Abstract
Backgrounds and aims
The role of hydroxychloroquine (HCQ) in the treatment of COVID-19 is not fully known. We studied the efficacy of HCQ compared to the control in COVID-19 subjects on - a. viral clearance measured by reverse transcriptase polymerase chain reaction (RT-PCR) and, b. death due to all cause.
Methods
PubMed, Scopus, Cochrane and MedRxiv database were searched using the specific keywords up to April 30, 2020. Studies that met our objectives were assessed for the risk of bias applying various tools as indicated. Three studies each that reported the outcome of viral clearance by RT-PCR and death due to all cause, were meta-analyzed by applying inverse variance-weighted averages of logarithmic risk ratio (RR) using a random effects model. Heterogeneity and publication bias were assessed using the I2 statistic and funnel plots, respectively.
Results
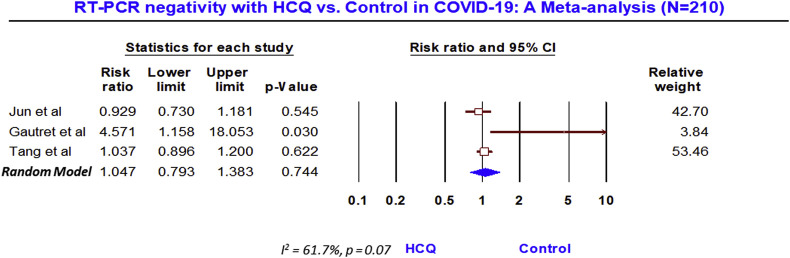
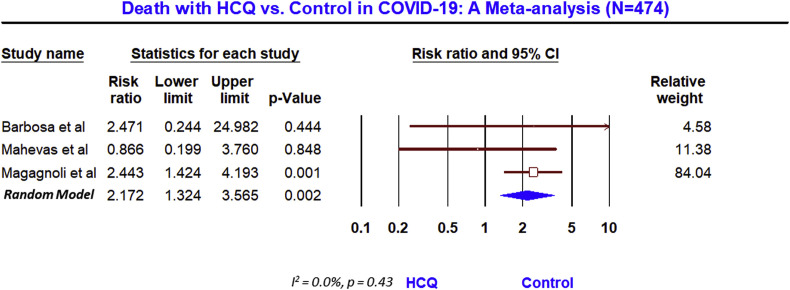
Meta-analysis of 3 studies (n = 210) on viral clearance assessed by RT-PCR showed no benefit (RR, 1.05; 95% CI, 0.79 to 1.38; p = 0.74), although with a moderate heterogeneity (I2 = 61.7%, p = 0.07). While meta-analysis of 3 studies (n = 474) showed a significant increase in death with HCQ, compared to the control (RR, 2.17; 95% 1.32 to 3.57; p = 0.002), without any heterogeneity (I2 = 0.0%, p = 0.43).
Conclusions
No benefit on viral clearance but a significant increase in mortality was observed with HCQ compared to control in patients with COVID-19.
Keywords: Hydroxychloroquine, COVID-19, Viral clearance, Outcomes, Death
1. Introduction
Scientist and physicians are working at heightened pace to research the treatment of coronavirus infection (COVID-19). Several potential repurposed candidate drugs have been tried in COVID-19. From these list of candidate drugs, two anti-malarial drugs came into limelight for following reasons. Initial studies found both chloroquine (CQ) and its derivative hydroxychloroquine (HCQ) inhibits SARS-CoV-2 effectively in vitro [[1], [2], [3]]. This led clinicians to believe that both drugs may have good potential in the treatment of COVID-19.
First report of human trial came from China. A commentary by Gao et al. [4] referring to 15 Chinese trials (whose complete results are still not available), claimed benefit with CQ in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus-negative conversion, and shortening the disease in more than 100 patients. One study from these 15 Chinese trials, conducted by Chen et al. [5] later showed data of 62 patients and found that HCQ significantly improved the clinical recovery (fever and cough) and pneumonia assessed by chest CT scan, compared to the control. However, a close look into this randomized control trial (RCT) found that the endpoints specified in the published protocol differed from those reported. First, the trial was originally supposed to report the results from two different dosage of HCQ on clinical and radiological outcome, although only the report of higher dose HCQ was reported finally. Second, the trial was stopped prematurely [6]. Another study from France, a non-randomized trial of HCQ (n = 36) by Gautret et al. [7] also reported a significant effect of HCQ and HCQ plus azithromycin (AZ) in lowering viral load and viral clearance compared to control, as measured by reverse-transcriptase polymerase chain reaction (RT-PCR). However, this study was widely criticized due to the poor trial design, unreliable conclusions, no clinical endpoints, assessments made on day 6 despite a planned 10 days trial, different value of Cycle threshold for RT-PCR, and derivation of results after excluding six patients from the HCQ arm [8]. The publishing journal’s society also subsequently declared that the trial by Gautret and Colleagues did “not meet the Society’s expected standard” [9].
Nevertheless, based on these limited observational and anecdotal evidence, several guidelines across the world allowed both these drugs in the treatment of COVID-19 [10]. Interestingly, Indian Council of Medical research hurriedly issued a guideline and additionally recommended the use of CQ and HCQ as a prophylactic agent in the close contacts, including the health care workers [11]. Surprisingly, based on these emerging developments, US President while addressing the nation on pandemic claimed CQ and HCQ as a “game changer” in the treatment of COVID-19. The consequence of this announcement resulted in FDA issuing an Emergency Use Authorization (EUA) to use both the drugs in the treatment of COVID-19 on March 30, 2020. Historically, this new EUA represents the second time when FDA has ever used any emergency authority to permit use of a medication for an unapproved indication. Earlier, an investigational neuraminidase inhibitor, peramivir was given similar EUA by FDA during the 2009–2010 for severely ill patients with H1N1 influenza. Although later an RCT failed to show any benefit of peramivir in severely ill hospitalized patients with influenza, compared to the placebo. Nonetheless, peramivir is approved only for uncomplicated influenza since 2014.
Since several newer studies of HCQ on COVID-19 have recently become available, we aimed to study its effect on COVID-19 on two important objective outcomes. These two important outcomes include – a. viral clearance by RT-PCR negativity and, b. death due to all cause. In addition, we have also compiled the results from all the studies that have studied the efficacy and safety of HCQ in COVID-19, including non-controlled trials.
2. Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12]. However, this study has not been registered in the International Prospective Register of Systematic Reviews (PROSPERO).
2.1. Search strategy and inclusion criteria
Three authors (AKS, AS and RS) systematically searched the PubMed, Scopus, Cochrane library and MedRxiv data base up to April 30, 2020. The key terms searched were ‘‘Hydroxychloroquine’’ OR ‘‘HCQ’’ (All Fields) OR “viral clearance” OR “death” OR “clinical recovery” AND COVID-19 OR SARS-CoV-2. We retrieved all the studies conducted with hydroxychloroquine in patients with COVID-19 that was compared to control, and explicitly reported at least one outcome of interest which include viral clearance by transcriptase polymerase chain reaction (RT-PCR) and or death due to all cause.
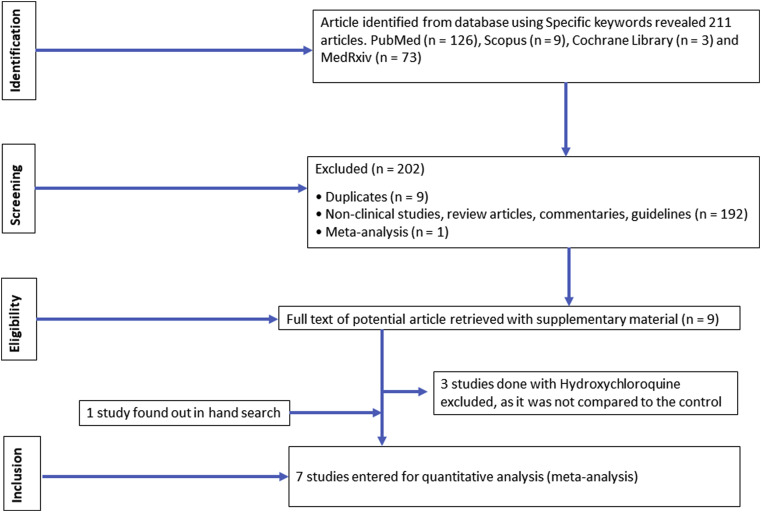
We excluded case reports, preclinical studies, studies that did not report outcomes with HCQ in COVID-19, and studies that did not compare the outcomes with HCQ compared to the placebo or control. The studies that met our predefined inclusion criteria were screened by three authors (AKS, RS and AS), and the studies that entirely fulfilled our inclusion criteria were retrieved with their supplementary appendix for further review. Any ambiguity during study selection was resolved by mutual discussion and consensus. One study whose full text was available in Chinese (abstract in English) was translated to English by Google translator and one study was retrieved through hand search. A detailed PRISMA flow-diagram for the search strategy is included in Fig. 1 .
Fig. 1.
PRISMA flow of study selection process.
2.2. Assessment of bias and statistical analysis
Four reviewers (AKS, AS, RS and AM) independently assessed the studies for risk of bias ascertained through Jadad checklist, ROBINS-I tool and Newcastle-Ottawa scale for randomized, non-randomized and observational studies, wherever appropriate [[13], [14], [15]] and any disagreements were resolved through mutual discussion and consensus. Scoring of these studies on risk of bias tools have been outlined in Supplementary Table 1. A detailed PRISMA checklist has been appended in Supplementary Table 2.
Comprehensive meta-analysis (CMA) software Version 3, Biostat Inc. Englewood, NJ, USA was used to calculate all the statistical analyses. Seven studies were retrieved that reported any outcome with HCQ compared to the control in COVID-19. Three studies each reported for viral clearance measured by RT-PCR and the outcome of death due to any cause. We meta-analyzed the pooled data of primary outcomes of 3 trials that reported the rate of PCR negativity, and 3 trials that reported the difference in mortality between HCQ and the control arm. Since one RCT by Chen et al. reported resorption of pneumonia on chest computed tomography (CT) as a primary outcome but neither reported RT-PCR negativity, nor the mortality outcome, we did not include this study in the meta-analysis, however the outcome of this study shall be discussed.
Estimates from all the eligible studies have been combined by applying inverse variance-weighted averages of logarithmic risk ratio (RR), using random-effects analysis. Heterogeneity was measured using Higgins I2 and Cochrane Q statistic [16]. Heterogeneity was considered as low (I2 <25%) or moderate (25–50%) or high (>50%). All the p reported here are two-sided and a p value of <0.05 is considered to be statistically significant. We also evaluated the potential publication bias by applying funnel plots using the “trim and fill” adjustment, rank correlation test and the Egger’s test.
3. Results
The overview of results including the risk of bias from all the 7 studies that compared HCQ to the control in COVID-19 have been summarized in Table 1 [5,7,[17], [18], [19], [20], [21]]. The meta-data that was used in this metanalysis has been also represented in Table 2 . Table 3 summarizes the safety and efficacy of all the 10 trials conducted with HCQ in COVID-19, to date [5,7,[17], [18], [19], [20], [21], [22], [23], [24]]. One RCT by Chen et al. [5] that is not included in this meta-analysis found “any improvement” in pneumonia were significantly higher in HCQ arm, compared to the control (80.6 vs. 54.8%, p = 0.048). Moreover, significant improvement in chest CT (more than 50% absorption of pneumonia) was increasingly observed in HCQ arm, compared to the control (61.3 vs. 16.1%, p = not reported).
Table 1.
Studies of HCQ compared to placebo in patients with COVID-19.
| Study | Types of studies | Country | Age (mean, years) | N | Case | Control | Severity of COVID-19 | HCQ dose/day X Days | Primary outcome | Secondary outcome | Improvement in Primary outcome | Improvement in Secondary outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen5 et al.∗ (ChiCTR 2000029559) |
RCT | China | 44.7 | 62 | 31 | 31 | Mild/moderate | 400 mg/d X 5D | Time to clinical recovery and improvement of pneumonia in chest CT | NR | Yes | NR |
| Jun17 et al.∗ (NCT04261517) | RCT | China | NR | 30 | 15 | 15 | Mild/moderate | 400 mg/d X 5D | Viral load by RT-PCR + vs. – at day 7 | NR | No | NR |
| Tang18 et al.∗ (ChiCTR 2000029868) |
RCT | China | 46 | 150 | 75 | 75 | Mild/Moderate (84%) | 1200 mg/d X 3D, followed by 800 mg/d X 2 wks (mild/moderate cases) or 3 wks (severe cases) | Viral load by RT-PCR + vs. – at day 28 | Clinical symptoms, normalization of laboratory parameters and chest radiology | No | No. However, reduction in CRP and symptoms noted in HCQ arm in post-hoc analysis |
| Gautret7 et al.∗∗ | nRCT | France | 45.1 | 36 | 20# | 16 | Mild/moderate | 600 mg/d X 10D | Viral load by RT-PCR + vs. – at day 6 | Improvement in symptoms, mortality | Yes | NR |
| Barbosa19 et al.∗∗ | qRCT | USA | 62.7 | 63 | 32 | 31 | Mild/moderate | 800 mg/d X 1-2D followed by 200–400 mg OD X 3-4D | Need to escalate respiratory support and rate of intubation at day 5 | Change in lymphocyte count, NLR, and mortality | No, rather harm in HCQ arm | No, direction towards harm |
| Mahevas20 et al.∗∗∗ | Retro | France | 60 | 181 | 84 | 97 | Pneumonia requiring O2 Rx | 600 mg/d X 7D | ICU transfer or death from any cause at day 7 | All-cause mortality at day 7, Occurrence of ARDS within 7 day |
No | No |
| Magagnoli21 et al.∗∗∗ | Retro | USA | 68 | 368 | 210## | 158 | Mild/moderate | NR | Need for MV and death from any cause | Death in patients on MV | No benefit. Risk of death due to any cause was higher in HCQ arm | No |
| Molina22 et al. | POS | France | 58.7 | 11 | 11 | 0 | Fever and O2 Rx (severe) | 600 mg/d X 10D + AZ 500 mg on day 1 and 250 mg 2–5 days | Viral load by RT-PCR + vs. – at day 5–6 | NR | No | NR |
| Gautret23 et al. | POS | France | 52.1 | 80 | 80 | 0 | Mild (92%) /moderate |
600 mg/d X 10D + AZ 500 mg on day 1 and 250 mg/d X 4D | Need for O2 therapy or ICU admission | Viral load, length of hospital stays | Yes | Yes |
| Million24 et al. | POS | France | 43.6 | 1061 | 1061 | 0 | Mild (95%) /moderate |
600 mg/d X 10D + AZ 500 mg on day 1 then 250 mg/d X 4D | death, negative RT-PCR | NR | Yes | NR |
∗Quality assessed as 5/8 on Jadad checklist, ∗∗Moderate quality on ROBINS I tool, ∗∗∗Quality assessed as 7/8 on Newcastle-Ottawa Scale, #6 patients received HCQ plus AZ, ##113 received HCQ plus AZ, HCQ-hydroxychloroquine, AZ-azithromycin, RCT – randomized controlled trial, nRCT- Non-randomized controlled trial, qRCT-quasi-randomized controlled trial, RT-PCR-reverse transcriptase polymerase chain reaction, ICU- intensive care unit, ARDS- acute respiratory distress syndrome, MV- mechanical ventilators, NR-not reported, CT-computed tomography, D-days, d-daily, O2- oxygen, Rx-treatment, POS- prospective observational studies.
Table 2.
Meta-data for analysis and results.
| Study | N | Types of outcome assessed | Outcome assessed for, N | Events in HCQ arm, n | Total case on HCQ arm, N | Events in control arm, n | Total control arm, N | Relative risk, 95% CI, p value |
|---|---|---|---|---|---|---|---|---|
| Chen et al. | 62 | Absorption pf pneumonia | 62 | 25 | 31 | 17 | 31 | 1.47, 1.02–2.11, p = 0.037 |
| Jun et al. | 30 | RT-PCR negativity | 30 | 13 | 15 | 14 | 15 | 0.93, 0.73–1.18, p = 0.55 |
| Gautret et al. | 36 | RT-PCR negativity | 30# | 8 | 14 | 2 | 16 | 4.57, 1.16–18.05, p = 0.03 |
| Tang et al. | 150 | RT-PCR negativity | 150 | 59 | 70 | 65 | 80 | 1.04, 0.90–1.20, p = 0.622 |
| Barbosa et al. | 63 | Death | 38∗ | 2 | 17 | 1 | 21 | 2.47, 0.24–24.98, p = 0.44 |
| Mahevas et al. | 181 | Death | 181 | 3 | 84 | 4 | 97 | 0.87, 0.20–3.76, p = 0.85 |
| Magagnoli et al. | 368 | Death | 255## | 27 | 97 | 18 | 158 | 2.44, 1.42–4.19, p = 0.001 |
#6 patients on HCQ plus AZ not analyzed, ## 131 patients on HCQ plus AZ not analyzed, ∗38 patients matched control analyzed, HCQ-hydroxychloroquine, AZ-azithromycin, CI- confidence interval.
Table 3.
Descriptive results, adverse events and limitation of all the trials done with Hydroxychloroquine as on April 30, 2020.
| Study | Details of primary and secondary outcome | Result of primary and secondary outcome | Adverse events noted | Limitations of the study |
|---|---|---|---|---|
| Chen5 et al. | i. Clinical recovery is defined as the return of body temperature (36.6 °C on the surface, ≤ 37.2 °C under the armpit and mouth or ≤ 37.8 °C in the rectum and tympanic Membrane) and cough relief, (slight or no cough) checked 3 times daily that maintained for more than 72 h. ii. Pulmonary recovery is defined at three levels as exacerbated, unchanged, and improved (moderately improved when less than 50% of pneumonia were absorbed, and significantly improved, when more than 50% pneumonia were absorbed in chest CT) |
i. Recovery time from fever significantly shortened in the HCQ arm compared to control (2.2 vs. 3.2 days, p = 0.0008). Cough remission time was significantly reduced in the HCQ arm compared to control (2.0 vs. 3.1 days, p = 0.0016) ii. Improvement in pneumonia were significantly higher in HCQ arm compared to control (80.6 vs 54.8%, p = 0.048). Significant improvement in chest CT were increasingly observed in HCQ arm compared to control (61.3 vs. 16.1%, p = nr). |
i. Mild adverse reactions noted in 2 patients from HCQ arm. one developed a rash, and one had headache. ii. Four of 62 patients progressed to severe COVID-19, all from control arm and none from HCQ arm. |
Protocol violation from original plan. Not reported the results from lower dose HCQ and premature stoppage of the trial. Detail use of other antivirals in control group is not available. |
| Jun17 et al. | Primary endpoint was negative RT-PCR of naso-pharyngeal for COVID-19 on days 7 after randomization | i. RT-PCR negativity at day 7 in throat swabs in HCQ arm versus control were similar (86.7 vs. 93.3% respectively, p > 0.05). ii. Median duration from hospitalization to PCR negative were similar in HCQ arm and placebo (4 vs. 2 days respectively, p > 0.05)]. The median time for fever normalization was similar (1 days) in both arms. iii. Radiological progression in CT chest was noted less in HCQ group compared to control (33.3 vs. 46.7% respectively, p = nr). . |
Transient diarrhea and abnormal liver function were seen in 26.7% cases in HCQ arm compared to 20% in controls (p > 0.05) | Manuscript available in Chinese language. |
| Tang18 et al. | i. The primary endpoint was PCR negativity for COVID-19 at day 28. ii. Secondary endpoints includes the improvement of clinical symptoms such as fever (axillary temperature of ≤36.6 °C), normalization of SpO2 (>94% on room air), disappearance of respiratory symptoms (nasal congestion, cough, sore throat, sputum production and shortness of breath), normalization of CRP, ESR, IL-6, TNF-α level and lymphocyte count within 28-days. In addition, PCR negativity at day 4, 7, 10, 14 or 21. |
i. No difference in PCR negative conversion rate between HCQ and control arm at day 28 (85.4 vs. 81.3%, p = 0.341). The negative conversion time in HCQ arm and control were same (median 8 vs. 7 days; HR 0.846; 0.580–1.234; p = 0.341). ii. No difference in symptoms between two arms within 28-days. No difference in PCR negativity between two arms at day 4, 7, 10, 14 or 21. iii. A significantly greater reduction of CRP observed in HCQ arm compared to control (6.986 vs. 2.723 mg/l, p = 0.045). A trend in more rapid recovery of lymphopenia also observed in HCQ arm compared to control. iv. Post-hoc analysis (confounding effects of anti-viral agents removed), found a significant improvement in symptoms in HCQ arm compared to control (HR 8.83, 1.09–71.3). |
Significantly higher adverse events noted in 30% of HCQ arm compared to 8.8% of control (p = 0.001). The most common adverse event was diarrhea in HCQ arm compared to control (10 vs. 0%, p = 0.004). Blurred vision seen in 1 patient on HCQ. | Selecting the virus negative conversion as the primary end-point might not be the most appropriate outcome. Issues to ensure the fidelity to the protocol by investigators. |
| Gautret7 et al. | i. Primary endpoint was negative RT-PCR for COVID-19 at day-6. ii. Secondary outcomes include virological clearance overtime, improvement in symptoms (temperature, respiratory rate, length of stay at hospital), mortality, and occurrence of side effects. |
i. Negative RT-PCR for COVID-19 was significantly higher in HCQ arm (70 vs. 12.5% p = 0.001) compared to control at day 6. Combination arm of HCQ plus AZ had significantly higher PCR negativity compared to HCQ alone and control (100 vs. 57.1 vs. 12.5%, p < 0.001) at day 6. ii. No other details available for secondary outcome |
One patient died in HCQ arm on day 3 despite negative RT-PCR. One patient stopped HCQ due to GI side effect | Poor trial design, assessments made on day 6 despite a planned 10 days trial, different value of Cycle threshold for RT-PCR, and derivation of results after excluding six patients from the HCQ arm |
| Barbosa19 et al. | i. Primary outcome - mortality, effect on escalation of respiratory support, ii. Secondary outcome - hematology benefits (absolute lymphocyte count and NLR) |
i. Significantly higher respiratory support needed at day 5 in HCQ arm compared to control (p = 0.013). HCQ treatment were independent predictors of escalation of respiratory support OR 7.18, (1.50–34.51, p = 0.014). In a matched subgroup analysis (n = 38) also shows escalated respiratory support in HCQ arm compared to control (p = 0.041). ii. Increased trend towards worsening of NLR in HCQ arm compared to control (p = 0.051). |
No torsade de pointes noted | i. Baseline requirement of O2 Rx or intubation were significantly higher in HCQ arm compared to control (p = 0.012). ii. Major errors in in Table 2. HCQ arm showing 31 patients and control arm 32 patients which is just reverse to Table 1. |
| Mahevas20 et al. | i. Primary outcome – composite of transfer to the ICU and or death from any cause within 7 days. ii. Secondary outcomes- all-cause mortality at day 7 and the occurrence of ARDS within 7 days. |
i. Transfer to the ICU or died within 7 days were similar in HCQ arm compared to control (20.2 vs 22.1%; RR 0.91, 0.47–1.80). ii. Percentage of all-cause death at day 7 were similar in HCQ arm compared to control (2.8 vs. 4.6%; RR, 0.61, 0.13–2.89). iii. Percentage of patients who developed ARDS within 7 days were similar in HCQ arm and control (27.4 vs. 24.1%; RR 1.14, 0.65–2.00). |
ECG changes were noted in 9.5% of cases in HCQ arm that caused HCQ discontinuation. ECG changes includes prolonged QTc, First-degree AV block and LBBB. | No random assignment, potential unmeasured confounders bias and no propensity match for some important prognostic variables. |
| Magagnoli21 et al. | i. Primary outcomes were death from any cause and the need for mechanical ventilation ii. Secondary outcome was death on those on mechanical ventilator |
i. Rates of death in the HCQ, HCQ + AZ, and control arm were 27.8%, 22.1%, 11.4%, respectively. Compared to control, the risk of death from any cause was higher in the HCQ group (adjusted HR 2.61, 1.10–6.17, p = 0.03) but not in the HCQ + AZ group (adjusted HR 1.14, 0.56–2.32, P = 0.72). ii. Rates of need of ventilation in HCQ, HCQ + AZ, and control arm were 13.3%, 6.9%, 14.1%, respectively. The risk of ventilation was similar in HCQ (adjusted HR 1.43, 0.53–3.79, p = 0.48), and HCQ + AZ arm (adjusted HR 0.43, 0.16–1.12, p = 0.09), compared to control. iii. Secondary outcome of death in patients who required mechanical ventilation was similar in HCQ (adjusted HR 4.08, 0.77–21.70, p = 0.10), and HCQ + AZ arm (adjusted HR 1.20, 0.25–5.77, p = 0.82), compared to the control. |
Nothing reported | Non-randomized, retrospective, selection bias, residual confounding, only men, median age >65 years and majority were of Black ethnicity. |
| Molina22 et al. | Primary outcome was RT-PCR negativity at day 5–6 | RT-PCR was positive in 80% of cases (95% CI 49–94) at days 5–6 after treatment. | One patient had prolonged QTc on HCQ + AZ and drug was stopped | Significant comorbidities present and majority of patient had severe COVID-19. |
| Gautret23 et al. | i. Primary outcome was need for O2 therapy or transfer to the ICU after at least three days of treatment. ii. Secondary outcome was PCR negativity and length of stay in the ID ward |
i. Majority of patients (81.3%) had favorable outcome and were discharged. Only 15% required oxygen therapy. ii. RT-PCR was negative in 83% of cases at day 7, and 93% of cases at day 8. iii. Mean time for discharge was 4.1 days with a mean length of stay of 4.6 days. |
Minor adverse events reported with HCQ including nausea, vomiting and blurred vision. | Results of six patients from previous trials by Gautret et al. were included in this study also. |
| Million24 et al. | Endpoints were death, negative RT-PCR | i. Good clinical outcome and negative RT-PCR were obtained in 91.7% within 10 days. Prolonged viral carriage was observed in 4.4% cases who had high viral load at diagnosis (p < 0.01), however viral culture was negative at day 10. All except one had negative PCR at day 15. ii. Poor outcome was observed in 4.3% and more associated with older age (OR 1.11), severe cases (OR 10.05) and use of selective beta-blockers and ARBs (p < 0.05). |
No cardiac toxicity was observed, although no details of assessment of cardiac toxicity is available. | Biased associated with all observational studies. Moreover, same groups of authors may have biased belief based on positive results from previous trials. |
RT-PCR – reverse-transcriptase-polymerase-chain-reaction, ARDS- acute respiratory syndrome, HCQ-hydroxychloroquine, AZ-azithromycin, CI- confidence interval, ICU- intensive care unit, MV- mechanical ventilator, HR-hazard ratio, RR-relative risk, OR-odds ratio, nr-not reported, CT-computed tomography, ESR-erythrocyte sedimentation rate, CRP- c-reactive protein, IL-interleukin, TNF- tumor necrosis factor, O2- oxygen therapy, ID-infectious disease, ECG-electrocardiogram, AV- atrioventricular, LBBB- left bundle branch block.
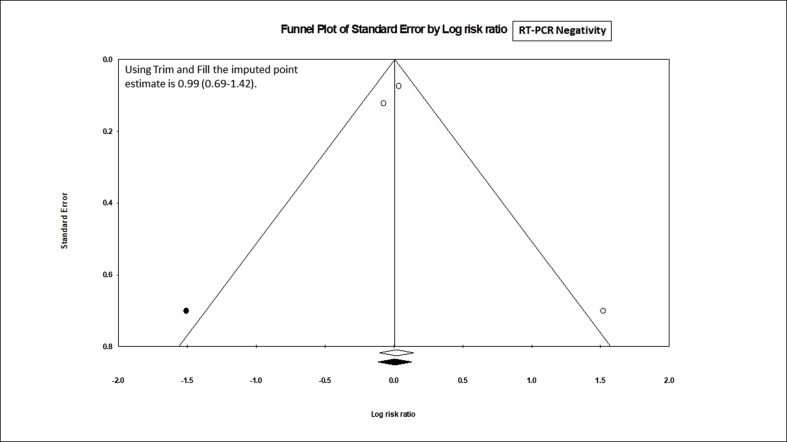
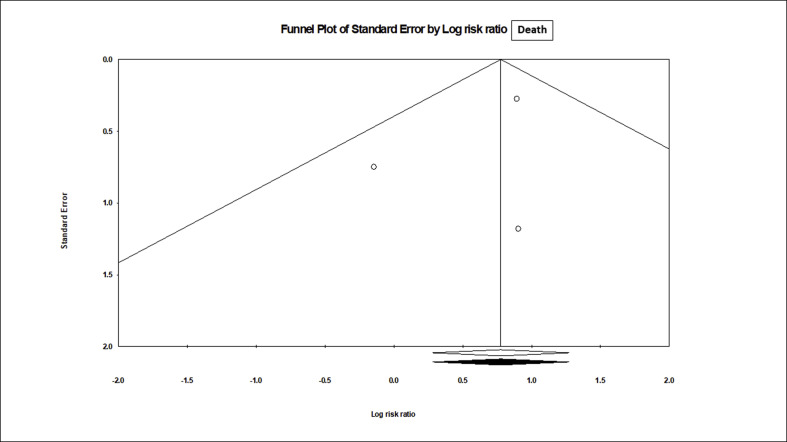
Nevertheless, the meta-analysis of 3 studies (n = 210) that reported the rate of PCR negativity (Fig. 2 ) found no benefit with HCQ, compared to the control (RR, 1.05; 95% CI, 0.79 to 1.38; p = 0.74), although with a moderate heterogeneity (I2 = 61.7%, p = 0.07). After the adjustment of publication bias, the Trim and Fill imputed the RR of 0.99 with 95% CI 0.69 to 1.42 (supplementary figure SF1). However, the meta-analysis of 3 trials (n = 474) that reported the mortality outcome, showed a significant (2-fold) increase in death in HCQ arm (Fig. 3 ), compared to the control (RR, 2.17; 95% 1.32 to 3.57; p = 0.002), without any heterogeneity (I2 = 0.0%, p = 0.43) and publication bias (supplementary figure SF2).
Fig. 2.
RT-PCR negativity with HCQ vs Control in COVID-19: A meta analysis (N = 210).
Fig. 3.
Death with HCQ vs Control in COVID-19: A meta analysis (N = 474).
4. Discussion
To our knowledge, this would be the most updated meta-analysis to report the effect of HCQ on viral clearance and mortality outcome, compared to the placebo that included 6 studies. Additionally, we have also analyzed the results from all the 10 studies available that have studied the efficacy and safety of HCQ in patients with COVID-19 (Table 3).
A recent meta-analysis published by Sarma et al. [25] have showed no difference in viral clearance and composite of death or clinical worsening with HCQ, while a significant improvement in radiological progression was observed, compared to the control. However, the meta-analysis by Sarma et al. seems to have overlooked the raw data and mistakenly included the wrong denominators. For example – they included number of patients for HCQ plus azithromycin (n = 20) in their analysis, rather than HCQ alone (n = 14), for the denominator for viral clearance. Similarly, the number of patients included for the composite of death or clinical worsening in HCQ arm was also overlooked and mistakenly reported in denominator (n = 20), rather than the actual number (n = 26). We believe that these differences could have changed the outcomes.
We do acknowledge a number of limitations in our analysis that include lesser number of patients overall, lack of individual patient data, combining the results of RCT with other non-randomized studies and the inclusion of pre-print version of some of the unpublished studies. Moreover, outcomes are not adjusted for multiple confounding factors and no sensitivity analysis were made. Besides, this metanalysis was not registered at PROSPERO.
While this meta-analysis found no benefit of HCQ in the treatment of COVID-19 on viral clearance and there was a 2-fold increase in death compared to the control arm, this could have been skewed by the one larger study that have shown a significant harm with HCQ, even when other smaller studies found no significant difference. For example, the study by Magagnoli et al. (n = 368) [21] found that there was no difference in the requirement of mechanical ventilator (MV) and death in patients who were on MV. However, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio 2.61, 1.10–6.17, p = 0.03), compared to the control. Since this study contributed more than 84% of weight in this pooled meta-analysis of 3 studies, the signal of significant death appears to emerge. Moreover, relatively elderly patients (mean age 68 year) and more sick (moderate to severe COVID-19) patients were studied in Magagnoli et al. study, compared to all other studies. Therefore, the purported benefit of HCQ in early or mild COVID-19 as observed in studies by Chen et al. [5] and Gautret et al. [7] cannot be entirely ruled out, from the result of this meta-analysis. It is also possible that HCQ may have some benefit in early and mild COVID-19 but possibly can be harmful in moderate to severe COVID-19.
Nevertheless, none of these studies attributed the harm of HCQ directly linked to the cardiac side effect. However, a recent double-blind RCT, Cloro-Covid-19 conducted by Borba et al. [26] hinted of high lethality with the higher dosage of chloroquine (CQ). Higher dose of CQ was associated with 39% death, compared to 15% death in lower dose arm. Fatality rate with high dose of CQ was as high as 60% in patients with underlying heart disease. QTc prolongation was significant in 19% of cases on high dose CQ compared to 11% in low dose CQ arm. Although no signals of torsade de pointes were noted in this trial, it is believed that increase in mortality in this trial could be attributed to the combination of CQ with azithromycin (AZ) and oseltamivir or lopinavir/ritonavir, all of which can prolong QTc interval [27]. Similarly, emerging studies from France and USA have increasingly cautioned for QTc prolongation with both HCQ and HCQ plus AZ. While Bessière et al. [28] reported (n = 40) a prolonged QTc in 93% of the patients receiving either HCQ or HCQ plus AZ; Mercuro et al. [29] reported QTc prolongation (n = 90) in 20% of patients treated with HCQ alone or HCQ plus AZ. These findings underscores the safety of HCQ in the light of negligible benefit observed in some of these studies.
Despite several limitations of this meta-analysis, we feel this finding would instill some degree of skepticism and shall help in curbing the exuberant use of over enthusiastically claimed “magical” drug. Hopefully, large randomized controlled trial such as DISCOVERY (EudraCT 2020-000936-23) and RECOVERY (UK), that is currently studying the effect of HCQ in COVID-19 and comparing it with other anti-viral drugs will finally decide its fate. Meanwhile, we believe that any prudent clinician would follow a pragmatic approach and shall apply these drugs only after assessing the potential risk versus uncertain benefit.
5. Conclusions
While no benefit on viral clearance demonstrated by HCQ compared to the control in patients with COVID-19, a significant 2-fold increase in mortality with the HCQ warrants its use if at all, with an extreme caution, until the results from larger randomized controlled trials are available.
Funding
Not funded
Ethical permission
Not required as this analysis do not involve patients directly.
Declaration of competing interest
Nothing to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2020.05.017.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020:5801998. doi: 10.1093/cid/ciaa237. pii:ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047.32074550. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z., Hu J., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. Version 2. medRxiv. 2020:20040758. doi: 10.1101/2020.03.22.20040758. 03.22. [Preprint.] [DOI] [Google Scholar]
- 6.Yan D., Zhang Z. Therapeutic effect of hydroxychloroquine on novel coronavirus pneumonia (COVID-19). Chinese Clinical Trials Registry. http://www.chictr.org.cn/showproj.aspx?proj=48880
- 7.Gautret P., Lagier J.C., Parola P., etal Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949.32205204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenzer J. Covid-19: US gives emergency approval to hydroxychloroquine despite lack of evidence. BMJ. 2020;369:m1335. doi: 10.1136/bmj.m1335.32238355. [DOI] [PubMed] [Google Scholar]
- 9.International Society of Antimicrobial Chemotherapy Statement on IJAA paper. April 23, 2020. https://www.isac.world/news-and-publications/official-isac-statement
- 10.Singh A.K., Singh A., Saikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020 May-June;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indian Council for Medical Research Recommendation for empiric use of hydroxychloroquine for prophylaxis of SARS-CoV-2 infection. https://icmr.nic.in/sites/default/files/upload_documents/HCQ_Recommendation_22March_final_MM_V2.pdf
- 12.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 13.Jadad A.R., Moore R.A., Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Sterne J.A.C., Hernan M.A., Reeves B.C., Savoic J., Berkman N.D., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun C., Danping L., Li L. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ. 2020 doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [Google Scholar]
- 18.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. doi: 10.1101/2020.04.10.20060558. [DOI]
- 19.Barbosa J., Kaitis D., Ryan F., Kim L., Xihui L. Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study. Biblio. 2020 https://bibliovid.org/clinical-outcomes-of-hydroxychloroquine-in-hospitalized-patients-with-covid-19-a-302 [Google Scholar]
- 20.Mahevas M., Tran V.-T., Roumier M. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv. 2020 2020.04.10.20060699. [Google Scholar]
- 21.Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin H.W., Sutton S.S. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. 2020. [DOI] [PMC free article] [PubMed]
- 22.Molina J.M., Delaugeree C., Goff J.L. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Maladies Infect. 2020 doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautret P., Lagier J.C., Parola P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study. IHU Méditerranée Infection. 2020;27(1) doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Million M., Lagier J.C., Gautret O. France. Bibliovid; Marseille: 2020. Early treatment of 1061 COVID-19 patients with hydroxychloroquine and azithromycin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarma P., Kaur H., Kumar H. Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25898. Published on April 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borba M.G.S., Val F.F.A., Sampaio V.S. CloroCovid-19 Team. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 27.Fihn S.D., Perencevich E., Bradley S.M. Caution needed on the use of chloroquine and hydroxychloroquine for coronavirus disease 2019. JAMA Network Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.9035. 23. [DOI] [PubMed] [Google Scholar]
- 28.Bessière F., Roccia H., Delinière A. Assessment of QT Intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1787. Published online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercuro N.J., Yen C.F., Shim D.J. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus 2019 (COVID-19) infection. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. Published online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.