Abstract
Introduction
As a result of the negative impact of bone metastases on patient quality of life, it is important to identify patients at increased risk of skeletal-related events (SREs). Biochemical markers produced by osteoblasts and osteoclasts may provide an early indicator of treatment response to antiresorptive therapy. We aimed to explore the relationship between change in the urinary bone turnover marker cross-linked N-terminal telopeptide of type 1 collagen (uNTX) at the earliest time of steady state and risk of SREs.
Methods
A comprehensive search of eight bibliographic databases and two trial registries was conducted (June 2017). We included randomized controlled trials of adults (≥18 years old) with bone metastases from solid tumors (including breast, lung, prostate) or bone lesions from multiple myeloma that compared denosumab or bisphosphonate(s) with each other or a placebo. Meta-analyses were used to evaluate the relationship between uNTX and SREs. The primary outcomes were based on uNTX at week 13 and SREs in those studies.
Results
Seventeen studies (12,130 patients) were included. The analysis results indicated a positive association between uNTX reduction, measured by the between-group difference of the natural logarithm of the ratio between uNTX at week 13 and baseline, and SRE risk reduction, measured by the natural logarithm of the hazard ratio (HR) for time to first SRE between the two groups (uNTX effect on SRE risk, defined as SRE HR increase corresponding to one unit smaller in the magnitude of uNTX reduction: 0.3560, 95% confidence interval 0.0249–0.6871; P = .0390, R2 = 0.7360). Results were similar for studies that reported change in uNTX from baseline to week 13 and to later than week 13. The limitation of this review is that it depends on how comprehensive study data were that could be included in the meta-regression.
Conclusions
Our findings support a positive relationship between reduction of bone turnover markers at the earliest time of steady state and reduction in longer-term risk of SREs.
Keywords: Meta-regression, Systematic review, Skeletal-related event, Bone turnover, Crosslinked N-telopeptide of type 1 collagen
Highlights
-
•
Identifying those at risk of skeletal-related events is key to ameliorate the impact of bone metastases on quality-of-life.
-
•
uNTX and bone-specific alkaline phosphatase are candidate surrogate markers for skeletal-related events.
-
•
We report a relationship between reduced markers of bone turnover and reduced longer-term risk of skeletal-related events.
1. Introduction
Bone metastases commonly occur during the progression of cancer, including both solid tumors and multiple myeloma. Although any malignancy may metastasize to bone, bone metastases are most commonly seen in advanced breast (70–80%), prostate (70–80%), thyroid (60%), lung (10–50%), and renal cancers (30%) (Grávalos et al., 2016). The bones of the pelvis, upper leg, skull, and hip are most commonly affected (National Institute for Health and Care Excellence, 2012). Estimates suggest that in 2012, approximately 330,000 adult patients in the USA were living with solid tumors and bone metastases (Hernandez et al., 2015). Bone metastases have a significant negative impact on the lives of patients and represent a significant resource burden for healthcare systems (Pockett et al., 2010).
Bone metastases disrupt the normal process of bone production by osteoblasts, as well as bone remodeling and resorption by osteoclasts, leading to symptoms such as bone pain (Grávalos et al., 2016). Other common clinical manifestations include pathological bone fractures, spinal cord compression, and the development of bone lesions, requiring the need for bone surgery and/or radiotherapy (Grávalos et al., 2016). Collectively, these clinical symptoms are often referred to as skeletal-related events (SREs) and are one of the most common complications of bone metastases in patients with solid tumors or multiple myeloma (Grávalos et al., 2016). The development of SREs is associated with poor long-term prognosis, negative impact on health-related quality of life, and higher probability of new bone events occurring (Grávalos et al., 2016). Therefore, it is important to identify which patients are at increased risk of SREs and which treatments effectively reduce the risk of SREs. However, conventional methods of measuring SREs, such as bone mineral densitometry, scintigraphy, and plain X-rays (Coleman et al., 2008), can be imprecise and rely on detecting changes after events have occurred.
Numerous biochemical markers produced by osteoblasts and osteoclasts may provide an alternative means of measuring skeletal health and treatment response in bone metastases. Assessments of serum or urinary levels of bone turnover markers may provide a simpler, more rapid, and more convenient way of detecting changes in skeletal health or bone lesion progression (Coleman et al., 2008). The ideal marker would need to be both sensitive and specific, and several candidate markers, including urinary N-telopeptide of type 1 collagen (uNTX) and bone-specific alkaline phosphatase (bone ALP), have been suggested (Grávalos et al., 2016; Coleman et al., 2008; Coleman et al., 2011). However, research is still required to establish the most appropriate surrogate marker for SREs (Coleman et al., 2011).
The mediation of bone destruction by osteoclasts requires the receptor activator of NFκB ligand (RANKL) (Lacey et al., 1998; Roodman, 2001). Preclinical models have shown that cancer cells increase RANKL and decrease osteoprotegerin expression by osteoclasts (Fizazi et al., 2003; Yang et al., 2001). Tumor cells induce osteoclast activation through RANKL, which subsequently releases growth factors and mediates bone resorption, resulting in a cycle of bone destruction and tumor proliferation (Roodman, 2004). Bone ALP hydrolyzes pyrophosphate, thus removing an inhibitor of osteogenesis while creating the inorganic phosphate required for development and deposition of hydroxyapatite (Balcerzak et al., 2003); it is therefore viewed as a reflection of osteogenesis (Coleman et al., 2011), whereas NTX is viewed as a reflection of osteolysis (Coleman et al., 2011). Crosslinked N-telopeptide is an amino-terminal peptide of mature type I collagen and is released during bone resorption (Watts, 1999). NTX molecules are mobilized by osteoclasts and subsequently excreted in the urine (as uNTX). NTX has been identified as a sensitive marker of bone metastases from prostate, breast, and lung cancer, as well as bone lesions associated with multiple myeloma (Coleman et al., 2008).
This systematic review explores the relationship between the change in uNTX at the earliest time of steady state and the longer-term risk of SREs; the same relationship for bone ALP was also investigated for exploratory purposes. Change in uNTX at week 13 was considered based on trials of intravenous bisphosphonates on levels of bone turnover and evidence of rapid and sustained suppression of bone turnover markers following treatment with denosumab suggesting a steady state (Fizazi et al., 2009; Lipton et al., 2007a). uNTX was our primary focus as most drugs affect markers of both osteoblasts and osteoclasts in the same direction, and it is hypothesized that osteoclast activity has a greater effect on the risk of SREs. Our analyses inform the feasibility of using bone markers as surrogate outcomes for SREs.
2. Materials and methods
The literature searches and systematic review adhered to published methods, including those recommended by the Cochrane Collaboration (Higgins and Green, 2011) and Centre for Reviews and Dissemination (CRD), UK (Centre for Reviews and Dissemination, 2009), in order to minimize the risk of bias and error.
2.1. Literature search strategy
Nine bibliographic databases were searched from inception to June 2017: MEDLINE; MEDLINE In-Process Citations and Daily Update; MEDLINE Epub Ahead of Print; EMBASE; Cochrane Database of Systematic Reviews (CDSR); Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; Database of Abstracts of Reviews of Effects (DARE); and Health Technology Assessment Database (HTA). The search strategies combined relevant terms comprising indexed keywords (eg Medical Subject Headings, MeSH in MEDLINE, EMTREE in EMBASE) and free-text terms appearing in the titles and/or abstracts of database records, and included search filters specifically designed to retrieve randomized controlled trials (RCTs) and observational studies. Full search strategies are provided in the Supplementary Material. Supplementary searches were conducted to identify completed and ongoing trials by searching the clinical trial registers, National Institutes of Health (NIH) ClinicalTrials.gov (http://www.clinicaltrials.gov/) and World Health Organization (WHO) International Clinical Trials Registry (http://www.who.int/ictrp/en/), up to June 9, 2017. Searches were not limited by language, publication status (unpublished or published), or date of publication. Additional unpublished material and copies of US Food and Drug Administration (FDA) reports were provided by Amgen International.
2.2. Selection criteria
Eligible studies met the following inclusion criteria: RCTs, placebo-controlled studies, single-arm studies with no comparator, and prospective cohort studies reporting data for adult patients (≥18 years old) with bone marrow metastases from solid tumors or bone lesions from multiple myeloma that compared one or more of the following treatments with each other, placebo, or no treatment:
-
•
Denosumab
-
•
Sodium clodronate, clodronic acid, or clodronate disodium
-
•
Disodium pamidronate, pamidronic acid, or pamidronate disodium pentahydrate
-
•
Ibandronic acid or ibandonate
-
•
Zoledronic acid or zoledronate
-
•
Etidronate or etidronate disodium
-
•
Risedronate
-
•
Alendronate
Studies had to report both SRE outcomes (pathological fracture, spinal cord compression, necessity for radiation to bone [for pain or impending fracture], surgery to bone) and change from baseline in uNTX or bone ALP at a minimum of a full 12 weeks of follow-up or uNTX/bone ALP results at baseline and Week ≥13. Relevant SRE outcomes were expressed as hazard ratio (HR), risk ratio (RR), or event data per patient. Studies that evaluated different dosing schedules for the same treatment (eg zoledronate 4 mg every 4 weeks [Q4W] vs every 12 weeks [Q12W]) were excluded from the analyses.
Titles and abstracts were independently screened for relevance by two reviewers, and full-text articles of studies considered potentially relevant were subsequently assessed for inclusion. Disagreements at any stage of study selection were resolved through discussion and consensus, or by consultation with a third reviewer.
2.3. Data extraction and management
The data extraction process was performed by two reviewers, with one reviewer extracting the study data and a second reviewer checking the data against the original study publication. Any discrepancies were resolved through discussion. If bone marker data were presented only in a figure, we estimated values reported closest to week 13 using the plot digitizer software DigizeIt (http://www.digitizeit.de/).
2.4. Quality assessment
The methodological quality of included studies was assessed using the Cochrane Collaboration's tool for assessing risk of bias in randomized trials (Higgins et al., 2011). This process was performed by two reviewers, with one reviewer completing the initial assessment and a second reviewer checking the assessment against the original study publication; for six studies, the assessment considered additional information in the form of study protocols supplied by Amgen International (Fizazi et al., 2009; Lipton et al., 2007a; Amgen, Daiichi Sankyo, Inc, n.d.; Fizazi et al., 2011; Henry et al., 2011; Stopeck et al., 2010).
2.5. Data synthesis and analysis
Meta-regression analyses were used to model the relationship between uNTX and SREs. A linear regression model weighted by the inverse variance of log (HR) of SRE was used to account for differences in trial sizes. The analyses were performed with Stata version 13.1 and SAS version 9.4. The estimate of uNTX effect, corresponding 95% confidence interval (CI), P value based on t-statistics, and weighted R2 were provided. As the statistical distribution of percentage change data was likely to deviate from normality, the change from baseline in natural logarithm (ln)-transformed values of uNTx (which are expected to fit normality) was used. Owing to the monotonic transformation between log ratio and percentage change, any percentage change data available for a study were easily transformed into log ratios.
The following meta-regression analyses were performed for uNTX values:
-
•
Log (HR) of SRE and difference in log (post-baseline/baseline) uNTX
-
•
Log (HR) of SRE and difference in log (post-baseline/baseline) uNTX with imputing of missing HR of SRE by RR of SRE
uNTX reduction was measured by the between-group difference in the natural logarithm of the ratio between uNTX at week 13 and baseline, which is log (week 13/baseline) for arm 1 – log (week 13/baseline) for arm 2, and can also be expressed as (log [week 13] – log [baseline]) for arm 1 – (log [week 13] – log [baseline]) for arm 2. As the statistical distribution of the serum concentration data might deviate from normality, the natural log-transformed values of uNTx (which are expected to fit normality) were used.
The study aimed to explore the relationship between change in the bone turnover marker uNTX at the earliest time of steady state and risk of SREs. Each study has its own uNTX reduction and corresponding RR/HR. The data from different studies were analyzed together. This is based on the assumption that a change in uNTX between groups, whether placebo or an active comparator, should result in a difference in the risk of SRE. Data were first analyzed at week 13. We also conducted a combined analysis as ‘log HR of SRE and difference in log (after week 13/baseline) uNTX’, which combined the week 13 data with data beyond week 13.
The analyses were repeated for exploratory purposes using bone ALP data in place of uNTX.
Data are illustrated using a circle to represent each study, and the size of the circle is proportional to the inverse of variance of log (HR) (or log [RR] of SRE if log [HR] is missing) for an individual study against the regression line.
3. Results
3.1. Overview of included studies
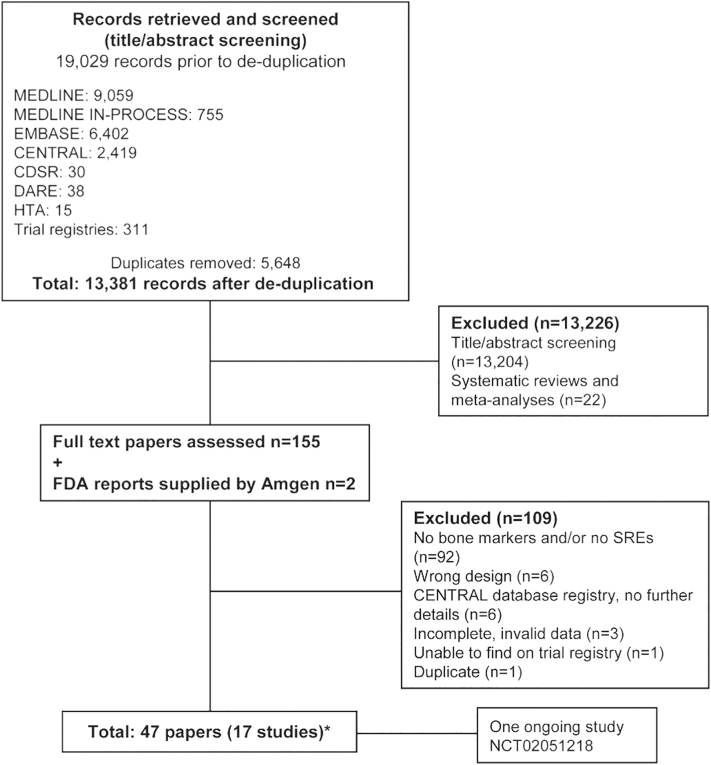
The searches identified 13,381 references. From these records, 155 full papers were obtained and screened in detail by two independent reviewers to determine whether they fulfilled the review inclusion criteria. After subsequent detailed review, 45 publications reporting 15 studies were selected as meeting all the inclusion criteria; a further two publications (FDA reports), supplied by Amgen, were also included. Therefore, 17 studies in total were included in this systematic review. We identified one ongoing study (Swiss Group for Clinical Cancer Research, n.d.). Details of the included studies are provided in Supplementary Table 1, and excluded publications and the reasons for exclusion are provided in Supplementary Table 2. A summary of the search, screening, and assessment process is presented in Fig. 1.
Fig. 1.
Summary of study flow and selection.
*One Amgen study was identified as a trial registry entry but was only included after additional information was provided by Amgen. Some unpublished data from Amgen trials are included: NCT00321464, NCT00330759, NCT00321620, NCT01345019, NCT00091832, NCT00104650
All 17 included studies were parallel RCTs with both SRE-related endpoints and either uNTX or bone ALP measurements; 15 studies were complete and published at the time of writing (Fizazi et al., 2009; Lipton et al., 2007a; Fizazi et al., 2011; Henry et al., 2011; Stopeck et al., 2010; Berenson et al., 1998; Berenson et al., 2001; Hortobagyi et al., 1996; Hortobagyi et al., 2017; Jiang et al., 2016; Rosen et al., 2001; Rosen et al., 2003; Saad et al., 2002; Theriault et al., 1999; Zhao et al., 2011), one study had been terminated because of poor accrual (Sweeney et al., n.d.), and unpublished data for one ongoing study were provided by Amgen (Amgen, Daiichi Sankyo, Inc, n.d.). Available data from all 17 studies were included in the analyses. Thirteen of the 17 included studies were worldwide, multicenter trials (Fizazi et al., 2009; Lipton et al., 2007a; Amgen, Daiichi Sankyo, Inc, n.d.; Fizazi et al., 2011; Henry et al., 2011; Stopeck et al., 2010; Berenson et al., 1998; Berenson et al., 2001; Hortobagyi et al., 1996; Rosen et al., 2001; Rosen et al., 2003; Saad et al., 2002; Theriault et al., 1999).
The outcome measures reported varied between studies, so none of the analyses included data from all studies. Two studies met the inclusion criteria for our systematic review but did not contribute data to any of the analyses: one study was a comparison of different dose schedules for a single intervention (Hortobagyi et al., 2017), and the other reported uNTX data only as median (standard deviation) at baseline and follow-up, so the percentage change from baseline could not be calculated (Sweeney et al., n.d.). Where reported, the SRE definition was consistent across the included studies, except for one study (Theriault et al., 1999) that included hypercalcemia of malignancy in its definition of SRE.
Overall, there was a low risk of bias associated with randomization, allocation concealment, and blinding of research staff, patients, and outcome assessors. There was evidence of unclear bias for selective outcome reporting, because results were not presented for all the outcome measures specified in the protocol or methods. Most of the included studies were rated as having an unclear risk of bias for incomplete outcome data because bone marker data were, or appeared to be, reported for only a subset of the study population. One study was classified as having a ‘high’ risk of bias for other criteria (Sweeney et al., n.d.) because, although results were reported in the trial registry entry, the study had been terminated early as a result of poor accrual.
3.2. Relationship between change in uNTX and risk of SRE
A summary of the results of studies that reported data for both SRE outcomes and uNTX and that contributed data to one or more analyses is provided in Table 1.
Table 1.
Summary of results for SRE outcomes versus change in uNTX.
| Study | Treatment 1 (number of subjects) | Treatment 2 (number of subjects) | Log (HR) SRE (SE) |
Log (RR) SRE (SE) | uNTX group difference of log (week 13/baseline) |
uNTX group difference of log (final/baseline) |
|---|---|---|---|---|---|---|
| Amgen 2017 (Amgen, Daiichi Sankyo, Inc, n.d.) | Denosumab 120 mg SC Q4W (859) |
Zoledronate 4 mg IV Q4W (859) |
−0.0156 (0.0733) |
−0.0185 (0.0542) |
−0.17 | – |
| Berenson 2001 (Berenson et al., 2001) | Zoledronate 4 mg IV Q4W (67) |
Pamidronate 90 mg IV Q4W (73) |
NR | 0.0858 (0.2496) |
−0.3848 | – |
| Fizazi 2009 (Fizazi et al., 2009) |
Denosumab, multiple doses combined (74) | Zoledronic acid/ pamidronate IV Q4W (37) |
−0.9586 (0.6728) |
−0.6932 (0.5411) |
−1.18 | – |
| Fizazi 2011 (Fizazi et al., 2011) |
Denosumab 120 mg SC Q4W (950) | Zoledronate 4 mg IV Q4W (951) |
−0.1970 (0.0749) |
−0.1229 (0.0585) |
−0.65 | – |
| Henry 2011 (Henry et al., 2011) | Denosumab 120 mg SC Q4W (886) |
Zoledronate 4 mg IV Q4W (890) |
−0.1777 (0.0823) |
−0.1455 (0.0666) |
−0.39 | – |
| Jiang 2016 (Jiang et al., 2016) | Denosumab 120 mg SC Q4W (326) |
Zoledronate 4 mg IV Q4W (159) |
NR | −0.2480 (0.3913) |
−0.3149 | – |
| Lipton 2007 (Lipton et al., 2007a) |
Denosumab, multiple doses combined (212) | Zoledronic acid/pamidronate/ ibandronate IV Q4W (43) |
−0.3585 (0.4260) |
−0.2832 (0.3916) |
−0.12 | – |
| Rosen 2001 (Rosen et al., 2001) | Zoledronate 4 mg IV Q3/4W (563) |
Pamidronate 90 mg IV Q3/4W (556) |
−0.0834 (0.0887) |
−0.0464 (0.0659) |
−0.3112 | – |
| Rosen 2003 (Rosen et al., 2003) |
Zoledronate 4 mg IV Q3W (254) |
Placebo IV Q4W (247) | −0.3106 (0.1402) |
−0.2133 (0.1039) |
NR | −0.9029 (week 36) |
| Saad 2002 (Saad et al., 2002) | Zoledronate 4 mg IV Q3W (214) |
Placebo IV Q3W (208) | −0.4050 (0.1497) |
−0.2876 (0.1244) |
−1.2448 | – |
| Stopeck 2010 (Stopeck et al., 2010) | Denosumab 120 mg SC Q4W (1026) |
Zoledronate 4 mg IV Q4W (1020) |
−0.1977 (0.0769) |
−0.1722 (0.0625) |
−0.43 | – |
| Theriault 1999 (Theriault et al., 1999) |
Pamidronate 90 mg IV Q4W (182) |
Placebo IV Q4W (189) | NR | −0.1815 (0.0830) |
NR | −0.3207 (week 96) |
| Zhao 2011 (Zhao et al., 2011) | Zoledronate 4 mg IV Q3W (30) |
Placebo IV Q3W (29) | NR | −0.0339 (0.6574) |
−1.2354 | – |
IV, intravenous; NR, not reported; Q3W, every 3 weeks; SC, subcutaneous; SE, standard error.
3.2.1. Log (HR) SRE versus week 13 data for change in uNTX
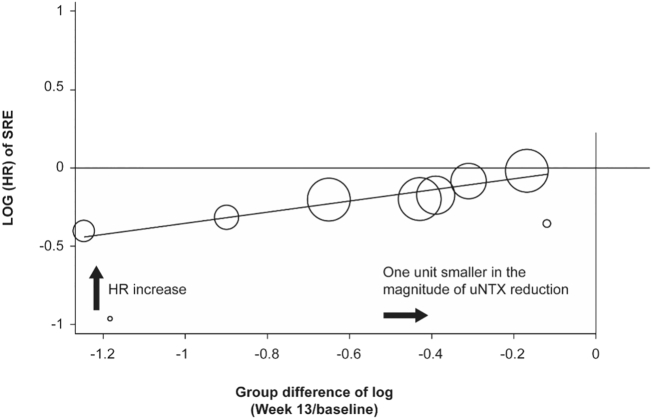
Eight studies contributed data to the analyses of HR of SRE versus week 13 data for change in uNTX (Fizazi et al., 2009; Lipton et al., 2007a; Amgen, Daiichi Sankyo, Inc, n.d.; Fizazi et al., 2011; Henry et al., 2011; Stopeck et al., 2010; Rosen et al., 2001; Saad et al., 2002). uNTX data were estimated from figures in three studies (Fizazi et al., 2009; Rosen et al., 2001; Saad et al., 2002). Meta-regression indicated a positive association between uNTX reduction, measured by the between-group difference of the natural logarithm of the ratio between uNTX at week 13 and baseline, and SRE risk reduction, measured by the natural logarithm of the HR for time to first SRE between the two groups (uNTX effect on SRE risk defined as SRE HR increase corresponding to one log unit smaller in the magnitude of uNTX reduction: 0.3560, 95% CI 0.0249–0.6871; P = .0390, R2 = 0.7360; Fig. 2). The positive slope indicates a positive relationship between HR and the log difference in uNTX, thus the closer the difference of uNTX is to zero, the larger the HR. A ‘one log unit smaller’ reduction for uNTX reflects a one unit increase towards zero for uNTX difference. Thus, as the value for uNTX decreases from baseline, the risk of SRE is lowered.
Fig. 2.
Log (HR) of SRE versus group difference of log (week 13/baseline) in uNTX*.
*Data are illustrated using a circle to represent an individual study, and the size of the circle is proportional to the inverse of variance of log (HR) (or log [RR] of SRE if log [HR] is missing) for an individual study.
Eleven studies contributed data to the sensitivity analyses of HR of SRE versus change in uNTX from baseline to week 13, with imputing of missing HR with RR. The analysis still indicated a statistically significant positive association between log (HR) of SRE and group difference of uNTX in log (week 13/baseline) (uNTX effect 0.3441; 95% CI 0.0424–0.6458; P = .0297, R2 = 0.6409).
3.2.2. Log (HR) SRE versus data after week 13 or final data for change in uNTX
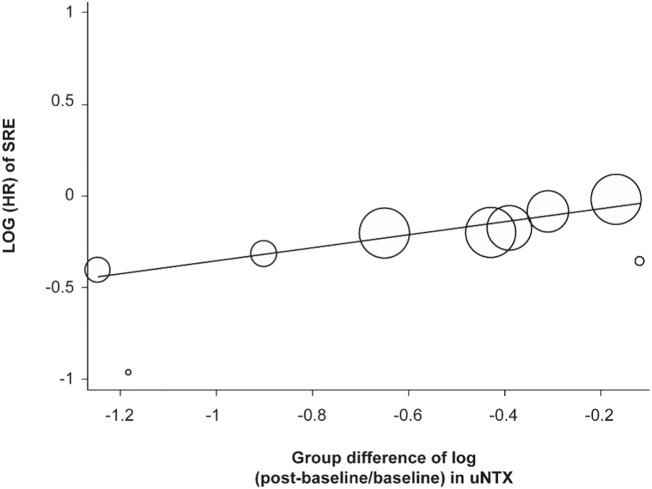
Nine studies contributed data to the analyses of HR of SRE versus change in uNTX, which included data for change at week 13 or later (Fizazi et al., 2009; Lipton et al., 2007a; Amgen, Daiichi Sankyo, Inc, n.d.; Fizazi et al., 2011; Henry et al., 2011; Stopeck et al., 2010; Rosen et al., 2001; Rosen et al., 2003; Saad et al., 2002). uNTX data were estimated from figures in three studies (Fizazi et al., 2009; Rosen et al., 2001; Saad et al., 2002). The results of the meta-regression analyses showed similar positive association to those of the analyses that included only week 13 data (uNTX effect 0.3537; 95% CI 0.0605–0.6469; P = .0246, R2 = 0.7660; Fig. 3).
Fig. 3.
Log (HR) of SRE versus group difference of log (after week 13 or final/baseline) in uNTX*.
*Data are illustrated using a circle to represent an individual study, and the size of the circle is proportional to the inverse of variance of log (HR) (or log [RR] of SRE if log [HR] is missing) for an individual study.
Thirteen studies (Fizazi et al., 2009; Lipton et al., 2007a; Amgen, Daiichi Sankyo, Inc, n.d.; Fizazi et al., 2011; Henry et al., 2011; Stopeck et al., 2010; Berenson et al., 2001; Jiang et al., 2016; Rosen et al., 2001; Rosen et al., 2003; Saad et al., 2002; Theriault et al., 1999; Zhao et al., 2011) contributed data to the sensitivity analyses of log (HR) of SRE versus change in uNTX from baseline to week 13 or final value by imputing missing HR with RR. Similar to the previous analyses, a statistically significant positive association was indicated (uNTX effect 0.3260; 95% CI 0.0614–0.5906; P = .0202, R2 = 0.6260).
3.3. Results of meta-regression for bone ALP data
Similar analyses were used to evaluate the association between bone ALP and SRE. A statistically significant positive association between bone ALP and SRE was observed, except for log (HR) of SRE versus change in bone ALP from baseline to week 13 or final value, with imputing of missing HR by RR (bone ALP effect 1.317; 95% CI –0.0163 to 0.5671; P = .0619, R2 = 0.3423).
4. Discussion
The results of the meta-regression analyses indicate that reduction from baseline in the bone turnover marker uNTX at the earliest time of steady state may be associated with reduced risk of an SRE. Statistically significant positive associations were seen between uNTX reduction, measured by the between-group difference of the natural logarithm of the ratio between uNTX at week 13 and baseline, and SRE risk reduction, measured by the natural logarithm of the HR for time to first SRE between the two groups. For two studies that compared multiple doses of the same treatment, combined results over all doses were used (data provided by Amgen); otherwise, only the licensed dose was included in the analyses (Supplementary Table 1). Sensitivity analyses imputing missing data for HRs with RRs also confirmed the association. The results suggest that uNTX may be a useful surrogate marker for the risk of SREs. Findings were similar for the exploratory analyses of the relationship between change in bone ALP and risk of SREs.
Previous primary studies have investigated bone turnover markers as prognostic factors in cancer patients with bone metastases through regression modeling. Baseline elevated uNTX (Rajpar et al., 2010), elevated NTX, C-telopeptides, bone ALP, and NTX at 3 months have been reported to be independent predictors of survival outcomes (Barnadas et al., 2014; Lipton et al., 2007b). However, where multivariate regression analyses considered SREs as the dependent variable, elevated NTX, C-telopeptides, and bone ALP at 3 months were not found to be a significant predictor (Rajpar et al., 2010; Barnadas et al., 2014). Our study was conducted to investigate the potential association between a reduction in markers of bone turnover at the earliest time of steady state and a reduction in the longer-term risk of SREs.
Extensive literature searches were conducted to maximize the retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trial registers to identify unpublished studies. There were no limitations on language or outcome measures. However, few studies were identified that compared two or more relevant interventions and reported both SREs and bone marker data. There is also a possibility of publication bias, that is, our search strategy may have excluded small studies. As can be seen from the details provided in Supplementary Table 2, most of the studies were excluded because they did not assess both SREs and bone marker outcomes. However, the review process followed recommended methods to minimize the potential for error and/or bias (Higgins and Green, 2011; Centre for Reviews and Dissemination, 2009). Studies included in this review were assessed for risk of bias using the Cochrane risk-of-bias tool (Higgins et al., 2011).
Several limitations exist in the present analyses. As this study was based on aggregated data derived from a literature-based review, while the standard also requires individual patient data, our meta-analytical approach can only be regarded as a first step towards this issue and must be confirmed at the individual patient level. Besides, because of the small number of published trials that required both uNTX and SRE data, we were not able to differentiate between the different mechanisms of drug activity. Therefore, re-evaluation according to the different mechanisms of drug activity is also necessary as soon as a larger set of studies becomes available.
From a clinical perspective, it is important that treatment should not be stopped based on reduction in bone turnover markers alone as this does not necessarily indicate maintenance of a clinically favorable outcome, and the time frame being explored in these analyses was only to week 13 for bone turnover markers, assuming that the markers reached steady state, given continuous treatment, at week 13.
5. Conclusions
The findings of this systematic review and meta-regression support the hypothesis that there is a statistically significant positive relationship between uNTX reduction at week 13 from baseline and SRE risk reduction between two groups of adult patients with bone metastases from solid tumors (including breast, lung, prostate) or bone lesions from multiple myeloma. This finding is consistent with the associations between the log ratio (week 13/baseline) of uNTX and log (HR) of an SRE, and between the log ratio (week 13 or later/baseline) of uNTX and log (HR) of an SRE. Imputation of missing HRs with RRs also confirmed this finding.
Based on the review of the literature, markers of bone turnover such as uNTX may be potential surrogate markers for SREs. Further research is warranted to confirm these findings.
Declaration of competing interest
Chuang Li, Tony Glennane are employees of Amgen. En-Tzu Tang, Biao Zhang and Li Zhu are former employees of Amgen. The other authors have no declaration of interests.
Acknowledgments
Acknowledgments
We acknowledge Kleijen Systematic Reviews Ltd. for support in literature screening and statistical analysis. Writing assistance was provided by Julie Brown, from Mudskipper Business Consulting (Shanghai) Limited, funded by Amgen China.
Funding
This work was supported by Amgen China. Amgen manufactures and commercializes denosumab (Xgeva).
Author contributions
En-Tzu Tang, Li Zhang, Zefei Jiang, Chuang Li, and Li Zhu were involved in the conception and design of the study. En-Tzu Tang, Li Zhang, Zefei Jiang, and Biao Zhang were involved in acquisition of data, and all authors contributed to the interpretation of results. All authors reviewed and approved the final manuscript. En-Tzu Tang takes responsibility for statistical integrity.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2020.100272.
Appendix A. Supplementary data
Supplementary material
References
- Amgen, Daiichi Sankyo, Inc Denosumab compared to zoledronic acid in the treatment of bone disease in subjects with multiple myeloma. https://ClinicalTrials.gov/show/NCT01345019 Available from.
- Balcerzak M., Hamade E., Zhang L., Pikula S., Azzar G., Radisson J., Bandorowicz-Pikula J., Buchet R. The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim. Pol. 2003;50(4):1019–1038. [PubMed] [Google Scholar]
- Barnadas A., Manso L., de la Piedra C., Meseguer C., Crespo C., Gomez P., Calvo L., Martinez P., Ruiz-Borrego M., Perello A., Anton A., Codes M., Margeli M., Murias A., Salvador J., Segui M.A., de Juan A., Gavila J., Luque M., Perez D., Zamora P., Arizcuma A., Chacon J.I., Heras L., Martin-Fernandez M., Mahillo-Fernandez I., Tusquets I. Bone turnover markers as predictive indicators of outcome in patients with breast cancer and bone metastases treated with bisphosphonates: results from a 2-year multicentre observational study (ZOMAR study) Bone. 2014;68:32–40. doi: 10.1016/j.bone.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Berenson J.R., Lichtenstein A., Porter L., Dimopoulos M.A., Bordoni R., George S., Lipton A., Keller A., Ballester O., Kovacs M., Blacklock H., Bell R., Simeone J.F., Reitsma D.J., Heffernan M., Seaman J., Knight R.D. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J. Clin. Oncol. 1998;16(2):593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- Berenson J.R., Rosen L.S., Howell A., Porter L., Coleman R.E., Morley W., Dreicer R., Kuross S.A., Lipton A., Seaman J.J. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91(7):1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination . University of York; York: 2009. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. [Google Scholar]
- Coleman R., Brown J., Terpos E., Lipton A., Smith M.R., Cook R., Major P. Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat. Rev. 2008;34(7):629–639. doi: 10.1016/j.ctrv.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Costa L., Saad F., Cook R., Hadji P., Terpos E., Garnero P., Brown J., Body J.J., Smith M., Lee K.A., Major P., Dimopoulos M., Lipton A. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol. 2011;80(3):411–432. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Fizazi K., Yang J., Peleg S., Sikes C.R., Kreimann E.L., Daliani D., Olive M., Raymond K.A., Janus T.J., Logothetis C.J., Karsenty G., Navone N.M. Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin. Cancer Res. 2003;9(7):2587–2597. [PubMed] [Google Scholar]
- Fizazi K., Lipton A., Mariette X., Body J.J., Rahim Y., Gralow J.R., Gao G., Wu L., Sohn W., Jun S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 2009;27(10):1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- Fizazi K., Carducci M., Smith M., Damiao R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H., Jiang Q., Tadros S., Dansey R., Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grávalos C., Rodríguez C., Sabino A., Seguí M.Á., Virizuela J.A., Carmona A., Cassinello J., Isla D., Jara C., Martin M. SEOM clinical guideline for bone metastases from solid tumours (2016) Clin Transl Oncol. 2016;18(12):1243–1253. doi: 10.1007/s12094-016-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan-Raj S., von Moos R., Willenbacher W., Woll P.J., Wang J., Jiang Q., Jun S., Dansey R., Yeh H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29(9):1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- Hernandez R.K., Adhia A., Wade S.W., O’Connor E., Arellano J., Francis K., Alvrtsyan H., Million R.P., Liede A. Prevalence of bone metastases and bone-targeting agent use among solid tumor patients in the United States. Clin Epidemiol. 2015;7:335–345. doi: 10.2147/CLEP.S85496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] [Google Scholar]
- Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi G.N., Theriault R.L., Porter L., Blayney D., Lipton A., Sinoff C., Wheeler H., Simeone J.F., Seaman J., Knight R.D. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N. Engl. J. Med. 1996;335(24):1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- Hortobagyi G.N., Van Poznak C., Harker W.G., Gradishar W.J., Chew H., Dakhil S.R., Haley B.B., Sauter N., Mohanlal R., Zheng M., Lipton A. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. JAMA Oncology. 2017;3(7):906–912. doi: 10.1001/jamaoncol.2016.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Shao Z., Zhang Q., Yao Y., He J., Liao W., Qin S., Cheng Y., Xu Y., Dong J., Zhang L. Efficacy and safety of denosumab from a phase III, randomized, active-controlled study compared with zoledronic acid in patients of Asian ancestry with bone metastases from solid tumors. J. Clin. Oncol. 2016;34(15):10116. Suppl. [Google Scholar]
- Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y.X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W.J. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lipton A., Steger G.G., Figueroa J., Alvarado C., Solal-Celigny P., Body J.J., de Boer R., Berardi R., Gascon P., Tonkin K.S., Coleman R., Paterson A.H., Peterson M.C., Fan M., Kinsey A., Jun S. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 2007;25(28):4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- Lipton A., Cook R.J., Major P., Smith M.R., Coleman R.E. Zoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activity. Oncologist. 2007;12(9):1035–1043. doi: 10.1634/theoncologist.12-9-1035. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2012. Denosumab for the Prevention of Skeletal-Related Events in Adults with Bone Metastases from Solid Tumours. NICE Technology Appraisal Guidance 265. [Google Scholar]
- Pockett R.D., Castellano D., McEwan P., Oglesby A., Barber B.L., Chung K. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care. 2010;19(6):755–760. doi: 10.1111/j.1365-2354.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpar S., Massard C., Laplanche A., Tournay E., Gross-Goupil M., Loriot Y., Di Palma M., Bossi A., Escudier B., Chauchereau A., Fizazi K. Urinary N-telopeptide (uNTx) is an independent prognostic factor for overall survival in patients with bone metastases from castration-resistant prostate cancer. Ann. Oncol. 2010;21(9):1864–1869. doi: 10.1093/annonc/mdq037. [DOI] [PubMed] [Google Scholar]
- Roodman G.D. Biology of osteoclast activation in cancer. J. Clin. Oncol. 2001;19(15):3562–3571. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- Rosen L.S., Gordon D., Kaminski M., Howell A., Belch A., Mackey J., Apffelstaedt J., Hussein M., Coleman R.E., Reitsma D.J., Seaman J.J., Chen B.L., Ambros Y. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7(5):377–387. [PubMed] [Google Scholar]
- Rosen L.S., Gordon D., Tchekmedyian S., Yanagihara R., Hirsh V., Krzakowski M., Pawlicki M., de Souza P., Zheng M., Urbanowitz G., Reitsma D., Seaman J.J. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial--the Zoledronic acid lung cancer and other solid tumors study group. J. Clin. Oncol. 2003;21(16):3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L., Chin J.L., Vinholes J.J., Goas J.A., Chen B. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., Fan M., Jiang Q., Dansey R., Jun S., Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- Sweeney C., Sanofi, Walther Cancer Institute, Hoosier Cancer Research Network Risedronate to prevent skeletal related events in patients with metastatic prostate cancer commencing hormonal therapy. https://ClinicalTrials.gov/show/NCT00216060 Available from.
- Swiss Group for Clinical Cancer Research Prevention of symptomatic skeletal events with denosumab administered every 4 weeks versus every 12 weeks. https://clinicaltrials.gov/ct2/show/NCT0251218 Available from.
- Theriault R.L., Lipton A., Hortobagyi G.N., Leff R., Gluck S., Stewart J.F., Costello S., Kennedy I., Simeone J., Seaman J.J., Knight R.D., Mellars K., Heffernan M., Reitsma D.J. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J. Clin. Oncol. 1999;17(3):846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- Watts N.B. Clinical utility of biochemical markers of bone remodeling. Clin. Chem. 1999;45(8):1359–1368. Pt 2. [PubMed] [Google Scholar]
- Yang J., Fizazi K., Peleg S., Sikes C.R., Raymond A.K., Jamal N., Hu M., Olive M., Martinez L.A., Wood C.G., Logothetis C.J., Karsenty G., Navone N.M. Prostate cancer cells induce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res. 2001;61(14):5652–5659. [PubMed] [Google Scholar]
- Zhao Y.Y., Xue C., Hou X., Liao H., Li S., Zhao H.Y., Huang Y., Chen L.K., Xu F., Liu J.L., Zhang L. Changes of bone resorption marker (NTX) in chemotherapy plus zoledronic acid versus chemotherapy alone for nasopharyngeal cancer patients with bone metastases. Eur. J. Cancer. 2011;47(6):848–853. doi: 10.1016/j.ejca.2010.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material