, Macrae F, Kamm MA, Bates GW, Cunningham G, Austin DW and Knowles SR (2015) Prevalence of mental health disorders in inflammatory bowel disease: an Australian outpatient cohort. Clinical and Experimental Gastroenterology 15, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen SPJ, Derksen JJL, Sadavoy J and Rosowsky E (2012) Features and challenges of personality disorders in late life. Aging and Mental Health 16, 805–810. [DOI] [PubMed] [Google Scholar]
- Vidal À, Gómez-Gil E, Sans M, Portella MJ, Salamero M, Piqué JM and Panés J (2008) Health-related quality of life in inflammatory bowel disease patients: the role of psychopathology and personality. Inflammatory Bowel Diseases 14, 977–983. [DOI] [PubMed] [Google Scholar]
- Wadsworth ME (2015) Development of maladaptive coping: a functional adaptation to chronic, uncontrollable stress. Child Development Perspectives 9, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westen D, Shedler J, Bradley B and DeFife JA (2012) An empirically derived taxonomy for personality diagnosis: bridging science and practice in conceptualizing personality. American Journal of Psychiatry 169, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Davis A, Badley E, Grewal R and Mohammed M (2010) Prevalence of Arthritis and Rheumatic Diseases Around the World: A Growing Burden and Implications for Health Care Needs. Arthritis Community Research and Evaluation Unit. Toronto, Ontario: Toronto Western Research Institute; Available at http://www.modelsofcare.ca/pdf/10-02.pdf [Google Scholar]
- Yarwood A, Han B, Raychaudhuri S, Bowes J, Lunt M, Pappas DA, Kremer J, Greenberg JD, Plenge R, Rheumatoid Arthritis Consortium International, Worthington J, Barton A and Eyre S (2015) A weighted genetic risk score using all known susceptibility variants to estimate rheumatoid arthritis risk. Annals of the Rheumatic Diseases 74, 170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig RA (2008) Personality disorder in older adults: assessment challenges and strategies. Professional Psychology: Research and Practice 39, 298–305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors received permission to access the data used in this study, however, they are unable to share the data as they are not the data custodians.