Abstract
Study Objectives
Major depressive disorder (MDD) is the leading cause of disability worldwide. Its high recurrence rate calls for prevention of first-onset MDD. Although meta-analysis suggested insomnia as the strongest modifiable risk factor, previous studies insufficiently addressed that insomnia might also occur as a residual symptom of unassessed prior depression, or as a comorbid complaint secondary to other depression risks.
Methods
In total, 768 participants from the Netherlands Study of Depression and Anxiety who were free from current and lifetime MDD were followed-up for four repeated assessments, spanning 6 years in total. We performed separate Cox proportional hazard analyses to evaluate whether baseline insomnia severity, short-sleep duration, and individual insomnia complaints prospectively predicted first-onset MDD during follow-up. The novel method of network outcome analysis (NOA) allowed us to sort out whether there is any direct predictive value of individual insomnia complaints among several other complaints that are associated with insomnia.
Results
Over 6-year follow-up, 141 (18.4%) were diagnosed with first-onset MDD. Insomnia severity but not sleep duration predicted first-onset MDD (HR = 1.11, 95% CI: 1.07–1.15), and this was driven solely by the insomnia complaint difficulty initiating sleep (DIS) (HR = 1.10, 95% CI: 1.04–1.16). NOA likewise identified DIS only to directly predict first-onset MDD, independent of four other associated depression complaints.
Conclusions
We showed prospectively that DIS is a risk factor for first-onset MDD. Among the different other insomnia symptoms, the specific treatment of DIS might be the most sensible target to combat the global burden of depression through prevention.
Keywords: insomnia, major depressive disorder, prevention, network outcome analysis, multivariate analysis
Statement of Significance.
The high prevalence and recurrence rate of major depressive disorder (MDD) stress the utmost importance of prevention of first-onset MDD. Using the novel method of network outcome analysis, we identified that, among insomnia complaints, only ‘difficulty initiating sleep (DIS) is an independent and primary predictor of first-onset MDD. Crucially, these findings are of high-clinical relevance, as cognitive behavioral therapy for insomnia is highly effective in improving DIS. Targeting specifically DIS in the treatment of insomnia might aid to combat the global costs of MDD by means of prevention.
Introduction
Major depressive disorder (MDD) is the leading cause of disability worldwide [1] and prevalence rates continue to rise [2, 3]. Because the probability of recurrence is high [4], the prevention of MDD would be more efficient than its treatment. It is therefore of utmost importance to determine primary modifiable risk factors for the development of first onset MDD [5]. Knowing these targets is a prerequisite to identify vulnerable individuals and to optimize attempts to prevent rather than treat depression [6]. Insomnia has often been suggested to be a primary modifiable risk factor of depression [7, 8]. The identification of insomnia as an independent risk factor for first-onset depression is however complicated by at least two challenges.
A first challenge, and shortcoming of most previous studies that investigated insomnia as a risk factor for depression, is that whereas current depression is often excluded, the lifetime history of a depression diagnosis is rarely assessed. Because prior depression is among the strongest risk factors for depression, it is difficult to disentangle de novo risk factors from residual symptoms. Importantly, insomnia is the most common residual symptom of depression [9]. Therefore, only if a lifetime history of a depression diagnosis can be excluded, it can be ruled out that prior depression rather than insomnia is predictive for a future depressive episode.
A second challenge is that insomnia complaints can be considered a symptom of both insomnia and MDD. Therefore, someone who suffers from insomnia also suffers from one symptom of MDD according to diagnostic criteria [10], which might explain its predictive effect. The predictive value of insomnia for first-onset MDD may thus be nonspecific, indistinguishable from other depression complaints. Although several studies investigated whether baseline depression symptoms predict MDD onset [e.g. [11–14]], none of these previous studies took into account that the depression symptoms are themselves related and that, as a consequence, some symptoms might only predict MDD via their relations to other symptoms. How to validly determine the importance of insomnia amidst this set of correlated symptoms has so far remained enigmatic.
To overcome these challenges we performed a 6-year study with three follow-up assessments in a large sample carefully selected to be free from both a current and a lifetime prior diagnosis of MDD. We moreover answered the pressing need to determine primary risk factors of first-onset MDD among multiple possibly predictive symptoms of insomnia and depression, by applying a novel method for symptom network analysis, which we will refer to as network outcome analysis (NOA).
Methods
Participants
We carefully selected participants from the Netherlands Study of Depression and Anxiety (NESDA), a multisite longitudinal study including four repeated assessments (T0–T3), spanning 6 years in total [15]. Included participants were strict without a current or prior lifetime MDD according to the DSM-IV, determined using the Composite International Diagnostic Interview (CIDI) [16], and for whom a CIDI at each of three follow-up measurements was completed. The 768 included participants were between 18 and 65 years of age (M = 41.1 years, SD = 14.4 years), and 482 (62.7%) were female.
Measures
At T0, insomnia severity, sleep duration, and severity of individual depression symptoms were assessed.
Insomnia severity was assessed using the Women’s Health Initiative Insomnia Rating Scale (IRS) [17]. The IRS contains five items on sleep problems (i.e. trouble falling asleep, waking up during the night, waking up earlier than planned, and troubles getting back to sleep) and sleep quality during the past month (scale 0–4), for which a total score can be computed (range 0–20).
Sleep duration was assessed by asking participants to estimate the average hours of sleep per night during the past month, where at most 6 h was coded as short sleep duration. The risk of depression by conveyed by short sleep is less clear than the risk imposed by insomnia. A meta-analysis of prospective studies suggests that short sleep increases the risk of depression with an odds ratio (OR) of 1.31 [18], whereas meta-analyses on the risk of MDD imposed by insomnia reported a range of OR = 2.10–2.60 [7, 8]. Despite lower risk, we still considered it important to include short sleep in our analyses, because short sleep has also been shown to predict insomnia chronicity [19], and chronic rather than acute insomnia adds to the risk of first-onset depression [20]. It has indeed been suggested that the risk of depression is largest for people suffering from both insomnia and short sleep [21].
Severity of nonclinical depression complaints at baseline was assessed using 30 items of the Inventory of Depressive Symptomatology (IDS), with items rated on a 4-point scale (0–3) [22]. Following van Borkulo et al. [23], we used the items of the IDS that mapped onto the nine criteria of a DSM-5 MDD diagnosis, see Table 1. Some DSM-5 criteria are represented by multiple IDS items, resulting in an initial selection of 16 items. However, two sets of two items, respectively inquiring changes in weight and appetite, were mutually exclusive, resulting in perfect negative relations between the two variables. We followed van Borkulo et al. [23] in recoding each of these two sets of two items into two single items on appetite and weight change, resulting in a total of 14 items. Finally, note that all nonclinical depression complaints at baseline, including insomnia, are assessed using the IDS, see Table 1.
Table 1.
DSM-5 criteria of MDD (left two columns), corresponding IDS items (middle two columns), and included nonclinical complaint abbreviation (right)
| DSM-5 MDD criteria | IDS item | Abbreviated symptom | ||
|---|---|---|---|---|
| Criterion | Description | Item | Description | |
| A1 | Depressed mood | 5 | Feeling sad | dep |
| A2 | Loss of interest/pleasure | 19 | General interest | int |
| A3 | Weight/appetite change | 11 | Decreased appetite | app |
| 12 | Increased appetite | |||
| 13 | Decreased weight | wei | ||
| 14 | Increased weight | |||
| A4-a | Insomnia | 1 | Falling asleep | dis |
| 2 | Sleep during the night | dms | ||
| 3 | Waking up too early | ema | ||
| A4-b | Hypersomnia | 4 | Sleeping too much | hyp |
| A5-a | Psychomotor retardation | 23 | Feeling slowed down | ret |
| A5-b | Psychomotor agitation | 24 | Feeling restless | agi |
| A6 | Fatigue or loss of energy | 20 | Energy level | ene |
| A7 | Guilt/worthlessness | 16 | View of myself | gui |
| A8 | Concentration | 15 | Concentration/decision making | con |
| A9 | Suicidality | 18 | Thoughts of death or suicide | sui |
Statistical analyses
Cox proportional hazard analysis. Using a Cox proportional hazard models we first investigated the effect of baseline insomnia severity (IRS) on first-onset MDD. Second, we investigated whether the short-sleep duration has an additional effect. Third, as insomnia is defined by different nocturnal complaints that are covered by separate IRS items, we assessed whether specific insomnia symptoms are most predictive of first-onset MDD using models with the individual IRS items instead of the IRS summary score. Analyses were performed in R (version 3.5.0) using the package ‘‘survival’’.
Network outcome analysis. We subsequently addressed the possible confounding issue that insomnia commonly co-occurs with a vast variety of other complaints. Therefore, the predictive value of insomnia complaints about first-onset MDD could be nonspecific, indistinguishable from the predictive value of other complaints. Network modeling techniques provide a unique framework to study the interactions among symptoms and their role in the development and maintenance of psychiatric disorders [24]. Using network analysis we can estimate the unique association between pairs of symptoms, whereas controlling for the state and associations of all other symptoms [25, 26]. Accordingly, a connection (edge) between two symptoms (nodes) in the network structure, provides evidence for a direct rather than indirect association. We recently innovated the network analysis framework by demonstrating the possibility and use of including the presence or absence of an intervention [25]. Here, we employed a similar key innovation [26], which we propose to call Network Outcome Analysis, in which we include the outcome ‘‘first-onset MDD’’ during the 6-year follow-up as a variable in the network. Accordingly, NOA allowed us to distinguish symptoms that predict first-onset MDD directly from symptoms that do so indirectly through their association with directly related symptoms.
Estimation. For the NOA we estimated a Mixed Graphical Model [27] in which we included all baseline depression symptoms (Table 1) as continuous and the first-onset MDD outcome as binary (0: no first-onset MDD; 1: first-onset MDD; FO-MDD). In the network, the symptoms are presented as nodes (IDS items as circles, FO-MDD indicator as a square) that are connected by edges that encode the unique association among two variables after controlling for all other variables in the network. In estimating the networks, we used LASSO regularization to prevent the inclusion of spurious edges due to sampling variation [28], see Supplementary material, Section 2.1 for details. Finally, we assessed the accuracy of the estimated networks [29, 30], see Supplementary material, Section 2.2 for details. These analyses were performed in R (version 3.5.0) using the packages ‘‘qgraph’’, ‘‘bootnet’’, and ‘‘mgm’’.
interpretation. In the network, the edges represent the unique association between two variables, whereas controlling for all the other variables in the network (i.e. conditional dependence relationships). Any edge between a symptom and the FO-MDD indicator variable is thus indicative of the unique predictive value of that symptom, whereas controlling for all the other baseline depression symptoms. Because the baseline depression symptoms temporally precede the first-onset depression during follow-up, we know that the symptoms can lead to depression onset but not vice versa (i.e. depression onset later in time cannot affect symptom levels at baseline). An edge between a symptom and the first-onset depression outcome thus indicates that symptom directly and uniquely predicts first-onset depression, whereas controlling for the other symptoms.
The presence and strength of an edge are proportionate to regression coefficients [28]. At the same time, and unlike regression analysis, the network also models how the different symptoms predict one another. The network thus maps out the linear prediction and multicollinearity among all variables. Accordingly, it can provide insight into predictive mediation: the network might reveal variables that are only indirectly related through a third variable (e.g. A–B–C), which indicates that, even though A and C might be correlated, any predictive effect from A to C or vice versa is mediated by B [28]. The network model thereby provides a unique opportunity to investigate direct and indirect effects from the baseline depression symptoms to first-onset MDD.
evaluation. To evaluate the symptoms that were identified by NOA as direct predictors of first-onset MDD, we compared the area under the receiver operator characteristic (AUC) curve predicting first-onset depression using the sum score of these direct predictors versus the sum score of all predictors. This analysis was performed in R (version 3.5.0) using the package ‘‘pROC’’.
Results
Of the 768 participants that were initially without current or prior lifetime MDD, 141 (18.4%) were later diagnosed with first-onset MDD: 75 between T0 and T1, 46 between T1 and T2, and 20 between T2 and T3. Participants who developed MDD during the 6 year follow-up period were slightly, but nonsignificantly, younger (39.1 versus 41.5 years, p = .09) and were more likely to be female (marginally significant; 70.2% versus 61.0% female, p = .05), as compared with participants who did not develop MDD.
Cox proportional hazard analysis showed that the odds of first-onset MDD increased by 11% (HR = 1.11, 95% CI: 1.07–1.15) with every 1-point increase of the IRS score (observed range: 0–20 points). Thus, as compared to participants without insomnia complaints (IRS = 0), participants scoring at the insomnia cutoff of IRS = 9 were 2.6 times more likely to develop first-onset MDD, and participants with the most severe insomnia complaints (IRS = 20) were 8.1 times more likely. This result did not change appreciably when adding age, sex, and presence of any anxiety disorder diagnosis at baseline as covariates (HR = 1.10, 95% CI: 1.04–1.16). Including short-sleep duration in the model did not affect the risk for first-onset depression: the 22% that indicated to have slept 6 h or less over the past month did not have an increased risk (HR = 0.95, 95% CI: 0.35–2.63), also not in interaction with insomnia severity (HR = 0.99, 95% CI: 0.91–1.10).
Performing Cox proportional hazard analysis using the individual IRS items revealed that the predictive effect of insomnia on first-onset MDD was driven solely by the item “did you have trouble falling asleep” (HR = 1.33, 95% CI: 1.12–1.57; observed range: 0–4). As compared to those without difficulty initiating sleep (DIS) (item score 0), people experiencing trouble falling asleep 3–4 times or more than 4 times a week were respectively 2.3 times or 3.2 more likely to develop first-onset MDD. Importantly, none of the other sleep complaints—including nocturnal and early morning awakening—significantly increased the risk of first-onset MDD.
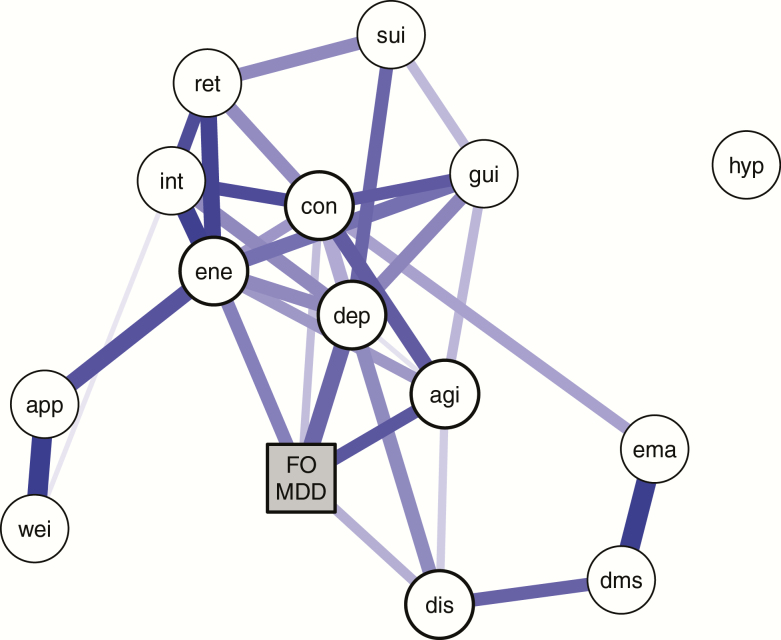
NOA identified five nonclinical complaints at baseline to directly predict first-onset MDD whereas controlling for the other complaints: energy level, concentration/decision making, feeling sad, feeling restless, and difficulty falling asleep. Predictive effects of other complaints were estimated to be indirect (see Figure 1). The results did not change when adding age, sex, and presence of any anxiety disorder diagnosis at baseline into the analyses, see Supplementary Material. The area under the receiver operating characteristic (AUC) curve revealed that the sum score of the five direct complaints captures all relevant information to predict first-onset MDD (AUC = 0.76, 95% CI: 0.71–0.81). Adding the other nine complaints did not improve the prediction accuracy (AUC = 0.75, 95% CI: 0.71–0.80).
Figure 1.
Regularized network showing the conditional dependence relations among the baseline depression symptoms (circles) and first-onset MDD (FO-MDD) during the 6 year follow-up period (yes or no; square). Edges represent conditional dependence relations among the variables and capture unique effects that remain after controlling for all the other variables in the network. The thickness and color saturation of the edges corresponds to the strength of the association. In this network, all associations are positive. The five symptom nodes that are directly predictive of first-onset MDD are indicated by thicker outlined circles. Abbreviations: agi = psychomotor agitation; app = appetite change; con = concentration problems; dep = depressed mood; dis = difficulty initiating sleep; dms = difficulty maintaining sleep; ene = fatigue or loss of energy; ema = early morning awakenings; FO-MDD = first-onset depression; gui = feelings of guilt or worthlessness; hyp = hypersomnia; int = loss of interest; ret = psychomotor retardation; sui = suicidal thoughts; wei = weight change.
Discussion
The ever increasing prevalence rate of MDD, already a major contributor to the global costs of disease today, stresses the utmost importance to identify modifiable risk factors. Although it has often been stated that insomnia is such a primary modifiable risk factor [7, 8], insomnia is also the most common residual symptom [9], and studies on risks have usually not assessed a prior diagnosis of MDD. Moreover, because insomnia often co-occurs secondary to other symptoms, insomnia might not necessarily be of primary importance. In the current study, we overcame these challenges by carefully selecting participants free from lifetime MDD and applying the novel NOA to identify primary risk factors amidst a large number of correlated predictors.
We could reveal that insomnia, and ‘‘DIS’’ in particular, is a primary and independent prospective risk factor for first-onset MDD. This predictive effect could not be attributed to an earlier depressive episode, nor to an indirect association via other depression symptoms. These results suggest that ‘‘DIS’’ might be a viable target for risk detection and prevention of MDD.
Although the relationship between insomnia and depression onset has been studied extensively [e.g. [7,8]], the current prospective study is the first to address both the possibility that insomnia complaints could represent a residual symptom from prior depression, as well as the issue of possible confounding through its connection to other baseline complaints. Another particular strength is that the gold standard and WHO-recommended CIDI was used to diagnose depression. A final strength of the current study is that we compared the prospective risk factor of insomnia to that of other depression symptoms.
The importance of our findings is stressed by the high prevalence of MDD [1], its increasing associated costs [31], and the pressing need to prevent first-onset MDD because of the high-recurrence rates [4, 6]. In order to prevent rather than treat MDD, this study meets the need to identify modifiable risk factors of first-onset depression. The identification of ‘‘DIS’’ as a risk factor is particularly promising, because a recent meta-analysis showed that cognitive-behavioral therapy, the treatment of choice for insomnia, is highly effective [32]. This intervention moreover improves mood [33–35] which we also identified as a direct risk factor. The current findings call for studies to evaluate whether treatment of ‘‘DIS’’ might prevent first-onset MDD.
We also evaluated whether short-sleep duration added to the risk to develop first-onset MDD. Contrary to suggestions that insomnia together with short sleep duration is the most severe phenotype, short sleep duration itself or in combination with insomnia did not increase the risk for developing first-onset MDD. Since we only used a self-reported measure of short-sleep duration, we cannot fully exclude the possibility that short-sleep duration adds to the risk of MDD. This finding does, however, align with earlier studies that showed that short-sleep duration was a weaker predictor of MDD than insomnia.
Another factor that might increase the prospective risk for depression is chronotype, as evening types are more prone to also suffer from complaints of both insomnia and depression [36, 37]. Unfortunately, chronotype was not assessed at baseline, and could therefore not be incorporated into the analysis. The assessment of chronotype in insomnia is however not without problems, not in the least because the calculation of the mid-sleep phase, which is the most common self-reported measure for chronotype, is intrinsically dependent on the subjectively experienced sleep onset, of which delays will be reported as difficulties initiating sleep [38]. Consequently, difficulties initiating sleep and chronotype will inevitably be correlated. Still, it is unlikely that the association of both a late chronotype and DIS with the risk of depression is merely due to confounding of these two predictors: in a study on 4948 adolescents, both evening type and insomnia were independently associated with an increased risk of having emotional problems [39]. Future prospective studies would ideally obtain different measures of chronotype [37, 40] to sort out the relative prospective risks conveyed by being an evening type and experiencing difficulties initiating sleep.
A notable finding is that particularly ‘‘DIS’’, and not the other sleep complaints difficulty maintaining sleep and early morning awakening, is predictive for first-onset MDD. This finding was robust across assessment formats and analyses. At least two earlier studies found ‘‘DIS’’ to be particularly predictive for depression [41, 42]. However, in these studies, lifetime history of depression was not assessed, so the possibility that sleep complaints represented residual symptoms of a prior depression could not be excluded. Moreover, none of these studies diagnosed depression using clinical interviews. We overcame these limitations and were able to confirm that the effect of DIS on first-onset MDD is robust.
The question may be posed why in particular DIS is directly predictive, whereas difficulty maintaining sleep and early morning awakening is only indirectly predictive. An intriguing possibility revealed by the novel NOA approach, is that DIS is the most direct indicator of underlying vulnerability. In this interpretation, it is not so much the waking up during the night or early in the morning that is bothersome, but rather the subsequent difficulty of getting back to sleep. Phrased differently, although insomnia undoubtedly involves abundant transitions from sleep to wake [43], the core experienced problem may rather be the difficulty of transitioning from wake to sleep, either at sleep onset or at any time later during the night. Interestingly, our results suggest that especially the presence of such difficulties at the onset of the night convey a vulnerability for first-onset MDD.
To conclude, we identified a robust direct contribution of DIS to the risk of first-onset MDD. The finding is of high clinical relevance, since this complaint can effectively be treated with cognitive-behavioral therapy [35]. Our identification of five primary predictive complaints will benefit the efficiency of studies on other preventive interventions, by defining the best outcome measures and allowing for selection of most vulnerable individuals. Treating difficulties initiating sleep could contribute significantly to combat the global costs of depression by means of prevention [6].
Supplementary Material
Author contributions
All authors contributed to the study design, critically reviewed the manuscript and approved the final manuscript. TFB and EJVWS drafted the manuscript. TFB conducted the analyses supervised by EJVWS and DB.
Funding
Support was provided by the European Research Council (ERC-ADG-2014-671084-INSOMNIA) and by a VU University Research Fellowship 2016-2017. The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht Program of the Netherlands Organisation for Health Research and Development (ZonMw, grant number 10-000-1002) and financial contributions by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Leiden University Medical Center, Leiden University, GGZ Rivierduinen, University Medical Center Groningen, University of Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Rob Giel Onderzoekscentrum).
Conflict of interest statement: None declared.
References
- 1. World Health Organization. 2018. Depression (www.who.int/en/news-room/fact-sheets/detail/depression). Accessed July 11, 2018.
- 2. Hidaka BH. Depression as a disease of modernity: explanations for increasing prevalence. J Affect Disord. 2012;140(3):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinberger AH, et al. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol Med. 2018;48(8):1308–1315. [DOI] [PubMed] [Google Scholar]
- 4. Burcusa SL, et al. Risk for recurrence in depression. Clin Psychol Rev. 2007;27(8):959–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ormel J, et al. Prevention of depression will only succeed when it is structurally embedded and targets big determinants. World Psychiatry. 2019;18(1):111–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuijpers P, et al. Preventing depression: a global priority. JAMA. 2012;307(10):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li L, et al. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. 2016;16(1):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baglioni C, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. [DOI] [PubMed] [Google Scholar]
- 9. McClintock SM, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31(2):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- 11. Boschloo L, et al. A prospective study on how symptoms in a network predict the onset of depression. Psychother Psychosom. 2016;85(3):183–184. [DOI] [PubMed] [Google Scholar]
- 12. Kouros CD, et al. Within-person changes in individual symptoms of depression predict subsequent depressive episodes in adolescents: a prospective study. J Abnorm Child Psychol. 2016;44(3):483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moazen-Zadeh E, et al. Depressive symptoms predict major depressive disorder after 15 years among whites but not blacks. Front Public Health. 2016;4:13. doi:10.3389/fpubh.2016.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts RE, et al. Sleep complaints and depression in an aging cohort: a prospective perspective. Am J Psychiatry. 2000;157(1):81–88. [DOI] [PubMed] [Google Scholar]
- 15. Penninx BW, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17(3):121–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Composite International Diagnostic Interview, Version 1.0. Geneva: World Health Organization; 1990. [Google Scholar]
- 17. Levine DW, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. [DOI] [PubMed] [Google Scholar]
- 18. Zhai L, et al. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress Anxiety. 2015;32(9):664–670. [DOI] [PubMed] [Google Scholar]
- 19. Vgontzas AN, et al. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep. 2012;35(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellis JG, et al. The natural history of insomnia: acute insomnia and first-onset depression. Sleep. 2014;37(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vgontzas AN, et al. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rush AJ, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. [DOI] [PubMed] [Google Scholar]
- 23. van Borkulo C, et al. Association of symptom network structure with the course of [corrected] depression. JAMA Psychiatry. 2015;72(12):1219–1226. [DOI] [PubMed] [Google Scholar]
- 24. Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blanken TF, et al. Introducing network intervention analysis to investigate sequential, symptom-specific treatment effects: a demonstration in co-occurring insomnia and depression. Psychother Psychosom. 2019;88(1):52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rouquette A, et al. Emotional and behavioral symptom network structure in elementary school girls and association with anxiety disorders and depression in adolescence and early adulthood: a network analysis. JAMA Psychiatry. 2018;75(11):1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haslbeck JM, et al. MGM: structure estimation for time-varying mixed graphical models in high-dimensional data. J Stat Softw. 2018; (in press). arXiv:1510.06871v5. [Google Scholar]
- 28. Epskamp S, et al. A tutorial on regularized partial correlation networks. Psychol Methods 2018;23(4):617–634.. doi:10.1037/met0000167 [DOI] [PubMed] [Google Scholar]
- 29. Borsboom D, et al. Robustness and replicability of psychopathology networks. World Psychiatry. 2018;17(2):143–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Epskamp S, et al. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods. 2018;50(1):195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greenberg PE, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162. [DOI] [PubMed] [Google Scholar]
- 32. van Straten A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3–16. doi:10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 33. Blom K, et al. Three-year follow-up comparing cognitive behavioral therapy for depression to cognitive behavioral therapy for insomnia, for patients with both diagnoses. Sleep. 2017;40(8). doi:10.1093/sleep/zsx108 [DOI] [PubMed] [Google Scholar]
- 34. Christensen H, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333–341. [DOI] [PubMed] [Google Scholar]
- 35. Manber R, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Merikanto I, et al. Relation of chronotype to sleep complaints in the general Finnish population. Chronobiol Int. 2012;29(3):311–317. [DOI] [PubMed] [Google Scholar]
- 37. Merikanto I, et al. Evening types are prone to depression. Chronobiol Int. 2013;30(5):719–725. [DOI] [PubMed] [Google Scholar]
- 38. Suh S, et al. Using mid-sleep time to determine chronotype in young adults with insomnia-related symptoms. Sleep Med Res. 2017;8(2):107–111. [Google Scholar]
- 39. Li SX, et al. Eveningness chronotype, insomnia symptoms, and emotional and behavioural problems in adolescents. Sleep Med. 2018;47:93–99. doi:10.1016/j.sleep.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 40. Van Someren EJ, et al. Improving melatonin circadian phase estimates. Sleep Med. 2007;8(6):590–601. [DOI] [PubMed] [Google Scholar]
- 41. Ikeda H, et al. The relationship between sleep disturbances and depression in daytime workers: a cross-sectional structured interview survey. Ind Health. 2017;55(5):455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yokoyama E, et al. Association between depression and insomnia subtypes: a longitudinal study on the elderly in Japan. Sleep. 2010;33(12):1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wei Y, et al. Sleep stage transition dynamics reveal specific stage 2 vulnerability in insomnia. Sleep. 2017;40(9). doi:10.1093/sleep/zsx117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.