The Plant Geminiviruses
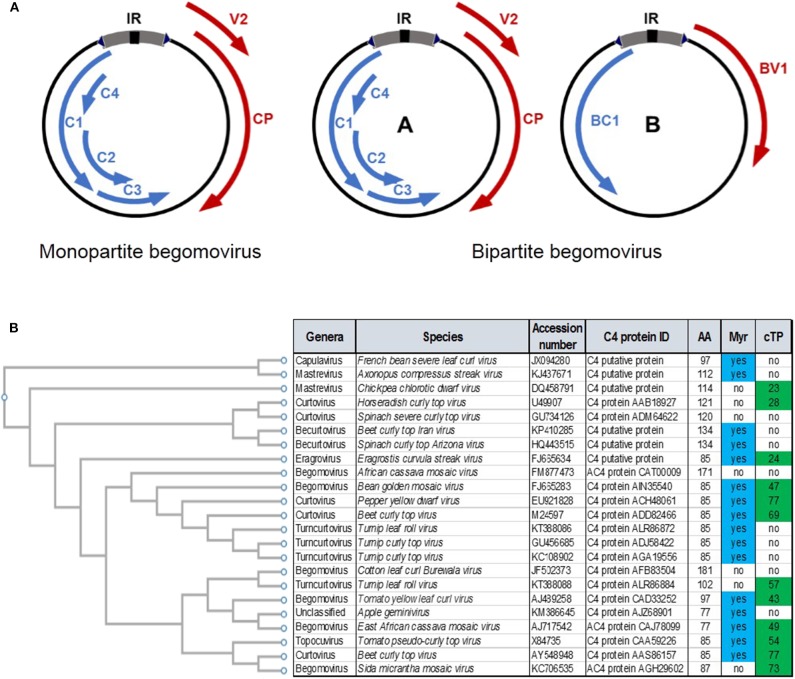
Geminiviruses are insect-transmitted plant viruses with circular, single-stranded (ss)DNA genomes that cause devastating diseases in major crops worldwide. The family Geminiviridae comprises more than 450 species divided in nine genera, based on genome organization, host range, and insect vector: Begomovirus, Mastrevirus, Curtovirus, Becurtovirus, Topocuvirus, Turncurtovirus, Capulavirus, Gablovirus, and Eragrovirus (Zerbini et al., 2017). The most diverse genus in this family is Begomovirus, which to date includes 409 different species (reviewed in Zhao et al., 2019). Begomoviruses can be further subdivided in monopartite, with one-molecule genomes, and bipartite, with two-molecule genomes (Figure 1A). Regardless of whether they are mono- or bi-partite, the size of each genomic DNA molecule is ~3 kb.
Figure 1.
(A) Geminivirus (begomovirus) genome structure in monopartite and bipartite species. Arrows represent open reading frames (ORFs). ORFs in the virion strand are in red; ORFs in the complementary strand are in blue. See text for details. (B) Comparison of the C4 proteins from different geminivirus species across genera. The presence of a predicted myristoylation site (Myr) or chloroplast transit peptide (cTP) in the protein sequence is indicated.
Apart from the obvious economic and practical interest propelling the study of geminiviruses, this virus family is an excellent model system to gain insight into plant processes. Geminiviruses replicate their DNA genomes in the nucleus by using the plant DNA replication machinery; the geminivirus genome forms minichromosomes that are subjected to epigenetic modifications; geminiviruses are both activators and suppressors of plant defense responses, and modulate plant developmental processes (reviewed in Hanley-Bowdoin et al., 2013). Therefore, geminiviruses can be used as probes to deepen our understanding not only of plant-virus interactions, but also of different aspects of plant biology.
Geminivirus-Encoded Proteins
As intracellular parasites, geminiviruses have to effectively manipulate plant cell functions to replicate, suppress anti-viral defense, and move throughout the plant, ultimately establishing a systemic infection; their evolved capacity to co-opt and modulate processes in a given host plant will determine the outcome of the plant-virus interaction. In order to hijack the host cell molecular machinery, geminiviruses produce a limited number (between 4 and 8) of small, fast-evolving, multifunctional proteins, encoded by bidirectional and partially overlapping open reading frames (ORFs) (Figure 1A). Monopartite begomoviruses encode six proteins, namely C1/Rep, C2/TrAP, C3/REn, C4, V2, and V1/CP. Homologs are encoded in one of the genomic component of bipartite begomoviruses, DNA A (in this case, named AC1/Rep, AC2/TrAP, AC3/REn, AC4, AV2, and AV1/CP); the other component in bipartite species, termed DNA B, encodes two additional proteins: the nuclear shuttle protein (NSP) and the movement protein (MP) (Figure 1A). Curiously, monopartite begomoviruses are often found in nature associated with satellite molecules, known as α- and β-satellites, which contribute to or even enable viral pathogenicity through the action of their encoded proteins (α-Rep and β-C1, respectively) (reviewed in Zhou, 2013).
In view of the fast pace of evolution of geminivirus genomes (reviewed in Zhao et al., 2019), it is expected that all proteins therein encoded are essential for the viral infection—since otherwise their coding sequence would be eventually lost. This idea is supported by the results obtained in the laboratory with artificially mutated viruses, which generally present a dramatically decreased virulence in their natural hosts and a high rate of reversion. Our current knowledge of the specific molecular function of individual geminivirus-encoded proteins derives from an ever-growing body of work, carried out by multiple research groups worldwide during the past few decades and resulting from the combination of molecular biology, cell biology, virology, and biochemistry.
Considering the biological properties and life cycle of geminiviruses and plant viruses in general, a series of functions that are conditio sine qua non for a successful viral infection can be inferred: these include manipulation of the cell cycle, DNA replication, intra- and inter-cellular movement, and suppression of gene silencing and other anti-viral defenses, such as the response to defense-related hormones. Virus-encoded proteins exerting these functions have indeed been identified in different geminivirus species, although in some cases the exact underlying molecular mechanisms remain to be unraveled (reviewed in Hanley-Bowdoin et al., 2013; Yang et al., 2016).
Positional Homologs in Geminiviruses
Genome structure is conserved among geminiviral species within the same genus, and in some cases even among species in different genera: genes in the same strand (virion or complementary) and position in different geminivirus species are therefore referred to as positional homologs, have the same name, and the resulting proteins show sequence similarity at the amino acid level (Figure 1). Given these shared properties, together with the observation that the biological requirements for a successful geminivirus infection are most likely common to all family members, positional homologs are frequently considered equivalent, and the properties identified for an individual gene are often extrapolated to others. This notion assumes that positional homologs are invariably and necessarily functional homologs; nonetheless, this is at odds with the idea of functional diversification that could result from the fast adaptation of different virus species to their hosts. Without the intention to be exhaustive, some specific examples are briefly discussed below.
Some functions of positional homologs seem indeed to be conserved across geminivirus species and genera: this is the case of Rep, which facilitates replication of the viral genome in all known species by reprogramming the cell cycle and mediating initiation, elongation, and termination of viral DNA replication (reviewed in Hanley-Bowdoin et al., 2013; Ruhel and Chakraborty, 2019); or that of V2, which acts as a suppressor of post-transcriptional gene silencing (PTGS) in all geminivirus species tested to date (Zrachya et al., 2007; Sharma and Ikegami, 2010; Amin et al., 2011; Zhang et al., 2012; Luna et al., 2017; Yang et al., 2018; Zhan et al., 2018; Mubin et al., 2019). Nevertheless, it has to be considered that geminivirus-encoded proteins are multifunctional: Rep, for example, promotes viral transcription (Kushwaha et al., 2017) and works as a suppressor of either transcriptional gene silencing (TGS) or PTGS in certain species (Rodríguez-Negrete et al., 2013; Liu et al., 2014); some V2 proteins act as suppressors of TGS (Wang et al., 2014, 2018, 2020; Mubin et al., 2019), and inhibit a host protease (Bar-Ziv et al., 2015). Therefore, at this point, whether functional homology among Rep or V2 proteins is complete or only partial is unclear.
On the other hand, examples of geminiviral positional homologs with proven partial functional homology are available in the literature. Perhaps the most illustrative case to date is that of the C2/AC2 proteins: in begomoviruses and curtoviruses, C2/AC2 proteins have a conserved zinc-finger motif, despite showing only limited similarity in the overall amino acid sequence; but while AC2, but perhaps not C2, from begomoviruses acts as a transcriptional activator for viral and some plant host genes (Sunter and Bisaro, 1992, 1997; Wartig et al., 1997; Trinks et al., 2005), C2 from curtoviruses lacks an obvious transcriptional activation domain and transcriptional activation activity (Sunter et al., 1994; Baliji et al., 2007). At least in two species, C2/AC2 interacts with and inactivates SNF1-related kinase (also known as Arabidopsis protein kinase 11 [AKIN11]), a global regulator of metabolism (Hao et al., 2003; Wang et al., 2003). Some C2/AC2 proteins are suppressors of PTGS (Voinnet et al., 1999; Vanitharani et al., 2004; Wang et al., 2005; Luna et al., 2012), but not others (Vanitharani et al., 2004; Luna et al., 2012). C2/AC2 has also been shown to suppress TGS by interfering with the methyl cycle in several species, but through at least two different mechanisms, namely the inhibition of adenosine kinase (ADK) (Buchmann et al., 2009; Jackel et al., 2015) and the attenuation of the proteasome-mediated degradation of S-adenosyl-methionine decarboxylase 1 (SAMDC1) (Zhang et al., 2011). A third strategy to suppress TGS is exhibited by the C2/AC2 protein encoded by at least two other species, of which the C2/AC2 proteins interact with and inhibit the H3K9 histone methyltransferase SUVH4/KYP (Castillo-González et al., 2015; Sun et al., 2015). The C2 protein encoded by a curtovirus creates a cellular environment permissive to DNA replication, but this function is not shared by the protein encoded by the position homologue in begomoviruses (Caracuel et al., 2012; Lozano-Duran et al., 2012) (Table 1).
Table 1.
Different C2/AC2 functions described in several geminiviral species.
| Virus | Function | References |
|---|---|---|
| Tomato golden mosaic virus (TGMV); Mungbean yellow mosaic virus (MYMV) | Transcriptional activator for viral and some plant host genes | Sunter and Bisaro, 1992, 1997; Trinks et al., 2005 |
| Tomato golden mosaic virus (TGMV) and Beet curly top virus (BCTV) | Inactivation of SNF1-related kinase (Arabidopsis protein kinase 11 [AKIN11]) | Hao et al., 2003; Wang et al., 2003 |
| African cassava mosaic virus (ACMV); Tomato yellow leaf curl virus (TYLCV); Tomato golden mosaic virus (TGMV); Beet curly top virus (BCTV); Indian cassava mosaic virus (ICMV) and East African cassava mosaic Cameroon virus (EACMCV) | Posttranscriptional gene silencing (PTGS) suppression | Voinnet et al., 1999; Vanitharani et al., 2004; Wang et al., 2005; Luna et al., 2012 |
| Tomato golden mosaic virus (TGMV); Cabbage leaf curl virus (CaLCuV), and Beet curly top virus (BCTV) | Transcriptional gene silencing (TGS) suppression by interfering with the methyl cycle through inhibition of adenosine kinase (ADK) | Buchmann et al., 2009; Jackel et al., 2015 |
| Beet severe curly top virus (BSCTV) | TGS suppression by interfering with the methyl cycle through attenuation of the proteasome-mediated degradation of S-adenosyl-methionine decarboxylase 1 (SAMDC1) | Zhang et al., 2011 |
| Tomato golden mosaic virus (TGMV); Cabbage leaf curl virus (CaLCuV) and Indian cassava mosaic virus (strains: ICMV-Dha and ICMV-SG) | TGS suppression by inhibiting the H3K9 histone methyltransferase SUVH4/KYP | Castillo-González et al., 2015; Sun et al., 2015 |
| Beet curly top virus (BCTV) | Creation of a cellular environment permissive to DNA replication | Caracuel et al., 2012; Lozano-Duran et al., 2012 |
The functions of the geminivirus-encoded C4/AC4 could be at least as varied in different species as those of C2/AC2. Several independent functions have been ascribed to C4/AC4 to date (e.g. Piroux et al., 2007; Teng et al., 2010; Luna et al., 2012; Sunitha et al., 2013; Ismayil et al., 2018; Li et al., 2018; Mei et al., 2018, 2020; Rosas-Diaz et al., 2018), and transgenic Arabidopsis thaliana plants expressing C4/AC4 from different geminiviruses display distinct developmental phenotypes (Mills-Lujan and Deom, 2010; Luna et al., 2012). Perhaps even more importantly, the C4/AC4 proteins encoded by different geminivirus species can have non-perfectly overlapping subcellular localizations, depending on specific targeting signals, namely acylation sites and a chloroplast transit peptide (e.g., Fondong et al., 2007; Carluccio et al., 2018; Mei et al., 2018; Rosas-Diaz et al., 2018; Zhan et al., 2018; Medina-Puche et al., 2019) (Figure 1B). These differences in subcellular distribution of different C4/AC4 proteins, which can be found associated to membranes, in the cytoplasm, in the nucleus, or in chloroplasts, will in all likelihood have a strong impact on their functionality during infection. Interestingly, C4 is seemingly under positive selection, in stark contrast to other geminiviral proteins (Sanz et al., 1999; Melgarejo et al., 2013; Yang et al., 2014).
In summary, a growing body of experimental data supports the idea that, although positional homologs have a common origin and frequently share functions, this functional overlap is not necessarily complete, since novel roles will have most likely been acquired during evolution. At the same time, not all geminiviral ORFs have positional counterparts (e.g., those in the DNA-B of bipartite geminiviruses), and therefore the essential virulence functions provided by the proteins they encode must be fulfilled by other, non-homologous geminiviral proteins. Hence, caution must be taken when extrapolating functional information to positional homologs, and uncovering the roles of each geminivirus-encoded protein in individual species will in all cases require experimental assessment.
Author Contributions
AL and RL-D conceived the idea and prepared the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Alberto P. Macho for critical reading of the manuscript, Laura Medina-Puche for her invaluable help in the preparation of Figure 1B, and Eduardo R. Bejarano, the authors' PhD supervisor, for nurturing their critical thinking abilities in their scientific infancy.
Footnotes
Funding. Work in the Lozano-Duran lab is supported by the Shanghai Center for Plant Stress Biology from the Chinese Academy of Sciences, the National Science Foundation China (NSFC grants 31671994 and 31870250), and the Chinese Academy of Sciences Strategic Pilot Science and Technology Special (B) funding (grant No. XDB27040206).
References
- Amin I., Hussain K., Akbergenov R., Yadav J. S., Qazi J., Mansoor S., et al. (2011). Suppressors of RNA silencing encoded by the components of the cotton leaf curl begomovirus-betasatellite complex. Mol. Plant Microbe. Interact. 24, 973–983. 10.1094/MPMI-01-11-0001 [DOI] [PubMed] [Google Scholar]
- Baliji S., Sunter J., Sunter G. (2007). Transcriptional analysis of complementary sense genes of Spinach curly top virus and functional role of C2 in pathogenesis. Mol. Plant Microbe. Interact. 20, 194–206. 10.1094/MPMI-20-2-0194 [DOI] [PubMed] [Google Scholar]
- Bar-Ziv A., Levy Y., Citovsky V., Gafni Y. (2015). The Tomato Yellow Leaf Curl Virus (TYLCV) V2 protein inhibits enzymatic activity of the host papain-like cysteine protease CYP1. Biochem. Biophys. Res. Commun. 460, 525–529. 10.1016/j.bbrc.2015.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann R. C., Asad S., Wolf J. N., Mohannath G., Bisaro D. M. (2009). Geminivirus AL2 and L2 proteins suppress transcriptional gene silencing and cause genome-wide reductions in cytosine methylation. J. Virol. 83, 5005–5013. 10.1128/jvi.01771-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracuel Z., Lozano-Durán R., Huguet S., Arroyo-Mateos M., Rodríguez-Negrete E. A., Bejarano E. R. (2012). C2 from Beet curly top virus promotes a cell environment suitable for efficient replication of geminiviruses, providing a novel mechanism of viral synergism. New Phytol. 194, 846–858. 10.1111/j.1469-8137.2012.04080.x [DOI] [PubMed] [Google Scholar]
- Carluccio A. V., Prigigallo M. I., Rosas-Diaz T., Lozano-Duran R., Stavolone L. (2018). S-acylation mediates mungbean yellow mosaic virus AC4 localization to the plasma membrane and in turns gene silencing suppression. PLoS Pathog. 14:e1007207. 10.1371/journal.ppat.1007207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-González C., Liu X., Huang C., Zhao C., Ma Z., Hu T., et al. (2015). Geminivirus-encoded TrAP suppressor inhibits the histone methyltransferase SUVH4/KYP to counter host defense. Elife 4:e0667. 10.7554/eLife.06671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondong V. N., Reddy R. V. C., Lu C., Hankoua B., Felton C., Czymmek K., et al. (2007). The consensus N-myristoylation motif of a geminivirus AC4 protein is required for membrane binding and pathogenicity. Mol. Plant. Microbe. Interact. 20, 380–391. 10.1094/MPMI-20-4-0380 [DOI] [PubMed] [Google Scholar]
- Hanley-Bowdoin L., Bejarano E. R., Robertson D., Mansoor S. (2013). Geminiviruses: masters at redirecting and reprogramming plant processes. Nat. Rev. Microbiol. 11, 777–788. 10.1038/nrmicro3117 [DOI] [PubMed] [Google Scholar]
- Hao L., Wang H., Sunter G., Bisaro D. M. (2003). Geminivirus AL2 and L2 proteins interact with and inactivate SNF1 kinase. Plant Cell. 15, 1034–1048. 10.1105/tpc.009530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismayil A., Haxim Y., Wang Y., Li H., Qian L., Han T., et al. (2018). Cotton leaf curl multan virus C4 protein suppresses both transcriptional and post-transcriptional gene silencing by interacting with SAM synthetase. PLoS Pathog. 14:e1007282. 10.1371/journal.ppat.1007282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackel J. N., Buchmann R. C., Singhal U., Bisaro D. M. (2015). Analysis of geminivirus AL2 and L2 proteins reveals a novel AL2 silencing suppressor activity. J. Virol. 89, 3176–3187. 10.1128/jvi.02625-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha N. K., Bhardwaj M., Chakraborty S. (2017). The replication initiator protein of a geminivirus interacts with host monoubiquitination machinery and stimulates transcription of the viral genome. PLoS Pathog. 13:e1006587. 10.1371/journal.ppat.1006587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zeng R., Chen Z., Liu X., Cao Z., Xie Q., et al. (2018). S-acylation of a geminivirus C4 protein is essential for regulating the CLAVATA pathway in symptom determination. J. Exp. Bot. 69, 4459–4468. 10.1093/jxb/ery228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jin W., Wang L., Wang X. (2014). Replication-associated proteins encoded by Wheat dwarf virus act as RNA silencing suppressors. Virus Res. 190, 34–39. 10.1016/j.virusres.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Lozano-Duran R., Caracuel Z., Bejarano E. R. (2012). C2 from beet curly top virus meddles with the cell cycle: a novel function for an old pathogenicity factor. Plant Signal. Behav. 7, 1705–1708. 10.4161/psb.22100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna A. P., Morilla G., Voinnet O., Bejarano E. R. (2012). Functional analysis of gene-silencing suppressors from tomato yellow leaf curl disease viruses. Mol. Plant. Microbe. Interact. 25, 1294–1306. 10.1094/MPMI-04-12-0094-R [DOI] [PubMed] [Google Scholar]
- Luna A. P., Rodríguez-Negrete E. A., Morilla G., Wang L., Lozano-Durán R., Castillo A. G., et al. (2017). V2 from a curtovirus is a suppressor of post-transcriptional gene silencing. J. Gen. Virol. 98, 2607–2614. 10.1099/jgv.0.000933 [DOI] [PubMed] [Google Scholar]
- Medina-Puche L., Tan H., Dogra V., Wu M., Rosas-Diaz T., Wang L., et al. (2019). A novel pathway linking plasma membrane and chloroplasts is co-opted by pathogens to suppress salicylic acid-dependent defences. bioRxiv 837955 10.1101/837955 [DOI] [Google Scholar]
- Mei Y., Ma Z., Wang Y., Zhou X. (2020). Geminivirus C4 antagonizes the HIR1-mediated hypersensitive response by inhibiting the HIR1 self-interaction and promoting degradation of the protein. New Phytol. 225, 1311–1326. 10.1111/nph.16208 [DOI] [PubMed] [Google Scholar]
- Mei Y., Wang Y., Hu T., Yang X., Lozano-Duran R., Sunter G., et al. (2018). Nucleocytoplasmic shuttling of geminivirus C4 protein mediated by phosphorylation and myristoylation is critical for viral pathogenicity. Mol. Plant 11, 1466–1481. 10.1016/j.molp.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Melgarejo T. A., Kon T., Rojas M. R., Paz-Carrasco L., Zerbini F. M., Gilbertson R. L. (2013). Characterization of a new world monopartite begomovirus causing leaf curl disease of tomato in Ecuador and Peru reveals a new direction in geminivirus evolution. J. Virol. 87, 5397–5413. 10.1128/JVI.00234-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Lujan K., Deom C. M. (2010). Geminivirus C4 protein alters arabidopsis development. Protoplasma 239, 95–110. 10.1007/s00709-009-0086-z [DOI] [PubMed] [Google Scholar]
- Mubin M., Briddon R. W., Mansoor S. (2019). The V2 protein encoded by a monopartite begomovirus is a suppressor of both post-transcriptional and transcriptional gene silencing activity. Gene 686, 43–48. 10.1016/j.gene.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Piroux N., Saunders K., Page A., Stanley J. (2007). Geminivirus pathogenicity protein C4 interacts with Arabidopsis thaliana shaggy-related protein kinase AtSKη, a component of the brassinosteroid signalling pathway. Virology 362, 428–440. 10.1016/j.virol.2006.12.034 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Negrete E., Lozano-Durán R., Piedra-Aguilera A., Cruzado L., Bejarano E. R., Castillo A. G. (2013). Geminivirus Rep protein interferes with the plant DNA methylation machinery and suppresses transcriptional gene silencing. New Phytol. 199, 464–475. 10.1111/nph.12286 [DOI] [PubMed] [Google Scholar]
- Rosas-Diaz T., Zhang D., Fan P., Wang L., Ding X., Jiang Y., et al. (2018). A virus-targeted plant receptor-like kinase promotes cell-to-cell spread of RNAi. Proc. Natl. Acad. Sci. U.S.A. 115, 1388–1393. 10.1073/pnas.1715556115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhel R., Chakraborty S. (2019). Multifunctional roles of geminivirus encoded replication initiator protein. VirusDisease 30, 66–73. 10.1007/s13337-018-0458-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A. I., Fraile A., Gallego J. M., Malpica J. M., García-Arenal F. (1999). Genetic variability of natural populations of cotton leaf curl geminivirus, a single-stranded DNA virus. J. Mol. Evol. 9, 672–81. 10.1007/PL00006588 [DOI] [PubMed] [Google Scholar]
- Sharma P., Ikegami M. (2010). Tomato leaf curl Java virus V2 protein is a determinant of virulence, hypersensitive response and suppression of posttranscriptional gene silencing. Virology 396, 85–93. 10.1016/j.virol.2009.10.012 [DOI] [PubMed] [Google Scholar]
- Sun Y.-W., Tee C.-S., Ma Y.-H., Wang G., Yao X.-M., Ye J. (2015). Attenuation of Histone Methyltransferase KRYPTONITE-mediated transcriptional gene silencing by Geminivirus. Sci. Rep. 5:16476. 10.1038/srep16476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunitha S., Shanmugapriya G., Balamani V., Veluthambi K. (2013). Mungbean yellow mosaic virus (MYMV) AC4 suppresses post-transcriptional gene silencing and an AC4 hairpin RNA gene reduces MYMV DNA accumulation in transgenic tobacco. Virus Genes 46, 496–504. 10.1007/s11262-013-0889-z [DOI] [PubMed] [Google Scholar]
- Sunter G., Bisaro D. M. (1992). Transactivation of geminivirus AR1 and BR1 gene expression by the viral AL2 gene product occurs at the level of transcription. Plant Cell 4, 1321–1331. 10.2307/3869417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter G., Bisaro D. M. (1997). Regulation of a geminivirus coat protein promoter by AL2 protein (TrAP): evidence for activation and derepression mechanisms. Virology 232, 269–280. 10.1006/viro.1997.8549 [DOI] [PubMed] [Google Scholar]
- Sunter G., Stenger D. C., Bisaro D. M. (1994). Heterologous complementation by geminivirus AL2 and AL3 genes. Virology 203, 203–210. 10.1006/viro.1994.1477 [DOI] [PubMed] [Google Scholar]
- Teng K., Chen H., Lai J., Zhang Z., Fang Y., Xia R., et al. (2010). Involvement of C4 protein of Beet severe curly top virus (Family Geminiviridae) in virus movement. PLoS ONE 5:e011280. 10.1371/journal.pone.0011280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinks D., Rajeswaran R., Shivaprasad P. V., Akbergenov R., Oakeley E. J., Veluthambi K., et al. (2005). Suppression of RNA silencing by a geminivirus nuclear protein, AC2, correlates with transactivation of host genes. J. Virol. 79, 2517–2527. 10.1128/JVI.79.4.2517-2527.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitharani R., Chellappan P., Pita J. S., Fauquet C. M. (2004). Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and suppression of posttranscriptional gene silencing. J. Virol. 78, 9487–9498. 10.1128/JVI.78.17.9487-9498.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O., Pinto Y. M., Baulcombe D. C. (1999). Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci.U.S.A. 96, 14147–14152. 10.1073/pnas.96.24.14147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li F., Huang C., Yang X., Qian Y., Xie Y., et al. (2014). V2 of Tomato yellow leaf curl virus can suppress methylation-mediated transcriptional gene silencing in plants. J. Gen. Virol. 95, 225–230. 10.1099/vir.0.055798-0 [DOI] [PubMed] [Google Scholar]
- Wang B., Yang X., Wang Y., Xie Y., Zhou X. (2018). Tomato yellow leaf curl virus V2 interacts with host histone deacetylase 6 to suppress methylation-mediated transcriptional gene silencing in plants. J. Virol. 92:e00036-18. 10.1128/JVI.00036-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Buckley K. J., Yang X., Buchmann R. C., Bisaro D. M. (2005). Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins. J. Virol. 79, 7410–7418. 10.1128/JVI.79.12.7410-7418.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Hao L., Shung C.-Y., Sunter G., Bisaro D. M. (2003). Adenosine kinase is inactivated by geminivirus AL2 and L2 proteins. Plant Cell 15, 3020–3032. 10.1105/tpc.015180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ding Y., He L., Zhang G., Zhu J.-K., Lozano-Duran R. (2020). A virus-encoded protein suppresses methylation of the viral genome in the Cajal body through its interaction with AGO4. bioRxiv 811091 10.1101/811091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartig L., Kheyr-Pour A., Noris E., De Kouchkovsky F., Jouanneau F., Gronenborn B., et al. (1997). genetic analysis of the monopartite tomato yellow leaf curl geminivirus: roles of V1, V2, and C2 ORFs in viral pathogenesis. Virology 228, 132–140. 10.1006/viro.1996.8406 [DOI] [PubMed] [Google Scholar]
- Yang X., Ren Y., Sun S., Wang D., Zhang F., Li D., et al. (2018). Identification of the potential virulence factors and RNA silencing suppressors of mulberry mosaic dwarf-associated geminivirus. Viruses 10:472. 10.3390/v10090472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wang B., Li F., Yang Q., Zhou X. (2016). “Research Advances in Geminiviruses,” in Current Research Topics in Plant Virology, eds Wang A., Zhou X. (Cham: Springer International Publishing; ), 251–269. 10.1007/978-3-319-32919-2_11 [DOI] [Google Scholar]
- Yang X. L., Zhou M. N., Qian Y. J., Xie Y., Zhou X. P. (2014). Molecular variability and evolution of a natural population of Tomato yellow leaf curl virus in Shanghai, China. J. Zhejiang Univ. Sci. B 15, 133–142. 10.1631/jzus.B1300110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbini F. M., Briddon R. W., Idris A., Martin D. P., Moriones E., Navas-Castillo J., et al. (2017). ICTV virus taxonomy profile: geminiviridae. J. Gen. Virol. 98, 131–133. 10.1099/jgv.0.000738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan B., Zhao W., Li S., Yang X., Zhou X. (2018). Functional scanning of apple geminivirus proteins as symptom determinants and suppressors of posttranscriptional gene silencing. Viruses 10:488. 10.3390/v10090488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Dong J., Xu Y., Wu J. (2012). V2 protein encoded by Tomato yellow leaf curl China virus is an RNA silencing suppressor. Virus Res. 163, 51–58. 10.1016/j.virusres.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Chen H., Huang X., Xia R., Zhao Q., Lai J., et al. (2011). BSCTV C2 attenuates the degradation of SAMDC1 to Suppress DNA methylation-mediated gene silencing in Arabidopsis. Plant Cell 23, 273–288. 10.1105/tpc.110.081695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Rosario K., Breitbart M., Duffy S. (2019). Eukaryotic circular rep-encoding single-stranded DNA (CRESS DNA) viruses: ubiquitous viruses with small genomes and a diverse host range. Adv. Virus Res. 103, 71–133. 10.1016/bs.aivir.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Zhou X. (2013). Advances in understanding begomovirus satellites. Annu. Rev. Phytopathol. 51, 357–381. 10.1146/annurev-phyto-082712-102234 [DOI] [PubMed] [Google Scholar]
- Zrachya A., Glick E., Levy Y., Arazi T., Citovsky V., Gafni Y. (2007). Suppressor of RNA silencing encoded by Tomato yellow leaf curl virus-Israel. Virology 358, 159–165. 10.1016/j.virol.2006.08.016 [DOI] [PubMed] [Google Scholar]