Abstract
In the present work, defatted corn germ was hydrolyzed by three proteases and further separated by sequential ultrafiltration with different molecular weight cutoff (100, 10, 2 kDa). Corn germ protein hydrolysate (CGPH) and their fractions were investigated for antioxidant activity, α‐glucosidase, α‐amylase, and DPP‐IV inhibitory activity. The degree of hydrolysis (DH) after 2 hr was 17.5%, 11.14%, and 2.05% for alcalase, trypsin, and flavourzyme, respectively. Trypsin hydrolysate showed the highest DPPH and ABTS+ radical scavenging and Fe2+ chelating activity, but a lower α‐glucosidase inhibitory activity. F1 fraction (<2 kDa) exhibited highest radical scavenging and α‐glucosidase inhibitory activity. While F2 fraction (2–10 kDa) showed the higher Fe2+ chelating and α‐amylase inhibitory activity, F1 fraction of flavourzyme showed the highest α‐glucosidase inhibitory and F2 fraction of alcalase and flavourzyme exhibited highest α‐amylase inhibitory activity. Hydrolysate and F1 fraction of alcalase and F2 fraction of trypsin showed the highest DPP‐IV inhibitory activity. RP‐HPLC results showed that trypsin hydrolysate had higher levels of high‐hydrophobic peptides. The amino acid composition of the F1 fractions showed high levels of hydrophobic amino acids. Thus, CGPHs may be used as a potential source of antioxidant and antidiabetic peptides in food industry and pharmaceutical application.
Keywords: antidiabetic potential, antioxidant, defatted corn germ, protein hydrolysates
Corn germ protein hydrolysate (CGPH) by three proteases and their fractions were investigated for antioxidant activity, α‐glucosidase, α‐amylase, and DPP‐IV inhibitory activity. F1 fraction (<2 kDa) exhibited highest radical scavenging and α‐glucosidase inhibitory activity. Hydrolysate and F1 fraction of alcalase and F2 fraction of trypsin showed the highest DPP‐IV inhibitory activity.

1. INTRODUCTION
In view of food industry and human health, prevention of lipid oxidation and generation of free radicals in the foodstuff and living body tissue are important for control of quality of food and diseases (Zhang et al., 2016, 2019). Antioxidants are often used in food formulation to eliminate the effects of free radicals and to inhibit lipid oxidation (Zhang et al., 2016). The use of synthetic antioxidants (BHA and BHT) has serious limitations due to the potential toxic effects on human health. Therefore, many studies are necessary on production and application of antioxidants from natural sources without side effects on human health (Jin, Liu, Zheng, Wang, & He, 2016; Nasri, 2017).
Diabetes mellitus is serious metabolic disease and characterized by high level of blood glucose (Bhandari, Jong‐Anurakkun, Hong, & Kawabata, 2008; Vilcacundo, Martínez‐Villaluenga, & Hernández‐Ledesma, 2017). One approach in the management of diabetes is slowing down the absorption of glucose through the inhibition of α‐glucosidase and α‐amylase in hydrolysis of carbohydrates (Alu'datt et al., 2012; Bhandari et al., 2008; Connolly, Piggott, & FitzGerald, 2014). α‐Amylase and α‐glucosidase inhibitors can inhibit both enzymes activities and decrease rate of digestion of starch and disaccharides to glucose, and as results, less glucose is absorbed in the small intestine (Vilcacundo et al., 2017). Another approaches for control of diabetes are through inhibition of dipeptidyl peptidase‐IV (DPP‐IV) activity that increases the half‐life of total circulating GLP‐1 (peptides that stimulates glucose‐dependent insulin secretion in pancreatic β‐cells) by preventing it from degradation and inactivation. Thereafter, prolonged secretion of insulin resulted in more absorption of glucose by the tissues and decreased in plasma glucose (Estrada‐Salas, Montero‐Morán, Martínez‐Cuevas, González, & Barba de la Rosa, 2014; Vilcacundo et al., 2017; Zambrowicz et al., 2015). Hence, in the past few years, enzyme inhibitors have developed for control of the type 2 diabetes. In this concept similar to α‐amylase and α‐glucosidase inhibitor, concern about synthetic drugs and toxicity and side effect should be noticed and using of natural and safe inhibitors as antidiabetic agents is considered (Wang et al., 2019).
Other than nutritional properties, protein hydrolysates and biopeptides have various biological activities such as antioxidant, antibacterial, antihypertensive, and antidiabetic potential depending on amino acid composition, sequencing, hydrophobicity, and chain length (Nasri, 2017). In recent years, various food protein hydrolysates have been reported to be antioxidant and antidiabetic potential in barley (Alu'datt et al., 2012), Palmaria palmata (Harnedy & FitzGerald, 2013), egg yolk protein (Zambrowicz et al., 2015), pinto beans (Ngoh & Gan, 2016), and cumin seeds (Siow & Gan, 2016).
Defatted corn germ (DCG) is by‐product of the corn oil industry. Its goes mainly into animal feed, and very low amount has been used as ingredient in food formulations (Barbieri & Casiraghi, 1983). Production of hydrolysate from corn germ and evaluation of antihypertensive activity has been studied (Parris, Moreau, Johnston, Dickey, & Aluko, 2008). However, limited information is available on antioxidant and antidiabetic properties of DCG hydrolysate. So, in this study, the ability of three proteases (alcalase, flavourzyme, trypsin) to generation hydrolysate from corn germ protein was evaluated and then antioxidant activity and DPP‐IV, α‐amylase and α‐glucosidase inhibitory activity of hydrolysates, and their fractions were investigated.
2. MATERIALS AND METHODS
2.1. Materials
Corn germ was obtained from Glucosan Ind Co. DPPH (2,2‐Diphenyl‐1‐picrylhydrazyl), ABTS (2,2′‐azino‐bis (3‐ethylbenzthiazoline‐6‐sulfonic acid) diammonium salt)), ferrozine (3‐(2‐Pyridyl)‐5,6‐diphenyl‐1,2,4‐triazine‐4′,4′′‐disulfonic acid sodium salt), PNPG (4‐nitrophenyl α‐d‐glucopyranoside), PAHBAH (4‐hydroxybenzhydrazide), trolox (6‐hydroxy‐2,5,7,8‐tetramethylchroman‐2‐carboxylic acid), 1,10‐phenanthroline, acarbose, chromogenic substrate Gly‐Pro‐p‐nitroaniline (Cat no. G0513) were purchased from Sigma‐Aldrich company. l‐Histidine (Cat no. 104351) was purchased from Merck company. Diprotin A (Ile‐Pro‐Ile) was purchased from Cayman chemical company. All other chemicals were of analytical grade.
Enzymes: Alcalase 2.4 L (Protease from Bacillus licheniformis), trypsin from porcine pancreas (Cat no. T4799), porcine pancreatic α‐amylase (Cat no. A3176), rat intestinal α‐glucosidase (Cat no. I1630), and dipeptidyl peptidase‐IV (DPP‐IV) (Cat no. 317640‐M) are obtained from Sigma‐Aldrich. Flavourzyme 500 MG from Aspergillus oryzae (Cat no. P6110) was purchased from Novozymes company.
2.2. Preparation of corn germ protein hydrolysate (CGPH)
Ground samples of corn germ were defatted with n‐hexane using soxhlet apparatus and dried at room temperature overnight. The dried material was ground and sieved through a 0.4 mm sieve (Mesh no. 40). The DCG was hydrolyzed according to the method of He, Girgih, Malomo, and Aluko (2013) with alcalase, flavourzyme, and trypsin enzymes. DCG suspension (5% w/v) was heated to the appropriate temperature and pH of each enzyme (alcalase pH 8 at 50°C, flavourzyme pH 7 at 50°C, and trypsin pH 7 at 50°C), and enzymes were added based on protein content of DCG at 1:20 ratio. The reaction continued with pH‐stat method for 2 hr, and then enzymes were inactivated by heating at 95°C for 15 min and finally, samples centrifuged at 10,000 g for 15 min and supernatant was freeze‐dried as CGPH.
2.3. Degree of hydrolysis (DH)
The pH‐stat method of Adler‐Nissen (1986) was used for measuring and calculating degree of hydrolysis (DH).
The htot of this equation was 7.75 meq/g (Zhang, Pang, & Xu, 2011).
2.4. Reversed‐phase chromatography separation of CGPH
The hydrophobicity of peptides from CGPHs was determined using an Azura HPLC system (Knauer). CGPHs were dispersed (10 mg/ml) and filtered through 0.2 μm cellulose acetate filters. RP‐HPLC was carried out following the procedure reported by Connolly et al. (2014) with some modifications. In brief, the samples were separated on Eurosil Bioselect column (250 × 4.6 mm ID, 5 μm particle size, 300 Å pore size) using solvent A (0.1% (v/v) trifluoroacetic acid (TFA) in water) and solvent B (0.1% (v/v) TFA in acetonitrile) under gradient conditions. The column was equilibrated using 100% A solvent. Elutions were performed as follows: 0–30 min, 0%–60% B; 30–35 min, 60% B; 35–45 min, 60%–10% B, and 45–50 min, 10% B. The UV‐Vis photodiode‐array detector (DAD 2. one langmuir, Knauer) was set at 214 nm for measuring absorbance.
2.5. Fractionation of hydrolysates
CGPH was fractionated by the ultrafiltration membranes with the molecular weight cutoff (MW) of 100, 10, and 2 kDa (Sartorius ‐VivaFlow 200) subsequently and namely F1, F2, F3, and F4. F1 corresponds to peptide with molecular weight lower than 2 kDa, F2 to peptide with MW between 2 and 10 kDa, F3 to peptides with MW between 10 and 100 kDa and F4 to undigested proteins and other compounds fragments with MW more than 100 kDa. All fractions were lyophilized and stored at −20°C for using all analysis.
2.6. Determination of antioxidant activities
2.6.1. DPPH radical scavenging activity
The DPPH radical scavenging effect of CGPH and fractions were measured according to the method of Zheng et al. (2015). The DPPH solution (500 μl) of each samples (0.5 mg/ml) was added to 500 μl DPPH in methanol (0.1 mM) and left for 30 min in dark place at 25°C. The ability of samples to scavenge of DPPH free radicals was measured at 517 nm with the UV–visible spectrophotometer (Agilent‐Carry 60). Trolox concentrations ranging from 20 to 200 µmol/L were used for calculation of scavenging activity of the hydrolysates and results expressed as µmol trolox/g dry matter of samples.
2.6.2. ABTS+ radical scavenging activity
The scavenging effect of CGPH and fractions on ABTS+ radical were performed according to the method of Ngoh and Gan (2016). The ABTS solution (980 µl) was added to 20 µl of samples (2.5 mg/ml) and mixed vigorously and then incubated in the dark at 25°C for 10 min and absorbance was measured at 734 nm. The result was expressed as µmol trolox equivalents (concentrations ranging from 50 to 1,100 µmol/L) per g dry matter of samples.
2.6.3. Hydroxyl radical scavenging activity
The hydroxyl radical scavenging effect of CGPH and fractions were measured following the method of Wang et al. (2014). Briefly, 200 μl samples (5 mg/ml), 100 μl FeSO4 (1.865 mM), and 100 μl of 1,10‐phenanthroline solution (1.865 mM) were mixed thoroughly and were allowed to stand for 10 min and then 100 μl of H2O2 (0.03% v/v) was added to the mixture. The mixture was then incubated at 37°C for 60 min, and after that, the absorbance was measured at 536 nm. Histidine was used as a standard curve (300–4,000 μmol/L). The result was expressed as µmol of histidine equivalents/g dry matter of samples.
2.6.4. Ferrous ion chelating activity
Chelation of Fe2+ ions by hydrolysates and fractions was estimated by the method of Sarteshnizi, Sahari, Gavlighi, Regenstein, and Nikoo (2019). The sample solutions of 5 mg/ml hydrolysate and fractions (500 μl) were mixed with 1,850 μl H2O and 50 μl FeCl2 (2 mM). After 3 min, 100 μl of 5 mM ferrozine aqueous solution was added and the mixture was allowed to react for 20 min. The absorbance of complex was measured at 562 nm. A standard curve was obtained by using EDTA (20–250 μmol/L). The result was expressed as µmol EDTA equivalents/g dry matter of samples.
2.6.5. α‐Glucosidase inhibition assay
Inhibition of α‐glucosidase (rat intestinal) by CGPH hydrolysates and fractions were measured using the method of Connolly et al. (2014) with some modifications. After extraction of the enzyme from rat intestinal acetone powders, the resulting solution was diluted to 90 mU/ml. One hundred microliter of sample solution (20 mg/ml) was mixed with 200 μl of α‐glucosidase and incubated at 37°C for 10 min. After preincubation, 5 mM PNPG solution (100 μl) was added and incubated at 37°C for 30 min and the absorbance of the solution was scanned every 2 min at 405 nm. The phosphate buffer was used as a control instead of sample solution. The IC50 value of acarbose was used as the positive control. The following equation was used to assess percentage inhibition of α‐glucosidase activity:
where As and Ac represent the slope of curve for absorbance of samples and control, respectively.
2.6.6. α‐Amylase inhibition assay
α‐Amylase inhibition was determined according to the method of Alu'datt et al. (2012) with some modifications. Briefly, 100 μl of sample solution (10 mg/ml) and 100 μl of α‐amylase solution (0.5 U/ml) were incubated at 37°C for 5 min. After preincubation, 100 μl of 0.5% (w/v) starch solution was added. Then, the reaction mixture was incubated for 20 min at 37°C. In the following, reaction mixture was heated at 100°C for 10 min and then cooled down to room temperature and centrifuged for 2 min at 16060 g to separate the undigested starch. Twenty microliters of supernatant was mixed with 1 ml of PAHBAH and heated to 70°C for 10 min. Finally, solution was cooled at room temperature and absorbance was measured at 410 nm. The IC50 value of acarbose was used as the positive control. The following equation was used to assess percentage inhibition of α‐amylase activity:
where As, Ab, and Ac represent the absorbance of sample, blank (phosphate buffer, enzyme, sample), and control (starch, buffer, enzyme), respectively.
2.6.7. Dipeptidyl peptidase‐IV (DPP‐IV) inhibition assay
DPP‐IV inhibition was determined following the procedure reported by Nongonierma and FitzGerald (2013). Briefly, 25 μl sample solution (5 mg/ml) was mixed by 25 μl of Gly‐Pro‐pNA, as substrate (0.2 mM) and incubated for 10 min at 37°C. The reaction was started by the addition of DPP‐IV (final concentration 0.0025 units/ml) for 1 hr at 37°C and the absorbance of p‐nitroaniline released was read at 405 nm. The IC50 value of diprotin A was used as positive control. The inhibitory activity of sample on DPP‐IV was calculated by the following equation:
where As, Asb, Ac, and Acb represent the absorbance of sample, sample blank (sample, buffer, substrate), control (enzyme, substrate, buffer), and control blank (buffer, substrate), respectively.
2.7. Amino acid composition
Samples were hydrolyzed with 6 M HCl at 110°C for 24 hr. Subsequently, the digested samples were lyophilized. Amino acid profile of the samples was determined using an OPA method by HPLC (Knauer), RP‐C18 Hypersil ODS column (250 × 4.6 mm, particle size 5 µm), and fluorometric detector RF‐530 (Shimadzu‐japan) (Nikoo, Benjakul, Yasemi, Gavlighi, & Xu, 2019).
2.8. Statistical analysis
All analyses were carried out in triplicate and data presented as mean ± SD. Data analysis was carried out with JMP 10 statistical software using one‐way analysis of variance and Tukey's test to compare differences between samples (p < .05).
3. RESULTS AND DISCUSSION
3.1. Degree of hydrolysis (DH)
DH of all enzymes treated DCG was increased sharply within the initial 30 min of reaction. Thereafter, the rate of DH was decreased (Figure 1). The reduction in hydrolysis rate over time may be related to decreased availability of cleavable peptide bonds within the substrate (Kumar, Chatli, Singh, Mehta, & Kumar, 2016). The highest DH value of hydrolysates was 17.5 ± 0.2%, which was obtained by alcalase, while that of the CGPH produced by flavourzyme was the lowest (2.05 ± 0.4%). Different DH value of each enzyme was due to different cleavage sites of the protease enzymes (Wang et al., 2019). Alcalase is an endo‐peptidase with a broad specificity, which favorably cleaves hydrophobic amino acid (tryptophan, phenylalanine, leucine, isoleucine, valine, and methionine) residues of peptide bonds, and higher DH value can be achieved with longer enzymolysis time. Flavourzyme is an exo‐peptidase that breaks the N‐terminal of peptide chains. While trypsin cleaves exclusively C‐terminal to arginine and lysine (Ambigaipalan, Al‐Khalifa, & Shahidi, 2015; Wang et al., 2019), this result indicates that alcalase is the most efficient for corn germ protein hydrolysis. Similar results were obtained for whey protein (Lin, Tian, Li, Cao, & Jiang, 2012), rapeseed protein (He et al., 2013), camel milk casein (Kumar et al., 2016), hemp seed protein (Ren et al., 2016), quinoa and amaranth proteins (Lina, Omar, Kamal, Kilari, & Maqsood, 2019), and buffalo and bovine caseins (Shazly et al., 2019). DH could be effect on functionality and bioactive properties of hydrolysate, so it is important to control DH in production of new food products (Mudgil, Omar, Kamal, Kilari, & Maqsood, 2019).
FIGURE 1.
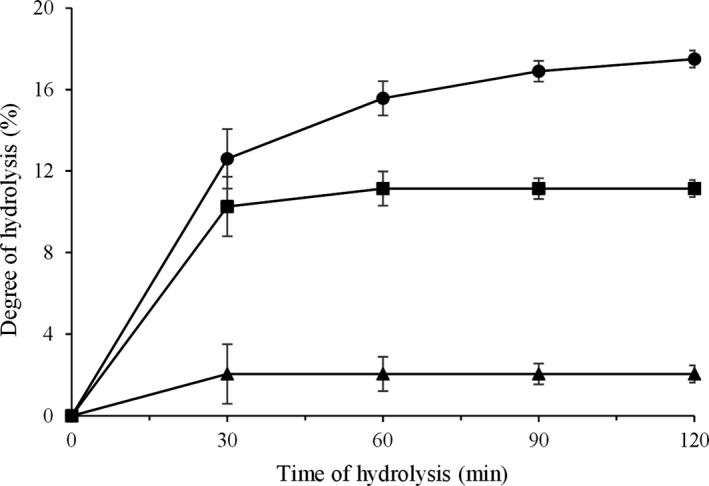
Degree of hydrolysis of corn germ with different enzymes (CGPH with alcalase ●, flavourzyme ▲, trypsin ■). Values represent the average of two independent hydrolysis
3.2. Reversed‐phase chromatography separation of CGPH
The ratio of hydrophilic to hydrophobic peptide groups is one of most important parameters that can effect on functional properties. Reversed‐phase HPLC was separated peptides based on the hydrophobic or hydrophilic character that resulted in later elution of hydrophobic peptide compared hydrophilic peptides (Zhang, Mu, & Sun, 2014). The RP‐HPLC profiles of CGPH (Figure 2) by trypsin showed a higher concentration of high‐hydrophobic peptides. Flavourzyme hydrolysate showed a lower concentration of high‐hydrophobic peptides. This effect is probably related to the specific function of each enzyme. The hydrophobicity of hydrolysates and peptides plays an important role in antioxidant activity, (Zambrowicz et al., 2015). An increase in hydrophobicity (As can be seen in Figure 2 for trypsin) will increase their solubility in hydrophobic phase and can improve their interaction with hydrophobic targets, thereby enhancing bioavailability and exhibiting enhanced interaction especially with radical species (Li, Jiang, Zhang, Mu, & Liu, 2008). Moreover, hydrophobic peptides tend to donate protons to reactive radicals and convert free radicals to more stable products and terminate radical chain reaction (Jin et al., 2016; Siow & Gan, 2016).
FIGURE 2.
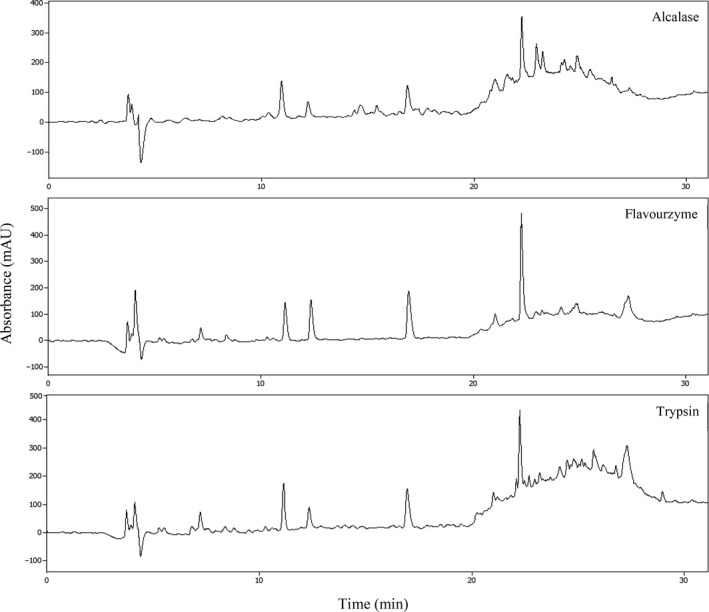
RP‐HPLC of CGPHs with different enzymes (alcalase, flavourzyme, trypsin). Samples (20 μl) were injected and separated on a Knauer 25EK Eurosil Bioselect column (C18, 250 × 4.6 mm, 5 μm)
3.3. Antioxidant activities of samples
Due to high turbidity of F3 and F4 fractions and made problem in antioxidant and antidiabetic tests, these fractions were excluded. Antioxidant peptides are new source of natural antioxidant compound that show activity through radical scavenging, metal chelating, hydroperoxide reduction, and inactivating reactive oxygen species (Zambrowicz et al., 2015). The DPPH radical scavenging activity (RSA) of CGPH and fractions showed distinct differences for the hydrolysates obtained by different proteases (Figure 3a). Trypsin hydrolysate was the most active against DPPH radical (64.7 µmol trolox eq/g sample) followed by flavourzyme and alcalase with least activity (p < .05). Hydrolysates prepared using trypsin had the highest ABTS+ RSA with 267 µmol trolox eq/g sample, while flavourzyme hydrolysates showed the lowest values (167 µmol trolox eq/g sample) (Figure 3b). The similar results were observed by Shazly et al. (2019) on higher ABTS+ RSA of buffalo and bovine caseins hydrolysates of trypsin than alcalase, papain, and pepsin. Hydrolysates prepared using flavourzyme showed the highest OH RSA (289 µmol histidine eq/g sample), while trypsin and alcalase hydrolysates significantly had the lower activity (Figure 3c). Hydrolysates prepared using flavourzyme showed the lowest chelation activity (39.2 µmol EDTA eq/g), while trypsin had the highest chelation activity (47.5 µmol EDTA eq/g) (Figure 3d). In general, the antioxidant activity of CGPHs was influenced by the type of enzymes in hydrolysates. Enzyme specificities differences, varying DH, size of molecules, amino acid composition, and sequence could be reason of varying in antioxidant activity observed among difference hydrolysate (Mudgil et al., 2019).
FIGURE 3.
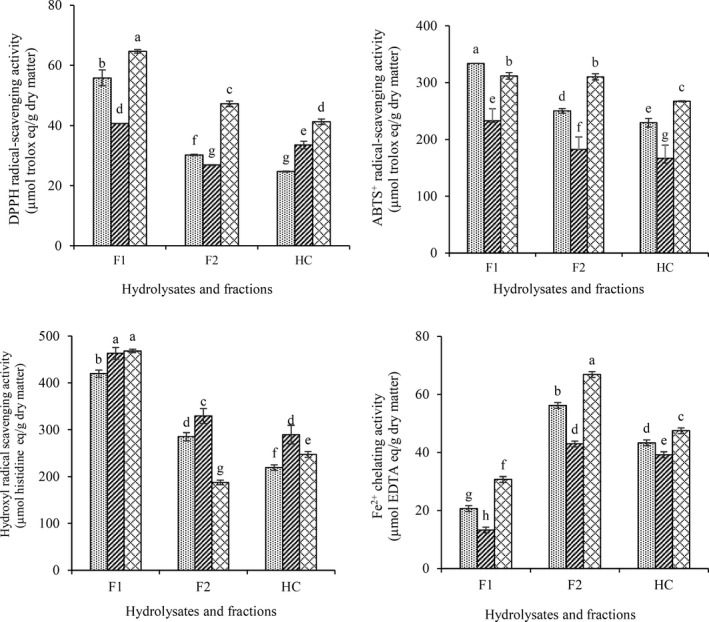
DPPH (a), ABTS+ (b), hydroxyl (c) radical scavenging, and Fe2+ chelating (d) activity of CGPH obtained from hydrolysis by different enzymes (alcalase  , flavourzyme
, flavourzyme  , trypsin
, trypsin  ) and their ultrafiltration fractions. F1, F2, and HC indicate fractions MW < 2, 2–10 kDa and unfractionated hydrolysate, respectively. Test concentration for DPPH, ABTS+, hydroxyl radical scavenging, and Fe2+ chelating activity was 0.5, 2.5, 5, and 5 mg/ml respectively. The data marked with different letters are significantly different (p < .05)
) and their ultrafiltration fractions. F1, F2, and HC indicate fractions MW < 2, 2–10 kDa and unfractionated hydrolysate, respectively. Test concentration for DPPH, ABTS+, hydroxyl radical scavenging, and Fe2+ chelating activity was 0.5, 2.5, 5, and 5 mg/ml respectively. The data marked with different letters are significantly different (p < .05)
Fractionation of the CGPHs led to improved DPPH RSA and fractioned of all enzymes with lower than 2 kDa (F1) had higher activity than the higher molecular weight. ABTS+ RSA was also increased after separation with UF membranes. ABTS+ RSA was observed in all fractions. F1 and followed by that F2 fraction showed the higher ABTS+ RSA than hydrolysates. F1 fraction of alcalase exhibited the highest activity against ABTS+ radical (333.84 µmol trolox eq/g) compared with other fractions (p < .05). Similar results were obtained and F1 fraction showed significantly higher (p < .05) OH RSA. However, there was no significant difference between trypsin and flavourzyme (Figure 3). The higher content of small peptides in hydrolysates may have higher radical scavenging activity than those with lower contents. This low molecule fractions can easily react with free radical and terminate the radical chain reaction (Agrawal, Joshi, & Gupta, 2019). Similar studies on chickpea protein (Li et al., 2008), corn gluten meal (Zhuang, Tang, & Yuan, 2013), rapeseed protein (He et al., 2013), wheat gluten (Choi, Lim, He, & Hwang, 2019), and perilla seed protein (Kim, Liceaga, & Yoon, 2019) had shown that short peptides are the most efficient antioxidants.
Hydrolysate and F2 fractions exhibited the stronger chelating capacity than F1 (p < .05). Decreasing chelating activity may be attributed to the inability of small peptides to form the complex with metals (Noman et al., 2019). Similar results were observed by He et al. (2013) and Tang, Wang, and Yang (2009) on hemp and rapeseed protein hydrolysate. The synergistic effects of higher number of amino acid residues from higher molecular weight of peptides compared with the lower molecular weight peptides could be reason of stronger metal chelating.
Type of amino acid present in hydrolysates also could influence antioxidant activity. The amino acid composition of the F1 of trypsin hydrolysate (the highest DPPH and OH RSA), F1 of alcalase hydrolysate (the highest ABTS+ RSA), and F1 of flavourzyme hydrolysate (the highest OH RSA) is described in Table 1. As can be seen, higher RSA of these fractions may be related to high levels of hydrophobic amino acids such as leucine, isoleucine, valine, and proline. Li et al. (2008) and Pownall, Udenigwe, and Aluko (2010) reported similar results with the strongest scavenging activity contained slightly higher amounts of hydrophobic amino acids. The presence of hydrophobic amino acids in peptides increases their solubility in hydrophobic or lipid phase, which simplify the interaction between peptides and donate protons to radical species. Hydrophobic interaction among hydrophobic amino acid residues might enhance the antioxidant activity of peptides (Jin et al., 2016; Ngoh & Gan, 2016; Zambrowicz et al., 2015).
TABLE 1.
Amino acid profile of protein hydrolysates produced by alcalase, flavourzyme, and trypsin (g/100 g protein)
| Amino acid | F1 (alcalase) | F1 (flavourzyme) | F1 (trypsin) | F2 (alcalase) | F2 (trypsin) |
|---|---|---|---|---|---|
| Hydrophilic | |||||
| Aspartic acid + asparagine | 4.69 ± 0.01 | 6.85 ± 0.22 | 5.1 ± 0.04 | 3.21 ± 0.04 | 2.42 ± 0.09 |
| Glutamic acid + glutamine | 22.01 ± 0.05 | 21.99 ± 0.27 | 24.08 ± 0.68 | 29.6 ± 0.92 | 28.01 ± 0.87 |
| Serine | 13.74 ± 0.15 | 12.05 ± 0.21 | 11.31 ± 0.09 | 10.1 ± 0.15 | 10.65 ± 0.12 |
| Glycine | 17.86 ± 0.01 | 15.23 ± 1.09 | 15.09 ± 0.09 | 18.21 ± 0.96 | 18.19 ± 0.27 |
| Histidine | 1.23 ± 0.04 | 1.27 ± 0.07 | 0.46 ± 0.06 | 0.67 ± 0.03 | 0.25 ± 0.08 |
| Arginine | 2.02 ± 0.04 | 2.78 ± 0.35 | 2.13 ± 0.04 | 3.01 ± 0.02 | 2.51 ± 0.04 |
| Threonine | 0.28 ± 0.03 | 0.55 ± 0.17 | 0.58 ± 0.05 | 0.07 ± 0.02 | 0.68 ± 0.06 |
| Cysteine | 0.48 ± 0.02 | 0.13 ± 0.07 | 1.2 ± 0.09 | 0.41 ± 0.02 | 0.3 ± 0.04 |
| Tyrosine | 3.01 ± 0.04 | 2.57 ± 0.04 | 2.84 ± 0.1 | 1.7 ± 0.03 | 2.61 ± 0.12 |
| Lysine | 4.41 ± 0.1 | 6.02 ± 0.38 | 5.18 ± 0.65 | 6.1 ± 0.01 | 6.53 ± 0.11 |
| 69.73 ± 0.88 | 69.44 ± 0.71 | 67.97 ± 0.69 | 73.08 ± 0.94 | 72.15 ± 0.74 | |
| Hydrophobic | |||||
| Alanine | 4.89 ± 0.02 | 3.65 ± 0.15 | 3.69 ± 0.22 | 4.04 ± 0.06 | 4.66 ± 0.16 |
| Proline | 5.8 ± 0.01 | 6.5 ± 0.12 | 7.06 ± 0.09 | 7.93 ± 0.07 | 7.16 ± 0.05 |
| Valine | 6.3 ± 0.19 | 6.45 ± 0.27 | 4.01 ± 0.05 | 5.1 ± 0.04 | 5.48 ± 0.08 |
| Methionine | 0.51 ± 0.05 | 0.69 ± 0.17 | 0.56 ± 0.07 | 0.43 ± 0.19 | 0.47 ± 0.03 |
| Isoleucine | 2.21 ± 0.03 | 3.43 ± 0.21 | 6.67 ± 0.1 | 1.39 ± 0.04 | 2.12 ± 0.09 |
| Leucine | 8.4 ± 0.04 | 8.44 ± 0.29 | 8.1 ± 0.13 | 6.01 ± 0.02 | 6.21 ± 0.08 |
| Phenylalanine | 2.16 ± 0.12 | 1.56 ± 0.09 | 1.89 ± 0.12 | 2.03 ± 0.03 | 1.89 ± 0.03 |
| Tryptophan | – | – | – | – | – |
| 30.27 ± 0.51 | 30.72 ± 0.49 | 32.08 ± 0.3 | 26.93 ± 1.12 | 27.99 ± 0.35 | |
The higher Fe2+ chelating abilities of peptides from F2 trypsin hydrolysate may return to high content of acidic and basic amino acids of peptides (glutamic acid and glutamine (Glx), aspartic acid, asparagine (Asx), arginine, and lysine) (Table 1). Ambigaipalan et al. (2015) reported that an increased concentration of carboxylic groups and amino groups in branches of the acidic and basic amino acids could enhance metal ion binding and removing metal ions from the system (Ambigaipalan et al., 2015).
3.4. α‐Glucosidase inhibition
As can be seen in Figure 4, all of the hydrolysates from three proteases had a significant difference inhibitory on the α‐glucosidase enzyme. The highest inhibition was related to flavourzyme hydrolysate (37.1%) whereas the lowest inhibitory was obtained by trypsin enzyme (12.8%). This can be due to the specificity of this enzyme and the presence of arginine in the peptide structure. Connolly et al. (2014) reported trypsin hydrolysate of brewers' spent grain protein had the highest amount of inhibition of α‐glucosidase enzyme. As can be expected, the α‐glucosidase inhibitory activity of acarbose (IC50 = 110 μg/ml) was higher than CGPH (20 mg/ml). Since synthetic inhibitors are pure compounds but CGPHs are mixtures of proteins and peptides (Connolly et al., 2014).
FIGURE 4.
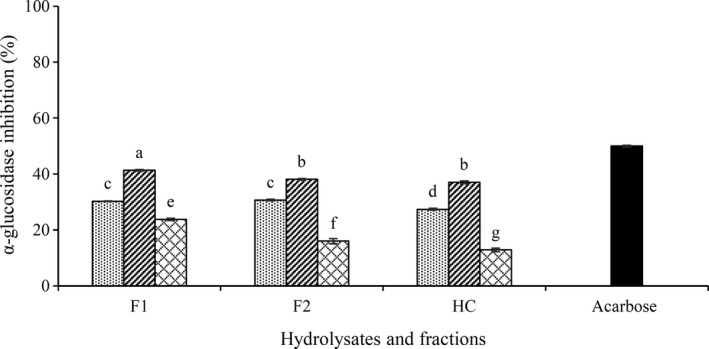
α‐Glucosidase inhibitory activity of CGPH obtained from hydrolysis by different enzymes (alcalase,  flavourzyme
flavourzyme  , trypsin
, trypsin  ) and ultrafiltration fractions. F1, F2, and HC indicate fractions MW < 2, 2–10 kDa, and unfractionated hydrolysate, respectively. Test concentration was 20 mg/ml. Acarbose was used as positive control (IC50 = 110 μg/ml). Different letters indicate significant differences among samples (p < .05)
) and ultrafiltration fractions. F1, F2, and HC indicate fractions MW < 2, 2–10 kDa, and unfractionated hydrolysate, respectively. Test concentration was 20 mg/ml. Acarbose was used as positive control (IC50 = 110 μg/ml). Different letters indicate significant differences among samples (p < .05)
Ultrafiltration fractions exhibited vary α‐glucosidase inhibitory activities and fractionation improved inhibitory activity. F1 of flavourzyme showed the highest inhibitory effect (41.3%). However, the least inhibition was related to F2 of trypsin (15.9%). These results indicate that short peptides are more potent inhibiting α‐glucosidase activity. Similar results were obtained by Uraipong and Zhao (2018) on rice bran proteins and Mejía, Batista, Fernández, and Fernandes (2019) on beans that reported smaller MW peptides in the digests had greater α‐glucosidase inhibitory activities than the larger peptides.
Inhibitory activity of peptides may be influenced by the composition of the amino acid residues (Di Stefano, Oliviero, & Udenigwe, 2018; Ibrahim, Bester, Neitz, & Gaspar, 2018). The inhibitory peptides are thought to interact more with the active site of α‐glucosidase via hydrogen bonds and electrostatic interactions. Therefore, the presence of amino acids with hydroxyl groups (serine, threonine and tyrosine) or basic amino acids (lysine and arginine) at the amino end of the peptides could play a critical role in alpha‐glucosidase inhibition. Hence, the effect of enzymatic inhibition of fractions in this study could be attributed to the presence of higher amount of amino acids such as serine and lysine (Table 1).
3.5. α‐Amylase inhibition
One of the effective approaches to control of type 2 diabetes is considered via inhibition of α‐amylase. CGPH obtained by all tree enzymes exhibited the strongest α‐amylase inhibitory activity in which higher than 50% (Figure 5). After ultrafiltration, maximum α‐amylase inhibition in the F2 fraction was observed, while F1 fraction showed the lowest inhibition. F2 fraction of alcalase showed the highest inhibitory effect (71.3%). However, the least inhibition was observed in F1 fraction of trypsin (37.50%). The proposed mechanism of action of the α‐amylase by bioactive peptide was suggested to interact or bind with the enzyme active site and inhibit the interaction between the enzyme and the substrate. Therefore, smaller interaction surface to the substrate occurred and less contact to the multiple‐attack action of amylases occurred (Ngoh & Gan, 2016). Another proposed inhibitory mechanism of biopeptides is the binding of peptides to the allosteric site of the enzyme in the enzyme structure (like calcium and chloride ion site) and create an unstable conformation. This conformation change can restrict the displacement of the enzyme on substrate (Admassu, Gasmalla, Yang, & Zhao, 2018; Siow & Gan, 2016). Calcium ions often have crucial roles in structure, function, and stability of α‐amylases, and the removal of calcium ions from some α‐amylase can inactivate the enzyme (Liao et al., 2019). Amylases are often inhibited by chelating reagents such as EDTA (Hagihara et al., 2001). The strong correlation between the metal ion chelating and α‐amylase inhibitory of this study (r = .7) could confirm that the interaction of metal ion chelator peptides with calcium ion in the enzyme structure is one of the reasons for its further decrease in enzyme activity by F2 fraction.
FIGURE 5.
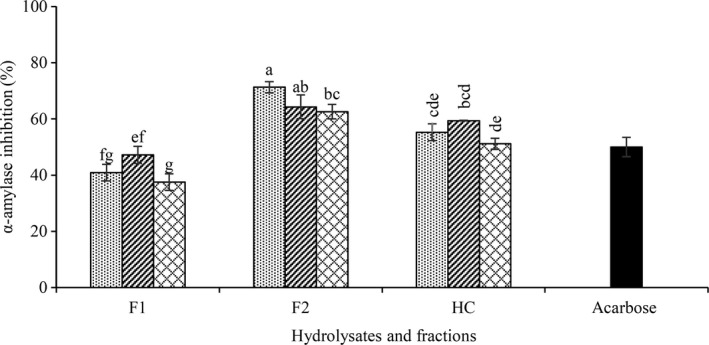
α‐Amylase inhibitory activity of CGPH obtained from hydrolysis by different enzymes (alcalase  , flavourzyme
, flavourzyme  , trypsin
, trypsin  ) and ultrafiltration fractions. F1, F2, and HC indicate fractions MW < 2, 2–10 kDa, and unfractionated hydrolysate, respectively. Test concentration was 10 mg/ml. Acarbose was used as positive control (IC50 = 30 μg/ml). Different letters indicate significant differences among samples (p < .05)
) and ultrafiltration fractions. F1, F2, and HC indicate fractions MW < 2, 2–10 kDa, and unfractionated hydrolysate, respectively. Test concentration was 10 mg/ml. Acarbose was used as positive control (IC50 = 30 μg/ml). Different letters indicate significant differences among samples (p < .05)
Amino acids found in peptides such as arginine, lysine, aspartic acid + asparagine, glutamic acid + glutamine, proline, leucine, glycine phenylalanine, serine, tryptophan, and tyrosine likely to bind to active domains and have a potential to inhibit the enzyme (Admassu et al., 2018; Ngoh & Gan, 2016; Siow & Gan, 2016). In our study, the higher enzymatic inhibitory of higher molecular weight fractions was probably due to the higher amounts of some amino acids such as proline, glutamic acid + glutamine, aspartic acid + asparagine, leucine, lysine, and glycine.
3.6. Dipeptidyl peptidase‐IV (DPP‐IV) inhibition
Inhibition of DPP‐IV proteinase and enhanced insulin secretion is another approach to lowering postprandial serum glucose. The DPP‐IV inhibitory ability of CGPH and its fractions is shown in Figure 6. Alcalase hydrolysate had the greatest DPP‐IV inhibition (45.9%) while trypsin and flavourzyme hydrolysates showed the lower inhibition (30.7% and 34.5%). Cheung and Li‐Chan (2017) and Mojica and de Mejia (2016) also obtained similar results that inhibition can vary by type of enzymes. In fact, fractions of alcalase and flavourzyme from CGPHs were not significantly higher DPP‐IV inhibitory activity. But the F2 fraction of trypsin hydrolysate showed the stronger inhibition than hydrolysate and F1 fraction. Li‐Chan, Hunag, Jao, Ho, and Hsu (2012) examined inhibitory activity of fractions of hydrolysate obtained from atlantic salmon skin gelatin hydrolyzed by different enzymes and reported that the fraction smaller than 1 kDa had the highest DPP‐IV‐inhibitory activity. Our results (Figure 6) shown that small molecular is not main reason for DPP‐IV inhibition effect and inhibitory activity varied by difference in amino acid composition and length. Similar findings were also reported for hydrolysates generated from Lesser mealworm (Lacroix, Dávalos Terán, Fogliano, & Wichers, 2019) and dairy protein (Lacroix & Li‐Chan, 2012).
FIGURE 6.
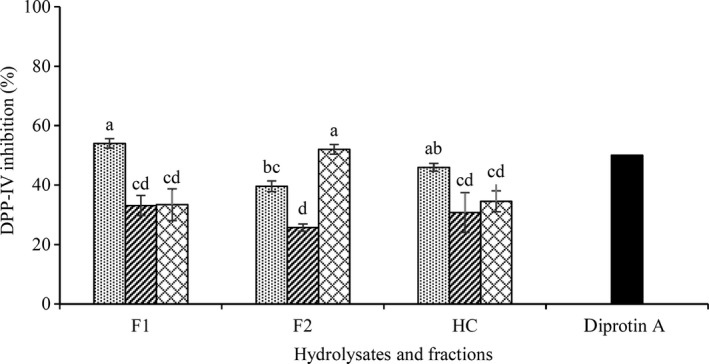
Dipeptidyl peptidase‐IV (DPP‐IV) inhibitory activity of CGPH obtained from hydrolysis by different enzymes (alcalase  , flavourzyme
, flavourzyme  , trypsin
, trypsin  ) and ultrafiltration fractions. F1, F2, and HC indicate fractions MW < 2, 2–10 kDa and unfractionated hydrolysate, respectively. Test concentration was 5 mg/ml. Diprotin A was used as positive control (IC50 = 4 μg/ml). Different letters indicate significant differences among samples (p < .05)
) and ultrafiltration fractions. F1, F2, and HC indicate fractions MW < 2, 2–10 kDa and unfractionated hydrolysate, respectively. Test concentration was 5 mg/ml. Diprotin A was used as positive control (IC50 = 4 μg/ml). Different letters indicate significant differences among samples (p < .05)
4. CONCLUSIONS
It was demonstrated that DH and antioxidant and antidiabetic potential of CGPHs can vary depending on the enzymes used. RP‐HPLC chromatograms showed that trypsin hydrolysate had higher levels of high‐hydrophobic peptides, which may be related to its higher antioxidant effect. F1 fraction exhibited highest radical scavenging and α‐glucosidase inhibitory activity. While F2 fraction showed the higher Fe2+ chelating and α‐amylase inhibitory activity, hydrolysate and F1 fraction of alcalase and F2 fraction of trypsin showed the highest DPP‐IV inhibitory activity. The amino acid composition of the F1 fractions showed high levels of hydrophobic amino acids such as valine, isoleucine, leucine, and proline. In general, CGPH could be considered as a natural source for ingredients in the functional food and medicinal industries with antioxidant and antidibetic potential.
CONFLICT OF INTEREST
The authors notify that there are no conflicts of interest.
ETHICAL APPROVAL
Study's protocols and procedures were ethically reviewed and approved by Tarbiat Modares Research Council.
ACKNOWLEDGMENTS
The authors would like to acknowledge Tarbiat Modares University for their financial support and the Glucosan Ind. Co to supply corn germ.
Karimi A, Azizi MH, Ahmadi Gavlighi H. Frationation of hydrolysate from corn germ protein by ultrafiltration: In vitro antidiabetic and antioxidant activity. Food Sci Nutr. 2020;8:2395–2405. 10.1002/fsn3.1529
Contributor Information
Mohammad Hossein Azizi, Email: azizit_m@modares.ac.ir.
Hassan Ahmadi Gavlighi, Email: ahmadi_ha@modares.ac.ir.
REFERENCES
- Adler‐Nissen, J. (1986). Enzymic hydrolysis of food proteins. Barking, UK: Elsevier Applied Science Publishers. [Google Scholar]
- Admassu, H. , Gasmalla, M. A. A. , Yang, R. , & Zhao, W. (2018). Identification of bioactive peptides with alpha‐amylase inhibitory potential from enzymatic protein hydrolysates of red seaweed (Porphyra spp). Journal of Agricultural and Food Chemistry, 66(19), 4872–4882. 10.1021/acs.jafc.8b00960 [DOI] [PubMed] [Google Scholar]
- Agrawal, H. , Joshi, R. , & Gupta, M. (2019). Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Research International, 120, 697–707. 10.1016/j.foodres.2018.11.028 [DOI] [PubMed] [Google Scholar]
- Alu'datt, M. H. , Ereifej, K. , Abu‐Zaiton, A. , Alrababah, M. , Almajwal, A. , Rababah, T. , & Yang, W. (2012). Antioxidant, antidiabetic, and antihypertensive effects of extracted phenolics and hydrolyzed peptides from barley protein fractions. International Journal of Food Properties, 15(4), 781–795. 10.1080/10942912.2010.503357 [DOI] [Google Scholar]
- Ambigaipalan, P. , Al‐Khalifa, A. S. , & Shahidi, F. (2015). Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. Journal of Functional Foods, 18, 1125–1137. 10.1016/j.jff.2015.01.021 [DOI] [Google Scholar]
- Barbieri, R. , & Casiraghi, E. (1983). Production of a food grade flour from defatted corn germ meal. International Journal of Food Science & Technology, 18(1), 35–41. 10.1111/j.1365-2621.1983.tb00242.x [DOI] [Google Scholar]
- Bhandari, M. R. , Jong‐Anurakkun, N. , Hong, G. , & Kawabata, J. (2008). α‐Glucosidase and α‐amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.). Food Chemistry, 106(1), 247–252. 10.1016/j.foodchem.2007.05.077 [DOI] [Google Scholar]
- Cheung, I. W. , & Li‐Chan, E. C. (2017). Enzymatic production of protein hydrolysates from steelhead (Oncorhynchus mykiss) skin gelatin as inhibitors of dipeptidyl‐peptidase IV and angiotensin‐I converting enzyme. Journal of Functional Foods, 28, 254–264. 10.1016/j.jff.2016.10.030 [DOI] [Google Scholar]
- Choi, Y. , Lim, T. , He, Y. , & Hwang, K. T. (2019). Chemical characteristics and antioxidant properties of wheat gluten hydrolysates produced by single and sequential enzymatic hydrolyses using commercial proteases and their application in beverage system. Journal of Food Measurement and Characterization, 13(1), 745–754. 10.1007/s11694-018-9987-x [DOI] [Google Scholar]
- Connolly, A. , Piggott, C. O. , & FitzGerald, R. J. (2014). In vitro α‐glucosidase, angiotensin converting enzyme and dipeptidyl peptidase‐IV inhibitory properties of brewers' spent grain protein hydrolysates. Food Research International, 56, 100–107. 10.1016/j.foodres.2013.12.021 [DOI] [Google Scholar]
- Di Stefano, E. , Oliviero, T. , & Udenigwe, C. C. (2018). Functional significance and structure‐activity relationship of food‐derived α‐glucosidase inhibitors. Current Opinion in Food Science, 20, 7–12. 10.1016/j.cofs.2018.02.008 [DOI] [Google Scholar]
- Estrada‐Salas, P. A. , Montero‐Morán, G. M. , Martínez‐Cuevas, P. P. , González, C. , & Barba de la Rosa, A. P. (2014). Characterization of antidiabetic and antihypertensive properties of canary seed (Phalaris canariensis L.) peptides. Journal of Agricultural and Food Chemistry, 62(2), 427–433. 10.1021/jf404539y [DOI] [PubMed] [Google Scholar]
- Hagihara, H. , Igarashi, K. , Hayashi, Y. , Endo, K. , Ikawa‐Kitayama, K. , Ozaki, K. , … Ito, S. (2001). Novel α‐amylase that is highly resistant to chelating reagents and chemical oxidants from the Alkaliphilic Bacillus isolate KSM‐K38. Applied and Environmental Microbiology, 67(4), 1744–1750. 10.1007/s13197-017-2898-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnedy, P. A. , & FitzGerald, R. J. (2013). In vitro assessment of the cardioprotective, antidiabetic and antioxidant potential of Palmaria palmata protein hydrolysates. Journal of Applied Phycology, 25(6), 1793–1803. 10.1007/s10811-013-0017-4 [DOI] [Google Scholar]
- He, R. , Girgih, A. T. , Malomo, S. A. , Ju, X. , & Aluko, R. E. (2013). Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. Journal of Functional Foods, 5(1), 219–227. 10.1016/j.jff.2012.10.008 [DOI] [Google Scholar]
- Ibrahim, M. A. , Bester, M. J. , Neitz, A. W. , & Gaspar, A. R. (2018). Structural properties of bioactive peptides with α‐glucosidase inhibitory activity. Chemical Biology & Drug Design, 91(2), 370–379. 10.1111/cbdd.13105 [DOI] [PubMed] [Google Scholar]
- Jin, D.‐X. , Liu, X.‐L. , Zheng, X.‐Q. , Wang, X.‐J. , & He, J.‐F. (2016). Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chemistry, 204, 427–436. 10.1016/j.foodchem.2016.02.119 [DOI] [PubMed] [Google Scholar]
- Kim, J. M. , Liceaga, A. M. , & Yoon, K. Y. (2019). Purification and identification of an antioxidant peptide from perilla seed (Perilla frutescens) meal protein hydrolysate. Food Science & Nutrition, 7(5), 1645–1655. 10.1002/fsn3.998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, D. , Chatli, M. K. , Singh, R. , Mehta, N. , & Kumar, P. (2016). Enzymatic hydrolysis of camel milk casein and its antioxidant properties. Dairy Science & Technology, 96(3), 391–404. 10.1007/s13594-015-0275-9 [DOI] [Google Scholar]
- Lacroix, I. M. , Dávalos Terán, I. , Fogliano, V. , & Wichers, H. J. (2019). Investigation into the potential of commercially available lesser mealworm (A. diaperinus) protein to serve as sources of peptides with DPP‐IV inhibitory activity. International Journal of Food Science & Technology, 54(3), 696–704. 10.1111/ijfs.13982 [DOI] [Google Scholar]
- Lacroix, I. M. , & Li‐Chan, E. C. (2012). Dipeptidyl peptidase‐IV inhibitory activity of dairy protein hydrolysates. International Dairy Journal, 25(2), 97–102. 10.1016/j.idairyj.2012.01.003 [DOI] [Google Scholar]
- Li, Y. , Jiang, B. , Zhang, T. , Mu, W. , & Liu, J. (2008). Antioxidant and free radical‐scavenging activities of chickpea protein hydrolysate (CPH). Food Chemistry, 106(2), 444–450. 10.1016/j.foodchem.2007.04.067 [DOI] [Google Scholar]
- Liao, S.‐M. , Liang, G. E. , Zhu, J. , Lu, B. O. , Peng, L.‐X. , Wang, Q.‐Y. , … Huang, R.‐B. (2019). Influence of calcium ions on the thermal characteristics of α‐amylase from thermophilic Anoxybacillus sp. GXS‐BL. Protein and Peptide Letters, 26(2), 148–157. 10.2174/0929866526666190116162958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li‐Chan, E. C. , Hunag, S.‐L. , Jao, C.‐L. , Ho, K.‐P. , & Hsu, K.‐C. (2012). Peptides derived from Atlantic salmon skin gelatin as dipeptidyl‐peptidase IV inhibitors. Journal of Agricultural and Food Chemistry, 60(4), 973–978. 10.1021/jf204720q [DOI] [PubMed] [Google Scholar]
- Lin, S. , Tian, W. , Li, H. , Cao, J. , & Jiang, W. (2012). Improving antioxidant activities of whey protein hydrolysates obtained by thermal preheat treatment of pepsin, trypsin, alcalase and flavourzyme. International Journal of Food Science & Technology, 47(10), 2045–2051. 10.1111/j.1365-2621.2012.03068.x [DOI] [Google Scholar]
- Lina, P. M. , Omar, S. , Kamal, H. , Kilari, B. P. , & Maqsood, S. (2019). Multi‐functional bioactive properties of intact and enzymatically hydrolysed quinoa and amaranth proteins. LWT‐Food Science and Technology, 110, 207–213. 10.1016/j.lwt.2019.04.084 [DOI] [Google Scholar]
- Mejía, E. V. , Batista, K. A. , Fernández, J. J. A. , & Fernandes, K. F. (2019). Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy‐to‐cook and hard‐to‐cook beans (Phaseolus vulgaris L.). Food Research International, 121, 238–246. 10.1016/j.foodres.2019.03.043 [DOI] [PubMed] [Google Scholar]
- Mojica, L. , & de Mejia, E. G. (2016). Optimization of enzymatic production of anti‐diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food & Function, 7(2), 713–727. 10.1039/c5fo01204j [DOI] [PubMed] [Google Scholar]
- Mudgil, P. , Omar, L. S. , Kamal, H. , Kilari, B. P. , & Maqsood, S. (2019). Multi‐functional bioactive properties of intact and enzymatically hydrolysed quinoa and amaranth proteins. LWT‐Food Science and Technology, 110, 207–213. 10.1016/j.lwt.2019.04.084 [DOI] [Google Scholar]
- Nasri, M. (2017). Protein hydrolysates and biopeptides: production, biological activities, and applications in foods and health benefits. A review In Fidel T. (Ed.), Advances in Food and Nutrition Research (Vol. 81, pp. 109‐159). London, UK: Elsevier; 10.1016/bs.afnr.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Ngoh, Y.‐Y. , & Gan, C.‐Y. (2016). Enzyme‐assisted extraction and identification of antioxidative and α‐amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chemistry, 190, 331–337. 10.1016/j.foodchem.2015.05.120 [DOI] [PubMed] [Google Scholar]
- Nikoo, M. , Benjakul, S. , Yasemi, M. , Gavlighi, H. A. , & Xu, X. (2019). Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by‐product with different pretreatments: Antioxidant activity and their effect on lipid and protein oxidation of raw fish emulsion. LWT‐Food Science and Technology, 108, 120–128. [Google Scholar]
- Noman, A. , Qixing, J. , Xu, Y. , Ali, A. H. , Al‐Bukhaiti, W. Q. , Abed, S. M. , & Xia, W. (2019). Influence of degree of hydrolysis on chemical composition, functional properties, and antioxidant activities of chinese sturgeon (Acipenser sinensis) hydrolysates obtained by using alcalase 2.4L. Journal of Aquatic Food Product Technology, 28(6), 583–597. 10.1080/10498850.2019.1626523 [DOI] [Google Scholar]
- Nongonierma, A. B. , & FitzGerald, R. J. (2013). Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein‐derived dipeptides and hydrolysates. Peptides, 39, 157–163. 10.1016/j.peptides.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Parris, N. , Moreau, R. A. , Johnston, D. B. , Dickey, L. C. , & Aluko, R. E. (2008). Angiotensin I converting enzyme‐inhibitory peptides from commercial wet‐and dry‐milled corn germ. Journal of Agricultural and Food Chemistry, 56(8), 2620–2623. 10.1021/jf072238d [DOI] [PubMed] [Google Scholar]
- Pownall, T. L. , Udenigwe, C. C. , & Aluko, R. E. (2010). Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. Journal of Agricultural and Food Chemistry, 58(8), 4712–4718. 10.1021/jf904456r [DOI] [PubMed] [Google Scholar]
- Ren, Y. , Liang, K. , Jin, Y. , Zhang, M. , Chen, Y. , Wu, H. , & Lai, F. (2016). Identification and characterization of two novel α‐glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. Journal of Functional Foods, 26, 439–450. 10.1016/j.jff.2016.07.024 [DOI] [Google Scholar]
- Sarteshnizi, R. A. , Sahari, M. A. , Gavlighi, H. A. , Regenstein, J. M. , & Nikoo, M. (2019). Antioxidant activity of Sind sardine hydrolysates with pistachio green hull (PGH) extracts. Food Bioscience, 27, 37–45. 10.1016/j.fbio.2018.11.007 [DOI] [Google Scholar]
- Shazly, A. B. , Mu, H. , Liu, Z. , El‐Aziz, M. A. , Zeng, M. , Qin, F. , … Chen, J. (2019). Release of antioxidant peptides from buffalo and bovine caseins: Influence of proteases on antioxidant capacities. Food Chemistry, 274, 261–267. 10.1016/j.foodchem.2018.08.137 [DOI] [PubMed] [Google Scholar]
- Siow, H.‐L. , & Gan, C.‐Y. (2016). Extraction, identification, and structure–activity relationship of antioxidative and α‐amylase inhibitory peptides from cumin seeds (Cuminum cyminum). Journal of Functional Foods, 22, 1–12. 10.1016/j.jff.2016.01.011 [DOI] [Google Scholar]
- Tang, C.‐H. , Wang, X.‐S. , & Yang, X.‐Q. (2009). Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chemistry, 114(4), 1484–1490. 10.1016/j.foodchem.2008.11.049 [DOI] [Google Scholar]
- Uraipong, C. , & Zhao, J. (2018). In vitro digestion of rice bran proteins produces peptides with potent inhibitory effects on alpha‐glucosidase and angiotensin I converting enzyme. Journal of Science of Food & Agriculture, 98(2), 758–766. 10.1002/jsfa.8523 [DOI] [PubMed] [Google Scholar]
- Vilcacundo, R. , Martínez‐Villaluenga, C. , & Hernández‐Ledesma, B. (2017). Release of dipeptidyl peptidase IV, α‐amylase and α‐glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. Journal of Functional Foods, 35, 531–539. 10.1016/j.jff.2017.06.024 [DOI] [Google Scholar]
- Wang, B. , Gong, Y.‐D. , Li, Z.‐R. , Yu, D. , Chi, C.‐F. , & Ma, J.‐Y. (2014). Isolation and characterisation of five novel antioxidant peptides from ethanol‐soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. Journal of Functional Foods, 6, 176–185. 10.1016/j.jff.2013.10.004 [DOI] [Google Scholar]
- Wang, R. , Zhao, H. , Pan, X. , Orfila, C. , Lu, W. , & Ma, Y. (2019). Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α‐glucosidase inhibitory peptides from soy protein. Food Science & Nutrition, 7(5), 1848–1856. 10.1002/fsn3.1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz, A. , Eckert, E. , Pokora, M. , Bobak, Ł. , Dąbrowska, A. , Szołtysik, M. , … Chrzanowska, J. (2015). Antioxidant and antidiabetic activities of peptides isolated from a hydrolysate of an egg‐yolk protein by‐product prepared with a proteinase from Asian pumpkin (Cucurbita ficifolia). RSC Advances, 5(14), 10460–10467. 10.1039/C4RA12943A [DOI] [Google Scholar]
- Zhang, H. , Wang, P. , Zhang, A.‐J. , Li, X. , Zhang, J.‐H. , Qin, Q.‐L. , & Wu, Y.‐J. (2016). Antioxidant activities of protein hydrolysates obtained from the housefly larvae. Acta Biologica Hungarica, 67(3), 236–246. 10.1556/018.67.2016.3.2 [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Mu, T.‐H. , & Sun, M.‐J. (2014). Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. Journal of Functional Foods, 7, 191–200. 10.1016/j.jff.2014.02.012 [DOI] [Google Scholar]
- Zhang, M. D. , Pang, Y. , & Xu, Z. J. (2011). Nutritive value and functional properties of corn germ protein hydrolysates. . Advanced Materials Research, 183‐185, 557–560. 10.4028/www.scientific.net/AMR.183-185.557 [DOI] [Google Scholar]
- Zhang, Z. , Su, G. , Zhou, F. , Lin, L. , Liu, X. , & Zhao, M. (2019). Alcalase‐hydrolyzed oyster (Crassostrea rivularis) meat enhances antioxidant and aphrodisiac activities in normal male mice. Food Research International, 120, 178–187. 10.1016/j.foodres.2019.02.033 [DOI] [PubMed] [Google Scholar]
- Zheng, X.‐Q. , Wang, J.‐T. , Liu, X.‐L. , Sun, Y. , Zheng, Y.‐J. , Wang, X.‐J. , & Liu, Y. (2015). Effect of hydrolysis time on the physicochemical and functional properties of corn glutelin by protamex hydrolysis. Food Chemistry, 172, 407–415. 10.1016/j.foodchem.2014.09.080 [DOI] [PubMed] [Google Scholar]
- Zhuang, H. , Tang, N. , & Yuan, Y. (2013). Purification and identification of antioxidant peptides from corn gluten meal. Journal of Functional Foods, 5(4), 1810–1821. 10.1016/j.jff.2013.08.013 [DOI] [Google Scholar]


