Abstract
Background
To compare the diagnostic performance of 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography (68Ga-PSMA PET/CT) with multi-parametric magnetic resonance imaging (mpMRI) on extracapsular extension (ECE) and seminal vesicle invasion (SVI) in primary prostate cancer and its impact on therapeutic decisions.
Methods
We retrospectively enrolled 54 patients with both PET/CT and mpMRI before radical prostatectomy. Diagnostic performance of mpMRI, PET/CT and their combination (com-MRI/PET) on ECE and SVI on a patient basis were analyzed. The impact of additional PET/CT scanning on therapeutic decisions were presented.
Results
Among the 54 patients, 17 had tumor limited in the prostate gland, 25 only had ECE and 12 patients had both SVI and ECE on pathology. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of ECE were 54%, 94%, 95%, 48% on mpMRI, 78%, 94%, 97%, 67% on PET/CT and 83%, 88%, 94%, 71% on com-MRI/PET. Both PET/CT and com-MRI/PET had a higher sensitivity than mpMRI on ECE diagnosis (78% vs. 54%, P<0.05 and 83% vs. 54%, P<0.05). No difference was observed between PET/CT and com-MRI/PET (78% vs. 83%, P=0.17). The Sensitivity, specificity, PPV and NPV of SVI were 67%, 93%, 72%, 91% on mpMRI, 75%, 95%, 82%, 93% on PET/CT and 75%, 88%, 64%, 93% on com-MRI/PET. No difference was found between the three scannings. After the additional evaluation of PET/CT, 18.5% (10/54) turned from nerve-sparing surgery to non-nerve sparing surgery.
Conclusions
68Ga-PSMA PET/CT has a higher sensitivity on ECE detection than mpMRI but shows no superiority on SVI.
Keywords: 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography (68Ga-PSMA PET/CT), multi-parametric magnetic resonance imaging (mpMRI), prostate cancer, extracapsular extension (ECE), seminal vesicle invasion (SVI)
Introduction
The latest cancer statistics revealed that there were 164,690 new cases and 29,430 death caused by prostate cancer in 2017 in the USA (1). The staging of prostate cancer is essential for categorizing the severity of disease, estimating prognosis and recommending proper treatment (2). For radical prostatectomy (RP), the extent of the primary tumor also decides whether the patient can be subjected to a nerve-sparing surgical technique or is there a necessity to perform an extended pelvic lymph node dissection (3). Digital rectal examination is traditionally performed to stage the prostate cancer before RP, but it cannot precisely evaluate the local extent of primary prostate cancer (4,5). Multi-parametric magnetic resonance imaging (mpMRI) has been commonly applied for the detection of prostate cancer. Despite the high specificity for local prostate cancer staging, its sensitivity of extra-prostatic growth is low and heterogeneous (6).
Prostate-specific membrane antigen (PSMA) is a transmembrane protein which is highly expressed on most of the prostate cancer cells, consistent with the stage and grade of prostate cancer (7). Depending on this, 68Ga-labeled PSMA positron emission tomography/computed tomography (68Ga-PSMA PET/CT) imaging has been developed for the detection of prostate cancer since 2012, showing high sensitivity for early detection of biochemical recurrence after radical treatment (8-11), as well as lymph node and bone metastasis before RP (12,13). Recently, a few articles analyzed its role in primary prostate cancer detection and staging (14,15). However, the performance of 68Ga-PSMA PET/CT in primary prostate cancer staging has never been compared with that of mpMRI in a separate procedure.
Here we compare the effectiveness of mpMRI, 68Ga-PSMA PET/CT (PET/CT) and their combination (com-MRI/PET) in analyzing primary staging including extracapsular extension (ECE) and seminal vesicle invasion (SVI) before RP. Furthermore, the impact of additional PET/CT scanning on therapeutic decisions was analyzed. All the results were correlated with whole-gland histology after RP.
Methods
Patients
Fifty-four consecutive patients who received both mpMRI and 68Ga-PSMA PET/CT scanning before robot-assisted laparoscopic RP were retrospectively enrolled. All patients underwent mpMRI before prostate biopsy, with median interval of 8 days (range, 5–13 days). Patients with biopsy-confirmed prostate cancer received 68Ga-PSMA PET/CT before RP, with a median interval of 13 days (range, 7–31 days) between biopsy and PET/CT. We excluded men diagnosed with lymph node or bone metastasis (n=5) or without a signature of informed consent (n=3) (Figure 1). Treatment decisions were based on mpMRI and PET/CT results. Only patients diagnosed by both mpMRI and PET/CT as T2 finally underwent nerve-sparing surgery. All patients were informed and signed consent for-anonymized evaluation and publication of their data. The retrospective analysis was approved by the Ethics Committee of the Drum Tower Hospital, Medical School of Nanjing University (approval 2017-147-01).
Figure 1.
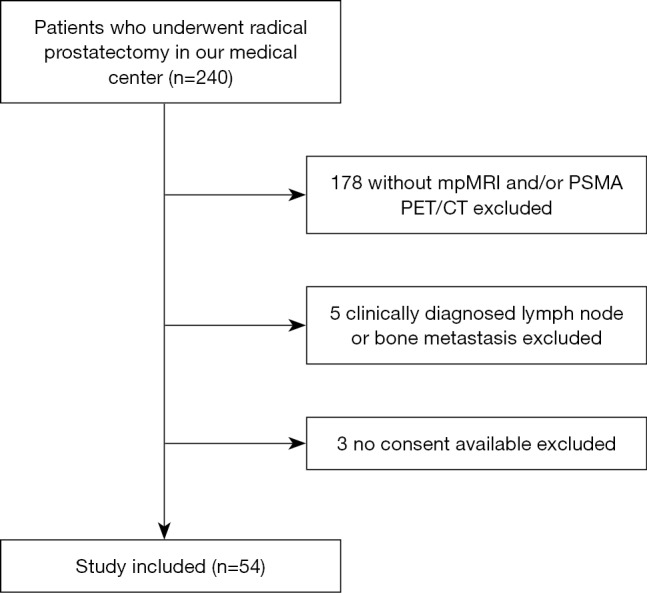
Study flow chart with excluded patients and reason for exclusion. mpMRI, multi-parametric magnetic resonance imaging; PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography/computed tomography.
mpMRI examination
A pelvic mpMRI was acquired using a 3.0-T MR scanner (Achieva 3.0 T TX, Philips Medical Systems, The Netherlands) by a 16-channel phased-array coil as described previously (16). Transverse/coronal/sagittal [18 slices, thickness 3 mm/gap 0.5 mm, repetition time (TR) 3,744 ms, echo time (TE) 120 ms, number of signals acquired 2, resolution 1.49 mm × 1.51 mm] T2-weighted turbo spin-echo (TSE) images were acquired. Diffusion-weighted imaging (DWI) (18 slices, thickness 3 mm, intersection gap 1 mm, TR 925/TE 41 ms, number of signals acquired 1) were acquired with b-factor 0/800/1,500 s/mm2. After the injection of gadolinium, a T1 high-resolution isotropic volume with fat suppression was employed for dynamic contrast enhanced (DCE) images. Mappings of the apparent diffusion coefficient (ADC) were generated from b 0, b 800 and b 1,500 images of DWI using the Philips WorkStation software (Extended Workspace, EWS).
68Ga-PSMA PET/CT examination
68Ga-PSMA PET/CT was performed by a uMI 780 PET-CT scanner [United Imaging Healthcare (UIH), Shanghai, China] 60 minutes after the injection of 68Ga-PSMA-11 (median, 135.72 MBq, range, 126.2–177.6 MBq). For the synthesis of 68Ga-PSMA-11, an ITG semi-automated module (Germany, Munich) was applied as described previously (17). 68Ga-PSMA-11 was stable in vitro, with radiochemical purity was >99% after 2 h of radiolabeling. To reduce tracer activity in the bladder, furosemide was injected 30 minutes before the tracer injection, and patients were asked to void before scanning. For image acquisition, a CT scan (130 keV, 80 mAs) was firstly obtained without using a contrast medium. Static emission scans, corrected for dead time, scatter and decay, were acquired from the vertex to the proximal legs. This required the patient assume 4 bed positions with 2 min per bed position. The images were iteratively reconstructed and included CT-based attenuation correction with the OSEM algorithm using 4 iterations with 8 subsets and Gaussian filtering to an in-plane spatial resolution of 5 mm at full-width at half-maximum.
Image evaluation
Two experienced radiologists intenerated all mpMRI images and all PET/CT were reviewed by two experienced nuclear medicine physicians independently, without the information of the other imaging and final pathological results. Different diagnostic results reached agreement through discussion. The stage of ECE and SVI on mpMRI were analyzed by the criterion described by Prostate Imaging Reporting and Data System Version 2 (PI-RADS V2) (18). The stage on PET/CT was analyzed as previously described (19). In detail, ECE was diagnosed with two criteria: angulated contour of the prostate gland or obliteration of the recto-prostatic angle. SVI was diagnosed if there is a focal or diffuse 68Ga-PSMA ligand accumulation above the background. For the combination of mpMRI and PET/CT, a positive diagnosis was defined as mpMRI positive and/or PET/CT positive while a negative diagnosis was mpMRI negative and PET/CT negative.
Whole-gland pathological evaluation
The whole-mount tissues were firstly fixed by 10% formalin after robotic-assisted RP. After that, two different dyes were applied to their surface to distinguish the side of the tissue (green on the right side and blue dye on the left). Tissue slices were then paraffin embedded, microtome cut into 4–5-mm slices and stained with hematoxylin-eosin. The staging of the tumors was based on the current UICC guidelines, 8th edition, 2017 (2). T staging results based on mpMRI, 68Ga-PSMA PET/CT and com-MRI/PET were correlated with final pathological results.
Statistical analysis
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) on the diagnosis of ECE and SVI were analyzed by Fisher’s exact test and 95% confidence interval (CI) were calculated. Differences in sensitivity and specificity on the diagnosis of ECE and SVI between mpMRI and 68Ga-PSMA PET/CT were compared by an extension to the McNemar test. A P value of 0.05 was used as statistical significance. Statistical evaluation and illustrations were performed using SPSS version 22.0 software (IBM Corp., Armonk, New York, USA).
Results
Patient characteristics
Relevant patient characteristics are summarized in Table 1. The median age of patients was 69 years (range, 55–84 years). The Gleason score (GS) among patients ranged from 6 to 10, of which 11.1% (6/54) were GS 3+3=6, 24.1% (13/54) were GS 3+4=7, 29.6% (16/54) were GS 4+3=7, 16.7% (9/54) were GS 4+4=8 or 5+3=8 and 18.5% (10/54) were Gleason 9–10. The median preoperative PSA level was 13.30 ng/mL (range, 4.04–110.00 ng/mL). Of the 54 patients, 17 (31.5%) had tumor limited in the prostate gland (T2) and 25 (46.3%) had pathological T3a on histopathological work-up. Twelve (22.2%) patients were proven as T3b on final pathology.
Table 1. Characteristics of 54 patients who underwent 68Ga-PSMA PET/CT and mpMRI prior to radical prostatectomy for adenocarcinoma of the prostate.
| Characteristics | Value |
|---|---|
| Age (years), median [range] | 69 [55–84] |
| Initial PSA (ng/dL), median (range) | 13.30 (4.04–110.00) |
| Gleason score, n (%) | |
| 3+3=6 | 6 (11.1) |
| 3+4=7 | 13 (24.1) |
| 4+3=7 | 16 (29.6) |
| 4+4=8/5+3=8 | 9 (16.7) |
| 9–10 point | 10 (18.5) |
| T stage, n (%) | |
| 2 | 17 (31.5) |
| 3a | 25 (46.3) |
| 3b | 12 (22.2) |
| 4 | 0 (0) |
PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography/computed tomography; mpMRI, multi-parametric magnetic resonance imaging; PSA, prostate-specific antigen.
68Ga-PSMA PET/CT improved the diagnostic performance of extra-prostatic expansion of prostate cancer compared with mpMRI
Diagnostic performance of mpMRI, PET/CT and com-MRI/PET on ECE (T3a) and SVI (T3b) are presented in Tables 2,3,S1. Totally, 37 patients had an ECE, with 25 patients had only ECE and 12 had both ECE and SVI. Sensitivity, specificity, PPV and NPV were 54% (95% CI, 37–71%), 94% (95% CI, 71–100%), 95% (95% CI, 76–100%) and 48% (95% CI, 31–66%) for ECE (T3a) on mpMRI, 78% (95% CI, 62–90%), 94% (95% CI, 71–100%), 97% (95% CI, 83–100%) and 67% (95% CI, 45–84%) on 68Ga-PSMA PET/CT and 83% (95% CI, 68–94%), 88% (95% CI, 64–99%), 94% (95% CI, 80–100%) and 71% (95% CI, 47–89%) on com-MRI/PET. Both 68Ga-PSMA PET/CT and com-MRI/PET had a higher sensitivity on ECE detection compared with mpMRI (78% vs. 54%, P=0.0125, 83% vs. 54%, P=0.0009). However, no difference was seen between 68Ga-PSMA PET/CT and com-MRI/PET (78% vs. 83%, P=0.1573). Figure 2 shows an example of T3a disease on pathology, which is positive on 68Ga-PSMA PET/CT but negative on mpMRI. To further analyze the impact of tumor position on the detection ECE, we performed a sub-analysis according to the tumor zonality. The results indicated that com-MRI/PET had a higher sensitivity to detect ECE for lesions in the peripheral zone while PET/CT alone or com-MRI/PET had a higher sensitivity than MRI along for detection of ECE of tumors located in the transition zone. No difference was found for mixed lesions (Table S2).
Table 2. Diagnostic accuracies for tumor expansion using multiparametric magnetic resonance imaging (MRI), 68Ga PSMA PET/CT and PET/MRI.
| Pathology | Tools | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) | Positive predictive value (95% CI) (%) | Negative predictive value (95% CI) (%) |
|---|---|---|---|---|---|
| ECE | MRI | 54 [37–71]*† | 94 [71–100] | 95 [76–100] | 48 [31–66] |
| PET/CT | 78 [62–90]* | 94 [71–100] | 97 [83–100] | 67 [45–84] | |
| PET/MRI | 83 [68–94]† | 88 [64–99] | 94 [80–100] | 71 [47–89] | |
| SVI | MRI | 67 [35–90] | 93 [81–99] | 72 [39–94] | 91 [78–97] |
| PET/CT | 75 [43–95] | 95 [84–99] | 82 [48–98] | 93 [81–99] | |
| PET/MRI | 75 [43–95] | 88 [74–96] | 64 [35–87] | 93 [80–98] |
*, mpMRI vs. PET/CT, P<0.05; †, mpMRI vs. PET/MRI, P<0.05. PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography computed tomography; PET/MRI, the combination of mpMRI and PET/CT; mpMRI, multiparametric magnetic resonance imaging; CI, confidence interval; ECE, extracapsular extension; SVI, seminal vesicle invasion.
Table 3. Diagnostic performance of the mpMRI and 68Ga-PSMA PET/CT on T staging compared to finial whole mount pathological T-stage.
| Pathology | Diagnostic results | mpMRI | PET/CT | PET/MRI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | ||||
| ECE | Positive | 20 | 17 | 29 | 8 | 31 | 6 | ||
| Negative | 1 | 16 | 1 | 16 | 2 | 15 | |||
| SVI | Positive | 8 | 4 | 9 | 3 | 9 | 3 | ||
| Negative | 3 | 39 | 2 | 40 | 5 | 37 | |||
mpMRI, multiparametric magnetic resonance imaging; PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography computed tomography; PET/MRI, the combination of mpMRI and PET/CT; ECE, extracapsular extension; SVI, seminal vesicle invasion.
Table S1. Comparison of sensitivity and specificity for tumor expansion using multiparametric magnetic resonance imaging (MRI), 68Ga-PSMA PET/CT and PET/MRI.
| Pathology | Tools | Sensitivity (95% CI) (%) | P value | Specificity (95% CI) (%) | P value |
|---|---|---|---|---|---|
| ECE | MRI | 54 [37–71] | 0.0125* | 94 [71–100] | 0.4795 |
| PET/CT | 78 [62–90] | 94 [71–100] | |||
| MRI | 54 [37–71] | 0.0009* | 94 [71–100] | 1 | |
| PET/MRI | 83 [68–94] | 88 [64–99] | |||
| PET/CT | 78 [62–90] | 0.1573 | 94 [71–100] | 1 | |
| PET/MRI | 83 [68–94] | 88 [64–99] | |||
| SVI | MRI | 67 [35–90] | 0.3173 | 93 [81–99] | 0.6171 |
| PET/CT | 75 [43–95] | 95 [84–99] | |||
| MRI | 67 [35–90] | 0.3173 | 93 [81–99] | 0.4795 | |
| PET/MRI | 75 [43–95] | 88 [74–96] | |||
| PET/CT | 75 [43–95] | NA | 95 [84–99] | 0.4795 | |
| PET/MRI | 75 [43–95] | 88 [74–96] |
*, P<0.05. PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography/computed tomography; PET/MRI, the combination of mpMRI and PET/CT; mpMRI, multiparametric magnetic resonance imaging; CI, confidence interval; ECE, extracapsular extension; SVI, seminal vesicle invasion.
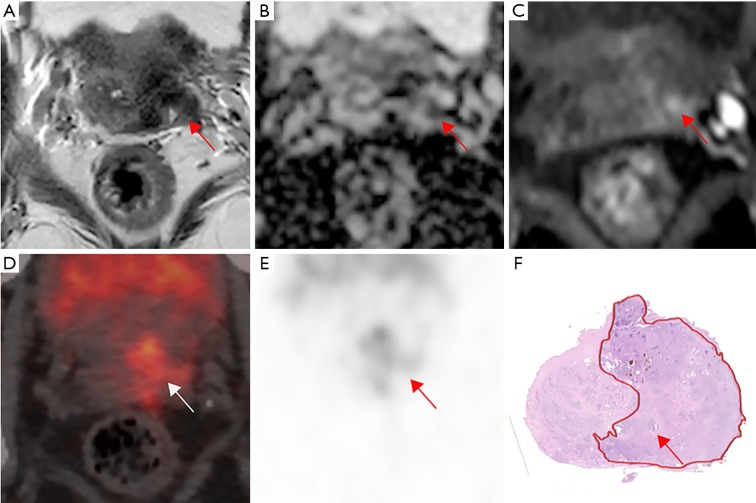
Figure 2.
A 71-year-old patient with a PSA level 15.06 ng/mL and a prostate cancer Gleason score 4+3=7 and pTNM stage T3aN0M0. (A) Transverse T2-weighted images show a lesion with slightly hypo-intense signal in the transitional zone on the right lobe (arrow). (B,C) Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) map show a restricted diffusion on that lesion (arrows). All these results in a Prostate Imaging Reporting and Data System (PI-RADS) scoring 5. No extracapsular extension (ECE) was seen on mpMRI. (D,E) The 68Ga-PSMA PET/CT image shows intense tracer accumulation (SUVmax 30.73, SUVmean 20.48) in the right lobe and an angulated contour of the prostate gland (arrows), consistent with extracapsular extension on whole mount histology (arrows) (F). PSA, prostate-specific antigen; mpMRI, multi-parametric magnetic resonance imaging; PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography/computed tomography.
Table S2. Diagnostic accuracies for tumor expansion with different location using multiparametric magnetic resonance imaging (MRI), 68Ga-PSMA PET/CT and PET/MRI.
| Pathology | Tools | Peripheral zone | Transitional zone | Combined lesions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (95% CI) (%) | P value | Specificity (95% CI) (%) | P value | Sensitivity (95% CI) (%) | P value | Specificity (95% CI) (%) | P value | Sensitivity (95% CI) (%) | P value | Specificity (95% CI) (%) | P value | ||||
| ECE | MRI | 52.6 (28.9–75.6) | 0.1025 | 100.0 (63.1–100.0) | 1 | 45.5 (16.8–76.6) | 0.0455* | 100.0 (59.0–76.6) | 1 | 71.4 (29.0–96.3) | 0.6537 | 100.0 (15.8–100.0) | NA | ||
| PET/CT | 73.7 (48.8–90.0) | 87.5 (47.4–97.7) | 81.8 (48.2–97.8) | 85.7 (42.1–99.6) | 85.7 (42.1–99.6) | 100.0 (15.8–100.0) | |||||||||
| MRI | 52.6 (28.9–75.6) | 0.0253* | 100.0 (96.1–100.0) | 1 | 45.5 (16.8–76.6) | 0.0455* | 100.0 (59.0–76.6) | 1 | 71.4 (29.0–96.3) | 0.1573 | 100.0 (15.8–100.0) | NA | |||
| PET/MRI | 78.9 (54.4–94.0) | 87.5 (47.4–97.7) | 81.8 (48.2–97.8) | 85.7 (42.1–99.6) | 100.0 (59.0–100.0) | 100.0 (15.8–100.0) | |||||||||
| PET/CT | 73.6 (48.8–90.0) | 0.3173 | 87.5 (47.4–97.7) | NA | 81.8 (48.2–97.8) | NA | 85.7 (42.1–99.6) | NA | 85.7 (42.1–99.6) | 0.3173 | 100.0 (15.8–100.0) | NA | |||
| PET/MRI | 78.9 (54.4–94.0) | 87.5 (47.4–97.7) | 81.8 (48.2–97.8) | 85.7 (42.1–99.6) | 100.0 (59.0–100.0) | 100.0 (15.8–100.0) | |||||||||
| SVI | MRI | 75.0 (19.4–99.4) | NA | 95.7 (78.1–99.9) | 1 | 66.7 (9.4–99.2) | NA | 86.7 (59.5–98.3) | 0.6171 | 60.0 (14.7–94.7) | 0.3173 | 100.0 (39.8–100.0) | NA | ||
| PET/CT | 75.0 (19.4–99.4) | 100.0 (29.2–100.0) | 66.7 (9.4–99.2) | 86.7 (59.5–98.3) | 80.0 (28.4–99.5) | 100.0 (39.8–100.0) | |||||||||
| MRI | 75.0 (19.4–99.4) | NA | 95.7 (78.1–99.9) | NA | 66.7 (9.4–99.2) | NA | 86.7 (59.5–98.3) | 0.4795 | 60.0 (14.7–94.7) | 0.3173 | 100.0 (39.8–100.0) | NA | |||
| PET/MRI | 75.0 (19.4–99.4) | 95.7 (78.1–99.9) | 66.7 (9.4–99.2) | 73.3 (50.1–93.2) | 80.0 (28.4–99.5) | 100.0 (39.8–100.0) | |||||||||
| PET/CT | 75.0 (19.4–99.4) | NA | 100.0 (29.2–100.0) | 1 | 66.7 (9.4–99.2) | NA | 86.7 (59.5–98.3) | 0.4795 | 60.0 (14.7–94.7) | NA | 100.0 (39.8–100.0) | NA | |||
| PET/MRI | 75.0 (19.4–99.4) | 95.7 (78.1–99.9) | 66.7 (9.4–99.2) | 73.3 (50.1–93.2) | 80.0 (28.4–99.5) | 100.0 (39.8–100.0) | |||||||||
*, P<0.05. PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography/computed tomography; PET/MRI, the combination of mpMRI and PET/CT; mpMRI, multiparametric magnetic resonance imaging; CI, confidence interval; ECE, extracapsular extension; SVI, seminal vesicle invasion; NA, not available.
For the diagnosis of SVI (T3b), sensitivity, specificity, PPV and NPV were 67% (95% CI, 35–90%), 93% (95% CI, 71–100%), 72% (95% CI, 39–94%) and 91% (95% CI, 78–97%) on mpMRI, 75% (95% CI, 43–95%), 95% (95% CI, 84–99%), 82% (95% CI, 48–98%) and 93% (95% CI, 81–99%) on PET/CT and 75% (95% CI, 43–95%), 88% (95% CI, 74–96%), 64% (95% CI, 35–87%) and 93% (95% CI, 80–98%) on com-MRI/PET. No statistically significant difference was observed between mpMRI, PET/CT and com-MRI/PET (Table S1). Figure 3 shows an example of T3b disease on pathology, who is recognized both on 68Ga-PSMA PET/CT and mpMRI. For SVI detection, no difference was observed in all subgroups according to tumor zonality (Table S2).
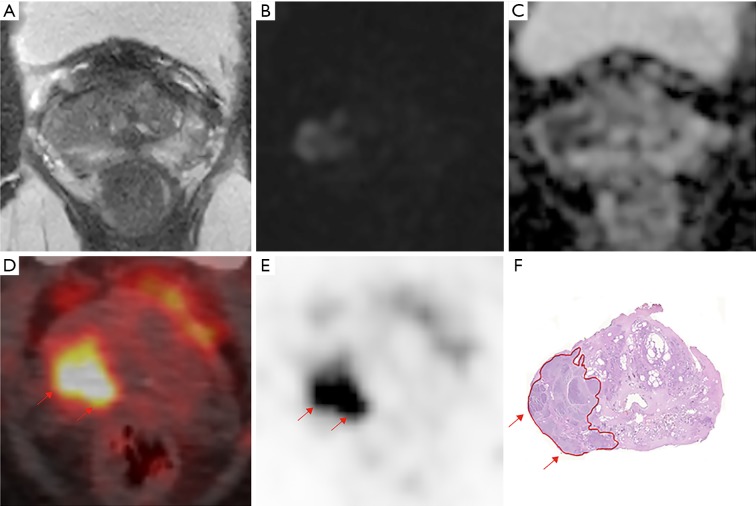
Figure 3.
A 71-year-old patient with a PSA level 13.76 ng/mL and a prostate cancer Gleason score 4+3=7 and TNM stage T3bN0M0. (A) Transverse T2-weighted images showed hypo intense signal on the left seminal vesicle. (B) Diffusion-weighted imaging (DWI) shows a restricted diffusion on the left seminal vesicle, with an enhancement on dynamic contrast-enhanced (C). All these leads to a diagnosis of seminal vesicle invasion (SVI) on mpMRI. (D,E) The 68Ga-PSMA PET/CT image shows intense tracer accumulation (SUVmax 9.27, SUVmean 2.18) on the left seminal vesicle (arrows) (F). Whole mount histology shows seminal vesicle invasion on pathology (arrows). PSA, prostate-specific antigen; mpMRI, multi-parametric magnetic resonance imaging; PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography/computed tomography.
68Ga-PSMA PET/CT changed treatment decisions on nerve-sparing surgery
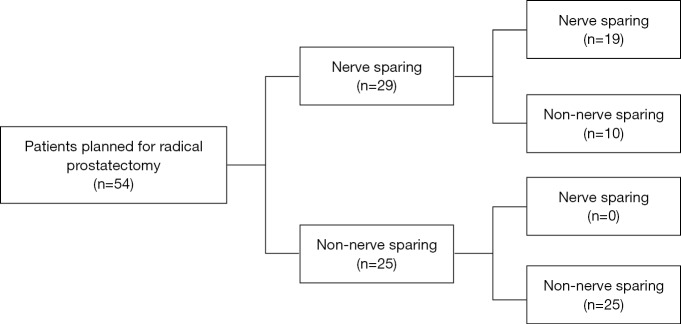
The treatment decision flow-chart was presented in Figure 4. Among the 54 patients planned for RP, 29 patients were assigned for nerve-sparing surgery and the rest 25 were non-nerve sparing surgery on mpMRI evaluation. With additional 68Ga-PSMA PET/CT scanning, 10 (18.5%) of the patients who were planned for nerve sparing surgery turned to non-nerve sparing surgery because of the additional diagnosis of ECE (nine patients) or SVI (one patients) on 68Ga-PSMA PET/CT. No decision was seen changed for patients planned for non-nerve sparing surgery. Final pathology results showed that nine of the patients who turned to non-nerve sparing surgery had extra-prostatic expansion and one was proven as pathological T2 stage.
Figure 4.
Therapeutic decision flow chart based on mpMRI alone and additional PSMA-PET/CT scanning, with final pathological results. Dark grey edging represents the patient who were reassigned after additional PSMA-PET/CT evaluation. mpMRI, multi-parametric magnetic resonance imaging; PSMA, prostate-specific membrane antigen; PET/CT, positron emission tomography/computed tomography.
Discussion
This clinical study demonstrated that 68Ga-PSMA PET/CT had a better diagnostic performance than mpMRI on the detection of ECE and similar performance on SVI, which could provide more information for therapeutic decision.
For patients with prostate cancer who are eligible for RP, it is always the major consideration for surgeons to achieve a balance between long-term cancer control and recovery of sexual and urinary function (20). Fortunately, with the technical refinements, the surgical complication continued to decrease (20). The accurate assessment of ECE and SVI is currently critical for urologist to decide whether the patient can receive a nerve-sparing surgical technique (3). However, the exact T staging based on current technology is not reliable enough (21). mpMRI, with high tissue contrast and sophisticated tissue characterization, has been explored for T staging for nearly one decade. Pooled data from a meta-analysis demonstrated that mpMRI has a low sensitivity on ECE (0.57, 95% CI, 0.49–0.64) but a high specificity (0.91, 95% CI, 0.88–0.93) (6), which is similar with our result (sensitivity, 54%, 95% CI, 37–71%, specificity 94%, 95% CI, 71–100%).
As 68Ga-PSMA PET/CT provides functional and molecular information, it is expected that 68Ga-PSMA PET/CT might have an outstanding performance not only on biochemical recurrence but also on primary prostate cancer detection and evaluation. Our results demonstrated that 68Ga-PSMA PET/CT could improve the diagnosis performance of ECE compared with mpMRI, with a significantly higher sensitivity (78% vs. 54%, P=0.013) and similar specificity (94% vs. 94%). This result is consistent with the results from the other two retrospective studies, which showed a sensitivity of 90% for the detection of ECE (15) and 71% accuracy for the detection of extraprostatic tumor spread by using 68Ga-PSMA PET/CT (22). No difference was observed between mpMRI and 68Ga-PSMA PET/CT on SVI assessment. The better performance of 68Ga-PSMA PET/CT allowed doctors to make a better therapeutic decision for nerve-sparing surgery, resulting in treatment strategy change in (10/54) 18.5% patients, among which 90.0% (9/10) were correctly assigned. As 68Ga-PSMA PET/CT has a better performance on lymph node metastasis than mpMRI before RP as well (12), it is possible that 68Ga-PSMA PET/CT has a potential value for local risk stratification and alteration of surgical approaches. Due to the better performance on T staging, PSMA PET/CT could be considered for those patients eligible for RP since precise clinical staging is the basis for decision making of surgery as well as the surgical technique, such as nerve sparing. However, the health economics and availability of PSMA PET/CT are still concerns.
A retrospective study demonstrated that PET/MRI has a higher sensitivity on ECE detection compared with mpMRI (23), which consistent with our results that com-MRI/PET has a higher sensitivity than mpMRI alone. A preoperative study explored the diagnostic value of the PET/MRI in primary prostate cancer staging and it showed an accuracy rates for predicting T3a stage of 79% (sensitivity 66.7%, specificity 91.5%) and T3b 86% (sensitivity 58%, specificity 96%) (24). Compared with PET/MRI, 68Ga-PSMA PET/CT alone in our study shows a similar performance both on T3a (sensitivity, 78% vs. 83% and specificity, 94% vs. 88%) and T3b (sensitivity, 75% vs. 75% and specificity, 95% vs. 88%). No difference was observed between PET/CT alone and PET/MRI both on ECE and SVI diagnosis. As PET/MRI remains expensive and unavailable for routine use, our study illustrated that 68Ga-PSMA PET/CT alone could be promising in primary staging for prostate cancer before treatment management.
There are some limitations in our study. First, patients included in our study were only those with limited ECE on imaging as only these patients are eligible for RP, which may be responsible for the low sensitivity for ECE and SVI detection. Second, the role of 68Ga-PSMA PET/CT in clinical practice and the impact of therapeutic decisions changed by PET/CT on patient outcomes need to be confirmed by further followed-up.
Conclusions
68Ga-PSMA PET/CT has a higher sensitivity on ECE detection than mpMRI but shows no superiority on SVI. This superiority of 68Ga-PSMA PET/CT may make up for the low sensitivity of ECE detection to help surgeons to select patients eligible for nerve sparing procedures to achieve both tumor control and the preservation of potency and continence. Further studies to explore the role of 68Ga-PSMA PET/CT on T staging in primary prostate cancer are warranted.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by a grants from the National Natural Science Foundation of China (No. 81772710, 81572519, 81602232, 81802535), Natural Science Foundation of Jiangsu Province (No. BK20150112, BK20150097), Nanjing Medical Science and technique Development Foundation (No. QRX17128) and Nanjing Health Distinguished Youth Fund (No. JQX16025), The Project of Invigorating Health Care through Science, Technology and Education, Jiangsu Provincial Key Medical Discipline (Laboratory) (No. ZDXKB2016014).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The retrospective analysis was approved by the Ethics Committee of the Drum Tower Hospital, Medical School of Nanjing University (approval 2017-147-01).
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.03.06). The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Buyyounouski MK, Choyke PL, McKenney JK, et al. Prostate cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:245-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeb S, Smith ND, Roehl KA, et al. Intermediate-term potency, continence, and survival outcomes of radical prostatectomy for clinically high-risk or locally advanced prostate cancer. Urology 2007;69:1170-5. 10.1016/j.urology.2007.02.054 [DOI] [PubMed] [Google Scholar]
- 4.Grossfeld GD, Chang JJ, Broering JM, et al. Under staging and under grading in a contemporary series of patients undergoing radical prostatectomy: results from the Cancer of the Prostate Strategic Urologic Research Endeavor database. J Urol 2001;165:851-6. 10.1016/S0022-5347(05)66543-3 [DOI] [PubMed] [Google Scholar]
- 5.Mullerad M, Hricak H, Kuroiwa K, et al. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol 2005;174:2158-63. 10.1097/01.ju.0000181224.95276.82 [DOI] [PubMed] [Google Scholar]
- 6.de Rooij M, Hamoen EH, Witjes JA, et al. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur Urol 2016;70:233-45. 10.1016/j.eururo.2015.07.029 [DOI] [PubMed] [Google Scholar]
- 7.Bostwick DG, Pacelli A, Blute M, et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma. Cancer 1998;82:2256-61. [DOI] [PubMed] [Google Scholar]
- 8.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med 2015;56:668-74. 10.2967/jnumed.115.154153 [DOI] [PubMed] [Google Scholar]
- 9.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2014;41:11-20. 10.1007/s00259-013-2525-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015;42:197-209. 10.1007/s00259-014-2949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachpekidis C, Kopka K, Eder M, et al. 68Ga-PSMA-11 Dynamic PET/CT Imaging in Primary Prostate Cancer. Clin Nucl Med 2016;41:e473-9. 10.1097/RLU.0000000000001349 [DOI] [PubMed] [Google Scholar]
- 12.Budäus L, Leyh-Bannurah SR, Salomon G, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol 2016;69:393-6. 10.1016/j.eururo.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 13.Sachpekidis C, Bäumer P, Kopka K, et al. 68Ga-PSMA PET/CT in the evaluation of bone metastases in prostate cancer. Eur J Nucl Med Mol Imaging 2018;45:904-12. 10.1007/s00259-018-3936-0 [DOI] [PubMed] [Google Scholar]
- 14.Simopoulos DN, Natarajan S, Jones TA, et al. Targeted Prostate Biopsy Using (68)Gallium PSMA-PET/CT for Image Guidance. Urol Case Rep 2017;14:11-4. 10.1016/j.eucr.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Klot CJ, Merseburger AS, Boker A, et al. (68)Ga-PSMA PET/CT Imaging Predicting Intraprostatic Tumor Extent, Extracapsular Extension and Seminal Vesicle Invasion Prior to Radical Prostatectomy in Patients with Prostate Cancer. Nucl Med Mol Imaging 2017;51:314-22. 10.1007/s13139-017-0476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Zang S, Zhang C, et al. Comparison of (68)Ga-PSMA-11 PET-CT with mpMRI for preoperative lymph node staging in patients with intermediate to high-risk prostate cancer. J Transl Med 2017;15:230. 10.1186/s12967-017-1333-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eder M, Schafer M, Bauder-Wust U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 2012;23:688-97. 10.1021/bc200279b [DOI] [PubMed] [Google Scholar]
- 18.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. 10.1016/j.eururo.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uprimny C, Kroiss AS, Decristoforo C, et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging 2017;44:941-9. 10.1007/s00259-017-3631-6 [DOI] [PubMed] [Google Scholar]
- 20.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function ("trifecta"). Urology 2005;66:83-94. 10.1016/j.urology.2005.06.116 [DOI] [PubMed] [Google Scholar]
- 21.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 22.Fendler WP, Schmidt DF, Wenter V, et al. 68Ga-PSMA PET/CT Detects the Location and Extent of Primary Prostate Cancer. J Nucl Med 2016;57:1720-5. 10.2967/jnumed.116.172627 [DOI] [PubMed] [Google Scholar]
- 23.Muehlematter UJ, Burger IA, Becker AS, et al. Diagnostic Accuracy of Multiparametric MRI versus 68Ga-PSMA-11 PET/MRI for Extracapsular Extension and Seminal Vesicle Invasion in Patients with Prostate Cancer. Radiology 2019;293:350-8. 10.1148/radiol.2019190687 [DOI] [PubMed] [Google Scholar]
- 24.Grubmüller B, Baltzer P, Hartenbach S, et al. PSMA Ligand PET/MRI for Primary Prostate Cancer: Staging Performance and Clinical Impact. Clin Cancer Res 2018;24:6300-7. 10.1158/1078-0432.CCR-18-0768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as