Abstract
This article provides an overview of the antibiotic era and discovery of earliest antibiotics until the present day state of affairs, coupled with the emergence of carbapenem-resistant bacteria. The ways of response to challenges of antibiotic resistance (AR) such as the development of novel strategies in the search of new antibiotics, designing more effective preventive measures as well as the ecology of AR have been discussed. The applications of plant extract and chemical compounds like nanomaterials which are based on recent developments in the field of antimicrobials, antimicrobial resistance (AMR), and chemotherapy were briefly discussed. The agencies responsible for environmental protection have a role to play in dealing with the climate crisis which poses an existential threat to the planet, and contributes to ecological support towards pathogenic microorganisms. The environment serves as a reservoir and also a vehicle for transmission of antimicrobial resistance genes hence, as dominant inhabitants we have to gain a competitive advantage in the battle against AMR.
Keywords: Biotechnology, Environmental science, Microbiology, Epidemiology, Environmental health, Environmental pollution, Carbapenem, Enterobacteriaceae, Klebsiella pneumoniae, Escherichia coli, Enterobacter cloacae, Salmonella spp.
Biotechnology; Environmental science; Microbiology; Epidemiology; Environmental health; Environmental pollution; Carbapenem; Enterobacteriaceae; Klebsiella pneumoniae; Escherichia coli; Enterobacter cloacae; Salmonella spp.
1. Introduction
The major worry for the release of antimicrobials into the aquatic milieu is associated with the development of antimicrobial resistance genes (ARGs) (Fletcher, 2015). The spread of antimicrobial resistance genes (ARGs) is mainly detected with the help of antibiotic susceptibility testing which can be constantly reassessed (Duriez et al., 2016). For instance, a common mechanism of antibiotic resistance (AR) is carbapenemase production in some members of the Enterobacteriaceae group. The acquisition of carbapenem-resistance genes (CRGs) by Enterobacteriaceae is of global interest and public health importance (Sara et al., 2017) but it is unfortunate that the African continent lags behind in the developments of new antibiotics to tackle this unrelenting menace. Whole-genome sequencing (WGS) can play a significant role in rapid and accurate differentiation of the existing and emerging CRGs, which will be essential for surveillance and control of their spread with its source mainly from hospital wastewater effluents (Tang et al., 2017).
Wastewater treatment plants (WWTPs) receive sewage from various sources, including hospitals, which are important sources of antibiotics and antibiotic resistant bacteria (ARB) (Devarajan et al., 2016) and this menace is not unknown to the Sub-Saharan aquatic environments (Laffite et al., 2016). The release of antimicrobials into the natural environments select for ARB and in many cases may be associated with plasmids that contain one or more ARGs (Wellington et al., 2013). Plasmids are mobile genetic elements which are involved in transmitting ARGs between different bacterial species. These plasmids may also transfer virulence genes (VGs) from pathogenic bacterial strains to others, rendering non-pathogenic ones with virulence capabilities (Leimbach et al., 2013). High amounts of sub-lethal doses of most antibiotics administered to humans and animals are defecated as active substances, inevitably reaching WWTPs via hospital wastewater (Jelic et al., 2012; Vieno and Sillanpää, 2014). Alternatively, during therapeutic treatment procedures, the human intestinal microbiota is exposed to high concentrations of antimicrobials that can stimulate the generation of resistance phenotypes before its discharged into sewage through human excreta (Servais and Passerat, 2009). Globally, several findings on the presence of antibiotics, as well as the detection of ARB and ARGs in WWTPs final effluents samples have been reported including the study of Schwermer et al. (2018). A recent study by Barancheshme and Munir (2018) suggested some strategies to combat antimicrobial resistance (AMR) in WWTPs such as equipment upgrades with new developments, and they also pointed out that urban wastewaters may not ideally undergo appropriate treatment procedures due to broken down facilities in developing countries. Hence, the receiving environments are negatively impacted by wastewater discharges and this poses a significant public health risk.
1.1. Black box as a predominant source of ARGs and its negative impact on receiving freshwater ecosystem in Sub-Saharan Africa
Blackbox (any habitat that consist of WWTP final effluent) is a typical example of a reservoir for clinically relevant carbapenem-resistance Enterobacteriaceae (CRE). Carbapenemases of clinical significance are those enzymes with corresponding ARGs harboured on specific plasmids consequently favouring their transfer and spread among various bacterial strains (Gama et al., 2018). Subsequently, the carbapenem-resistance genes (CRGs) can easily be transferred via plasmids into other species, thus increasing the prospects of possible dissemination of new or emerging ARGs (Carattoli et al., 2018). WWTPs are regarded as hotspots for the procurement and spread of ARB into aquatic environments and according to several studies there are three main reasons set forth to sustain this idea: i) the chronic release of antibiotic residues, ARB, and ARGs collected in the community and hospital sewer systems; ii) the environmental conditions that are favourable for selection and/or transfer of ARGs among various bacterial species in WWTPs; and iii) the extensive observation that WWTP final effluents encompass high concentrations of diverse ARGs conferring AR against widely used antimicrobials and drugs of last resort (Lorenzo et al., 2018; Proia et al., 2018; Yee et al., 2019).
Receiving aquatic ecosystems (especially municipal rivers) may well play an imperative part in motivating the importunity and spread of ARB and ARGs as well as mobile genetic elements (MGEs) within bacterial communities. Szekeres et al. (2017) reported high levels of ARGs in river water samples hence, rivers offer a situation in which the horizontal exchange of MGEs encoding AR can occur between bacterial strains. Accordingly, WWTP final effluents are considered to be among the most significant conduits for the spread of ARGs into the environs. Several studies have investigated the occurrence of antimicrobials from wastewater (WW) treatment (Gothwal and Shashidhar, 2015; Margot et al., 2015), whereas countless other studies including the studies of Rizzo et al. (2013); Czekalski et al. (2014); Verlicchi et al. (2015); Ben et al. (2017) have focused on responses of ARGs to WW treatment and their impacts on the receiving surface waters. Likewise, some other studies have also analysed the incidence of ARB in final effluent samples of WWTPs using metagenomics analyses (Karkman et al., 2018; Chu et al., 2018). In spite of the significant number of studies carried out combining the investigation of antimicrobials and ARGs (Tong et al., 2019) along the WW treatment process (Lupan et al., 2017), comprehensive researches evaluating the fate of antibiotics, ARB, ARGs in WWTPs and their subsequent impacts on receiving water bodies are still deficient in South Africa. The continent Africa has its own share of the stigma with ARGs detected in different bacterial species. Bakour et al. (2015) reported the occurrence of blaNDM-1 and blaKPC genes in other bacterial species like Acinetobacter baumannii and Pseudomonas aeruginosa.
Clinically, the most relevant carbapenemases are Klebsiella pneumonia carbapenemases (KPC), New Delhi metallo-β-lactamase-1 (NDM-1), and Oxacillinase-type carbapenemases (for instance, OXA-48-like) enzymes, whose genetic factors have been progressively reported globally in Enterobacter isolates recovered from different environmental samples such as rivers (Singh-Moodley and Perovic, 2016). A study by Proia et al. (2018) provides evidence of the occurrence of CRGs in samples from health-care centres and WWTPs and their further dissemination in receiving surface water. Concomitantly, a study in Tunisia carried out by Ben et al. (2017) reported the incidence of blaKPC and blaOXA-48-like genes in Escherichia coli and Klebsiella pneumoniae isolates respectively. Unfortunately, many African countries employ the use of conventional WWT processes which inefficiently eliminate ARB (Di Cesare et al., 2016; Rafraf et al., 2016; Yang et al., 2016); it is paramount to evaluate the extent to which hospital wastewater effluents contribute to the distribution of ARGs, specifically those conferring resistance against carbapenems.
For several years, most developing countries make use of the conventional WWTPs and neighbouring water-bodies serve as receiving watersheds directly from hospitals and manufacturing companies (Shutes, 2001; Zhang et al., 2008). Many hospitals release effluents directly into water-bodies and even when effluents pass via WWTPs, they are poorly treated hence, this increases the likelihood of detecting ARGs. This unhygienic practice has resulted in the prevalence and dissemination of multidrug resistant (MDR) bacterial strains and this is of global concern to public health risk. Studies have confirmed that hospital WW effluents are major sources of ARGs, notwithstanding, conventional WWTPs do not have the capability to eliminate ARB. The dissemination of ARGs becomes even more perturbing because ARB strains are not constrained to the hospital environment but they are also found in other environmental niches (Dziri et al., 2018).
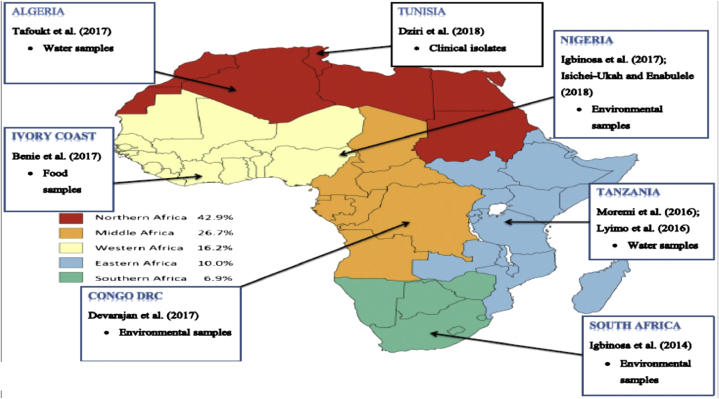
The acquisition rates of ESBL-Producing Enterobacteriaceae (ESBL-PE) as reported by Lääveri et al. (2018) proves the presence of another relevant ARGs detected in some sub-regions in Africa. Several investigations that focused on the prevalence of CRE have been carried out in some African countries including studies by Benie et al. (2017); Devarajan et al. (2017); Igbinosa et al. (2014), (2017); Isichei-Ukah and Enabulele (2018); Karkman et al. (2018); Lyimo et al. (2016); Moremi et al. (2016) and Tafoukt et al. (2017) as seen in Figure 1. However, there is still a lack of information that evaluates CRE from different environmental samples in other regions of Africa thus, more studies in this area should be encouraged.
Figure 1.
(a) ESBL-PE acquisition rates in five African sub-regions; joint data on 396 travellers from Finland and the Netherlands; Source: Lääveri et al. (2018) (b) prevalence of CRE in some African countries from different samples (2014–2018).
1.2. Global epidemiology of carbapenem-resistant Enterobacteriaceae infections
The first report on CRE was in 1993 (Nordmann et al., 1993), a study that reported carbapenem resistance in E. coli and Enterobacter cloacae strains isolated from hospital samples in Paris, France. Enterobacteria harbouring carbapenem-resistance genes (CRG) have been increasingly reported for more than 2 decades now. Klebsiella pneumoniae carbapenemases also known as KPC have been reported in the United States of America as well as in Greece (Cuzon et al., 2008; Poirel et al., 2010). Studies on metalloenzymes such as Verona integron–encoded metallo-β-lactamase (VIM), Imipenemase (IMP) have been reported globally, with an increased incidence in Europe and also in Asia (Nordmann et al., 2011). Carbapenemase-producing genes of the oxacillinase-48-like type were previously reported in India, Mediterranean and some parts of Europe (Bakthavatchalam et al., 2016; Sharma et al., 2016). ARGs specifically blaNDM-1 has also been reported in the United Kingdom, Pakistan and India (Perry et al., 2011; Walsh and Toleman, 2011; Dimou et al., 2012) and are presently one of the most important carbapenemase of worldwide concern (Patel and Bonomo, 2013; Wang et al., 2015).
A study in South Africa carried out by Lowman et al. (2011) was the first reported case of blaNDM-1 gene detected in Enterobacteria isolated from clinical samples taken from patients that arrived from India. The gene blaKPC was originally identified in bacterial strains recovered in USA in 1996 (Apisarnthanarak et al., 2013). Since then, these ARGs have been able to transfer genetic components and have demonstrated the tendency to spread rapidly among different bacterial species (Lawrence and Roth, 1996; Yong et al., 2009; Cuzon et al., 2008). Carbapenems remain the reliable last line of defence however, AMR is a major concern to researchers in view to uncovering ways to combat this public health challenge. Generally, resistance against antimicrobial agents is like a recurring nuisance to the health of humans and this has subsequently led to consistent research in this field. Due to indiscriminate use of antibiotics, the spread of AR has become a subject matter of trepidation with hospitals and WWTPs playing vital roles worldwide. The rapid emergence of CRE is presently a worry because the comparatively high minimum inhibition concentration (MIC) of carbapenems in these pathogenic bacteria along with exhibiting multiple ARGs make them difficult to eliminate (Seiffert et al., 2014). Moreover, some pathogenic strains of bacteria can acquire a silent gene, while exhibiting phenotypic resistance they are observed to lack ARGs (Abbassi et al., 2008).
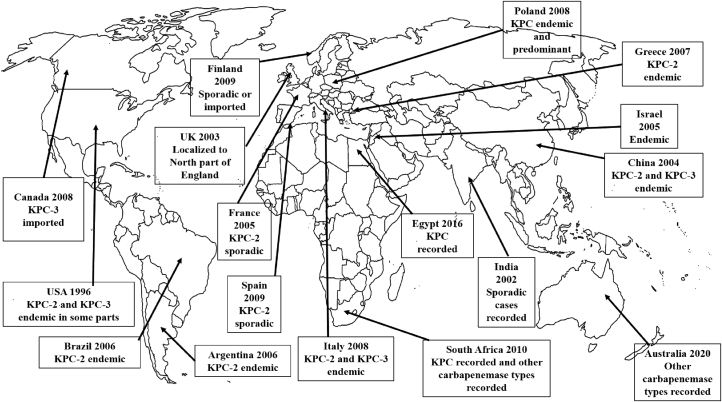
A recent study by Mahon et al. (2017) in Ireland reported NDM-1-producing Enterobacteriaceae recovered from beach water samples. This study pointed out that it appears that there is a potential for CRE to exist in harsh environments thereby contributing to a transition of CRE from largely health care-associated to organisms affecting the general population and also the veterinary sector. Several reports on the predominance of Enterobacteriaceae isolates harbouring KPC-producing genes and other key CRGs have been on the rise in every continent even in Africa. Figure 2 represents a world map showing the global expansion of KPC over the years.
Figure 2.
Clinical epidemiology of the universal expansion of Klebsiella pneumoniae carbapenemases (KPC).
1.3. Diversity of antimicrobial resistant bacteria in major hotspots and the removal efficiency
There is an abundance of carbon sources and other important nutrients which are favourable to the diversity of bacteria in WWTP final effluent (Mielczarek et al., 2013). The sewage microbiome is made up of environmental bacterial species (human and animal sources) with several of them harbouring ARGs (Li et al., 2018). Although there are various contributing factors that influence this microbiome (i.e. microbes in a particular environment including the human body), the consequence of unambiguous prospective discerning pressures like antimicrobial residues or metals, are apparently considered determinants that order the fate of ARB and ARGs during WWT processes (Manaia et al., 2018). Microbial pathogens cause infections (such as gonorrhoea) which remain virtually untreatable because of an increase in the strains harbouring emerging ARGs (Penchovsky and Traykovska, 2015). Coexistence of pathogenic microorganisms, antimicrobials and ARGs within WWTPs are responsible for the conducive conditions that enhance emerging ARB and facilitate horizontal genomic transfer amongst other species and it has been established that WWTP also serves as a hotspot for the transmission of ARB into the environment (Devarajan et al., 2015; Miller et al., 2016: Nnadozie et al., 2017). Advanced facilities of some WWTPs are effective in getting rid of pathogenic microbes and antibiotic residues largely during secondary and tertiary treatment processes (Uribe et al., 2015).
There have been reports of increased variations among ARB species in WWTP final effluents (Aali et al., 2014). Extended sludge retention time (SRT) along with hydraulic retention time (HRT) all through secondary treatment processes ease the removal of antibiotics by adsorption and biodegradation (Polesel et al., 2016). Nevertheless, those conditions previously mentioned can lead to the development of antimicrobial resistance (AMR) processes in microorganisms. Hence, there is lack of ideal conditions for the operation of conventional WWTPs for the effective elimination of antibiotics in developing countries (Michael-Kordatou et al., 2018). The removal of antibiotic deposits can be accelerated by means of coalescing conventional activated sludge (CAS) processes with a supplementary treatment technology that involves dosing with ozone treatment techniques (Helbling et al., 2012; Gerrity et al., 2013). The unconventional biological treatment system using membrane bioreactors (MBR) combined with coagulation seemingly possess the best removal efficiency (RE) of ARGs, and confiscate equally ARB and extracellular ARGs. Although some studies have predicted the fate of ARGs in WWTPs, the mechanisms of ARGs acquisition are still inconclusively established (Karam et al., 2016). RE represents the percentage of the number of molecules of a compound removed or destroyed (ARGs in this case). According to the report of Rafraf et al. (2016), the RE of WWTPs ranges from 45.5 to 100%. A report by Amábile-Cuevas (2016) stated that 90% of wastewater in US and Canada is treated while 66% of wastewater in Europe is treated. Additionally, 35% of wastewater in Asia is treated and 14% in Latin America but in Africa, less than 1% of wastewater is treated.
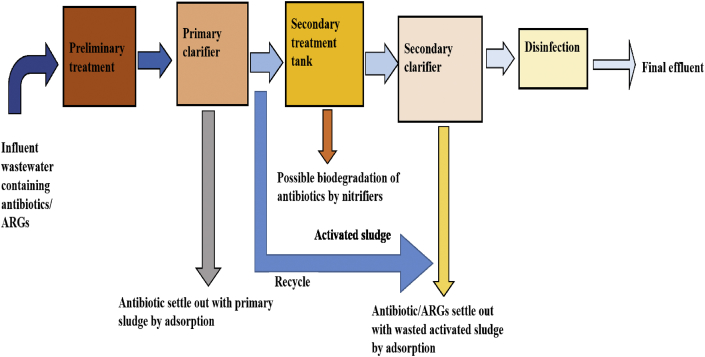
The spread of CRGs by CRE from hospital wastewater and WWTP final effluent to other non-pathogenic bacteria in the environment calls for a great deal of urgency to introduce new methods of elimination such as nucleic acid removal (Kardos, 2017). Figure 3 shows the design of a typical WWTP but unfortunately it is incapable of eliminating multidrug pathogens due to the mutations in genetic elements that lead to alterations in the target sites of antimicrobials. Out of all the stages involved in the WW treatment processes, both the final effluent and the activated sludge impact the environment negatively via farm practices as they are used for irrigation purposes and fertilizer respectively.
Figure 3.
A flowchart of treatment stages involved in a typical conventional wastewater treatment plant.
2. Enterobacteriaceae taxonomic history
The Family Enterobacteriaceae belongs to the Phylum: Proteobacteria > Class: Gamma Proteobacteria > Order: Enterobacteriales. Enterobacteriaceae group consists of several bacterial strains that are found in various environmental niches. Enterobacteriaceae members are generally rod-shaped, Gram-negative bacteria that are typical inhabitants of the abdominal flora, amid the most common pathogens to mankind that can cause infections ranging from pneumonia, meningitis, cystitis to pyelonephritis, septicaemia, peritonitis, as well as device-associated infections. They are the main causative agents of both community- and hospital-acquired infections (HAI), with E. coli being the most significant pathogen to man (Dramowski et al., 2016). For several years, Enterobacteriaceae members have succeeded in spreading easily among humans either by hand carriage or contaminated food/water and possess a proclivity to acquire genetic materials via horizontal gene transfer (HGT), usually facilitated by means of plasmids along with transposons (Xu et al., 2015).
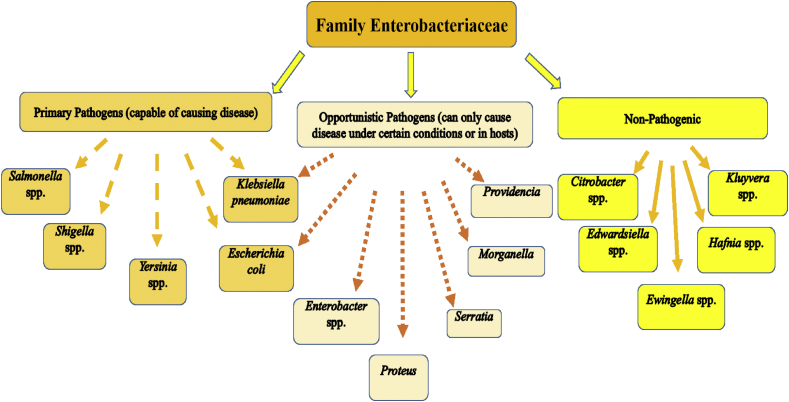
Antimicrobial resistant (AMR) has remained a prolonged challenge to global therapeutic options and this menace has existed and persisted since the 1960's, ever since then AMR has spread swiftly worldwide. AMR bacterial strains are able to withstand attack by antimicrobial agents and this ability threatens the effective prevention of infections (Alanis, 2005). Figure 4 represents examples of the common members of family Enterobacteriaceae.
Figure 4.
Common members of the Family Enterobacteriaceae.
3. Common members of Enterobacteriaceae
The family Enterobacteriaceae includes many genera and species with members being the most commonly encountered microorganisms previously isolated from clinical specimens. There are about 176 named species among 44 different genera however, clinically relevant species comprise primarily of E. coli, K. pneumoniae and Proteus mirabilis (Coudron et al., 2000). Other Enterobacteria include Enterobacter species, Klebsiella spp., Morganella spp., Proteus spp., Providencia spp., and Serratia spp. The obligate pathogens include Salmonella species, Shigella spp. and Yersinia spp (Kocsis and Szabó, 2013). Members of Enterobacteriaceae are distributed worldwide and are naturally found in soil, water, farm crops, fruits, meats, flowering plants and trees.
3.1. Salmonella spp.
The genus Samonella are rod-shaped, Gram-negative bacteria which are characterized into two species namely Salmonella enterica and Salmonella bongori. S. enterica is further classified into six subspecies that comprise over 2,500 serotypes. Generally, Salmonella spp. reside in the digestive tract of human and gut of warm-blooded animals (Wen et al., 2017). However, less than 100 serotypes (predominantly S. typhi, and S. paratyphi A and B) are responsible for human infections (Schellack et al., 2018).
Salmonella enterica serovar Typhimurium is regarded as a major foodborne pathogen and can cause Salmonellosis with several reports of the detection of antibiotic resistant (AR) strains. A specific strain of S. typhi was reported by Kopecko et al. (2009) to exhibit a silent gene. Some studies reported that some genes were compared and found to be similar to 11 genome sequences of various Salmonella serovars (Allard et al., 2013; Alikhan et al., 2018). Typhoid and paratyphoid fever are severe and frequently lethal feverish sicknesses caused by the enteric Salmonella enterica, serotype typhii and paratyphii. Typhoid remains a contagious ailment that spreads via the faecal–oral route and consists of various ways of occurrence which include: (i) the consumption of food/drinks (if handled by an asymptomatic carrier of the pathogen), (ii) oral transmission (faecal contaminated water), (iii) the ingestion of shellfish (from contaminated water), (iv) poor sanitation practices and (v) the consumption of raw farm produce such as fruits and vegetables (Schellack et al., 2018).
A study carried out in India by Guerra et al. (2014) reported that Salmonella spp. harboured carbapenem-resistance gene (CRG) blaNDM-1 in clinical samples from faeces and urine specimens recovered from some patients. Nevertheless, studies by Le Hello et al. (2013) and Seiffert et al. (2014) reported the detection of OXA-48-producing Salmonella enterica in different regions across the world. Multidrug resistant (MDR) Salmonella spp. may silently spread into the environment with the probability of causing illness, and this poses a grave risk to the health of the general public (Guerra et al., 2014).
3.2. Escherichia coli
Escherichia was named after Theodore Escherich, a German paediatrician who was the first to define the colon bacillus under the previous name Bacterium coli commune in the year 1885 (Shulman et al., 2007). Escherichia has five common human pathogenic species including E. coli, E. blattae, E. fergusonii, E. hermanii and E. vulneris. Escherichia coli can naturally be found in the intestines (Sokurenko et al., 1998). Several studies across the world including the studies of An et al. (2002); Edge and Hill (2005); Servais et al. (2009) and Chigor et al. (2010) have reported the occurrence of E. coli in surface water samples. Generally, E. coli exists as a non-pathogenic microorganism and essentially plays an important role in the gut of a healthy human. However, some pathogenic strains of E. coli harbour virulence genes (VGs) thus causing common infections like chronic diarrhoea, gastroenteritis, urinary tract infections (UTI), sepsis and also respiratory illnesses (Sidhu et al., 2013).
Pathogenic strains of E. coli comprise a varied group of bacteria consisting of strains which are further categorized into respective pathotypes (Chapman et al., 2006). According to Gomi et al. (2015), E. coli consists of intestinal pathogenic E. coli strains i.e. InPEC and extra-intestinal pathogenic E. coli i.e. ExPEC. Extra-intestinal infections are majorly caused by three E. coli pathotypes: (i) uropathogenic E. coli (UPEC) strains that cause UTI in humans and other domestic animals (ii) neonatal meningitis E. coli (NMEC); and (iii) strains responsible for septicemia in both humans and animals.
Another six E. coli pathotypes are related to diarrhoea and are referred to as diarrheagenic E. coli (DEC) (Akbar and Anal, 2011) and these comprise the following (i) Shiga toxin-producing E. coli (STEC) also known as Verocytotoxin-producing E. coli (VTEC) or enterohemorrhagic E. coli (EHEC) (ii) Enteroinvasive E. coli (EIEC) as well as (iii) diffusely adherent E. coli (DAEC) and (iv) Enterotoxigenic E. coli (ETEC) along with (v) Enteropathogenic E. coli (EPEC) (vi) Enteroaggregative E. coli (EAEC).
ETEC is one of the important bacterial agent that causes chronic diarrhoea which can lead to death (Walker et al., 2007). Specific virulence factors like enterotoxins are responsible for its pathogenicity. Amongst E. coli pathotypes, ETEC harbours at least lt1 and lt2 (heat-labile) or sta and stb (heat-stable) toxins and reports have linked it with various infections affecting humans (Suez et al., 2013).
EPEC is another significant pathotype of DECs and has been linked to child diarrhoea. EPEC strain is one of the major DEC that acquires a plasmid called EAF (EPEC adherence factor) which encrypts a type IV pilus known as the bundle-forming pilus (bfp) (Miri et al., 2017) and many types of EPEC have the eae chromosomal genes (Alikhani et al., 2013).
EHEC causes infections such as bloody or non-haemorrhagic diarrhoea and haemolytic uremic syndrome (HUS) (Rivas et al., 2006). A wide variety of food items have been linked with these infections (Akbar and Anal, 2011). The main virulence gene of EHEC is called Shiga toxins (stx genes) also known as Verotoxin (Vtx) and it contains two subdivisions namely stx1 and stx2 (Dänicke et al., 2015). However, EHEC strains of O157:H7 serotypes are the commonest serotypes of this group and Miri et al. (2017) reported that EHEC O157:H7 arose from the locus of enterocyte effacement-containing (LEE-containing) O55 EPEC strains that developed bacteriophage encrypting stx. The majority of these serotypes lacks the LEE pathogenicity island (Nielsen and Andersen, 2003). EHEC causes traveller's diarrhoea in both children and adults in developing and developed countries. This pathologic group is made up of intestinal bacteria that caused several global outbreaks (Thapar and Sanderson, 2004). EAEC is another E. coli pathotype which clings to HEp-2 cells and abdominal mucosa by virtue of fimbria structure aggregative adherence fimbriae (AAf) encoded by aggR gene which is located in the main virulence plasmids of typical EAEC (Kaper et al., 2004; Miri et al., 2017).
EIEC is the causative agent of invasive inflammatory colitis and dysentery, but usually, it causes watery diarrhoea and can be fatal among immunosuppressed individuals. The ability to invade the epithelium of the colon is dependent on a 220kb plasmid, called pInv, which harbours the genes meant for a type III discharge system that is used as the virulence factor. EIEC strains are very similar to pathogenic Shigella strains (Berglund, 2015). Although many strains of E. coli used to be considered as harmless commensals other strains of EIEC harbour ARGs and a study by Gülmez et al. (2008) reported carbapenem-resistance genes (CRGs) in E. coli.
3.3. Enterobacter spp.
Pathogenic strains of Enterobacter spp. can cause an extensive variety of nosocomial infections which include invasive and also device-associated diseases (Rosenthal, 2008). MDR strains have been reported to be responsible for several outbreaks in clinics and intensive care units (ICU) worldwide. Enterobacter spp. can cause community-acquired infections while becoming resistant against antimicrobials because of their colonization within clinical environments. AR against carbapenems impends our preceding effective therapeutic options and this phenomenon constitutes a concern to public health globally (Paterson, 2006).
Enterobacter genus has 13 species although E. aerogenes and E. cloacae are the two key species. Additionally, both species are significant nosocomial pathogens that can cause various opportunistic infections such as bacteremia, skin infections, soft-tissue infections, UTIs, endocarditis, intra-abdominal infections, lower respiratory tract infections, septic arthritis, osteomyelitis, ophthalmic infections, among many others (Foxman, 2010). There are four other species that can be found in the environment or as plant pathogens but have not been found in human clinical specimens and these include E. intermedius, E. dissolvens, E. nimipressuralis and E. pyrinus (Brady et al., 2013). Although reports on carbapenem resistance (CR) are increasingly reported among Enterobacteriaceae members, there are a few reports on ARGs harboured by Enterobacter spp. despite being the first member reported to harbour CRGs (Neuwirth et al., 1996; Coudron et al., 1997).
An alternative CR strategy for these pathogens is to combine ESBLs, increased efflux pump, porin modification and a sturdily expressed (de-repressed) endogenous AmpC enzyme (Flury et al., 2016). In E. cloacae, an extensive variety of carbapenem MICs have been ascribed to these options. However, the loss of porins appeared to be the foremost contributor to CR (Stürenburg et al., 2002).
3.4. Klebsiella spp.
Klebsiella was initially classified into three main species based on their biochemical reactions. Presently seven major species which demonstrated similarities in DNA homology have been identified. The other six species include K. ornithinolytica, K. oxytoca, K. ozaenae, K. rhinoscleromatis, K. planticola, and K. terrigena (Brisse and Verhoef, 2001). Klebsiella pneumoniae is the main species existing as a facultative anaerobic, non-motile bacillus and its natural habitat comprises surface water, soil and plants. K. pneumoniae can also live as commensal resident of the mammalian nasopharynx and digestive tract (Flemming and Wingender, 2010).
K. pneumoniae is presently considered as one of the most imperative opportunistic microorganism because it can cause nosocomial and community infections, predominantly amongst immune-compromised people, with amplified rates in health-care settings associated with antibiotic use (Holt et al., 2015). The gastrointestinal tract serves as a reservoir for certain microbes and is often regarded as the concealed source of common infections (Martens and Demain, 2017).
K. pneumoniae can potentially cause respiratory infections, UTI, blood and wound infections. In addition, K. pneumonia has been reported to cause diabetes related liver abscess syndrome in some Asian countries (Siu et al., 2012; van Crevel et al., 2017). Klebsiella spp. has been reported to be responsible for severe nosocomial infections with few pharmacological therapeutic options which can bring about a high mortality rate (Ackerman et al., 2018). The pathogenicity of K. pneumoniae is as a result of several virulence elements that permit it to overwhelm innate host immunity maintaining infections in a mammalian host.
In some developed and developing countries, carbapenem-resistant Klebsiella pneumonia (CRKP) has been reported with molecular epidemiology based on multilocus sequence typing (MLST) and this proves that several sequence types (STs) are common in this microorganism (Karampatakis et al., 2016; Alotaibi et al., 2017; uz Zaman et al., 2018). For instance, K. pneumoniae ST258 is the most commonly related strain with CR in the US and Greece (Kitchel et al., 2009; Andrade et al., 2014). The ST258 clone has been reported to belong to the MDR clonal complex (CC) 258 of K. pneumoniae which is associated with KPC-producing K. pneumoniae strains (KPC-Kp).
Interestingly, some studies carried out in Brazil and several Asian countries reported other members of CC258 (especially ST11) to be the major clones in dissemination of blaKPC and blaNDM-1 genes (Peirano et al., 2017). A study carried out in Russia also described ST340 as another member of CC258 which can be potentially linked with NDM-1-producing K. pneumoniae (Netikul and Kiratisin, 2015). As previously discussed, these AR profiles can as well be attributable to the expression of different enzymes like Extended-spectrum β-lactamases (ESBL) along with the overexpression of efflux schemes. Colistin remains one of the rare antimicrobials still effective against KPC-Kp, but regrettably, evolving data on colistin-resistance have further constrained therapeutic alternatives (Bassetti et al., 2017).
4. Antibiotic resistance in water-food nexus
The epidemiologic report and phenotypic findings of carbapenem-resistant Enterobacteriaceae (CRE) are complex probably because Enterobacteriaceae members may well be unsusceptible (i.e. intermediate or resistant) against carbapenems. Moreover, studies have reported various microbes isolated from the aquatic environment with tendencies of harbouring silent genes (Lehn et al., 1996; Nguyen et al., 2019), these are inactive genes considered to be part of cryptic genetic systems. Alternatively, studies have shown plasmids carrying the blaOXA-48 gene in different enterobacterial strains investigated in different countries but shared very dissimilar features (Falagas et al., 2014). Ribot et al. (2006), Mediavilla et al. (2016), Poirel et al. (2016) and Fernandes et al. (2016) reported the occurrence of some Enterobacteriaceae members that harboured mcr-1 colistin resistance genes and if not contained may spread to other strains thereby compounding the existing concerns to public health.
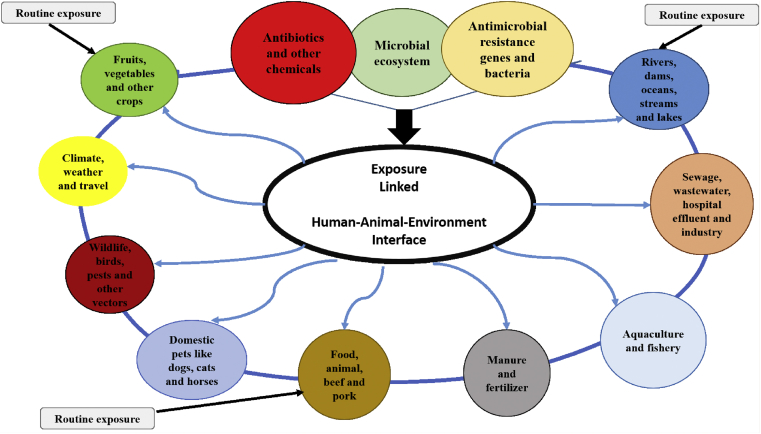
In 2014, in a workshop organized by the World Health Organization (WHO), the speakers explored microbial and genomic movements across agriculture, health and environmental compartments. A call was made to generate a real-time worldwide databank of prevailing data sets that included antibiotic resistance (AR), infections, sequencing, climate, wildlife movement, and migrating birds. The databank was assembled, assimilated, and analysed to recognise gaps, encourage novelty, and take collective action against AR. A recommendation was made to frame this work inside a new paradigm—the Collective Antimicrobial Resistance Ecosystem (CARE). The paradigm was established on incessant exposures to numerous forms of resistance determining factors at the interface of humans, animals, and also the environment. Slayton et al. (2015) reported on a similar approach intended for the reduction of AR infections in hospitals which are considered as major hotspots for antibiotic resistant bacteria (ARB).
Every gram of soil sample inside the native resistome (i.e. the collection of all the ARGs and their precursors present in the chromosomes of both pathogenic and non-pathogenic bacteria), is a peculiar collection of all the potential ARB in a microbial milieu and can be driven towards a problematic direction through anthropogenic factors for instance aquaculture, animal husbandry, antibiotic production, WWTPs and general pollution. Horizontal gene exchange (HGE) arises in the commensal relationship that briefly occurs out of this environmental selection leading to the transfer of ARGs and spread of mobile genetic elements (MGE) between strains (Lozupone et al., 2012). Nevertheless, the rudiments involved that remain vital in driving the rapid emergence of ARB are selection, growth conditions and cell contact as well as cell density. Management of these elements is crucial in order to abate HGE and ARB (von Wintersdorff et al., 2016). Ample evidence demonstrates this process of HGE. A study carried out by Li et al. (2015) reported on the abundance of ARGs in the metagenome of diverse environs stating the loads in natural environmental niches, such as soils and river water. Contrariwise, more studies are necessary to analyse hospital wastewater effluents which is the key source of ARB. Figure 5 represents the CARE model showing antimicrobial agents exposure linkage with the human-animal-environment interface.
Figure 5.
The CARE model (Collective Antimicrobial Resistance Ecosystem).
4.1. Occurrence of carbapenem resistance Enterobacteriaceae (CRE) in different environmental niches
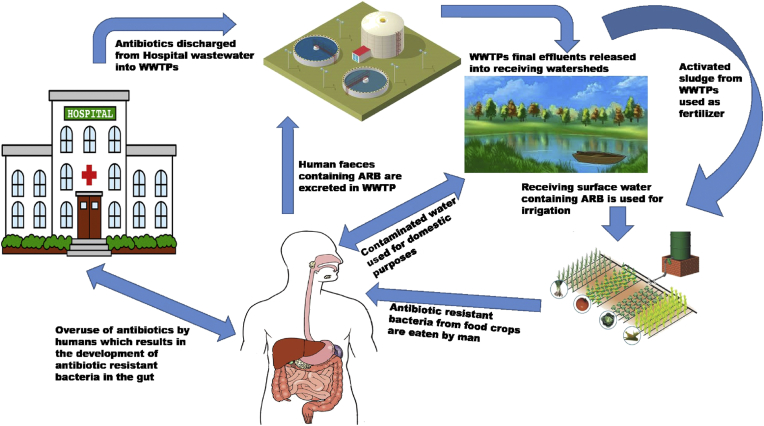
The reuse of wastewater for irrigation purposes in order to improve soil fertility may lead to various public health implications (Castro et al., 2015). River water serves as a reservoir for different bacterial species and pollution may be from direct contact with either wastewater or animal dung (Araújo et al., 2017). Undercooked or raw farm products such as vegetables and fruits eventually serve as reservoirs of pathogenic microorganisms due to a switch in ecological niche. Enterobacteria are the most common causative agents of food poisoning and a host of various infections. However, there is a need for more studies investigating CRE isolates recovered from different environmental niches in South Africa. Certainly, surface waters are exposed to discharges from various sources, in receipt of chemical and microbial contaminants that originate from agricultural, domestic and industrial origins. Pollution of waterbodies has been reported to modulate the antibiotic resistome (Tacão et al., 2012; Tafoukt et al., 2017). Another source of contamination from WWTPs arises from the use of activated sludge as organic fertilizer to treat farm soil and the use of WWTPs final effluents and/or receiving watersheds as irrigation sources for vegetable farms and also drinking for farm animals (Kümmerer, 2004; Vaz-Moreira, 2014). Effluents from WWTPs are made up of diverse wastewater which comprises those from households, pharmaceutical industries and hospitals (Hernández et al., 2011; Birch et al., 2015; Bueno et al., 2016). AR is a major and growing public health concern and surveillance of the rise of this AR phenomenon in the environments is improbably inadequate (Marti et al., 2014). As earlier mentioned, several studies have shown that most WWTPs final effluents consist of MDR bacterial strains and another study of Marti et al. (2013) reported the detection of ARGs and concentration of antibiotics in both WWTP effluent samples and river water samples. Figure 6 is a schematic diagram showing the process involved in the spread of antibiotic resistant bacteria.
Figure 6.
A schematic diagram showing the process involved in the spread of antibiotic resistant bacteria.
5. Mechanisms of carbapenem resistance in Enterobacteriaceae
Bacterial strains can exhibit antibiotic resistance (AR) against carbapenems through several mechanisms and these include production of β-lactamases (carbapenemases), efflux pumps and mutations that alter the expression and/or function of porins and penicillin-binding proteins (PBPs). Carbapenemase producing (CP) microorganisms are usually only susceptible to polymyxins (e.g. colistin), fosfomycin and variably susceptible to tigecycline, although colistin resistance in CP Klebsiella pneumoniae isolates has also been reported (Nordmann et al., 2009). Carbapenemases are able to hydrolyze carbapenems effectively, while other β-lactamases hydrolyze them very slowly (Temkin et al., 2014). Furthermore, carbapenemases hydrolyze a variety of β-lactams, including cephalosporins, penicillins and aztreonam but these enzymes can be inhibited by β-lactamase inhibitors, such as clavulanic acid, tazobactam and sulbactam (Drawz and Bonomo, 2010). Associated with the rise in carbapenem resistance is the deficiency of new antimicrobial agents. This has triggered different initiatives globally to develop novel or more effective antimicrobial compounds and also to develop novel delivery-targeting strategies (Baptista et al., 2018). Among the common mechanisms of resistance (MOR) are target protection, decreased cell permeability, target overproduction, altered target site/enzyme, enzyme inactivation, increased efflux due to over-expression of efflux pumps, amongst others (Baptista et al., 2018). There are limitations in the development of new antibiotics, nevertheless combination therapy has been considered and there are ongoing studies to analyse the effectiveness of polytherapy. Every class of antibiotics has its mode of action and sequentially the target pathogens develop resistance mechanisms thereby conferring AR against the existing antibiotics. Table 1 summarizes different classes of antibiotics, the modes of action and common MOR exhibited by ARB.
Table 1.
Antibiotics class, modes of action and common resistance mechanisms (Burch et al., 2013; Butaye et al., 2015).
| Antimicrobial class | Mode of action | Mechanism of resistance (MOR) |
|---|---|---|
|
Beta-lactam antibiotics Penicillins: Penicillin G, Penicillin V Methicillin, Oxacillin Ampicillin, Amoxycillin Piperacillin |
Hinder cell wall production. Binds enzymes (Penicillin-Binding Proteins-PBPs) which help form peptidoglycans |
|
|
Cephalosporins: 1stand 2ndgeneration Cephalexin, Cephradine 3rdand 4thgeneration Ceftiofur, Cefquinome Cefotaxime, Ceftazidime Monobactams: Aztreonam Carbapenems: Imipenem, Meropenem, Doripenem, Ertapenem Beta-lactamase inhibitors Clavulanic acid, Sulbactam Tazobactam |
Inhibits/binds to beta-lactamase enzymes | Extended-spectrum beta lactamases (ESBLs) CTX-M beta-lactamase (bla) genes- transfer of plasmid (better term- expanded spectrum cephalosporinases – ESCs)
|
|
Polymixin Colistin |
Action on cell membrane-disrupts permeability | Unclear – reduction of bacterial permeability |
|
Tetracyclines Chlortetracycline, oxytetracycline, Doxycycline, minocycline |
rRNA-binds to 30S subunit and interferes with amino acid transfer Prevents protein production |
Inducible efflux in E. coli etc (tetA, tetB, tetC) Binding site mutations (tetO, tetM genes) Rare, changes to tetracycline molecule |
|
Aminoglycosides Streptomycin, Neomycin, Kanamycin, Apramycin, Gentamicin, Amikacin Aminocyclitol Spectinomycin |
rRNA-binds to 30S subunit, so misreads genetic code. Inhibits protein production. Effect on permeability of cell membrane | Phosphorylation, adenylation and acetylation of aminoglycoside (aph, aad, aac genes) inhibits binding. Streptomycin – single binding site Others – multiple binding sites, slower resistance, primarily plasmid transfer |
Microorganisms usually exhibit bioreduction, which involves accumulation of metallic ions in order to reduce their toxicity. They can either bioreduce intracellularly with the support of various reducing species present inside the cell and on the cell wall, or bioreduce extracellularly by different metabolites (Singh et al., 2018). Some bacterial strains exhibit MOR in the form of other more complex phenotypes (such as biofilm formation and quorum sensing) which can also be induced by antibiotics. AR against carbapenems can arise as a result of modifications in membrane absorptivity through mutations in bacterial efflux pumps otherwise porins attached with ESβL expression, or by the procurement of carbapenem-resistance genes (CRGs) which encode specific carbapenemase (Potter et al., 2016). The latter mechanism has been reported globally in Enterobacteriaceae isolated from clinical samples. Nonetheless, the perseverance of the corresponding ARGs in environmental bacterial strains is of great concern as a result of its implication to public wellbeing (Walsh, 2010).
According to Nordmann et al. (2012), in some members of Enterobacteriaceae group, CR arises as a result of two main mechanisms: (i) acquisition of CRGs encoding for enzymes that can degrade carbapenems, or (ii) a reduction in the uptake of carbapenems by a qualitative or quantitative deficit of porin expression associated with overexpression of carbapenemases that have very low affinity for carbapenems (Netikul and Kiratisin, 2015; Shields et al., 2017).
Studies have reported other non-enzymatic mechanisms associated with CR such as reduction in the expression of outer membrane proteins (OMPs) (particularly OmpK35 and OmpK36 porins) to be related to an increase in MICs of both cephalosporins and carbapenems when exposed to K. pneumonia (Arora and Jagdale, 2009; Poulou et al., 2013; uz Zaman et al., 2014; Hamzaoui et al., 2018). Nevertheless, there may be other factors responsible for CR, factors such as carbapenemase production with no defective porin expression can strongly raise carbapenem MICs (Gomez-Simmonds et al., 2018). CR also encompasses numerous collective mechanisms which could include alterations or mutations in OMP and up-regulation of efflux systems—linked with hyper-production of AmpC β-lactamases or ESBLs—or creation of particular carbapenem-hydrolysing-β-lactamases (Rubin and Pitout, 2014; Russotto et al., 2015; Van Duin and Doi, 2017).
The widespread use of prescribed drugs such as antibiotics and antivirals including anti-retrovirals (ARVs) culminates in WWTP final effluents across the world (K'oreje et al., 2018; Myllyniemi Maldonado, 2018) and this is a major health concern due to its potential to encourage AMR. Mosekiemang et al. (2019) also investigated the occurrence of ARVs in environmental samples. Recent studies by Hocquet et al. (2016) and Christou et al. (2017) described AMR as a result of the ineffectiveness of different antibiotics which is a consequence of the mutations developed at the various target sites in bacterial cell organelles. The aftermath of this scourge is disastrous hence swift actions are needed to tackle this consistent phenomenon.
6. Non-carbapenemase mediated CRE
The outer membrane (OM) of bacteria has the ability to form a hydrophobic barrier that gives protection to the cell against external agents like detergents, heavy metals and other antimicrobials (Spengler et al., 2017). This OM contains definite proteins, known as porins that can also form hydrophilic channels in order to permit the selective uptake of crucial nutrients and other compounds (e.g. antibiotics), moreover, the principal porins in Enterobacteriaceae involved in uptake of antimicrobials belong to the OmpF/OmpC families (James et al., 2009). Hence, any fluctuations in the amount or activity of porins might be reliant on AR. Chromosomal mutations of the central channel or gatekeeping loop in the OMP, shift in the types of porins or a loss of porin expression can increase resistance against antimicrobials (Nordmann et al., 2012). Additionally, breakdown of porin can be controlled in swift response to antimicrobials or aromatic products thru numerous cascades including the mar and sox operons, by means of a successive reduction in the quantity of porins in the bacterial OM (Shimizu, 2015). CR was first observed among Enterobacter spp. which over-expressed a chromosomal ampC gene encrypting an inherent cephalosporinase and displayed alterations in the OmpC or OmpF porins and according to a study by Bello and Dingle (2018), similar mechanisms were reported in Serratia spp., Citrobacter freundii, and Morganella morganii. Furthermore, CR mechanisms have also been reported in other members of Enterobacteriaceae that do not exhibit an intrinsic cephalosporinase (Poole, 2004; Su et al., 2008). From the several studies reviewed, various reports have necessitated the urgency to act fast in order to curtail the emergence and spread of ARGs in the environment. There is the need to develop new antibiotics or new ways to combat the defence metabolic mechanisms displayed by CRE.
Different approaches, such as the use of nanostructured materials, are being developed to overcome the various MOR. Nanostructured materials can possess antimicrobial activity by themselves. In addition, nanoparticles which include metallic, organic and carbon nanotubes may circumvent MOR in pathogenic bacterial strains and, associated with their antimicrobial potential, inhibit biofilm formation or other important microbial processes (Singh et al., 2018). Other strategies to oppose AR, for instance, the combined use of plant-based antimicrobials and nanoparticles to overcome toxicity issues, are also being investigated. On the other hand, the use of nanoparticles still presents a challenge to treatment options and more studies in this area are also needed.
7. Conclusion
Antimicrobial resistance (AR) has been increasing globally and bacterial infections are predominantly treated with antibiotics but the antibacterial activities have been compromised. The major routes of transmission of carbapenem-resistant bacteria to humans are generally through consumption of contaminated foods or water as well as poor domestic hygiene in many developing countries. The introduction of more metagenomics studies and use of risk assessment techniques in analysing different environmental samples will be essential for the prevention of infections caused by carbapenem-resistant Enterobacteriaceae. This calls for proper treatment of WWTP final effluent before being discharged into receiving watersheds and the development of new antibiotics that will assist in tackling AR strains. The manufacture of new and effective antibiotics for better treatment of bacterial infections will also help to reduce the prevalence of CRE and the dissemination of antimicrobial resistance genes.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the South Africa Medical Research Council (SAMRC) under the grant number SAMRC/UFH/P790 and University of Fort Hare, South Africa.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aali R., Nikaeen M., Khanahmad H., Hassanzadeh A. Monitoring and comparison of antibiotic resistant bacteria and their resistance genes in municipal and hospital wastewaters. Int. J. Prev. Med. 2014;5(7):887. [PMC free article] [PubMed] [Google Scholar]
- Abbassi M.S., Torres C., Achour W., Vinué L., Sáenz Y., Costa D., Bouchami O., Hassen A.B. Genetic characterisation of CTX-M-15-producing Klebsiella pneumoniae and Escherichia coli strains isolated from stem cell transplant patients in Tunisia. Int. J. Antimicrob. Agents. 2008;32(4):308–314. doi: 10.1016/j.ijantimicag.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Ackerman A.L., Parameshwar P.S., Anger J.T. Diagnosis and treatment of patients with prostatic abscess in the post-antibiotic era. Int. J. Urol. 2018;25(2):103–110. doi: 10.1111/iju.13451. [DOI] [PubMed] [Google Scholar]
- Akbar A., Anal A.K. Food safety concerns and food-borne pathogens, Salmonella, Escherichia coli and Campylobacter. FUUAST J. Biol. 2011;1(1 June):5–17. [Google Scholar]
- Alanis A.J. Resistance to antibiotics: are we in the post-antibiotic era? Arch. Med. Res. 2005;36(6):697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Alikhan N.F., Zhou Z., Sergeant M.J., Achtman M. A genomic overview of the population structure of Salmonella. PLoS Genet. 2018;14(4) doi: 10.1371/journal.pgen.1007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhani M.Y., Hashemi S.H., Aslani M.M., Farajnia S. Prevalence and antibiotic resistance patterns of diarrheagenic Escherichia coli isolated from adolescents and adults in Hamedan, Western Iran. Iran. J. Microbiol. 2013;5(1):42. [PMC free article] [PubMed] [Google Scholar]
- Allard M.W., Luo Y., Strain E., Pettengill J., Timme R., Wang C., Li C., Keys C.E., Zheng J., Stones R., Wilson M.R. On the evolutionary history, population genetics and diversity among isolates of Salmonella Enteritidis PFGE pattern JEGX01. 0004. PloS One. 2013;8(1) doi: 10.1371/journal.pone.0055254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi F.E., Bukhari E.E., Al-Mohizea M.M., Hafiz T., Essa E.B., AlTokhais Y.I. Emergence of carbapenem-resistant Enterobacteriaceae isolated from patients in a university hospital in Saudi Arabia. Epidemiology, clinical profiles and outcomes. J. Infect. Publ. Health. 2017;10(5):667–673. doi: 10.1016/j.jiph.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amábile-Cuevas C. Society must seize control of the antibiotics crisis. Nature. 2016;533(7604):439. doi: 10.1038/533439a. [DOI] [PubMed] [Google Scholar]
- An Y.J., Kampbell D.H., Breidenbach G.P. Escherichia coli and total coliforms in water and sediments at lake marinas. Environ. Pollut. 2002;120(3):771–778. doi: 10.1016/s0269-7491(02)00173-2. [DOI] [PubMed] [Google Scholar]
- Andrade L.N., Vitali L., Gaspar G.G., Bellissimo-Rodrigues F., Martinez R., Darini A.L.C. Expansion and evolution of a virulent, extensively drug-resistant (polymyxin B-resistant), QnrS1-, CTX-M-2-and KPC-2-producing Klebsiella pneumoniae ST11 international high-risk clone. J. Clin. Microbiol. 2014:JCM-00088. doi: 10.1128/JCM.00088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apisarnthanarak A., Hsu L.Y., Khawcharoenporn T., Mundy L.M. Carbapenem-resistant Gram-negative bacteria: how to prioritize infection prevention and control interventions in resource-limited settings? Expert Rev. Anti-infect. Ther. 2013;11(2):147–157. doi: 10.1586/eri.12.164. [DOI] [PubMed] [Google Scholar]
- Araújo S., Silva I.A., Tacão M., Patinha C., Alves A., Henriques I. Characterization of antibiotic resistant and pathogenic Escherichia coli in irrigation water and vegetables in household farms. Int. J. Food Microbiol. 2017;257:192–200. doi: 10.1016/j.ijfoodmicro.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Arora B., Jagdale T. Altered membrane permeability in multidrug resistant Escherichia coli isolated from extra-intestinal infections. Afr. J. Biotechnol. 2009;8(21):5995–5999. [Google Scholar]
- Bakour S., Garcia V., Loucif L., Brunel J.M., Gharout-Sait A., Touati A., Rolain J.M. Rapid identification of carbapenemase-producing Enterobacteriaceae Pseudomonas aeruginosa and Acinetobacter baumannii using a modified Carba NP test. New Microb. New Infect. 2015;7:89–93. doi: 10.1016/j.nmni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthavatchalam Y.D., Anandan S., Veeraraghavan B. Laboratory detection and clinical implication of oxacillinase-48 like carbapenemase: the hidden threat. J. Global Infect. Dis. 2016;8(1):41. doi: 10.4103/0974-777X.176149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista P.V., McCusker M.P., Carvalho A., Ferreira D.A., Mohan N.M., Martins M., Fernandes A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018;9:1441. doi: 10.3389/fmicb.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barancheshme F., Munir M. Strategies to combat antibiotic resistance in the wastewater treatment plants. Front. Microbiol. 2018;8:2603. doi: 10.3389/fmicb.2017.02603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M., Carnelutti A., Peghin M. Patient specific risk stratification for antimicrobial resistance and possible treatment strategies in gram-negative bacterial infections. Expert Rev. Anti-infect. Ther. 2017;15(1):55–65. doi: 10.1080/14787210.2017.1251840. [DOI] [PubMed] [Google Scholar]
- Bello A., Dingle T.C. What's that resistance mechanism? Understanding genetic determinants of gram-negative bacterial resistance. Clin. Microbiol. Newsl. 2018;40(20):165–174. [Google Scholar]
- Ben W., Wang J., Cao R., Yang M., Zhang Y., Qiang Z. Distribution of antibiotic resistance in the effluents of ten municipal wastewater treatment plants in China and the effect of treatment processes. Chemosphere. 2017;172:392–398. doi: 10.1016/j.chemosphere.2017.01.041. [DOI] [PubMed] [Google Scholar]
- Ben Tanfous F., Alonso C.A., Achour W., Ruiz-Ripa L., Torres C., Ben Hassen A. First description of KPC-2-producing Escherichia coli and ST15 OXA-48-positive Klebsiella pneumoniae in Tunisia. Microb. Drug Resist. 2017;23(3):365–375. doi: 10.1089/mdr.2016.0090. [DOI] [PubMed] [Google Scholar]
- Benie C.K.D., Nathalie G., Adjéhi D. Prevalence and antibiotic resistance of Pseudomonas aeruginosa isolated from bovine meat, fresh fish and smoked fish. Arch. Clin. Microbiol. 2017;8:3. doi: 10.1556/1886.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund B. Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infect. Ecol. Epidemiol. 2015;5(1):28564. doi: 10.3402/iee.v5.28564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch G.F., Drage D.S., Thompson K., Eaglesham G., Mueller J.F. Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia. Mar. Pollut. Bull. 2015;97(1-2):56–66. doi: 10.1016/j.marpolbul.2015.06.038. [DOI] [PubMed] [Google Scholar]
- Brady C., Cleenwerck I., Venter S., Coutinho T., De Vos P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as . Syst. Appl. Microbiol. 2013;36(5):309–319. doi: 10.1016/j.syapm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Brisse S., Verhoef J. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int. J. Syst. Evol. Microbiol. 2001;51(3):915–924. doi: 10.1099/00207713-51-3-915. [DOI] [PubMed] [Google Scholar]
- Bueno M.M., Herrera S., Munaron D., Boillot C., Fenet H., Chiron S., Gómez E. POCIS passive samplers as a monitoring tool for pharmaceutical residues and their transformation products in marine environment. Environ. Sci. Pollut. Control Ser. 2016;23(6):5019–5029. doi: 10.1007/s11356-014-3796-5. [DOI] [PubMed] [Google Scholar]
- Burch T.R., Sadowsky M.J., LaPara T.M. Aerobic digestion reduces the quantity of antibiotic resistance genes in residual municipal wastewater solids. Front. Microbiol. 2013;4:17. doi: 10.3389/fmicb.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butaye P., Argudín M.Á., Threlfall J. Antimicrobial Resistance and Food Safety. Academic Press; 2015. Introduction to antimicrobial-resistant foodborne pathogens; pp. 1–17. [Google Scholar]
- Carattoli A., Villa L., Fortini D., García-Fernández A. Contemporary IncI1 plasmids involved in the transmission and spread of antimicrobial resistance in Enterobacteriaceae. Plasmid. 2018 doi: 10.1016/j.plasmid.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Castro C.B., Lopes A.R., Moreira I.V., Silva M.E., Manaia C.M., Nunes O.C. 2015. Wastewater Reuse in Irrigation: A Microbiological Perspective on Implications in Soil Fertility and Human and Environmental Health. [DOI] [PubMed] [Google Scholar]
- Chapman T.A., Wu X.Y., Barchia I., Bettelheim K.A., Driesen S., Trott D., Wilson M., Chin J.J.C. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 2006;72(7):4782–4795. doi: 10.1128/AEM.02885-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigor V.N., Umoh V.J., Smith S.I., Igbinosa E.O., Okoh A.I. Multidrug resistance and plasmid patterns of Escherichia coli O157 and other E. coli isolated from diarrhoeal stools and surface waters from some selected sources in Zaria, Nigeria. Int. J. Environ. Res. Publ. Health. 2010;7(10):3831–3841. doi: 10.3390/ijerph7103831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou A., Agüera A., Bayona J.M., Cytryn E., Fotopoulos V., Lambropoulou D., Manaia C.M., Michael C., Revitt M., Schröder P., Fatta-Kassinos D. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: the knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes–A review. Water Res. 2017;123:448–467. doi: 10.1016/j.watres.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Chu B.T., Petrovich M.L., Chaudhary A., Wright D., Murphy B., Wells G., Poretsky R. Metagenomics reveals the impact of wastewater treatment plants on the dispersal of microorganisms and genes in aquatic sediments. Appl. Environ. Microbiol. 2018;84(5) doi: 10.1128/AEM.02168-17. e02168-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudron P.E., Moland E.S., Sanders C.C. Occurrence and detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae at a veterans medical center: seek and you may find. J. Clin. Microbiol. 1997;35(10):2593–2597. doi: 10.1128/jcm.35.10.2593-2597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudron P.E., Moland E.S., Thomson K.S. Occurrence and detection of AmpC beta-lactamases among Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates at a veteran’s medical center. J. Clin. Microbiol. 2000;38(5):1791–1796. doi: 10.1128/jcm.38.5.1791-1796.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon G., Naas T., Demachy M.C., Nordmann P. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC-2 in Klebsiella pneumoniae isolate from Greece. Antimicrob. Agents Chemother. 2008;52(2):796–797. doi: 10.1128/AAC.01180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekalski N., Díez E.G., Bürgmann H. Wastewater as a point source of antibiotic-resistance genes in the sediment of a freshwater lake. ISME J. 2014;8(7):1381. doi: 10.1038/ismej.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dänicke S., Meyer U., Geue L. Dynamics of total and Shiga-toxin (Stx) forming Escherichia coli shedding with faeces of dairy cows experiencing a change from a low to a high caloric diet. J. für Verbraucherschutz und Lebensmittelsicherheit. 2015;10(1):23–28. [Google Scholar]
- Devarajan N., Laffite A., Graham N.D., Meijer M., Prabakar K., Mubedi J.I., Elongo V., Mpiana P.T., Ibelings B.W., Wildi W., Poté J. Accumulation of clinically relevant antibiotic-resistance genes, bacterial load, and metals in freshwater lake sediments in Central Europe. Environ. Sci. Technol. 2015;49(11):6528–6537. doi: 10.1021/acs.est.5b01031. [DOI] [PubMed] [Google Scholar]
- Devarajan N., Laffite A., Mulaji C.K., Otamonga J.P., Mpiana P.T., Mubedi J.I., Prabakar K., Ibelings B.W., Poté J. Occurrence of antibiotic resistance genes and bacterial markers in a tropical river receiving hospital and urban wastewaters. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0149211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan N., Köhler T., Sivalingam P., Van Delden C., Mulaji C.K., Mpiana P.T., Ibelings B.W., Poté J. Antibiotic resistant Pseudomonas spp. in the aquatic environment: a prevalence study under tropical and temperate climate conditions. Water Res. 2017;115:256–265. doi: 10.1016/j.watres.2017.02.058. [DOI] [PubMed] [Google Scholar]
- Di Cesare A., Eckert E.M., D'Urso S., Bertoni R., Gillan D.C., Wattiez R., Corno G. Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res. 2016;94:208–214. doi: 10.1016/j.watres.2016.02.049. [DOI] [PubMed] [Google Scholar]
- Dimou V., Dhanji H., Pike R., Livermore D.M., Woodford N. Characterization of Enterobacteriaceae producing OXA-48-like carbapenemases in the UK. J. Antimicrob. Chemother. 2012;67(7):1660–1665. doi: 10.1093/jac/dks124. [DOI] [PubMed] [Google Scholar]
- Dramowski A., Whitelaw A., Cotton M.F. Burden, spectrum, and impact of healthcare-associated infection at a south African children's hospital. J. Hosp. Infect. 2016;94(4):364–372. doi: 10.1016/j.jhin.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drawz S.M., Bonomo R.A. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 2010;23(1):160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriez E., Armengaud J., Fenaille F., Ezan E. Mass spectrometry for the detection of bioterrorism agents: from environmental to clinical applications. J. Mass Spectrom. 2016;51(3):183–199. doi: 10.1002/jms.3747. [DOI] [PubMed] [Google Scholar]
- Dziri O., Alonso C.A., Dziri R., Gharsa H., Maraoub A., Torres C., Chouchani C. Metallo-β-lactamases and class D carbapenemases in South-East of Tunisia: implication of mobile genetic elements in their dissemination. Int. J. Antimicrob. Agents. 2018 doi: 10.1016/j.ijantimicag.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Edge T.A., Hill S. Occurrence of antibiotic resistance in Escherichia coli from surface waters and fecal pollution sources near Hamilton, Ontario. Can. J. Microbiol. 2005;51(6):501–505. doi: 10.1139/w05-028. [DOI] [PubMed] [Google Scholar]
- Falagas M.E., Tansarli G.S., Karageorgopoulos D.E., Vardakas K.Z. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg. Infect. Dis. 2014;20(7):1170. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M.R., Moura Q., Sartori L., Silva K.C., Cunha M.P., Esposito F., Lopes R., Otutumi L.K., Gonçalves D.D., Dropa M., Matté M.H. Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Euro Surveill. 2016;21(17) doi: 10.2807/1560-7917.ES.2016.21.17.30214. [DOI] [PubMed] [Google Scholar]
- Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8(9):623. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Fletcher S. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ. Health Prev. Med. 2015;20(4):243. doi: 10.1007/s12199-015-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flury B.B., Ellington M.J., Hopkins K.L., Turton J.F., Doumith M., Loy R., Staves P., Hinic V., Frei R., Woodford N. Association of novel nonsynonymous single nucleotide polymorphisms in ampD with cephalosporin resistance and phylogenetic variations in ampC, ampR, ompF, and ompC in Enterobacter cloacae isolates that are highly resistant to carbapenems. Antimicrob. Agents Chemother. 2016;60(4):2383–2390. doi: 10.1128/AAC.02835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010;7(12):653. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- Gama J.A., Zilhão R., Dionisio F. Impact of plasmid interactions with the chromosome and other plasmids on the spread of antibiotic resistance. Plasmid. 2018 doi: 10.1016/j.plasmid.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Gerrity D., Holady J.C., Mawhinney D.B., Quinones O., Trenholm R.A., Snyder S.A. The effects of solids retention time in full-scale activated sludge basins on trace organic contaminant concentrations. Water Environ. Res. 2013;85(8):715–724. doi: 10.2175/106143012x13560205144533. [DOI] [PubMed] [Google Scholar]
- Gomi R., Matsuda T., Fujimori Y., Harada H., Matsui Y., Yoneda M. Characterization of pathogenic Escherichia coli in river water by simultaneous detection and sequencing of 14 virulence genes. Environ. Sci. Technol. 2015;49(11):6800–6807. doi: 10.1021/acs.est.5b00953. [DOI] [PubMed] [Google Scholar]
- Gomez-Simmonds A., Stump S., Giddins M.J., Annavajhala M.K., Uhlemann A.C. clonal background, resistance gene profile, and porin gene mutations modulate in vitro susceptibility to imipenem/relebactam in diverse Enterobacteriaceae. Antimicrob. Agents Chemother. 2018:AAC-00573. doi: 10.1128/AAC.00573-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothwal R., Shashidhar T. Antibiotic pollution in the environment: a review. Clean. 2015;43(4):479–489. [Google Scholar]
- Guerra B., Fischer J., Helmuth R. An emerging public health problem: acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet. Microbiol. 2014;171(3-4):290–297. doi: 10.1016/j.vetmic.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Gülmez D., Woodford N., Palepou M.F.I., Mushtaq S., Metan G., Yakupogullari Y., Kocagoz S., Uzun O., Hascelik G., Livermore D.M. Carbapenem-resistant Escherichia coli and Klebsiella pneumoniae isolates from Turkey with OXA-48-like carbapenemases and outer membrane protein loss. Int. J. Antimicrob. Agents. 2008;31(6):523–526. doi: 10.1016/j.ijantimicag.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Hamzaoui Z., Ocampo-Sosa A., Maamar E., Fernandez Martinez M., Ferjani S., Hammami S., Harbaoui S., Genel N., Arlet G., Saidani M., Slim A. An outbreak of NDM-1-producing Klebsiella pneumoniae, associated with OmpK35 and OmpK36 porin loss in Tunisia. Microb. Drug Resist. 2018 doi: 10.1089/mdr.2017.0165. [DOI] [PubMed] [Google Scholar]
- Helbling D.E., Johnson D.R., Honti M., Fenner K. Micropollutant biotransformation kinetics associate with WWTP process parameters and microbial community characteristics. Environ. Sci. Technol. 2012;46(19):10579–10588. doi: 10.1021/es3019012. [DOI] [PubMed] [Google Scholar]
- Hernández F., Ibáñez M., Gracia-Lor E., Sancho J.V. Retrospective LC-QTOF-MS analysis searching for pharmaceutical metabolites in urban wastewater. J. Separ. Sci. 2011;34(24):3517–3526. doi: 10.1002/jssc.201100540. [DOI] [PubMed] [Google Scholar]
- Hocquet D., Muller A., Bertrand X. What happens in hospitals does not stay in hospitals: antibiotic-resistant bacteria in hospital wastewater systems. J. Hosp. Infect. 2016;93(4):395–402. doi: 10.1016/j.jhin.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Holt K.E., Wertheim H., Zadoks R.N., Baker S., Whitehouse C.A., Dance D., Jenney A., Connor T.R., Hsu L.Y., Severin J., Brisse S. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112(27):E3574–E3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://amr-review.org/ (accessed: 18/12/2018).https://www.cdc.gov/drugresistance/about.html (accessed: 07/07/2018).https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70190-7/fulltext (accessed: 27/5/2018).
- Igbinosa I.H., Igbinosa E.O., Okoh A.I. Molecular detection of metallo-β-lactamase and putative virulence genes in environmental isolates of Pseudomonas species. Pol. J. Environ. Stud. 2014;23(6) [Google Scholar]
- Igbinosa I.H., Beshiru A., Odjadjare E.E., Ateba C.N., Igbinosa E.O. Pathogenic potentials of Aeromonas species isolated from aquaculture and abattoir environments. Microb. Pathog. 2017;107:185–192. doi: 10.1016/j.micpath.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Isichei-Ukah O.B., Enabulele O.I. Prevalence and antimicrobial resistance of Pseudomonas aeruginosa recovered from environmental and clinical sources in Benin City, Nigeria. IFE J. Sci. 2018;20(3):547–555. [Google Scholar]
- James C.E., Mahendran K.R., Molitor A., Bolla J.M., Bessonov A.N., Winterhalter M., Pagès J.M. How β-lactam antibiotics enter bacteria: a dialogue with the porins. PloS One. 2009;4(5):e5453. doi: 10.1371/journal.pone.0005453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelić A., Gros M., Petrović M., Ginebreda A., Barceló D. Springer; Berlin, Heidelberg: 2012. Occurrence and elimination of pharmaceuticals during conventional wastewater treatment; pp. 1–23. (Emerging and Priority Pollutants in Rivers). [Google Scholar]
- Kaper J.B., Nataro J.P., Mobley H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2(2):123. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Karam G., Chastre J., Wilcox M.H., Vincent J.L. Antibiotic strategies in the era of multidrug resistance. Crit. Care. 2016;20(1):136. doi: 10.1186/s13054-016-1320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karampatakis T., Antachopoulos C., Iosifidis E., Tsakris A., Roilides E. Molecular epidemiology of carbapenem-resistant Klebsiella pneumonia in Greece. Future Microbiol. 2016;11(6):809–823. doi: 10.2217/fmb-2016-0042. [DOI] [PubMed] [Google Scholar]
- Kardos N. Overuse of antibiotics and antibiotic resistance in medical applications featuring carbapenemase resistant Enterobacteriaceae. SOJ Microbiol. Infect. Dis. 2017;5(5):1–21. Overuse of Antibiotics and Antibiotic Resistance in Medical Applications Featuring Carbapenemase Resistant Enterobacteriaceae (CRE) [Google Scholar]
- Karkman A., Do T.T., Walsh F., Virta M.P. Antibiotic-resistance genes in waste water. Trends Microbiol. 2018;26(3):220–228. doi: 10.1016/j.tim.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Kitchel B., Rasheed J.K., Patel J.B., Srinivasan A., Navon-Venezia S., Carmeli Y., Brolund A., Giske C.G. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 2009;53(8):3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B., Szabó D. Microbial Pathogens and Strategies for Combating them: science, technology and education, 1. 2013. Antibiotic resistance mechanisms in Enterobacteriaceae. [Google Scholar]
- Kopecko D.J., Sieber H., Ures J.A., Fürer A., Schlup J., Knof U., Collioud A., Xu D., Colburn K., Dietrich G. Genetic stability of vaccine strain Salmonella typhi Ty21a over 25 years. Int. J. Med. Microbiol. 2009;299(4):233–246. doi: 10.1016/j.ijmm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- K'oreje K.O., Kandie F.J., Vergeynst L., Abira M.A., Van Langenhove H., Okoth M., Demeestere K. Occurrence, fate and removal of pharmaceuticals, personalcare products and pesticides in wastewater stabilization ponds and receiving rivers in the Nzoia Basin, Kenya. Sci. Total Environ. 2018;637:336–348. doi: 10.1016/j.scitotenv.2018.04.331. [DOI] [PubMed] [Google Scholar]
- Kümmerer K. Resistance in the environment. J. Antimicrob. Chemother. 2004;54(2):311–320. doi: 10.1093/jac/dkh325. [DOI] [PubMed] [Google Scholar]
- Lääveri T., Vlot J.A., van Dam A.P., Häkkinen H.K., Sonder G.J., Visser L.G., Kantele A. Extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) among travellers to Africa: destination-specific data pooled from three European prospective studies. BMC Infect. Dis. 2018;18(1):341. doi: 10.1186/s12879-018-3245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffite A., Kilunga P.I., Kayembe J.M., Devarajan N., Mulaji C.K., Giuliani G., Slaveykova V.I., Poté J. Hospital effluents are one of several sources of metal, antibiotic resistance genes, and bacterial markers disseminated in Sub-Saharan urban rivers. Front. Microbiol. 2016;7:1128. doi: 10.3389/fmicb.2016.01128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J.G., Roth J.R. Selfish operons: horizontal transfer may drive the evolution of gene clusters. Genetics. 1996;143(4):1843–1860. doi: 10.1093/genetics/143.4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hello S., Harrois D., Bouchrif B., Sontag L., Elhani D., Guibert V., Zerouali K., Weill F.X. Highly drug-resistant Salmonella enterica serotype Kentucky ST198-X1:a microbiological study. Lancet Infect. Dis. 2013;13(8):672679. doi: 10.1016/S1473-3099(13)70124-5. [DOI] [PubMed] [Google Scholar]
- Lehn N., Stower-Hoffmann J., Kott T., Strassner C., Wagner H., Kronke M., Schneider-Brachert W. Characterization of clinical isolates of Escherichia coli showing high levels of fluoroquinolone resistance. J. Clin. Microbiol. 1996;34(3):597–602. doi: 10.1128/jcm.34.3.597-602.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimbach A., Hacker J., Dobrindt U. Between Pathogenicity and Commensalism. Springer; Berlin, Heidelberg: 2013. E. coli as an all-rounder: the thin line between commensalism and pathogenicity; pp. 3–32. [DOI] [PubMed] [Google Scholar]
- Li B., Yang Y., Ma L., Ju F., Guo F., Tiedje J.M., Zhang T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME J. 2015;9(11):2490. doi: 10.1038/ismej.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Dechesne A., He Z., Madsen J.S., Nesme J., Sørensen S.J., Smets B.F. Estimating the transfer range of plasmids encoding antimicrobial resistance in a wastewater treatment plant microbial community. Environ. Sci. Technol. Lett. 2018;5(5):260–265. [Google Scholar]
- Lorenzo P., Adriana A., Jessica S., Carles B., Marinella F., Marta L., Luis B.J., Pierre S. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem. Chemosphere. 2018;206:70–82. doi: 10.1016/j.chemosphere.2018.04.163. [DOI] [PubMed] [Google Scholar]
- Lowman W., Sriruttan C., Nana T., Bosman N., Duse A., Venturas J., Clay C., Coetzee J. NDM-1 has arrived: first report of a carbapenem resistance mechanism in South Africa. SAMJ: S. Afr. Med. J. 2011;101(12):873–875. [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupan I., Carpa R., Oltean A., Kelemen B.S., Popescu O. Release of antibiotic resistant bacteria by a waste treatment plant from Romania. Microb. Environ. 2017;32(3):219–225. doi: 10.1264/jsme2.ME17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyimo B., Buza J., Subbiah M., Temba S., Kipasika H., Smith W., Call D.R. IncF plasmids are commonly carried by antibiotic resistant Escherichia coli isolated from drinking water sources in northern Tanzania. Int. J. Microbiol. 2016 doi: 10.1155/2016/3103672. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon B.M., Brehony C., McGrath E., Killeen J., Cormican M., Hickey P., Keane S., Hanahoe B., Dolan A., Morris D. Indistinguishable NDM-producing Escherichia coli isolated from recreational waters, sewage, and a clinical specimen in Ireland, 2016 to 2017. Euro Surveill. 2017;22(15) doi: 10.2807/1560-7917.ES.2017.22.15.30513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaia C.M., Rocha J., Scaccia N., Marano R., Radu E., Biancullo F., Cerqueira F., Fortunato G., Iakovides I.C., Zammit I., Kampouris I. Antibiotic resistance in wastewater treatment plants: tackling the black box. Environ. Int. 2018;115:312–324. doi: 10.1016/j.envint.2018.03.044. [DOI] [PubMed] [Google Scholar]
- Margot J., Rossi L., Barry D.A., Holliger C. A review of the fate of micropollutants in wastewater treatment plants. Wiley Interdisciplin. Rev.: Water. 2015;2(5):457–487. [Google Scholar]
- Martens E., Demain A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017;70(5):520. doi: 10.1038/ja.2017.30. [DOI] [PubMed] [Google Scholar]
- Marti E., Jofre J., Balcazar J.L. Prevalence of antibiotic resistance genes and bacterial community composition in a river influenced by a wastewater treatment plant. PloS One. 2013;8(10) doi: 10.1371/journal.pone.0078906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E., Variatza E., Balcazar J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014;22(1):36–41. doi: 10.1016/j.tim.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. The role of operating parameters and oxidative damage mechanisms of advanced chemical oxidation processes in the combat against antibiotic-resistant bacteria and resistance genes present in urban wastewater. Water Res. 2018;129:208–230. doi: 10.1016/j.watres.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Mielczarek A.T., Nguyen H.T.T., Nielsen J.L., Nielsen P.H. Population dynamics of bacteria involved in enhanced biological phosphorus removal in Danish wastewater treatment plants. Water Res. 2013;47(4):1529–1544. doi: 10.1016/j.watres.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Miller J.H., Novak J.T., Knocke W.R., Pruden A. Survival of antibiotic resistant bacteria and horizontal gene transfer control antibiotic resistance gene content in anaerobic digesters. Front. Microbiol. 2016;7:263. doi: 10.3389/fmicb.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miri S.T., Dashti A., Mostaan S., Kazemi F., Bouzari S. Identification of different Escherichia coli pathotypes in north and north-west provinces of Iran. Iran. J. Microbiol. 2017;9(1):33. [PMC free article] [PubMed] [Google Scholar]
- Mediavilla J.R., Patrawalla A., Chen L., Chavda K.D., Mathema B., Vinnard C., Dever L.L., Kreiswirth B.N. Colistin-and carbapenem-resistant Escherichia coli harbouring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. mBio. 2016;7(4) doi: 10.1128/mBio.01191-16. e01191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremi N., Manda E.V., Falgenhauer L., Ghosh H., Imirzalioglu C., Matee M., Chakraborty T., Mshana S.E. Predominance of CTX-M-15 among ESBL producers from environment and fish gut from the shores of Lake Victoria in Mwanza, Tanzania. Front. Microbiol. 2016;7:1862. doi: 10.3389/fmicb.2016.01862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosekiemang T.T., Stander M.A., de Villiers A. Simultaneous quantification of commonly prescribed antiretroviral drugs and their selected metabolites in aqueous environmental samples by direct injection and solid phase extraction liquid Chromatography-Tandem mass spectrometry. Chemosphere. 2019 doi: 10.1016/j.chemosphere.2018.12.205. [DOI] [PubMed] [Google Scholar]
- Myllyniemi Maldonado J. 2018. Occurrence of Selected Pharmaceuticals in Groundwater, Surface Water, Wastewater and Source-Separated Urine in a Peri-Urban Area of Lusaka, Zambia. [Google Scholar]
- Netikul T., Kiratisin P. Genetic characterization of carbapenem-resistant enterobacteriaceae and the spread of carbapenem-resistant Klebsiella pneumonia ST340 at a university hospital in Thailand. PloS One. 2015;10(9) doi: 10.1371/journal.pone.0139116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwirth C., Siebor E., Lopez J., Pechinot A., Kazmierczak A. Outbreak of TEM-24-producing Enterobacter aerogenes in an intensive care unit and dissemination of the extended-spectrum beta-lactamase to other members of the family enterobacteriaceae. J. Clin. Microbiol. 1996;34(1):76–79. doi: 10.1128/jcm.34.1.76-79.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.N., Kasuga I., Liu M., Katayama H. Vol. 266. IOP Publishing; 2019. Occurrence of antibiotic resistance genes as emerging contaminants in watersheds of Tama River and Lake Kasumigaura in Japan. (IOP Conference Series: Earth and Environmental Science). 1. [Google Scholar]
- Nielsen E.M., Andersen M.T. Detection and characterization of verocytotoxin-producing Escherichia coli by automated 5′ nuclease PCR assay. J. Clin. Microbiol. 2003;41(7):2884–2893. doi: 10.1128/JCM.41.7.2884-2893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnadozie C.F., Kumari S., Bux F. Status of pathogens, antibiotic resistance genes and antibiotic residues in wastewater treatment systems. Rev. Environ. Sci. Biotechnol. 2017;16(3):491–515. [Google Scholar]
- Nordmann P., Mariotte S., Naas T., Labia R., Nicolas M.H. Biochemical properties of a carbapenem-hydrolyzing beta-lactamase from Enterobacter cloacae and cloning of the gene into Escherichia coli. Antimicrob. Agents Chemother. 1993;37(5):939–946. doi: 10.1128/aac.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Cuzon G., Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 2009;9(4):228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- Nordmann P., Naas T., Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011;17(10):1791. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Dortet L., Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol. Med. 2012;18(5):263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Patel G., Bonomo R. “Stormy waters ahead”: global emergence of carbapenemases. Front. Microbiol. 2013;4:48. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D.L. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Contr. 2006;34(5):S20–S28. doi: 10.1016/j.ajic.2006.05.238. [DOI] [PubMed] [Google Scholar]
- Peirano G., Bradford P.A., Kazmierczak K.M., Chen L., Kreiswirth B.N., Pitout J.D. The importance of clonal complex 258 and IncFK2-like plasmids among a global collection of Klebsiella pneumoniae with blaKPCs. Antimicrob. Agents Chemother. 2017:AAC-02610. doi: 10.1128/AAC.02610-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penchovsky R., Traykovska M. Designing drugs that overcome antibacterial resistance: where do we stand and what should we do? Expet Opin. Drug Discov. 2015;10(6):631–650. doi: 10.1517/17460441.2015.1048219. [DOI] [PubMed] [Google Scholar]
- Perry J.D., Naqvi S.H., Mirza I.A., Alizai S.A., Hussain A., Ghirardi S., Orenga S., Wilkinson K., Woodford N., Zhang J., Livermore D.M. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J. Antimicrob. Chemother. 2011;66(10):2288–2294. doi: 10.1093/jac/dkr299. [DOI] [PubMed] [Google Scholar]
- Poirel L., Nordmann P., Lagrutta E., Cleary T., Munoz-Price L.S. Emergence of KPC-producing Pseudomonas aeruginosa in the United States. Antimicrob. Agents Chemother. 2010;54(7):3072. doi: 10.1128/AAC.00513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Kieffer N., Liassine N., Thanh D., Nordmann P. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect. Dis. 2016;16(3):281. doi: 10.1016/S1473-3099(16)00006-2. [DOI] [PubMed] [Google Scholar]
- Polesel F., Andersen H.R., Trapp S., Plosz B.G. Removal of antibiotics in biological wastewater treatment systems. A critical assessment using the activated sludge modeling framework for xenobiotics (ASM-X) Environ. Sci. Technol. 2016;50(19):10316–10334. doi: 10.1021/acs.est.6b01899. [DOI] [PubMed] [Google Scholar]
- Poole K. Resistance to β-lactam antibiotics. Cell. Mol. Life Sci. CMLS. 2004;61(17):2200–2223. doi: 10.1007/s00018-004-4060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter R.F., D’souza A.W., Dantas G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist. Updates. 2016;29:30–46. doi: 10.1016/j.drup.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulou A., Voulgari E., Vrioni G., Koumaki V., Xidopoulos G., Chatzipantazi V., Markou F., Tsakris A. Outbreak caused by an ertapenem-resistant, CTX-M-15-producing Klebsiella pneumoniae ST101 clone carrying an OmpK36 porin variant. J. Clin. Microbiol. 2013:JCM-01244. doi: 10.1128/JCM.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia L., Anzil A., Borrego C., Farrè M., Llorca M., Sanchis J., Bogaerts P., Balcázar J.L., Servais P. Occurrence and persistence of carbapenemases genes in hospital and wastewater treatment plants and propagation in the receiving river. J. Hazard Mater. 2018;358:33–43. doi: 10.1016/j.jhazmat.2018.06.058. [DOI] [PubMed] [Google Scholar]
- Rafraf I.D., Lekunberri I., Sànchez-Melsió A., Aouni M., Borrego C.M., Balcázar J.L. Abundance of antibiotic resistance genes in five municipals wastewater treatment plants in the Monastir Governorate, Tunisia. Environ. Pollut. 2016;219:353–358. doi: 10.1016/j.envpol.2016.10.062. [DOI] [PubMed] [Google Scholar]
- Ribot E.M., Fair M.A., Gautom R., Cameron D.N., Hunter S.B., Swaminathan B., Barrett T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157: H7, Salmonella, and Shigella for PulseNet. Foodb. Pathog. Dis. 2006;3(1):59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- Rivas M., Miliwebsky E., Chinen I., Roldan C.D., Balbi L., Garcia B., Fiorilli G., Sosa-Estani S., Kincaid J., Rangel J., Griffin P.M. Characterization and epidemiologic subtyping of Shiga toxin–producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodb. Pathog. Dis. 2006;3(1):88–96. doi: 10.1089/fpd.2006.3.88. [DOI] [PubMed] [Google Scholar]
- Rizzo L., Manaia C., Merlin C., Schwartz T., Dagot C., Ploy M.C., Michael I., Fatta-Kassinos D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci. Total Environ. 2013;447:345–360. doi: 10.1016/j.scitotenv.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Rosenthal V.D. Device-associated nosocomial infections in limited-resources countries: findings of the international nosocomial infection control consortium (INICC) Am. J. Infect. Contr. 2008;36(10) doi: 10.1016/j.ajic.2008.10.009. S171-e7. [DOI] [PubMed] [Google Scholar]
- Rubin J.E., Pitout J.D. Extended-spectrum β-lactamase, carbapenemase and AmpC producing Enterobacteriaceae in companion animals. Vet. Microbiol. 2014;170(1-2):10–18. doi: 10.1016/j.vetmic.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Russotto V., Cortegiani A., Graziano G., Saporito L., Raineri S.M., Mammina C., Giarratano A. Bloodstream infections in intensive care unit patients: distribution and antibiotic resistance of bacteria. Infect. Drug Resist. 2015;8:287. doi: 10.2147/IDR.S48810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara L., Matthew C., Christine L., Ruth T., Kevin A., Dave H., Segaran P., Stephen M., Erum K., Molly H., Marc A. 7th ARAE–Symposium on Antimicrobial Resistance in Animals and the Environment. 2017. Resistome of multidrug resistant Klebsiella pneumoniae; pp. 1–167. [Google Scholar]
- Schellack N., Bronkhorst E., Maluleka C., Hunt L., Srinivas P., Grootboom W., Goff D., Naicker P., Modau T., Babarinde O. Fluoroquinolone-resistant Salmonella typhi infection: a report of two cases in South Africa. Southern Afr. J. Infect. Dis. 2018;33(2):54–56. [Google Scholar]
- Schwermer C.U., Krzeminski P., Wennberg A.C., Vogelsang C., Uhl W. Removal of antibiotic resistant E. coli in two Norwegian wastewater treatment plants and by nano-and ultra-filtration processes. Water Sci. Technol. 2018;77(4):1115–1126. doi: 10.2166/wst.2017.642. [DOI] [PubMed] [Google Scholar]
- Seiffert S.N., Perreten V., Johannes S., Droz S., Bodmer T., Endimiani A. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob. Agents Chemother. 2014;58(4):2446–2449. doi: 10.1128/AAC.02417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais P., Passerat J. Antimicrobial resistance of faecal bacteria in waters of the Seine river watershed (France) Sci. Total Environ. 2009;408(2):365–372. doi: 10.1016/j.scitotenv.2009.09.042. [DOI] [PubMed] [Google Scholar]
- Servais P., Prats J., Passerat J., Garcia-Armisen T. Abundance of culturable versus viable Escherichia coli in freshwater. Can. J. Microbiol. 2009;55(7):905–909. doi: 10.1139/w09-043. [DOI] [PubMed] [Google Scholar]
- Sharma A., Bakthavatchalam Y.D., Gopi R., An S., Verghese V.P., Veeraraghavan B. Mechanisms of carbapenem resistance in K. pneumoniae and E. coli from bloodstream infections in India. J. Infect. Dis. Ther. 2016 [Google Scholar]
- Shields R.K., Chen L., Cheng S., Chavda K.D., Press E.G., Snyder A., Pandey R., Doi Y., Kreiswirth B.N., Nguyen M.H., Clancy C.J. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob. Agents Chemother. 2017;61(3) doi: 10.1128/AAC.02097-16. e02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K. Bioreactor Engineering Research and Industrial Applications I. Springer; Berlin, Heidelberg: 2015. Metabolic regulation and coordination of the metabolism in bacteria in response to a variety of growth conditions; pp. 1–54. [DOI] [PubMed] [Google Scholar]
- Shulman S.T., Friedmann H.C., Sims R.H. Theodor Escherich: the first pediatric infectious diseases physician? Clin. Infect. Dis. 2007;45(8):1025–1029. doi: 10.1086/521946. [DOI] [PubMed] [Google Scholar]
- Shutes R.B.E. Artificial wetlands and water quality improvement. Environ. Int. 2001;26(5-6):441–447. doi: 10.1016/s0160-4120(01)00025-3. [DOI] [PubMed] [Google Scholar]
- Sidhu J.P., Ahmed W., Hodgers L., Toze S. Occurrence of virulence genes associated with diarrheagenic pathotypes in Escherichia coli isolates from surface water. Appl. Environ. Microbiol. 2013;79(1):328–335. doi: 10.1128/AEM.02888-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Moodley A., Perovic O. Antimicrobial susceptibility testing in predicting the presence of carbapenemase genes in Enterobacteriaceae in South Africa. BMC Infect. Dis. 2016;16(1):536. doi: 10.1186/s12879-016-1858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Garg A., Pandit S., Mokkapati V.R.S.S., Mijakovic I. Antimicrobial effects of biogenic nanoparticles. Nanomaterials. 2018;8(12):1009. doi: 10.3390/nano8121009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu L.K., Yeh K.M., Lin J.C., Fung C.P., Chang F.Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 2012;12(11):881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- Slayton R.B., Toth D., Lee B.Y., Tanner W., Bartsch S.M., Khader K., Wong K., Brown K., McKinnell J.A., Ray W., Miller L.G. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities—United States. MMWR. Morbidit. Mortal. Weekly Rep. 2015;64(30):826. [PMC free article] [PubMed] [Google Scholar]
- Sokurenko E.V., Chesnokova V., Dykhuizen D.E., Ofek I., Wu X.R., Krogfelt K.A., Struve C., Schembri M.A., Hasty D.L. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc. Natl. Acad. Sci. Unit. States Am. 1998;95(15):8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler G., Kincses A., Gajdács M., Amaral L. New roads leading to old destinations: efflux pumps as targets to reverse multidrug resistance in bacteria. Molecules. 2017;22(3):468. doi: 10.3390/molecules22030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürenburg E., Sobottka I., Mack D., Laufs R. Cloning and sequencing of Enterobacter aerogenes OmpC-type osmoporin linked to carbapenem resistance. Int. J. Med. Microbiol. 2002;291(8):649–654. doi: 10.1078/1438-4221-00175. [DOI] [PubMed] [Google Scholar]
- Su L.H., Chu C., Cloeckaert A., Chiu C.H. An epidemic of plasmids? Dissemination of extended-spectrum cephalosporinases among Salmonella and other Enterobacteriaceae. FEMS Immunol. Med. Microbiol. 2008;52(2):155–168. doi: 10.1111/j.1574-695X.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Suez J., Porwollik S., Dagan A., Marzel A., Schorr Y.I., Desai P.T., Agmon V., McClelland M., Rahav G., Gal-Mor O. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PloS One. 2013;8(3) doi: 10.1371/journal.pone.0058449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres E., Baricz A., Chiriac C.M., Farkas A., Opris O., Soran M.L., Andrei A.S., Rudi K., Balcázar J.L., Dragos N., Coman C. Abundance of antibiotics, antibiotic resistance genes and bacterial community composition in wastewater effluents from different Romanian hospitals. Environ. Pollut. 2017;225:304–315. doi: 10.1016/j.envpol.2017.01.054. [DOI] [PubMed] [Google Scholar]
- Tacão M., Correia A., Henriques I. Applied and Environmental Microbiology; 2012. Resistance to Broad-Spectrum Antibiotics in Aquatic Systems: Anthropogenic Activities Modulate the Dissemination of blaCTX-M-like Genes; p. AEM-00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafoukt R., Touati A., Leangapichart T., Bakour S., Rolain J.M. Characterization of OXA-48-like-producing Enterobacteriaceae isolated from river water in Algeria. Water Res. 2017;120:185–189. doi: 10.1016/j.watres.2017.04.073. [DOI] [PubMed] [Google Scholar]
- Tang P., Croxen M.A., Hasan M.R., Hsiao W.W., Hoang L.M. Infection control in the new age of genomic epidemiology. Am. J. Infect. Contr. 2017;45(2):170–179. doi: 10.1016/j.ajic.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Temkin E., Adler A., Lerner A., Carmeli Y. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann. N. Y. Acad. Sci. 2014;1323(1):22–42. doi: 10.1111/nyas.12537. [DOI] [PubMed] [Google Scholar]
- Thapar N., Sanderson I.R. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004;363(9409):641–653. doi: 10.1016/S0140-6736(04)15599-2. [DOI] [PubMed] [Google Scholar]
- Tong J., Tang A., Wang H., Liu X., Huang Z., Wang Z., Zhang J., Wei Y., Su Y., Zhang Y. Microbial community evolution and fate of antibiotic resistance genes along six different full-scale municipal wastewater treatment processes. Bioresour. Technol. 2019;272:489–500. doi: 10.1016/j.biortech.2018.10.079. [DOI] [PubMed] [Google Scholar]
- Uribe I.O., Mosquera-Corral A., Rodicio J.L., Esplugas S. Advanced technologies for water treatment and reuse. AIChE J. 2015;61(10):3146–3158. [Google Scholar]
- uz Zaman T., Aldrees M., Al Johani S.M., Alrodayyan M., Aldughashem F.A., Balkhy H.H. Multi-drug carbapenem-resistant Klebsiella pneumoniae infection carrying the OXA-48 gene and showing variations in outer membrane protein 36 causing an outbreak in a tertiary care hospital in Riyadh, Saudi Arabia. Int. J. Infect. Dis. 2014;28:186–192. doi: 10.1016/j.ijid.2014.05.021. [DOI] [PubMed] [Google Scholar]
- uz Zaman T., Alrodayyan M., Albladi M., Aldrees M., Siddique M.I., Aljohani S., Balkhy H.H. Clonal diversity and genetic profiling of antibiotic resistance among multidrug/carbapenem-resistant Klebsiella pneumoniae isolates from a tertiary care hospital in Saudi Arabia. BMC Infect. Dis. 2018;18(1):205. doi: 10.1186/s12879-018-3114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Crevel R., van de Vijver S., Moore D.A. The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? The Lancet Diabet. Endocrinol. 2017;5(6):457–468. doi: 10.1016/S2213-8587(16)30081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin D., Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8(4):460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz-Moreira I., Nunes O.C., Manaia C.M. Bacterial diversity and antibiotic resistance in water habitats: searching the links with the human microbiome. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 2014;38(4):761–778. doi: 10.1111/1574-6976.12062. [DOI] [PubMed] [Google Scholar]
- Verlicchi P., Al Aukidy M., Zambello E. What have we learned from worldwide experiences on the management and treatment of hospital effluent?—an overview and a discussion on perspectives. Sci. Total Environ. 2015;514:467–491. doi: 10.1016/j.scitotenv.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieno N., Sillanpää M. Fate of diclofenac in municipal wastewater treatment plant—a review. Environ. Int. 2014;69:28–39. doi: 10.1016/j.envint.2014.03.021. [DOI] [PubMed] [Google Scholar]
- von Wintersdorff C.J., Penders J., van Niekerk J.M., Mills N.D., Majumder S., van Alphen L.B., Savelkoul P.H., Wolffs P.F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016;7:173. doi: 10.3389/fmicb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R.I., Steele D., Aguado T., Ad Hoc ETEC Technical Expert Committee Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine. 2007;25(14):2545–2566. doi: 10.1016/j.vaccine.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Walsh T.R. Emerging carbapenemases: a global perspective. Int. J. Antimicrob. Agents. 2010;36:S8–S14. doi: 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- Walsh T.R., Toleman M.A. The new medical challenge: why NDM-1? Why Indian? Expert Rev. Anti-infect. Ther. 2011;9(2):137–141. doi: 10.1586/eri.10.159. [DOI] [PubMed] [Google Scholar]
- Wang X., Chen G., Wu X., Wang L., Cai J., Chan E.W., Chen S., Zhang R. Increased prevalence of carbapenem resistant Enterobacteriaceae in hospital setting due to cross-species transmission of the blaNDM-1 element and clonal spread of progenitor resistant strains. Front. Microbiol. 2015;6:595. doi: 10.3389/fmicb.2015.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington E.M., Boxall A.B., Cross P., Feil E.J., Gaze W.H., Hawkey P.M., Johnson-Rollings A.S., Jones D.L., Lee N.M., Otten W., Thomas C.M. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 2013;13(2):155–165. doi: 10.1016/S1473-3099(12)70317-1. [DOI] [PubMed] [Google Scholar]
- Wen S.C., Best E., Nourse C. Non-typhoidal Salmonella infections in children: review of literature and recommendations for management. J. Paediatr. Child Health. 2017;53(10):936–941. doi: 10.1111/jpc.13585. [DOI] [PubMed] [Google Scholar]
- Xu Y., Gu B., Huang M., Liu H., Xu T., Xia W., Wang T. Epidemiology of carbapenem resistant enterobacteriaceae (CRE) during 2000-2012 in Asia. J. Thorac. Dis. 2015;7(3):376. doi: 10.3978/j.issn.2072-1439.2014.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Pignatello J.J., Ma J., Mitch W.A. Effect of matrix components on UV/H 2 O 2 and UV/S 2 O 8 2− advanced oxidation processes for trace organic degradation in reverse osmosis brines from municipal wastewater reuse facilities. Water Res. 2016;89:192–200. doi: 10.1016/j.watres.2015.11.049. [DOI] [PubMed] [Google Scholar]
- Yee R.A., Leifels M., Scott C., Ashbolt N.J., Liu Y. Evaluating microbial and chemical hazards in commercial struvite recovered from wastewater. Environ. Sci. Technol. 2019 doi: 10.1021/acs.est.8b03683. [DOI] [PubMed] [Google Scholar]
- Yong D., Toleman M.A., Giske C.G., Cho H.S., Sundman K., Lee K., Walsh T.R. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumonia sequence type 14 from India. Antimicrob. Agents Chemother. 2009;53(12):5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Geißen S.U., Gal C. Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere. 2008;73(8):1151–1161. doi: 10.1016/j.chemosphere.2008.07.086. [DOI] [PubMed] [Google Scholar]