Abstract
Acetylcholinesterase inhibitors (AChEIs) have been noted to increase bone density and quality in mice. Human studies are limited but suggest an association with improved bone healing after hip fracture. We examined the relationship between AChEI use and fracture risk in a national cohort of 360,015 male veterans aged 65 to 99 years with dementia but without prior fracture using Veterans Affairs (VA) hospital, Medicare, and pharmacy records from 2000 to 2010. Diagnosis of dementia, any clinical fracture (excluding facial and digital), comorbidities, and medications were identified using ICD-9 and drug class codes. Cox proportional hazard models considering AChEI use as a time-varying covariate and adjusting for fall and fracture risk factors compared the time-to-fracture in AChEI users versus non-AChEI users. Potential confounders included demographics (age, race, body mass index), comorbidities associated with fracture or falls (diabetes, lung disease, stroke, Parkinson’s, seizures, etc.) and medications associated with fracture or falls (bisphosphonates, glucocorticoids, androgen deprivation therapy [ADT], proton pump inhibitors [PPIs], selective serotonin receptor inhibitors [SSRIs], etc.). Competing mortality risk was considered using the methods of Fine and Gray. To account for persistent effects on bone density or quality that might confer protection after stopping the medication, we completed a secondary analysis using the medication possession ratio (MPR) as a continuous variable in logistic regression models and also compared MPR increments of 10% to minimal/no use (MPR 0 to <0.10). Among older veterans with diagnosis of dementia, 20.1% suffered a fracture over an average of 4.6 years of follow-up. Overall, 42.3% of the cohort were prescribed AChEIs during the study period. The hazard of any fracture among AChEI users compared with those on other/no dementia medications was significantly lower in fully adjusted models (hazard ratio [HR] = 0.81; 95% confidence interval [CI] 0.75–0.88). After considering competing mortality risk, fracture risk remained 18% lower in veterans using AChEIs (HR = 0.82; 95% CI 0.76–0.89).
Keywords: CORTICOSTEROID OSTEOPOROSIS, AGING, CLINICAL TRIALS, EPIDEMIOLOGY, OSTEOPOROSIS, HORMONES AND RECEPTORS
Introduction
Alzheimer’s disease (AD), the most common cause of dementia, is characterized by presynaptic cholinergic deficits and reduced acetylcholine concentrations and function.(1) Patients with AD have elevated fracture rates, which have been linked in part to vitamin D deficiency, increased falls, and lower average bone density.(2,3) The lower bone density is hypothesized to be related to bone density loss occurring before the diagnosis of dementia. The mechanism is not yet understood but has been hypothesized to be mediated by central cholinergic and serotonergic pathways.(4–6)
Acetylcholinesterase inhibitors (AChEIs) such as donepezil are approved by the United States Food and Drug Administration (USFDA) for the treatment of AD, and have clinical trial data supporting their use in vascular dementia and Parkinson’s disease.(7–9) in vivo studies show increase in overall bone mass and whole-body energy among donepezil-treated mice,(10) possibly by inhibiting receptor activator of NF-κB ligand (RANKL)-induced osteoclast differentiation.(11) Studies in human subjects have been very few but suggest that AChEIs may confer protection against fractures, accelerate bone healing, and reduce both fracture complications and all-cause mortality post-hip fracture.(12–14) Concerns about small sample sizes and risk of bias have limited the application and generalizability of these initial studies. Nevertheless, these observations have inspired new basic and clinical inquiries into the effect of cholinergic stimulation on bone health in older adults.
The goal of this study is to examine the relationship between the use of AChEIs and fracture risk in a large cohort of older male veterans with dementia.
Materials and Methods
This is a secondary analysis of a national cohort study designed to study osteoporosis screening and treatment in men using linked administrative data. All male veterans aged 65 to 99 years receiving primary care in any US Veterans Affairs facility from 2000 to 2010 were included. Details about the original cohort, data collection, and variable definitions have been previously described.(15–17) Administrative data were drawn from all 146 Veterans Health Administration (VHA) centers including inpatient and outpatient treatment records, fee-basis records, and Pharmacy Benefits Management Services records merged with Centers for Medicare and Medicaid Services (CMS) Parts A and B claims. Approval from the Institutional Review Board (IRB) of the Durham Veterans Administration (VA) Medical Center was obtained for all analyses.
Eligible subjects in the original cohort included men aged 65 to 99 years with at least 2 primary care visits within 2 consecutive calendar years between 2000 and 2010 and who had no diagnosis of osteoporosis or fracture in the 2 years before baseline (n = 2,539,812). We included only those who receive primary care in the VA because almost all of their prescriptions for chronic disease management come from the VA. However, because many veterans seek additional care outside the VA, both CMS and VA records were used to ascertain fractures, dementia, and fracture-related comorbidities. Veterans without linked CMS records were excluded such that all subjects had both VA and CMS administrative data for analysis.
For this analysis, we selected veterans with at least one ICD-9 code for dementia or who received at least one prescription for a dementia medication. Patients were excluded if they were receiving hospice services (Fig. 1). Dementia and other fall and fracture-related comorbidities listed in Table 1 were defined using ICD-9 codes in inpatient and outpatient encounters and validated algorithms where available.(18–20) Two medication classes are commonly recommended for dementia treatment: AChEIs (donepezil, galantamine, and rivastigmine) and an N-methyl-D-aspartate (NMDA) receptor antagonist (memantine), which does not impact the adrenergic system.(7–9,21) Prescription dispensing dates were identified for all medication classes prescribed for dementia treatment (AChEIs, memantine) as well as medications known to increase fracture risk (glucocorticoids, androgen deprivation therapy, antiepileptic drugs, proton pump inhibitors, selective serotonin reuptake inhibitors), and medications commonly prescribed in dementia that are known to increase falls (other antidepressants, antipsychotics, opiates, and highly anticholinergic medications). The primary outcome variable was any clinical fracture as identified by ICD-9 code, excluding facial and digital fractures.
Fig. 1.
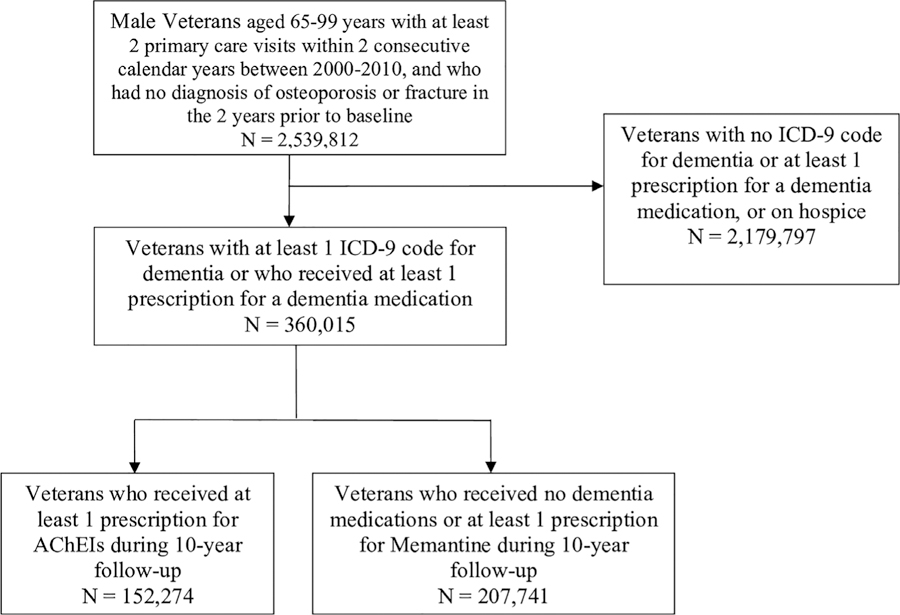
Consort diagram. All veterans aged 65 to 99 years with an ICD-9 diagnosis code for dementia or at least one prescription for a dementia medication were included in this analysis.
Table 1.
Baseline Characteristics of Study Population
| Characteristics | Ever prescribed acetylcholinesterase inhibitors | No medication/other dementia medications | p Value |
|---|---|---|---|
| n = 152,274 | n = 207,741 | ||
| Age (years), mean ± SD | 75.8 (6.1) | 75.4 (6.5) | <0.001 |
| White (%) | 70.8 | 69.9 | <0.001 |
| Body mass index (kg/m2), mean ± SD | 27.5 (4.4) | 28.0 (4.8) | <0.001 |
| Current smoker (%) | 16.6 | 20.7 | <0.001 |
| Chronic alcohol use (%) | 19.0 | 20.0 | <0.001 |
| Rheumatoid arthritis (%) | 0.5 | 0.6 | 0.03 |
| Malabsorption (%) | 0.4 | 0.6 | <0.001 |
| Chronic lung disease (%) | 26.1 | 35.9 | <0.001 |
| Chronic liver disease (%) | 6.8 | 11.2 | <0.001 |
| Stage 3–4 kidney disease (%) | 1.0 | 1.9 | <0.001 |
| Congestive heart failure (%) | 16.9 | 24.9 | <0.001 |
| Hyperthyroidism (%) | 0.9 | 1.8 | <0.001 |
| Diabetes (%) | 32.9 | 37.2 | <0.001 |
| Parkinson’s disease (%) | 7.1 | 5.4 | <0.001 |
| Osteoarthritis (%) | 33.2 | 38.9 | <0.001 |
| Stroke (%) | 1.2 | 2.2 | <0.001 |
| Glucocorticoid (%) | 1.2 | 1.7 | <0.001 |
| Androgen deprivation therapy (%) | 0.0 | 0.0 | 0.56 |
| Antiepileptic drugs (%) | 9.8 | 13.3 | <0.001 |
| Proton pump inhibitors (%) | 40.7 | 40.4 | 0.06 |
| Antidepressants (%) | |||
| SSRIs (%) | 12.6 | 9.7 | <0.001 |
| Others (%) | 10.3 | 8.9 | <0.001 |
| Opiates (%) | 2.5 | 2.0 | <0.001 |
| Any psychoactive medication (5) | 27.5 | 15.3 | <0.001 |
| FRAX score with BMI (10-year probability of major fracture), mean ± SD | 10.1 (3.4) | 10.0 (3.6) | <0.001 |
SD = standard deviation; SSRIs = selective serotonin reuptake inhibitors; BMI = body mass index.
The p value refers to comparison between veterans on acetylcholinesterase inhibitors and other medications.
Reference group is “no medication or other dementia medications.”
Statistical analysis
Comparisons for baseline characteristics between the groups (AChEI users versus memantine or no medication users) were assessed using the chi-square test for categorical variables or the Student’s t test for continuous variables. To evaluate the risk for fracture associated with using AChEIs, we used repeated measures Cox proportional hazards regression models adjusted for all fracture risk factors listed above. Repeated measures analysis was employed because subjects often received multiple courses of AChEIs for various lengths of time. Prescription fill dates were used to define periods of AChEIs use; each individual could go on and off treatment multiple times over the study period. Repeated measures survival analysis was used to manage the time-varying effects of being on AChEIs. The fracture outcome of interest was any clinical fracture assessed as described above. To account for the competing risk of death, we used the Fine and Gray methods to estimate the subdistribution relative hazards of fracture. Kaplan–Meier curves were generated using conventional nonparametric survival analysis.
Because the repeated measures analysis does not account for persistent effects on bone density or quality that might confer protection after stopping the medication, we also completed a secondary analysis using the medication possession ratio (MPR). The MPR is defined as the number of days’ supply of AChEIs dispensed to the subjects divided by the total number of days they were followed. In a fully adjusted logistic regression model, we considered MPR as a continuous variable between 0 and 1 and also compared MPR increments of 10% to minimal/no use (MPR 0 to <0.10). All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
In total, 360,015 older veterans with dementia were included in this analysis. The average age of the population was 75.6 years. Forty-two percent (n = 152,274) of this cohort received at least one prescription for AChEIs during follow-up. Baseline characteristics of the cohort stratified by use of AChEIs are presented in Table 1; clinically important differences included a higher proportion of current smokers among non-AChEI users compared with AChEI users (20.7% versus 16.6%). Non-AChEI users were also more likely to have coexisting medical conditions such as chronic lung disease (35.9% versus 26.1%), congestive heart failure (24.9% versus 16.9%), chronic liver disease (11.2% versus 6.8%), and diabetes (37.2% versus 32.9%). Veterans with AChEI prescriptions were more likely to be using antidepressants (SSRIs and non-SSRIs) and other psychoactive medications but less likely to be prescribed glucocorticoids or antiepileptics compared with non-AChEI users. FRAX scores calculated with body mass index (BMI) for both groups were similar, with 10-year risk for major osteoporotic fracture at approximately 10%.
Over the 10-year period of follow-up (mean 4.6 years), 20.1% of older veterans with dementia suffered a fracture. Of the study cohort, 42.3% were ever-prescribed an AChEI, 1.7% were prescribed only an NMDA receptor antagonist, and 56.0% were prescribed neither. Among AChEI users, the average days prescribed was 629. The proportion of veterans who experienced a fracture was 14.5% (n = 22,089) among AChEI users compared with 34.5% (n = 71,700) among non-AChEIs users. The distribution of fracture types was similar between AChEI users and nonusers, with 23.8% and 25.0% hip and femur fractures, 19.9% and 18.5% rib or clavicle fractures, 13.7% and 14.6% tibial/fibular fractures, 4.5% and 6.5% humerus fractures, 6.5% and 6.4% forearm fractures, 3.8% and 3.2% pelvic fractures, and 27.8% and 25.9% other fracture types. Compared with those on other dementia medications or no medication, the hazard of any fracture among AChEI users was significantly lower in fully adjusted models (hazard ratio [HR] = 0.81; 95% confidence interval [CI] 0.75–0.88). After considering competing mortality risk, fracture risk remained 18% lower in veterans using AChEIs (HR = 0.82; CI 0.76–0.89). The Kaplan–Meier survival plot (Fig. 2) for the cohort shows a lower risk for fracture among the AChEI group, with the risk separation becoming more apparent around the third to fourth year of AChEI use. However, the separation time in this analysis is difficult to interpret because individuals are counted in both groups as they go on or off the medication over time.
Fig. 2.
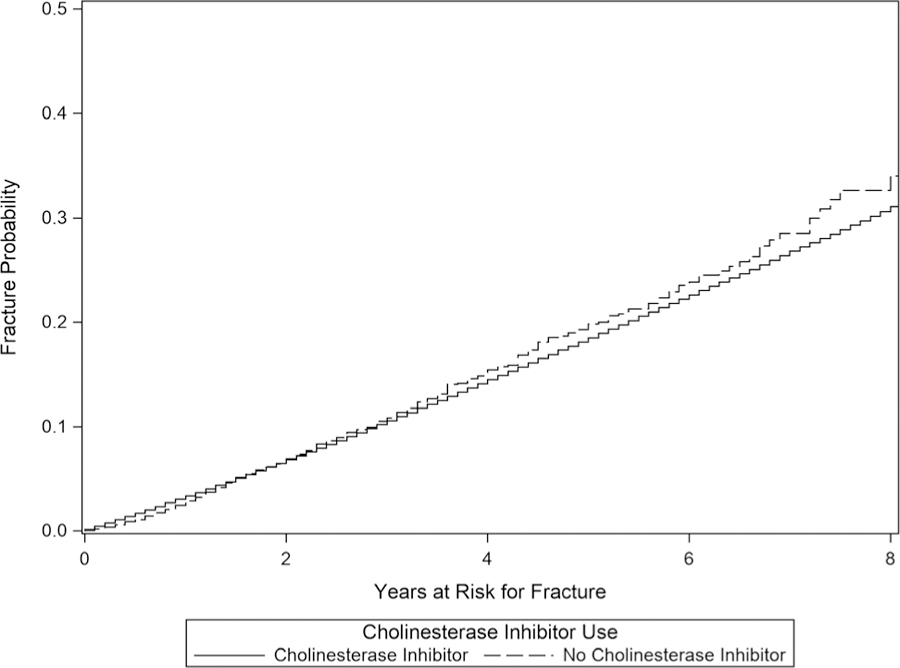
Comparison of fracture-free survival stratified by AChEIs using Kaplan–Meier and competing risk methods. In this tracing, the y axis has been flipped to highlight the lower fracture risk in the AChEIs-use group and the prominent risk separation at the 4-year mark.
Analyses using the MPR as a reflection of the proportion of follow-up time an individual received AChEIs showed an even stronger protective effect, with OR = 0.61 (0.58–0.64). Higher MPRs were associated with greater fracture protection in a dose–response pattern, as depicted in Table 2.
Table 2.
Hazard of Any Fracture by Medication Possession Ratio (MPR)1 Category Compared With Subjects With MPR 0–10%
| MPR category | Hazard ratio compared with MPR 0–10% | 95% Confidence limits |
|---|---|---|
| 10–<20% | 0.79 | 0.75–0.84 |
| 20–<30% | 0.74 | 0.70–0.79 |
| 30–<40% | 0.71 | 0.67–0.75 |
| 40–<50% | 0.69 | 0.65–0.73 |
| 50–<60% | 0.65 | 0.62–0.70 |
| 60–<70% | 0.64 | 0.60–0.68 |
| 70–<80% | 0.67 | 0.63–0.71 |
| 80–<90% | 0.63 | 0.59–0.67 |
| 90–100% | 0.56 | 0.52–0.59 |
MPR is calculated by the number of days’ supply of AChEI dispensed to the subject divided by the total number of days they were followed.
Discussion
Evidence suggesting a benefit of AChEI use on bone health has slowly emerged.(11,13,14,22) Bone homeostasis is regulated by local autocrine and paracrine mechanisms and neuronal signals from the autonomic nervous system; similarly, the autonomic nervous system is also implicated in dementia.(23) Preclinical studies on bone remodeling have shown cholinergic activity induces bone mass accrual by stimulating osteoblast proliferation via muscarinic receptors, and apoptosis in osteoclasts via nicotinic receptors,(24–28) whereas adrenergic stimulations promote osteoclast formation and bone resorption via beta adrenergic receptors.(10,29,30)
These observations have prompted consideration of whether medications impacting the cholinergic system might impact bone-related outcomes. Evidence from in vivo studies shows that administration of a peripherally acting AChEI, pyridostigmine, raises acetylcholine levels and increased trabecular bone mass in mice by inhibiting osteoclasts and bone resorption.(24) Available clinical studies, though few, support improved bone health with use of AChEIs.(12–14,22) In older adults with AD, use of centrally acting AChEIs such as donepezil and rivastigmine is associated with reduced hip fracture risks.(13) A cohort study examining AChEI use in AD patients with hip fracture described a reduction in all-cause mortality by 56% and risk of a second hip fracture by 41%.(12) In a retrospective study examining the effect of AChEI use on fracture healing among 49 AD patients, AChEI users showed better radiographic union at fracture sites (relative risk [RR] = 2.7; CI 0.9–7.8), fewer healing complications (RR = 0.8, CI 0.7–1.0), and overall better bone quality (RR = 2; CI 1.2–3.3).(22) Similarly, in a nested case-control study on 1190 cases and 4760 controls, any prior use of AChEIs was associated with reduced fracture risk (OR = 0.8; CI 0.70–0.91).(14)
This study extends these findings by demonstrating a clinically important association between AChEI exposure and a reduced hazard of any clinical fracture, etc., over a 10-year follow-up period. Although prior human studies have been limited to those with Alzheimer’s dementia, our study included subjects with all types of dementia (vascular, Parkinson’s, dementia with Lewy bodies, etc.) who were prescribed AChEIs, thus widening the population in whom there is a suggestion of benefit. The use of competing risk methods in this analysis further reduces concerns about biased estimates that could have arisen using traditional time-to-fracture in survival analysis or case-control studies. Our primary analysis does not assume a carry-over effect on fracture risk when an individual stops the medication; because available data suggest an impact on bone density and quality, this analysis would underestimate any reduction of fracture risk. The secondary analyses using the MPR show an even stronger effect and a suggestion of a “dose response” such that higher adherence and proportion of time treated are associated with lower fracture rates.
Another finding of note from this national study is the extremely high fracture rate in this population. The high proportion of older male veterans with dementia with incident fracture reported in this cohort of older male veterans with dementia in less than 5 years’ average follow-up (20.4%) compared with the 10-year probability for a major fracture predicted by the FRAX model (10%) suggests a limitation of FRAX in the fracture-risk assessment of this population and demonstrates a need for better short-and long-term fracture risk calculators in older patients with comorbidities, particularly for men. The high incidence of fractures also reinforces the need for better fall prevention measures and osteoporosis treatment in this population.
An important limitation of this study is the effect of unmeasured confounders. These include variables not available in administrative data such as number of falls, vitamin D levels, dementia types and stages, and frailty. In this nonrandomized trial, there were important differences in men who were prescribed AChEIs and those who were not, with a higher comorbidity burden observed in nonusers. Conversely, users had substantially higher use of psychoactive medications known to increase fall and fracture risk, including SSRIs and antipsychotics, suggesting that they had greater levels of behavioral disturbance, which is also known to increase fracture risk. To reduce these imbalances, we controlled for a substantial number of factors that may impact fracture risks, including comorbid conditions and medications associated with both falls and fractures. However, inaccuracies in coding in administrative data and the known low sensitivity of ICD-9 codes in many chronic illnesses(31) limit our ability to adjust for imbalance in these risk factors. There were insufficient numbers of individuals prescribed memantine only to compare their fracture rates with those prescribed only AChEIs, which would reduce the concern about selection bias. Some veterans may have been prescribed AChEIs outside the VA system, and we did not capture this use; however, this under-counting would suggest that the true fracture reduction is even larger than our estimates. Conversely, veterans prescribed non-VA medications that negatively impact bone such as steroids or androgen deprivation therapy would inflate our estimates. Although vitamin D is an important factor involved in both falls and fractures, prescriptions of supplements are not reliably ascertained through the VA pharmacy database and are not included in our models.
These study findings have clinical implications for drug selection for dementia treatment. Dementia and fractures share a complex bidirectional relationship beyond the common impact of advancing age on their incidences.(32) Dementia independently increases hip fracture risk 2.7-fold.(33) Available study outcomes on the association between dementia and risk of fractures at other sites (wrist and vertebral) are inconsistent.(34) Because of modest effect sizes, inconsistent study results, and side effect profiles of the medications, clinical uptake of dementia medications has been limited.(35,36) The possibility of a reduction in fracture risk with AChEI use introduces an important factor to consider when discussing the risks and benefits of pharmacologic dementia treatment in view of the high coexisting fracture risk. This study, along with emerging preclinical evidence, makes a strong collective case for a phase 2 clinical study of AChEI use on bone density, quality, and fracture risk in older adults with dementia.
In summary, we found that AChEI use is associated with a clinically important reduction in fracture risk in men with dementia, controlling for many other fall and fracture risk factors. Additional clinical studies on the impact of AChEIs on bone in older adults are needed.
Acknowledgments
The original work from which data were drawn for this study was supported by the Department of Defense grants W81XWH-12-2-0093, NIAK24AG049077-0IAI, and NIA2P30AG028716-06.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Frances PT. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr 2005;10(11 Suppl 18):6–9. [DOI] [PubMed] [Google Scholar]
- 2.Kipen E, Helme RD, Wark JD, Flicker L. Bone density, vitamin D nutrition, and parathyroid hormone levels in women with dementia. J Am Geriatr Soc 1995;43(10):1088–91. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SM, Menzies IB, Bukata SV, Mendelson DA, Kates SL. Dementia and hip fractures: development of a pathogenic framework for understanding and studying risk. Geriatr Orthop Surg Rehabil 2010;1(2):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stark SL, Roe CM, Grant EA, et al. Preclinical Alzheimer disease and risk of falls. Neurology 2013;81(5):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eimar H, Tamimi I, Murshed M, Tamimi F. Cholinergic regulation of bone. J Musculoskelet Neuronal Interact 2013;13(2):124–32. [PubMed] [Google Scholar]
- 6.Dengler-Crish CM, Smith MA, Wilson GN. Early evidence of low bone density and decreased serotonergic synthesis in the dorsal raphe of a tauopathy model of Alzheimer’s disease. J Alzheimers Dis. 2017;55(4): 1605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaji KS, Sivakumar PT, Rao GP, Paul N. Clinical practice guidelines for management of dementia. Indian J Psychiatry 2018;60:S312–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koller D, Hua T, Bynum JP. Treatment patterns with antidementia drugs in the United States: Medicare cohort study. J Am Geriatr Soc 2016;64(8):1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008;148(5):370–8. [DOI] [PubMed] [Google Scholar]
- 10.Eimar H, Alebrahim S, Manickam G, et al. Donepezil regulates energy metabolism and favors bone mass accrual. Bone 2016;84:131–8. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Enoki Y, Sakamoto Y, et al. Donepezil prevents RANK-induced bone loss via inhibition of osteoclast differentiation by downregulating acetylcholinesterase. Heliyon 2015;1(1):e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamimi I, Madathil SA, Kezouh A, Nicolau B, Karp I, Tamimi F. Effect of acetylcholinesterase inhibitors on post-surgical complications and mortality following a hip fracture: a cohort study. J Musculoskelet Neuronal Interact 2017;17(2):69–77. [PMC free article] [PubMed] [Google Scholar]
- 13.Tamimi I, Ojea T, Sanchez-Siles J, et al. Acetylcholinesterase inhibitors and the risk of hip fracture in Alzheimer’s disease patients: a case-control study. J Bone Miner Res 2012;27(7):1518–27. [DOI] [PubMed] [Google Scholar]
- 14.Tamimi I, Nicolau B, Eimar H, et al. Acetylcholinesterase inhibitors and the risk of osteoporotic fractures: nested case-control study. Osteoporos Int 2018;29(4):849–57. [DOI] [PubMed] [Google Scholar]
- 15.Colon-Emeric CS, Pieper CF, Van Houtven CH, et al. Limited osteoporosis screening effectiveness due to low treatment rates in a national sample of older men. Mayo Clin Proc 2018;93(12):1749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall RK, Sloane R, Pieper C, et al. Competing risks of fracture and death in older adults with chronic kidney disease. J Am Geriatr Soc 2018;66(3):532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee RH, Sloane R, Pieper C, et al. Clinical fractures among older men with diabetes are mediated by diabetic complications. J Clin Endocrinol Metab 2018;103(1):281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006;40 (7–8):1280–8. [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 20.Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res 2003;38(4):1103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birks J Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006;(1):CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eimar H, Perez Lara A, Tamimi I, et al. Acetylcholinesterase inhibitors and healing of hip fracture in Alzheimer’s disease patients: a retrospective cohort study. J Musculoskelet Neuronal Interact 2013;13(4):454–63. [PubMed] [Google Scholar]
- 23.Takeda S Central control of bone remodelling. J Neuroendocrinol 2008;20(6):802–7. [DOI] [PubMed] [Google Scholar]
- 24.Bajayo A, Bar A, Denes A, et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci USA 2012;109(38):15455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, Oury F, Yadav VK, et al. Signaling through the M(3) muscarinic receptor favors bone mass accrual by decreasing sympathetic activity. Cell Metab 2010;11(3):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mito K, Sato Y, Kobayashi T, et al. The nicotinic acetylcholine receptor alpha7 subunit is an essential negative regulator of bone mass. Sci Rep 2017;7:45597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haupt M, Kauschke V, Sender J, et al. Bone status of adult female butyrylcholinesterase gene-deficient mice. Int Immunopharmacol 2015;29(1):208–14. [DOI] [PubMed] [Google Scholar]
- 28.Kliemann K, Kneffel M, Bergen I, et al. Quantitative analyses of bone composition in acetylcholine receptor M3R and alpha7 knockout mice. Life Sci 2012;91(21–22):997–1002. [DOI] [PubMed] [Google Scholar]
- 29.Togari A Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech 2002;58(2):77–84. [DOI] [PubMed] [Google Scholar]
- 30.Arai M, Nagasawa T, Koshihara Y, Yamamoto S, Togari A. Effects of beta-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim Biophys Acta 2003;1640(2–3):137–42. [DOI] [PubMed] [Google Scholar]
- 31.Singh JA. Accuracy of Veterans Affairs databases for diagnoses of chronic diseases. Prev Chronic Dis 2009;6(4):A126. [PMC free article] [PubMed] [Google Scholar]
- 32.Vun JSH, Ahmadi M, Panteli M, Pountos I, Giannoudis PV. Dementia and fragility fractures: issues and solutions. Injury 2017;48:S10–6. [DOI] [PubMed] [Google Scholar]
- 33.Melton LJ, Beard CM, Kokmen E, Atkinson EJ, O’Fallon WM. Fracture risk in patients with Alzheimer’s disease. J Am Geriatr Soc 1994;42 (6):614–9. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Hung C, Lin S, et al. Increased risk of hip fractures in patients with dementia: a nationwide population-based study. BMC Neurol 2014;14:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomized controlled trials. Lancet Neurol 2007;6(9): 782–92. [DOI] [PubMed] [Google Scholar]
- 36.Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008;148 (5):379–97. [DOI] [PubMed] [Google Scholar]


