A recent report from the World Health Organisation highlights the lack of treatment options available for multidrug-resistant Gram-negative pathogens, including carbapenem-resistant Enterobacteriaceae (such as Klebsiella and Escherichia coli), Pseudomonas and Acinetobacter, which can cause severe and often life-threatening infections.1 Polymyxins remain one of the last classes of antibiotics with activity against these pathogens, and this, despite concerns over nephrotoxicity leading to poor therapeutic index, has led to a resurgence in their clinical use as a therapy of last resort.2 Strains with reduced susceptibility to polymyxins are however, beginning to emerge in the clinic, although this is currently at a low level.3 An increased understanding of the structure-activity relationships (SAR) of this important class of compounds is required to further the development of the next generation of polymyxin-related antibiotics.
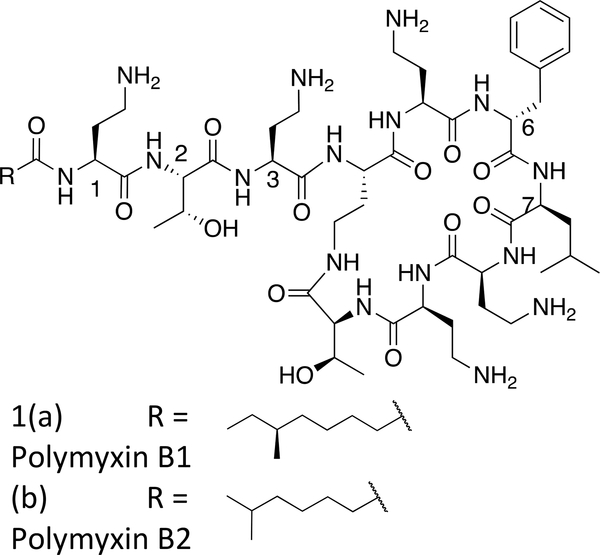
Polymyxin B (PMB, Figure 1) is a naturally-occurring lipopeptide antibiotic comprising a cyclic heptapeptide core, with a linear exocyclic tripeptide chain capped at the N-terminus with a fatty acyl group. PMB demonstrates bactericidal activity via a mechanism involving specific electrostatic and hydrophobic interactions with the lipid A component of lipopolysaccharide (LPS) in the outer membrane of Gram-negative bacteria. Nuclear magnetic resonance (NMR) studies of PMB with LPS indicate that in addition to the electrostatic interactions of the positively charged Dab (diaminobutyric acid) residues with the negatively charged phosphate and carboxylate groups of lipid A, the hydrophobic residues of PMB – phenylalanine, leucine, and fatty acyl group – align with the lipid chains of the LPS.4
Figure 1:
Polymyxin B major components
Commercially available sources of PMB comprise two major components polymyxin B1 and polymyxin B2 (Figure 1a and b), which have been shown to have similar activity to each other and to the commercial natural product mixture.5,6 Over the last ten years, our knowledge of the SAR of polymyxins has steadily developed and has been the subject of recent reviews.7–10
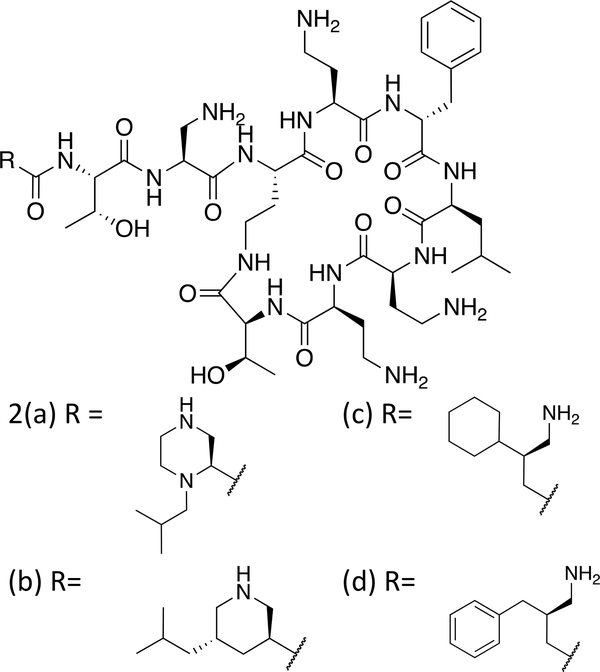
We have recently reported a series of novel polymyxins with improved antibacterial potency and reduced renal cell cytotoxicity, achieved by replacing the amino acid at position 1 and the fatty acyl chain with branched amine-containing acyl moieties of specific regio- and stereochemistry.11 These compounds are based on a nonapeptide scaffold with the amino acid Dap (diaminopropionic acid) at the position adjacent to the cyclic core. Examples include the N-terminal heterocycles, and beta branched amino butyrates shown in Figure 2. As part of our SAR programme we sought to modify the lipophilicity of the cyclic core of polymyxins by modification of position 6 (D-Phe). To date, modified core Polymyxins have been prepared via solid phase peptide synthesis on resin, often requiring an off-resin cyclisation step.12–14 We sought to develop methodology which would enable modification of the D-Phe position on the polymyxin molecule itself, utilising the readily available and low-cost starting material, PMB.
Figure 2:
Amino acyl nonapeptides11
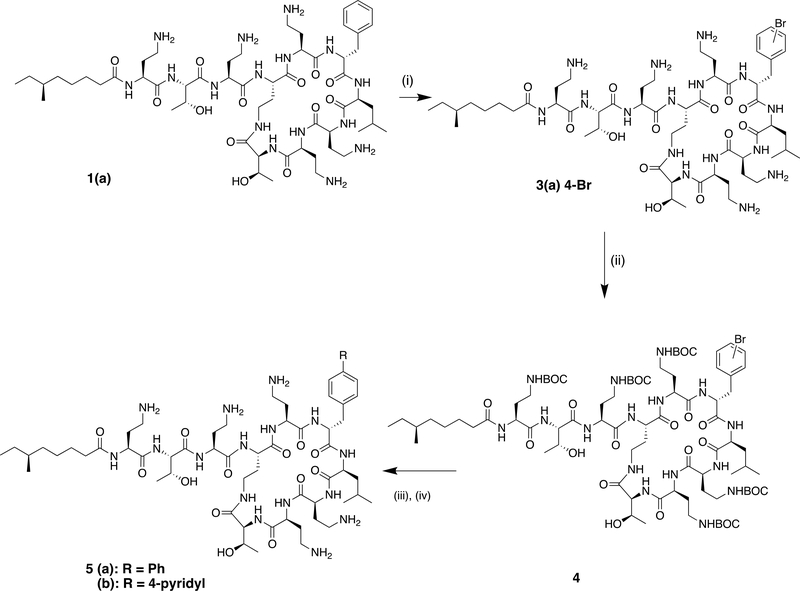
Our initial approach was to attempt to introduce a bromine substituent on the phenylalanine ring, to afford an intermediate amenable to palladium-catalysed cross coupling reactions. While the phenylalanine system was resistant to bromination with N-bromosuccinimide, harsher acidic conditions (60% sulphuric acid, bromoisocyanuric acid)15 led to decomposition of the peptide. However, we were encouraged by the reports of the Olah group on the mild electrophilic bromination of deactivated aromatic rings with Nbromo succinimide in the presence of boron trifluoride monohydrate.16 Indeed we found that boron trifluoride, used as the commercially-available dihydrate served as both a solvent for the cyclic peptide and a Lewis-acid catalyst for the bromination reaction (Scheme 1). Bromination proceeded at room temperature to afford mono-brominated material, from which the major component was isolated and purified for antibacterial testing. 1H nmr confirmed this to be the (4-bromo)phenylalanine derivative 3(a).
Scheme 1:
Reagents and conditions: (i) N-bromosuccinimide, BF3.2H2O; (ii) BOC2O, Et3N, CH3CN, H2O, 27% over 2 steps; (iii) PhB(OH)2 (or (4-pyridyl)B(OH)2), PdCl2(Ph3P)2, Na2CO3, DMF, H2O; (iv) TFA, DCM, followed by prep HPLC, 10% over 2 steps
For further derivatisation, protection of the amino groups was required, thus the crude brominated material was protected as the penta-BOC derivative (4), before being used as a substrate in palladium catalysed cross-coupling. Initial attempts at Suzuki coupling with phenyl boronic acid in the presence of bis(triphenylphosphine)palladium(II)dichloride gave the desired cross-coupled product. After deprotection with TFA and purification by preparative HPLC the 4-phenyl phenylalanine derivative 5a was obtained. Suzuki coupling of 4 with 4-pyridyl-boronic acid under the same conditions led to 5b.
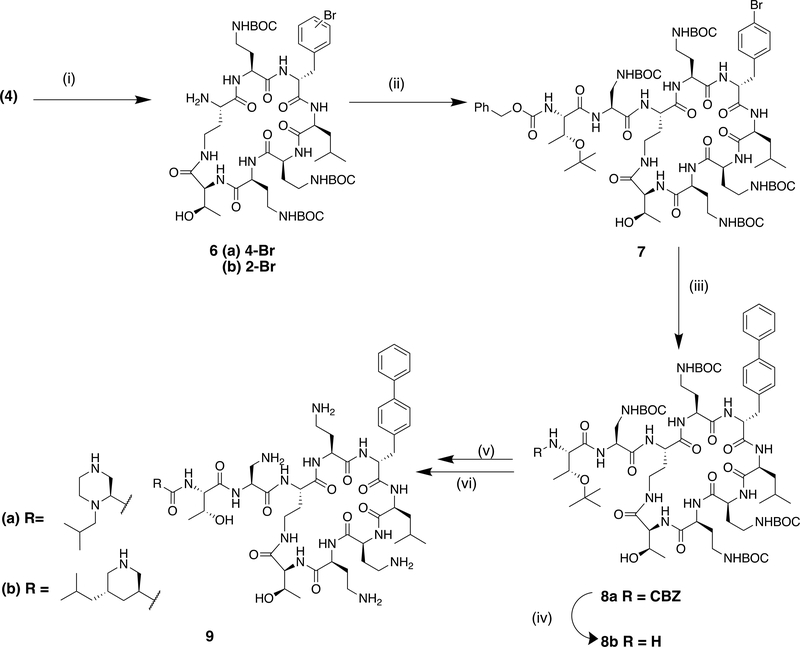
A key intermediate in the semi-synthetic route to polymyxin derivatives with a modified exocyclic chain is the corresponding cyclic heptapeptide, produced by enzymatic cleavage of the natural product.11, 17 We were pleased to find that the crude BOC-protected bromo derivative 4 was a substrate for the commercially-available protease Savinase®, enabling the synthesis of the brominated heptapeptide core (Scheme 2). Purification at this stage enabled the isolation of the brominated isomers 6(a) and (b) in a 6:1 ratio. The major component, 6(a) was assigned the (4-bromo)phenylalanine regiochemistry based on the 1H nmr in the aromatic region, while the minor isomer was consistent with the (2-bromo)phenylalanine.15 (Supplementary). The 4-bromo regioisomer 6a was then coupled to an appropriate dipeptide fragment as previously described11 to form the nonapeptide 7 containing the protected 1,3-diaminopropionic acid at the position adjacent to the core. An improved Suzuki reaction with phenyl boronic acid, utilising X-Phos (2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl) in the presence of palladium acetate proceeded in 50% yield to the biphenyl nonapeptide 8a. N-terminal deprotection of the CBZ group was effected by catalytic transfer hydrogenolysis in the presence of ammonium formate to give 8b. Coupling to the corresponding BOC protected N-terminal acid followed by deprotection and purification gave the biphenyl nonapeptide analogues 9a and b.
Scheme 2:
Reagents and conditions: (i) Savinase, 1,4-butanediol, pH 8, 27%; (ii) CBZThr (O-tBu)-Dap(BOC)OH, HATU, NEtiPr2, DCM, quant.; (iii) PhB(OH)2, Pd(OAc)2, X-Phos, K3PO4,toluene, 100°C, 50%; (iv) NH3+HCOO-, 10% Pd/C, MeOH; 93%; (v) RCO2H (N-BOC protected), HATU, NEtiPr2, DCM, DMF; (vi) TFA, DCM, followed by prep HPLC, 23% over 2 steps.
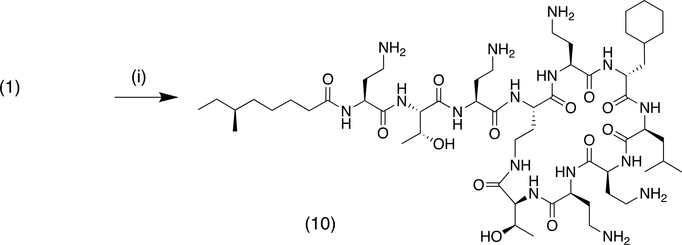
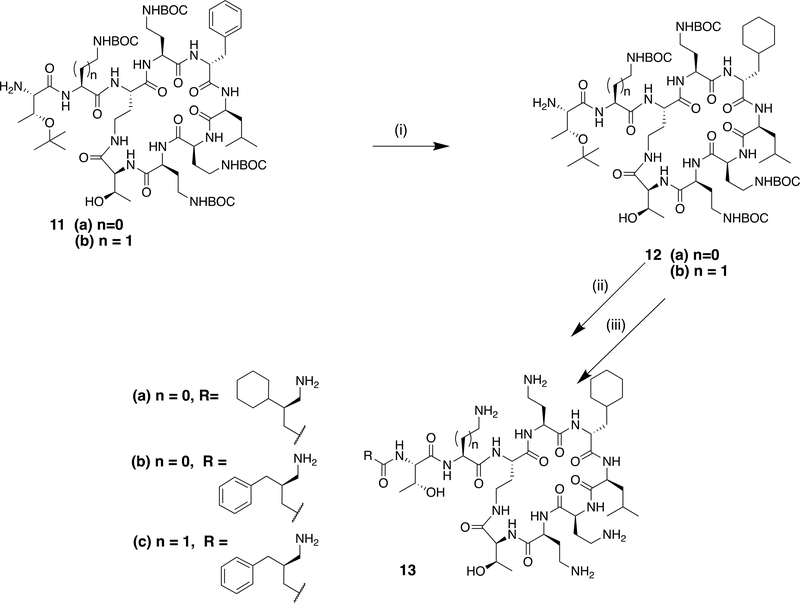
A second approach to the modification of the C-6 position of polymyxins was the conversion of the D-phenylalanine residue to cyclohexyl alanine (Cha) by reduction (Scheme 3). Hydrogenation at atmospheric pressure over platinum oxide catalyst afforded clean conversion to the cyclohexyl derivative 10. For the synthesis of nonapeptide derivatives, reduction of the previously described key nonapeptide intermediates 11a and 11b11 under similar conditions gave excellent conversion to the cyclohexyl derivatives 12a and 12b (Scheme 4). These could then be elaborated further to the desired aminoacyl nonapeptides, for example 13a-c as previously described.11
Scheme 3:
Reagents and conditions: (i) H2/PtO2, CH3CO2H, followed by prep HPLC, 29%
Scheme 4.
Reagents and conditions with example yields (i) H2/PtO2, CH3CO2H, 97%; (ii) RCO2H (N-BOC protected), HATU, NEtiPr2, DCM; (iii) TFA, DCM, followed by prep HPLC, 29% over 2 steps.
Compounds 3a, 5a-b, 9a-b, 10, and 13a-c were tested against a panel of Gram-negative bacteria including clinical strains with reduced susceptibility to PMB (Table 1). Cytotoxicity was measured against the human kidney proximal tubule epithelial cell line (HK-2) and is presented as the ratio of the IC50 of test compound to that of PMB19. PMB and previously reported nonapeptides 2a-d11 were included for comparison. Introduction of a bromine or additional phenyl ring to the phenylalanine (position 6) of polymyxin (3, 5a) served to enhance the activity against resistant strains. This is consistent with previous reports on analogues of PMB where increased lipophilicity of the core of polymyxins at positions 6 or 7 has been indicated to increase activity against resistant strains.12,13 The 4-pyridyl phenylalanine compound 5b, however, was less active against all strains. This may be due to unfavourable interactions with the lipid chain of LPS. In the nonapeptide series with a heterocyclic N-terminus, the biphenyl compounds 9a and 9b also showed enhanced activity against resistant strains compared to the corresponding phenylalanine analogues 2a and 2b. While the conversion of phenylalanine to the more bulky cyclohexyl alanine (Cha) at position 6 was tolerated, the effect on activity was less dramatic than the bromo or biphenyl modifications. In the DCha-6 polymyxin analogue 10, clear improvement in activity was noted against the less-sensitive Klebsiella strain. The cytotoxicity of the novel derivatives was, however, increased compared to their counterparts with the polymyxin scaffold, likely due to the overall increased lipophilicity. Nevertheless, the synthetic approach described herein, allowing separate modification of the core and N-terminus from a common starting material, could enable independent modification of the lipophilicity of the cyclic core and the N-terminus without the need for total synthesis. The systematic evaluation of the effect of lipophilicity on activity and cytotoxicity of polymyxin derivatives will be the subject of a further communication.
Table 1.
MIC (μg/ml) and in vitro cytotoxicity of test and reference compounds
| E.coli | K.pneumoniae | P.aeruginosa | A.baumannii | Cytotox (HK-2 IC50 relative to PMB) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cpd | ATCC 25922 | IHMA 940398 | ATCC 4352 | IHMA 652780 | ATCC 27853 | IHMA 517175 | NCTC 13424 | IHMA 863866 | |
| 1 | 0.25 | 16 | 0.125 | 4.0 | 0.5 | 8.0 | 0.25 | 32 | 1 |
| 2a | 0.25 | 32 | 0.25 | 16 | 0.5 | 32 | 0.5 | >64 | 16.6 |
| 2b | 0.03 | 2.0 | 0.03 | 2.0 | 0.25 | 2.0 | 0.125 | 2.0 | 8.8 |
| 2c | 0.06 | 16 | 0.125 | 4.0 | 0.06 | 8.0 | 0.25 | >64 | 6.9 |
| 2d | 0.25 | 32 | 0.125 | ND | 0.125 | 8.0 | 0.125 | >64 | 12.0 |
| 3a | 0.5 | 4.0 | 0.5 | 0.5 | 0.5 | 2.0 | 0.5 | 4.0 | ND |
| 5a | 1.0 | 8.0 | 0.5 | 0.5 | 1.0 | 2.0 | 1.0 | 16 | 0.14 |
| 5b | 1.0 | 32 | 1.0 | ND | 1.0 | 32 | 2.0 | ND | 0.7 |
| 9a | 0.125 | 8.0 | 0.25 | 0.5 | 0.5 | 4.0 | 0.125 | 4.0 | 2.4 |
| 9b | 0.25 | 2.0 | 0.25 | 0.5 | 0.5 | 1.0 | 0.25 | 0.5 | 1.2 |
| 10 | 0.5 | 8.0 | 0.5 | 1.0 | 1.0 | 4.0 | 0.5 | 32 | ND |
| 13a | 0.5 | 8.0 | ND | 2.0 | 0.5 | 2.0 | 0.125 | 32 | ND |
| 13b | 0.25 | 16 | 0.06 | 4.0 | 0.25 | 4.0 | 0.125 | 64 | 5.2 |
| 13c | 0.125 | 32 | ND | 4.0 | 0.25 | 8.0 | 0.06 | 64 | 2.4 |
MICs determined by broth microdilution using cation-adjusted Mueller-Hinton broth (Oxoid, CM0405) according to CLSI guidelines.18 In vitro cytotoxicity was measured against the human kidney proximal tubule epithelial cell line (HK-2) and is presented as the ratio of the IC50 of test compound to that of polymyxin B19.
In conclusion, we have described synthetic methodology enabling the exploration of modifications at the C-6 position of polymyxins and leading to increased knowledge of SAR in this region.
Supplementary Information provides details of the synthesis and characterisation of final compounds 3a, 5a-b, 9a-b, 10, 13a-c, and intermediates 6a and b.
Supplementary Material
Acknowledgements
This work was partially supported by a grant awarded to Cantab Anti-infectives Ltd by Innovate UK under the Biomedical Catalyst scheme. This work has been funded in part with US Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract HHSN272201500014C. Strains with reduced susceptibility to Polymyxin B were obtained from IHMA Europe Sàrl, Monthey, Switzerland. Cytotoxicity assays were performed at Horizon Discovery Ltd, Cambridge, UK. High resolution mass spectrometry was carried out at the National Mass Spectrometry Facility at Swansea University, UK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Prioritisation of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections including tuberculosis. Geneva: World Health Organisation; 2017(WHO/EKP/IAU/2017.12). [Google Scholar]
- 2.Garg SK, Singh O, Juneja D, Tyagi N, Khurana AS, Qamra A, Motlekar S, Barkate H. Resurgence of Polymyxin B for MDR/XDR Gram-Negative Infections: An Overview of Current Evidence. Crit Care Res Pract. 2017; 2017: 3635609; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaye KS, Pogue JM, Tran TB, Nation RL, Li J. Agents of last resort: polymyxin resistance. Infect Dis Clin N Am. 2016; 30(2): 391–414. [DOI] [PubMed] [Google Scholar]
- 4.Mares J, Kumaran S, Gobbo M, and Zerbe O. Interactions of lipopolysaccharide and polymyxin studied by NMR spectroscopy. J. Biol. Chem. 2009; 284: 11498–11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam VH, Cao H, Ledesma KR, Hu M. In vitro potency of various Polymyxin B components. Antimicrob. Agents. Chemother. 2011; 55: 4490–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts KD, Azad MAK, Wang J, Horne AJ, Thompson PE, Nation RL, Velkov T, Li J. Antimicrobial activity and toxicity of the major lipopeptide components of Polymyxin B and Colistin: last-line antibiotics against multi-drug resistant Gram negative bacteria. ACS Inf Dis. 2015; 1: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velkov T, Thompson PE, Nation RL, Li J. Structure-activity relationships of Polymyxin Antibiotics. J. Med. Chem; 2010: 53, 1898–1916. DOI: 10.1021/jm900999h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaara M Novel derivatives of polymyxins. J. Antimicrob. Chemother. 2013; 68: 1213–1219. DOI: 10.1093/jac/dkt039 [DOI] [PubMed] [Google Scholar]
- 9.Brown P, Dawson MJ. Development of new polymyxin derivatives for multi-drug resistant Gram-negative infections. J. Antibiotics 2017; 70: 386–394. DOI: 10.1038/ja.2016.146 [DOI] [PubMed] [Google Scholar]
- 10.Rabanal F, Cajal Y. Recent advances and perspectives in the design and development of polymyxins. Nat. Prod. Rep. 2017; 34: 886–908. DOI: 10.1039/c7np00023e [DOI] [PubMed] [Google Scholar]
- 11.Brown P, Abbott E, Abdulle O, Boakes S, Coleman S, Divall N, Duperchy E, Moss S, Rivers D, Simonovic Singh J, Stanway S, Wilson A, Dawson MJ. Design of Next Generation Polymyxins with Lower Toxicity: The Discovery of SPR206. ACS Inf. Dis. 2019; 5: 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velkov T et al. Teaching ‘old’ Polymyxins new tricks: New-generation lipopeptides targeting Gram-negative ‘superbugs’. ACS Chemical Biology. 2014; 9: 1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallardo-Godoy A, Muldoon C, Becker B, Elliott AG, Lash LH, Huang JX, Butler M, Pelingon R, Kavanagh AM, Ramu S, Phetsang W, Blaskovitch MAT, and Cooper M. Activity and predicted nephrotoxicity of synthetic analogues based on Polymyxin B. J. Med. Chem. 2016; 59: 1068–1077. DOI: 10.1021/acs.jmedchem.5b01593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui A-L, Hu X-X, Gao Y, Jin J, Yi H, Wang X-K, Nie T-Y, Chen Y, He Q-Y, Guo H-F, Jiang JD, You X-F, Li Z-R. Synthesis and Bioactivity Investigation of the Individual Components of Cyclic Lipopeptide Antibiotics. J.Med.Chem. 2018; 61: 1845–1857 [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama Y, Yamaguchi T, Sato M, Kobayashi E, Murakami Y, Okuno H. Chemistry of unprotected amino acids in aqueous solution: direct bromination of aromatic amino acids with bromoisocyanuric acid sodium salt under strong acidic condition. Chem. Pharm. Bull. 2006; 54: 1715–1719. [DOI] [PubMed] [Google Scholar]
- 16.Prakash GKS, Mathew T, Hoole D, Esteves PM, Wang Q, Rasul G, Olah GA. N-Halosuccinimide/BF3-H2O, Efficient electrophilic halogenating systems for aromatics. J.Amer Chem Soc. 2004; 126: 15770–15776 [DOI] [PubMed] [Google Scholar]
- 17.Li B, Akin A, Magee TV, Martinez C, Szeliga J, Vuong DV. Syntheses of Dap-3 Polymyxin Analogues via a Tris-Boc-Protected Polymyxin B Heptapeptide. Synthesis; 2015; 47: 2088–92. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically: approved standard, 9th ed. CLSI document. M07–A9; 2012. CLSI, Wayne, PA [Google Scholar]
- 19.Mammalian cell toxicity was measured using confluent monolayers of the human HK-2 proximal tubule epithelial cell line. Compounds were incubated with cells for 24h at 37°C in 5% CO2 using a top concentration of 1,000 or 3,000 μg/mL with semi-log dilutions to give a 9-point concentration range. Cell viability was measured using resazurin blue. Compound concentration values were plotted as log values to enable a dose-response curve to be fitted. The bottom of the curve was constrained to zero and IC50 values were determined using GraphPad Prism. The relative cytotoxicity is reported as the ratio of the IC50 of test compound to that of PMB in the same experiment.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.