Abstract
Ancylostoma caninum is the most prevalent intestinal nematode of dogs, and has a zoonotic potential. Multiple-drug resistance (MDR) has been confirmed in a number of A. caninum isolates, including isolate Worthy 4.1F3P, against all anthelmintic drug classes approved for hookworm treatment in dogs in the United States (US). The cyclooctadepsipeptide emodepside is not registered to use in dogs in the US, but in a number of other countries/regions. The objective of this study was to evaluate the efficacy of emodepside + praziquantel, as well as three commercial products that are commonly used in the US for treatment of hookworms, against a suspected (subsequently confirmed) MDR A. caninum isolate Worthy 4.1F3P. 40 dogs infected on study day (SD) 0 with 300 third-stage larvae, were randomly allocated to one of five treatment groups with eight dogs each: pyrantel pamoate (Nemex®-2), fenbendazole (Panacur® C), milbemycin oxime (Interceptor®), emodepside + praziquantel tablets and non-treated control. Fecal egg counts (FEC) were performed on SDs 19, 20, 22, 27, 31 and 34. All treatments were administered as per label requirements on SD 24 to dogs in Groups 1 through 4. Two additional treatments were administered on SDs 25 and 26 to dogs in Group 2 as per label requirements. Dogs were necropsied on SD 34 and the digestive tract was removed/processed for worm recovery and enumeration. The geometric mean (GM) worm counts for the control group was 97.4, and for the pyrantel pamoate, fenbendazole, milbemycin oxime, and emodepside + praziquantel groups were 74.8, 72.0, 88.9, and 0.4, respectively. These yielded efficacies of 23.2%, 26.1%, and 8.8%, and 99.6%, respectively. These data support previous findings of the MDR status of Worthy 4.1F3P as treatments with pyrantel pamoate, fenbendazole and milbemycin oxime lacked efficacy. In sharp contrast, Worthy 4.1F3P was highly susceptible to treatment with emodepside + praziquantel.
Keywords: Ancylostoma caninum, Hookworms, Lack of efficacy, Multiple-drug resistance (MDR), Treatment, Emodepside
Graphical abstract
1. Introduction
The canine hookworm, Ancylostoma caninum is the most prevalent and important intestinal nematode parasite of dogs in the United States (US) with the prevalence depending on age, level of care and geographic location of the dog (Little et al., 2009). A recent study evaluating over 39 million fecal samples from 2012 to 2018, showed evidence of a steady yearly increase in prevalence from 2015 onwards, with an overall increase of 47% (Drake and Carey, 2019). Anthelmintic drugs currently approved for treatment of A. caninum in the United States include, febantel and fenbendazole of the benzimidazole class, moxidectin and milbemycin oxime of the macrocyclic lactone class (sub-class milbemycins), and pyrantel of the tetrahydropyrimidine class. In registration studies, febantel, moxidectin and milbemycin oxime all demonstrated efficacies >99% (F.D.A, 1994, 1998, 2006), fenbendazole demonstrated an efficacy >98% (F.D.A, 1983) and pyrantel demonstrated a somewhat variable efficacy, with a mean across studies of approximately 94% with more than half of those studies with efficacies greater than 99% (F.D.A, 1993).
Hookworms are blood-feeding nematodes that use a cutting apparatus to attach to the intestinal mucosa and submucosa, and contract their muscular esophagus to create negative pressure, which sucks a plug of tissue into their buccal capsules (Hotez et al., 2004). Bleeding is facilitated by both mechanical damage and chemical action by hydrolytic enzymes that cause rupture of capillaries and arterioles. Additionally, hookworms release an assortment of anticlotting agents to ensure blood flow (Stassens et al., 1996). Pathological consequences of infection include iron-deficiency anemia, hypoalbuminemia, and an enteritis characterized by diarrhea, which may contain fresh (hematochezia) or digested blood (melena) (Kalkofen, 1987).
In the past few years, there is empirical evidence that veterinarians are diagnosing increasing numbers of cases of persistent hookworm infections that appear refractory to standard anthelmintic therapy available in the United States. Recently retired racing greyhounds are highly over-represented among the cases of persistent/recurrent hookworm infection being reported to our laboratory (University of Georgia). To further investigate these observations, three isolates of A. caninum from clinical cases with persistent infections were established in laboratory beagles and evaluated using in vitro (egg hatch assay, larval development assay), molecular (deep amplicon sequencing), and in vivo testing (fecal egg count reduction). Data from these studies confirmed that all three isolates were resistant to the benzimidazole, avermectin/milbemycin and tetrahydropyrimidine classes of anthelmintics (Jimenez Castro et al., 2019). One isolate that was characterized in the above-mentioned studies by Jimenez Castro et al. (2019) was Worthy 4.1F3P. Though still unproven, clinical evidence strongly suggests that the MDR status of these A. caninum isolates evolved on greyhound breeding farms and kennels (Jimenez Castro et al., 2019). Spread of these isolates could pose a serious threat to dogs in the US.
These recent findings are particularly interesting given the history of anthelmintic resistance in A. caninum, where only very few cases are reported. The first report of anthelmintic resistance in A. caninum was to pyrantel in a greyhound puppy that was imported from Australia (Jackson et al., 1987). Several additional cases of resistance to pyrantel in dogs were subsequently diagnosed in Australia (Hopkins et al., 1988; Hopkins and Gyr, 1991; Kopp et al., 2007, 2008a, 2008b). However, subsequent to 2008 there were no further cases of anthelmintic resistance reported in A. caninum until 2019, when a report provided evidence of a case of resistance to benzimidazoles and macrocyclic lactones in an isolate of A. caninum obtained from a greyhound dog originating from Florida, USA (Kitchen et al., 2019). This was followed shortly thereafter by the report mentioned above, which demonstrated MDR against all three of the major drug classes most commonly used for the treatment of this parasite in dogs (Jimenez Castro et al., 2019).
Additionally, this could also pose a public health concern, as A. caninum is a zoonotic parasite that can cause cutaneous larva migrans (Leeming and Oxon, 1966; Bowman et al., 2010; Del Giudice et al., 2019), eosinophilic enteritis (Prociv and Croese, 1996), as well as patent infections in humans (Ngcamphalala et al., 2019; Furtado et al., 2020). Long-term treatment protocols composed of triple drug combinations of febantel, pyrantel and moxidectin, combined with strict environmental hygiene, were recently reported as being effective against persistent hookworm infections in greyhound dogs (Hess et al., 2019). However, diagnostic surveillance performed by our laboratory (University of Georgia) over the past year on actively racing and recently retired greyhounds originating from different locations has shown that this same triple anthelmintic combination most often fails to treat and control infections.
Emodepside is a drug in the cyclooctadepsipeptide class, a semisynthetic derivative of PF1022A (Sasaki et al., 1992). PF1022A itself is a fermentation product of the fungus Rosellinia spp. PF1022, which is found on the leaves of the plant Camellia japonica (Terada, 1992; Harder et al., 2003; Kulke, 2014). Historically the presynaptic latrophilin-like receptor (LAT-1), called depsiphilin in A. caninum (Krüger et al., 2009), and the ionotropic GABAA receptors (Chen et al., 1996; Miltsch et al., 2012) have been considered as putative molecular targets of emodepside in nematodes. However, various studies confirmed the calcium-activated and voltage-gated potassium nematode channels, SLO-1, as the most relevant and direct molecular drug target (Guest et al., 2007; Welz et al., 2011; Kulke et al., 2014; Crisford et al., 2015).
Emodepside has proven efficacy against a large and diverse number of nematode parasites infecting multiple different hosts, including A. caninum (Akyol et al., 1993; Zahner and Schares, 1993; Kachi et al., 1998; von Samson-Himmelstjerna et al., 2000; Zahner et al., 2001; Reinemeyer et al., 2005; Schmahl et al., 2007; Schimmel et al., 2009; Schroeder et al., 2009). Furthermore, due to its unique mechanism of action, emodepside has proven effective against nematode isolates with resistance to drugs from the other major classes of anthelmintics (von Samson-Himmelstjerna et al., 2005). Emodepside is sold for dogs as two separate products in several markets outside the USA; Profender® tablets for Dogs, containing emodepside and praziquantel and Procox® oral suspension containing emodepside and toltrazuril. A field study performed in several European countries in dogs ranging from one month to 11 years old using Profender® Tablets for Dogs demonstrated an efficacy of 99.9% against A. caninum and U. stenocephala, (Altreuther et al., 2011).
The objective of this study was to evaluate the efficacy of emodepside + praziquantel, as well as three other anthelmintic products commonly used for the treatment of canine hookworms, against A. caninum isolate Worthy 4.1F3P. This isolate was later confirmed as an MDR isolate of A. caninum (Jimenez Castro et al., 2019).
2. Materials and methods
2.1. Study design
The study was performed as a randomized, blinded, controlled efficacy study. The study protocol was approved by the study site Institutional Animal Care and Use Committee. All dogs were individually identified by ear tattoo, individually housed throughout the study, fed a balanced commercial dry dog food once daily, and provided with water ad libitum. Physical examinations were performed during the acclimation period to ensure dogs were healthy and eligible for enrolment into the study. The inclusion criteria included three negative fecal egg counts (FECs) performed on SDs −7, - 6, and −3 and good health. All personnel that were involved in data collection or assessment were blinded to the treatment assignment of the animals throughout the entire study period.
2.2. Ancylostoma caninum isolate
The A. caninum isolate used in this study was designated as Worthy 4.1F3P, the fourth laboratory passage of an isolate that was originally isolated from a retired racing greyhound in October 2017 (Jimenez Castro et al., 2019). During the course of laboratory passage, this isolate underwent treatment selection with fenbendazole and pyrantel on the first and third passages, respectively.
2.3. Parasitological methods
Two laboratory beagles were infected with the A. caninum isolate Worthy 3.1F3P to provide the infective larvae used in this study. Feces containing hookworm eggs were then cultured by mixing with activated charcoal (Black Diamond Media, Tinley Park, IL), and incubated at 76–80 °F and 56–92% relative humidity for at least five days. Third-stage larvae were then harvested using the Baermann technique (Baermann, 1917), placed into gelatin capsules and administered to the dogs. Fecal egg counts were performed on SD -7, −6, −3, 19, 20, 22, 27, 31 and 34, with all FECs performed using the McMaster procedure with a lower limit of detection of 25 eggs per gram (EPG) (Gordon and Whitlock, 1939).
2.4. Experimental inoculations
Forty-two purpose-bred Beagles sourced from a USDA licensed vendor (mix of male/female; 2.5 months of age at infection; 2.6–4.6 kg) were acclimated for 7 days. On SD 0, 42 dogs were inoculated orally both in the morning and in the afternoon, with 150 third-stage infective larvae (a total of 300 L3) of the Worthy 4.1F3P A. caninum isolate. Just prior to inoculation, L3 were placed into gelatin capsules to insure uniformity of inoculation. At the time of inoculation, capsules were placed in the back of the throat, and the dog was then administered 20–30 mls of deionized water to ensure that the capsule was swallowed. All dogs were checked for vomiting at 1 h ± 15 min post-inoculation and were examined at least once daily for any abnormal clinical signs or adverse events. On SD 22, all dogs were weighed followed by a physical examination, then 40 of the 42 dogs confirmed as hookworm-infection positive by having FEC higher than 25 EPG on SDs 19, 20 and 22, were completely randomized to one of five treatment groups, each composed of eight dogs. The two dogs not included in the study went back to the colony maintained at the study facility.
2.5. Drug administrations
All dogs were weighed using a certified scale two days before their scheduled treatment on SD 24. All treatments were administered orally as per the label requirements. Treatment groups were as follows: Group 1: pyrantel pamoate (Nemex®-2: Zoetis, Kalamazoo, MI) at a minimum of 5 mg/kg bodyweight (BW), Group 2: fenbendazole (Panacur® C: Merck Animal Health, Madison, NJ) at a minimum of 50 mg/kg BW for three consecutive days, Group 3: milbemycin oxime (Interceptor®: Elanco, Greenfield, IN) at a minimum of 0.5 mg/kg BW, Group 4: emodepside + praziquantel tablets at a minimum of 1 mg + 5 mg/kg BW, and Group 5: non-treated control.
2.6. Necropsy/worm counts
All dogs were humanely euthanized on SD 34, and the entire gastrointestinal tract, from the stomach to the rectum, was removed and processed in accordance with the relevant laboratory standard operating and parasitological procedures from TRS Labs, Inc. Briefly, the tract was opened longitudinally and the mucosa was scraped twice with 12” tissue forceps with gross serrated jaws, rinsed with tap water and left to soak for 2–3 h in plastic containers. The entire content of the container was then passed through a standard testing sieve #60 with a pore size of 250 μm. All recovered worms were placed into a labelled container containing warm normal saline, left overnight at room temperature and counted and sexed on SD 35, and then placed in 70% ETOH for storage.
2.7. Efficacy calculation/statistical analysis
The adult hookworm counts at necropsy were used to evaluate the efficacy of the treatment groups against A. caninum. Percent efficacy was calculated using the formula:
Effectiveness would be claimed against the parasite (i.e., calculated for both sexes combined on the basis of the addition of small and large intestinal counts) if the following criteria were met:
-
1.
At least six adequately infected non-treated dogs. From the parasitological perspective, a number ≥5 worms per dog was considered adequate.
-
2.
Calculated percent efficacy of at least 90% for each treatment group.
-
3.
Significant difference between the treatment group and the non-treated control group using a 5% level of significance and appropriate statistical analyses.
A non-parametric statistical method (Wilcoxon's Rank Sum test) was used to test for group differences in worm counts using a 5% significance level. Only pair-wise comparisons using the non-treated group were analyzed and reported. All analyses were performed using programs in SAS® version 9.4.
3. Results
No treatment related adverse events were recorded. The geometric mean (GM) worm count for the control group was 97.4, whereas GM worm counts for the pyrantel pamoate, fenbendazole, milbemycin oxime, and emodepside + praziquantel treatment groups were 74.8, 72.0, 88.9, and 0.4, respectively. These yielded efficacies of 23.2%, 26.1%, and 8.8%, and 99.6% respectively. The control group had significantly higher worm counts compared to the fenbendazole, pyrantel pamoate and emodepside + praziquantel treatment groups. The emodepside + praziquantel treated group had a higher efficacy when compared with each of the other three treatment groups (Table 1).
Table 1.
Numbers of worms recovered and percent efficacy for each treatment group. All dogs were infected with 300 A. caninum L3 on day 0, were treated on day 24, and were euthanized and worms recovered on day 34. Statistical comparisons were performed using the Wilcoxon's Rank Sum test.
| Treatment Groupa | No. ofdogs | Numbers of worms per dog (range) | Geometric mean b number of worms per dog | % Efficacy c |
|---|---|---|---|---|
| Pyrantel pamoate | 8 | 63–105 | 74.8a | 23.2 |
| Fenbendazole | 8 | 57–94 | 72.0a | 26.1 |
| Milbemycin oxime | 8 | 55–115 | 88.9 | 8.8 |
| Emodepside + praziquantel | 8 | 0–1 | 0.4a | 99.6 |
| Non-treated control | 8 | 71–132 | 97.4 | NA |
NA: Not applicable.
Statistically significant compared to the non-treated control group (p < 0.05).
Nemex®-2 (pyrantel pamoate), Panacur® C (fenbendazole), Interceptor® (milbemycin oxime).
Worm counts were logarithmically transformed (ln [count + 1]), averaged and then back-transformed to approximate the geometric means.
Efficacy was calculated using the formula: [(geometric mean worm count control group – geometric mean worm count treated group)/(geometric mean worm count control group)] x 100.
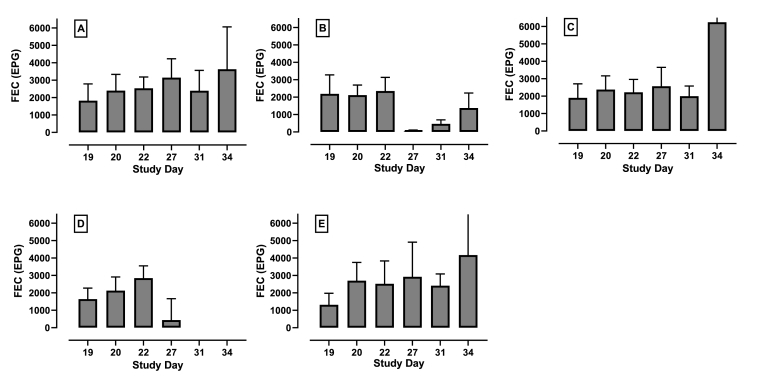
FEC were recorded for each dog on SD 19, 20, 22, 27, 31 and 34, and are shown in Table 2, with averages for each day shown in Fig. 1. For the pyrantel and milbemycin groups, the post-treatment FECs increased in a similar fashion as observed in the non-treated control group. Interestingly, the pattern of FEC reduction was very different for fenbendazole compared to the other drugs. Dogs treated with fenbendazole initially showed a high level of FEC reduction on SD 27, but this effect was temporary. In contrast, only one dog in the emodepside + praziquantel group was positive for hookworm eggs on SD 27, and on SD 31 and 34 all dogs were negative for hookworm eggs.
Table 2.
Fecal egg counts (FEC) in eggs per gram for each dog on each study day (SD) that FEC were performed.
| Post-infection | Post-treatment | |||||
|---|---|---|---|---|---|---|
| Treatment Groupa | SD 19 | SD 20 | SD 22 | SD 27 | SD 31 | SD 34 |
| Pyrantel pamoate | 2375 | 2450 | 3900 | 3850 | 1875 | 5575 |
| 2775 | 2250 | 2025 | 2075 | 1825 | 4850 | |
| 2875 | 3450 | 2550 | 2050 | 2675 | 1475 | |
| 1275 | 2325 | 2200 | 1950 | 1475 | 2275 | |
| 1200 | 3875 | 2450 | 2800 | 3025 | 7450 | |
| 2725 | 2125 | 1750 | 4425 | 4950 | 5150 | |
| 625 | 1875 | 2500 | 4600 | 1525 | 1050 | |
| 725 | 825 | 2875 | 3425 | 1775 | 1175 | |
| Fenbendazole | 1150 | 1425 | 1975 | 0 | 425 | 375 |
| 4250 | 2425 | 2550 | 25 | 500 | 2550 | |
| 1450 | 1350 | 1125 | 225 | 700 | 2100 | |
| 2375 | 3075 | 2350 | 25 | 700 | 875 | |
| 1700 | 1750 | 1450 | 0 | 650 | 1325 | |
| 3050 | 2350 | 3275 | 0 | 475 | 2350 | |
| 1025 | 2425 | 3075 | 0 | 50 | 425 | |
| 2500 | 2100 | 3000 | 0 | 225 | 1025 | |
| Milbemycin oxime | 3625 | 3500 | 1875 | 4775 | 2425 | 17875 |
| 1225 | 2925 | 2300 | 3575 | 2025 | 1375 | |
| 2400 | 2200 | 1550 | 2100 | 1725 | 2075 | |
| 2025 | 3200 | 3800 | 2575 | 1575 | 2375 | |
| 1175 | 1525 | 2350 | 1850 | 1425 | 3875 | |
| 1625 | 2075 | 2175 | 2200 | 2475 | 6075 | |
| 1475 | 1250 | 1375 | 1950 | 2975 | 13400 | |
| 1650 | 2300 | 2325 | 1500 | 1325 | 2875 | |
| Emodepside + praziquantel | 1475 | 2275 | 3950 | 0 | 0 | 0 |
| 1575 | 1750 | 3250 | 0 | 0 | 0 | |
| 925 | 875 | 2000 | 0 | 0 | 0 | |
| 3050 | 3475 | 3125 | 0 | 0 | 0 | |
| 1725 | 2525 | 2025 | 0 | 0 | 0 | |
| 1300 | 1825 | 3200 | 0 | 0 | 0 | |
| 1800 | 2650 | 3025 | 0 | 0 | 0 | |
| 1250 | 1650 | 2175 | 3475 | 0 | 0 | |
| Non-treated control | 500 | 1225 | 2075 | 1625 | 3175 | 4850 |
| 925 | 3300 | 2775 | 4250 | 3450 | 6225 | |
| 1650 | 4225 | 2125 | 2325 | 2375 | 3050 | |
| 950 | 2950 | 2000 | 275 | 1250 | 1475 | |
| 925 | 2575 | 5550 | 6825 | 2350 | 11550 | |
| 2150 | 3725 | 2650 | 2425 | 2325 | 2775 | |
| 2375 | 1650 | 1525 | 3650 | 2000 | 2100 | |
| 1025 | 1925 | 1450 | 2000 | 2350 | 1300 | |
Nemex®-2 (pyrantel pamoate), Panacur® C (fenbendazole), Interceptor® (milbemycin oxime).
Fig. 1.
Arithmetic mean fecal egg counts (FEC) and standard deviation for groups of eight dogs infected with the Worthy 4.1F3P isolate of Ancylostoma caninum on study days (SD) 19, 20, 22, 27, 31 and 34. Treatments with pyrantel pamoate (A), fenbendazole (B), milbemycin oxime (C), emodepside + praziquantel (D), and non-treated control (E) were administered on SD 24 per approved label instructions.
4. Discussion
The present study supports previous findings of the MDR status of the A. caninum Worthy 4.1F3P as treatments with pyrantel pamoate, fenbendazole and milbemycin oxime lacked efficacy. Additionally, we demonstrate that emodepside + praziquantel tablets were highly effective against this isolate, yielding an efficacy of 99.6%. Emodepside has already demonstrated high efficacy against MDR nematode isolates of Haemonchus contortus and Cooperia oncophora in ruminants (von Samson-Himmelstjerna et al., 2005).
The benzimidazoles are one of the most important broad-spectrum classes of anthelmintics available to control parasitic nematodes of both animals and humans (Stepek et al., 2006). An interesting observation in this study was that following treatment with fenbendazole (Panacur® C), there was a large reduction in egg counts one day after completion of the three-day treatment regimen (SD 27), but FEC gradually increased on SDs 31 and 34 (Table 2). We also reported similar findings in the two isolates tested in our other recent work (Jimenez Castro et al., 2019). These data demonstrate that treatment with fenbendazole caused a temporary suppression in egg shedding. Interestingly, this phenomenon appears to be relatively unique to A. caninum. Benzimidazole anthelmintics have been used for many decades in a multitude of hosts against numerous parasite species, and high prevalences of resistance to benzimidazoles are reported worldwide in many species of gastrointestinal nematode parasites (Kaplan, 2004; Kaplan and Vidyashankar, 2012). Yet, to our knowledge, this egg suppression phenomenon has only been reported once previously, in H. contortus in sheep (Scott et al., 1991). In contrast, egg output suppression has been reported on several occasions following treatment with ivermectin and moxidectin in several different parasite species (McKellar et al., 1988; Sutherland et al., 1999; Condi et al., 2009; Macrelli et al., 2019).
The milbemycin oxime (Interceptor®) treated group had the lowest efficacy of all the treatments (8.8%). This clearly demonstrates that milbemycin oxime lacks efficacy against this MDR isolate of A. caninum, and based on previously published data (Jimenez Castro et al., 2019), it is highly likely that this isolate is also less susceptible to all other macrocyclic lactone anthelmintics. Macrocyclic lactones, particularly ivermectin, have a long history of being used intensively by the greyhound industry for parasite control (Ridley et al., 1994). Though, there are no recent citable publications confirming this ongoing intensive use, multiple communications with people familiar with the greyhound industry, including one of the authors of the article cited above, have confirmed the ongoing nature of this practice. In contrast, to our knowledge, moxidectin, a substantially more potent member of this drug class (Prichard et al., 2012), has only recently started to be used on greyhound farms and kennels. The dog from which the Worthy isolate was originally isolated was a recently adopted retired racing greyhound that had multiple failed treatments with moxidectin prior to us collecting the parasites from this dog. However, after being established in the laboratory this isolate received no further treatment selection with macrocyclic lactones (Jimenez Castro et al., 2019).
A further interesting observation was the large increase in egg production per female worm in the milbemycin group compared to the control and pyrantel-treated groups. We have made similar observations on multiple occasions in both small ruminants and cattle infected with macrocyclic-resistant isolates of Haemonchus following treatment with ivermectin (unpublished observations). Currently, we do not have an explanation for this increased egg output, but it seems to be a common occurrence and warrants further investigation.
Previous work with both drug-susceptible and pyrantel-resistant A. caninum demonstrated a density dependent fecundity of the female worms, whereby female worms increased their individual egg output due to reductions in the number of worms in the lumen of the small intestine (Kopp et al., 2007). This phenomenon was not seen in this study.
Based on work in our laboratory, both published and unpublished, as well as frequent communications with veterinarians dealing with cases of persistent hookworm infections, Worthy 4.1F3P appears to be representative of the worms currently circulating in greyhounds. The lack of efficacy demonstrated by the most commonly used products in the US for the treatment of hookworms in dogs therefore portends a very serious situation, and threatens not just canine health, but also human health due to its zoonotic potential. Further research investigating the molecular epidemiology is warranted in order to gain a deeper understanding of the origin(s) of this MDR to commercial products used in the US, as well as its prevalence and geographic distribution.
Declaration of competing interest
The study reported here was funded by Bayer US LLC, Animal Health, Research and Development, Shawnee, KS, USA and was performed at TRS Labs Inc.
Acknowledgements
We thank the staff at TRS Labs Inc, for their assistance with this project.
References
- Akyol C.V., Kino H., Terada M. Effects of PF1022A, a newly developed gabergic anthelmintic, on adult stage of Angiostrongylus cantonensis in rats. Jpn. J. Parasitol. 1993;42:220–226. [Google Scholar]
- Altreuther G., Gasda N., Adler K., Hellmann K., Thurieau H., Schimmel A., Hutchens D., Krieger K.J. Field evaluations of the efficacy and safety of Emodepside plus toltrazuril (Procox(R) oral suspension for dogs) against naturally acquired nematode and Isospora spp. infections in dogs. Parasitol. Res. 2011;109(Suppl. 1):S21–S28. doi: 10.1007/s00436-011-2399-z. [DOI] [PubMed] [Google Scholar]
- Baermann G. A simple method for detecting ankylostomum (nematodes) larvae in soil samples. Med. J. Dutch East Indie. 1917;57:131–137. [Google Scholar]
- Bowman D.D., Montgomery S.P., Zajac A.M., Eberhard M.L., Kazacos K.R. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010;26:162–167. doi: 10.1016/j.pt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Chen W., Terada M., Cheng J. Characterization of subtypes of gamma-aminobutyric acid receptors in an Ascaris muscle preparation by binding assay and binding of PF1022A, a new anthelmintic, on the receptors. Parasitol. Res. 1996;82:97–101. doi: 10.1007/s004360050077. [DOI] [PubMed] [Google Scholar]
- Condi G.K., Soutello R.G., Amarante A.F. Moxidectin-resistant nematodes in cattle in Brazil. Vet. Parasitol. 2009;161:213–217. doi: 10.1016/j.vetpar.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Crisford A., Ebbinghaus-Kintscher U., Schoenhense E., Harder A., Raming K., O'Kelly I., Ndukwe K., O'Connor V., Walker R.J., Holden-Dye L. The cyclooctadepsipeptide anthelmintic emodepside differentially modulates nematode, insect and human calcium-activated potassium (SLO) channel alpha subunits. PLoS Negl Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice P., Hakimi S., Vandenbos F., Magana C., Hubiche T. Autochthonous cutaneous larva migrans in France and Europe. Acta Derm. Venereol. 2019;99:805–808. doi: 10.2340/00015555-3217. [DOI] [PubMed] [Google Scholar]
- Drake J., Carey T. Seasonality and changing prevalence of common canine gastrointestinal nematodes in the USA. Parasites Vectors. 2019;12:430. doi: 10.1186/s13071-019-3701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- F.D.A . Food and Drug Administration; 1983. NADA 121-473 Panacur. [Google Scholar]
- F.D.A . Food and Drug Administration; 1993. NADA 141-008 Drontal. [Google Scholar]
- F.D.A . Food and Drug Administration; 1994. NADA 141-007 Drontal Plus. [Google Scholar]
- F.D.A . Food and Drug Administration; 1998. NADA 140-915 Interceptor. [Google Scholar]
- F.D.A . Food and Drug Administration; 2006. NADA 141-251 Advantage Multi. [Google Scholar]
- Furtado L.F.V., Dias L.T.d.O., Rodrigues T.d.O., Silva V.J.d., Oliveira V.N.G.M.d., Rabelo É.M.L. Egg genotyping reveals the possibility of patent Ancylostoma caninum infection in human intestine. Sci. Rep. 2020;10:3006. doi: 10.1038/s41598-020-59874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H.M., Whitlock H. A new technique for counting nematode eggs in sheep faeces. J. Council. Scient. Indu. Res. 1939;12:50–52. [Google Scholar]
- Guest M., Bull K., Walker R.J., Amliwala K., O'Connor V., Harder A., Holden-Dye L., Hopper N.A. The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int. J. Parasitol. 2007;37:1577–1588. doi: 10.1016/j.ijpara.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Harder A., Schmitt-Wrede H.P., Krucken J., Marinovski P., Wunderlich F., Willson J., Amliwala K., Holden-Dye L., Walker R. Cyclooctadepsipeptides--an anthelmintically active class of compounds exhibiting a novel mode of action. Int. J. Antimicrob. Agents. 2003;22:318–331. doi: 10.1016/s0924-8579(03)00219-x. [DOI] [PubMed] [Google Scholar]
- Hess L.B., Millward L.M., Rudinsky A., Vincent E., Marsh A. Combination anthelmintic treatment for persistent Ancylostoma caninum ova shedding in greyhounds. J. Am. Anim. Hosp. Assoc. 2019;55:160–166. doi: 10.5326/JAAHA-MS-6904. [DOI] [PubMed] [Google Scholar]
- Hopkins T., Gyr P. Synergism of a combination of febantel and pyrantel embonate against Ancylostoma caninum on dogs. Vet. Med. Rev. 1991;61:3–9. [Google Scholar]
- Hopkins T., Gyr P., Hedemann P. Nematocidal and cesticidal efficacy of a tablet formulation containing febantel, pyranted embonate and praziquantel in dogs. Vet. Med. Rev. 1988;59:71–75. [Google Scholar]
- Hotez P.J., Brooker S., Bethony J., Bottazzi M.E., Loukas A., Xiao S. Hookworm infection. N. Engl. J. Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- Jackson R., Lance D., Townsend K., Stewart K. Isolation of anthelmintic resistant Ancylostoma caninum. N. Z. Vet. J. 1987;35:215–216. doi: 10.1080/00480169./1987.35456. [DOI] [PubMed] [Google Scholar]
- Jimenez Castro P.D., Howell S.B., Schaefer J.J., Avramenko R.W., Gilleard J.S., Kaplan R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasites Vectors. 2019;12:576. doi: 10.1186/s13071-019-3828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachi S., Terada M., Hashimoto H. Effects of amorphous and polymorphs of PF1022A, a new antinematode drug, on Angiostrongylus costaricensis in mice. Jpn. J. Pharmacol. 1998;235 doi: 10.1254/jjp.77.235. [DOI] [PubMed] [Google Scholar]
- Kalkofen U.P. Hookworms of dogs and cats. Vet Clin North Am Small Anim Pract. 1987;17:1341–1354. doi: 10.1016/s0195-5616(87)50005-5. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Kitchen S., Ratnappan R., Han S., Leasure C., Grill E., Iqbal Z., Granger O., O'Halloran D.M., Hawdon J.M. Isolation and characterization of a naturally occurring multidrug-resistant strain of the canine hookworm, Ancylostoma caninum. Int. J. Parasitol. 2019;49:397–406. doi: 10.1016/j.ijpara.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp S.R., Coleman G.T., McCarthy J.S., Kotze A.C. Application of in vitro anthelmintic sensitivity assays to canine parasitology: detecting resistance to pyrantel in Ancylostoma caninum. Vet. Parasitol. 2008;152:284–293. doi: 10.1016/j.vetpar.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Kopp S.R., Coleman G.T., McCarthy J.S., Kotze A.C. Phenotypic characterization of two Ancylostoma caninum isolates with different susceptibilities to the anthelmintic pyrantel. Antimicrob. Agents Chemother. 2008;52:3980–3986. doi: 10.1128/AAC.00523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp S.R., Kotze A.C., McCarthy J.S., Coleman G.T. High-level pyrantel resistance in the hookworm Ancylostoma caninum. Vet. Parasitol. 2007;143:299–304. doi: 10.1016/j.vetpar.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Krüger N., Harder A., von Samson-Himmelstjerna G. The putative cyclooctadepsipeptide receptor depsiphilin of the canine hookworm Ancylostoma caninum. Parasitol. Res. 2009;105(Suppl. 1):S91–S100. doi: 10.1007/s00436-009-1500-3. [DOI] [PubMed] [Google Scholar]
- Kulke D. Thesis. Biology, Chemistry and Pharmacy. Freïe Universitat Berlin; 2014. Analysis of the efficacy of aminophenylamidines and cyclooctadepsipeptides and their mode of action, Ph.D. [Google Scholar]
- Kulke D., von Samson-Himmelstjerna G., Miltsch S.M., Wolstenholme A.J., Jex A.R., Gasser R.B., Ballesteros C., Geary T.G., Keiser J., Townson S., Harder A., Krücken J. Characterization of the Ca2+-gated and voltage-dependent K+-channel Slo-1 of nematodes and its interaction with emodepside. PLoS Negl Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming J.A.L., Oxon D.M. Cutaneous larva migrans. S. Afr. Med. J. 1966:403–405. [PubMed] [Google Scholar]
- Little S.E., Johnson E.M., Lewis D., Jaklitsch R.P., Payton M.E., Blagburn B.L., Bowman D.D., Moroff S., Tams T., Rich L., Aucoin D. Prevalence of intestinal parasites in pet dogs in the United States. Vet. Parasitol. 2009;166:144–152. doi: 10.1016/j.vetpar.2009.07.044. [DOI] [PubMed] [Google Scholar]
- Macrelli M., Williamson S., Mitchell S., Pearson R., Andrews L., Morrison A.A., Nevel M., Smith R., Bartley D.J. First detection of ivermectin resistance in Oesophagostomum dentatum in pigs. Vet. Parasitol. 2019;270:1–6. doi: 10.1016/j.vetpar.2019.05.002. [DOI] [PubMed] [Google Scholar]
- McKellar Q.A., Bogan J.A., Horspool L., Reid K. Effect of ivermectin on the reproductive potential of Cooperia curticei. Vet. Rec. 1988;122:444. doi: 10.1136/vr.122.18.444. [DOI] [PubMed] [Google Scholar]
- Miltsch S.M., Krucken J., Demeler J., Janssen I.J., Kruger N., Harder A., von Samson-Himmelstjerna G. Decreased emodepside sensitivity in unc-49 gamma-aminobutyric acid (GABA)-receptor-deficient Caenorhabditis elegans. Int. J. Parasitol. 2012;42:761–770. doi: 10.1016/j.ijpara.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Ngcamphalala P.I., Lamb J., Mukaratirwa S. Molecular identification of hookworm isolates from stray dogs, humans and selected wildlife from South Africa. J. Helminthol. 2019:1–9. doi: 10.1017/S0022149X19000130. [DOI] [PubMed] [Google Scholar]
- Prichard R., Ménez C., Lespine A. Moxidectin and the avermectins: consanguinity but not identity. Int. J. Parasitol.: Drugs Drug Resist. 2012;2:134–153. doi: 10.1016/j.ijpddr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prociv P., Croese J. Human enteric infection with Ancylostoma caninum: hookworms reappraised in the light of a "new" zoonosis. Acta Trop. 1996;62:23–44. doi: 10.1016/s0001-706x(96)00016-2. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C.R., Charles S.D., Buch J., Settje T., Altreuther G., Cruthers L., McCall J.W., Young D.R., Epe C. Evaluation of the efficacy of emodepside plus praziquantel topical solution against ascarid infections (Toxocara cati or Toxascaris leonina) in cats. Parasitol. Res. 2005;97(Suppl. 1):S41–s50. doi: 10.1007/s00436-005-1443-2. [DOI] [PubMed] [Google Scholar]
- Ridley R.K., Dryden M.W., Gabbert N.H., Schoning P. Epidemiology and control of helminth-parasites in greyhound breeding farms. Comp Cont Educ Pract. 1994;16:585–596. [Google Scholar]
- Sasaki T., Takagi M., Yaguchi T., Miyadoh S., Okada T., Koyama M. A new anthelmintic cyclodepsipeptide, PF1022A. J. Antibiot. 1992;45:692–697. doi: 10.7164/antibiotics.45.692. [DOI] [PubMed] [Google Scholar]
- Schimmel A., Altreuther G., Schroeder I., Charles S., Cruthers L., Kok D.J., Kraemer F., Krieger K.J. Efficacy of Emodepside Plus Praziquantel Tablets (Profender® Tablets for Dogs) against Mature and Immature Adult Trichuris vulpis Infections in Dogs, SUPP/1. ed. Springer; 2009. p. 17. [DOI] [PubMed] [Google Scholar]
- Schmahl G., Mehlhorn H., Harder A., Klimpel S., Krieger K.J. Efficacy of a Combination of Emodepside Plus Praziquantel against Larval and Adult Stages of Nematodes (Trichuris muris, Angiostrongylus cantonensis) in Rodents. SUPP/1 ed. Springer; 2007. p. 77. [Google Scholar]
- Schroeder I., Altreuther G., Schimmel A., Deplazes P., Kok D.J., Schnyder M., Krieger K.J. Efficacy of Emodepside Plus Praziquantel Tablets (Profender® Tablets for Dogs) against Mature and Immature Cestode Infections in Dogs, SUPP/1. ed. Springer; 2009. p. 31. [DOI] [PubMed] [Google Scholar]
- Scott E.W., Baxter P., Armour J. Fecundity of anthelmintic resistant adult Haemonchus contortus after exposure to ivermectin or benzimidazoles in vivo. Res. Vet. Sci. 1991;50:247–249. doi: 10.1016/0034-5288(91)90117-7. [DOI] [PubMed] [Google Scholar]
- Stassens P., Bergum P.W., Gansemans Y., Jespers L., Laroche Y., Huang S., Maki S., Messens J., Lauwereys M., Cappello M., Hotez P.J., Lasters I., Vlasuk G.P. Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc. Natl. Acad. Sci. Unit. States Am. 1996;93:2149–2154. doi: 10.1073/pnas.93.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepek G., Buttle D.J., Duce I.R., Behnke J.M. Human gastrointestinal nematode infections: are new control methods required? Int. J. Exp. Pathol. 2006;87:325–341. doi: 10.1111/j.1365-2613.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I.A., Leathwick D.M., Brown A.E. Moxidectin: persistence and efficacy against drug-resistant Ostertagia circumcincta. J. Vet. Pharmacol. Therapeut. 1999;22:2–5. doi: 10.1046/j.1365-2885.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- Terada M. Neuropharmacological mechanism of action of Pf1022A, an antinematode anthelmintic with a new structure of cyclic depsipeptide, on Angiostrongylus cantonensis and isolated frog rectus. Jpn. J. Parasitol. 1992;41:108–117. [Google Scholar]
- von Samson-Himmelstjerna G., Harder A., Sangster N.C., Coles G.C. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology. 2005;130:343–347. doi: 10.1017/s0031182004006523. [DOI] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G., Harder A., Schnieder T., Kalbe J., Mencke N. In vivo activities of the new anthelmintic depsipeptide PF 1022A. Parasitol. Res. 2000;86:194–199. doi: 10.1007/s004360050031. [DOI] [PubMed] [Google Scholar]
- Welz C., Kruger N., Schniederjans M., Miltsch S.M., Krucken J., Guest M., Holden-Dye L., Harder A., von Samson-Himmelstjerna G. SLO-1-channels of parasitic nematodes reconstitute locomotor behaviour and emodepside sensitivity in Caenorhabditis elegans slo-1 loss of function mutants. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner H., Schares G. Experimental chemotherapy of filariasis: comparative evaluation of the efficacy of filaricidal compounds in Mastomys coucha infected with Litomosoides carinii, Acanthocheilonema viteae, Brugia malayi and B. pahangi. Acta Trop. 1993;52 doi: 10.1016/0001-706x(93)90010-9. [DOI] [PubMed] [Google Scholar]
- Zahner H., Taubert A., Harder A., von Samson-Himmelstjerna G. Effects of Bay 44-4400, a new cyclodepsipeptide, on developing stages of filariae (Acanthocheilonema viteae, Brugia malayi, Litomosoides sigmodontis) in the rodent Mastomys coucha. Acta Trop. 2001;80:19–28. doi: 10.1016/s0001-706x(01)00144-9. [DOI] [PubMed] [Google Scholar]