Abstract
Aims
To systematically assess the level of evidence for psychotropic drugs approved by the European Medicines Agency (EMA).
Methods
Cross-sectional analysis of all European Public Assessment Reports (EPARs) and meta-analyses of the many studies reported in these EPARs. Eligible EPARs were identified from the EMA's website and individual study reports were requested from the Agency when necessary. All marketing authorisation applications (defined by the drug, the route of administration and given indications) for psychotropic medications for adults (including drugs used in psychiatry and addictology) were considered. EPARs solely based on bioequivalence studies were excluded. Our primary outcome measure was the presence of robust evidence of comparative effectiveness, defined as at least two ‘positive’ superiority studies against an active comparator. Various other features of the approvals were assessed, such as evidence of non-inferiority v. active comparator and superiority v. placebo. For studies with available data, effect sizes were computed and pooled using a random effect meta-analysis for each dose of each drug in each indication.
Results
Twenty-seven marketing authorisations were identified. For one, comparative effectiveness was explicitly considered as not needed in the EPAR. Of those remaining, 21/26 (81%) did not provide any evidence of superiority against an active comparator, 2/26 (8%) were based on at least two trials showing superiority against active comparator and three (11%) were based on one positive trial; 1/26 provided evidence for two positive non-inferiority analyses v. active comparator and seven (26%) provided evidence for one. In total, 20/27 (74%) evaluations reported evidence of superiority v. placebo with two or more trials. Among the meta-analyses of initiation studies against active comparator (57 available comparisons), the median effect size was 0.051 (range −0.503; 0.318). Twenty approved evaluations (74%) reported evidence of superiority v. placebo on the basis of two or more initiation trials and seven based on a single trial. Among meta-analyses of initiation studies against placebo (125 available comparisons), the median effect size was −0.283 (range −0.820; 0.091). Importantly, among the 89 study reports requested on the EMA website, only 19 were made available 1 year after our requests.
Conclusions
The evidence for psychiatric drug approved by the EMA was in general poor. Small to modest effects v. placebo were considered sufficient in indications where an earlier drug exists. Data retrieval was incomplete after 1 year despite EMA's commitment to transparency. Improvements are needed.
Key words: Psychopharmacology, psychotropic drugs, randomised controlled trials, research design and methods, systematic reviews
Introduction
Since early 1995, European Union authorisations for many new medicinal products has been obtained through a centralised procedure. This procedure, managed by the European Medicines Agency (EMA), is mandatory for products derived from biotechnology and other high-technology procedures, those aimed at the treatment of human immunodeficiency virus/acquired immunodeficiency syndrome, cancer, diabetes, neurodegenerative diseases, autoimmune disorders, viral diseases and also orphan medicines used to treat rare diseases. The centralised authorisation application also can be submitted whenever the medicinal product involved is a major therapeutic, scientific or technical innovation, or is relevant in any other way for population health. In this context the EMA assesses the evidence presented by drug companies requesting marketing authorisations, and judges whether the known (and possibly unknown) adverse effects of a new drug are acceptable when set against the expected benefits, demonstrated in ad hoc randomised controlled trials (RCTs) (Bighelli and Barbui, 2012). This type of evaluation involves a complex interplay of clinical practice, pharmacology, epidemiology, policy and politics (Avorn, 2012) and comes under pressure from major conflicting interests between companies, patients, doctors and public health advocates who have sometimes very divergent opinions. Therefore, regulatory agencies' decisions are scrutinised and are often criticised, especially when based on weak evidence. In this instance, care that is offered to patients is liable to be questioned and discredited, with the risk of decreasing public trust in medicine.
Psychotropic medication is a perfect example. While these drugs have gone through strict regulatory controls, their risk–benefit balance is still the subject of heated debate (Gotzsche et al., 2015). Specifically, new drugs are often criticised (1) for being no more or even less effective than more long-established drugs, (2) because of questionable differences v. placebo in terms of clinical relevance, (3) sometimes because of specific safety concerns and (4) because of higher cost. Some argue that these concerns are merely a manifestation of the recurrent attacks on psychiatry (Nutt et al., 2014). On the other hand, many well-described examples, such as the approvals of nalmefene (Naudet et al., 2016) and, more recently, paliperidone in 3-monthly injections (Ostuzzi et al., 2017), suggest that the EMA's thresholds are too lenient, especially for comparative effectiveness (Garattini and Bertele, 2005).
Interestingly, the EMA has been committed for many years to promoting transparency about drug efficacy and safety data (EMA, 2018b). The EMA publishes a European Public Assessment Report (EPAR) for each marketing authorisation application it grants. An EPAR presents the evidence considered, describes the discussion of the Committee for Medicinal Products for Human use (CHMP), and the final recommendation of the CHMP. While the exhaustiveness of the EPARs has been criticised for not providing enough detailed information for the purpose of meta-analyses (Barbui et al., 2011), these documents describe all arguments retained for approving the drug. On the basis of these EPARs, we aimed to systematically assess the level of evidence on which drug approvals in psychiatry are based.
Methods
A standard protocol was developed and registered before the beginning of the study in the PROSPERO database (systematic review registration – PROSPERO 2017:CRD42017059930).
Eligibility criteria
We surveyed all available EPARs concerning all classes of approved psychotropic medication (drugs used in both psychiatry and addictology) for adults. Since some EPARs presented various evaluations of different applications, we focused on the different marketing authorisation applications defined by the drug, the route of administration and the indication. All years were considered. We excluded EPARs solely based on bioequivalence studies, because we were only interested in clinical evidence.
Search strategy and selection process
Eligible EPARs were identified by two independent reviewers (Florian Erhel and Florian Naudet) from the European Medicines Agency website (http://www.ema.europa.eu/ema/) using the following path: Find medicine→Human medicines→European public assessment reports→Browse by therapeutic area→Psychiatry and Psychology. All disagreements between the two reviewers were resolved by consensus.
Data collection
Two independent reviewers (Florian Erhel and Alexandre Scanff) collected data from all the selected EPARs using a data extraction sheet that was pilot-tested on two EPARS. For each EPAR, information was extracted on (1) characteristics of the EPAR (year, drug, route of administration, disease, indication and manufacturer of the drug) and (2) all outcomes measured as specified below.
In addition, the two reviewers collected information on all the RCTs included in these EPARs. Studies were retrieved and extracted exclusively from the EPARs (no search of the literature was performed in parallel). When there was missing information concerning these RCTs, one reviewer (Florian Erhel) contacted the EMA to obtain the corresponding study reports, which were also assessed by the two independent reviewers.
All disagreements were first resolved by consensus and then by consulting a third reviewer for arbitration (Florian Naudet).
Outcome measures
Description of the EPARs
Our primary outcome measure was the presence of robust evidence of comparative effectiveness defined as at least two ‘positive’ superiority studies in an indication where an active comparator was already approved. The criterion of two positive studies was retained since it is used to establish effectiveness in the Food and Drug Administration's Guidance for Industry (FDA, 1998). In addition, we noted whether the evaluation reported (1) evidence from only one positive study (alone, or associated with one or more negative studies) or (2) no evidence at all (only negative studies, no comparison reported, or no study at all) or (3) no requirement for evidence of comparative effectiveness.
We used on the same method to describe the presence of evidence of non-inferiority against active comparator.
We described the primary outcomes used in all the EPARs demonstrating comparative effectiveness, and detailed whether these EPARs investigated a list of possible outcomes (symptom severity scale, clinical global impression, global functioning, quality of life, relapse, response, remission or any other outcome).
As secondary outcomes, we adopted the same method to describe (1) evidence of efficacy against active comparator in continuation trials (defined as studies among patients who have responded to initial treatment with the same drug and route), (2) evidence of efficacy against placebo in superiority trials and (3) evidence of efficacy v. placebo in continuation trials.
In addition, we reported whether the definition of the target population in the approval was based on a subgroup analysis (with a difference between pre-specified and a posteriori subgroup analyses). Approvals where subgroup analyses were presented but without any impact on the target population definition were considered as not based on subgroup analyses.
We checked whether the EMA reported an assessment of the risk of bias concerning the trials considered within the EPAR (and if it did, whether it was with or without a systematic assessment). If an assessment was provided, we noted whether any bias was identified.
Concerning the description of possible safety issues, we described (1) the number of patients exposed to the drug before submission for market approval and (2) the presence or absence of a reported comparison with active comparator and with placebo (yes/no/only qualitative) and the explicit mention of a safety issue in the report (yes/possible/no).
Concerning the description of possible tolerance issues, we described the rates of discontinuation due to adverse events, the presence or absence of a comparison with active comparator and with placebo (yes/no/only qualitative) and the mention of a tolerance issue (yes/possible/no).
We described the number of approvals granted but accompanied by the requirement to conduct specific post-marketing studies (with the reasons), the need for further opinion (e.g. scientific advisory group) and the possible expression of divergent positions within the CHMP in the EMA.
Description of the studies included in different EPARs
For each EPAR, we noted the number of studies. For each study listed in the efficacy chapter, we extracted information on study duration, design ((1) superiority/non-inferiority with non-inferiority boundaries, (2) initial, continuation), comparator(s), number of arms, number of participants per arm, effect sizes measured for the primary outcomes, type of primary outcome, numbers of withdrawals, numbers of discontinuation due to adverse events, inclusion of suicidal participants. In the case of multiple-arm studies, every arm was extracted.
Strategy for data synthesis
A descriptive analysis was performed. It consisted of estimates for the different outcomes (numbers and percentages for categorical outcomes and means, standard error or medians and interquartile intervals for quantitative outcomes).
In addition to the descriptive analysis, effect sizes (Cohen's d) and their corresponding p values were plotted against one other for different outcomes (and for each study design) both at the study level and at the approval level for each dose of each drug (i.e. pooled effect size obtained with a random effect meta-analysis). The formulas applied to calculate the effect sizes are provided in the e-methods. Although not always intuitive, and oversimplified, it is admitted that a Cohen's d value of 0.2 is small, 0.5 is medium and 0.8 is large (Cohen, 2013). All analyses were performed using R language and the packages tidyverse, compute.es and meta.
Results
On 22nd March 2017, we had identified 25 EPARs corresponding to 29 marketing authorisation applications. Two of them resulted in a refusal: agomelatine (orally) for major depressive disorder and asenapine (sublingual) for schizophrenia; 27 applications resulting in an approval were therefore retained for further analysis. Their release dates ranged between 1996 and 2016 (median = 2008). One of these was a second application for agomelatine (orally) for major depressive disorder. The applications concerned 13 drugs, among which aripiprazole, olanzapine, paliperidone and duloxetine have been the subject of numerous authorisation applications. Sixteen applications concerned the oral route, eight concerned the intra-muscular route and three concerned other routes (sublingual and inhalation). More details, including the manufacturers' descriptions, are provided in Table 1.
Table 1.
Details of the different evaluations leading to approval
| EPAR | Year | Drug | Route | Disease | Manufacturer |
|---|---|---|---|---|---|
| EMEA/H/C/000916 | 2008 | Agomelatine | O | Depressive disorders | Servier |
| a | 2004 | Aripiprazole | O | Schizophrenia | Otsuka |
| EMEA/H/C/000471/II/0015 | 2006 | Aripiprazole | IM | Agitation in patients with schizophrenia and/or bipolar disorders | Otsuka |
| EMEA/H/C/471/II/0039 | 2008 | Aripiprazole | O | Bipolar disorders | Otsuka |
| EMEA/H/C/471/II/0041 | 2008 | Aripiprazole | IM | Agitation in patients with schizophrenia and/or bipolar disorders | Otsuka |
| EMEA/H/C/002755/0000 | 2013 | Aripiprazole | IM | Schizophrenia | Otsuka |
| EMEA/H/C/001177 | 2010 | Asenapine | Sublingual | Bipolar disorders | Organon |
| a | 2006 | Buprenorphine/naloxone | Sublingual | Addictive disorders | Schering-Plough Europe |
| a | 2004 | Duloxetine | O | Depressive disorders | Eli Lilly Nederland BV |
| EMEA/H/C/572/II/27 | 2008 | Duloxetine | O | Anxiety disorders | Eli Lilly Nederland BV |
| EMEA/H/C/572/II/0036 | 2009 | Duloxetine | O | Depressive disorders | Eli Lilly Nederland BV |
| EMEA/H/C/002400 | 2012 | Loxapine | INH | Agitation in patients with schizophrenia and/or bipolar disorders | AlexzaUk |
| EMEA/H/C/002713/0000 | 2014 | Lurasidone | O | Schizophrenia | Takeda Pharma A/S |
| EMEA/H/C/002583/0000 | 2012 | Nalmefene | O | Addictive disorders | Lundbeck |
| EMEA/H/C/000890 | 2008 | Olanzapine | IM | Schizophrenia | Eli Lilly Nederland BV |
| CPMP/0646/96 | 1996 | Olanzapine | O | Schizophrenia | Eli Lilly Nederland BV |
| CPMP/0646/96 | 2003 | Olanzapine | O | Bipolar disorders | Eli Lilly Nederland BV |
| CPMP/0646/96 | 2001 | Olanzapine | IM | Agitation in patients with schizophrenia and/or bipolar disorders | Eli Lilly Nederland BV |
| CPMP/0646/96 | 2002 | Olanzapine | IM | Agitation in patients with schizophrenia and/or bipolar disorders | Eli Lilly Nederland BV |
| a | 2007 | Paliperidone | O | Schizophrenia | Janssen-Cilag |
| EMEA/H/C/000746/II/0023 | 2010 | Paliperidone | O | Schizoaffective disorder | Janssen-Cilag |
| EMEA/H/C/000746/II/0043 | 2015 | Paliperidone | O | Schizoaffective disorder | Janssen-Cilag |
| EMEA/H/C/004066/X/0007/G | 2016 | Paliperidone palmitate long acting injection | IM | Schizophrenia | Janssen-Cilag |
| EMEA/H/C/2105 | 2011 | Paliperidone palmitate long acting injection | IM | Schizophrenia | Janssen-Cilag |
| EMEA/H/C/000546/II/0004 | 2006 | Pregabalin | O | Anxiety disorders | Pfizer |
| a | 2006 | Varenicline | O | Addictive disorders | Pfizer |
| EMEA/H/C/002717 | 2013 | Vortioxetine | O | Depressive disorders | Lundbeck |
EPAR, European Public Assessment Report; O, oral route; IM, intramuscular; INH, inhalation.
These EPARs had no identifying number on EMA's website.
Description of the EPARs
Evidence in trials v. active comparator
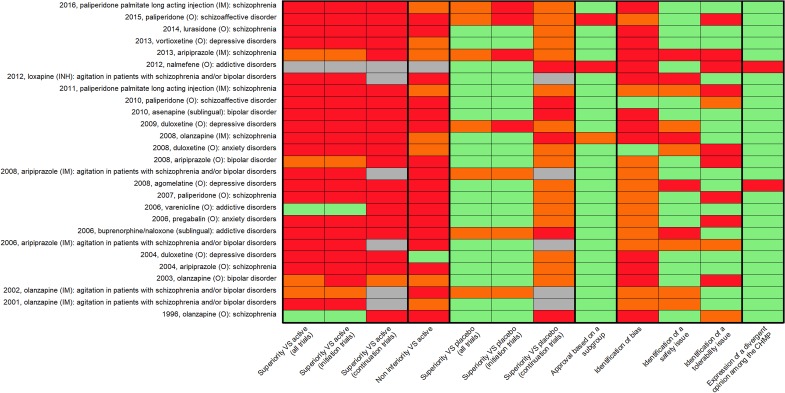
A description of all the EPARs is summarised in Fig. 1. For one evaluation (nalmefene, oral – (O), in alcohol use disorders), the comparative effectiveness was explicitly considered as not needed by the CHMP. Among the remaining approvals, 2/26 (8%) applications leading to approval were based on at least two initiation trials showing superiority against active comparator (olanzapine (O), for schizophrenia and varenicline (O), for smoking cessation), and 3/26 (12%) were based on one positive superiority study against active comparator. In total, 21/26 (80%) did not present any evidence of superiority against an active comparator: for 9 (35%) an active comparator arm was presented without any comparison with the investigation drug (this was described in the EPARs as ‘internal positive control for assay sensitivity’), for 9 (35%) no superiority study including an active comparator was presented, and for 3 (11%) superiority trials against active comparator presented a negative (i.e. non-significant) result.
Fig. 1.
Heatmap presenting a descriptive analysis of the evaluations leading to approval. Columns 1–7: green = 2 or more studies; orange = one study; red = no study; column 8: green = not based on subgroup analyses; red = based on a posteriori subgroup analyses; column 9: green = no bias was identified; orange = bias assessment not presented; red = identification of bias; columns 10–11: green = no issue was identified; orange = issue presented as possible; red = identification of an issue; column 12: green = no divergent opinion; red = divergent opinion.
In total, 1/26 (4%) of the applications gaining approval (duloxetine (O), for major depressive episode) provided evidence of two positive non-inferiority analyses v. active comparator. However, these two analyses were based on pooled analyses of different superiority trials. In total, 8/26 (31%) approved applications providing evidence of one positive non-inferiority study against active comparator, including one with one positive trial but two negative trials (paliperidone (IM), in maintenance treatment of schizophrenia) and another one based on a pooled analysis of two non-inferiority trials (duloxetine (O), for generalised anxiety disorder). In total, 17/26 (65%) evaluations provided no non-inferiority study against active comparator.
We considered that evidence from continuation trials was not needed in the five evaluations concerning agitation. Only one of the remaining 21 approvals (5%) provided evidence of non-inferiority against active comparator. It was based on only one study. None provided evidence of superiority against active comparator in continuation trials. In total, 2/21 (9.5%) reported one negative continuation trial v. active comparator. Because it was sometimes arbitrary to differentiate continuation and initiation trials (e.g. for long-acting injectable treatments the initiation of the IM route often follows stabilisation with the same drug given orally), we decided to group these two categories for descriptive purpose (Fig. 1). Details of these approvals in terms of study design and comparators explored are presented in Table 2. Details of outcomes presented in these approvals are presented in online appendix, e-Tables 1a and 1b.
Table 2.
Approvals with evidence of superiority or non-inferiority against active comparator
| Evaluation | Study: design (primary outcome/arms) | Comparator | Study durationa | Number of subjects |
|---|---|---|---|---|
| Positive superiority initiation trials v. active comparator | ||||
| Aripiprazole (O): bipolar disorders | CN138008: response/aripiprazole flexible dose 15–30 mg/day and comparator | Haloperidol, flexible dose, 10–15 mg/day | 12 weeks | 347 (randomised) |
| Olanzapine (O): schizophrenia | FID-EW-E003: not specified/olanzapine fixed doses 1–17.5 mg/day and comparator | Haloperidol, fixed doses, 10–20 mg/day | 6 weeks | 431 (initial) |
| Olanzapine (O): schizophrenia | FID-MC-HGAJ: not specified/olanzapine 5–20 mg/day and comparator | Haloperidol, 5–20 mg/day | 6 weeks | 1996 (included) |
| Olanzapine (IM): agitation in manic episode | HGHW: PANSS excited component/olanzapine fixed dose 10 mg, placebo and comparator | Lorazepam IM, fixed dose, 2 mg | 2 h | 228 (screened) |
| Varenicline (O): smoking cessation | A3051028: 4 weeks continuous quit rate/varenicline, fixed dose, 2 mg/day, placebo and comparator | Bupropion, fixed dose, 300 mg/day | 12 weeks | 1025 (randomised) |
| Varenicline (O): smoking cessation | A3051036: 4 weeks continuous quit rate/varenicline, fixed dose, 2 mg/day, placebo and comparator | Bupropion, fixed dose, 300 mg/day | 12 weeks | 1027 (randomised) |
| Positive non-inferiority continuation trials v. active comparator | ||||
| Olanzapine (O): bipolar disorder | HGHT: relapse/olanzapine and comparator. NIB: 7.3% | Lithium | 12 months | 431 (randomised) |
| Positive non-inferiority initiation trials v. active comparator | ||||
| Aripiprazole (IM): schizophrenia | 31-07-247: relapse/aripiprazole IM depot flexible dose 300–400 mg/month, aripiprazole IM depot flexible dose 25–50 mg/month and comparator. NIB : 11.5% | Oral aripiprazole flexible dose 10–30 mg/day | 26 weeks | 662 (randomised) |
| Duloxetine (O): depressive disorder (pooled results) | Pooled F1J-MC-HMAT(a,b): HAMD17/duloxetine fixed doses 40 and 80/day, placebo and comparator. NIB: 2.2 | Paroxetine fixed dose 20 mg | 8 weeks | 707 (randomised) |
| Duloxetine (O): depressive disorder (pooled results) | Pooled F1J-MC-HMAY(a,b): HAMD17/duloxetine fixed doses 80 and 120 mg/day, placebo and comparator. NIB: 2.2 | Paroxetine fixed dose 20 mg | 8 weeks | 759 (randomised) |
| Duloxetine (O): anxiety disorders (pooled results) | Pooled HMDU, HMDW: HAMA/duloxetine flexible dose 60–120 mg/day, placebo and comparator. NIB: −1.5 | Venlafaxine flexible dose 75–225 mg/day | 10 weeks | 1068 (randomised) |
| Olanzapine (IM): schizophrenia | F1D-MC-HGKA: relapse/olanzapine IM depot fixed dose 150 and 300 mg/2 weeks and comparator. NIB: 5% | Oral olanzapine fixed dose 10–20 mg/day | 24 weeks | 1065 (randomised) |
| Olanzapine (O): bipolar disorder | HGHQ: Y-MRS/olanzapine 5–20 mg/day and comparator | Valproate 500–2500 mg/day | 3 weeks | 251 (randomised) |
| Olanzapine (IM): agitation (in schizophrenia) | F1D-MC-HGHB: PANSS excitement component/olanzapine fixed dose, placebo and comparator | Haloperidol IM | 2 h | 311 (type of population not given) |
| Paliperidone (IM): schizophrenia (3-month injection) | PSY-3011: relapse/paliperidone IM depot fixed doses 175–525/12 weeks and comparator. NIB: 15% | Paliperidone fixed dose 50–150 mg/month | 48 weeks | 1016 (randomised) |
| Paliperidone (IM): schizophrenia | PSY-3006: PANSS/paliperidone IM depot flexible dose 50–150 mg/month and comparator. NIB: 5 | Risperidone IM flexible dose 25–50 mg/2 weeks | 13 weeks | 1220 (randomised) |
| Vortioxetine (O): depressive disorders | 14178A: MADRS/vortioxetine flexible dose 10–0 mg/day and comparator. NIB: 2 | Agomelatine flexible dose 25–50 mg/day | 12 weeks | 501 (randomised) |
NIB, non-inferiority boundary.
Missing data (for doses, route, NIB) in the table are due to missing data in the EPARs.
Duration between randomisation and endpoint.
Quantitative information on the comparison of safety outcomes with an active comparator was presented for 17/26 (65%). In addition, for 2/26 (8%) evaluations the comparison was described qualitatively. A comparison of tolerance outcomes with active comparator was provided for 22/26 (85%) evaluations (21 quantitatively and 1 qualitatively).
Evidence v. placebo
In total, 20/27 (74%) evaluations resulting in approval reported evidence of superiority v. placebo with two or more initiation trials. In 3/27 (11%) superiority v. placebo was based on one initiation trial. In total, 16/22 (73%) evaluations reported evidence from one positive continuation study against placebo (two of these also reported failed trials) and six did not report evidence of such studies.
We also decided to group these two categories for descriptive purpose (Fig. 1) resulting in 20/27 evaluations resulting in approval with evidence of superiority v. placebo with two or more trials and seven with a single positive trial.
Quantitative information on the comparison of safety outcomes v. placebo was presented for 21/27 (78%) evaluations. In addition, for 2/27 (7%) evaluations the comparison was described qualitatively.
A comparison of tolerance outcomes v. placebo was provided for 26/27 (96%) evaluations (25 quantitatively and 1 qualitatively).
Other characteristics of the evidence presented in the EPARs
All other characteristics of the evidence presented in the EPARs are detailed in the online appendix (e-results, e-Tables 2, 3 and 4).
Description of the studies included in EPARs for approved medications
Missing information
We identified 137 eligible RCTs in EPARs for approved medications. Data needed to determine effect sizes were fully available for these studies in seven EPARs, corresponding to 41 studies. Complete data were lacking for 18 EPARs, where 24 studies were adequately reported and 89 lacked sufficient data for direct calculation of effect size. All the study reports of these 89 studies were requested from the EMA via its website. All the receipt confirmations had been obtained on 3rd July 2017. We stopped data collection 1 year later, on 21st July 2018. At that time, three other EPARs had been completed, which corresponded to 19 additional studies. We also used the data concerning nalmefene obtained for previous work by of one of the present authors (Palpacuer et al., 2015), corresponding to five additional studies.
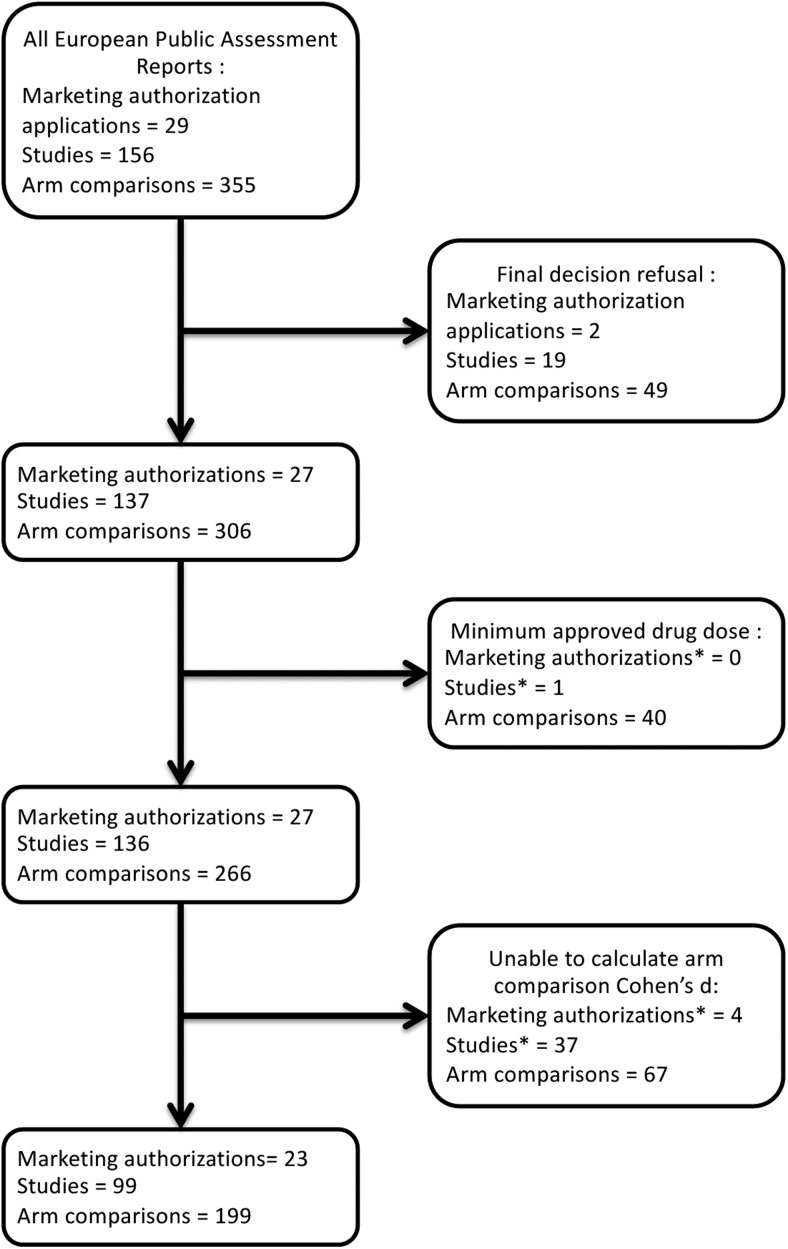
Effect size calculations and meta-analyses were performed only for approved dosages for approved medications. For one EPAR-approved study (Olanzapine (O), in schizophrenia), all dosages presented were below the minimum approved dosage, and the study was not included in meta-analyses. Therefore, we analysed 266 arm comparisons between the drug tested and placebo or active comparator in 136 studies. For these comparisons, 158/266 accounting for 78 studies (57%) presented sufficient information to calculate effect sizes and p values from arm results (proportions or mean changes and variance of mean changes), 29 enabled calculation of differences in arm results, adding five studies (4%) and 12 only presented p values and numbers of subjects, adding seven more studies (5%). Finally, 67/266 (25%) comparisons in 46 studies (34%) had insufficient information to calculate Cohen's d. For 37 studies (26%), none of the arm comparisons had sufficient information to calculate Cohen's d. After imputation of p values and numbers of subjects, we were able to calculate the effect sizes for at least one study in 23 (85%) EPARs, and for all studies in 12 (48%) EPARs. Results sufficient for meta-analysis concerned 199 comparisons (75%) in 99 studies (73%) (see the flowchart presented in Fig. 2).
Fig. 2.
Flowchart of marketing authorisation application, individual studies and arm comparisons, for EPARs on psychiatric drugs. *Study/marketing authorisation totally excluded because all of its arm comparisons were excluded.
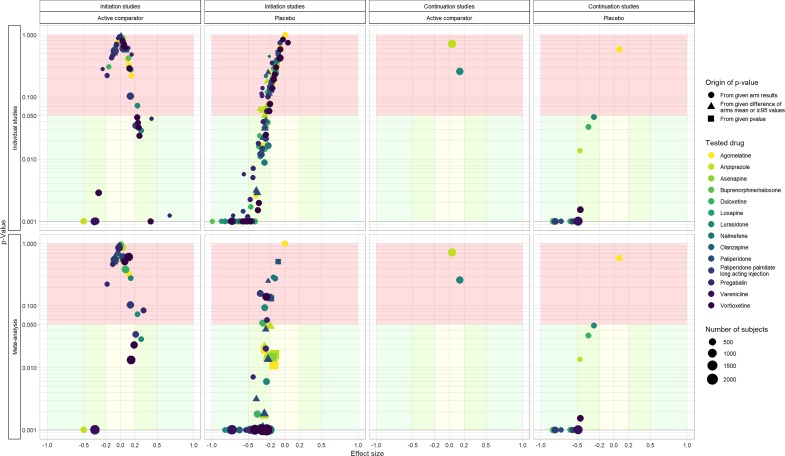
The relationship between effect-size and p values in the studies included and the results of the meta-analyses are presented in Fig. 3. Details per drug class are provided in online e-table 5.
Fig. 3.
Effect sizes and p values observed in individual studies and meta-analyses pooled by drug and daily dose, for each study design. For each plot, the x-axis presents effect sizes and the y-axis (log scale) presents p values. Top plots present data at the study level and bottom plots present data at the meta-analysis level (data were pooled by drug and daily dose).
Effect sizes in initiation studies against active comparator
For initiation studies against active comparator, we describe 57 different comparisons. Of these 13 (23%) results were considered statistically significant, 4 (7%) were in favour of the investigation drug and 9 (16%) were in favour of the active comparator. These comparisons involved 31 different combinations of drug and daily dose, 16 of which were explored only once. When results were pooled (meta-analyses) per drug and daily dose, we found 25 (81%) drug/dose comparisons with no evidence of superiority v. active comparator, 2 (7%) drug/dose comparisons with results in favour of the investigation drug and 4 (13%) drug/dose comparisons with results in favour of the active comparator. In these meta-analyses, the median effect size was 0.051 (range −0.503; 0.318).
Effect sizes in initiation studies against placebo
For initiation studies against placebo, we describe 125 different comparisons for 54 different combinations of drug and daily dose, 16 explored only once. Of these, 66 (53%) results were considered statistically significant. When results were pooled (meta-analyses) per drug and dose, we found 12 (23%) drug/dose comparisons with no evidence of superiority and 41 (77%) drug/dose comparisons with results in favour of the investigation drug. In these meta-analyses, the median effect size v. placebo was −0.283 (range −0.820; 0.091).
Effect sizes in continuation studies against active comparator
For continuation studies against active comparator, we describe two different comparisons. No results were considered statistically significant. These two comparisons concerned two different drugs. The effect sizes were 0.036 and 0.143.
Effect sizes in continuation studies against placebo
For continuation studies against placebo, we describe 15 different comparisons. Of these 14 (93%) results were considered statistically significant. Each combination of drug and daily dose was noted only once. In these comparisons, the median effect size was −0.528 (range −0.831; 0.074).
Exclusion of suicidal participants
Finally, in 48/137 studies (35%) we noted that suicidal participants were not included. For the other studies, no information was given concerning inclusion or not of suicidal participants.
Discussion
Statement of principal findings
Our critical appraisal of 25 EPARS revealed that EMA's standards for psychiatric drug approvals were low, especially for the consideration of comparative effectiveness issues. Only two applications receiving approval were based on at least two initiation trials showing superiority against active comparator (8%) and two others were based on only one study (8%). In one case, nalmefene (O), for reduction of alcohol consumption, the applicant considered that comparative effectiveness results were not needed, as the therapeutic goal was new, consisting of reducing alcohol consumption rather than maintaining alcohol abstinence. This has proved to be controversial, as nalmefene is a very similar compound to naltrexone, a drug already used off-label to reduce alcohol consumption (Naudet et al., 2016).
Interestingly, some EPARs presented studies with an active comparator but did not report the results of the comparison, and the CHMP did not consider this evidence in the decision process. In these cases, the active comparator was only compared to the placebo control to demonstrate ‘the sensitivity of the trial’. In other words, if the active comparator showed no difference v. placebo, the trial was considered as ‘a failed trial’, thus disqualifying any absence of difference observed with the drug under investigation. This is a serious issue as it is insufficient to consider a trial as ‘failed’ when an active comparator was not significantly better than placebo, especially given the large number of ‘negative’ trials of this sort (Turner et al., 2008).
Conversely, in certain non-inferiority studies such as study 14178A, comparing vortioxetine with agomelatine in major depressive episode, one would have expected a placebo arm to make it clear whether the two drugs were equally effective or equally ineffective. This example is of concern because agomelatine could be considered as a very poor comparator, since its approval was controversial: it was granted after a second application (the first application was refused) with a mention of divergent opinions among the CHMP members (Barbui and Cipriani, 2012; Koesters et al., 2013). While in the recent network meta-analysis by Cipriani et al. (2018), agomelatine ranked first if one considered the ratio between efficacy and tolerability, another meta-analysis suggested ‘that a clinically important difference between agomelatine and placebo in patients with unipolar major depression is unlikely’ (Koesters et al., 2013).
In fact, most of the approvals were solely based on evidence of superiority against placebo, which appears to be the standard for EMA. As a consequence, our meta-analyses revealed that in most of the cases, there was no added benefit with the most recent drugs and some approved doses for some drugs were less effective than those of already marketed drugs. It can be noted that these differences can be considered as small or very small in terms of effect size.
In addition, almost half of the comparisons v. placebo we considered did not reach statistical significance, a result observed in the antidepressant trials submitted to the FDA (Turner et al., 2008), suggesting that most of the pivotal trials lack power given the actual efficacy of the drug studied. Importantly, some approved doses for some drugs did not demonstrate effectiveness against placebo in our meta-analyses. When compared with placebo, the median effect size was small for all the doses of each drug explored. Larger effect sizes were observed in continuation studies, a design that is known to inflate the true treatment effect (Kopec et al., 1993), possibly in part because of a withdrawal effect and/or a selection of enriched sample of drug responders.
Further to this, while some evaluations identified safety issues that were general and related to drug classes (e.g. cardiovascular risks for antipsychotics or suicide risk for antidepressants) other evaluations reported issues that were specific to a given drug. For instance, long-acting IM depot olanzapine, besides the classic metabolic risks, presents a risk of inadvertent intravascular (IAIV) injection, resulting in a reduced level of consciousness, and coma in the worst cases. As the approval is based on a non-inferiority trial against oral olanzapine, it seems that the only identified difference between these two treatments was the IAIV injection risk. Other specific risk domains were liver function for agomelatine (a drug with poor evidence of effectiveness) and bronchospasm for inhaled loxapine. Inhaled loxapine was only compared to placebo while the oral and IM routes for loxapine were long available without this specific safety issue.
While in most EPARs the definition of the target population was not based on subgroup analyses, two evaluations used a posteriori subgroup analyses to define the target population: nalmefene (O) for reduction of alcohol consumption and paliperidone (O) for the depressive symptom domain of schizoaffective disorder. These analyses could increase the probability of false-positive findings (Wallach et al., 2017) and have no place in defining a target population.
Strengths and weaknesses of the study
The criteria we used could be considered as controversial. For instance, the criterion of ‘at least two positive studies’ is classic, but it has been criticised, as it can still cover a weak level of evidence (van Ravenzwaaij and Ioannidis, 2017). It can be considered as too restrictive, since there are other benefits of new approved drugs (such as better observance, or better tolerance). Nevertheless, we extracted every primary outcome in every RCT and we found no trial exploring and demonstrating this kind of benefit. Primary outcomes are most of the time related to symptom severity, in a quantitative manner (symptom severity scale) or in a binary manner (relapse, response), depending on the study design. In addition, other outcomes like global functioning or quality of life, if present, are systematically assessed as secondary outcomes. In addition, because our aim was to assess the broad picture of drug approvals in psychiatry, we focused on very broad criteria and did not retain more qualitative outcomes involving the subtleties of day to day therapeutic practice. For example, we did not collect inclusion criteria for different studies and we were not able to discuss the representativeness of the samples included (Zimmerman et al., 2005; Naudet et al., 2013).
Data extraction in different EPARs proved to be difficult in certain situations, due to a lack of standardisation among EPARs. This was all the more so for adverse events that could be described quantitatively or qualitatively. In addition, EPARs summarise all the evidence analysed for granting marketing approval but they do not report the exact content of the market approval meetings. In addition, gathering data proved to be difficult, since details of studies were inconsistently reported in the EPARs (Barbui et al., 2011) and requests for study reports resulted in a long and still ongoing process, despite EMA's commitment to transparency. Our analyses therefore give an incomplete picture of the evidence base for these approvals. The next steps in our project are to finish data collection and extraction, to share an online interactive tool monitoring EMA's approvals of psychotropic medication and to update our database with the most recent approvals. Following the spirit of living systematic reviews (Elliott et al., 2017), this tool will be regularly updated, as we are operating in a fast-changing environment. For instance, since our searches, brexpiprazole and cariprazine have been approved for schizophrenia and esketamine for depression. Interestingly, the approval of esketamine has proved controversial (Cristea and Naudet, 2019, Gastaldon et al., 2019) suggesting the need for a continuous assessment of this type.
We could only assess published EPARs and found only two evaluations resulting in a refusal: one for agomelatine (the drug was approved later) and one for asenapine in schizophrenia (it was approved for bipolar disorder in the same EPAR). We had no access to unpublished and/or refused applications. Butlen-Ducuing et al. had access to applications of this type and found 46 applications in psychiatry between 1995 and 2014 (Butlen-Ducuing et al., 2016). We were unable to compare the applications that resulted in an approval with refused applications. In addition, we did not systematically appraise the EMA guidelines that are available online (EMA, 2018a). This is currently being done by another ongoing undertaking (Boesen et al., 2020), and it will be interesting to confront findings of this study with ours to explore whether EMA's guidelines were followed or not.
Finally, our study is not exhaustive for all psychotropic drugs approved in Europe, as these drugs can be authorised via other procedures (e.g. national procedure and decentralised/mutual recognition). And indeed, the situation may be even worse, as suggested by the example of reboxetine, an antidepressant that was approved for marketing in many European countries (e.g. the United Kingdom and Germany) in 1997 while it was an ineffective and potentially harmful antidepressant (Eyding et al., 2010). More recently, baclofen was approved by the French national agency for medicine and health product safety (the Agence Nationale de Sécurité du Médicament et des Produits de Santé [ANSM]), despite the evidence-based assessment by its Temporary Special Scientific Committee of independent experts who concluded that the risk–benefit ratio of baclofen in alcohol use disorder was negative (Naudet and Braillon, 2018).
Conclusions
The evidence for psychiatric drug approvals by the EMA is in general low, especially when comparative effectiveness issues are considered. This result compounds many others that raise criticisms of EMA's criteria, not only in the field of psychopharmacology (Barbui et al., 2011; Banzi et al., 2014a, b). In addition, despite its commitment to sharing study report data, retrieval of these data proved to be difficult, combining a slow process of retrieval along with the concealment of EPARs reporting non-approval. These doubtful approvals and the lack of transparency could incentivise ineffective drug development and waste research efforts (Chalmers and Glasziou, 2009; Glasziou et al., 2014; Ioannidis et al., 2014). Output from independent and continuous monitoring by health authorities (such as the EMA) should motivate the definition of high standards, so as to drive the research agenda and avoid wasted efforts, and to reduce persistent uncertainties. Simple solutions can be proposed. First, EPARs should include proper meta-analyses (Chalmers and Glasziou, 2009; Glasziou et al., 2014), with data sharing of all aggregated data in a structured form allowing a complete overview of the evidence base for approved drugs. Ideally, to provide complete transparency, these meta-analyses should be prospective (prior to the RCTs). In the spirit of Open Science, we have suggested a model of ‘registered drug approvals’ following the data-blind peer-review model of registered reports (Naudet and Cristea, 2020). With suitable success criteria in place, this model would guarantee transparency in establishing the evidence required for approving a new drug.
Acknowledgements
Angela Verdier (angela.verdier@wanadoo.fr) is acknowledged for her help with translation.
Data
The R session information, code and data used are available on Open Science Framework at https://osf.io/j9c26, DOI 10.17605/OSF.IO/J9C26.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S2045796020000359.
click here to view supplementary material
Conflict of interest
None.
References
- Avorn J (2012) Two centuries of assessing drug risks. The New England Journal of Medicine 367, 193–197. [DOI] [PubMed] [Google Scholar]
- Banzi R, Bertele V, Demotes-Mainard J, Garattini S, Gluud C, Kubiak C and Ohmann C (2014a) Fostering EMA's transparency policy. European Journal of Internal Medicine 25, 681–684. [DOI] [PubMed] [Google Scholar]
- Banzi R, Bertele V and Garattini S (2014b) EMA's transparency seems to be opaque. Lancet (London, England) 384, 1847. [DOI] [PubMed] [Google Scholar]
- Barbui C and Cipriani A (2012) Agomelatine and the brave old world of narrative-based medicine. Evidence-based Mental Health 15, 2–3. [DOI] [PubMed] [Google Scholar]
- Barbui C, Baschirotto C and Cipriani A (2011) EMA Must improve the quality of its clinical trial reports. BMJ (Clinical Research Edition) 342, d2291. [DOI] [PubMed] [Google Scholar]
- Bighelli I and Barbui C (2012) What is the European Medicines Agency? Epidemiology and Psychiatric Sciences 21, 245–247. [DOI] [PubMed] [Google Scholar]
- Boesen K, Gotzsche P C and Ioannidis J P (2020) FDA and EMA clinical research guidelines: Assessment of trial design recommendations for pivotal psychiatric drug trials (Protocol). 2020.01.22.20018499.
- Butlen-Ducuing F, Petavy F, Guizzaro L, Zienowicz M, Haas M, Alteri E, Salmonson T and Corruble E (2016) Regulatory watch: challenges in drug development for central nervous system disorders: a European Medicines Agency perspective. Nature Reviews. Drug Discovery 15, 813–814. [DOI] [PubMed] [Google Scholar]
- Chalmers I and Glasziou P (2009) Avoidable waste in the production and reporting of research evidence. Obstetrics and Gynecology 114, 1341–1345. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA and Geddes JR (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet (London, England) 391, 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (2013) Statistical Power Analysis for the Behavioral Sciences. New York: Taylor & Francis. [Google Scholar]
- Cristea IA and Naudet F (2019) US Food and Drug Administration approval of esketamine and brexanolone. The Lancet Psychiatry 6, 975–977. [DOI] [PubMed] [Google Scholar]
- Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, Mcdonald S, Salanti G, Meerpohl J, Maclehose H, Hilton J, Tovey D, Shemilt I and Thomas J (2017) Living systematic review: 1. Introduction-the why, what, when, and how. Journal of Clinical Epidemiology 91, 23–30. [DOI] [PubMed] [Google Scholar]
- EMA (2018a) Clinical Efficacy and Safety : Central Nervous System. London: EMA; Available at https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/clinical-efficacy-safety/clinical-efficacy-safety-nervous-system. [Google Scholar]
- EMA (2018b) Transparency. London: EMA; Available: https://www.ema.europa.eu/en/about-us/how-we-work/transparency. [Google Scholar]
- Eyding D, Lelgemann M, Grouven U, Harter M, Kromp M, Kaiser T, Kerekes MF, Gerken M and Wieseler B (2010) Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ (Clinical Research Edition) 341, c4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (1998) Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products. FDA; Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/providing-clinical-evidence-effectiveness-human-drug-and-biological-products. [Google Scholar]
- Garattini S and Bertele V (2005) The impact of European regulatory policies on psychotropic drug prescribing patterns. International Review of Psychiatry (Abingdon, England) 17, 199–204. [DOI] [PubMed] [Google Scholar]
- Gastaldon C, Papola D, Ostuzzi G and Barbui C (2019) Esketamine for treatment resistant depression: a trick of smoke and mirrors? Epidemiology and Psychiatric Sciences 29, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, Michie S, Moher D and Wager E (2014) Reducing waste from incomplete or unusable reports of biomedical research. Lancet (London, England) 383, 267–276. [DOI] [PubMed] [Google Scholar]
- Gotzsche PC, Young AH and Crace J (2015) Does long term use of psychiatric drugs cause more harm than good? BMJ (Clinical Research Edition) 350, h2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, Schulz KF and Tibshirani R (2014) Increasing value and reducing waste in research design, conduct, and analysis. Lancet (London, England) 383, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koesters M, Guaiana G, Cipriani A, Becker T and Barbui C (2013) Agomelatine efficacy and acceptability revisited: systematic review and meta-analysis of published and unpublished randomised trials. The British Journal of Psychiatry: The Journal of Mental Science 203, 179–187. [DOI] [PubMed] [Google Scholar]
- Kopec JA, Abrahamowicz M and Esdaile JM (1993) Randomized discontinuation trials: utility and efficiency. Journal of Clinical Epidemiology 46, 959–971. [DOI] [PubMed] [Google Scholar]
- Naudet F and Braillon A (2018) Baclofen and alcohol in France. The Lancet. Psychiatry 5, 961–962. [DOI] [PubMed] [Google Scholar]
- Naudet F and Cristea IA (2020) Approval of esketamine for treatment-resistant depression – Authors’ reply. The Lancet. Psychiatry 7, 235–236. [DOI] [PubMed] [Google Scholar]
- Naudet F, Millet B, Reymann JM and Falissard B (2013) Improving study design for antidepressant effectiveness assessment. International Journal of Methods in Psychiatric Research 22, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudet F, Palpacuer C, Boussageon R and Laviolle B (2016) Evaluation in alcohol use disorders - insights from the nalmefene experience. BMC Medicine 14, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Goodwin GM, Bhugra D, Fazel S and Lawrie S (2014) Attacks on antidepressants: signs of deep-seated stigma? The Lancet. Psychiatry 1, 102–104. [DOI] [PubMed] [Google Scholar]
- Ostuzzi G, Papola D, Gastaldon C and Barbui C (2017) New EMA report on paliperidone 3-month injections: taking clinical and policy decisions without an adequate evidence base. Epidemiology and Psychiatric Sciences 26, 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpacuer C, Laviolle B, Boussageon R, Reymann JM, Bellissant E and Naudet F (2015) Risks and benefits of nalmefene in the treatment of adult alcohol dependence: a systematic literature review and meta-analysis of published and unpublished double-blind randomized controlled trials. PLoS Medicine 12, e1001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner EH, Matthews AM, Linardatos E, Tell RA and Rosenthal R (2008) Selective publication of antidepressant trials and its influence on apparent efficacy. The New England Journal of Medicine 358, 252–260. [DOI] [PubMed] [Google Scholar]
- Van Ravenzwaaij D and Ioannidis JP (2017) A simulation study of the strength of evidence in the recommendation of medications based on two trials with statistically significant results. PLoS One 12, e0173184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach JD, Sullivan PG, Trepanowski JF, Sainani KL, Steyerberg EW and Ioannidis JP (2017) Evaluation of evidence of statistical support and corroboration of subgroup claims in randomized clinical trials. JAMA Internal Medicine 177, 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I and Posternak MA (2005) Generalizability of antidepressant efficacy trials: differences between depressed psychiatric outpatients who would or would not qualify for an efficacy trial. The American Journal of Psychiatry 162, 1370–1372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S2045796020000359.
click here to view supplementary material