Abstract
The validity and clinical utility of the concept of “clinical high risk” (CHR) for psychosis have so far been investigated only in risk‐enriched samples in clinical settings. In this population‐based prospective study, we aimed – for the first time – to assess the incidence rate of clinical psychosis and estimate the population attributable fraction (PAF) of that incidence for preceding psychosis risk states and DSM‐IV diagnoses of non‐psychotic mental disorders (mood disorders, anxiety disorders, alcohol use disorders, and drug use disorders). All analyses were adjusted for age, gender and education. The incidence rate of clinical psychosis was 63.0 per 100,000 person‐years. The mutually‐adjusted Cox proportional hazards model indicated that preceding diagnoses of mood disorders (hazard ratio, HR=10.67, 95% CI: 3.12‐36.49), psychosis high‐risk state (HR=7.86, 95% CI: 2.76‐22.42) and drug use disorders (HR=5.33, 95% CI: 1.61‐17.64) were associated with an increased risk for clinical psychosis incidence. Of the clinical psychosis incidence in the population, 85.5% (95% CI: 64.6‐94.1) was attributable to prior psychopathology, with mood disorders (PAF=66.2, 95% CI: 33.4‐82.9), psychosis high‐risk state (PAF=36.9, 95% CI: 11.3‐55.1), and drug use disorders (PAF=18.7, 95% CI: –0.9 to 34.6) as the most important factors. Although the psychosis high‐risk state displayed a high relative risk for clinical psychosis outcome even after adjusting for other psychopathology, the PAF was comparatively low, given the low prevalence of psychosis high‐risk states in the population. These findings provide empirical evidence for the “prevention paradox” of targeted CHR early intervention. A comprehensive prevention strategy with a focus on broader psychopathology may be more effective than the current psychosis‐focused approach for achieving population‐based improvements in prevention of psychotic disorders.
Keywords: Psychosis, ultra‐high risk, clinical high risk, mood disorders, drug use disorders, early intervention, prevention, at risk mental states
Early intervention in psychosis has been an active area of investigation in the mental health field over the past quarter century. Compelling evidence indicates that specialized early intervention services for first‐episode psychosis yield better short‐term clinical outcomes in all measurable domains compared to usual treatment 1 . In addition, it has been suggested that shortening the duration of untreated psychosis leads to a better prognosis over the course of the illness 2 . The field has thus moved forward with the idea of intervening even earlier by detecting psychosis at the preclinical phase of “ultra‐high risk” (UHR), also known as “clinical high risk” (CHR).
Over the last decade, the validity and clinical utility of the CHR paradigm have been widely investigated in help‐seeking participants sampled in clinical settings (risk‐enriched samples) 3 . The CHR paradigm relies on the frequency and severity of positive psychotic symptoms to identify the at‐risk state and determine the risk of transition to psychosis 3 .
Early studies reported up to 40% transition rates in CHR samples, but these rates consistently decreased as data accumulated over time, with recent meta‐analytical estimates showing less than half of the initially reported rates: 15% over a mean period of 38 months 4 , or 4.7% per year. This sizeable reduction in the transition rates may be due to a dilution effect, which is the by‐product of the increased awareness of subtle psychotic states and broader outreach of early intervention services, leading to an increase in self‐referrals, and thereby inflating false positives in more recent CHR samples.
Following our critical perspective papers on the CHR concept3, 5, an intense debate has started, splitting the field into proponents6, 7, 8, opponents9, 10, 11, 12, and those with ambivalent attitudes toward that concept13, 14, 15, 16.
In parallel with the growing interest in understanding early stages of psychopathology for early detection and intervention in clinical settings, the psychosis phenotype has been widely studied in general population datasets.
These population‐based epidemiological studies have revealed two important findings. First, subtle positive psychotic experiences (PEs) are not as rare as once assumed, with prevalence rates varying between 5 and 8% 17 . Second, PEs are temporally associated with help‐seeking 18 , suicidal behavior19, 20, poor functioning21, 22, decline in cognitive capacity 23 , affective dysregulation, and a multitude of mental disorders, including but not limited to psychosis spectrum disorder24, 25, 26. In that sense, PEs in the general population appear to be clinically valuable as a severity marker, but they do not imply diagnostic specificity.
With the exception of the cross‐sectional Bern Epidemiological At‐Risk (BEAR) study, these two lines of research – clinical and population‐based – have yet to be crossed. Particularly relevant is the issue of help‐seeking behavior of individuals, which is included in the CHR concept but not in the population studies of PEs. The BEAR study demonstrated that the CHR is not a frequent but a clinically relevant state, which is associated with increased odds for present mental disorder diagnosis and impaired functioning 27 . Further, the CHR entity shares the same etiological factors with PEs in community studies and psychotic disorders in the clinical samples, providing support for the notion of etiological continuity across the psychosis spectrum.
Although the findings from the cross‐sectional BEAR study may provide some insight into the characteristics of the CHR state in an epidemiologically representative sample, the core issue of progression of psychosis in the framework of the CHR‐transition paradigm has not been longitudinally tested to date in an unbiased general population cohort.
In this study, we aimed to explore the notions of “risk” and “transition” in the general population, for the first time, by estimating the population attributable fraction (PAF) of clinical psychosis incidence (the proportion of clinical psychosis outcome that would have been avoided, had the risk factors been eliminated) for the preceding psychosis risk states and DSM‐IV diagnoses of non‐psychotic mental disorders.
METHODS
Study cohort
The Netherlands Mental Health Survey and Incidence Study‐2 (NEMESIS‐2) was designed to investigate the prevalence, incidence, course and consequences of mental disorders in the Dutch general population. The study was approved by the Medical Ethics Review Committee for Institutions on Mental Health Care, and written informed consent was collected from participants at each wave28, 29.
A multistage random sampling procedure was applied to ensure sample representativeness in regard to age (between 18 and 65 years), region, as well as population density. Participants were excluded if they were not proficient in Dutch.
The NEMESIS‐2 cohort includes four waves. The baseline data (T0) were assessed from 2007 to 2009, and were followed up at year 3 (T1), year 6 (T2) and year 9 (T3). The first wave (T0) enrolled 6,646 participants (response rate 65.1%; average interview duration: 95 min). Response rates at T1, T2 and T3 were 80.4% (N=5,303; average interview duration: 84 min), 87.8% (N=4,618; average interview duration: 83 min), and 86.8% (N=4,007; average interview duration: 102 min), respectively 30 .
Non‐clinician, trained interviewers applied the Composite International Diagnostic Interview (CIDI) version 3.031, 32 and additional questionnaires during home visits. Rates at baseline reflect lifetime occurrence; rates at T1, T2 and T3 reflect 3‐year interval occurrence. Attrition between T0 and T3 was not significantly associated with any of the individual 12‐month mental disorders at T0 after controlling for socio‐demographic characteristics 33 .
Psychosis risk strata
In accordance with the clinical high‐risk framework 3 and previous analyses conducted in the NEMESIS‐2 cohort34, 35, psychosis risk strata were defined based on the degree of positive psychotic symptomatology, help‐seeking attempt, antipsychotic treatment, and service use and admission for psychotic symptomatology.
At each time point, positive psychotic symptoms were assessed using a 20‐item binary‐response questionnaire that is based on CIDI 1.1 and specifically developed for evaluating psychotic symptoms36, 37, since previous studies have demonstrated that earlier CIDI versions were not adequately capturing positive psychotic symptomatology. Positive reports (positive response to at least one item) were reassessed and validated over a clinical telephone interview conducted by trained graduate psychologists and discussed with a clinically experienced psychiatrist 38 , and participants were asked whether they had sought help for these symptoms. At each time point, antipsychotic prescription, service use and admission were explored using an adaptation of the self‐constructed NEMESIS‐1 questionnaire 39 .
Psychosis risk strata consisted of the following non‐overlapping categories: reference group (no psychosis expression), low‐risk (endorsement of a single positive psychotic item that did not require help‐seeking or treatment), moderate‐risk (endorsement of multiple positive psychotic items that did not require help‐seeking or treatment), high‐risk (endorsement of at least one positive psychotic item that required help‐seeking but not antipsychotic treatment or admission), and clinical psychosis (endorsement of at least one positive psychotic item that required help‐seeking and antipsychotic treatment or admission to a health care service). The primary outcome of the study was the category of clinical psychosis. The low‐risk, moderate‐risk, and high‐risk strata served as risk states.
Preceding diagnosis of DSM‐IV mental disorders
The CIDI 3.0 31 was used to assess the following four domains of DSM‐IV mental disorders at each follow‐up visit (diagnosis over the last 3‐year period, such that T1 assessment covers the period between T0 and T1; T2 assessment covers the period from T1 to T2, and so on): mood disorders (major depressive disorder, bipolar disorder, dysthymia); anxiety disorders (social phobia, specific phobia, panic disorder, generalized anxiety disorder, agoraphobia without panic disorder); alcohol use disorders (alcohol abuse and dependence); and drug use disorders (drug abuse and dependence).
Statistical analyses
Analyses were conducted using Stata version 16.0. Participants diagnosed with psychotic disorders (N=43, 0.7%) or bipolar disorder I (N=73, 1.1%) at baseline were excluded from the analysis.
A priori defined psychosis risk strata were validated by using cumulative measures of environmental and genetic liability to schizophrenia.
Adopting our previously validated estimates for constructing cumulative environmental load in a Dutch cohort (GROUP) 40 , we generated the exposome score for schizophrenia (ES‐SCZ) by summing log‐odds weighted environmental exposures, including cannabis use, hearing impairment, winter birth, and five childhood adversity domains (sexual, physical and psychological abuse, emotional neglect and bullying). Analyses were carried out using the dichotomous environmental risk state: the highest quartile, ES‐SCZ >75%, was considered the binary environmental vulnerability for schizophrenia, guided by the definition in our previous study (hereafter: ES‐SCZ75) 41 .
The validation of the psychosis risk strata using polygenic risk score for schizophrenia (PRS‐SCZ) was performed in the genotyped sample (N=3,104). Analyses were carried out using the molecular genetic risk state, guided by the definition in our previous study 41 : the highest quartile of PRS‐SCZ >75% was considered the binary genetic liability for schizophrenia (hereafter: PRS‐SCZ75).
Multinomial logistic regression models using the MLOGIT command were performed to analyze the association of psychosis risk strata (“no‐risk” group as the reference) with ES‐SCZ75 and PRS‐SCZ75, respectively. Consistent with our previous work in NEMESIS‐2, the validation analysis of the strata included observations from all assessment points, that were analyzed multi‐cross‐sectionally in the “long format” (each participant contributing four observations: T0, T1, T2 and T3). To correct for the clustering of multiple observations within participants, the CLUSTER option was used to estimate cluster‐robust standard errors (SEs).
The relative risk ratios (RRRs) at each psychosis risk stratum for ES‐SCZ75 and PRS‐SCZ75 were compared using the Wald test. All analyses were adjusted for gender, age (continuous), and four‐level education (1‐ primary school, 2‐ lower secondary education, 3‐ higher secondary education, 4‐ higher professional education). Analyses of PRS‐SCZ75 were additionally corrected for population stratification adjusted using the first three principal components.
The crude incidence rates with 95% CIs of each psychosis risk stratum per 100,000 person‐years were estimated in participants with at least one follow‐up interview. Two‐sided exact significance tests were applied to compare incidence rates over and below 35 years of age at the study entry.
The Cox proportional hazards models, with the time‐on‐study as the time scale over the whole study period from T0 to T3, were used to estimate the adjusted (age, gender and education) and multivariable adjusted hazard ratios (HRs) and 95% CIs for the associations of clinical psychosis outcome with the time‐varying factors of preceding psychosis risk states and diagnoses of anxiety, mood, alcohol use, and drug use disorders, respectively.
Efron's method was used for handling ties 42 . To take into account clustering of multiple observations within participants, a robust Hubert/White sandwich estimator was applied 43 . The proportional‐hazards assumptions were confirmed using the Schoenfeld residuals and −ln(−ln[survival]) plots, also adjusted for covariates 44 . Potential bias due to unmeasured confounders was assessed using the E‐value, which is the minimum strength of association that an unmeasured confounder must have with both the exposure and the outcome to negate the observed association 45 .
By using the PUNAFCC command 46 with the UNCONDITIONAL option that accounts for the sampling variability of the covariates, the attributable fraction and the PAF with 95% CIs for each risk factor were estimated. Under the assumption that the different risk groups are causally associated with the clinical psychosis outcome, the PAF shows the proportion of clinical psychosis disease burden that might be prevented if the risk were eliminated 47 . The nominal significance threshold was set two‐sided at p=0.05.
RESULTS
Table 1 shows the demographic features and the frequency of preceding psychosis risk states and DSM‐IV diagnoses of non‐psychotic mental disorders (as assessed at T0) in participants with at least one follow‐up interview (N=5,303).
Table 1.
Sample characteristics (N=5,303 participants with at least one follow‐up interview)
| Age at T1 (years, mean±SD) | 47.7±12.4 |
|---|---|
| Gender (% female) | 55.1 |
| Education at T1 (%) | |
| Primary school | 4.3 |
| Lower secondary | 25.9 |
| Higher secondary | 32.6 |
| Higher professional | 37.2 |
| Preceding psychopathology (%, as assessed at T0) | |
| Psychosis low‐risk state | 7.1 |
| Psychosis moderate‐risk state | 4.2 |
| Psychosis high‐risk state | 3.7 |
| Mood disorders | 7.2 |
| Anxiety disorders | 7.2 |
| Drug use disorders | 0.9 |
| Alcohol use disorders | 3.5 |
Table 2 reports the validation of the psychosis risk strata by using the ES‐SCZ75 and PRS‐SCZ75. In comparison to the reference group, ES‐SCZ75 and PRS‐SCZ75 showed a progressively greater magnitude of association with increasing psychosis risk strata, with RRRs ranging between 1.44 and 3.49 for the ES‐SCZ75, and between 0.85 and 3.63 for the PRS‐SCZ75.
Table 2.
Validation of the psychosis risk strata
| Reference group (“no‐risk”) | Psychosis low‐risk state | Psychosis moderate‐risk state | Psychosis high‐risk state | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RRR | 95% CI | p | Wald χ2 | p | Wald χ2 | p | Wald χ2 | p | |
| ES‐SCZ 75 a | |||||||||
| Psychosis low‐risk state | 1.44 | 1.22‐1.69 | <0.001 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Psychosis moderate‐risk state | 2.06 | 1.63‐2.61 | <0.001 | 7.40 | 0.007 | ‐ | ‐ | ‐ | ‐ |
| Psychosis high‐risk state | 2.72 | 2.17‐3.41 | <0.001 | 23.15 | <0.001 | 3.26 | 0.071 | ‐ | ‐ |
| Clinical psychosis | 3.49 | 1.80‐6.79 | <0.001 | 6.52 | 0.011 | 2.17 | 0.141 | 0.53 | 0.469 |
| PRS‐SCZ 75 a , b | |||||||||
| Psychosis low‐risk state | 0.85 | 0.66‐1.10 | 0.217 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Psychosis moderate‐risk state | 1.25 | 0.88‐1.79 | 0.215 | 3.77 | 0.052 | ‐ | ‐ | ‐ | ‐ |
| Psychosis high‐risk state | 1.55 | 1.11‐2.16 | 0.010 | 9.07 | 0.003 | 0.87 | 0.350 | ‐ | ‐ |
| Clinical psychosis | 3.63 | 1.23‐10.71 | 0.020 | 6.62 | 0.010 | 3.43 | 0.064 | 2.33 | 0.127 |
RRR – relative risk ratio, ES‐SCZ75 – exposome score for schizophrenia (75% cut‐point), PRS‐SCZ75 – polygenic risk score for schizophrenia (75% cut‐point)
adjusted for age, gender and education;
adjusted for three principal components
The ES‐SCZ75 was significantly associated with the low‐risk, moderate‐risk, high‐risk, and clinical psychosis strata. The PRS‐SCZ75 was significantly associated with the high‐risk and clinical psychosis strata, which were therefore validated. Additional post‐hoc group comparisons of the ES‐SCZ75 across strata showed significant differences in low‐risk vs. moderate‐risk, low‐risk vs. high‐risk, and low‐risk vs. clinical psychosis; while analysis of the PRS‐SCZ75 across strata showed significant differences in low‐risk vs. high‐risk, and low‐risk vs. clinical psychosis.
The incidence rate of clinical psychosis was 63.0 per 100,000 person‐years (95% CI: 42.9‐92.6), with comparable rates for individuals under 35 years (50.1 per 100,000 person‐years, 95% CI: 20.9‐120.5) and 35 years of age and above (67.1 per 100,000 person‐years, 95% CI: 43.8‐103.0; incidence rate ratio=1.34, 95% CI: 0.49‐4.55, p=0.58).
Figures 1 and 2 show the HRs for psychosis risk categories and diagnoses of non‐psychotic mental disorders. Preceding diagnoses of mood, drug use, and anxiety disorders, along with psychosis high‐risk state, showed an increased risk for clinical psychosis incidence in the age, gender and education‐adjusted model. In the multivariable adjusted model, the preceding diagnoses of mood disorders (HR=10.67, 95% CI: 3.12‐36.49), psychosis high‐risk state (HR=7.86, 95% CI: 2.76‐22.42) and drug use disorders (HR=5.33, 95% CI: 1.61‐17.64) were associated with an increased risk for clinical psychosis incidence.
Figure 1.
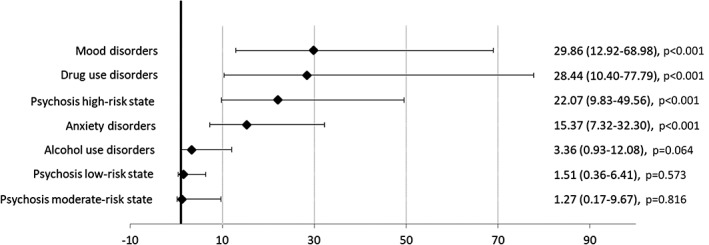
Hazard ratios (95% CI) for clinical psychosis incidence in the age, gender and education‐adjusted model
Figure 2.
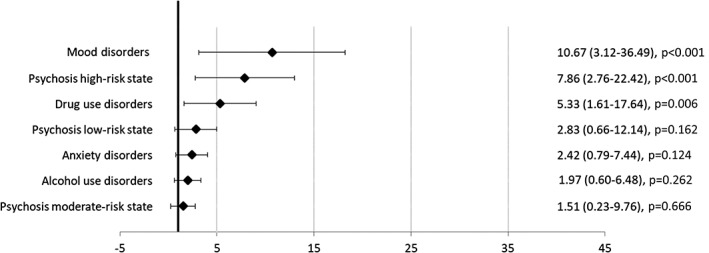
Hazard ratios (95% CI) for clinical psychosis incidence in the multivariable adjusted model
The E‐values for the association of incident clinical psychosis with preceding diagnoses and risk states were 20.8 for mood disorders, 15.2 for psychosis high‐risk state, 10.1 for drug use disorders, 5.1 for psychosis low‐risk state, 4.3 for anxiety disorders, 3.4 for alcohol use disorders, and 2.4 for psychosis moderate‐risk state.
Figures 3 and 4 show the PAFs for psychosis risk categories and diagnoses of non‐psychotic mental disorders. The estimation of the PAFs in the multivariable adjusted model indicated that 85.5% (95% CI: 64.6‐94.1) of the clinical psychosis incidence could have been avoided if all psychosis risk states and non‐psychotic mental disorders had been prevented. The most important factors were mood disorders (PAF=66.2, 95% CI: 33.4‐82.9), psychosis high‐risk state (PAF=36.9, 95% CI: 11.3‐55.1), and drug use disorders (PAF=18.7, 95% CI: –0.9 to 34.6).
Figure 3.
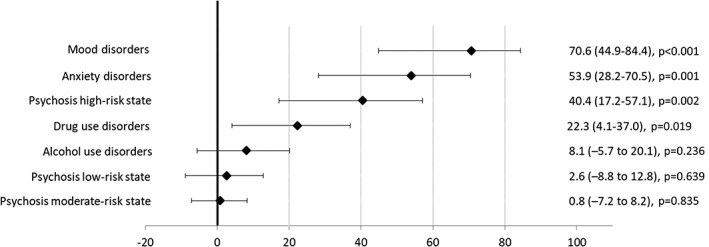
Population attributable fractions (95% CI) for clinical psychosis incidence in the age, gender and education‐adjusted model
Figure 4.
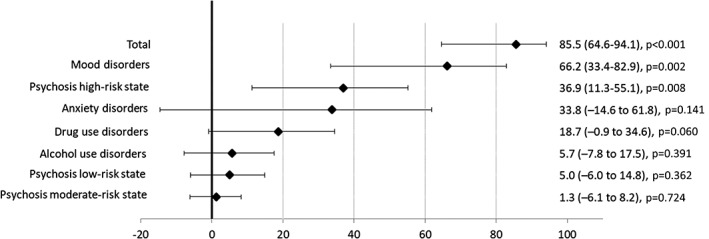
Population attributable fractions (95% CI) for clinical psychosis incidence in the multivariable adjusted model
Further, we estimated the PAF for the subpopulation of the psychosis high‐risk state. This restricted analysis revealed that 87.3% (95% CI: 63.7‐95.5) of the clinical psychosis incidence could have been avoided if the psychosis high‐risk state had been prevented when other psychopathology remained the same; while the combined PAF for non‐psychotic DSM diagnoses was 71.8% (95% CI: 33.6‐88.0) when all other factors remained as observed.
DISCUSSION
The main findings of this first population‐based study of longitudinal risk for clinical psychosis as a function of the preceding psychosis risk states and DSM‐IV diagnoses of non‐psychotic mental disorders were as follows: a) prior psychopathology accounted for a total of 85.5% of the incidence of clinical psychosis outcome in the multivariable analysis, with mood disorders, psychosis high‐risk state, and drug use disorders independently contributing to clinical psychosis risk; b) the significant reduction of mutually‐adjusted HRs in the multivariable model put the importance of comorbidity in perspective. These findings have important public health implications for early intervention strategies.
The PAFs for each psychopathology measure estimated in the final model were considerably lower than those estimated in the individual models, which were adjusted only for age, gender and education. The substantial differences in estimates between models demonstrate the importance of accounting for comorbidity beyond isolated measures of psychosis risk to yield more accurate PAF estimates for mental disorders.
We observed relatively large PAFs, except those for psychosis low‐risk state, psychosis moderate‐risk state, and alcohol use disorders, which were negligible. Preceding diagnosis of mood disorders was strongly associated with clinical psychosis outcome, and by far had the largest PAF, followed by psychosis high‐risk state, anxiety disorders, and drug use disorders. In addition to the marked reduction of PAF estimates in the final model, PAF for anxiety disorders, although still noteworthy, was not statistically significant anymore.
From a public health perspective, a 10‐fold increase in risk for clinical psychosis incidence attributable to mood disorders highlights the importance of addressing the prevention of these disorders to reduce the burden of psychosis in the general population.
Given the fact that non‐psychotic disorders are highly prevalent among individuals with CHR and likely to influence the longitudinal outcomes48, 49, 50, we estimated the risk attributable to these disorders in the subpopulation of participants with psychosis high‐risk state. The joint PAF for all non‐psychotic mental disorders was noteworthy but still lower than the individual PAF for psychosis high‐risk state when everything else remained the same in this subpopulation.
Even though the psychosis high‐risk state group displayed a high relative risk for clinical psychosis outcome even after adjusting for other psychopathology, the PAF was comparatively low. In contrast, anxiety disorders had a high PAF with respect to HR. This discrepancy between PAF and HR can be understood by examining the estimation method of PAF, which accounts for the prevalence of the risk factor in the population in addition to the strength of the association between outcome and risk factor.
In this regard, addressing the psychosis high‐risk state in a sample enriched for clinical psychosis risk may appear to be an effective strategy at first glance. However, an early intervention strategy targeting high‐risk state only will have minimal impact on reducing the population burden of psychotic disorders, because of the low prevalence of that state in the general population 27 . Further, efforts to case‐finding will require major resources, given the rarity of psychosis high‐risk state in the population. These findings provide empirical evidence for the “prevention paradox” and echo our concerns over the effectiveness and the economic feasibility of targeted CHR early intervention programs at the population level3, 5.
In this first study investigating the PAFs of psychopathology categories for clinical psychosis in the general population, we used multivariable modeling to yield more accurate estimates 51 . The large and representative population cohort collected at four time‐points over 9 years was a major strength. The clinical psychosis outcome incidence and the point prevalence of psychosis high‐risk state were comparable to the population estimates in the literature27, 52, thereby providing further support for the validity of our psychosis risk stratification approach in this population, that was guided by our previous work and verified using cumulative measures of environmental and genetic liability to schizophrenia. Nevertheless, future studies could benefit from a detailed clinical assessment and multi‐source data including electronic health records to minimize measurement bias. Finally, the high E‐values (20.8 for mood disorders, 15.2 for psychosis high‐risk state, 10.1 for drug use disorders) show that unmeasured confounding is unlikely to influence the current significant findings. Notwithstanding, strong causal inferences should be avoided, considering the observational nature of the study.
Our results provide initial empirical evidence that a comprehensive prevention strategy with a focus on broader measures of psychopathology may be more effective than the current psychosis‐focused approach in achieving population‐based improvements for prevention of psychotic disorders. Guided by a public health approach, a fully‐integrated universal mental health care system that ensures low‐threshold entry and rapid access may serve as a more efficient strategy for improving population‐based estimates of mental health, including psychosis prevention, and may counter the trend of balkanizing mental health care to smaller and competing units 53 .
Acknowledgement
S. Guloksuz and L.‐K. Pries contributed equally to this work.
REFERENCES
- 1. Correll CU, Galling B, Pawar A et al. Comparison of early intervention services vs treatment as usual for early‐phase psychosis: a systematic review, meta‐analysis, and meta‐regression. JAMA Psychiatry 2018;75:555‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Srihari VH, Shah J, Keshavan MS. Is early intervention for psychosis feasible and effective? Psychiatr Clin North Am 2012;35:613‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Os J, Guloksuz S. A critique of the “ultra‐high risk” and “transition” paradigm. World Psychiatry 2017;16:200‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fusar‐Poli P, Schultze‐Lutter F, Cappucciati M et al. The dark side of the moon: meta‐analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr Bull 2015;42:732‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guloksuz S, van Os J. Need for evidence‐based early intervention programmes: a public health perspective. Evid Based Ment Health 2018;21:128‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yung AR, Wood SJ, Malla A et al. The reality of at risk mental state services: a response to recent criticisms. Psychol Med (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McHugh MJ, McGorry P, Yuen H et al. The ultra‐high‐risk for psychosis groups: evidence to maintain the status quo. Schizophr Res 2018;195:543‐8. [DOI] [PubMed] [Google Scholar]
- 8. Schultze‐Lutter F, Klosterkötter J, Gaebel W et al. Psychosis‐risk criteria in the general population: frequent misinterpretations and current evidence. World Psychiatry 2018;17:107‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perez J, Jones PB. Breaking the web: life beyond the at‐risk mental state for psychosis. Psychol Med (in press). [DOI] [PubMed] [Google Scholar]
- 10. Raballo A, Poletti M, Carpenter WT. Rethinking the psychosis threshold in clinical high risk. Schizophr Bull 2019;45:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moritz S, Gawe˛da Ł, Heinz A et al. Four reasons why early detection centers for psychosis should be renamed and their treatment targets reconsidered: we should not catastrophize a future we can neither reliably predict nor change. Psychol Med 2019;49:2134‐40. [DOI] [PubMed] [Google Scholar]
- 12. Ajnakina O, David AS, Murray RM. ‘At risk mental state’ clinics for psychosis – an idea whose time has come – and gone! Psychol Med 2019;49:529‐34. [DOI] [PubMed] [Google Scholar]
- 13. Nelson B, Amminger GP, McGorry PD. Recent meta‐analyses in the clinical high risk for psychosis population: clinical interpretation of findings and suggestions for future research. Front Psychiatry 2018;9:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGorry PD, Mei C. Ultra‐high‐risk paradigm: lessons learnt and new directions. Evid Based Ment Health 2018;21:131‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fusar‐Poli P. The hype cycle of the clinical high risk state for psychosis: the need of a refined approach. Schizophr Bull 2018;44:250‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGorry PD, Hartmann JA, Spooner R et al. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry 2018;17:133‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linscott R, Van Os J. An updated and conservative systematic review and meta‐analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med 2013;43:1133‐49. [DOI] [PubMed] [Google Scholar]
- 18. Hanssen M, Bak M, Bijl R et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol 2005;44:181‐91. [DOI] [PubMed] [Google Scholar]
- 19. Bromet EJ, Nock MK, Saha S et al. Association between psychotic experiences and subsequent suicidal thoughts and behaviors: a cross‐national analysis from the World Health Organization World Mental Health Surveys. JAMA Psychiatry 2017;74:1136‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yates K, Lång U, Cederlöf M et al. Association of psychotic experiences with subsequent risk of suicidal ideation, suicide attempts, and suicide deaths: a systematic review and meta‐analysis of longitudinal population studies. JAMA Psychiatry 2019;76:180‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oh H, Koyanagi A, Kelleher I et al. Psychotic experiences and disability: findings from the Collaborative Psychiatric Epidemiology Surveys. Schizophr Res 2018;193:343‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rössler W, Riecher‐Rössler A, Angst J et al. Psychotic experiences in the general population: a twenty‐year prospective community study. Schizophr Res 2007;92:1‐14. [DOI] [PubMed] [Google Scholar]
- 23. Fonville L, Cohen Kadosh K, Drakesmith M et al. Psychotic experiences, working memory, and the developing brain: a multimodal neuroimaging study. Cereb Cortex 2015;25:4828‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGrath JJ, Saha S, Al‐Hamzawi A et al. The bidirectional associations between psychotic experiences and DSM‐IV mental disorders. Am J Psychiatry 2016;173:997‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirli U, Binbay T, Drukker M et al. DSM outcomes of psychotic experiences and associated risk factors: 6‐year follow‐up study in a community‐based sample. Psychol Med 2019;49:1346‐56. [DOI] [PubMed] [Google Scholar]
- 26. Kelleher I, Keeley H, Corcoran P et al. Clinicopathological significance of psychotic experiences in non‐psychotic young people: evidence from four population‐based studies. Br J Psychiatry 2012;201:26‐32. [DOI] [PubMed] [Google Scholar]
- 27. Schultze‐Lutter F, Michel C, Ruhrmann S et al. Prevalence and clinical relevance of interview‐assessed psychosis‐risk symptoms in the young adult community. Psychol Med 2018;48:1167‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Graaf R, ten Have M, van Dorsselaer S. The Netherlands Mental Health Survey and Incidence Study‐2 (NEMESIS‐2): design and methods. Int J Methods Psychiatr Res 2010;19:125‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Graaf R, ten Have M, van Gool C et al. Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study‐2. Soc Psychiatry Psychiatr Epidemiol 2012;47:203‐13. [DOI] [PubMed] [Google Scholar]
- 30. de Graaf R, Van Dorsselaer S, Tuithof M et al. Sociodemographic and psychiatric predictors of attrition in the third wave of the Netherlands Mental Health Survey and Incidence Study‐2 (NEMESIS‐2). Compr Psychiatry 2013;54:1131‐9. [DOI] [PubMed] [Google Scholar]
- 31. de Graaf R, Ormel J, Ten Have M et al. Mental disorders and service use in The Netherlands. Results from the European Study of the Epidemiology of Mental Disorders (ESEMeD). New York: Cambridge University Press, 2008. [Google Scholar]
- 32. Alonso J, Angermeyer MC, Bernert S et al Sampling and methods of the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand 2004;109:8‐20. [DOI] [PubMed] [Google Scholar]
- 33. Nuyen J, Tuithof M, de Graaf R et al. The bidirectional relationship between loneliness and common mental disorders in adults: findings from a longitudinal population‐based cohort study. Soc Psychiatry Psychiatr Epidemiol (in press). [DOI] [PubMed] [Google Scholar]
- 34. Radhakrishnan R, Guloksuz S, Ten Have M et al. Interaction between environmental and familial affective risk impacts psychosis admixture in states of affective dysregulation. Psychol Med 2019;49:1879‐89. [DOI] [PubMed] [Google Scholar]
- 35. Reininghaus U, Rauschenberg C, ten Have M et al. Reasoning bias, working memory performance and a transdiagnostic phenotype of affective disturbances and psychotic experiences in the general population. Psychol Med 2019;49:1799‐809. [DOI] [PubMed] [Google Scholar]
- 36. van Nierop M, Viechtbauer W, Gunther N et al. Childhood trauma is associated with a specific admixture of affective, anxiety, and psychosis symptoms cutting across traditional diagnostic boundaries. Psychol Med 2015;45:1277‐88. [DOI] [PubMed] [Google Scholar]
- 37. Pries L‐K, Guloksuz S, ten Have M et al. Evidence that environmental and familial risks for psychosis additively impact a multidimensional subthreshold psychosis syndrome. Schizophr Bull 2018;44:710‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bak M, Myin‐Germeys I, Hanssen M et al. When does experience of psychosis result in a need for care? A prospective general population study. Schizoph Bull 2003;29:349‐58. [DOI] [PubMed] [Google Scholar]
- 39. Bijl RV, Ravelli A. Psychiatric morbidity, service use, and need for care in the general population: results of The Netherlands Mental Health Survey and Incidence Study. Am J Public Health 2000;90:602‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pries LK, Lage‐Castellanos A, Delespaul P et al. Estimating exposome score for schizophrenia using predictive modeling approach in two independent samples: the results from the EUGEI study. Schizophr Bull 2019;45:960‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guloksuz S, Pries LK, Delespaul P et al. Examining the independent and joint effects of molecular genetic liability and environmental exposures in schizophrenia: results from the EUGEI study. World Psychiatry 2019;18:173‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Efron B. The efficiency of Cox's likelihood function for censored data. J Am Stat Assoc 1977;72:557‐65. [Google Scholar]
- 43. Lin DY, Wei L‐J. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989;84:1074‐8. [Google Scholar]
- 44. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239‐41. [Google Scholar]
- 45. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med 2017;167:268‐74. [DOI] [PubMed] [Google Scholar]
- 46. Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J 2013;13:672‐98. [Google Scholar]
- 47. Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88:15‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Albert U, Tomassi S, Maina G et al. Prevalence of non‐psychotic disorders in ultra‐high risk individuals and transition to psychosis: a systematic review. Psychiatry Res 2018;270:1‐12. [DOI] [PubMed] [Google Scholar]
- 49. Fusar‐Poli P, Nelson B, Valmaggia L et al. Comorbid depressive and anxiety disorders in 509 individuals with an at‐risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull 2012;40:120‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Addington J, Piskulic D, Liu L et al. Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophr Res 2017;190:90‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tanuseputro P, Perez R, Rosella L et al. Improving the estimation of the burden of risk factors: an illustrative comparison of methods to measure smoking‐attributable mortality. Popul Health Metr 2015;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jongsma HE, Turner C, Kirkbride JB et al. International incidence of psychotic disorders, 2002‐17: a systematic review and meta‐analysis. Lancet Public Health 2019;4:e229‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Os J, Guloksuz S, Vijn TW et al. The evidence‐based group‐level symptom‐reduction model as the organizing principle for mental health care: time for change? World Psychiatry 2019;18:88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]


