Abstract
Background
Hepatocellular carcinoma (HCC) is a common cancer worldwide and prognosis for patients with the disease remains poor. Most patients are diagnosed at an advanced stage and are only eligible for palliative therapy. As a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor (VEGFR2-TKI), apatinib has a certain antitumor effect for a variety of solid tumors. In clinical practice, clinicians have attempted to treat intermediate- to advanced-stage HCC patients with a combination of transcatheter arterial chemoembolization (TACE) and apatinib. However, a consensus on the therapeutic effects of this treatment is yet to be reached. This meta-analysis was conducted to compare the therapeutic efficacy and clinical safety of the combination therapy of TACE plus apatinib with that of TACE alone in patients with intermediate- to advanced-stage HCC.
Methods
Relevant studies were identified by searching PubMed, Embase, Web of Science, the Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Biomedical Literature Database (CBM), Chinese Science and Technology Periodical Database (VIP) and the reference lists of retrieved articles up to July 31, 2019. Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated to express the therapeutic effects of TACE plus apatinib versus TACE on survival, objective response rate, disease control rate, progressive disease rate and adverse events using a mixed-effect model. Subgroup analyses of study type, dosage of apatinib, TACE regimen, study sample size between treatment groups and control groups were performed. Publication bias was assessed using fail-safe N, Begg-Mazumdar test and Egger’s test.
Results
From 23 eligible studies, a total of 1,342 patients were included in this review and meta-analysis. Among these studies, 18 were randomized clinical trials and 5 were case-control studies. Compared with those being treated with TACE alone, patients receiving TACE plus apatinib showed significantly better half-year survival (OR, 2.741, 95% CI, 1.745–4.306) and 1-year survival (OR, 2.284, 95% CI, 1.442–3.620). The superiority of TACE and apatinib over TACE monotherapy was evident in the disease control rate (OR, 2.919, 95% CI, 2.184–3.903), objective response rate (OR, 2.683, 95% CI, 2.099–3.429) and progressive disease rate (OR, 0.341, 95% CI, 0.255–0.456), respectively.
Conclusions
The combination treatment of apatinib and TACE provides better survival benefits for intermediate- to advanced-stage HCC patients when compared to TACE monotherapy and should be recommended for suitable patients with unresectable HCC. However, further investigation into future prospective clinical studies is warranted.
Keywords: Transcatheter arterial chemoembolization (TACE), apatinib, hepatocellular carcinoma (HCC), meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy. Additionally, due to its poor outcome for patients, it was the second most lethal cancer worldwide in 2018 (1). Despite massive efforts to find novel serum biomarkers and advanced imaging methods to improve sensitivity and specificity in detecting early-stage HCC, up until now, no particularly satisfying marker or imaging technique has been found (2-4). Moreover, on account of the highly aggressive nature and hidden character of HCC, a large portion of patients are classified as being at the intermediate- to advanced-stage of the disease at the time of their diagnosis, placing them beyond the indications for curative treatments including hepatectomy, radiofrequency or microwave ablation, and liver transplantation (5).
According to the Barcelona Clinic Liver Cancer (BCLC) staging system, transarterial chemoembolization (TACE) is the most common treatment for unresectable HCC (6-8). However, high recurrence rate and poor survival restrict the clinical use of TACE monotherapy. It has been reported that TACE may increase the expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) and that repeated TACE may aggravate liver dysfunction (9). Gradually, owing to the development of molecular targeted therapy, apatinib has been applied to patients at the intermediate and advanced stages of HCC (10). Apatinib has been verified as being effective for HCC patients, with mild and tolerable toxicity (11). The combination of apatinib with TACE as a therapy has been considered promising. According to a case report by Han et al., a 41-year-old Chinese man with a history of chronic hepatitis B underwent an emergency partial hepatectomy for a ruptured tumor which treatment by transcatheter arterial chemoembolization and sorafenib had failed to control (12). Due to the failure of sorafenib and positive expression of VEGF, the patient’s drug regimen was changed and, through anti-angiogenic therapy with apatinib, there were unexpected and positive effects. However, due to the relatively small sample size of related studies and a lack of multi-center and large-sample randomized controlled trials, there has been no definite conclusion made regarding the efficacy of apatinib combined with TACE in the treatment of intermediate- and advanced-stage HCC. Therefore, this meta-analysis was carried out in order to evaluate the efficacy and safety of combined therapy and to provide evidence for clinical decision-making.
Methods
Retrieval of published studies
The following databases were comprehensively searched to identify relevant studies in the period up to July 31, 2019: PubMed, Embase, Web of Science, the Cochrane Library, China National Knowledge Infrastructure and China Biology Medicine. Different combinations of the following key terms were used: “transcatheter arterial chemoembolization” or “transarterial chemoembolization” or “TACE” “apatinib,” and “hepatocellular carcinoma” or “primary liver cancer” or “HCC”. No language restrictions were applied. Additionally, the references of retrieved articles were also searched until no new potential articles could be found.
Selection criteria
The studies included in our meta-analysis satisfied all of the following criteria: (I) patients should be clearly diagnosed with intermediate- to advanced-stage HCC by computed tomography (CT), magnetic resonance imaging (MRI) or pathology; (II) studies should include an experimental group and a control group, with the experimental group having received apatinib combined with TACE and the control group having received TACE monotherapy; (III) evaluation indicators should include complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the mRECIST to evaluate tumor response. Other evaluation indicators such as adverse events (AEs), half-year survival rate and one-year survival rate were also assessed if the number of included studies was more than three.
The exclusion criteria eliminated studies with the following characteristics: (I) repetitive studies, narrative reviews, systematic reviews, letters, comments, case reports or studies unrelated to our topic; (II) studies in which patients had other malignancies or had received other interventions; (III) studies where no available data was extracted or no control group was established.
Quality assessment
The included studies were independently evaluated by two researchers (Shoujie Zhao and Desha Zheng). To avoid subjectivity, the authors’ names and institutions were kept from the researchers. All discrepancies were re-examined and discussed with the third researcher (Lei Liu) to reach a consensus.
The quality of each included randomized clinical trial was assessed in accordance with the Cochrane format, using a grading scheme for each of the 7 main aspects: (I) random sequence generation; (II) allocation concealment; (III) blinding of participants and personnel; (IV) blinding of outcome assessment; (V) incomplete outcome data; (VI) selective reporting; (VII) other bias (13). These above were further graded as (A) adequate, with correct procedures; (B) unclear, without a description of methods; (C) inadequate procedures, methods or information. The overall quality of the studies was then assessed and classified into 3 groups as follows: (I) low risk of bias for studies with A grades for all items; (II) moderate risk of bias for studies with B grades; (III) high risk of bias for studies with C grades. The quality of case-control studies was assessed using the Newcastle-Ottawa scoring system (NOS). With the NOS, the maximum scores are four points for selection, two for comparability (reconstruction method and the extent of lymphadenectomy), and three for outcome assessment (14).
Data extraction and statistical analysis
General information including the author’s name, year of publication and intervention was recorded into a pre-designed electronic data sheet. The parametric data, including therapeutic response rate, adverse events and overall survival rate, was collected for quantitative analyzing. Comprehensive Meta Analysis V2 software was used in the meta-analysis. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to express therapeutic effects, which were identified to be statistically significant if P<0.05. Heterogeneity was assessed by means of Cochran’s Q test. Statistical heterogeneity was considered to exist among the studies if I2>50.00% or P<0.10. A random effects model was used to analyze the results if the heterogeneity existed, otherwise, a fixed effect model was used. Egger’s test and Begg’s test were applied to assess publication bias. Publication bias was not assessed if the number of included studies was less than 5. Sensitivity analyses were conducted to evaluate the influences of the study type, dosage of apatinib, TACE regimen and sample size.
Results
Literature search and selection
A total of 1,342 patients, including 662 patients from the experimental group and 680 patients from the control group, were enrolled. Of the included patients, 862 were male and 347 were female. Detailed information of the 23 relevant citations is presented in Table 1.
Table 1. Baseline characteristics of the included studies.
| Reference | Year | Trial | Treatment | Reference | Gender of patients (M/F) | Age of patients (mean ± SD) | Tumor diameter (mean ± SD) | Child-Pugh classification |
|---|---|---|---|---|---|---|---|---|
| Yang et al. | 2019 | R | Treatment group: TACE-apatinib | (15) | 12/11 | 45.03±5.62 | NR | NR |
| Control group: TACE | 12/11 | 45.53±5.85 | NR | NR | ||||
| Xiu et al. | 2019 | R | Treatment group: TACE-apatinib | (16) | 12/11 | 57.62±12.57 | NR | NR |
| Control group: TACE | 13/10 | 54.75±10.38 | NR | NR | ||||
| Wu et al. | 2018 | C | Treatment group: TACE-apatinib | (17) | 20/8 | 57.70±8.30 | 7.56±2.33 | A or B |
| Control group: TACE | 18/10 | 56.40±4.20 | 6.45±5.34 | A or B | ||||
| Wu et al. | 2019 | R | Treatment group: TACE-apatinib | (18) | 27/15 | 55.43±3.69 | 6.84±0.71 | NR |
| Control group: TACE | 25/16 | 55.16±3.48 | 6.08±0.85 | NR | ||||
| Wu et al. | 2019 | R | Treatment group: TACE-apatinib | (19) | 18/10 | 57.20±7.00 | 7.70±2.40 | A or B |
| Control group: TACE | 23/8 | 58.00±7.10 | 7.40±2.50 | A or B | ||||
| Wang et al. | 2017 | R | Treatment group: TACE-apatinib | (20) | 25/18 | 58.28±5.21 | NR | A or B or C |
| Control group: TACE | 24/19 | 58.29±5.22 | NR | A or B or C | ||||
| Song et al. | 2018 | C | Treatment group: TACE-apatinib | (21) | NR | NR | NR | NR |
| Control group: TACE | NR | NR | NR | NR | ||||
| Shen et al. | 2019 | R | Treatment group: TACE-apatinib | (22) | 16/3 | NR | NR | A or B |
| Control group: TACE | 17/2 | NR | NR | A or B | ||||
| Lu et al. | 2019 | R | Treatment group: TACE-apatinib | (23) | 12/10 | 56.00±12.00 | 5.70±0.40 | A or B |
| Control group: TACE | 14/7 | 58.00±10.00 | 5.50±0.40 | A or B | ||||
| Li et al. | 2017 | R | Treatment group: TACE-apatinib | (24) | 10/10 | 43.90±5.10 | NR | NR |
| Control group: TACE | 12/8 | 45.20±5.20 | NR | NR | ||||
| Li et al. | 2018 | R | Treatment group: TACE-apatinib | (25) | 28/26 | 52.5±9.10 | 5.20±1.80 | A or B |
| Control group: TACE | 29/23 | 51.6±6.90 | 5.10±1.30 | A or B | ||||
| Jin et al. | 2017 | R | Treatment group: TACE-apatinib | (26) | 17/5 | 58.90±9.40 | 7.12±2.15 | A or B |
| Control group: TACE | 16/6 | 56.1±10.8 | 6.86±2.12 | A or B | ||||
| Huang et al. | 2018 | R | Treatment group: TACE-apatinib | (27) | 23/7 | 51.60±9.80 | NR | A or B |
| Control group: TACE | 22/8 | 55.20±12.1 | NR | A or B | ||||
| Huang et al. | 2017 | R | Treatment group: TACE-apatinib | (28) | 22/16 | 43.80±4.90 | 5.01±1.27 | NR |
| Control group: TACE | 21/17 | 43.70±5.00 | 5.08±1.20 | NR | ||||
| He et al. | 2018 | R | Treatment group: TACE-apatinib | (29) | 24/26 | 52.70±1.30 | NR | NR |
| Control group: TACE | 28/22 | 53.10±1.50 | NR | NR | ||||
| Cui et al. | 2019 | C | Treatment group: TACE-apatinib | (30) | 17/8 | NR | 6.20±1.30 | A or B |
| Control group: TACE | 16/9 | NR | 5.30±1.70 | A or B | ||||
| Zeng et al. | 2018 | R | Treatment group: TACE-apatinib | (31) | 12/8 | 56.20±4.10 | NR | NR |
| Control group: TACE | 14/6 | 56.40±3.80 | NR | NR | ||||
| Zeng et al. | 2018 | R | Treatment group: TACE-apatinib | (32) | 18/20 | 56.40±8.80 | NR | NR |
| Control group: TACE | 21/17 | 58.82±7.50 | NR | NR | ||||
| Bai et al. | 2018 | R | Treatment group: TACE-apatinib | (33) | 20/5 | 58.34±5.67 | 6.91±0.83 | NR |
| Control group: TACE | 17/8 | 59.22±5.17 | 6.29±1.56 | NR | ||||
| Yang et al. | 2018 | C | Treatment group: TACE-apatinib | (34) | 20/5 | NR | 12.11±3.98 | NR |
| Control group: TACE | 18/4 | NR | 10.59±4.30 | NR | ||||
| Zhu et al. | 2019 | R | Treatment group: TACE-apatinib | (35) | 32/12 | NR | 7.12±2.15 | A or B |
| Control group: TACE | 34/10 | NR | 6.86±2.12 | A or B | ||||
| Lu et al. | 2017 | R | Treatment group: TACE-apatinib | (36) | 16/4 | 56.10±10.79 | 7.12±2.15 | A or B |
| Control group: TACE | 17/5 | 58.90±9.38 | 6.86±2.12 | A or B | ||||
| Chen et al. | 2018 | C | Treatment group: TACE-apatinib | (37) | 23/4 | 45.80±11.00 | NR | A or B |
| Control group: TACE | 43/10 | 54.40±11.90 | NR | A or B |
NR, not reported; M, male; F, female; SD, standard deviation; TACE, transarterial chemoembolization; R, randomized controlled trial; C, case-control study.
Identification of eligible studies
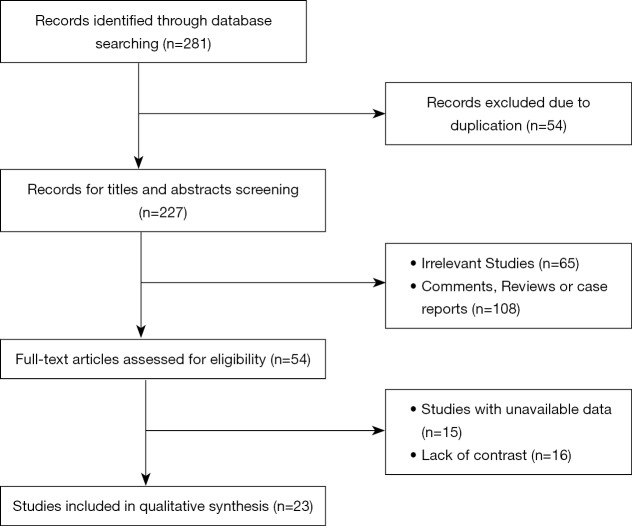
After searching for literature within several databases, 281 studies were initially identified as potentially relevant. Of these, 54 studies were excluded on account of duplication. After the examination of titles and abstracts, 108 studies were excluded because they were systematic reviews or case reports and 65 studies were unrelated to our topic. After carefully reading the full text, 15 studies were excluded for lack of important data and 16 studies were lack of contrast. Ultimately, 23 studies met our inclusion criteria and were included in our meta-analysis, with 18 randomized clinical trials and 5 case-control studies (15) (Figure 1).
Figure 1.
Flow diagram of the process of including and excluding studies for this meta-analysis.
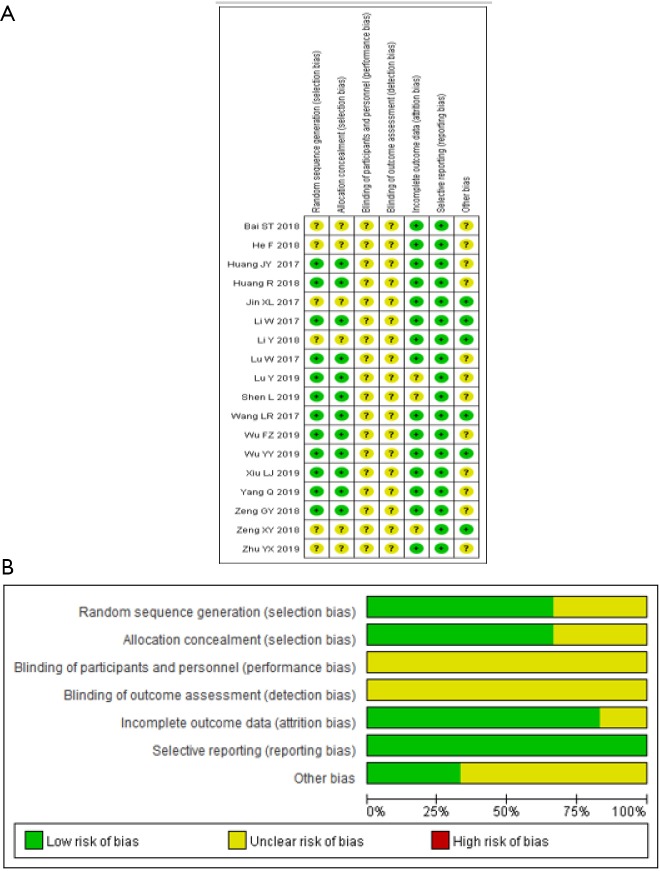
Methodological quality assessment
The included randomized clinical trials underwent a quality assessment using the risk of bias tool of the Review Manager software 5.3, and the outcome is shown in Figure 2. The quality of the included case-control studies was assessed by NOS, and the outcome is shown in Table 2.
Figure 2.
Assessment of risk of bias in this meta-analysis. (A) Summary of risk of bias for each trial, assessed using the Cochrane Collaboration’s risk of bias tool: a plus sign was for a judgment of “Yes” or low risk of bias, and a question mark was for a judgment of “Unclear”, or uncertain risk of bias, which means there was insufficient information to permit a judgment of “Yes” or “No”. (B) Risk of bias graph about each risk of bias item, presented as percentages across all included studies.
Table 2. Results of quality assessment using the Newcastle-Ottawa Scale for case-control studies.
| Study (year) | Selection | Comparability of cases and controls on the basis of the design or analysis | Exposure | Scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adequate definition of cases | Representativeness of the cases | Selection of controls | Definition of controls | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | ||||||
| Wu 2017 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | ||||
| Song 2018 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | ||||
| Cui 2019 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | ||||
| Yang 2018 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | ||||
| Chen 2018 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | ||||
Disease control rate
Disease control rate was reported in 21 studies. No statistical heterogeneity was found among the studies and a fixed effect model was used (P=0.998, I2=0.00%). The results showed that the disease control rate in the combined therapy group (TACE + apatinib) was significantly higher than that of the monotherapy group (OR, 2.919, 95% CI, 2.184–3.903, P<0.001). Neither Egger’s test (P=0.33038) nor Begg’s test (P=0.36498) revealed publication bias (Figure 3).
Figure 3.
Disease control rate of TACE plus apatinib in comparison with TACE monotherapy in intermediate- to advanced-stage HCC patients. CI, confidence interval; TACE, transcatheter arterial chemoembolization; HCC, hepatocellular carcinoma.
Objective response rate
Objective response rate was reported in 21 studies. No statistical heterogeneity was found among the studies and a fixed effect model was used for meta-analysis (P=0.995, I2=0.00%). The results showed that the objective response rate of the combined therapy group (TACE + apatinib) was significantly higher than that of the monotherapy group (OR, 2.683, 95% CI, 2.099–3.429, P<0.001). Neither Egger’s test (P=0.167) nor Begg’s test (P=0.156) revealed publication bias (Figure 4).
Figure 4.
Objective response rate of TACE plus apatinib compared to TACE monotherapy in intermediate- to advanced-stage HCC patients. TACE, transcatheter arterial chemoembolization; HCC, hepatocellular carcinoma.
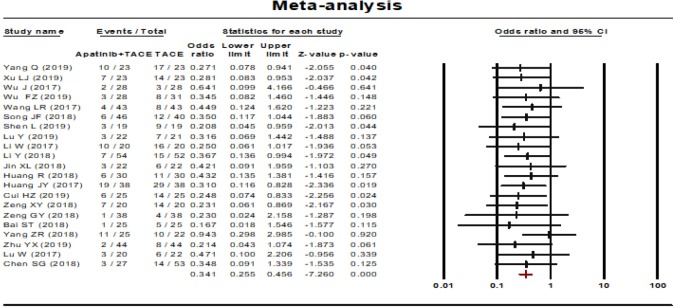
Progressive disease rate
Progressive disease rate was reported in 21 studies. No statistical heterogeneity was found among the studies and a fixed effect model was selected (P=0.998, I2=0.00%). The results showed that the progressive disease rate of the combined therapy group (TACE + apatinib) was higher than that of the TACE monotherapy group (OR, 0.341, 95% CI, 0.255–0.456, P<0.001). Neither Egger’s test (P=0.305) nor Begg’s test (P=0.349) revealed publication bias (Figure 5).
Figure 5.
Progressive disease rate of TACE plus apatinib compared to TACE monotherapy for intermediate- to advanced-stage HCC patients. TACE, transcatheter arterial chemoembolization; HCC, hepatocellular carcinoma.
Half-year survival rate
Half-year survival rate was reported in 6 studies. No statistical heterogeneity was found among the studies and a fixed effect model was used (P=0.993, I2=0.00%). The results showed that the half-year survival rate of the combined therapy group (TACE + apatinib) was significantly higher than that of the monotherapy group (OR, 2.741, 95% CI, 1.745–4.306, P<0.001). Neither Egger’s test (P=0.264) nor Begg’s test (P=0.452) revealed publication bias (Figure 6).
Figure 6.
Half-year survival rate of TACE plus apatinib compared to TACE monotherapy for intermediate- to advanced-stage HCC patients. TACE, transcatheter arterial chemoembolization; HCC, hepatocellular carcinoma.
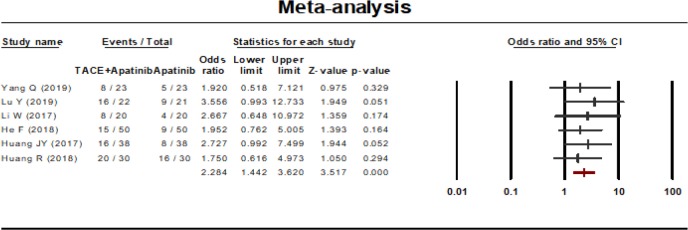
One-year survival rate
One-year survival rate was reported in 6 studies. A fixed effect model was used to analyze the result on account of the statistical heterogeneity which was found among the studies (P=0.958, I2=00.00%). The results showed that the 1-year survival rate of the combined therapy group (TACE + apatinib) was significantly higher than that of the monotherapy group (OR, 2.284, 95% CI, 1.442–3.620, P<0.001). Neither Egger’s test (P=0.425) nor Begg’s test (P=0.707) revealed publication bias (Figure 7).
Figure 7.
One-year survival rate of TACE plus apatinib compared to TACE monotherapy for intermediate- to advanced-stage HCC patients. TACE, transcatheter arterial chemoembolization; HCC, hepatocellular carcinoma.
Adverse events
Adverse events between treatment groups and control groups were performed (Table 3).
Table 3. TACE plus apatinib vs. TACE alone: a meta-analysis of adverse events (AEs) and survival rate.
| Study group | Number of studies | OR (95% CI) | P value | I2 | Heterogeneity P value |
|---|---|---|---|---|---|
| Adverse events | |||||
| Fever | 12 | 1.057 (0.749–1.492) | 0.752 | 21.854 | 0.229 |
| Abdominal pain | 9 | 1.080 (0.748–1.558) | 0.681 | 0 | 0.494 |
| Nausea/vomit | 12 | 1.099 (0.778–1.554) | 0.591 | 1.97 | 0.425 |
| Myelosuppression | 9 | 1.119 (0.682–1.835) | 0.656 | 0 | 0.645 |
| Hypertension | 13 | 10.867 (6.319–18.688) | <0.001 | 82.653 | <0.001 |
| Hand-foot syndrome | 11 | 20.681 (9.399–45.503) | <0.001 | 69.326 | <0.001 |
| Proteinuria | 11 | 9.830 (4.685–20.625) | <0.001 | 61.255 | 0.004 |
| Diarrhea | 12 | 3.375 (1.932–5.897) | <0.001 | 30.243 | 0.15 |
| Oral ulcer | 4 | 3.843 (0.834–17.720) | 0.084 | 45.939 | 0.136 |
TACE, transcatheter arterial chemoembolization.
Fever
Twelve trials were identified with outcome measurements of fever. The pooled analysis showed that, in comparison with TACE alone, TACE plus apatinib did not significantly increase the incidence rate of fever (OR, 1.057, 95% CI, 0.749–1.492).
Abdominal pain
Nine trials were identified with outcome measurements of abdominal pain. The pooled analysis showed that, in comparison with TACE alone, TACE plus apatinib did not significantly cause abdominal pain (OR, 1.080, 95% CI, 0.748–1.558).
Nausea/vomit
Twelve trials were identified with outcome measurements of nausea/vomit. The pooled analysis showed that, in comparison with TACE alone, TACE plus apatinib did not significantly increase the incidence of nausea/vomit (OR, 1.099, 95% CI, 0.778–1.554).
Myelosuppression
Nine trials were identified with outcome measurements of myelosuppression. The pooled analysis showed that, in comparison with TACE alone, TACE plus apatinib did not significantly increase the incidence of myelosuppression (OR, 1.119, 95% CI, 0.682–1.835).
Hypertension
Thirteen trials were identified with outcome measurements of hypertension. The pooled analysis showed that, in comparison to TACE alone, TACE plus apatinib significantly increased the incidence of hypertension (OR, 10.867, 95% CI, 6.319–18.688).
Hand-foot syndrome
Eleven trials were identified with outcome measurements of hand-foot syndrome. The pooled analysis showed that, in comparison to TACE alone, TACE plus apatinib significantly increased the incidence of hand-foot syndrome (OR, 20.681, 95% CI, 9.399–45.503).
Proteinuria
Eleven trials were identified with outcome measurements of proteinuria. The pooled analysis showed that, in comparison to TACE alone, TACE plus apatinib significantly increased the incidence of proteinuria (OR, 9.830, 95% CI, 4.685–20.625).
Diarrhea
Twelve trials were identified with outcome measurements of diarrhea. The pooled analysis showed that, in comparison to TACE alone, TACE plus apatinib significantly increased the incidence of diarrhea (OR, 3.375, 95% CI, 1.932–5.897).
Oral ulcer
Four trials were identified with outcome measurements of oral ulcer. The pooled analysis showed that, in comparison to TACE alone, TACE plus apatinib significantly increased the incidence of oral ulcer (OR, 3.843, 95% CI, 0.834–17.720).
Subgroup analysis
Subgroup analyses of study type, dosage of apatinib, TACE regimen, study sample size between treatment groups and control groups were performed (Table 4).
Table 4. TACE plus apatinib vs. TACE alone: a meta-analysis of tumor response rate and adverse events.
| Study group | Description | No. of studies | OR (95% CI) | Significance P value |
I2 | Heterogeneity P value |
|---|---|---|---|---|---|---|
| Objective response rate | ||||||
| Study type | Randomized clinical trials | 17 | 2.817 (2.144–3.701) | <0.001 | 0 | 0.999 |
| Case-control studies | 4 | 2.185 (1.249–3.823) | 0.006 | 0 | 0.447 | |
| Dosage of apatinib | 850 mg/d | 4 | 3.072 (1.642–5.749) | <0.001 | 0 | 0.997 |
| 500 mg/d | 14 | 2.681 (2.021–3.555) | <0.001 | 0 | 0.975 | |
| 250 mg/d | 3 | 2.152 (0.957–4.842) | 0.064 | 0 | 0.375 | |
| TACE regimen | 5-fluorouracil + adriamycin | 4 | 3.120 (1.492–6.522) | 0.002 | 0 | 0.998 |
| Adriamycin + platinol | 7 | 2.707 (1.860–3.940) | <0.001 | 0 | 0.993 | |
| 5-fluorouracil + adriamycin + platinol | 4 | 2.584 (1.441–4.632) | 0.001 | 0 | 0.77 | |
| Adriamycin | 3 | 2.389 (1.305–4.376) | 0.005 | 0 | 0.518 | |
| Study sample size | ≥50 | 12 | 2.582 (1.929–3.456) | <0.001 | 0 | 0.997 |
| <50 | 9 | 2.994 (1.870–4.637) | <0.001 | 0 | 0.756 | |
| Disease control rate | ||||||
| Study type | Randomized clinical trials | 16 | 3.180 (2.268–4.458) | <0.001 | 0 | 0.999 |
| Case-control studies | 5 | 2.295 (1.302–4.046) | 0.004 | 0 | 0.561 | |
| Dosage of apatinib | 850 mg/d | 4 | 3.553 (1.867–6.760) | <0.001 | 0 | 0.992 |
| 500 mg/d | 13 | 2.864 (1.955–4.195) | <0.001 | 0 | 0.999 | |
| 250 mg/d | 3 | 2.425 (1.139–5.164) | 0.022 | 42.572 | 0.175 | |
| TACE regimen | 5-fluorouracil + adriamycin | 4 | 3.531 (1.956–6.377) | <0.001 | 0 | 0.995 |
| Adriamycin + platinol | 7 | 2.877 (1.675–4.941) | <0.001 | 0 | 0.992 | |
| 5-fluorouracil + adriamycin + platinol | 4 | 3.050 (1.587–5.861) | 0.001 | 0 | 0.788 | |
| Adriamycin | 2 | 3.046 (1.102–8.418) | 0.032 | 0 | 0.404 | |
| Study sample size | ≥50 | 10 | 2.802 (1.871–4.195) | <0.001 | 0 | 0.999 |
| <50 | 11 | 3.051 (2.009–4.632) | <0.001 | 0 | 0.884 | |
| Progressive disease | ||||||
| Study type | Randomized clinical trials | 16 | 0.313 (0.223–0.439) | <0.001 | 0 | 0.999 |
| Case-control studies | 5 | 0.436 (0.247–0.768) | 0.004 | 0 | 0.561 | |
| Dosage of apatinib | 850 mg/d | 4 | 0.281 (0.148–0.536) | <0.001 | 0 | 0.992 |
| 500 mg/d | 13 | 0.347 (0.237–0.508) | <0.001 | 0 | 0.999 | |
| 250 mg/d | 3 | 0.412 (0.194–0.878) | 0.022 | 42.572 | 0.175 | |
| TACE regimen | 5-fluorouracil + adriamycin | 4 | 0.283 (0.157–0.511) | <0.001 | 0 | 0.995 |
| Adriamycin + platinol | 7 | 0.343 (0.200–0.589) | <0.001 | 0 | 0.994 | |
| 5-fluorouracil + adriamycin + platinol | 3 | 0.294 (0.138–0.627) | 0.002 | 0 | 0.69 | |
| Adriamycin | 2 | 0.328 (0.119–0.907) | 0.032 | 0 | 0.404 | |
| Study sample size | ≥50 | 10 | 0.357 (0.238–0.535) | <0.001 | 0 | 0.999 |
| <50 | 11 | 0.325 (0.214–0.494) | <0.001 | 0 | 0.891 |
TACE, transcatheter arterial chemoembolization.
Study type
Randomized clinical trials and case-control studies. Subgroup analyses showed that patients who received TACE plus apatinib had significantly better objective response rates, disease control rates and progressive disease rates than those receiving TACE alone.
Dosage of apatinib
A dosage of 850, 500 and 250 mg/d. Except in the analysis 250 mg/d of apatinib in the study group of objective response rate, subgroup analyses showed that patients who received TACE plus apatinib had significantly better objective response rates, disease control rates and progressive disease rates than those receiving TACE alone.
TACE regimen
5-fluorouracil + adriamycin, adriamycin, adriamycin + platinum, and 5-fluorouracil + adriamycin + platinol. Subgroup analyses showed that patients who received TACE plus apatinib had significantly better objective response rates, disease control rates and progressive disease rates than those receiving TACE alone.
Study sample size
Fifty patients or more vs. less than 50 patients. Subgroup analyses showed that patients who received TACE plus apatinib had a significantly better objective response rates, disease control rates and progressive disease rates than those receiving TACE alone.
Discussion
This meta-analysis provided evidence that, in comparison with treatment by TACE alone, TACE plus apatinib significantly improved the half-year and 1-year survival rates as well as disease control rate and objective response rate in patients with intermediate- to advanced-HCC. In relation to adverse events, TACE plus apatinib was associated with a greater incidence of hypertension, hand-foot syndrome, proteinuria, diarrhea, and oral ulcer, while having similar frequencies of nausea and/or vomiting, fever, abdominal pain and myelosuppression, when compared to treatment with TACE alone. Subgroup analyses showed slight or no differences were seen between study types, dosage of apatinib and TACE regimen.
According to the BCLC criteria, TACE is recognized as an alternative treatment option for intermediate- to advanced-HCC patients. However, tumor tissues cannot be completely eliminated through TACE for three main reasons (38). Firstly, some infiltrating cells and liver metastatic cells remain alive even after TACE, and repeated treatment can result in a certain resistance to chemotherapy drugs. Secondly, the clinical efficacy of TACE is influenced by the damage caused to liver tissue by hypoxia and ischemia, embolization agents and chemotherapy drugs. Thirdly, part of the tumor tissue recovers blood supply following TACE. Therefore, although the short-term efficacy of TACE is justifiable, it still has limitations, and its long-term efficacy remains unsatisfactory.
Angiogenesis acts as an important role in the process of tumor growth because it responds to the request for increased oxygen and nutrient supply, which is mediated by VEGF and the VEGF receptor. VEGFR family proteins are membrane receptor tyrosine kinases, including VEGFR-1, VEGFR-2, VEGFR-3. Sorafenib has been used as the first-line of therapy for advanced HCC or as an adjuvant therapy for many years. However, its high cost, as well as the toxicities of sorafenib limits its utilization. Furthermore, some studies have shown that high expression of VEGF in HCC is closely related to sorafenib resistance and a worse prognosis (39,40). Apatinib is a new inhibitor of VEGFR-2 tyrosine kinase that targets the intracellular ATP blinding site of the receptor. As a highly selective VEGFR-2 blocker, apatinib can block the migration and proliferation of vascular endothelial cells, decrease tumor microvessel density, and inhibit tumor growth with an affinity 10 times that of sorafenib. As a result, apatinib may become a future substitute for HCC patients who have sorafenib resistance, especially for those with high expression of VEGF.
The therapeutic role of apatinib combined with TACE for intermediate- to advanced-stage HCC has, of late, received more recognition than before. We speculated that the improved survival and tumor response rates of combination therapy in comparison to TACE alone was because apatinib can block neoangiogenesis and ultimately help to inhibit HCC growth. In relation to adverse events, TACE plus apatinib was associated with a greater incidence of hypertension, hand-foot syndrome, proteinuria, diarrhea, and oral ulcers than treatment by TACE alone, at the same time as having similar frequencies of nausea and/or vomiting, fever, abdominal pain, and myelosuppression. These adverse reactions were easily managed and gradually alleviated or disappeared within 1 or 2 weeks without the need for dose reduction or suspension of medication. Additional studies to examine the rate of adverse effects in different treatment regimen of TACE plus apatinib are required.
However, this analysis has obvious methodological limitations that compel us to be cautious when interpreting the results. Firstly, the included studies provided incomplete data with regard to safety and efficacy. For example, most of the studies did not provide data on vascular endothelial growth factor (VEGF) or alpha fetoprotein (AFP), and the follow-up time was short. All of these factors may have led to the statistical analysis having a reduced power. Secondly, all of the included studies came from the East, which may have resulted in some regional bias. Further studies are needed to verify the safety and effectiveness of the combined therapy in Western practice. Thirdly, as with all systematic reviews, there is potential for publication bias, as studies with positive findings are more likely to be published than those with negative findings. We attempted to remedy this bias by including searches in gray literature; however, none of the studies found with this resource met our search criteria. This could have potentially affected the calculated pooled odds ratios.
In conclusion, for the treatment of intermediate to advanced HCC, apatinib plus TACE was more therapeutically beneficial than TACE alone. TACE plus apatinib may provide an additional option for the treatment of suitable patients with unresectable HCC. In order to confirm the advantageous effects of the combined therapy and to clarify the optimal dosage of the apatinib and TACE regimen, adequately powered and high-quality randomized clinical trials with short- and long-term follow-ups are recommended in future.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.125). SZ serves as the unpaid editorial board member of Annals of Translational Medicine from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.De Stefano F, Chacon E, Turcios L, et al. Novel biomarkers in hepatocellular carcinoma. Dig Liver Dis 2018;50:1115-23. 10.1016/j.dld.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 3.Farag RMA, Al Ayobi D, Alsaleh KA, et al. Studying the Impact of Golgi Protein 73 Serving as a Candidate Biomarker in Early Diagnosis for Hepatocellular Carcinoma among Saudi Patients. Asian Pac J Cancer Prev 2019;20:215-20. 10.31557/APJCP.2019.20.1.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renzulli M, Biselli M, Brocchi S, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: a new diagnostic algorithm. Gut 2018;67:1674-82. 10.1136/gutjnl-2017-315384 [DOI] [PubMed] [Google Scholar]
- 5.Muaddi H, AI-Adra DP, Beecroft R, et al. Liver Transplantation is Equally Effective as a Salvage Therapy for Patients with Hepatocellular Carcinoma Recurrence Following Radiofrequency Ablation or Liver Resection with Curative Intent. Ann Surg Oncol 2018;25:991-9. 10.1245/s10434-017-6329-x [DOI] [PubMed] [Google Scholar]
- 6.Labeur TA, Takkenberg RB, Klümpen HJ, et al. Reason of Discontinuation After Transarterial Chemoembolization Influences Survival in Patients with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2019;42:230-8. 10.1007/s00270-018-2118-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu JI, Park HC, Jung SH, et al. Combination treatment with transarterial chemoembolization, radiotherapy, and hyperthermia (CERT) for hepatocellular carcinoma with portal vein tumor thrombosis: Final results of a prospective phase II trial. Oncotarget 2017;8:52651-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan P, Zhang Z, Kuai J. Analysis on efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J BUON 2019;24:163-70. [PubMed] [Google Scholar]
- 9.Wu J, Li A, Yang J, et al. Efficacy and safety of TACE in combination with sorafenib for the treatment of TACE-refractory advanced hepatocellular carcinoma in Chinese patients: a retrospective study. Onco Targets Ther 2017;10:2761-8. 10.2147/OTT.S131022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhen L, Jiali C, Yong F, et al. The Efficacy and Safety of Apatinib Treatment for Patients with Unresectable or Relapsed Liver Cancer: a retrospective study. J Cancer 2018;9:2773-7. 10.7150/jca.26376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kou P, Zhang Y, Shao W, et al. Significant efficacy and well safety of apatinib in an advanced liver cancer patient: a case report and literature review. Oncotarget. 2017;8:20510-5. 10.18632/oncotarget.14724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Z, He Z, Wang C, et al. The effect of apatinib in the treatment of sorafenib resistant metastatic hepatocellular carcinoma: A case report. Medicine (Baltimore) 2018;97:e13388. 10.1097/MD.0000000000013388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Naunyn Schmiedebergs Arch Pharmakol 2011;5:S38. [Google Scholar]
- 14.Shea BJ, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Appl Eng Agric 2014;18:727-34. [Google Scholar]
- 15.Yang Q, Pan SS, Shi CS, et al. Effect of apatinib mesylate combined with TACE in the treatment of advanced hepatocellular carcinoma and its influence on serum levels of VEGF and MMP-9. Chin J Inter Rad 2019;7:111-6. [Google Scholar]
- 16.Xiu LJ, Zhou X, Zhou WJ. The safety and effectiveness of TACE combined apatinib in the treatment of intermediate and advanced hepatocellular carcinoma. Pharmaceutical Research 2018;14:223. [Google Scholar]
- 17.Wu J, Yin F, Luo GH, et al. Clinical effect and safety of transarterial chemoembolization combined with apatinib in the treatment of advanced primary liver cancer. J Clin Hepatol 2018;34:775-8. [Google Scholar]
- 18.Wu YY. Effect of transcatheter arterial chemoembolization combined with apatinib on serum alpha-fetoprotein heterogeneity in patients with advanced primary liver cancer. Clinical Journal of Traditional Chinese Medicine 2019;31:736-8. [Google Scholar]
- 19.Wu FZ, Wu XL, Song JJ, et al. Clinical efficacy of apatinib combined with transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma and its effect on tumor angiogenesis. Journal of Wenzhou Medical University 2019;49:423-31. [Google Scholar]
- 20.Wang LR, Wang Y, Cao Y, et al. Apatinib combined with arterial embolism intervention for liver cancer and its effect on vascular endothelial growth factors and apoptosis related factors. IMHGN 2017;23:1015-7. [Google Scholar]
- 21.Song JF, Song C, Li CL, et al. Expression of Hsp90αand AFP in liver cancer and its correlation with the clinical outcome of TACE combined apatinib. Chinese Hepatology 2018;23:432-5. [Google Scholar]
- 22.Shen L, Yang XL, Ya GW, et al. Clinical observation on low-dose apatinib combined with TACE for advanced hepatocellular carcinoma. Guangzhou Medical Journal 2019;50:72-5. [Google Scholar]
- 23.Lu Y, Jiang YN, Wan C, et al. Curative effect of apatinib combined with TACE for advanced hepatocellular carcinoma. J Intervent Radiol 2019;28:162-5. [Google Scholar]
- 24.Li W, Man WL, Guo HQ, et al. Clinical research of apatinib with transcatheter arterial chemoembolization in the treatment of advanced hepatocellular carcinoma. Anti-tumor Pharmacy 2017;7:74-8. [Google Scholar]
- 25.Li Y, Yang DY, Lai XR, et al. Apatinib combined with TACE for liver cancer and its effect on AFP, VEGF and CEA. Chinese Hepatology 2018;23:426-30. [Google Scholar]
- 26.Jin XL, Wei Lu. TACE combined with apatinib in the treatment of advanced hepatocellular carcinoma. Chin J Interv Imaging Ther 2017;14:200-4. [Google Scholar]
- 27.Huang R, Yao LZ, Li WH, et al. Efficacy and safety of TACE combined with apatinib mesylate tablets in the treatment of intermediate and advanced hepatoma. Clin Res 2018;38:965-72. [Google Scholar]
- 28.Huang JY, Huang W, Zhu QH. Transcatheter arterial chemoembolization combined with mesylate apatinib in the treatment of advanced hepatocellular carcinoma. China Journal of Liver Disease 2017;9:78-81. [Google Scholar]
- 29.He F, Chen XD, Lin ZW, et al. Effects of hepatic artery chemoembolization combined with apatinib in patients with hepatocellular carcinoma. Anti-tumor Pharmacy 2018;8:383-6. [Google Scholar]
- 30.Cui HZ, Ge W, Cao DD, et al. Efficacy of Apatinib mesylate combined with hepatic artery embolization chemotherapy in the treatment of advanced primary liver cancer. China Medical Herald 2019;16:88-91. [Google Scholar]
- 31.Zeng XY. Apatinib combined with TACE in the treatment of primary liver cancer. Public Medical Forum Magazine 2018;22:1442-4. [Google Scholar]
- 32.Zeng GY, WG LL, Zheng W, et al. Clinical trial of apatinib tables combined with transcatheter arterial chemoembolization in the treatment of advanced primary liver cancer. Chin J Clin Pharmacol 2018;34:2693-6. [Google Scholar]
- 33.Bai ST, Zhang YM. Clinical effect of TACE combined apatinib on advanced liver cancer. China Modern Doctor 2018;56:90-2. [Google Scholar]
- 34.Yang Z, Chen G, Cui Y, et al. The safety and efficacy of TACE combined with apatinib on patients with advanced hepatocellular carcinoma: a retrospective study. Cancer Biol Ther 2019;20:321-7. 10.1080/15384047.2018.1529099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Feng B, Mei L, et al. Clinical efficacy of TACE combined with Apatinib in the treatment of advanced hepatocellular carcinoma. J BUON 2019;24:608-14. [PubMed] [Google Scholar]
- 36.Lu W, Jin XL, Yang C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single-center randomized controlled trial. Cancer Biol Ther 2017;18:433-8. 10.1080/15384047.2017.1323589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, Yu W, Zhang K, et al. Comparison of the efficacy and safety of transarterial chemoembolization with and without Apatinib for the treatment of BCLC stage C hepatocellular carcinoma. BMC Cancer 2018;18:1131. 10.1186/s12885-018-5081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni JY, Kong J, Sun HL, et al. Prognostic Factors for Survival After Transarterial Chemoembolization Combined with Sorafenib in the Treatment of BCLC Stage B and C Hepatocellular Carcinomas. Acad Radiol 2018;25:423-9. 10.1016/j.acra.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 39.Choi SB, Han HJ, Kim WB, et al. VEGF overexpression predicts poor survival in hepatocellular carcinoma. Open Med (Wars) 2017;12:430-9. 10.1515/med-2017-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuchiya K, Asahina Y, Matsuda S, et al. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer 2014;120:229-37. 10.1002/cncr.28384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as