Abstract
Resilience – a key topic in clinical science and practice – still lacks a clear conceptualization that integrates its evolutionary and human‐specific features, refrains from exclusive focus on fear physiology, incorporates a developmental approach, and, most importantly, is not based on the negation (i.e., absence of symptoms following trauma). Building on the initial condition of mammals, whose brain matures in the context of the mother's body and caregiving behavior, we argue that systems and processes that participate in tuning the brain to the social ecology and adapting to its hardships mark the construct of resilience. These include the oxytocin system, the affiliative brain, and biobehavioral synchrony, all characterized by great flexibility across phylogenesis and ontogenesis. Three core features of resilience are outlined: plasticity, sociality and meaning. Mechanisms of sociality by which coordinated action supports diversity, endurance and adaptation are described across animal evolution. Humans' biobehavioral synchrony matures from maternal attuned behavior in the postpartum to adult‐adult relationships of empathy, perspective‐taking and intimacy, and extends from the mother‐child relationship to other affiliative bonds throughout life, charting a fundamental trajectory in the development of resilience. Findings from three high‐risk cohorts, each tapping a distinct disruption to maternal‐infant bonding (prematurity, maternal depression, and early life stress/trauma), and followed from birth to adolescence/young adulthood, demonstrate how components of the neurobiology of affiliation confer resilience and uniquely shape the social brain.
Keywords: Resilience, oxytocin system, affiliative brain, biobehavioral synchrony, mother‐child relationship, neurobiology of affiliation, sociality, plasticity, meaning
Resilience, usually defined as positive outcome despite adversity1, 2, 3, is likely the ultimate goal of human maturity and the single most important target of prevention and intervention science. Individuals who are able to face life's hardships with courage and perseverance, maintain positive outlook under difficult circumstances, enjoy both intimate bonds and a wider social circle, express empathy and compassion to others' misfortune, foster industry and a sense of agency toward long‐term autonomous goals, live a life of creativity, vitality and meaning, and are free of debilitating symptoms despite early adversity or current trauma, define the hallmark of human achievement and the main goal of clinical effort since Freud. It is thus surprising that, despite decades of research, a comprehensive biobehavioral perspective on resilience has not yet been formulated.
Current empirical work on resilience typically focuses on the neurobiology of stress and fear regulation, or employs epidemiological/clinical research in the aftermath of trauma. In both lines, resilience is conceptualized as the “absence of symptoms” or the “maintenance of mental health” following adversity or trauma 4 . A recent interdisciplinary panel 5 , while emphasizing the urgent need to shift the focus from psychopathology to resilience in the field of mental health, and highlighting the immense economic burden and personal suffering caused by stress‐related disorders, concluded that resilience can only be defined ex post facto after the trauma has passed and some individuals do not succumb and remain symptom‐free.
From a scientific standpoint, such position is problematic. Without a clear definition of a construct, empirical evidence cannot accumulate nor can it guide intervention effort. In particular, it is critical to identify whether resilience involves processes that gate deterioration following physical or mental insult, or those that uniquely foster strength and stamina6, 7.
Positive psychology focused on resilience as a key component of well‐being 8 and launched the well‐known resiliency training in the US army 9 . Some aspects of resilience are also echoed in the writing of post‐Freudian psychoanalysts who emphasized the functioning, growing and relating aspects of the self and its embeddedness in the social milieu, such as Sullivan 10 , Fromm 11 and Erickson 12 ; in the work of Maslow 13 on self‐actualization; and in the formulations of humanistic psychology 14 . Yet, these authors did not focus on resilience per se but on personal growth, did not integrate systematic research into their models, and did not incorporate neurobiological findings into their conceptualizations, or even negated the relevance of any neuroscientific evidence15, 16. A human‐specific model of resilience, which on the one hand is attentive to internal reality and man's higher faculties, but on the other draws on evolutionary models and incorporates neuroscientific findings into its core concepts, has not been constructed.
Two major issues may further complicate the construction of a comprehensive biobehavioral model of human resilience. First, with most current effort directed toward understanding the neurobiological underpinnings of mental disorders, research in psychiatry has generally focused on features that can be readily tested from a cross‐species perspective. This has led to an almost exclusive focus on the neurobiology of fear – the neural, endocrine, genetic and molecular processes that sustain the fear response and enable stress management4, 17, 18, 19, 20, 21. Accordingly, studies often utilize cross‐species stress‐related paradigms, particularly fear conditioning, and this has resulted in a fear‐focused view of resilience22, 23.
Second, a true focus on development as a core component in understanding mental health, particularly resilience, has often been missing, despite the fact that all models of the self are, in essence, developmental (that is, describe stage‐like progression from immature to mature states). Resilient individuals are not only born, but are (critically) raised. It has been advocated 24 that, in order to study resilience, we must follow children from infancy and over lengthy periods to detect age‐specific biological, behavioral and social markers that tip children toward a resilient pathway. However, such longitudinal effort is extremely rare.
These two issues have led to a rather limited, one‐sided view of resilience. When asked, in a discussion on resilience, “what have you changed your mind about…”, a panel of leading researchers 25 all pinpointed the narrow focus on fear physiology and stress neurobiology in resilience theory and research as the main issue they had changed their mind about.
A new conceptualization of resilience must be evolutionary‐based, enable a thorough cross‐species research, and set the stage for meticulous data collection that tests its specific expression across developmental stages, contexts and psychopathological conditions. Most critical for science, it should be verifiable (i.e., open to proof and falsifiability).
In the following, we propose a model of resilience that is based on the neurobiology of affiliation and offers a biobehavioral, evolutionary‐based and developmentally‐sensitive conceptualization, which is not constructed on the neurobiology of fear on the one hand or on the pursuit of happiness on the other. Our model takes into account the fundamental condition of mammals, whose brain matures in the context of the mother's body and caregiving behavior, and contends that maturation of all neurobiological processes that foster resilience are embedded in the provisions afforded by the mother's body and species‐typical caregiving.
Moreover, the model argues that any understanding of resilience must consider the initial dependence of the infant on its mother and the immense impact that this dependence has on brain structure and function. Mammalian young are born with an immature brain, and their brain is shaped by the mature maternal brain through physical proximity, lactation, and the assemblage of species‐typical well‐adapted caregiving via processes that provide external regulation from mother to young in a system‐specific manner26, 27.
Such external regulation of the immature brain by the mature brain charts the core mechanism of brain development in mammals and functions to fine‐tune the infant's neurobiological and behavioral systems to life within the social ecology and its unique features28, 29. We argue that the tuning of the infant's brain to life within the ecological niche and its distinct hardships marks the very essence of resilience and that processes that participate in such tuning define what resilience “is”, and should become the focus in resilience theory and research.
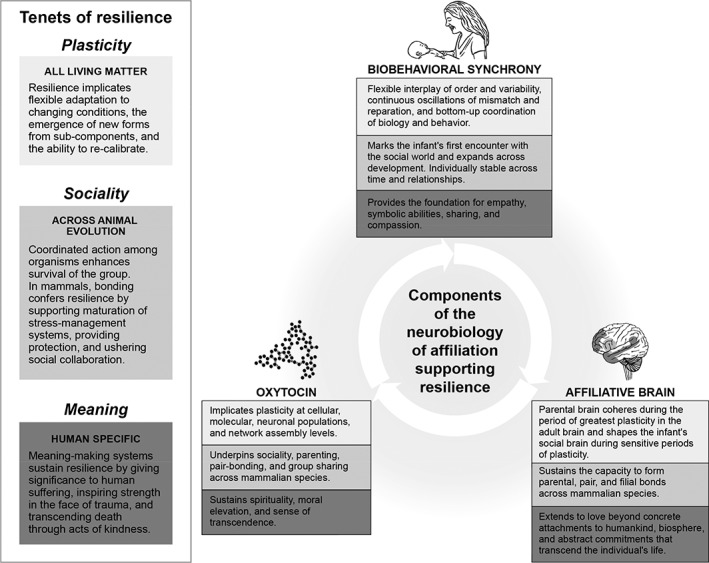
CORE COMPONENTS OF THE NEUROBIOLOGY OF AFFILIATION
Our model draws on three core components of the neurobiology of affiliation: the oxytocin system, the affiliative brain, and biobehavioral synchrony.
Oxytocin
The ancient oxytocin system, evolving approximately 500 million years ago, functioned to mediate organisms’ response to environmental challenges by supporting the regulation of basic life functions, such as water conservation, thermoregulation and energy balance across the phylogenetic scale31, 32. Hence, its initial involvement in endurance, organism‐ecology adaptation, and resilience.
With the evolution of mammals, oxytocin has been incorporated into labor and lactation. For mammalian young, then, the mother‐infant bond has become the key context for the maturation of systems that support stress reduction 32 . Life‐sustaining functions no longer develop in the context of the group, like in fish or ants, but within the intimacy of the “nursing dyad”, via provisions embedded in the mother's body.
In mammals, the oxytocin system became the key one supporting the resilience‐by‐affiliation mechanisms, where robustness, plasticity and tolerance of ecological hardship is achieved by social contact in processes that span a single cell to human cultural communities29, 33. Overall, the role of oxytocin in resilience stems from three sources, associated with its involvement in neural plasticity, sociality and immunity.
Oxytocin is implicated in neural plasticity at the molecular, cellular and network‐assembly levels34, 35, 36. Oxytocin neurons can co‐express with various neurotransmitters, including dopamine, serotonin and opioids. Oxytocin‐expressing neurons include a wide variety of cell types, such as GABAergic interneurons, glutamatergic pyramidal cells, and other peptidergic cells34, 37, 38. Oxytocin integrates brain and periphery, incorporates massive epigenetic inputs, and is particularly related to attachment experiences39, 40. It increases plasticity in the hippocampal network to increase salience of the attachment target 41 , and attachment experiences shape oxytocin receptors availability 42 .
Oxytocin's pulsatile mode of release is particularly important for neural plasticity, by which it shapes environment‐dependent neurobiological systems 43 . Its pulsatile release coordinates birth according to favorable environmental conditions, charting the first integration of brain and environment in human life 44 . Its surge during birth causes gamma‐aminobutyric acid (GABA) signaling to change from excitatory to inhibitory, synchronizing the fetus' hippocampal neurons with the transition from prenatal to postnatal life45, 46, setting the lifelong excitation‐to‐inhibition balance. Optimal balance of excitation and inhibition is critical for adaptive functioning and buttresses the “sensitive period” effect, which is critical to the robustness of all living organisms 47 .
Oxytocin plays a key role in sociality. The neural systems that enable attachment and bonding evolved through oxytocin's sensitivity to the recurring elements in the environment, imbuing mother and surrounding with incentive value48, 49, 50. Oxytocin availability at core limbic sites guides infants to prefer cues associated with their mother, leading to the formation of dyad‐specific attachments51, 52. During first post‐birth days, oxytocin receptors become connected to specific social cues via oxytocin's links with the brain dopamine reward system53, 54, 55, olfactory‐amygdala pathways56, 57, innervation of sensory cortices 54 , and sharpening signal‐to‐noise ratio in hippocampal pyramidal cells 58 . These program the brain's social perception, preferences and memory, and connect them to the attachment target.
Oxytocin supports the integration of individuals into social groups59, 60. Across evolution, it has been implicated in social functions: in courting rhythmic movement in nematodes 61 , social processes in worms 62 , mate selection and flocking in birds 63 , exclusive bonding in herding animals 64 , and social affiliation in rodents 65 , primates 66 and humans29, 67. Evolutionary constraints led this flexible environment‐dependent system to direct young to bond with their parents, function within their social ecology, and engage in the social structure of their species 64 . Notably, greater social support and a sense of belonging to the social group have been repeatedly associated with greater resilience68, 69, 70.
The infant's oxytocin system is shaped by caregiving. Animal studies indicate that maternal behavior programs oxytocin receptor availability in the brain 71 , and longitudinal human studies show that peripheral oxytocin is programmed by sensitive parenting repeatedly experienced throughout childhood72, 73, 74. Oxytocin induces a physiological state of quiescence that affords participation in the world without fear and stimulates the desire for social contact through its links with dopamine in striatal neurons75, 76, 77. This unique state provides the basis for the individual's sense of security upon which resilience can develop.
Finally, oxytocin plays an important role in functionality of the immune system. Human studies show associations between oxytocin and immune biomarkers78, 79. In cell culture, oxytocin reduces oxidative stress and interleukin‐6 (IL‐6) secretion from stimulated macrophages 80 . In vivo, it decreases inflammatory cytokines, IL‐6 and tumor necrosis factor (TNF)‐α 81 . During periods of bond formation, including the period of becoming a parent and falling in love, both oxytocin and IL‐6, an immune biomarker, increase their activity 82 , and oxytocin is implicated in quicker wound healing 83 . Recently, an oxytocin‐producing gut bacterium (Lactobacillus reuteri) was found to play a role in resilience, stress management, and quicker wound healing in the host, suggesting not only an additional gut‐brain axis of oxytocin production, but also a microbiome‐host link that promotes resilience 84 .
The affiliative brain
The “affiliative brain” charts the network of inter‐connected structures that enable humans to form and maintain close relationships 85 .
The human affiliative brain, which evolved from the rodent maternal brain, expanded to include several higher‐order cortical networks that integrate the immediacy and subconscious motivation with the cognitive aspects of human parenting30, 86. This global human caregiving network has been further repurposed to sustain human social affiliations with lovers, close friends, and fellow humans, all shaped in the infant's brain by maternal provisions during early sensitive periods30, 85.
Studies of the maternal brain in animal models date back to the 1950s, and describe the critical role of the medial pre‐optic area of the hypothalamus in initiating the subcortical network that enables mammalian mothers (and fathers in bi‐parental species) to care for their infants 87 . Primed by oxytocin release during pregnancy and labor, the medial pre‐optic area sends projections to the amygdala, to increase maternal vigilance for infant safety, and to the ventral tegmental area, to increase maternal reward from infant stimuli, sensitizing a limbic network underpinning maternal care (also including the nucleus accumbens, lateral septum, ventral pallidum, bed nucleus of stria terminalis, and globus pallidus).
In humans, this subcortical network expanded to include higher‐order networks that enable empathy, simulation, mentalization, and emotion regulation, forming a global network that supports attachments 30 . In the 3‐5% of mammalian species that show bi‐parental care, the same system underpins father care. However, recent molecular and system‐level findings show that different neuronal populations underpin maternal and paternal caregiving 88 , and, while the same network supports human mothering and fathering, the pathway to fatherhood is more cognitive and relies on concrete paternal childcare activities87, 89.
Oxytocin plays an important role in tuning and function of the affiliative brain. Humans are wired for social behavior via activity of the mammalian caregiving network, which contains abundant oxytocin receptors 90 . Oxytocin causes long‐term depression in the amygdala 91 to attenuate amygdalar response to aversive social stimuli, increasing network connectivity and enabling response specificity to social targets92, 93.
Following the attenuation of social avoidance, oxytocin enhances motivation for social bonding through its crosstalk with dopamine receptors in striatum, particularly nucleus accumbens. Dopamine acts in nucleus accumbens to organize goal‐directed reward‐related behavior by inhibiting the output of GABAergic (inhibitory) neurons94, 95, 96, 97, which enables activation of glutamate (excitatory) inputs, leading to energetic, vigorous, goal‐directed action98, 99.
Nucleus accumbens shell contains oxytocin receptors that form heteromers (neurons expressing for both oxytocin and dopamine 100 ) and this enables dopamine neurons specifically suited to identify sensory‐motor reward to encode the temporal patterns of social action49, 101. This allows the brain to internalize the social partner, encode bond‐specific patterns, and draw reward from social synchrony96, 101.
The tighter oxytocin‐dopamine crosstalk during bond formation enables the flexible incorporation of the new bond into the self 102 and the formation of sensory‐motor memories of attachment experiences 103 . Thus, while dopamine affords motivation and vigor, oxytocin provides the tranquility necessary for bond formation.
While this brain network sustains human parenting, it also provides the neural support for the formation of other affiliative bonds throughout life; hence the term “affiliative brain”. Animal 104 and human 85 studies indicate that the mammalian parental brain also sustains pair‐bonds in monogamous mammals 77 , and romantic attachment and close friendships in humans 85 . This affiliative network develops in the infant's brain during early sensitive periods through attuned caregiving, and enables the child to form close relationships, fall in love, become a member in social groups from sports team to nations, and eventually nurture his/her own children.
It has long been noted by Darwin 105 that evolutionary adaptations take place at the parent‐infant interface and its inherent plasticity enables the emergence of new behaviors which, over time, alter gene expression. Consistently, our model – which places the parent‐child interface at its core – highlights how the affiliative brain utilizes its inherent plasticity for resilience, endurance and recalibration.
The affiliative brain confers resilience in multiple ways. Optimal activation of this network enables individuals to form and maintain social bonds throughout life, manage stress by relationships, and, through the crosstalk of oxytocin and dopamine, draw their deepest reward from affiliations, rather than non‐social sources (e.g., drugs of abuse). Indeed, disruptions in the integration of oxytocin and dopamine is found in addiction, when reward disconnects from its social targets and disruptions are found in both oxytocin 106 and neural plasticity107, 108.
The parental brain shapes the child's social abilities. We found that parental brain activations in infancy predicted the child's emotion regulation, stress management, and symptom formation across the first seven years of life109, 110, 111. In parallel, sensitive and synchronous parenting longitudinally shaped the child's affiliative brain in adolescence112, 113. Finally, humans' large associative cortex enables humans to find meaning through love to abstract ideas, such as homeland or God, and extend affiliations to fellow‐humans, pets, or the Earth's flora and fauna, all supported by the same network 85 .
Biobehavioral synchrony
Biobehavioral synchrony is the core mechanism sustaining human sociality and affiliation. It is defined as “the coordination of biological and behavioral signals between social partners during moments of social contact”, and it describes the mechanism by which the parent's mature brain externally regulates the infant's immature brain and tunes it to social life29, 114, 115.
Biobehavioral synchrony creates a template for the coordination of the biological with the social and mental; the merging of autonomous self with autonomous other; and the integration of moments of interpersonal match with moments of mismatch, alone states, and reparation, all within a secure dialogue.
In multiple studies spanning infancy to adulthood, and across a wide range of healthy and high‐risk populations in various cultures, we showed that these “precious social moments”, when parent and child coordinate their non‐verbal behavior, frame moments of biological coordination. For instance, only during these episodes there was synchrony between mother and infant's heart rhythms 116 , coordinated release of oxytocin 117 , and brain‐to‐brain synchrony in the social brain 118 .
Synchrony links with better stress management 73 , higher respiratory sinus arrhythmia 119 , and better immune functions 120 , depicting a mechanism by which coordinated social behaviors reduce stress and enhance resilience.
The linkage of behavioral and biological synchrony originates in utero 121 , incorporating the infant's biological rhythms into a social dialogue that transforms the biological into relational and the intra‐individual into interpersonal. Patterns of non‐verbal synchrony reverberate in the dyadic relationship across time, while expanding in symbolic and interpersonal complexity 122 , and such increased diversity of repertoire amidst a core order charts a mechanism of resilience, as suggested by dynamic systems' theory 123 . Notably, all forms of physiological synchrony (neural, endocrine and autonomic) are embedded within behavioral coordination, supporting our main hypothesis that behavioral synchrony frames physiological connection and that resilience is behavior‐based29, 124, 125.
Biobehavioral synchrony experienced in the first months of life marks a critical experience during a sensitive period that predicts a host of resilience‐related outcomes from birth to young adulthood, including emotion regulation, symbolic competence, stress management, lower externalizing and internalizing symptoms, and social brain development30, 114, 126, 127.
Across development, the non‐verbal affect matching of infancy morphs into reciprocal exchanges that incorporate, like expanding ripples, the child's growing symbolic, linguistic and social competencies and evolves to include empathy, perspective taking, and intimacy, all built upon the rhythmic non‐verbal core in the service of resilience (see below). This echoes Maslow's notions 13 that the “self” includes both what the person is and what the person can become. Furthermore, while charting a human‐specific mechanism that develops across human life, biobehavioral synchrony draws on a long evolutionary line of socially‐based survival‐related mechanisms in mammals and other eusocial (hyper‐social) species that sustain endurance and resilience.
Across evolution, from bacteria to human, synchrony builds on processes that bind two organisms (or entities) into a coupled biology. Recent advances in quantum physics suggest that such coupling began even before the emergence of life, as seen in the phenomenon of “quantum entanglement”, the connection of particles across time and space that locks two units together, giving their union immeasurable strength and endurance.
THE THREE TENETS OF RESILIENCE
Taking into consideration the aforementioned foundations of affiliative neuroscience (oxytocin, the affiliative brain, and biobehavioral synchrony), our model highlights three tenets that define what resilience is. While all three are required for the making of the resilient individual, they come in different combinations across individuals and cultures, and express differently across ages and stages (Figure 1).
Figure 1.
The three tenets of resilience as integrated into the core components of the neurobiology of affiliation
Resilience implicates plasticity
At the outset, resilience involves mechanisms that promote flexible adaptation to changing conditions, resourceful use of contextual provisions in the service of personal growth, and the capacity to persist toward long‐term goals tempered by the ability to modify and recalibrate. That is, resilience implies plasticity.
Plasticity relies on neurobiological systems that underpin social fittedness, physical stamina and endurance as they flexibly adapt to diverse conditions128, 129. Bonding is likely the process exuding the greatest plasticity in mammals. Great neural plasticity has led to the evolution of viviparity (internal gestation) and to physiological reorganization in mother and young that enabled the maturation of the fetus within the maternal body 130 . Immense neural plasticity is also required to make that newborn the most salient object to its mother to the exclusion of all other focus 131 .
As noted, the oxytocin system plays a key role in neural plasticity, which is critical to the formation of attachments, and the period after childbirth marks the time of greatest plasticity in the adult brain 132 .
The “plasticity” component of resilience comprises two features: a) resilience is integrative and regulatory; b) resilience is time‐based.
Resilience is integrative and regulatory
Regulation promotes flexible integration of system components into a functional whole, shaping self, individuality, agency and well‐being through the formation of new, person‐specific, dyad‐specific and culture‐specific configurations. Much developmental research has been directed to the construct of “regulation”, with some suggesting that this is the single most important concept in understanding developmental disruptions133, 134.
Across multiple fields, “regulation” adopts a system perspective. It describes how various components of the system dynamically coalesce into a functional whole; how higher and lower elements hierarchically organize over time; and how components from within the system integrate online with those in the immediate environment123, 135, 136, 137. Conceptual models suggest that regulatory processes mature on top of each other from biological to emotional to attentional to self‐regulatory processes 138 , and parent‐child co‐regulation (synchrony) supports maturation of higher‐order regulatory skills, such as attention modulation and self‐control139, 140, 141.
Resilience is time‐based
Resilience is time‐bound and process‐based, and develops from simple to complex and from biological to mental. The “timeness” component of resilience is critical not only across evolution (phylogenesis) and from infancy to adulthood (ontogenesis), but also at the level of concrete social experiences.
Social moments always unfold in time when two or more participants create a novel “dance” of matched and mismatched moments that coordinate behavior, physiology and mental states. The timeness of these encounters enables the formation of new forms from existing units. Time, therefore, is an indispensable component of resilience (the ability to re‐calibrate) and this is captured by “synchrony”, a time‐based construct.
Resilience is social
Sociality underpins survival and adaptation, and species that can better utilize social mechanisms of coordinated action have a significant survival advantage. This is elegantly described by the entomologist E. Wilson 142 in The social conquest of earth, where he argues that humans achieved supremacy among vertebrates and ants among invertebrates, in terms of population size, spread across earth, and durability, due to their eusociality (hyper‐sociality), which involves the capacity for collaborated action among group members and social organization across generations.
Primitive mechanisms of synchrony are found in ants, fish and birds, and are underpinned by the coordination of biology and behavior through vasotocin, the parent molecule of the mammalian oxytocin and vasopressin32, 62, 143. Humans' biobehavioral synchrony, therefore, relies on a long history of social mechanisms that promoted resilience via action coordination. Consistent with the behavior‐based principle of affiliative neuroscience, these mechanisms were selected with a focus on behavior: social behavior in the group in non‐mammalian species and affiliative bonds in mammals. Notably, however, while loneliness is hazardous to the well‐being of any living organism 144 , the “social” component of resilience is highly variable, and wide variability is observed across the animal kingdom, paralleled by great variability in the density and localization of oxytocin receptors145, 146.
Social monogamy
Social monogamy marks the first extension of the mother‐infant bond to other attachments within the family, specifically mating and fathering. Studies on social monogamy utilized several primate species (cotton‐top tamarins, marmosets and lamurs) 147 , and five rodent species, all originating from a single rodent lineage (prairie voles, mandarin voles, California mice, Campbell's dwarf hamsters, and Mongolian gerbils) 148 .
Monogamy provides the basis for fatherhood. Direct paternal care is found mainly in socially monogamous species 149 , where fathering occurs in the context of maternal care and parents coordinate their caregiving in relation to each other 150 . Paternal care contributes to confer resilience to mammalian young, increasing offspring survival, litter size, and growth rates151, 152, 153, 154, 155, 156.
While the specific ecological pressures that led to bi‐parental caregiving and to humans' cooperative breeding are unknown, paternal caregiving stabilized monogamous mating systems. Once social monogamy has been established in a species, it fosters the emergence of complex social behaviors, that foster resilience154, 157.
Both father care and pair bonds involve the extension of the mother‐infant bond, repurposing the same neural networks and molecular processes and providing the first expression of both consistency and diversity in the neurobiology of affiliation. Monogamy also necessitates coordination of the three intra‐family attachments (mothering, fathering, and the pair‐bond) in the formation of a family unit, and such coordination paved the way for the evolution of the human family and, eventually, of complex socio‐cultural organizations, leading to humans' supreme resilience in the animal kingdom.
In humans, involved fatherhood confers substantial resilience. Throughout human history, fathers have been the main source of indirect care, controlling the material resources, physical conditions, and social status with which infants develop158, 159. Historical accounts point to close associations between paternal provisioning and child mortality in pre‐industrial US and Europe 160 , and anthropological studies indicate that men with more land or higher social status show greater reproductive success161, 162.
In modern societies, greater father involvement enhances child resilience, in terms of better mental health, higher academic achievement and professional attainment, and better self‐regulatory abilities163, 164. Children of involved fathers are less aggressive and resolve conflicts with more respect and dialogue 165 , and epidemiological studies show that fatherless children are more prone to aggression, law‐breaking, and conduct problems166, 167.
Complex social organizations
While social monogamy marks the first extension of the mother‐infant bond to the family unit, complex and hierarchical social organization was thought to evolve only in hominins and expand in parallel to the increase in brain size 168 . Recent research in Western gorillas discovered hierarchical social modularity, defining not only complex affiliative behavior within extended groups of kin, but also reciprocity and cooperation among non‐kin groups toward goal‐directed seasonal coalitions, in ways that mirror the social structure of a small human village 169 .
Such behavior‐based organizations enable the joint gathering of widely‐dispersed foods and protection from predators, enhancing resilience through collaborated actions outside the family. Among primates living in groups, such as chimpanzees, post‐conflict reconciliation behaviors were observed, which enable group members to amicably resolve conflict and maintain social ties, and these affiliative post‐aggression acts involve increase in urinary oxytocin 170 .
A study in marmosets showed that the greater the bonding among an affiliative pair (of same or opposite sex), measured in terms of relationship duration, time spent together, and amount of affiliative behavior, the greater the endocrine synchrony of urinary oxytocin fluctuation 171 , pointing to biobehavioral links in non‐human primates that preceded humans' biobehavioral synchrony.
Biobehavioral synchrony – a human‐specific mechanism
Building on these mechanisms of sociality that sustain stress management, group cohesion, and sensory‐motor coordination, biobehavioral synchrony is a human‐specific mechanism through which two individuals can mutually impact each other's physiology without physical contact, but via the coordination of facial socio‐affective signals, which is not found in non‐human primates and rodents 29 .
Human synchrony develops throughout life into an increasingly complex human social exchange that involves the co‐construction of a joint narrative, the capacity to assume multiple perspectives, and the ability to empathize with others' pain, actions, emotions, and mental states. The development of synchrony begins with the mother's recognition of the infant's biological rhythms in utero and culminates in adult‐adult relationship of mutual care and intimacy.
Resilience involves meaning
While the first two tenets of resilience build on species‐general foundations and add a human dimension, the meaning‐making element is exclusively human. For a conceptualization of human resilience, we must integrate the species‐general foundations of endurance, diversity, adaptation and stress‐management with the human ability to give meaning to hardship, adversity and trauma.
Humans' ability to give meaning to trauma often utilizes collective cultural or religious myths and, at other times, builds on forming personal meaning through actions, typically those that involve the strengthening of affiliative bonds or acts of altruism that extend beyond the individual.
Much research has underscored the role of spirituality in the capacity to bounce back from trouble or in the ability to use trauma for growth172, 173, 174. Studies have also pointed to the importance of generosity in resilience 175 , and to the consoling function of religious affiliations that give collective meanings but also generate community support176, 177. W. James, in The will to believe 178 , considered belief as an intentional choice that confers resilience and enables the individual to create a personally‐meaningful view of reality that gives significance to trauma and hardship. His famous metaphor of turning discrete experiences into a meaningful whole as resembling “alive electrical wires” that light and shine versus “dead wires” that remain diffuse and unlit, elegantly describes this resilience‐promoting function of belief.
Meaning‐making introduces a future dimension into the concept of resilience, adding a temporal horizon beyond the “remembered presence” 179 of other primates. This underscores the goal‐directed function by which humans create cultural myths that transcend the individual's life and fuel internal reserves of resilience in the face of hardship.
The attribution of meaning that transcends the individual's life is not only a core feature of resilience, but also relies on the two systems of the neurobiology of affiliation. Carter 76 suggested that the oxytocin system provides the neurobiological substrate for spirituality, via its role in sustaining love, caring, empathy, and moral elevation, and, specifically, as the oxytocin system enables mammals to experience “a state of vigilance without fear”, that is, to be fully aware of the present moment without vigilance of potential danger. Similarly, the neural structures that cohere into the “affiliative brain” and are formed during early sensitive periods enable humans to extend love to unfamiliar strangers, social groups, and abstract ideas, bestowing generosity beyond the individual's immediate bonds.
However, intense cross‐generational cultural myths, meaning systems, and religious beliefs run the risk of overlooking the first tenet of resilience – flexibility – by tightening habits, obligations, and submissive attitudes and increasing surveillance and rigidity. Such close‐knit groups often function through tight in‐group cohesion, achieved by tightening the neural and behavioral synchrony among in‐group members to a hyper‐social level in the face of real or perceived danger. For instance, throughout human history, soldiers receive intense training for coordinated action, and this motor synchrony enables the removal of cognitive empathy during battle in order to fight and destroy out‐group members. The social component of resilience becomes significantly tighter for the in‐group and is abolished for the out‐group.
Notably, both oxytocin and neural synchrony participate in such in‐group/out‐group division, built on ancient mechanisms that immediately distinguish friend from foe to protect loved ones. For instance, we studied the neural response of Israeli and Palestinian youth using magnetoencephalography (MEG) while viewing in‐group and out‐group protagonists in pain. For the first 500 ms, representing the brain's automatic response to vicarious pain, youth responded to the pain of both in‐group and out‐group members. However, after this half‐second of grace, top‐down processes blocked the brain's natural empathic response to the out‐group, displaying only response to the pain of in‐group 180 .
Two processes assisted in shutting down the evolutionary‐ancient empathic response to a conspecific in distress: increase in oxytocin levels and tightening brain‐to‐brain synchrony among group members. Thus, oxytocin and neural synchrony functioned in the service of a superordinate meaning system not supporting empathy, but out‐group derogation.
Studies on the involvement of oxytocin in out‐group derogation 181 open the question of how to integrate the role of meaning systems which, on the one hand, can increase resilience by building communities and giving cross‐generational meaning to trauma, while, on the other, induce out‐group aggression and prejudice. Perhaps one solution should focus on directing constant effort to imbue ancient meaning systems with flexibility and humanity, so that old rituals do not become rigid and extend to all fellow humans.
SYNCHRONY FROM INFANCY TO ADULTHOOD: THE UNFOLDING OF RESILIENCE
Synchrony does not only mature across animal evolution, but also throughout the lives of individuals. Synchrony's main development occurs within the mother‐child relationship, the primary mammalian bond, and from there it expands to other social bonds, including fathers, mentors, close friends, and romantic partners, to humankind, and to a sense of synchrony with nature, art, and sacred experiences.
These notions provide biological and scientific evidence to Winnicott's conceptualization in Playing and Reality 182 on the mother's non‐impinging presence as the basis for symbol formation, play, creativity, and spiritual experiences. Synchrony increases in complexity, diversity of repertoire, symbolic level, and degree of mutuality across childhood and adolescence, tuning the experience‐dependent social brain to understanding others' mind, showing empathy to others' distress, and participating in relationships 183 . The rootedness of synchrony in evolutionary‐ancient patterns and in the fetus' biological rhythms grounds this experience in the physical and the concrete and enables the entire history of the relationship to resonate within a human moment of meeting.
While philosophical perspectives on “embodiment” suggest that the “self” constructs from micro‐identities that unfold during concrete daily experiences, synchrony adds the element that the self assembles from concrete patterns with a significant human. Our model details the maturation of this phenomenon across both evolution and human life, and charts its contribution to resilience in the face of condition‐specific adversity.
Mother‐infant synchrony originates from the mother's recognition of the infant's first biological rhythms in utero, such as heart rhythms and sleep‐wake cycles, which send signals to the placenta and the maternal brain184, 185, 186. Following birth, mothers entrain these familiar rhythms into the dyadic exchange.
Studies from the 1970s described how mother‐infant face‐to‐face interactions build on the “burst‐pause” pattern of biological periodicities, such as sucking or crying187, 188. From the entrainment of these biological rhythms, synchrony progresses through distinct stages into an empathic, adult‐adult relationship that is dialogical and empathic.
We followed mother‐child synchrony from birth up to age 25 and observed how interactions maintained the same non‐verbal rhythmic patterns, arousal fluctuations, and positive peaks across a quarter of a century. For instance, some dyads cycle steadily between low and medium arousal, while others engage in quick peaks of positive arousal. Such stability gives order while complex and creative patterns are incorporated into the dialogue and form a familiar and unified event.
Apart from providing the “rhythm of safety”, two additional features of synchrony are particularly important in fostering resilience. First, the micro‐structure of the synchronous experiences is that of a constant shift between rupture and repair. According to Tronick 189 , mothers synchronize with the infant only about 30% of the time; thus, dyads spend more time in mis‐coordinated states that are framed by precious moments of synchrony. Psychoanalytic and developmental authors189, 190 emphasize the importance of such match‐mismatch cycles for teaching infants how to tolerate moments of non‐attunement and how to repair the misunderstanding inherent in human dialogue.
Two types of deviations from the long‐mismatch‐shorter‐match pattern are described. The first, hypersynchrony, is found in anxious mothers and expresses in heightened episodes of matching (above 45% of the time); the second, withdrawal, observed in depressed mothers, involves a near total lack of attunement. Both result in regulatory difficulties in infants191, 192. Synchrony, therefore, creates a series of micro‐events consisting of constant rupture and repair, training infants for social frustrations within a safe context. At around 9 months of age, infants begin to assume responsibility for interactive “repair” 193 , which prepares them for the equal relationships with friends and partners.
A second resilience‐promoting feature of synchrony is its role as the first context for the development of predictions in the brain. Recent models on “predictive coding”194, 195 view the brain as a computational device whose main role is to increase adaptation by minimizing entropy and augmenting certainty. Neural oscillations play an important role in predictive coding: alpha oscillations participate in building predictions, beta oscillations in assessing the accuracy of these predictions, and gamma oscillations in prediction error, the constant pitting of the brain's predictions with incoming information196, 197. Synchronous experiences provide a template for polyrhythmic coherence that enables multisensory representation of the body in the world 197 and involves the integration of alpha, beta and gamma rhythms in formation of social predictions during real‐life events 112 .
Using ecological paradigm and hyperscanning techniques, we found brain‐to‐brain coupling of gamma rhythms between both mothers and children 118 and romantic partners 198 during moments of behavioral synchrony. Gamma rhythms have been shown in both animal199, 200 and human201, 202 studies to index brain maturity, highlighting the role of synchrony in fine‐tuning this maturity. Gamma rhythms and prediction error in visceromotor cortex and motivation areas amplify feelings but blur the distinction of self and other, due to the agranularity of these regions 203 . Thus, the experience of synchrony can provide a new vantage point on social brain maturation in real‐life contexts.
Developmental stages of synchrony
In multiple longitudinal and cross‐sectional studies, we detected five distinct stages in the development of synchrony from pregnancy to young adulthood, and showed individual stability among these stages and sensitivity to specific adverse conditions.
Preparation for synchrony relates to the mother's increasing familiarity with the fetus' biological rhythms: the sleep‐wake cycle, consolidating at around 31‐32 weeks of gestation204, 205, followed by the organization of heart rhythms at around 33 weeks of gestation206, 207. These cycles coordinate with placenta response 208 , and better organization of these biological rhythms predicted greater mother‐infant synchrony at 3 months 121 .
Neonatal period: maternal postpartum behavior
Immediately after birth and across the first 6 weeks of life, human mothers – like any mammalian mothers – express the species‐specific repertoire of maternal behavior, which in humans involve gaze at the infant's face and body, expression of positive affect, “motherese” high‐pitched vocalizations, and affectionate touch. However, unlike other mammals, human mothers coordinate their behavior with the neonate's scant moments of alertness. Thus, in health, the human infant experiences at birth a coordination between his/her inner state and the response of the social world.
The expression of maternal postpartum behavior in the neonatal period provides the foundation for the development of symbolic competence in the toddler years 209 , and better cognitive development and less externalizing and internalizing symptoms across early childhood 210 , and correlates with parental oxytocin 211 .
Infancy: affect synchrony
During the third month of life, mothers and infants begin to engage in an interactive “dance”, where they coordinate their gaze, affective expressions, co‐vocalizations, and touch patterns into a dyad‐specific rhythmic dialogue. This non‐verbal experience plays a key role in social, emotional, cognitive, and brain development 114 . Mothers and fathers engage in parent‐specific forms of synchrony, more rhythmic in mothers and object‐focused in fathers 212 .
Parent‐child affect synchrony is associated with multiple hormones that support bonding, such as oxytocin, vasopressin, beta‐endorphin, prolactin, cortisol and salivary alpha amylase, as well as immune biomarkers, including salivary IgA and IL‐6 213 . Similarly, it is linked with activation of the affiliative brain in both mothers 214 and fathers 89 . Non‐verbal synchrony is also found during triadic mother‐father‐infant interaction 215 , setting the stage for children's social participation in cultural and group activities.
Toddler/preschool: symbolic play sequences and co‐construction of imaginary narratives
At the second and third years of life, toddlers begin to engage in symbolic play and start to imbue objects with symbolic meaning and “story‐like” symbolic sequences. Children's symbolic complexity is not only predicted by synchrony with mother and father in infancy 216 , but the temporal contour of the infant's rhythmic exchange with mother and father predicts the organization of symbolic play sequences – brief, random and numerous with father, and longer, slower‐to‐build and fewer with mother 126 .
During the preschool years, children begin to co‐construct a dialogue that contain future and past events, imaginary scenarios, and alternate reality, in which they can immerse themselves. These playful creative abilities draw on the non‐verbal synchrony of the first months of life 210 and transform the synchronous dialogue into a social event involving creativity, language and emerging theory‐of‐mind skills, that express inner reality. Preschoolers' reciprocal interactions with mother and father predict children's theory‐of‐mind abilities and the development of a moral stance across childhood and adolescence 114 .
At this stage, children begin to have “best friends” and enter into social institutions built by the culture. The experience of affect synchrony shapes the child's social competencies with peers in culture‐specific ways 217 . Parental oxytocin levels, OXTR genes, and early synchrony predict children's synchrony with their first best friend 72 .
Later‐childhood/adolescence: empathic dialogue
Beginning at around 9‐10 years, and continuing into adolescence, children markedly reduce the amount of “play” interactions with their parents, and the dialogue becomes a verbal one: interactions that require the resolution of conflicts, exchange of information, and, in health, parent‐child discussion of experiences, ideas, feelings, opinions, and plans for the future.
The synchronous dialogue at this stage incorporates the child's emerging capacity for behavioral, emotional and cognitive empathy; the ability to plan ahead, elaborate, cooperate, and show motivation; and the capacity to see the other person's point of view. Such social abilities, particularly at this stage when the attachment focus shifts from parents to friends, are crucial for children's well‐being, and are associated with resilience in the face of adversity and with maturation of the social brain113, 218, 219, 220.
Adulthood: mutuality, intimacy and perspective‐taking
When the mother‐child bond was “good enough” and synchrony progressed along developmental lines, creating space for both resonance and reparation, mother and young adult are able to face each other as two adults who still maintain their roles, but are able to incorporate them into a dialogue that respect their maturity yet reverberates their entire relationship. It rests on the early familiar rhythms and echoes all developmental stages, but it is a dialogue that is mutual and respectful, intimate and autonomous, familiar and secure, and still differs from a couple.
Such dependable synchrony enables individuals to enter with trust and mutuality other relationships and build the bridge to the next generation, that can transcend the parent's life through the adult child's ability to evoke the dyadic experiences with the parent in his/her own brain in the parent's absence.
Overall, synchrony, which gradually enriches the infant's social repertoire with the maturation of more complex mental abilities, enables variability within order, diversity within familiarity, and creativity within stability. Synchrony bears on the “stuff” of life, where the biological integrates with the social to give meaning, form bonds, and withstand hardship.
THE MAKING OF THE RESILIENT CHILD: THREE LONGITUDINAL HIGH‐RISK COHORTS
Our model suggests that biological and social provisions embedded in the mother‐infant bond provide the foundation for life‐long resilience. For many children across the globe, however, these provisions are compromised. To make progress in understanding resilience, we must tease apart one adverse condition from the next, examine the specific provisions impaired by each, and test how these omissions affect outcome.
We have suggested that human studies must begin at birth or as close to it as possible, employ longitudinal designs, and examine the “missing component” in the maternal provisions on the basis of specific research programs in animal models, that manipulate these provisions and test their sequalae on offspring brain and behavior 30 .
There are three main sources of disruptions to maternal‐infant bonding, stemming from mother, child and context, each affecting millions of children worldwide.
Maternal postpartum depression impacts 15‐18% of parturient mothers in industrial societies, and up to 30% in the developing world 221 . We have suggested that Meaney's work 222 on the long‐term effects of low maternal licking‐and‐grooming on the brain oxytocin and stress response in rat pups may provide insights into the long‐term consequences of maternal depression.
Premature birth occurs in 10.5% of live birth in industrial societies 223 , and its well‐known negative impact relates, in part, to maternal separation following incubation, and its effects on environment‐dependent life‐sustaining systems, resonating Hofer's “maternal proximity” model 27 .
Early life stress bears long‐term negative consequences on development. One in five children worldwide are growing up in the context of chaos, immigration, food or shelter insecurity, tribal or ethnic war, poverty, and violence. The animal model that may parallel these disruptions is the “varying foraging demands”224, 225, in which bonnet macaque mothers are exposed to episodes of available food versus unavailable and difficult to find food, alternating unpredictably between times when mother is available and periods of minimal caregiving. Such conditions were found to carry the worst effect on offspring – in terms of brain growth, stress response, and behavior – compared to the high or low conditions, suggesting that the inconsistency embedded in early life stress is the most detrimental to children's resilience.
To understand resilience from a developmental neuroscience perspective, we followed three cohorts of mothers and infants from birth (or infancy) up to adolescence/young adulthood, focusing on how the components of the neurobiology of affiliation differentiated children on a risk versus resilient trajectory. Each cohort tapped one of the aforementioned disruptions to maternal‐infant bonding, and hypotheses were based on the parallel animal models.
The postpartum depression cohort utilized a community birth‐cohort to tease out mothers who were chronically depressed across the child's first years. The war‐exposed cohort involved mothers and children living in a zone of continuous war‐related trauma, and the premature cohort included low‐birthweight but neurologically intact premature infants, half of whom received maternal‐infant skin‐to‐skin contact (“kangaroo care”) in the neonatal period. Repeated assessments of synchrony, regulatory skills, oxytocin, stress hormones, and psychopathology were conducted across childhood, and at the final time‐point we imaged the social brain.
Maternal postpartum depression
Our birth cohort included only physically healthy, cohabitating mothers who were above 21 years and above poverty line, to tease apart the effects of depression per se from frequently co‐occurring conditions (single parenthood, teenage mothers, poverty). Women were assessed for depression repeatedly across the first year, and again at 6 and 10 years. We formed two cohorts: children growing up in the context of chronic maternal depression from birth to 6 years, and healthy controls.
Maternal depression increases psychopathology
Exposure to early and chronic maternal depression markedly increased child propensity for psychopathology, even when families were at low risk. At six years, 60% of children to mothers who were diagnosed with major depression at both 9 months and 6 years, and reported being generally depressed throughout the child's early years, received a full‐blown Axis I psychiatric diagnosis (compared with 15% of controls), with the most prevalent disorders being anxiety and conduct disorders 226 . At 10 years and pre‐adolescence, more than 50% of these children still received a psychiatric diagnosis, even when mothers remitted, highlighting the long‐term effect of early exposure. Higher externalizing and internalizing symptoms were also reported in children of depressed mothers 227 .
Synchrony fosters resilience
Depressed mothers failed to provide the age‐appropriate co‐regulatory caregiving required to support development. At 9 months, micro‐analysis of non‐verbal behavior indicated that depressed mothers showed minimal social gaze, positive affect, and affectionate touch, and engaged in minimal synchrony with their infant 191 . As synchrony extended over time, depressed mothers were unable to develop more mature forms of reciprocal dialogue.
Synchrony was individually stable from birth to adolescence, and the lower synchrony in children of depressed mothers predicted increased psychopathology and greater social withdrawal. At 6 years, children of depressed mothers showed little behavioral empathy 228 . At 10 years, they showed lower executive functions and reduced emotion understanding. These aberrant socio‐emotional outcomes were predicted by the lower synchrony.
At the same time, synchrony functioned as a resilience component. Among children of depressed mothers who still received more synchrony (either from their fathers, due to greater functionality of the oxytocin system, or because of the child's inborn sociability), it served as a protective factor.
Children's ability to function more adequately in the social world, form friendships, and engage in peer activity, all triggered by synchrony, markedly reduced the effects of early maternal depression on the propensity for mental disorders, executive abilities, and emotion knowledge. This effect was particularly salient in late childhood, a period when peer relationships begin to assume a greater impact on children's lives, lending support to our argument that resilience components function differently at various stages and that development should become a focus in the conceptualization and research of resilience.
Altered stress response is mediated by mothers' negative parenting
Effects of maternal depression on children's stress response were complex, depending on developmental stage, type of measurement, and resilience indicators. At 9 months, infants of depressed mothers showed greater cortisol reactivity to a social stressor and diminished recovery 229 . At 6 years, maternal depression impacted cortisol variability, but this was found only among children who received tense, critical and negative parenting 228 . These findings highlight the importance of the plasticity/flexibility component of resilience for stress reactivity. At 10 years, only children of depressed mothers who received more negative parenting exhibited higher cortisol, and such over‐activation of the hypothalamic‐pituitary‐adrenal (HPA) axis mediated the effects of depression on psychopathology 221 .
We also measured salivary IgA, a biomarker of the immune system, and found higher levels in children of depressed mothers in late childhood, indicating greater stress, but this was found only among children receiving minimal synchrony, attesting to the resilience role of synchrony on the stress and immune systems.
Fathers enhance resilience
In the context of the minimal synchrony provided by the depressed mother, a synchronous father‐child relationship served an important resilience function. When fathers showed sensitive and reciprocal parenting, the propensity for psychopathology among children of depressed mothers markedly decreased 230 . It appears that one mechanism by which sensitive fathering promotes resilience is by altering the family atmosphere, making family interactions more cohesive, harmonious and involved even when mothers are depressed 231 . These findings echo the “social monogamy” mechanism described above, and suggest that opening the maternal‐infant bond to other affiliative bonds within the family confers resilience.
In another study, we followed parents and their first‐born child in the Israeli and Palestinian societies from infancy to preschool. We found that maternal depression carried a less toxic effect on child psychopathology and symbolic competencies in the Palestinian society, and this was related to the extended‐family living arrangements in this culture, which enabled children ample opportunities for synchronous interactions with other adults of kin relationship 232 .
Oxytocin promotes resilience
At both 6 and 10 years, depressed mothers and their children had lower oxytocin production, as measured in both saliva 226 and urine 233 . Both mothers and children had greater prevalence of the GG genotype on the OXTR gene (rs2254298), associated with greater vulnerability for mental disorders 234 . When mothers had the A allele on the OXTR gene, the child's propensity to receive an Axis I diagnosis at 6 years was reduced by half 226 . At 10 years, when children's salivary oxytocin was high, this attenuated the effects of maternal depression on child externalizing and internalizing symptoms 227 .
Adolescents' affiliative brain
In early adolescence, we measured children's neural empathic response to others' pain and the brain basis of attachment using MEG. Among children of depressed mothers, we found disruptions to the neural empathic response in the superior temporal sulcus, a hub of the social brain, which showed diminished alpha activation and quicker abortion of neural response at around 900‐1100 ms post‐stimulus. Such aborted response was predicted by the augmented intrusive and negative parenting and diminished synchrony that these adolescents experienced in infancy, highlighting the detrimental effects of the depressed mother's style on brain development over time 219 .
To assess the brain basis of attachment, we employed the typical paradigm of exposing children to videos of their own interaction with their mother at an earlier stage as compared to unfamiliar interaction. The typical neural activation to attachment cues involved a multi‐rhythmic response of alpha, beta and gamma, including alpha suppression in posterior region, and beta and gamma activations in a large right cluster including the superior temporal sulcus, fusiform gyrus, and insula. However, children of depressed mothers, but only those who developed an affective disorder themselves, showed an aberrant response involving both reduced response to social cues and attenuation of the differentiation between attachment and non‐attachment stimuli. These disruptions were predicted by the lower functionality of the oxytocin system and the reduced mother‐child synchrony across childhood.
While these findings specify the risk for later attachments in children of depressed mothers, they also show that some children growing up in the context of chronic maternal depression are more resilient, and that components of the neurobiology of affiliation are markers of resilience.
Early life stress and trauma
Our early life stress and trauma cohort included children and their mothers living in a zone of continuous war who were exposed to repeated and unpredictable missile and rocket attacks for nearly 20 years. We assessed children in infancy, middle childhood (5‐7 years) and late childhood (10 years), and imaged the social brain in early adolescence.
Comorbid mental disorders following chronic early trauma
Children growing in such a traumatic and chaotic environment exhibited a 3 to 4‐fold increase in the prevalence of Axis I mental disorders and a marked increase in internalizing and externalizing symptoms. In comparison with the depressed mothers cohort, a special feature of this cohort was that two thirds of the diagnosed children showed more than one diagnosis, with some presenting three or even four mental disorders, suggesting that trauma expresses in multiple dysfunctions across the entire psychopathological spectrum 235 .
Assessing the trajectories of risk and resilience across the first decade of life in trauma‐exposed children, we found that children who never exhibited mental disorders or remitted after early psychopathology had mothers who were less symptomatic, experienced more synchrony, and showed greater social competence at late childhood (10 years) 236 .
Oxytocin buffers stress
In this cohort, oxytocin functionality was associated with resilience in the face of trauma. Greater functionality in the oxytocin receptor gene in child, mother and father differentiated children who developed chronic post‐traumatic stress disorder (PTSD) from those who remitted by middle childhood 235 .
At 10 years, unlike the children of depressed mothers, we found no group differences in children's oxytocin levels, indicating that not all children growing up within a war zone show fundamental disruptions to the biological basis of affiliation, and that some mothers are able, by recruiting significant effort, to buffer the hazardous effects of war on their child. Oxytocin levels in war‐exposed mothers, however, were lower, attesting to the immense burden of raising a child in the context of unpredictability and trauma, and such burden was found across multiple maternal hormonal and neural systems.
Endocrine synchrony was found between mother and child. When maternal oxytocin was low and synchronous parenting reduced, children exhibited significantly more symptoms. But this was not the case when mothers maintained high oxytocin levels and exhibited sensitive, non‐intrusive parenting 120 .
The stress response
We measured mothers' and children's chronic and phasic cortisol in early childhood, late childhood, and early adolescence, by assessing both hair and salivary levels of the hormone. In early childhood, cortisol and salivary alpha amylase, a marker of the sympathetic arm of the stress response, differentiated exposed children with and without PTSD. The exposed no‐PTSD children had significantly higher levels, while the PTSD children had low and flat levels 236 . These findings suggest that, in the context of chronic trauma and during early childhood, greater activation of the HPA axis marks resilience, not risk.
At 10 years, again, both chronic and phasic markers of the HPA axis were elevated only in war‐exposed children who developed psychopathology, and those were children of mothers with higher HPA axis activation and lower synchrony 73 . We suggest that “mothers stand between war and the child” and that, when mothers are able to contain their own stress and protect the child from the external trauma, they are capable to buffer the child's stress response.
In early adolescence, however, exposed children as a group, as well as their mothers, showed higher and less variable cortisol levels, suggesting that chronic exposure to unpredictable stress marks a risk factor in itself, regardless of the relationship. Possibly, such vulnerability is expressed during key developmental periods, such as the transition to adolescence 237 . Immune biomarkers were higher in war‐exposed mothers and children, highlighting the great wear‐and‐tear on the immune system in the context of chronic adversity and supporting models on allostatic load and the stress response 238 .
Children's and mothers' brain
In this cohort, unlike the other two, we imaged both mother's and child's brain in identical paradigms, in an attempt to assess how chronic stress impacts neural systems in both sides of the caregiving dyad. Across paradigms, we found that alterations in brain functioning were predicted by the history of the relationship, differentiating children on risk or resilience trajectories for maturation of the social brain.
We assessed connectivity and power of the default mode network (DMN), the neural system that sustains the sense of self, switch of internal and external attention, and autobiographical memory239, 240, 241. In both mothers and children, disruptions were found to DMN connectivity, not power, highlighting again the role of the plasticity component in resilience and the reduced ability of the discrete structures to cohere into a unified system that provides a foundation for the sense of self.
Disruptions to maternal DMN were found in alpha rhythms, the main rhythm of the awake mature brain, whereas disruptions to children's DMN occurred in the theta band, a biomarker of the developing brain 242 . Children with PTSD showed the greatest disruption to theta connectivity. Disruption in theta connectivity patterns were predicted by maternal intrusive, anxiety‐provoking parenting across childhood and by higher cortisol production in later childhood, underscoring the long‐term effects of unpredictable rearing combined with uncontained parenting on the core system sustaining neural functions 243 .
We found no group differences between exposed and non‐exposed children in the neural empathic response to others' distress. This response involved alpha activation in a large cluster including the supplementary motor area, part of the embodied‐simulation network, and the middle cingulate cortex, a node of the DMN. Synchrony, which was diminished in the war‐exposed cohort, mediated the effects of early trauma on the neural empathic response, and children receiving more synchrony across childhood showed greater activation to others' distress 244 . Mothers' neural empathic response similarly showed disruptions, but those were specific to the adult brain 245 .
Prematurity
Our “kangaroo care” project is the only existing study testing the effects of maternal separation and structured contact on the maturation of life‐sustaining functions over time in human infants. Mothers of low‐birthweight premature infants (<1,750 g) were randomized to the experimental intervention (skin‐to‐skin contact for at least one hour per day for at least 14 consecutive days during the incubation period) or to standard incubation care.
Dyads were followed seven times across the first decade (before the intervention, at discharge, at 3, 6, 12 and 24 months corrected age, and at 5 and 10 years). In young adulthood (18‐20 years), we home‐visited young adults and observed their relationship with their mothers, assessed hormonal indices and executive functions, and within the next month imaged the social brain using functional magnetic resonance imaging.
We found that provision of maternal bodily contact impacted the same systems in humans as it did in young mammals. Kangaroo care improved autonomic functioning and organized the sleep‐wake cycle, and improved newborn orientation and information processing. At the same time, it improved mothering and the provision of maternal behavior in the neonatal period 246 .
Consistent with our model of the staged development of regulatory functions 138 , these improvements in physiological regulation and mothering enhanced resilience and dynamically impacted development. Neonates showed better arousal modulation in the processing of highly‐aroused stimuli at 3 months, better exploratory behavior at 6 months, and better abilities for self‐control at 1 and 2 years. Mental, but not motor, abilities were improved in the experimental group at 6, 12 and 24 months247, 248. At the same time, mother‐infant synchrony improved, and mothers also expressed more breast milk, triggering an oxytocin response 249 . Following kangaroo contact, synchrony was greater at any observation across the first years, and the higher social reciprocity linked with better cognitive and regulatory abilities 210 .
At 10 years, we found that the improved regulatory capacities of the kangaroo care subjects persisted. We found higher respiratory sinus arrhythmia and better responsivity of this arrhythmia to emotional stress, indicating more adaptive functioning of the autonomic nervous system. Sleep was measured by actigraphy worn across five consecutive nights, and children who received kangaroo care as neonates showed better sleep organization and shorter wake bouts. Furthermore, the kangaroo children's HPA axis response to social stressor exhibited diminished cortisol stress response and quicker recovery 250 . As to cognitive abilities, by 5 years there were no longer differences in general IQ, but kangaroo care subjects had improved executive abilities, working memory, and cognitive flexibility at 5 and 10 years.
Overall, our findings underscore the systems impacted by the resilience components embedded in the maternal body and well‐adapted caregiving, as those related to the management of stress, flexible response to environmental conditions, modulation of arousal and attention, and the capacity to engage in reciprocal dialogue.
In young adulthood, we imaged the brain's empathic response to others' emotions in the kangaroo care group and the controls, assessing how the brain sustains “empathic accuracy”, an important determinant of the empathic response251, 252, and differentiates response to others' distress, sadness and joy. Using complex analysis, we detected three structures that showed highly dissimilar activations across emotions: the amygdala, anterior insula, and temporal pole. Synchrony measured across development, from infancy to young adulthood, mediated the links between group membership and social brain's flexible empathic response to others' emotions. Thus, the kangaroo care increased synchrony provided a pathway by which early attachment experiences shaped the flexible neural response to others' affective states.
CONCLUSIONS
Resilience is a core construct in clinical theory and research that is yet to receive a comprehensive, biobehavioral conceptualization. Two main lacunas in current models on resilience involve the exclusive focus on the neurobiology of fear and the lack of empirical attention to development. Moreover, most models define resilience on the negation (i.e., absence of symptoms following trauma) rather than addressing what resilience is.
We argue that the initial condition of mammals should be taken into consideration in understanding resilience. Mammalian young are born with two important constrains: their brain is immature at birth, and young maintain close proximity to a nursing mother. As such, all systems that support resilience, stress management, adaptation and endurance mature in mammals in relation to the provisions afforded by mother's body and caregiving behavior.
We propose a model of resilience based on the neurobiology of affiliation, the emerging scientific field that describes the neural, endocrine, genetic and molecular processes which underpin our capacity to bond, love, care, empathize and belong to social groups.
Our model highlights three core components of the neurobiology of affiliation that sustain resilience. These include the oxytocin system, the affiliative brain, and biobehavioral synchrony.
The oxytocin system is implicated in plasticity at the cellular, molecular and network assembly levels, wires the brain toward attachments, underpins the mammalian capacity to manage hardships through relationships, and plays a role in the immune system.
The affiliative brain evolved in humans from the rodent maternal brain, expanded to include higher‐order structures that enable empathy, simulation and mentalization, and extended to support all other affiliative bonds, including romantic attachment, close friendship and mentorship. It is marked by great plasticity, cross‐generationally transmits to infant during early sensitive periods, and shapes socio‐emotional competencies.
Biobehavioral synchrony involves the coordination of biological and behavioral processes during social interaction, and it is the mechanism by which the maternal mature brain externally regulates the infant's immature brain and tunes it to social life. Humans' biobehavioral synchrony draws on mechanisms by which coordinated social behavior fosters diversity and adaptation across animal evolution, and develops within the mother‐infant bond on the basis of the fetus' biological rhythms in utero, upon which the mother builds a social non‐verbal “dance” during the first months of life. This synchronous exchange expands across development into a dialogue of mutuality, intimacy, and acknowledgement of multiple perspectives, and transfers from the mother‐child relationship to other human affiliation and encounters throughout life, charting a key trajectory in the development of resilience.
Our model proposes three tenets that address what resilience is. These include plasticity, sociality and meaning. While the first two are animal‐general, the latter is human‐specific. All three tenets are supported by oxytocin, the affiliative brain, and biobehavioral synchrony, due to their involvement in neural and behavioral plasticity, their role in attachment and sociality, and their support of the capacity to attribute meaning to trauma through cultural and spiritual systems and affiliative acts that transcend the individual.
This model is supported by evidence from three longitudinal cohorts, each followed from birth/infancy up to adolescence/young adulthood. Each cohort addressed one type of disruption to maternal‐infant bonding, originating in mother, child or context (maternal depression, premature birth, and chronic exposure to war‐related trauma), which bears long‐term impact on the child's brain, behavior and well‐being. In each cohort, hypotheses were built on a specific research program in animal models that describes the “missing component” in each condition (liking‐and‐grooming, variable foraging demands, and maternal proximity). We repeatedly measured psychopathology, parenting, synchrony, oxytocin and stress hormones, cognition and regulatory functions, particularly looking for factors that separate children on risk versus resilient trajectories. In adolescence/young adulthood, we imaged the social brain.
Disruptions to development emerged across conditions; yet, outcomes were condition‐specific and mainly expressed in interaction effects, with some children showing significant resilience. Components of the neurobiology of affiliation – synchrony and oxytocin – functioned as resilience factors across development in condition‐specific ways. Endocrine synchrony (the hormonal concordance between mother and child oxytocin and stress hormones) functioned to increase risk or resilience, attesting to the mother's continuous biological external‐regulatory impact on risk and resilient trajectories. In late childhood, children's social competencies, buttressed by synchrony, functioned as important resilience markers. Regulatory functions matured on top of one another, and greater regulation improved later functioning, particularly alterations during early sensitive periods, as, for instance, resulted from mother‐infant skin‐to‐skin contact to premature infants.
In imaging the social brain, we found alterations pending on risk and resilience status. While children reared by chronically depressed mothers aborted the neural empathic response, not all children growing in traumatic contexts showed disruptions; only those who received minimal synchrony. The brain basis of attachment was disrupted in children of depressed mothers, but only among those who developed affective disorder. Similarly, when assessing the brain basis of empathic accuracy, premature infants who received synchrony showed an adequate social neural response.
In sum, drawing on 20th century philosophical and neuroscientific models that formulated a concrete, behavior‐based approach to cognition and action and blurred the distinction of brain and mind, our model aims to direct attention to systems that sustain our capacity to form affiliative bonds, enter into social groups, and use relationships to manage stress, as core features of the human capacity to withstand, even thrive, in the face of trauma.
REFERENCES
- 1. Bonanno GA, Diminich ED. Annual research review: Positive adjustment to adversity – Trajectories of minimal‐impact resilience and emergent resilience. J Child Psychol Psychiatry Allied Discip 2013;54:378‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Masten AS. Global perspectives on resilience in children and youth. Child Dev 2014;85:6‐20. [DOI] [PubMed] [Google Scholar]
- 3. Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science 2012;338:79‐82. [DOI] [PubMed] [Google Scholar]
- 4. Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci 2009;10:446‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalisch R, Cramer AOJ, Binder H et al. Deconstructing and reconstructing resilience: a dynamic network approach. Perspect Psychol Sci 2019;14:765‐77. [DOI] [PubMed] [Google Scholar]
- 6. Charney D, Russo SJ, Murrough JW et al. Neurobiology of resilience. Nat Neurosci 2012;15:1475‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holz NE, Tost H, Meyer‐Lindenberg A. Resilience and the brain: a key role for regulatory circuits linked to social stress and support. Mol Psychiatry 2020;25:379‐96. [DOI] [PubMed] [Google Scholar]
- 8. Seligman M, Csziksentmihaly M. Positive psychology. An introduction. Am Psychol 2000;55:5‐14. [DOI] [PubMed] [Google Scholar]
- 9. Reivich KJ, Seligman MEP, McBride S. Master resilience training in the U.S. army. Am Psychol 2011;66:25‐34. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan HS. Conceptions of modern psychiatry. New York: Norton, 1940. [Google Scholar]
- 11. Fromm E. The nature of man. New York: Macmillan, 1968. [Google Scholar]
- 12. Erikson EH. Childhood and society. New York: Norton, 1963. [Google Scholar]
- 13. Maslow AH. A theory of human motivation. Psychol Rev 1943;50:370‐96. [Google Scholar]
- 14. Friedman HL, Robbins BD. The negative shadow cast by positive psychology: contrasting views and implications of humanistic and positive psychology on resiliency. Humanist Psychol 2012;40:87‐102. [Google Scholar]
- 15. Blass RB, Carmeli Z. Further evidence for the case against neuropsychoanalysis: how Yovell, Solms, and Fotopoulou's response to our critique confirms the irrelevance and harmfulness to psychoanalysis of the contemporary neuroscientific trend. Int J Psychoanal 2015;96:1555‐73. [DOI] [PubMed] [Google Scholar]
- 16. Blass RB, Carmeli Z. The case against neuropsychoanalysis: on fallacies underlying psychoanalysis’ latest scientific trend and its negative impact on psychoanalytic discourse. Int J Psychoanal 2007;88:19‐40. [DOI] [PubMed] [Google Scholar]
- 17. Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron 2012;75:747‐61. [DOI] [PubMed] [Google Scholar]
- 18. Karatsoreos IN, McEwen BS. Annual research review: The neurobiology and physiology of resilience and adaptation across the life course. J Child Psychol Psychiatry Allied Discip 2013;54:337‐47. [DOI] [PubMed] [Google Scholar]
- 19. Yehuda R, Flory JD, Southwick S et al. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann NY Acad Sci 2006;1071:379‐96. [DOI] [PubMed] [Google Scholar]
- 20. Han MH, Nestler EJ. Neural substrates of depression and resilience. Neurotherapeutics 2017;14:677‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Averill LA, Averill CL, Kelmendi B et al. Stress response modulation underlying the psychobiology of resilience. Curr Psychiatry Rep 2018;20:27. [DOI] [PubMed] [Google Scholar]
- 22. Oken BS, Chamine I, Wakeland W. A systems approach to stress, stressors and resilience in humans. Behav Brain Res 2015;282:144‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schiller D, Monfils M‐H, Raio CM et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 2010;463:49‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rutter M. Annual research review: Resilience – clinical implications. J Child Psychol Psychiatry 2013;54:474‐87. [DOI] [PubMed] [Google Scholar]
- 25. Southwick SM, Bonanno GA, Masten AS et al. Resilience definitions, theory, and challenges: interdisciplinary perspectives. Eur J Psychotraumatol 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abraham E, Feldman R. The neurobiology of human allomaternal care; implications for fathering, coparenting, and children's social development. Physiol Behav 2018;193:25‐34. [DOI] [PubMed] [Google Scholar]
- 27. Hofer MA. Hidden regulators: implication for a new understanding of attachment, separation, and loss In: Goldberg S, Muir R, Kerr J. (eds). Attachment theory: social, developmental, and clinical perspectives. Hillsdale: Analytic Press, 1995:203‐30. [Google Scholar]
- 28. Feldman R. Mutual influences between child emotion regulation and parent‐child reciprocity support development across the first 10 years of life: implications for developmental psychopathology. Dev Psychopathol 2015;27:1007‐23. [DOI] [PubMed] [Google Scholar]
- 29. Feldman R. The neurobiology of mammalian parenting and the biosocial context of human caregiving. Horm Behav 2016;77:3‐17. [DOI] [PubMed] [Google Scholar]
- 30. Feldman R. Sensitive periods in human social development: new insights from research on oxytocin, synchrony, and high‐risk parenting. Dev Psychopathol 2015;27:369‐95. [DOI] [PubMed] [Google Scholar]
- 31. Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin‐releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol 1999;115:1‐22. [DOI] [PubMed] [Google Scholar]
- 32. Feldman R, Monakhov M, Pratt M et al Pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry 2016;79:174‐84. [DOI] [PubMed] [Google Scholar]
- 33. Pratt M, Apter‐Levi Y, Vakart A et al. Mother‐child adrenocortical synchrony; moderation by dyadic relational behavior. Horm Behav 2017;89:167‐75. [DOI] [PubMed] [Google Scholar]
- 34. Althammer F, Jirikowski G, Grinevich V. The oxytocin system of mice and men – similarities and discrepancies of oxytocinergic modulation in rodents and primates. Peptides 2018;109:1‐8. [DOI] [PubMed] [Google Scholar]
- 35. Grinevich V, Knobloch‐Bollmann HS, Eliava M et al. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol Psychiatry 2016;79:155‐64. [DOI] [PubMed] [Google Scholar]
- 36. Hurlemann R, Scheele D. Dissecting the role of oxytocin in the formation and loss of social relationships. Biol Psychiatry 2016;79:185‐93. [DOI] [PubMed] [Google Scholar]
- 37. Grinevich V, Stoop R. Interplay between oxytocin and sensory systems in the orchestration of socio‐emotional behaviors. Neuron 2018;99:887‐904. [DOI] [PubMed] [Google Scholar]
- 38. Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005;308:245‐8. [DOI] [PubMed] [Google Scholar]
- 39. Baker M, Lindell SG, Driscoll CA et al. Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proc Natl Acad Sci 2017;114:11769‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumsta R, Hummel E, Chen FS et al. Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Front Neurosci 2013;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tirko NN, Eyring KW, Carcea I et al. Oxytocin transforms firing mode of CA2 hippocampal neurons. Neuron 2018;100:593‐608.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Froemke RC, Carcea I. Oxytocin and brain plasticity In: Legato M. (ed). Principles of gender‐specific medicine. Cambridge: Academic Press, 2017:161‐82. [Google Scholar]
- 43. Baumgartner T, Heinrichs M, Vonlanthen A et al. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 2008;58:639‐50. [DOI] [PubMed] [Google Scholar]
- 44. Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Neurosci 2008;9:11‐25. [DOI] [PubMed] [Google Scholar]
- 45. Bali B, Kovacs KJ. GABAergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Eur J Neurosci 2003;18:1518‐26. [DOI] [PubMed] [Google Scholar]
- 46. Blyth BJ, Hauger RL, Purdy RH et al. The neurosteroid allopregnanolone modulates oxytocin expression in the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 2000;278:R684‐91. [DOI] [PubMed] [Google Scholar]
- 47. Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005;6:877‐88. [DOI] [PubMed] [Google Scholar]
- 48. Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010;65:768‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol 2009;30:534‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feldman R. Oxytocin and social affiliation in humans. Horm Behav 2012;61:380‐91. [DOI] [PubMed] [Google Scholar]
- 51. Carter CS. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol 2014;65:17‐39. [DOI] [PubMed] [Google Scholar]
- 52. Kendrick KM. Oxytocin regulation of sheep social and maternal behavior In: Choleris E, Pfaff D, Kavaliers M. (eds). Oxytocin, vasopressin and related peptides in the regulation of behavior. Cambridge: Cambridge University Press, 2013:183‐91. [Google Scholar]
- 53. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 2001;81:629‐83. [DOI] [PubMed] [Google Scholar]
- 54. Zheng J‐J, Li S‐J, Zhang X‐D et al. Oxytocin mediates early experience‐dependent cross‐modal plasticity in the sensory cortices. Nat Neurosci 2014;17:391‐9. [DOI] [PubMed] [Google Scholar]
- 55. Cameron NM, Shahrokh D, Del Corpo A et al. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J Neuroendocrinol 2008;20:795‐801. [DOI] [PubMed] [Google Scholar]
- 56. Ferguson JN, Aldag JM, Insel TR et al. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 2001;21:8278‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hurlemann R, Patin A, Onur OA et al. Oxytocin enhances amygdala‐dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 2010;30:4999‐5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Owen SF, Tuncdemir SN, Bader PL et al. Oxytocin enhances hippocampal spike transmission by modulating fast‐spiking interneurons. Nature 2013;500:458‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anacker AMJ, Beery AK. Life in groups: the roles of oxytocin in mammalian sociality. Front Behav Neurosci 2013;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Dreu CKW, Kret ME. Oxytocin conditions intergroup relations through upregulated in‐group empathy, cooperation, conformity, and defense. Biol Psychiatry 2016;79:165‐73. [DOI] [PubMed] [Google Scholar]
- 61. Beets I, Temmerman L, Janssen T et al. Ancient neuromodulation by vasopressin/oxytocin‐related peptides. Worm 2013;2:e24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008;322:900‐4. [DOI] [PubMed] [Google Scholar]
- 63. Adkins‐Regan E. Hormonal mechanisms of mate choice. Integr Comp Biol 1998;38:166‐78. [Google Scholar]
- 64. Keverne EB, Kendrick KM. Oxytocin facilitation of maternal behavior in sheep. Ann NY Acad Sci 1992;652:83‐101. [DOI] [PubMed] [Google Scholar]
- 65. Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci 2001;2:129‐36. [DOI] [PubMed] [Google Scholar]
- 66. Maestripieri D, Hoffman CL, Anderson GM et al. Mother‐infant interactions in free‐ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol Behav 2009;96:613‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Feldman R. Bio‐behavioral synchrony: a model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting 2012;12:154‐64. [Google Scholar]
- 68. Pinkerton J, Dolan P. Family support, social capital, resilience and adolescent coping. Child Fam Soc Work 2007;12:219‐28. [Google Scholar]
- 69. Scarf D, Moradi S, McGaw K et al. Somewhere I belong: long‐term increases in adolescents’ resilience are predicted by perceived belonging to the in‐group. Br J Soc Psychol 2016;55:588‐99. [DOI] [PubMed] [Google Scholar]
- 70. DeLongis A, Holtzman S. Coping in context: the role of stress, social support, and personality in coping. J Pers 2005;73:1633‐56. [DOI] [PubMed] [Google Scholar]
- 71. Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci 2007;121:1353‐63. [DOI] [PubMed] [Google Scholar]
- 72. Feldman R, Gordon I, Influs M et al. Parental oxytocin and early caregiving jointly shape children's oxytocin response and social reciprocity. Neuropsychopharmacology 2013;38:1154‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Halevi G, Djalovski A, Kanat‐Maymon Y et al. The social transmission of risk: maternal stress physiology, synchronous parenting, and well‐being mediate the effects of war exposure on child psychopathology. J Abnorm Psychol 2017;126:1087‐103. [DOI] [PubMed] [Google Scholar]
- 74. Pasco Fearon RM, Tomlinson M, Kumsta R et al. Poverty, early care, and stress reactivity in adolescence: findings from a prospective, longitudinal study in South Africa. Dev Psychopathol 2017;29:449‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev 2006;30:173‐87. [DOI] [PubMed] [Google Scholar]
- 76. Carter CS. The role of oxytocin and vasopressin in attachment. Psychodyn Psychiatry 2017;45:499‐517. [DOI] [PubMed] [Google Scholar]
- 77. Walum H, Young LJ. The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci 2018;19:643‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li T, Wang P, Wang SC et al. Approaches mediating oxytocin regulation of the immune system. Front Immunol 2017;7:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Morhenn V, Beavin LE, Zak PJ. Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern Ther Health Med 2012;18:11‐8. [PubMed] [Google Scholar]
- 80. Szeto A, Nation DA, Mendez AJ et al. Oxytocin attenuates NADPH‐dependent superoxide activity and IL‐6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab 2008;295:E1495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Clodi M, Vila G, Geyeregger R et al. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab 2008;295:E686‐91. [DOI] [PubMed] [Google Scholar]
- 82. Ulmer‐Yaniv A, Avitsur R, Kanat‐Maymon Y et al. Affiliation, reward, and immune biomarkers coalesce to support social synchrony during periods of bond formation in humans. Brain Behav Immun 2016;56:130‐9. [DOI] [PubMed] [Google Scholar]
- 83. Gouin J‐P, Carter CS, Pournajafi‐Nazarloo H et al. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology 2010;35:1082‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Varian BJ, Poutahidis T, DiBenedictis BT et al. Microbial lysate upregulates host oxytocin. Brain Behav Immun 2017;61:36‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Feldman R. The neurobiology of human attachments. Trends Cogn Sci 2017;21:80‐99. [DOI] [PubMed] [Google Scholar]
- 86. Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol 2008;20:858‐65. [DOI] [PubMed] [Google Scholar]
- 87. Feldman R, Braun K, Champagne FA. The neural mechanisms and consequences of paternal caregiving. Nat Rev Neurosci 2019;20:1‐20. [DOI] [PubMed] [Google Scholar]
- 88. Kohl J, Babayan BM, Rubinstein ND et al. Functional circuit architecture underlying parental behaviour. Nature 2018;556:326‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abraham E, Hendler T, Shapira‐Lichter I et al. Father's brain is sensitive to childcare experiences. Proc Natl Acad Sci USA 2014;111:9792‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sokolowski K, Corbin JG. Wired for behaviors: from development to function of innate limbic system circuitry. Front Mol Neurosci 2012;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gur R, Tendler A, Wagner S. Long‐term social recognition memory is mediated by oxytocin‐dependent synaptic plasticity in the medial amygdala. Biol Psychiatry 2014;76:377‐86. [DOI] [PubMed] [Google Scholar]
- 92. Bosch OJ, Waldherr M, Nair HP et al. Viral vector‐mediated overexpression of oxytocin receptors in the amygdala of virgin rats increases aggression and reduces anxiety. Front Neuroendocrinol 2006;27:124‐5. [Google Scholar]
- 93. Bosch OJ, Meddle SL, Beiderbeck DI et al. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci 2005;25:6807‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Aggarwal M, Hyland BI, Wickens JR. Neural control of dopamine neurotransmission: implications for reinforcement learning. Eur J Neurosci 2012;35:1115‐23. [DOI] [PubMed] [Google Scholar]
- 95. Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci 2000;1:199‐207. [DOI] [PubMed] [Google Scholar]
- 96. Schultz W. Reward functions of the basal ganglia. J Neural Transm 2016;123:679‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 2015;66:25‐52. [DOI] [PubMed] [Google Scholar]
- 98. Grillner S, Hellgren J, Ménard A et al. Mechanisms for selection of basic motor programs – roles for the striatum and pallidum. Trends Neurosci 2005;28:364‐70. [DOI] [PubMed] [Google Scholar]
- 99. Maldonado‐Irizarry CS, Kelley AE. Differential behavioral effects following microinjection of an NMDA antagonist into nucleus accumbens subregions. Psychopharmacology 1994;116:65‐72. [DOI] [PubMed] [Google Scholar]
- 100. Olazábal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 2006;141:559‐68. [DOI] [PubMed] [Google Scholar]
- 101. Báez‐Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci 2013;7:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dölen G, Darvishzadeh A, Huang KW et al. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013;501:179‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ross HE, Cole CD, Smith Y et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 2009;162:892‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Numan M, Young LJ. Neural mechanisms of mother‐infant bonding and pair bonding: similarities, differences, and broader implications. Horm Behav 2015;77:98‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Darwin C. On the origin of the species. London: Murray, 1859. [Google Scholar]
- 106. Buisman‐Pijlman FTA, Sumracki NM, Gordon JJ et al. Individual differences underlying susceptibility to addiction: role for the endogenous oxytocin system. Pharmacol Biochem Behav 2014;119:22‐38. [DOI] [PubMed] [Google Scholar]
- 107. Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine‐dependent synaptic plasticity. Biol Psychiatry 2014;76:927‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron 2015;86:1145‐57. [DOI] [PubMed] [Google Scholar]
- 109. Abraham E, Raz G, Zagoory‐Sharon O et al. Empathy networks in the parental brain and their long‐term effects on children's stress reactivity and behavior adaptation. Neuropsychologia 2018;116:75‐85. [DOI] [PubMed] [Google Scholar]
- 110. Abraham E, Hendler T, Zagoory‐Sharon O et al. Network integrity of the parental brain in infancy supports the development of children's social competencies. Soc Cogn Affect Neurosci 2016;11:1707‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Abraham E, Gilam G, Kanat‐Maymon Y et al. The human coparental bond implicates distinct corticostriatal pathways: longitudinal impact on family formation and child well‐being. Neuropsychopharmacology 2017;42:2301‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pratt M, Goldstein A, Feldman R. Child brain exhibits a multi‐rhythmic response to attachment cues. Soc Cogn Affect Neurosci 2018;13:957‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pratt M, Zeev‐Wolf M, Goldstein A et al. Exposure to early and persistent maternal depression impairs the neural basis of attachment in preadolescence. Prog Neuro‐Psychopharmacol Biol Psychiatry 2019;93:21‐30. [DOI] [PubMed] [Google Scholar]
- 114. Feldman R. Parent‐infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry Allied Discip 2007;48:329‐54. [DOI] [PubMed] [Google Scholar]
- 115. Feldman R. Parent‐infant synchrony: a biobehavioral model of mutual influences in the formation of affiliative bonds. Monogr Soc Res Child Dev 2012;77:42‐51. [Google Scholar]
- 116. Feldman R, Magori‐Cohen R, Galili G et al. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav Dev 2011;34:569‐77. [DOI] [PubMed] [Google Scholar]
- 117. Feldman R, Gordon I, Zagoory‐Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent‐infant synchrony: considering stress and affiliation components of human bonding. Dev Sci 2011;14:752‐61. [DOI] [PubMed] [Google Scholar]
- 118. Levy J, Goldstein A, Feldman R. Perception of social synchrony induces mother‐child gamma coupling in the social brain. Soc Cogn Affect Neurosci 2017;12:1036‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Feldman R, Singer M, Zagoory‐Sharon O et al. Touch attenuates infants’ physiological reactivity to stress. Dev Sci 2010;13:271‐8. [DOI] [PubMed] [Google Scholar]
- 120. Ulmer‐Yaniv A, Djalovski A, Yirmiya K et al. Maternal immune and affiliative biomarkers and sensitive parenting mediate the effects of chronic early trauma on child anxiety. Psychol Med 2018;48:1020‐33. [DOI] [PubMed] [Google Scholar]
- 121. Feldman R. From biological rhythms to social rhythms: physiological precursors of mother‐infant synchrony. Dev Psychol 2006;42:175‐88. [DOI] [PubMed] [Google Scholar]
- 122. Feldman R. The relational basis of adolescent adjustment: trajectories of mother‐child interactive behaviors from infancy to adolescence shape adolescents’ adaptation. Attach Hum Dev 2010;12:173‐92. [DOI] [PubMed] [Google Scholar]
- 123. Oyama S. The ontogeny of information: developmental systems and evolution. Durham: Duke University Press, 2000. [Google Scholar]
- 124. Davis M, West K, Bilms J et al. A systematic review of parent‐child synchrony: it is more than skin deep. Dev Psychobiol 2018;60:674‐91. [DOI] [PubMed] [Google Scholar]
- 125. Noy L, Levit‐Binun N, Golland Y. Being in the zone: physiological markers of togetherness in joint improvisation. Front Hum Neurosci 2015;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Feldman R. On the origins of background emotions: from affect synchrony to symbolic expression. Emotion 2007;7:601‐11. [DOI] [PubMed] [Google Scholar]
- 127. Feldman R. Mother‐infant synchrony and the development of moral orientation in childhood and adolescence: direct and indirect mechanisms of developmental continuity. Am J Orthopsychiatry 2007;77:582‐97. [DOI] [PubMed] [Google Scholar]
- 128. Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry 2011;20:265‐76. [PMC free article] [PubMed] [Google Scholar]
- 129. Kolb B, Gibb R, Robinson TE. Brain plasticity and behavior. Curr Dir Psychol Sci 2003;12:1‐5. [Google Scholar]
- 130. Webb AR, Heller HT, Benson CB et al. Mother's voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci USA 2015;112:3152‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pereira M. Structural and functional plasticity in the maternal brain circuitry. New Dir Child Adolesc Dev 2016;2016:23‐46. [DOI] [PubMed] [Google Scholar]
- 132. Leuner B, Glasper ER, Gould E. Parenting and plasticity. Trends Neurosci 2010;33:465‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Scoglio AAJ, Rudat DA, Garvert D et al. Self‐compassion and responses to trauma: the role of emotion regulation. J Interpers Violence 2018;33:2016‐36. [DOI] [PubMed] [Google Scholar]
- 134. Schore AN. Attachment affect regulation, and the developing right brain: linking developmental neuroscience to pediatrics. Pediatr Rev 2005;26:204‐17. [DOI] [PubMed] [Google Scholar]
- 135. Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Dev 2004;75:317‐33. [DOI] [PubMed] [Google Scholar]
- 136. Fogel A. Developing through relationships: origins of communication, self and culture. Chicago: University of Chicago Press, 1993. [Google Scholar]
- 137. Thelen E, Smith LB. A dynamic systems approach to the development of cognition and action. Cambridge: MIT Press, 1994. [Google Scholar]
- 138. Feldman R. The development of regulatory functions from birth to 5 years: insights from premature infants. Child Dev 2009;80:544‐61. [DOI] [PubMed] [Google Scholar]
- 139. Tucker DM, Derryberry D, Luu P. Anatomy and physiology of human emotion: vertical integration of brainstem, limbic, and cortical systems In: Borod J. (ed). Handbook of the neuropsychology of emotion. New York: Oxford University Press, 2000:56‐79. [Google Scholar]
- 140. Eisenberg N. Emotion, regulation, and moral development. Annu Rev Psychol 2000;51:665‐97. [DOI] [PubMed] [Google Scholar]
- 141. McRae K, Gross JJ, Weber J et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci 2012;7:11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wilson EO. The social conquest of earth. New York: Liveright, 2013. [Google Scholar]
- 143. Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev 2018;98:1805‐908. [DOI] [PubMed] [Google Scholar]
- 144. Holt‐Lunstad J, Smith TB, Baker M et al. Loneliness and social isolation as risk factors for mortality. Perspect Psychol Sci 2015;10:227‐37. [DOI] [PubMed] [Google Scholar]
- 145. Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Curr Opin Neurobiol 2000;10:784‐9. [DOI] [PubMed] [Google Scholar]
- 146. Stevens FL, Wiesman O, Feldman R et al. Oxytocin and behavior: evidence for effects in the brain. J Neuropsychiatry Clin Neurosci 2013;25:96‐102. [DOI] [PubMed] [Google Scholar]
- 147. Nunes S, Fite JE, Patera KJ et al. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii). Horm Behav 2001;39:70‐82. [DOI] [PubMed] [Google Scholar]
- 148. Saltzman W, Harris BN, De Jong TR et al. Paternal care in biparental rodents: intra‐ and inter‐individual variation. Integr Comp Biol 2017;57:589‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lukas D, Clutton‐Brock TH. The evolution of social monogamy in mammals. Science 2013;341:526‐30. [DOI] [PubMed] [Google Scholar]
- 150. Kleiman DG. Monogamy in mammals. Q Rev Biol 1977;52:39‐69. [DOI] [PubMed] [Google Scholar]
- 151. Emlen ST. An evolutionary theory of the family. Proc Natl Acad Sci USA 1995;92:8092‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Huber S, Millesi E, Dittami JP. Paternal effort and its relation to mating success in the European ground squirrel. Anim Behav 2002;63:157‐64. [Google Scholar]
- 153. Smith HG, Hardling R. Clutch size evolution under sexual conflict enhances the stability of mating systems. Proc R Soc B Biol Sci 2000;267:2163‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Stockley P, Hobson L. Paternal care and litter size coevolution in mammals. Proc R Soc B Biol Sci 2016;283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wright HWY. Paternal den attendance is the best predictor of offspring survival in the socially monogamous bat‐eared fox. Anim Behav 2006;71:503‐10. [Google Scholar]
- 156. Wright SL, Brown RE. The importance of paternal care on pup survival and pup growth in Peromyscus californicus when required to work for food. Behav Processes 2002;60:41‐52. [DOI] [PubMed] [Google Scholar]
- 157. Opie C, Atkinson QD, Dunbar RIM et al. Male infanticide leads to social monogamy in primates. Proc Natl Acad Sci USA 2013;110:13328‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lamb ME. The role of the father in child development. Chichester: Wiley, 2010. [Google Scholar]
- 159. Hewlett BS. Father‐child relations: cultural and biosocial contexts. Abingdon‐on‐Thames: Routledge, 1992. [Google Scholar]
- 160. Parker G, Simmons LW. Parental investment and the control of sexual selection: predicting the direction of sexual competition. Proc R Soc Lond B 1996;263:315‐21. [Google Scholar]
- 161. Flinn MV. Correlates of reproductive success in a Caribbean village. Hum Ecol 1986;14:225‐43. [DOI] [PubMed] [Google Scholar]
- 162. Flinn MV, Low BS. Resource distribution, social competition, and mating patterns in human societies In: Rubenstein DI, Wrangham R. (eds). Ecological aspects of social evolution. Princeton: Princeton University Press, 1986:217‐43. [Google Scholar]
- 163. Coley RL. Children's socialization experiences and functioning in single‐mother households: the importance of fathers and other men. Child Dev 1998;69:219‐30. [PubMed] [Google Scholar]
- 164. Sarkadi A, Kristiansson R, Oberklaid F et al. Fathers’ involvement and children's developmental outcomes: a systematic review of longitudinal studies. Acta Paediatr 2008;97:153‐8. [DOI] [PubMed] [Google Scholar]
- 165. Feldman R, Bamberger E, Kanat‐Maymon Y. Parent‐specific reciprocity from infancy to adolescence shapes children's social competence and dialogical skills. Attach Hum Dev 2013;15:407‐23. [DOI] [PubMed] [Google Scholar]
- 166. Nelson C, Valliant PM. Personality dynamics of adolescent boys where the father was absent. Percept Mot Skills 1993;76:435‐43. [DOI] [PubMed] [Google Scholar]
- 167. Sigle‐Rushton W, McLanahan S. Father absence and child wellbeing: a critical review. New York: Russell Sage Foundation, 2004. [Google Scholar]
- 168. Dunbar RI, Shultz S. Understanding primate brain evolution. Philos Trans R Soc B Biol Sci 2007;362:649‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Morrison RE, Groenenberg M, Breuer T et al. Hierarchical social modularity in gorillas. Proc R Soc B Biol Sci 2019;286:20190681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Preis A, Samuni L, Mielke A et al. Urinary oxytocin levels in relation to post‐conflict affiliations in wild male chimpanzees (Pan troglodytes verus). Horm Behav 2018;105:28‐40. [DOI] [PubMed] [Google Scholar]
- 171. Finkenwirth C, Burkart JM. Long‐term‐stability of relationship structure in family groups of common marmosets, and its link to proactive prosociality. Physiol Behav 2017;173:79‐86. [DOI] [PubMed] [Google Scholar]
- 172. Park CL. Making sense of the meaning literature: an integrative review of meaning making and its effects on adjustment to stressful life events. Psychol Bull 2010;136:257‐301. [DOI] [PubMed] [Google Scholar]
- 173. Brewer‐Smyth K, Koenig HG. Could spirituality and religion promote stress resilience in survivors of childhood trauma? Issues Ment Health Nurs 2014;35:251‐6. [DOI] [PubMed] [Google Scholar]
- 174. Bryant‐Davis T, Ellis MU, Burke‐Maynard E et al. Religiosity, spirituality, and trauma recovery in the lives of children and adolescents. Prof Psychol Res Pract 2012;43:306‐14. [Google Scholar]
- 175. Athukorala P. Indian Ocean tsunami: disaster, generosity and recovery. Asian Econ J 2012;26:211‐31. [Google Scholar]
- 176. Landau J. Enhancing resilience: families and communities as agents for change. Fam Process 2007;46:351‐65. [DOI] [PubMed] [Google Scholar]
- 177. Aldrich DP, Meyer MA. Social capital and community resilience. Am Behav Sci 2015;59:254‐69. [Google Scholar]
- 178. James W. The will to believe. New York: Dover, 1956. [Google Scholar]
- 179. Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt, 1999. [Google Scholar]
- 180. Levy J, Goldstein A, Influs M et al. Adolescents growing up amidst intractable conflict attenuate brain response to pain of outgroup. Proc Natl Acad Sci USA 2016;113:13696‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. De Dreu CKW, Greer LL, Handgraaf MJJ et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 2010;328:1408‐11. [DOI] [PubMed] [Google Scholar]
- 182. Winnicott DW. Playing and reality. Abingdon‐on‐Thames: Routledge, 2012. [Google Scholar]
- 183. Levy J, Feldman R. Synchronous interactions foster empathy. J Exp Neurosci 2019;13:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Serón‐Ferré M, Richter HG, Valenzuela GJ et al. Circadian rhythms in the fetus and newborn: significance of interactions with maternal physiology and the environment In: Walker D. (ed). Prenatal and postnatal determinants of development. New York: Humana Press, 2016:147‐65. [Google Scholar]
- 185. Bronson SL, Bale TL. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 2016;41:207‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Yen SS. The placenta as the third brain. J Reprod Med 1994;39:277‐80. [PubMed] [Google Scholar]
- 187. Boston M. Recent research in developmental psychology. J Child Psychother 1975;4:15‐34. [Google Scholar]
- 188. Tronick E, Als H, Brazelton TB. Early development of neonatal and infant behavior In: Falkner F, Tanner JM. (eds). Human growth. Boston: Springer, 1979:305‐28. [Google Scholar]
- 189. Tronick EZ. Emotions and emotional communication in infants. Am Psychol 1989;44:112‐9. [DOI] [PubMed] [Google Scholar]
- 190. Stern DN. One way to build a clinically relevant baby. Infant Ment Health J 1994;15:9‐25. [Google Scholar]
- 191. Granat A, Gadassi R, Gilboa‐Schechtman E et al. Maternal depression and anxiety, social synchrony, and infant regulation of negative and positive emotions. Emotion 2017;17:11‐27. [DOI] [PubMed] [Google Scholar]
- 192. Beebe B, Lachmann F. Maternal self‐critical and dependent personality styles and mother‐infant communication. J Am Psychoanal Assoc 2017;65:491‐508. [DOI] [PubMed] [Google Scholar]
- 193. Feldman R, Greenbaum CW, Yirmiya N. Mother‐infant affect synchrony as an antecedent of the emergence of self‐control. Dev Psychol 1999;35:223‐31. [DOI] [PubMed] [Google Scholar]
- 194. Friston KJ. Waves of prediction. PLoS Biol 2019;17:e3000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kilner JM, Friston KJ, Frith CD. Predictive coding: an account of the mirror neuron system. Cogn Process 2007;8:159‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Sedley W, Gander PE, Kumar S et al. Neural signatures of perceptual inference. Elife 2016;5:e11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Fries P. Rhythms for cognition: communication through coherence. Neuron 2015;88:220‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kinreich S, Djalovski A, Kraus L et al. Brain‐to‐brain synchrony during naturalistic social interactions. Sci Rep 2017;7:17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Cao W, Lin S, Xia Q et al. Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron 2018;97:1253‐60.e7. [DOI] [PubMed] [Google Scholar]
- 200. Cho KKA, Hoch R, Lee AT et al. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6+/− mice. Neuron 2015;85:1332‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Levy J, Goldstein A, Pratt M et al. Maturation of pain empathy from child to adult shifts from single to multiple neural rhythms to support interoceptive representations. Sci Rep 2018;8:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Gireesh ED, Plenz D. Neuronal avalanches organize as nested theta‐ and beta/gamma‐oscillations during development of cortical layer 2/3. Proc Natl Acad Sci USA 2008;105:7576‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Gendron M, Barrett LF. Emotion perception as conceptual synchrony. Emot Rev 2018;10:101‐10. [Google Scholar]
- 204. Mirmiran M, Maas YGH, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev 2003;7:321‐34. [DOI] [PubMed] [Google Scholar]
- 205. Okai T, Kozuma S, Shinozuka N et al. A study on the development of sleep‐wakefulness cycle in the human fetus. Early Hum Dev 1992;29:391‐6. [DOI] [PubMed] [Google Scholar]
- 206. Pildner von Steinburg S, Boulesteix A‐L, Lederer C et al. What is the “normal” fetal heart rate? Peer J 2013;1:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Mulder EJH, Visser GHA. Fetal behavior: clinical and experimental research in the human In: Reissland N, Kisilevsky BS. (eds). Fetal development. Cham: Springer, 2016:87‐105. [Google Scholar]
- 208. Waddell BJ, Wharfe MD, Crew RC et al. A rhythmic placenta? Circadian variation, clock genes and placental function. Placenta 2012;33:533‐9. [DOI] [PubMed] [Google Scholar]
- 209. Feldman R, Eidelman AI, Rotenberg N. Parenting stress, infant emotion regulation, maternal sensitivity, and the cognitive development of triplets: a model for parent and child influences in a unique ecology. Child Dev 2004;75:1774‐91. [DOI] [PubMed] [Google Scholar]
- 210. Feldman R, Eidelman AI. Biological and environmental initial conditions shape the trajectories of cognitive and social‐emotional development across the first years of life. Dev Sci 2009;12:194‐200. [DOI] [PubMed] [Google Scholar]
- 211. Gordon I, Zagoory‐Sharon O, Leckman JF et al. Oxytocin and the development of parenting in humans. Biol Psychiatry 2010;68:377‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Feldman R. Infant‐mother and infant‐father synchrony: the coregulation of positive arousal. Infant Ment Health J 2003;24:1‐23. [Google Scholar]
- 213. Feldman R. The social neuroendocrinology of human parenting In: Bornstein MH. (ed). Handbook of parenting. London: Routledge, 2019:220‐49. [Google Scholar]
- 214. Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology 2011;36:2603‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Gordon I, Feldman R. Synchrony in the triad: a microlevel process model of coparenting and parent‐child interactions. Fam Process 2008;47:465‐79. [DOI] [PubMed] [Google Scholar]
- 216. Feldman R, Greenbaum CW. Affect regulation and synchrony in mother‐infant play as precursors to the development of symbolic competence. Infant Ment Health J 1997;18:4‐23. [Google Scholar]
- 217. Feldman R, Masalha S. Parent‐child and triadic antecedents of children's social competence: cultural specificity, shared process. Dev Psychol 2010;46:455‐67. [DOI] [PubMed] [Google Scholar]
- 218. Halevi G, Djalovski A, Vengrober A et al. Risk and resilience trajectories in war‐exposed children across the first decade of life. J Child Psychol Psychiatry 2016;57:1183‐93. [DOI] [PubMed] [Google Scholar]
- 219. Pratt M, Goldstein A, Levy J et al. Maternal depression across the first years of life impacts the neural basis of empathy in preadolescence. J Am Acad Child Adolesc Psychiatry 2017;56:20‐29.e3. [DOI] [PubMed] [Google Scholar]
- 220. Ulmer‐Yaniv A, Djalovski A, Priel A et al. Maternal depression alters stress and immune biomarkers in mother and child. Depress Anxiety 2018;35:1145‐57. [DOI] [PubMed] [Google Scholar]
- 221. Kessler RC, Petukhova M, Sampson NA et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 2012;21:169‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev 2010;81:41‐79. [DOI] [PubMed] [Google Scholar]
- 223. Beck S, Wojdyla D, Say L et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010;88:31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Coplan J, Andrews M, Rosenblum L et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin‐releasing factor in adult nonhuman primates exposed to early‐life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci USA 1996;93:1619‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Coplan JD, Smith EL, Altemus M et al. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin‐releasing factor concentrations in adult primates. Biol Psychiatry 2001;50:200‐4. [DOI] [PubMed] [Google Scholar]
- 226. Apter‐Levy Y, Feldman M, Vakart A et al. Impact of maternal depression across the first 6 years of life on the child's mental health, social engagement, and empathy: the moderating role of oxytocin. Am J Psychiatry 2013;170:1161‐8. [DOI] [PubMed] [Google Scholar]
- 227. Priel A, Djalovski A, Zagoory‐Sharon O et al. Maternal depression impacts child psychopathology across the first decade of life: oxytocin and synchrony as markers of resilience. J Child Psychol Psychiatry Allied Discip 2019;60:30‐42. [DOI] [PubMed] [Google Scholar]
- 228. Apter‐Levi Y, Pratt M, Vakart A et al. Maternal depression across the first years of life compromises child psychosocial adjustment; relations to child HPA‐axis functioning. Psychoneuroendocrinology 2016;64:47‐56. [DOI] [PubMed] [Google Scholar]
- 229. Feldman R, Granat A, Pariante C et al. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry 2009;48:919‐27. [DOI] [PubMed] [Google Scholar]
- 230. Vakrat A, Apter‐Levy Y, Feldman R. Sensitive fathering buffers the effects of chronic maternal depression on child psychopathology. Child Psychiatry Hum Dev 2018;49:779‐85. [DOI] [PubMed] [Google Scholar]
- 231. Vakrat A, Apter‐Levy Y, Feldman R. Fathering moderates the effects of maternal depression on the family process. Dev Psychopathol 2018;30:27‐38. [DOI] [PubMed] [Google Scholar]
- 232. Feldman R, Masalha S. The role of culture in moderating the links between early ecological risk and young children's adaptation. Dev Psychopathol 2007;19:1‐21. [DOI] [PubMed] [Google Scholar]
- 233. Pratt M, Apter‐Levi Y, Vakart A et al. Maternal depression and child oxytocin response; moderation by maternal oxytocin and relational behavior. Depress Anxiety 2015;32:635‐46. [DOI] [PubMed] [Google Scholar]
- 234. Brüne M. Does the oxytocin receptor polymorphism (rs2254298) confer “vulnerability” for psychopathology or “differential susceptibility”? Insights from evolution. BMC Med 2012;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Feldman R, Vengrober A, Ebstein RP. Affiliation buffers stress: cumulative genetic risk in oxytocin‐vasopressin genes combines with early caregiving to predict PTSD in war‐exposed young children. Transl Psychiatry 2014;4:e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Feldman R, Vengrober A, Eidelman‐Rothman M et al. Stress reactivity in war‐exposed young children with and without posttraumatic stress disorder: relations to maternal stress hormones, parenting, and child emotionality and regulation. Dev Psychopathol 2013;25:943‐55. [DOI] [PubMed] [Google Scholar]
- 237. Yirmiya K, Djalovski A, Motsan S et al. Stress and immune biomarkers interact with parenting behavior to shape anxiety symptoms in trauma‐exposed youth. Psychoneuroendocrinology 2018;98:153‐60. [DOI] [PubMed] [Google Scholar]
- 238. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007;87:873‐904. [DOI] [PubMed] [Google Scholar]
- 239. Axelrod V, Rees G, Bar M. The default network and the combination of cognitive processes that mediate self‐generated thought. Nat Hum Behav 2017;1:896‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Li W, Mai X, Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci 2014;8:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Satpute AB, Lindquist KA. The default mode network's role in discrete emotion. Trends Cogn Sci 2019;23:851‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Schäfer CB, Morgan BR, Ye AX et al. Oscillations, networks, and their development: MEG connectivity changes with age. Hum Brain Mapp 2014;35:5249‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Zeev‐Wolf M, Levy J, Goldstein A et al. Chronic early stress impairs default mode network connectivity in preadolescents and their mothers. Biol Psychiatry Cogn Neurosci Neuroimaging 2019;4:72‐80. [DOI] [PubMed] [Google Scholar]
- 244. Levy J, Goldstein A, Feldman R. The neural development of empathy is sensitive to caregiving and early trauma. Nat Commun 2019;10:1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Levy J, Yirmiya K, Goldstein A et al. Chronic trauma impairs the neural basis of empathy in mothers: relations to parenting and children's empathic abilities. Dev Cogn Neurosci 2019;38:100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Feldman R, Eidelman AI, Sirota L et al. Comparison of skin‐to‐skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics 2002;110:16‐26. [DOI] [PubMed] [Google Scholar]
- 247. Feldman R, Weller A, Sirota L et al. Skin‐to‐skin contact (kangaroo care) promotes self‐regulation in premature infants: sleep‐wake cyclicity, arousal modulation, and sustained exploration. Dev Psychol 2002;38:194‐207. [DOI] [PubMed] [Google Scholar]
- 248. Feldman R. Mother‐infant skin‐to‐skin contact (kangaroo care): theoretical, clinical, and empirical aspects. Infants Young Child 2004;2:145‐61. [Google Scholar]
- 249. Feldman R, Eidelman AI. Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Dev Psychobiol 2003;43:109‐19. [DOI] [PubMed] [Google Scholar]
- 250. Feldman R, Rosenthal Z, Eidelman AI. Maternal‐preterm skin‐to‐skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry 2014;75:56‐64. [DOI] [PubMed] [Google Scholar]
- 251. Zaki J, Weber J, Bolger N et al. The neural bases of empathic accuracy. Proc Natl Acad Sci USA 2009;106:11382‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Mackes NK, Golm D, O'Daly OG et al. Tracking emotions in the brain – revisiting the Empathic Accuracy Task. Neuroimage 2018;178:677‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]


