Abstract
Background
This study aims to explore and project the temporal trends in incidence and mortality of testicular cancer. Moreover, it can provide theoretical guidance for the rational allocation of health resources.
Methods
This study analyzed existing data on testicular cancer morbidity and mortality from 1990 to 2016 and predicted time-varying trends of age-standardized incidence rate (ASIR) and age-standardized death rate (ASDR) from 2017 to 2030 in different ages, regions and sociodemographic index (SDI) quintile sub-groups.
Result
Globally, numbers of testicular cancer cases in 2016 [66,833; 95% uncertainty interval (UI), 64,487–69,736] are 1.8 times larger than in 1990 (37,231; 95% UI, 36,116–38,515). The testicular cancer-related death cases increased slightly from 8,394 (95% UI, 7,980–8,904) in 1990 to 8,651 (95% UI, 8,292–9,027) in 2016. In aspect of ASIR, the data showed an up-trend from 0.74 (95% UI, 0.72–0.77) in 1990 to 0.88 (95% UI, 0.85–0.92) in 2016. The ASDR of testicular cancer declined from 0.18 (95% UI, 0.17–0.19) in 1990 to 0.12 (95% UI, 0.11–0.12) in 2016. From 2017 to 2030, predictions of trends in testicular cancer indicate that the ASIRs of most SDI countries are rising, but the ASDRs trends in testicular cancer will decrease.
Conclusions
By analyzing the available and reliable data in different ages, regions and SDI, this study shows a significant upward trend in incidence and a slow upward trend in mortality of testicular cancer from 1990 to 2016, and simultaneously, predicts the increase of ASIR and the downward trend of ASDR in 2017–2030.
Keywords: Testicular cancer, incidence, mortality, trend, projection
Introduction
Testicular cancer, especially testicular germ cell tumor (TGCT), often happens in young and middle-aged men, and has a higher treatable rate than other cancer (1). The high survival rates contribute to the long-term burden of this cancer: patients often suffer from infertility, sexual dysfunction, and many other unknown treatment complications (2-4). In 2019, according to the latest data from 2002 to 2016, it is estimated that there will be 9,560 new cases of testicular cancer worldwide, of which 410 may be killed (5). In general, the incidence rates of testicular cancer appear to increase over time: for example, the incidence in the United States had increased steadily for about 10 years, and this growth trend will continue in the next 10 years (6).
Some studies researched this disease by analyzing the burden of testicular cancer in which is primarily confined to a country or a certain region (7,8). We collected data from the Global Burden of Disease data base (GBD), analyzed the temporal trends and simultaneously, estimated the incidence and death rates of testicular cancer in the next few years by 2030. Moreover, this study analyzed the temporal trends in several subgroups, including age, region, and sociodemographic index (SDI: a summary indicator of income per capita, educational attainment, and fertility). Understanding these factors is essential to provide the information about testicular cancer etiologies and the different changes in different subgroups.
Due to enormous cancer-relate economic burden, appropriate policies need to be developed to address this health issue so that health resource can be reasonably allocated. For this reason, the results of this study are sufficient to guide healthcare decisions and adjust implement plans.
Methods
Our research team collected the reliable data on testicular cancer in GBD. Many methods of analysis and estimation of existing data have been previously reported (9-14). The current study complies with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) (15). This time series is re-estimated overall compared to the published studies (16,17), and the results revealed by this study may replace previous studies. Testicular cancer is divided into 4 groups in incidence including C62−C62.92, Z80.43, Z85.47−Z85.48 in ICD-10 by International Classification of Diseases (ICD), and 4 cancer groups in mortality including C61-C61.9, D07.5, D29.1, D40.0 in ICD-10. Based on the collected data, we predict the disease burden of testicular cancer in different SDI countries and regions. The rates are calculated per 100,000 person-years. Age-standardized rates are calculated according to the GBD world population standard (18). Uncertainty intervals (UIs) are also reported.
Estimation process reported by this study begins with cancer mortality. Data sources of cancer mortality include vital registration systems (84% of data in 2016), cancer registries (16% of data in 2016), and verbal autopsy data (0.7% of data in 2016). Cancer incidence data are used to estimate mortality in some places where do not contain cancer death data by multiplying incidence by mortality-to incidence ratio. Cause of death ensemble model (CODEm) also can be appropriate for the death estimated data (11,19). Using the mortality-incidence rate (MIR) divides final cancer-specific mortality estimates to estimate cancer incidence. Statistical programming was done using the R statistical program version 3.4 and SAS version 9.3. Related methodological details can be found in Supplementary materials.
Results
Over-time trends in incidence cases of testicular cancer from 1990 to 2016
Globally, there were 66,833 (95% UI, 64,486–69,736) incident cases of testicular cancer in 2016, 1.8 times the numbers of new cases in 1990 (66,833; 95% UI, 64,487–69,736). Overall, 2.3% of this increase was due to changes in the population age structure, 12.4% was due to changes in the population size, and 15.8% was due to changes in the incidence rates (Tables S1-S9 in Supplementary materials and table online: http://fp.amegroups.cn/cms/236a29d20a4eb14f040340fbf5255b3b/tau.2020.02.22-1.docx). Among regions, the largest increase in incident cases from 1990 to 2016 occurred in Central Latin America, increased by 576% from 718 (95% UI, 682–7,598) in 1990 to 4,856 (95% UI, 4,571–5,151) in 2016. In terms of absolute numbers, high-income North America had the most cases of testicular cancer for males in 2016 (14,680; 95% UI, 13,694–15,690), followed by Western Europe (14,417; 95% UI, 13,251–16,174) and East Asia (5,381; 95% UI, 4,977–5,782). In Oceania, the number was only 48 (95% UI, 41–54). Among SDI countries, the largest increase in incident cases (224%) happened in middle SDI countries [from 3,629 (95% UI, 3,423–4,031) in 1990 to 11,740 (95% UI, 11,334–12,177) in 2016], followed by high-middle SDI, middle SDI, low-middle SDI and low SDI (Table 1).
Table S1. GATHER Guidelines checklist.
| Objectives and funding | Reported in the manuscript/Supplementary materials |
|---|---|
| 1. Define the indicator(s), populations (including age, sex, and geographic entities), and time period(s) for which estimates were made | – |
| 2. List the funding sources for the work | See main manuscript |
| Data inputs | |
| For all data inputs from multiple sources that are synthesized as part of the study | |
| 3. Describe how the data were identified and how the data were accessed | – |
| 4. Specify the inclusion and exclusion criteria. Identify all ad-hoc exclusions | – |
| 5. Provide information about all included data sources and their main characteristics. For each data source used, report reference information or contact name/institution, population represented, data collection method, year(s) of data collection, sex and age range, diagnostic criteria or measurement method, and sample size, as relevant | http://ghdx.healthdata.org/gbd-2016/data-input-sources |
| 6. Identify and describe any categories of input data that have potentially important biases (e.g., based on characteristics listed in item 5) | – |
| For data inputs that contribute to the analysis but were not synthesized as part of the study | |
| 7. Describe and give sources for any other data inputs | http://ghdx.healthdata.org/gbd-2016/data-input-sources |
| For all data inputs | |
| 8. Provide all data inputs in a file format from which data can be efficiently extracted (e.g., a spreadsheet rather than a PDF), including all relevant meta-data listed in item 5. For any data inputs that cannot be shared because of ethical or legal reasons, such as third-party ownership, provide a contact name or the name of the institution that retains the right to the data | http://ghdx.healthdata.org/gbd-2016/data-input-sources |
| Data analysis | |
| 9. Provide a conceptual overview of the data analysis method. A diagram may be helpful | – |
| 10. Provide a detailed description of all steps of the analysis, including mathematical formulae. This description should cover, as relevant, data cleaning, data pre-processing, data adjustments and weighting of data sources, and mathematical or statistical model(s) | – |
| 11. Describe how candidate models were evaluated and how the final model(s) were selected | See Supplementary materials “CODEm models”; see Table S2: Covariates selected for CODEm for each GBD testicular cancer group and expected direction of covariate |
| 12. Provide the results of an evaluation of model performance, if done, as well as the results of any relevant sensitivity analysis | See Table S3: Results for CODEm model testing |
| 13. Describe methods of calculating uncertainty of the estimates. State which sources of uncertainty were, and were not, accounted for in the uncertainty analysis | – |
| 14. State how analytic or statistical source code used to generate estimates can be accessed | http://ghdx.healthdata.org/gbd-2016-code |
| Results and discussion | |
| 15. Provide published estimates in a file format from which data can be efficiently extracted | GBD 2016 estimates are available online (http://vizhub.healthdata.org/gbdcompare). |
| 16. Report a quantitative measure of the uncertainty of the estimates (e.g., uncertainty intervals) | Done |
| 17. Interpret results in light of existing evidence. If updating a previous set of estimates, describe the reasons for changes in estimates | Table S4: Comparison of GBD 2015 and GBD 2016 covariates used and level of covariates; table online: http://fp.amegroups.cn/cms/236a29d20a4eb14f040340fbf5255b3b/tau.2020.02.22-1.docx |
| 18. Discuss limitations of the estimates. Include a discussion of any modelling assumptions or data limitations that affect interpretation of the estimates | See main manuscript “Limitations” |
GATHER, Guidelines for Accurate and Transparent Health Estimates Reporting; CODEm, cause of death ensemble model; GBD, Global Burden of Disease data base.
Table S2. Covariates selected for CODEm for each GBD testicular cancer group and expected direction of covariate.
| Cause | Sex | Age start | Age end | Direction | Covariate |
|---|---|---|---|---|---|
| Testicular cancer | Male | 15–19 years | 95+ years | 1 | Cumulative cigarettes (10 years) |
| Testicular cancer | Male | 15–19 years | 95+ years | 1 | Cumulative cigarettes (15 years) |
| Testicular cancer | Male | 15–19 years | 95+ years | 1 | Cumulative cigarettes (5 years) |
| Testicular cancer | Male | 15–19 years | 95+ years | −1 | Education (years per capita) |
| Testicular cancer | Male | 15–19 years | 95+ years | −1 | Fruits (kcal per capita) |
| Testicular cancer | Male | 15–19 years | 95+ years | −1 | Health System Access 2 (unitless) |
| Testicular cancer | Male | 15–19 years | 95+ years | −1 | LDI (I$ per capita) |
| Testicular cancer | Male | 15–19 years | 95+ years | −1 | Vegetables (kcal per capita) |
| Testicular cancer | Male | 15–19 years | 95+ years | 0 | Sociodemographic index |
| Testicular cancer | Male | 15–19 years | 95+ years | −1 | Healthcare access and quality index |
CODEm, cause of death ensemble model; GBD, Global Burden of Disease data base.
Table S3. Results for CODEm model testing.
| Cause | Sex | Age start | Age end | Predictive validity | |||||
|---|---|---|---|---|---|---|---|---|---|
| RMSE in | RMSE out | Trend in | Trend out | Coverage in | Coverage out | ||||
| Testicular cancer (global) | Male | 15–19 years | 95+ years | 0.328371 | 0.529164 | 0.255569 | 0.25659 | 0.999375 | 0.995125 |
| Testicular cancer (data rich) | Male | 15–19 years | 95+ years | 0.283022 | 0.371326 | 0.232189 | 0.243099 | 0.999645 | 0.999282 |
CODEm, cause of death ensemble model; RMSE, root mean square of errors.
Table S4. Comparison of GBD 2015 and GBD 2016 covariates used and level of covariates.
| Cause | Sex | Covariate | GBD 2015 | GBD 2016 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | ||||
| Testicular cancer | Male | Cumulative cigarettes (10 years) | X | X | |||||
| Testicular cancer | Male | Cumulative cigarettes (15 years) | X | X | |||||
| Testicular cancer | Male | Cumulative cigarettes (5 years) | X | X | |||||
| Testicular cancer | Male | Education (years per capita) | X | X | |||||
| Testicular cancer | Male | Fruits (kcal per capita) | X | X | |||||
| Testicular cancer | Male | Health System Access 2 (unitless) | X | X | |||||
| Testicular cancer | Male | LDI (I$ per capita) | X | X | |||||
| Testicular cancer | Male | Vegetables (kcal per capita) | X | X | |||||
| Testicular cancer | Male | Sociodemographic index | X | X | |||||
GBD, Global Burden of Disease data base.
Table S5. List of International Classification of Diseases (ICD) codes mapped to the Global Burden of Disease cause list for testicular cancer incidence and mortality data.
| Cause | ICD-10 | ICD9 |
|---|---|---|
| Incidence | C62−C62.9, D29.2−D29.8, D40.1−D40.8 | 186−186.9, 222.0, 222.3, 236.4 |
| Mortality | C62−C62.92, Z80.43, Z85.47−Z85.48 | 186−186.9, V10.47−V10.48, V16.43 |
Table S6. Sociodemographic Index groupings by geography, based on 2016 values.
| Location | SDI quintile |
|---|---|
| Andorra | High SDI |
| Australia | High SDI |
| Austria | High SDI |
| Belgium | High SDI |
| Brunei | High SDI |
| Canada | High SDI |
| Croatia | High SDI |
| Cyprus | High SDI |
| Czech Republic | High SDI |
| Denmark | High SDI |
| Estonia | High SDI |
| Finland | High SDI |
| France | High SDI |
| Georgia | High SDI |
| Germany | High SDI |
| Greece | High SDI |
| Iceland | High SDI |
| Ireland | High SDI |
| Italy | High SDI |
| Japan | High SDI |
| Latvia | High SDI |
| Lithuania | High SDI |
| Luxembourg | High SDI |
| Malta | High SDI |
| Netherlands | High SDI |
| New Zealand | High SDI |
| Norway | High SDI |
| Poland | High SDI |
| Puerto Rico | High SDI |
| Singapore | High SDI |
| Slovakia | High SDI |
| Slovenia | High SDI |
| South Korea | High SDI |
| Sweden | High SDI |
| Switzerland | High SDI |
| Taiwan | High SDI |
| United Kingdom | High SDI |
| United States | High SDI |
| Virgin Islands, U.S. | High SDI |
| Antigua and Barbuda | High-middle SDI |
| Argentina | High-middle SDI |
| Armenia | High-middle SDI |
| Azerbaijan | High-middle SDI |
| Barbados | High-middle SDI |
| Belarus | High-middle SDI |
| Bermuda | High-middle SDI |
| Bulgaria | High-middle SDI |
| Chile | High-middle SDI |
| Cuba | High-middle SDI |
| Georgia | High-middle SDI |
| Greenland | High-middle SDI |
| Guam | High-middle SDI |
| Hungary | High-middle SDI |
| Iran | High-middle SDI |
| Israel | High-middle SDI |
| Kazakhstan | High-middle SDI |
| Kuwait | High-middle SDI |
| Lebanon | High-middle SDI |
| Libya | High-middle SDI |
| Macedonia | High-middle SDI |
| Malaysia | High-middle SDI |
| Mauritius | High-middle SDI |
| Montenegro | High-middle SDI |
| Northern Mariana Islands | High-middle SDI |
| Panama | High-middle SDI |
| Portugal | High-middle SDI |
| Qatar | High-middle SDI |
| Romania | High-middle SDI |
| Russia | High-middle SDI |
| Saudi Arabia | High-middle SDI |
| Serbia | High-middle SDI |
| Spain | High-middle SDI |
| The Bahamas | High-middle SDI |
| Trinidad and Tobago | High-middle SDI |
| Turkey | High-middle SDI |
| Turkmenistan | High-middle SDI |
| Ukraine | High-middle SDI |
| United Arab Emirates | High-middle SDI |
| Albania | Middle SDI |
| Algeria | Middle SDI |
| American Samoa | Middle SDI |
| Bahrain | Middle SDI |
| Bosnia and Herzegovina | Middle SDI |
| Botswana | Middle SDI |
| Brazil | Middle SDI |
| China | Middle SDI |
| Colombia | Middle SDI |
| Costa Rica | Middle SDI |
| Dominica | Middle SDI |
| Dominican Republic | Middle SDI |
| Ecuador | Middle SDI |
| Egypt | Middle SDI |
| El Salvador | Middle SDI |
| Equatorial Guinea | Middle SDI |
| Fiji | Middle SDI |
| Grenada | Middle SDI |
| Guyana | Middle SDI |
| Indonesia | Middle SDI |
| Jamaica | Middle SDI |
| Jordan | Middle SDI |
| Maldives | Middle SDI |
| Mexico | Middle SDI |
| Moldova | Middle SDI |
| Mongolia | Middle SDI |
| Oman | Middle SDI |
| Paraguay | Middle SDI |
| Peru | Middle SDI |
| Philippines | Middle SDI |
| Saint Lucia | Middle SDI |
| Saint Vincent and the Grenadines | Middle SDI |
| Seychelles | Middle SDI |
| South Africa | Middle SDI |
| Sri Lanka | Middle SDI |
| Suriname | Middle SDI |
| Thailand | Middle SDI |
| Tunisia | Middle SDI |
| Uruguay | Middle SDI |
| Uzbekistan | Middle SDI |
| Venezuela | Middle SDI |
| Vietnam | Middle SDI |
| Bangladesh | Low-middle SDI |
| Belize | Low-middle SDI |
| Bhutan | Low-middle SDI |
| Bolivia | Low-middle SDI |
| Cambodia | Low-middle SDI |
| Cameroon | Low-middle SDI |
| Cape Verde | Low-middle SDI |
| Congo | Low-middle SDI |
| Federated States of Micronesia | Low-middle SDI |
| Gabon | Low-middle SDI |
| Ghana | Low-middle SDI |
| Guatemala | Low-middle SDI |
| Honduras | Low-middle SDI |
| India | Low-middle SDI |
| Iraq | Low-middle SDI |
| Kenya | Low-middle SDI |
| Kyrgyzstan | Low-middle SDI |
| Laos | Low-middle SDI |
| Lesotho | Low-middle SDI |
| Marshall Islands | Low-middle SDI |
| Mauritania | Low-middle SDI |
| Morocco | Low-middle SDI |
| Myanmar | Low-middle SDI |
| Namibia | Low-middle SDI |
| Nepal | Low-middle SDI |
| Nicaragua | Low-middle SDI |
| Nigeria | Low-middle SDI |
| North Korea | Low-middle SDI |
| Pakistan | Low-middle SDI |
| Samoa | Low-middle SDI |
| Sudan | Low-middle SDI |
| Swaziland | Low-middle SDI |
| Syria | Low-middle SDI |
| Tajikistan | Low-middle SDI |
| Timor-Leste | Low-middle SDI |
| Tonga | Low-middle SDI |
| Vanuatu | Low-middle SDI |
| Zambia | Low-middle SDI |
| Zimbabwe | Low-middle SDI |
| Afghanistan | Low SDI |
| Angola | Low SDI |
| Benin | Low SDI |
| Burkina Faso | Low SDI |
| Burundi | Low SDI |
| Central African Republic | Low SDI |
| Chad | Low SDI |
| Comoros | Low SDI |
| Cote d’Ivoire | Low SDI |
| Democratic Republic of the Congo | Low SDI |
| Djibouti | Low SDI |
| Eritrea | Low SDI |
| Ethiopia | Low SDI |
| Guinea | Low SDI |
| Guinea-Bissau | Low SDI |
| Haiti | Low SDI |
| Kiribati | Low SDI |
| Liberia | Low SDI |
| Madagascar | Low SDI |
| Malawi | Low SDI |
| Mali | Low SDI |
| Mozambique | Low SDI |
| Niger | Low SDI |
| Palestine | Low SDI |
| Papua New Guinea | Low SDI |
| Rwanda | Low SDI |
| Sao Tome and Principe | Low SDI |
| Senegal | Low SDI |
| Sierra Leone | Low SDI |
| Solomon Islands | Low SDI |
| Somalia | Low SDI |
| South Sudan | Low SDI |
| Tanzania | Low SDI |
| The Gambia | Low SDI |
| Togo | Low SDI |
| Uganda | Low SDI |
| Yemen | Low SDI |
SDI, sociodemographic index.
Table S7. Disability weights.
| Health state | Lay description | Estimate | Uncertainty interval | |
|---|---|---|---|---|
| Cancer, diagnosis and primary therapy | Has pain, nausea, fatigue, weight loss and high anxiety | 0.288 | 0.193 | 0.399 |
| Cancer, controlled phase | Has a chronic disease that requires medication every day and causes some worry but minimal interference with daily activities | 0.049 | 0.031 | 0.072 |
| Cancer, metastatic | Has severe pain, extreme fatigue, weight loss and high anxiety | 0.451 | 0.307 | 0.600 |
| Terminal phase, with medication | Has lost a lot of weight and regularly uses strong medication to avoid constant pain. The person has no appetite, feels nauseous, and needs to spend most of the day in bed | 0.540 | 0.377 | 0.687 |
Table S8. Decomposition analysis of testicular cancer incidence trends at the global and regional levels, and by SDI quintile, both sexes, 2006 to 2016.
| Location | Cancer | Incidence cases, No. | Expected incidence cases, 2016, No. | Change in incidence cases, 2006 to 2016, % | Overall change, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2016 | Given population growth alone | Given population growth and aging | Due to population growth | Due to change in age structure | Due to change in incidence rate | |||||
| Global | Testicular cancer | 51,202 (50,063 to 52,400) | 66,833 (64,487 to 69,736) | 57,565 | 58,744 | 12.4 | 2.3 | 15.8 | 30.5 | ||
| High SDI | Testicular cancer | 29,422 (28,417 to 30,391) | 34,681 (32,921 to 36,935) | 30,993 | 29,738 | 5.3 | −4.3 | 16.8 | 17.9 | ||
| High-middle SDI | Testicular cancer | 11,199 (10,758 to 11,673) | 15,610 (14,831 to 16,376) | 12,441 | 12,739 | 11.1 | 2.7 | 25.6 | 39.4 | ||
| Middle SDI | Testicular cancer | 6,982 (6,768 to 7,280) | 11,740 (11,334 to 12,177) | 7,494 | 7,715 | 7.3 | 3.2 | 57.6 | 68.1 | ||
| Low-middle SDI | Testicular cancer | 3,269 (3,101 to 3,471) | 4,198 (3,965 to 4,480) | 3,812 | 4,035 | 16.6 | 6.8 | 5 | 28.4 | ||
| Low SDI | Testicular cancer | 538 (479 to 613) | 651 (590 to 734) | 711 | 730 | 32.3 | 3.5 | −14.8 | 21 | ||
Data in the parentheses indicates 95% uncertainty interval (95% UI). SDI, sociodemographic index.
Table S9. Probability of developing testicular cancer within selected age intervals, global, and by SDI quintile, by sex, 2006–2016 in % (odds).
| Location/SDI quintile | Cancer | Birth to age 49 | Age 50 to 59 | Age 60 to 69 | Age 70 to 79 | Age 30 to 70 | Birth to age 79 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |||||||
| Global | Testicular cancer | 0.10 (1 in 1,010) | NA | 0.01 (1 in 7,006) | NA | 0.01 (1 in 8,191) | NA | 0.01 (1 in 7,480) | NA | 0.10 (1 in 1,018) | NA | 0.14 (1 in 720) | NA | |||||
| High-middle SDI | Testicular cancer | 0.13 (1 in 748) | NA | 0.02 (1 in 6,624) | NA | 0.02 (1 in 5,655) | NA | 0.02 (1 in 4,851) | NA | 0.13 (1 in 780) | NA | 0.19 (1 in 535) | NA | |||||
| High SDI | Testicular cancer | 0.43 (1 in 232) | NA | 0.04 (1 in 2,226) | NA | 0.02 (1 in 4,538) | NA | 0.01 (1 in 7,198) | NA | 0.33 (1 in 306) | NA | 0.51 (1 in 195) | NA | |||||
| Low-middle SDI | Testicular cancer | 0.02 (1 in 4,905) | NA | 0.00 (1 in 27,489) | NA | 0.00 (1 in 20,556) | NA | 0.01 (1 in 13,863) | NA | 0.03 (1 in 3,535) | NA | 0.04 (1 in 2,770) | NA | |||||
| Low SDI | Testicular cancer | 0.01 (1 in 9,844) | NA | 0.00 (1 in 33,316) | NA | 0.00 (1 in 22,247) | NA | 0.01 (1 in 16,688) | NA | 0.02 (1 in 5,007) | NA | 0.02 (1 in 4,229) | NA | |||||
| Middle SDI | Testicular cancer | 0.05 (1 in 1,942) | NA | 0.01 (1 in 16,097) | NA | 0.01 (1 in 11,812) | NA | 0.01 (1 in 7,482) | NA | 0.05 (1 in 1,892) | NA | 0.08 (1 in 1,258) | NA | |||||
SDI, sociodemographic index.
Table 1. Global and regional testicular cancer incident and death cases by geography, gender and SDI quintile, 1990 and 2016.
| Location | Incident cases, global and regional | Death cases, global and regional | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 2016 | 1990 | 2016 | ||||||||||||
| Male | Female | Both | Male | Female | Both | Male | Female | Both | Male | Female | Both | ||||
| Global | 37,231 [36,116–38,515] | NA | 37,231 [36,116–38,515] | 66,833 [64,487–69,736] | NA | 66,833 [64,487–69,736] | 8,394 [7,980–8,904] | NA | 8,394 [7,980–8,904] | 8,651 [8,292–9,027] | NA | 8,651 [8,292–9,027] | |||
| High SDI | 23,333 [22,497–24,216] | NA | 23,333 [22,497–24,216] | 34,681 [32,921–36,935] | NA | 34,681 [32,921–36,935] | 1,692 [1,612–1,751] | NA | 1,692 [1,612–1,751] | 1,359 [1,279–1,448] | NA | 1,359 [1,279–1,448] | |||
| High-middle SDI | 7,190 [6,708–7,875] | NA | 7,190 [6,708–7,875] | 15,610 [14,831–16,376] | NA | 15,610 [14,831–16,376] | 1,932 [1,796–2,099] | NA | 1,932 [1,796–2,099] | 1,749 [1,578–1,925] | NA | 1,749 [1,578–1,925] | |||
| Low SDI | 413 [356–522] | NA | 413 [356–522] | 651 [590–734] | NA | 651 [590–734] | 385 [328–497] | NA | 385 [328–497] | 651 [574–746] | NA | 651 [574–746] | |||
| Low-middle SDI | 2,914 [2,729–3,223] | NA | 2,914 [2,729–3,223] | 4,198 [3,965–4,480] | NA | 4,198 [3,965–4,480] | 2,097 [1,896–2,330] | NA | 2,097 [1,896–2,330] | 2,389 [2,198–2,619] | NA | 2,389 [2,198–2,619] | |||
| Middle SDI | 3,629 [3,423–4,031] | NA | 3,629 [3,423–4,031] | 11,740 [11,334–12,177] | NA | 11,740 [11,334–12,177] | 2,285 [2,123–2,536] | NA | 2,285 [2,123–2,536] | 2,500 [2,378–2,641] | NA | 2,500 [2,378–2,641] | |||
| High-income Asia Pacific | 1,851 [1,759–1,946] | NA | 1,851 [1,759–1,946] | 2,478 [2,256–2,870] | NA | 2,478 [2,256–2,870] | 149 [137–157] | NA | 149 [137–157] | 114 [102–127] | NA | 114 [102–127] | |||
| Western Europe | 11,339 [10,613–12,090] | NA | 11,339 [10,613–12,090] | 14,417 [13,251–16,174] | NA | 14,417 [13,251–16,174] | 886 [823–929] | NA | 886 [823–929] | 613 [553–678] | NA | 613 [553–678] | |||
| Andean Latin America | 191 [167–221] | NA | 191 [167–221] | 362 [318–422] | NA | 362 [318–422] | 111 [97–128] | NA | 111 [97–128] | 124 [102–149] | NA | 124 [102–149] | |||
| Central Latin America | 718 [682–759] | NA | 718 [682–759] | 4,856 [4,572–5,151] | NA | 4,856 [4,572–5,151] | 334 [307–376] | NA | 334 [307–376] | 749 [679–848] | NA | 749 [679–848] | |||
| Southern Latin America | 920 [808–1061] | NA | 920 [808–1061] | 3,049 [2,690–3,430] | NA | 3,049 [2,690–3,430] | 310 [272–356] | NA | 310 [272–356] | 319 [267–381] | NA | 319 [267–381] | |||
| Tropical Latin America | 402 [378–427] | NA | 402 [378–427] | 1,849 [1,720–1,985] | NA | 1,849 [1,720–1,985] | 183 [160–201] | NA | 183 [160–201] | 342 [309–385] | NA | 342 [309–385] | |||
| North Africa and Middle East | 876 [743–1017] | NA | 876 [743–1017] | 3,082 [2,755–3,436] | NA | 3,082 [2,755–3,436] | 495 [430–576] | NA | 495 [430–576] | 643 [571–725] | NA | 643 [571–725] | |||
| High-income North America | 8,466 [8,098–8,851] | NA | 8,466 [8,098–8,851] | 14,680 [13,694–15,690] | NA | 14,680 [13,694–15,690] | 425 [401–456] | NA | 425 [401–456] | 462 [428–498] | NA | 462 [428–498] | |||
| Oceania | 22 [19–25] | NA | 22 [19–25] | 48 [41–54] | NA | 48 [41–54] | 12 [10–15] | NA | 12 [10–15] | 20 [16–24] | NA | 20 [16–24] | |||
| Central sub-Saharan Africa | 79 [58–94] | NA | 79 [58–94] | 139 [105–166] | NA | 139 [105–166] | 61 [43–73] | NA | 61 [43–73] | 114 [82–141] | NA | 114 [82–141] | |||
| Eastern sub-Saharan Africa | 207 [176–295] | NA | 207 [176–295] | 334 [310–373] | NA | 334 [310–373] | 198 [158–291] | NA | 198 [158–291] | 318 [273–372] | NA | 318 [273–372] | |||
| Central Asia | 365 [302–456] | NA | 365 [302–456] | 656 [596–725] | NA | 656 [596–725] | 131 [109–164] | NA | 131 [109–164] | 133 [118–149] | NA | 133 [118–149] | |||
| Southern sub-Saharan Africa | 100 [90–109] | NA | 100 [90–109] | 261 [246–279] | NA | 261 [246–279] | 49 [43–55] | NA | 49 [43–55] | 84 [76–93] | NA | 84 [76–93] | |||
| Western sub-Saharan Africa | 163 [142–183] | NA | 163 [142–183] | 273 [250–300] | NA | 273 [250–300] | 149 [126–173] | NA | 149 [126–173] | 263 [226–303] | NA | 263 [226–303] | |||
| East Asia | 1,881 [1,683–2,177] | NA | 1,881 [1,683–2,177] | 5,381 [4,977–5,782] | NA | 5,381 [4,977–5,782] | 1,160 [1,018–1,345] | NA | 1,160 [1,018–1,345] | 683 [640–727] | NA | 683 [640–727] | |||
| South Asia | 2,835 [2,666–3,084] | NA | 2,835 [2,666–3,084] | 4,134 [3,856–4,398] | NA | 4,134 [3,856–4,398] | 2,024 [1,837–2,238] | NA | 2,024 [1,837–2,238] | 2,064 [1,878–2,285] | NA | 2,064 [1,878–2,285] | |||
| Southeast Asia | 892 [809–1134] | NA | 892 [809–1134] | 2,055 [1,914–2,418] | NA | 2,055 [1,914–2,418] | 549 [487–665] | NA | 549 [487–665] | 667 [608–765] | NA | 667 [608–765] | |||
| Australasia | 537 [474–606] | NA | 537 [474–606] | 955 [811–1,120] | NA | 955 [811–1,120] | 37 [34–40] | NA | 37 [34–40] | 29 [25–33] | NA | 29 [25–33] | |||
| Caribbean | 130 [110–150] | NA | 130 [110–150] | 254 [229–286] | NA | 254 [229–286] | 38 [33–43] | NA | 38 [33–43] | 42 [37–48] | NA | 42 [37–48] | |||
| Central Europe | 2,524 [2,365–2,680] | NA | 2,524 [2,365–2,680] | 4,217 [3,856–4,645] | NA | 4,217 [3,856–4,645] | 497 [472–526] | NA | 497 [472–526] | 387 [359–419] | NA | 387 [359–419] | |||
| Eastern Europe | 2,733 [2,399–3,300] | NA | 2,733 [2,399–3,300] | 3,353 [2,987–3,786] | NA | 3,353 [2,987–3,786] | 591 [511–703] | NA | 591 [511–703] | 478 [346–626] | NA | 478 [346–626] | |||
Data in the parentheses indicates 95% uncertainty interval (95% UI). SDI, sociodemographic index; NA, not available.
Over-time trends in mortality cases of testicular cancer from 1990 to 2016
Globally, testicular cancer caused 8,651 deaths (95% UI, 8,292–9,027) in 2016, but in 1990, that number was 8,394 (95% UI, 7,980–8,904). In terms of regions, regions with the highest number of deaths are South Asia (2,064; 95% UI, 1,878–2,285), Central Latin America (749; 95% UI, 679–848), and South Asia (683; 95% UI, 640–727). The mortality rates of low SDI, low-middle SDI and middle SDI increased slightly, but the mortality rates of high SDI and high-middle SDI decreased from 1990 to 2016. The Middle SDI countries had the greatest number of death cases followed by low-middle SDI, high-middle SDI, high SDI and low SDI countries. Deaths from high and high-middle SDI countries were declined by 333 and 193, respectively (Table 1).
Over-time trends in age-standardized incidence (ASIR) of testicular cancer from 1990 to 2016
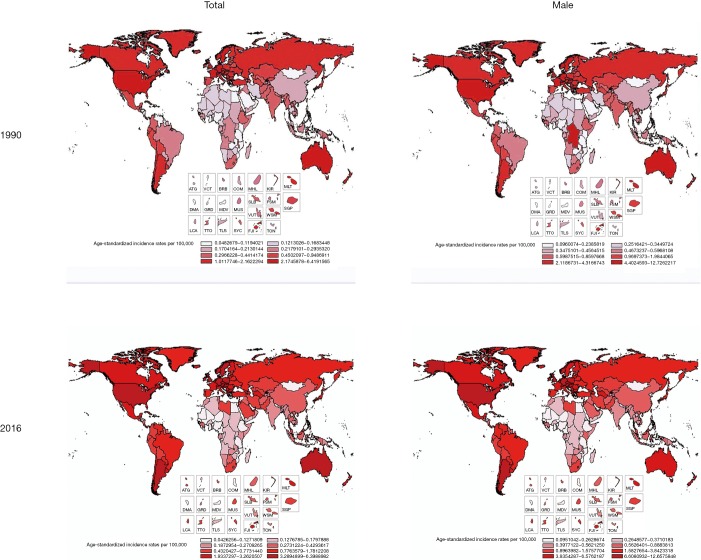
The ASIR increased by 18.92% from 0.74 (95% UI, 0.72–0.77) in 1990 to 0.88 (95% UI, 0.85–0.92) in 2016 all over the world (Table 2). Among regions, the 3 highest ASIR of testicular cancer were Southern Latin America (9.11; 95% UI, 8.04–10.24), high-income North America (8.26; 95% UI, 7.70–8.84), and Central Europe (7.05; 95% UI, 6.43–7.77). Between 1990 and 2016, the highest changes in ASIR occurred in middle SDI countries and Central Latin America. For SDI countries, the rapid change is in Middle SDI countries, reaching 113%, and the growth in other SDI countries also appeared obviously (Figures 1,2 and Table 2).
Table 2. Global and regional age-standardized testicular cancer incidence and death rates with 95% uncertainty interval and percent change by SDI and sex between 1990 and 2016.
| Location | Sex | Age-standardized incidence rates per 100,000 | Age-standardized death rates per 100,000 | |||||
|---|---|---|---|---|---|---|---|---|
| 1990 | 2016 | Change (%) | 1990 | 2016 | Change (%) | |||
| Global | Both | 0.74 (0.72–0.77) | 0.88 (0.85–0.92) | 18.92 | 0.18 (0.17–0.19) | 0.12 (0.11–0.12) | −57.14 | |
| Male | 1.50 (1.45–1.55) | 1.75 (1.69–1.83) | 16.67 | 0.39 (0.37–0.41) | 0.25 (0.24–0.26) | −35.9 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| High SDI | Both | 2.46 (2.38–2.56) | 3.48 (3.30–3.71) | 41.46 | 0.17 (0.17–0.18) | 0.11 (0.11–0.12) | −35.29 | |
| Male | 4.95 (4.77–5.13) | 6.92 (6.56–7.38) | 39.8 | 0.37 (0.35–0.38) | 0.24 (0.22–0.25) | −35.14 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| High-middle SDI | Both | 0.79 (0.74–0.86) | 1.19 (1.13–1.25) | 50.63 | 0.23 (0.21–0.24) | 0.13 (0.12–0.15) | −43.48 | |
| Male | 1.63 (1.52–1.78) | 2.35 (2.24–2.46) | 44.17 | 0.49 (0.46–0.53) | 0.28 (0.25–0.31) | −42.86 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Middle SDI | Both | 0.23 (0.22–0.26) | 0.49 (0.47–0.50) | 113.04 | 0.16 (0.15–0.18) | 0.11 (0.10–0.12) | −31.25 | |
| Male | 0.48 (0.45–0.53) | 0.97 (0.94–1.00) | 102.08 | 0.34 (0.32–0.38) | 0.23 (0.22–0.24) | −32.35 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Low-middle SDI | Both | 0.28 (0.27–0.32) | 0.22 (0.21–0.24) | −21.43 | 0.22 (0.19–0.24) | 0.14 (0.13–0.15) | −36.36 | |
| Male | 0.57 (0.53–0.63) | 0.45 (0.43–0.48) | −21.05 | 0.43 (0.39–0.48) | 0.29 (0.27–0.32) | −32.56 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Low SDI | Both | 0.17 (0.15–0.22) | 0.12 (0.11–0.14) | −29.41 | 0.17 (0.14–0.22) | 0.13 (0.12–0.15) | −23.53 | |
| Male | 0.35 (0.30–0.45) | 0.25 (0.23–0.28) | −28.57 | 0.35 (0.30–0.45) | 0.27 (0.24–0.31) | −22.86 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| High-income Asia Pacific | Both | 1.06 (1.00–1.11) | 1.47 (1.34–1.7) | 38.68 | 0.08 (0.08–0.09) | 0.05 (0.05–0.06) | −37.5 | |
| Male | 2.11 (2.00–2.22) | 2.89 (2.63–3.33) | 36.97 | 0.18 (0.16–0.19) | 0.11 (0.10–0.12) | −38.89 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Western Europe | Both | 2.82 (2.64–3.02) | 3.48 (3.18–3.93) | 23.4 | 0.21 (0.19–0.22) | 0.11 (0.10–0.13) | −47.62 | |
| Male | 5.66 (5.3–6.04) | 6.96 (6.35–7.86) | 22.97 | 0.44 (0.41–0.46) | 0.24 (0.21–0.27) | −45.45 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Andean Latin America | Both | 0.59 (0.53–0.68) | 0.62 (0.55–0.72) | 5.08 | 0.39 (0.35–0.45) | 0.24 (0.20–0.29) | −38.46 | |
| Male | 1.22 (1.09–1.39) | 1.27 (1.13–1.46) | 4.1 | 0.82 (0.73–0.93) | 0.50 (0.42–0.61) | −39.02 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Central Latin America | Both | 0.47 (0.45–0.50) | 1.79 (1.69–1.90) | 280.85 | 0.25 (0.23–0.27) | 0.30 (0.27–0.34) | 20 | |
| Male | 0.97 (0.92–1.02) | 3.62 (3.41–3.83) | 273.2 | 0.52 (0.48–0.57) | 0.62 (0.57–0.69) | 19.23 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Southern Latin America | Both | 1.92 (1.69–2.21) | 4.56 (4.02–5.13) | 137.5 | 0.66 (0.59–0.76) | 0.47 (0.39–0.56) | −28.79 | |
| Male | 3.92 (3.45–4.49) | 9.11 (8.04–10.24) | 132.4 | 1.38 (1.23–1.58) | 0.97 (0.82–1.16) | −29.71 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Tropical Latin America | Both | 0.28 (0.27–0.30) | 0.80 (0.74–0.85) | 185.71 | 0.15 (0.13–0.16) | 0.15 (0.14–0.17) | 0 | |
| Male | 0.59 (0.56–0.62) | 1.62 (1.50–1.73) | 174.58 | 0.32 (0.29–0.35) | 0.33 (0.30–0.37) | 3.13 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| North Africa and Middle East | Both | 0.31 (0.27–0.36) | 0.52 (0.47–0.58) | 67.74 | 0.19 (0.17–0.21) | 0.12 (0.11–0.14) | −36.84 | |
| Male | 0.62 (0.54–0.71) | 1.02 (0.93–1.13) | 64.52 | 0.38 (0.33–0.43) | 0.25 (0.22–0.28) | −34.21 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| High-income North America | Both | 2.78 (2.66–2.91) | 4.14 (3.86–4.44) | 48.92 | 0.14 (0.13–0.15) | 0.12 (0.11–0.13) | −14.29 | |
| Male | 5.60 (5.36–5.86) | 8.26 (7.70–8.84) | 47.50 | 0.29 (0.27–0.31) | 0.24 (0.22–0.26) | −17.24 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Oceania | Both | 0.44 (0.38–0.49) | 0.51 (0.45–0.58) | 15.91 | 0.57 (0.49–0.67) | 0.49 (0.41–0.59) | −14.04 | |
| Male | 0.88 (0.77–0.98) | 1.05 (0.93–1.18) | 19.32 | 0.51 (0.42–0.58) | 0.63 (0.59–0.66) | 23.53 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Central sub-Saharan Africa | Both | 0.20 (0.15–0.23) | 0.16 (0.13–0.19) | −20.00 | 0.17 (0.12–0.20) | 0.14 (0.11–0.17) | −17.65 | |
| Male | 0.42 (0.33–0.49) | 0.33 (0.26–0.38) | −21.43 | 0.35 (0.26–0.41) | 0.29 (0.22–0.36) | −17.14 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Eastern sub-Saharan Africa | Both | 0.19 (0.16–0.28) | 0.14 (0.13–0.16) | −26.32 | 0.20 (0.16–0.29) | 0.15 (0.13–0.17) | −25.00 | |
| Male | 0.40 (0.34–0.58) | 0.29 (0.27–0.34) | −27.5 | 0.41 (0.33–0.61) | 0.31 (0.27–0.36) | −24.39 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Central Asia | Both | 0.57 (0.48–0.71) | 0.70 (0.64–0.77) | 22.81 | 0.23 (0.19–0.29) | 0.16 (0.14–0.18) | −30.43 | |
| Male | 1.22 (1.02–1.51) | 1.45 (1.33–1.60) | 18.85 | 0.51 (0.42–0.64) | 0.34 (0.31–0.38) | −33.33 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Southern sub-Saharan Africa | Both | 0.27 (0.24–0.29) | 0.41 (0.39–0.43) | 51.85 | 0.16 (0.14–0.17) | 0.16 (0.15–0.18) | 0 | |
| Male | 0.58 (0.53–0.63) | 0.91 (0.86–0.97) | 56.9 | 0.35 (0.31–0.39) | 0.40 (0.36–0.43) | 14.29 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Western sub-Saharan Africa | Both | 0.13 (0.11–0.14) | 0.11 (0.10–0.12) | −15.38 | 0.13 (0.11–0.15) | 0.11 (0.10–0.13) | −15.38 | |
| Male | 0.27 (0.23–0.30) | 0.22 (0.2–0.24) | −18.52 | 0.27 (0.23–0.31) | 0.24 (0.20–0.27) | −11.11 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| East Asia | Both | 0.18 (0.16–0.20) | 0.34 (0.31–0.36) | 88.89 | 0.12 (0.11–0.14) | 0.04 (0.04–0.05) | −66.67 | |
| Male | 0.36 (0.33–0.42) | 0.67 (0.62–0.72) | 86.11 | 0.26 (0.23–0.30) | 0.09 (0.09–0.10) | −65.38 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| South Asia | Both | 0.33 (0.31–0.36) | 0.26 (0.24–0.28) | −21.21 | 0.16 (0.14–0.20) | 0.11 (0.1–0.13) | −31.25 | |
| Male | 0.64 (0.61–0.70) | 0.52 (0.49–0.55) | −18.75 | 0.34 (0.30–0.42) | 0.24 (0.22–0.27) | −29.41 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Southeast Asia | Both | 0.24 (0.22–0.30) | 0.31 (0.29–0.37) | 29.17 | 0.16 (0.14–0.20) | 0.11 (0.10–0.13) | −31.25 | |
| Male | 0.50 (0.45–0.63) | 0.65 (0.61–0.76) | 30.00 | 0.34 (0.30–0.42) | 0.24 (0.22–0.27) | −29.41 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Australasia | Both | 2.44 (2.16–2.76) | 3.28 (2.75–3.85) | 34.43 | 0.17 (0.15–0.18) | 0.09 (0.08–0.10) | −47.06 | |
| Male | 4.88 (4.33–5.51) | 6.57 (5.52–7.72) | 34.63 | 0.35 (0.32–0.38) | 0.18 (0.16–0.21) | −48.57 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Caribbean | Both | 0.37 (0.32–0.42) | 0.55 (0.5–0.62) | 48.65 | 0.12 (0.11–0.14) | 0.09 (0.08–0.11) | −25.00 | |
| Male | 0.76 (0.65–0.87) | 1.12 (1.01–1.25) | 47.37 | 0.25 (0.22–0.28) | 0.19 (0.17–0.22) | −24.00 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Central Europe | Both | 2.02 (1.90–2.15) | 3.55 (3.23–3.91) | 75.74 | 0.39 (0.37–0.42) | 0.29 (0.26–0.31) | −26.19 | |
| Male | 4.07 (3.82–4.32) | 7.05 (6.43–7.77) | 73.22 | 0.83 (0.79–0.87) | 0.60 (0.55–0.65) | −25.29 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
| Eastern Europe | Both | 1.18 (1.03–1.42) | 1.43 (1.27–1.60) | 21.19 | 0.25 (0.22–0.30) | 0.19 (0.14–0.25) | −24 | |
| Male | 2.54 (2.24–3.03) | 2.97 (2.64–3.32) | 16.93 | 0.60 (0.52–0.71) | 0.43 (0.31–0.56) | −28.33 | ||
| Female | NA | NA | NA | NA | NA | NA | ||
Data in the parentheses indicates 95% uncertainty interval (95% UI). SDI, sociodemographic index; NA, not available.
Figure 1.
Global and regional testicular cancer ASIR by geography and gender, 1990 and 2016. ASIR, age-standardized incidence rate; ATG, Antigua and Barbuda; VCT, Saint Vincent and the Grenadines; BRB, Barbados; COM, Comoros; MHL, Marshall Islands; KIR, Kiribati; MLT, Malta; DMA, Dominica; GRD, Grenada; MDV, Maldives; MUS, Mauritius; SLB, Solomon Islands; FSM, Federated States of Micronesia; VUT, Vanuatu; WSM, Samoa. SGP, Singapore; LCA, Saint Lucia; TTO, Trinidad and Tobago; TLS, Timor-Leste; SYC, Seychelles; FJI, Fiji; TON, Tonga.
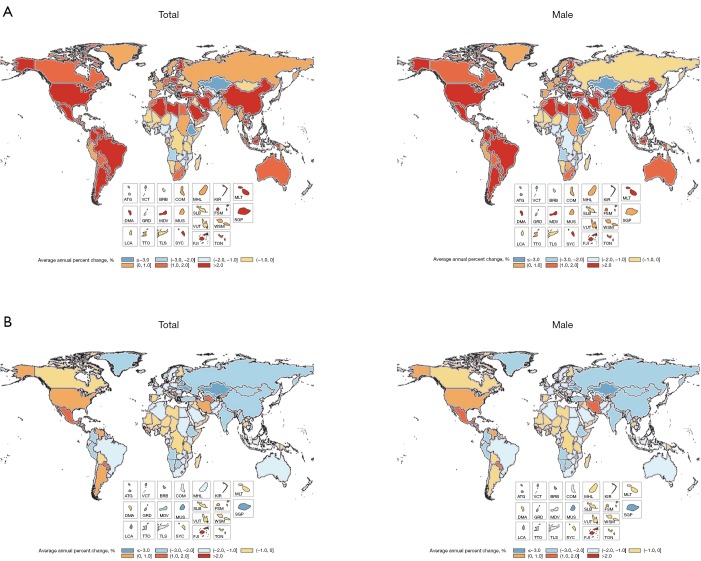
Figure 2.
Global and regional average annual percent change in age-standardized incidence and death rates for testicular cancer by geography and gender, 1990–2016. (A) Average annual percent change in age-standardized incidence rates for testicular cancer by geography and gender, 1990-2016; (B) average annual percent change in age-standardized death rates for testicular cancer by geography and gender, 1990–2016. ATG indicates Antigua and Barbuda; VCT, Saint Vincent and the Grenadines; BRB, Barbados; COM, Comoros; MHL, Marshall Islands; KIR, Kiribati; MLT, Malta; DMA, Dominica; GRD, Grenada; MDV, Maldives; MUS, Mauritius; SLB, Solomon Islands; FSM, Federated States of Micronesia; VUT, Vanuatu; WSM, Samoa. SGP, Singapore; LCA, Saint Lucia; TTO, Trinidad and Tobago; TLS, Timor-Leste; SYC, Seychelles; FJI, Fiji; TON, Tonga.
Over-time trends in age-standardized death rate (ASDR) of testicular cancer from 1990 to 2016
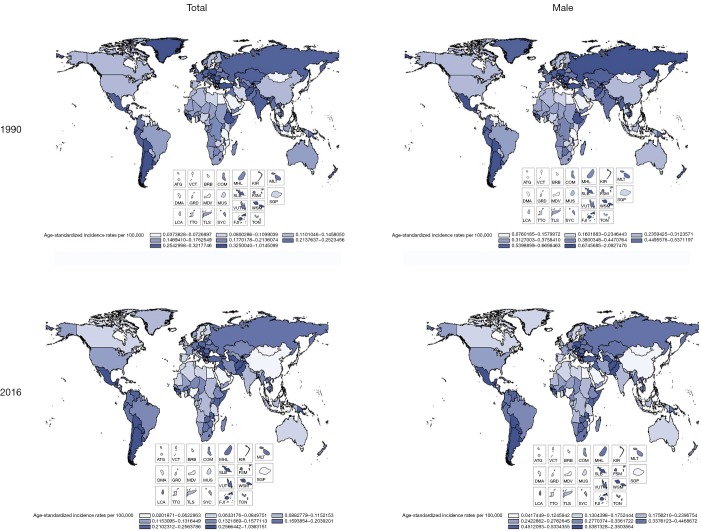
Globally, the change in ASDR decreased by 57.14% from 0.18 (95% UI, 0.17–0.19) in 1990 to 0.12 (95% UI, 0.11–0.12) in 2016 (Table 2). Among regions, the 3 highest ASDR happened in Southern Latin America (0.97; 95% UI, 0.82–1.16), Central Latin America (0.62; 95% UI, 0.57–0.69), and Central Europe (0.60; 95% UI, 0.55–0.65). However, ASDR has decreased steeply in most regions, and most of this change in East Asia is about 66.67%. The highest increasing changes of ASDR occurred in Central Latin America. In addition, the average annual percentage change in ASDR of testicular cancer by geography and gender displayed a significant increase in North America, South America and Central Africa. Compared with China, the rise of ASDR in America was higher than in China in both sexes and only males. In SDI countries, High-middle SDI showed the largest reduction in ASDR, about 43.48%, and other SDI countries have changed by about 30% from 1990 to 2016 (Figures 2,3 and Table 2).
Figure 3.
Global and regional testicular cancer ASDR by geography and gender, 1990 and 2016. ASDR, age-standardized death rate; ATG, Antigua and Barbuda; VCT, Saint Vincent and the Grenadines; BRB, Barbados; COM, Comoros; MHL, Marshall Islands; KIR, Kiribati; MLT, Malta; DMA, Dominica; GRD, Grenada; MDV, Maldives; MUS, Mauritius; SLB, Solomon Islands; FSM, Federated States of Micronesia; VUT, Vanuatu; WSM, Samoa. SGP, Singapore; LCA, Saint Lucia; TTO, Trinidad and Tobago; TLS, Timor-Leste; SYC, Seychelles; FJI, Fiji; TON, Tonga.
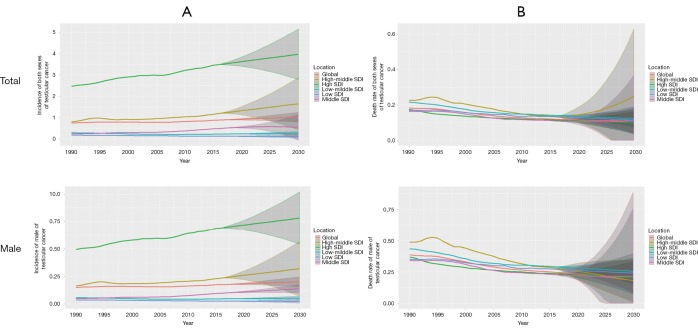
Over-time trends in projection of testicular cancer from 2017 to 2030
This study also forecasts the trends in the ASIR and ASDR of testicular cancer from 2017 to 2030. At the global level, ASIR will maintain steady growth, while ASDR will fall more sharply. Testicular cancer will most often occur in developed countries by 2030, and at the same time, the incidence will continue to increase in male. From 2017 to 2030, ASIR will rise in most of SDI countries while the low SDI and low-middle SDI countries will be different, where they will remain stable. However, the largest ASDR decrease will occur in high-middle SDI, followed by low SDI, low-middle SDI, and the high SDI, high-middle SDI future trend also remain stable. It should be noted that the changing trend within 95% UI can’t be ignored. Actual changes are likely to fluctuate within this range and may differ from the trends predicted above (Figure 4).
Figure 4.
Global and regional trends and predictions in age-standardized incidence and death rates for testicular cancer by SDI quintile, 1990–2030. (A) Trends and predictions in age-standardized incidence rates for testicular cancer by SDI quintile, 1990–2030; (B) trends and predictions in age-standardized death rates for testicular cancer by SDI quintile, 1990–2030. SDI, sociodemographic index.
Discussion
Testicular cancer is the most common cancer in young and middle-aged men. Although curable, illness or treatment or both can impose huge physical, psychological and financial burdens on patients, especially young people. Although the incidence of testicular cancer accounts for only 1% of all cancers, it has received increasing attention due to its severe consequences. However, many prior researches lacked attention to trends in the incidence and mortality rates of testicular. According to literature, this is the first study to systematically analyze the incidence and death rates between 1990 and 2016 by ages, SDI countries, and regions. The testicular cancer incidence trend indicates that from 1990 through 2016, especially in adolescence and young people (<49 years old), the incidence increased significantly. The key reason for the increase in incident cases is the change in incidence rate, followed by population growth and change in age structure. However, with the growth of the economy and the advancement of medical technology, the trend of death worldwide is falling sharply. In this study, Among SDI countries, the largest increase in incident cases was in middle SDI and high SDI countries. Among the regions, incident cases in America and many Europe countries increased commonly from 1990 to 2016. In 2016, there were 66,833 testicular cancer events worldwide. Its incidence may not be enough to cause more concern than other common cancers, but TGCT is the most common solid tumor in men between the ages of 20 and 34. Overall, research related to testicular cancer is insufficient.
It has been reported in previous study that the cancer outcomes are strongly correlated with the health care expenditures, adequate diagnosis and treatment services (20). Generally, developed countries invest more in health and also have more advanced treatment and diagnostic methods. Moreover, with increasing investment in health and advances in medical technology in developing countries, the future trend of ASDR worldwide and in each country is declining. These conjectures explain the above situation well, while it is not easy to explain it perfectly.
The two most influential factors are population growth and changes in age structure during the development of testicular cancer incidence. But, these two reasons contribute differently at different ages, regions and SDI countries. There are many other risk factors for the occurrence of seminoma or nonseminoma in germ cell tumors (GCTs). For example, several environmental risk factors are independently associated with testicular cancer. The most common include cryptorchidism, low birth weight and short gestational age (21). Cryptorchidism can induce ipsilateral and contralateral testicular cancer (22), and Testicular microlithiasis may often coexists with this disease (23). The genetic factors also play a role in testicular cancer, while only 5% of patients diagnosed with this disease are considered to have inherent relation. People whose father has testicular cancer are 4 to 6 times more likely to develop the disease than normal people, and if a brother has this cancer, the risk would become 8 to 10 times (24). Furthermore, Down syndrome, Klinefelter’s syndrome and testicular dysgenesis syndrome are also associated with increased risks of testicular cancer (25-27). In addition, mother’s weight gain during pregnancy, estrogen level, race, birth weight, social life status, education levels, serum cholesterol level and lifestyle are all associated with testicular neoplasms (28-34). Screening has not yet taken place globally, and prevention recommendations are also not enough in all regions.
Testicular cancer mortality may be impacted by multiple factors. For example, the living environment change is an important influencing factor. But the harmful factors are still existence in lifestyles such as tobacco smoking, obesity, hypertension and high fat diet (35). Furthermore, the high body mass index (BMI), sport absence and sedentariness were also considered to increase the mortality in this cancer, while the evidences were insufficient compared to other urologic cancers. However, the most established risk factor remains cryptorchidism. The favorable trends in mortality are largely due to the introduction (since the 1970s) of effective treatments, mainly platinum-derived chemotherapy.
This study shows future trends in testicular cancer. Trends are predicted by statistical methods and professional tools, and the feasibility ensured by analyzing. As mentioned above, the reason for the increasing incidence is likely to be related to the risk factors brought about by social development. However, the reason for the declining mortality rate can still be explained by development of economic and medical technological, but the specific reasons for the small change in the incidence of low SDI and low middle-SDI countries may be related to population size and local customs. To the best of my knowledge, this research is the first to analyze the temporal trends of testicular cancer incidence and mortality from 1990 to 2030. This article analyzes the trends of different subgroups including ages, regions and SDI from 1990 to 2030 by combining existing data and estimated data. The time trends presented the testicular cancer epidemiology and can guide intervention programs and instruct cancer determinants and outcomes research. Trends in cancer incidence will assist with resource allocation as a window into the future, which is essential for health policy, screening guidelines, and resource allocation decisions.
Conclusions
After detailed analysis of temporal trends in collecting and predicting data on future testicular cancer incidence and death rate in 2030, the outcomes show that the global incidence increased significantly in terms of population expansion and age structure changes, but not for multifactor mortality. This has led to serious economic problems in treatment and supportive therapies, and challenges to all segments of society. As the first systematic summary of testicular cancer, this study has a great reference for the designation of testicular cancer prevention and health policies in various regions.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank the Global Burden of Disease Study for collection of the GBD data.
Funding: This study was funded by the National Natural Science Foundation of China (No. 91746205), IBM Global University Programs (2018 IBM Shared University Research Award), Tianjin Technical Expert Project and Hospital Innovation & Management Research Project of Tianjin Medical University (No. 2019YG08).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.02.22). The authors have no conflicts of interest to declare.
References
- 1.Tandstad T, Dahl O, Cohn-Cedermark G, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. J Clin Oncol 2009;27:2122-8. Erratum in: J Clin Oncol. 2009 Jul 1;27(19):3263; J Clin Oncol. 2010 Mar 10;28(8):1438. Dosage error in article text. 10.1200/JCO.2008.18.8953 [DOI] [PubMed] [Google Scholar]
- 2.Magelssen H, Brydøy M, Fosså SD. The effects of cancer and cancer treatments on male reproductive function. Nat Clin Pract Urol 2006;3:312-22. 10.1038/ncpuro0508 [DOI] [PubMed] [Google Scholar]
- 3.Kim C, McGlynn KA, McCorkle R, et al. Sexual functioning among testicular cancer survivors: a case-control study in the U.S. J Psychosom Res 2012;73:68-73. 10.1016/j.jpsychores.2012.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pühse G, Wachsmuth JU, Kemper S, et al. Chronic pain has a negative impact on sexuality in testis cancer survivors. J Androl 2012;33:886-93. 10.2164/jandrol.110.012500 [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 6.Ghazarian AA, Kelly SP, Altekruse SF, et al. Future of testicular germ cell tumor incidence in the United States: forecast through 2026. Cancer 2017;123:2320-8. 10.1002/cncr.30597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministry of Health (2017). Cancer: Historical summary 1948-2015. Available online: https://www.health.govt.nz/publication/cancer-historical-summary-1948-2015
- 8.Association of the Nordic Cancer Registries (2018). Cancer stat fact sheets: Nordic countries- Testis. Available online: http://www-dep.iarc.fr/NORDCAN/english/frame.asp
- 9.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1260-344. 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzmaurice C, Allen C, Barber RM, et al. Global Burden of Disease Cancer Collaboration . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioral, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:2287-323. 10.1016/S0140-6736(15)00128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151-210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Global Burden of Disease Cancer Collaboration , Fitzmaurice C, Dicker D, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505-27. 10.1001/jamaoncol.2015.0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens GA, Alkema L, Black RE, et al. GATHER Working Group . Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. PLoS Med 2016;13:e1002056. 10.1371/journal.pmed.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioral, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1345-422. 10.1016/S0140-6736(17)32366-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Burden of Disease Cancer Collaboration , Fitzmaurice C, Akinyemiju TF, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2018;4:1553-68. 10.1001/jamaoncol.2018.2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730-56. 10.1016/S1470-2045(08)70179-7 [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. SEER Stat Fact Sheets: Testis Cancer. Available online: http://seer.cancer.gov/statfacts/html/testis.html. Accessed May 28, 2015.
- 21.Cook MB, Akre O, Forman D, et al. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer-experiences of the son. Int J Epidemiol 2010;39:1605-18. 10.1093/ije/dyq120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith ZL, Werntz RP, Eggener SE. Testicular Cancer: Epidemiology, Diagnosis, and Management. Med Clin North Am 2018;102:251-64. 10.1016/j.mcna.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 23.Dogra VS, Gottlieb RH, Oka M, et al. Sonography of the scrotum. Radiology 2003;227:18-36. 10.1148/radiol.2271001744 [DOI] [PubMed] [Google Scholar]
- 24.Hemminki K, Li X. Familial risk in testicular cancer as a clue to a heritable and environmental aetiology. Br J Cancer 2004;90:1765-70. 10.1038/sj.bjc.6601714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med 1997;337:242-53. 10.1056/NEJM199707243370406 [DOI] [PubMed] [Google Scholar]
- 26.Turnbull C, Rahman N. Genome-wide association studies provide new insights into the genetic basis of testicular germ-cell tumour. Int J Androl 2011;34:e86-96; discussion e96-7. [DOI] [PubMed]
- 27.Greene MH, Kratz CP, Mai PL, et al. Familial testicular germ cell tumors in adults: 2010 summary of genetic risk factors and clinical phenotype. Endocr Relat Cancer 2010;17:R109-21. 10.1677/ERC-09-0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson BE, Benton B, Jing J, et al. Risk factors for cancer of the testis in young men. Int J Cancer 1979;23:598. 10.1002/ijc.2910230503 [DOI] [PubMed] [Google Scholar]
- 29.Petridou E, Roukas KI, Dessypris N, et al. Baldness and other correlates of sex hormones in relation to testicular cancer. Int J Cancer 1997;71:982. [DOI] [PubMed] [Google Scholar]
- 30.Coupland CA, Forman D, Chilvers CE, et al. Maternal risk factors for testicular cancer: a population-based case-control study(UK). Cancer Causes Control 2004;15:277. 10.1023/B:CACO.0000024257.49409.1f [DOI] [PubMed] [Google Scholar]
- 31.Møller H, Skakkebaek NE. Testicular cancer and cryptorchidism in relation to prenatal factors: case-control studies in Denmark. Cancer Causes Control 1997;8:904. 10.1023/A:1018472530653 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Graubard BI, Klebanoff MA. Maternal hormone levels among populations at high and low risk of testicular germ cell cancer. Br J Cancer 2005;92:1787. 10.1038/sj.bjc.6602545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aschim EL, Grotmol T, Tretli S, et al. Is there an association between maternal weight and the risk of testicular cancer? An epidemiologic study of Norwegian data with emphasis on World War II. Int J Cancer 2005;116:327. 10.1002/ijc.21044 [DOI] [PubMed] [Google Scholar]
- 34.Swerdlow AJ, De Stavola BL, Swanwick MA, et al. Risks of breast and testicular cancers in young adult twins in England and Wales:evidence on prenatal and genetic aetiology. Lancet 1997;350:1723. 10.1016/S0140-6736(97)05526-8 [DOI] [PubMed] [Google Scholar]
- 35.Swerdlow AJ, Skeet RG. Occupational associations of testicular cancer in southeast England. Br J Ind Med 1988;45:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as