Abstract
Adrenal myelolipoma is a benign tumor of the adrenal cortex composed predominantly of fat and hematopoietic tissue. These lesions are usually asymptomatic, and most often incidentally detected on imaging. Uncommonly, they present with retroperitoneal hemorrhage, and these have been traditionally treated with emergent surgery. Although, transarterial embolization has been effectively and safely used in patients presenting with active hemorrhage from acute traumatic and nontraumatic causes, literature specifically pertaining to adrenal artery embolization is scant, perhaps due to smaller size and variability of adrenal arteries. With recent advances in endovascular techniques and imaging, there are emerging case reports and series of adrenal artery embolization in acute and nonacute settings. We report a case of spontaneous hemorrhage within an adrenal myelolipoma in a 43-year-old male patient, successfully treated with transarterial embolization, thereby avoiding major surgery. Our report adds to the growing body of literature pertaining to adrenal artery embolization.
Keywords: Adrenal myelolipoma, Transarterial embolization, Adrenal hemorrhage, Pheochromocytoma, Coils, Trauma
Introduction
Acute adrenal hemorrhage is a rare but catastrophic event. Most adrenal hemorrhage is related to trauma or ruptured neoplasms [1].
Transarterial embolization is a minimally invasive, safe, and effective option for acute bleeding emergencies in the abdomen and pelvis [2,3]. Literature on adrenal artery embolization in the setting of acute adrenal hemorrhage is generally scant [4], [5], [6], [7], [8]. We describe a case of adrenal myelolipoma (AML) with spontaneous hemorrhage, successfully treated with transarterial embolization, thus avoiding major surgery.
Case report
A 43-year-old male with a past medical history significant for hypertension and obesity presented to the emergency department at an outside hospital with sudden onset of sharp, severe right flank pain. The pain was continuous, did not radiate, and was associated with nausea and vomiting. He denied history of recent trauma, dysuria, or gross hematuria and had no history of renal stones or tumors. Review of systems was otherwise unremarkable. The only significant genitourinary history was urethral disruption following motor vehicle crash at age 21, with subsequent pelvic realignment and multiple procedures to treat resultant strictures. The last complication from this event occurred over 20 years prior to presentation. Family history was significant for kidney stones and prostate cancer.
On examination, the patient appeared diaphoretic and pale, with stable vitals. The patient was started on pain medication, which helped improve his pain. Lab results showed a hemoglobin of 12.5 g/dL and elevated random glucose at 125 mg/dL.
As a part of his initial abdominal pain work up, a computed tomography (CT) of the abdomen and pelvis without contrast was reportedly remarkable for a large fatty mass from the upper pole of the right kidney with perinephric and retroperitoneal hemorrhage. Based on the results of the CT scan, the patient was transferred to our hospital, a tertiary care facility, for management of his bleeding retroperitoneal mass.
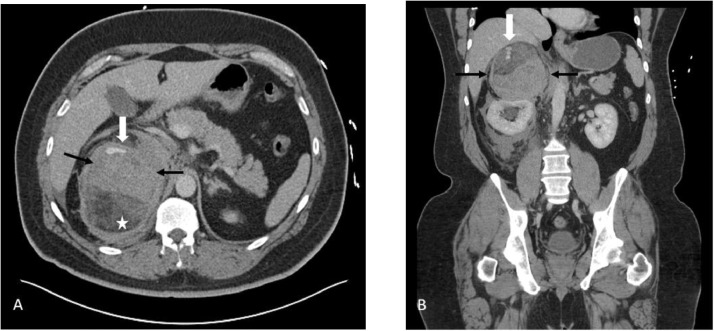
Upon arrival, he was somnolent with mild pallor. His SpO2 was markedly low at 88%, but his vital signs were otherwise normal. His abdomen was soft, nondistended, with mild flank tenderness. His labs were as follows; WBC: 21.18 × 10(9) /L, Hb: 10.6 g/dL, anion gap: 22 mmol/L, and glucose: 177 mg/dL. A contrast-enhanced CT of the abdomen and pelvis was performed to evaluate for continued hemorrhage, which showed the known fatty mass in the right suprarenal area with active extravasation/enlarging hematoma (Fig. 1). The mass was clearly separate from the right kidney and was diagnosed as an AML with spontaneous hemorrhage.
Fig. 1.
Contrast-enhanced axial (A) and coronal (B) CT of the abdomen showing hemorrhage (black arrows) in a fatty mass (white star) with a focus of contrast extravasation in the anterior and superior aspect (white arrow). This mass is located above the kidney and appears to indent it rather than arise from it.
Interventional radiology was consulted to perform an angiogram and embolization.
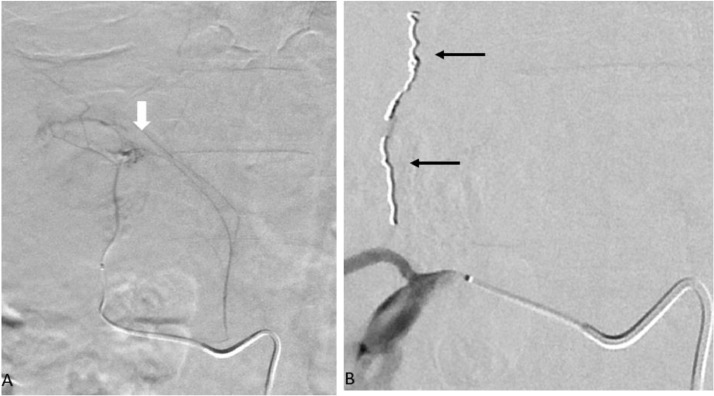
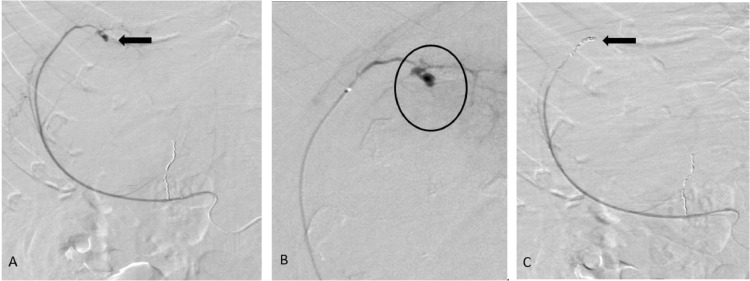
After obtaining appropriate consent, the patient was moved to the IR suite for an angiogram. The right renal artery was selectively catheterized with a 5-French reverse curve catheter via right femoral access. A selective right renal angiogram demonstrated 2 adrenal arteries (Fig. 2A). The more lateral adrenal artery appeared to supply a cluster of abnormal vessels (Fig. 2A). A microcatheter was used to select the abnormal adrenal artery. Super-selective arteriogram demonstrated a diffuse cluster of abnormal vessels (Fig. 2B). Embolization was performed using 100-300-micron embospheres. This was followed by coiling of the branch with 2-mm detachable coils to achieve stasis of flow in the vessel (Fig. 2B). Following this a repeat renal angiogram showed a lateral capsular branch with a small pseudoaneurysm/active extravasation (Fig. 3A,B). This branch was selectively catheterized with a microcatheter and embolization was performed using 100-300-micron embosphere particles followed by multiple detachable coils (Fig. 3C) to achieve complete stasis in the vessel.
Fig 2.
Digital subtraction angiogram: Selective catheterization (A) of the laterally located adrenal vessel with a microcatheter showing abnormal vasculature (white arrow). Postcoil embolization of the adrenal vessels (B) with nonfilling of the abnormal vasculature (black arrows).
Fig: 3.
Selective catheterization and angiogram of the capsular branch from the right renal artery (A) showing a small aneurysm in the superior aspect of the mass (black arrow). This vessel was super selectively catheterized (B) with a microcatheter. The angiogram shows the aneurysm more conspicuously (black circle). Coil embolization was performed using microcoils (C) with no flow in the postembolization images (black arrow).
His postprocedure period was uneventful except for mild right testicular pain which responded to IV pain medication. After the procedure, the patient's hemoglobin stabilized with eventual improvement in pain. He was discharged 2 days postprocedure in a stable condition.
Discussion
AML is a rare, benign, and nonfunctioning tumor composed of hematopoietic and adipose tissues, with peak incidence in the fifth to the seventh decades of life [9,10]. AMLs are the second most common incidentalomas of the adrenal gland after adrenal adenomas, and account for 2%-4% of all adrenal tumors [9].
AMLs are usually asymptomatic, incidentally detected, and when large enough present with hemorrhage and shock [11,12]. Rapid tumor growth with elevated intracapsular pressure is the proposed mechanism for hemorrhage in neoplastic tumors [4,13]. The location of the right adrenal gland also makes it more prone to trauma [14], [15], [16].
The diagnosis of AML is generally made on cross sectional imaging. On CT, it typically appears as a well-circumscribed, round or elliptical fatty mass within the adrenal gland with varied attenuation from −20 to −120 HU depending upon the myeloid: adipose ratio in the mass and the presence of atypical features such as hemorrhage and calcifications [9,[17], [18], [19]]. On MRI, the mass is hyperintense on T1, demonstrates macroscopic fat suppression and enhancement on postcontrast images [9,20]. The differential diagnosis should include renal angiomyolipoma, retroperitoneal lipoma, and liposarcoma [21], [22], [23]. Management varies with clinical presentation and local practices. For asymptomatic masses, the American association of endocrine surgery recommends imaging follow-up annually for a duration of 5 years [19]. However, some series have demonstrated a growth in a majority of asymptomatic AML [24], [25], [26]. Given the growth pattern and the cost involved in follow-up, elective surgery has been favored as a treatment option at some centers [27]. The AMLs presenting with spontaneous hemorrhage reported in literature have been traditionally treated with surgery [11,[28], [29], [30]]. Literature on adrenal artery embolization is scant, perhaps due to the small size of the arteries, and complexity/variability of the blood supply [14]. With recent advances in endovascular techniques and imaging, there are emerging reports and case series of adrenal artery embolization in pain palliation, tumor debulking, preoperative embolization, treatment of hyperaldosteronism, and in hemostasis of acute retroperitoneal hemorrhage caused by ruptured tumors, traumatic injuries and adrenal artery aneurysms [14,[31], [32], [33], [34], [35]]. The most common primary adrenal tumor to present with spontaneous hemorrhage is a pheochromocytoma, possibly form massive release of catecholamines with associated vasoconstriction, necrosis, and hemorrhage [36], [37], [38], [39]. Lung cancer is the most common primary tumor to present with hemorrhagic adrenal metastasis [38,39]. Based on our extensive data base search, this is the only case of AML with spontaneous hemorrhage, successfully treated by transarterial embolization. This relatively uncommon adrenal gland tumor with its unique presentation, and the nontraditional nature of the treatment employed warrants reporting of this case.
Author contribution
Study conception (AB,AA,AG), Data collection (KK, JM,AB), Data analysis (KK,AB,AA,AG), Manuscript writing (KK,AG,AA,RB,JM,AB), Critical revision (JM,AG,RB,AB), Final approval (KK,AA,RB,AG,JM,AB).
Footnotes
Competing interests: The authors declare no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data sharing: Data used in this study are not shared publicly.
Contributor Information
Khalid Kabeel, Email: kak9cr@missouri.edu.
Jasraj Marjara, Email: jsmxv3@health.missouri.edu.
Roopa Bhat, Email: bhatro@health.missouri.edu.
Ayman H. Gaballah, Email: gaballaha@health.missouri.edu.
Amr Abdelaziz, Email: abdelaziza@health.missouri.edu.
Ambarish P. Bhat, Email: bhatap@health.missouri.edu.
References
- 1.Giurazza F., Corvino F., Silvestre M., Cangiano G., Cavaglià E., Amodio F. Adrenal glands hemorrhages: embolization in acute setting. Gland Surg. 2019;8(2):115–122. doi: 10.21037/gs.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velmahos G.C., Chahwan S., Falabella A., Hanks S.E., Demetriades D. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg. 2000;24(5):539–545. doi: 10.1007/s002689910087. [DOI] [PubMed] [Google Scholar]
- 3.Patel P.J., Hieb R.A., Bhat A.P. Percutaneous revascularization of chronic total occlusions. Tech Vasc Interv Radiol. 2010;13(1):23–36. doi: 10.1053/j.tvir.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Fowler A.M., Burda J.F., Kim S.K. Adrenal artery embolization: anatomy, indications, and technical considerations. Am J Roentgenol. 2013;201(1):190–201. doi: 10.2214/AJR.12.9507. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda O., Urata J., Araki Y., Yoshimatsu S., Kume S., Torigoe Y. Acute adrenal hemorrhage after blunt trauma. Abdom Imaging. 2007;32(2):248–252. doi: 10.1007/s00261-006-9046-7. [DOI] [PubMed] [Google Scholar]
- 6.Dinc H, Simşek A, Ozyavuz R, Ozgür GK, Gümele HR. Endovascular treatment of massive retroperitoneal haemorrhage due to inferior adrenal artery injury. A case report. Acta Radiol. 2002;43(3) doi: 10.1080/J.1600-0455.2002.430316.X. [DOI] [PubMed] [Google Scholar]
- 7.Igwilo O.C., Sulkowski R.J., Shah M.R., Messink W.F., Kinnas N.C. Embolization of traumatic adrenal hemorrhage. J Trauma. 1999;47(6):1153–1155. doi: 10.1097/00005373-199912000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Bhat A.P., Pimpalwar A., Dyke P.C. Ultrasonography and X-ray guided drain placement to evacuate a pneumopericardium/pneumomediastinum in a 1-day-old infant. Indian J Radiol Imaging. 2019;29(1):94–97. doi: 10.4103/ijri.IJRI_447_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shenoy V.G., Thota A., Shankar R., Desai M.G. Adrenal myelolipoma: controversies in its management. Indian J Urol. 2015;31(2):94–101. doi: 10.4103/0970-1591.152807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasin J., Thimmappa N., Kaifi J.T., Avella D.M., Davis R., Tewari S.O. CT-guided cryoablation for post-thoracotomy pain syndrome: A retrospective analysis. Diagn Interv Radiol. 2020;26(1):53–57. doi: 10.5152/dir.2019.19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H.P., Chang W.Y., Chien S.T., Hsu C.W., Wu Y.C., Kung W.C. Intra-abdominal bleeding with hemorrhagic shock: A case of adrenal myelolipoma and review of literature. BMC Surg. 2017;17(1):74. doi: 10.1186/s12893-017-0270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thimmappa N., Bhat A.P., Bishop K., Nagpal P., Prince M.R., Saboo S.S. Preoperative cross-sectional mapping for deep inferior epigastric and profunda artery perforator flaps. Cardiovasc Diagn Ther. 2019;9(S1):S131–S142. doi: 10.21037/cdt.2018.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehrberg A., Kharbutli B. Isolated unilateral adrenal gland hemorrhage following motor vehicle collision: a case report and review of the literature. J Med Case Rep. 2017;11(1):358. doi: 10.1186/s13256-017-1506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giurazza F., Corvino F., Silvestre M., Cangiano G., Cavaglià E., Amodio F. Adrenal glands hemorrhages: embolization in acute setting. Gland Surg. 2019;8(2):115–122. doi: 10.21037/gs.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhat A., Layfield L.J., Tewari S.O., Gaballah A.H., Davis R., Wu Z. Solitary fibrous tumor of the ischioanal fossa—a multidisciplinary approach to management with radiologic-pathologic correlation. Radiol Case Rep. 2018;13(2) doi: 10.1016/j.radcr.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuchardt P., Yasin J., Davis R.M., Thimmappa N., Bhat A.P. Pelvic trauma. Contemp Diagn Radiol. 2019;42(21):1–6. doi: 10.1097/01.CDR.0000582600.38333.f4. [DOI] [Google Scholar]
- 17.Pinto A., Scaglione M., Guidi G., Farina R., Acampora C., Romano L. Role of multidetector row computed tomography in the assessment of adrenal gland injuries. Eur J Radiol. 2006;59(3):355–358. doi: 10.1016/j.ejrad.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 18.To'o K.J., Duddalwar V.A. Imaging of traumatic adrenal injury. Emerg Radiol. 2012;19(6):499–503. doi: 10.1007/s10140-012-1063-y. [DOI] [PubMed] [Google Scholar]
- 19.Sreenivasan N., Kalyanpur A., Bhat A., Sridhar P., Singh J. CT diagnosis of cecal diverticulitis. Indian J Radiol Imaging. 2006;16(4) doi: 10.4103/0971-3026.32244. [DOI] [Google Scholar]
- 20.Verma M., Yarlagadda B., Hendrani A., Bhat A.P., Kumar S. Simplified rapid protocol for assessing the thoracic aortic dimensions and pathology with noncontrast MR angiography. Int J Angiol. 2019;28(2) doi: 10.1055/s-0039-1688473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell C., Goodacre B.W., vanSonnenberg E., Orihuela E. Spontaneous rupture of adrenal myelolipoma: spiral CT appearance. Abdom Imaging. 2000;25(4):431–434. doi: 10.1007/s002610000061. [DOI] [PubMed] [Google Scholar]
- 22.Répássy D.L., Csata S., Sterlik G., Iványi A. Giant adrenal myelolipoma. Pathol Oncol Res. 2001;7(1):72–73. doi: 10.1007/BF03032610. [DOI] [PubMed] [Google Scholar]
- 23.Schuchardt P.A., Yasin J.T., Davis R.M., Tewari S.O., Bhat A.P. The role of an IVC filter retrieval clinic—a single center retrospective analysis. Indian J Radiol Imaging. 2019;29(4) doi: 10.4103/ijri.IJRI_258_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han M., Burnett A.L., Fishman E.K., Marshall F.F. The natural history and treatment of adrenal myelolipoma. J Urol. 1997;157(4):1213–1216. [PubMed] [Google Scholar]
- 25.JP Meaglia J.S. Natural history of an adrenal myelilipoma. J Urol. 1992;147:1089. doi: 10.1016/s0022-5347(17)37482-7. [DOI] [PubMed] [Google Scholar]
- 26.Han M., Burnett A.L., Fishman E.K., Marshall F.F. The natural history and treatment of adrenal myelolipoma. J Urol. 1997;157(4):1213–1216. doi: 10.1016/S0022-5347(01)64926-7. [DOI] [PubMed] [Google Scholar]
- 27.Melck A.L., Rosengart M.R., Armstrong M.J., Stang M.T., Carty S.E., Yip L. Immediate laparoscopic adrenalectomy versus observation: cost evaluation for incidental adrenal lesions with atypical imaging characteristics. Am J Surg. 2012;204(4):462–467. doi: 10.1016/j.amjsurg.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Bhat A.P., Davis R.M., Bryan W.D. A rare case of bleeding duodenal varices from superior mesenteric vein obstruction-treated with transhepatic recanalization and stent placement. Indian J Radiol Imaging. 2019;29(3) doi: 10.4103/ijri.IJRI-21-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman H.B., Howard R.C., Patterson A.L. Spontaneous retroperitoneal hemorrhage from a giant adrenal myelolipoma. J Urol. 1996;155(2):639. doi: 10.1016/S0022-5347(01)66474-7. [DOI] [PubMed] [Google Scholar]
- 30.Albala D.M., Chung C.J., Sueoka B.L., Memoli V.A., Heaney J.A. Hemorrhagic myelolipoma of adrenal gland after blunt trauma. Urology. 1991;38(6):559–562. doi: 10.1016/0090-4295(91)80180-F. [DOI] [PubMed] [Google Scholar]
- 31.Bhat A.P., Schuchardt P.A., Bhat R., Davis R.M., Singh S. Metastatic appendiceal cancer treated with Yttrium 90 radioembolization and systemic chemotherapy: a case report. World J Radiol. 2019;11(13):116–125. doi: 10.4329/wjr.v11.i9.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ierardi A.M., Petrillo M., Patella F., Biondetti P., Fumarola E.M., Angileri S.A. Interventional radiology of the adrenal glands: Current status. Gland Surg. 2018;7(2):147–165. doi: 10.21037/gs.2018.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunhivalappil F.T., Hefny A.F., Abu-Zidan F.M. Management of blunt adrenal gland injury in a community-based hospital. Injury. 2019;50(5):1049–1052. doi: 10.1016/j.injury.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 34.Senne J., Davis R., Yasin J., Brimmo O., Evenski A., Bhat A.P. Computed tomography guided radio-frequency ablation of osteoid osteomas in atypical locations. Indian J Radiol Imaging. 2019;29(3) doi: 10.4103/ijri.IJRI-259-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghouri M.A., Gupta N., Bhat A.P., Thimmappa N.D., Saboo S.S., Khandelwal A. CT and MR imaging of the upper extremity vasculature: pearls, pitfalls, and challenges. Cardiovasc Diagn Ther. 2019;9(S1):S152–S173. doi: 10.21037/cdt.2018.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habib M., Tarazi I., Batta M. Arterial embolization for ruptured adrenal pheochromocytoma. Curr Oncol. 2010;17(6):65–70. doi: 10.3747/co.v17i6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambika S., Melton A., Lee D., Hesketh P.J. Massive retroperitoneal adrenal hemorrhage secondary to lung cancer metastasis treated by adrenal artery embolization. Clin Lung Cancer. 2009;10(5):E1. doi: 10.3816/CLC.2009.n.053. [DOI] [PubMed] [Google Scholar]
- 38.Marti J.L., Millet J., Sosa J.A., Roman S.A., Carling T., Udelsman R. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75–82. doi: 10.1007/s00268-011-1338-6. [DOI] [PubMed] [Google Scholar]
- 39.Lam K.Y., Lo C.Y. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56(1):95–101. doi: 10.1046/j.0300-0664.2001.01435.x. [DOI] [PubMed] [Google Scholar]