Abstract
Study Objectives
The bases for sex disparities in obstructive sleep apnea (OSA), is poorly understood. We quantified the influences of event definitions, sleep-state, and body position on apnea–hypopnea indices (AHIs) in men and women, and evaluated sex differences in pathophysiological endotypes.
Methods
Polysomnography (PSG) data were analyzed from 2057 participants from the multi-ethnic study of atherosclerosis. Alternative AHIs were compared using various desaturation and arousal criteria. Endotypes (loop gain, airway collapsibility, arousal threshold) were derived using breath-by-breath analysis of PSG signals. Regression models estimated the extent to which endotypes explained sex differences in AHI.
Results
The sample (mean 68.5 ± 9.2 years) included 54% women. OSA (AHI4P ≥15/h, defined by events with ≥4% desaturations) was found in 41.1% men and 21.8% women. Compared to AHI4P, male/female AHI ratios decreased by 5%–10% when using 3%-desaturation and/or arousal criteria; p < 0.05. REM-OSA (REM-AHI ≥15/h) was similar in men and women regardless of event desaturation criteria. REM-AHI4P ≥15/h was observed in 57% of men and women each. In NREM, AHI4P in men was 2.49 (CI95: 2.25, 2.76) of that in women. Women demonstrated lower loop gain, less airway collapsibility, and lower arousal threshold in NREM (ps < 0.0005). Endotypes explained 30% of the relative sex differences in NREM-AHI4P.
Conclusions
There are significant sex differences in NREM-AHI levels and in physiological endotypes. Physiological endotypes explained a significant portion of the relative sex differences in NREM-AHI. Definitions that use 4%-desaturation criteria under-estimate AHI in women. Combining NREM and REM events obscures OSA prevalence in REM in women.
Keywords: OSA, OSA—clinical assessment, OSA—pathogenesis, sleep disordered breathing, sleep in women
Statement of Significance.
This is the first study to quantify the influences of desaturations, arousals, sleep-state, and position on apnea–hypopnea index (AHI) in men and women, and to evaluate sex differences in obstructive sleep apnea (OSA) physiological endotypes in a large, diverse population. Our results suggest that sex differences in OSA are influenced by state-specific mechanisms that are reflected by polysomnographic estimates of airway collapsibility, loop gain, and arousal threshold. Protective mechanisms during NREM sleep do not substantively protect women from airway collapse during REM sleep. The relatively high REM-AHI in women is of clinical significance given growing evidence of the association between REM-OSA and adverse cardiovascular outcomes. Since current guidelines do not directly address REM-AHI for treatment, our data suggest women may be disproportionately under-treated for OSA based on the total AHI, which predominantly reflects NREM-AHI.
Introduction
Obstructive sleep apnea (OSA) is a heterogeneous disorder influenced and manifested by varying clinical, polysomnographic, and mechanistic factors. Its prevalence is reported to be three to five times greater in men compared to women [1]. However, there likely are sex differences in polysomnographic phenotypes, and disease recognition may vary by how OSA is identified. Use of a global apnea–hypopnea index (AHI), which does not fully capture OSA-related physiological stresses, may contribute to the differential recognition of the disorder across groups, such as between sexes. Specifically, the AHI weighs all respiratory events similarly, regardless of duration, level of desaturation, state-specificity, or mechanism, and without consideration of potential differences in clinical impact [2].
Although polysomnography (PSG) characterizes numerous physiological parameters, most of this information is not routinely used for diagnosis, prognosis, or therapy. Metrics that describe position or stage-dependence [3], event-related stresses (such as hypoxic burden [4]), or endotypes (e.g. apnea duration [5], a marker of low arousal threshold), may better prognosticate health outcomes than the AHI, particularly in women. Recently validated methods are able to extract measures from PSG to quantify mechanistic indices such as arousal threshold (ArT), pharyngeal collapsibility (Vpassive), and loop gain (LG) [6–8]. At present, there has been limited investigation of whether these endotypes vary by sex.
This study offers a comprehensive and rigorous analysis of physiologic and polysomnographic difference in OSA by sex, by (1) analyzing the impact of different desaturation and arousal criteria on relative sex differences in AHI; (2) quantifying REM and positional dependencies in men and women; and (3) quantifying the extent to which sex differences in AHI values relate to sex differences in the mechanistic traits of LG, ArT, and upper airway collapsibility. Together, this information may help inform future approaches for improving sleep apnea diagnosis, prognosis and treatments in both women and men.
Methods
Participants
The multi-ethnic study of atherosclerosis (MESA) is a multi-site cohort of community-dwelling men and women aged 45–84 years initially enrolled between 2000 and 2002 [9]. Because the MESA study aimed to understand progression of atherosclerosis and cardiovascular disease, only men and women initially free of disease were included. The study also aimed to increase the representation of minorities and ensure close to equal numbers of men and women. As a result, the MESA cohort is generally healthier and more ethnically diverse than the age-matched US population. Participants were studied about every 1.5–5 years with in-clinic examinations to identify risk factors for cardiovascular disease [9]. The current analyses used data from the MESA Exam 5 and an associated MESA Sleep ancillary study. The research protocols were approved by the Institutional Review Boards at each participating institution, and all participants provided written informed consent. This study is registered with ClinicalTrials.gov (NCT00005487).
Polysomnography data
As described before [10], between 2010 and 2013, 2057 MESA participants underwent successful 15-channel PSG (Somté PSG, Compumedics Ltd., Abbotsford, AU). Respiratory measurements included thoracic and abdominal inductance plethysmography, and airflow via oral/nasal thermistor and nasal pressure transducer. See online data supplement for detailed PSG procedures. The main AHI derivations included AHI3P, AHI3PA, AHI4P, AHI4PA, AHI4P-REM, AHI4P-NREM, AHI4P supine, and AHI4P non-supine, reflecting requirements for each apnea and hypopnea to be linked with minimal desaturation criteria (noted by 3P or 4P) or arousal (noted by an “A”) (see Supplementary Table S1 for definitions).
Endotype measures from polysomnography
Advanced signal processing methods [6–8] which employ computer-automated (expert-supervised) breath-by-breath analysis of PSG signals from available data in NREM sleep were used to generate physiologic endotypes: LG, ArT, and Vpassive [11, 12]. Measurements were made using a minimum of three 7-min windows that each included at least one respiratory event. These measurements from non-invasive PSG provide indices that correlate with gold standard in-laboratory measurements [12, 13]. Additional detail on the method for making these measurements is provided in an online data supplement.
Statistical analysis
Student’s t-tests were used to test the unadjusted sex differences among various characteristics. Linear regression was used to estimate covariate-adjusted sex differences in PSG indices where covariates included age (years), race/ethnicity, BMI, waist circumference (cm), and smoking status (never, former, current).
AHI measures were skewed and analyzed using a log transformation. Therefore, the effect estimates of interest are given as the ratio of apneas plus hypopneas per hour in men compared to women. Under the null of no sex differences, the ratio of log-transformed AHI in men compared to women is one.
To evaluate the influence of varying AHI definitions on relative sex differences in AHI, we estimated the change in effect estimate as the ratio between the two effect estimates derived for models using alternative AHI definitions (e.g. (AHI4P in males/AHI4P in females) divided by (AHI3P in males/AHI3P in females)). Statistical significance was based on the confidence intervals for these ratios generated using 1000 bootstrap samples, where we fit all models for each bootstrap sample. The 95%-confidence intervals were based on Bootstrap percentiles [14]; a confidence interval that does not contain the null value corresponds to two-sided p-value less than 0.05.
To estimate the influence of endotypes on sex differences, we used splines to allow for a flexible, non-parametric model of the association between the endotype and log-AHI in males divided by log-AHI in females. Each endotype was modeled using cubic splines with four knots, selected automatically, using the SAS function glmselect (SAS 9.4 statistical software). Confidence intervals for the change in ratios for AHI across models with and without a given set of endotypes were calculated using a bootstrap method described above.
Results
Participant characteristics
Women comprised 54% of the sample. There were no differences between men and women in age or race/ethnicity. (Table 1) The majority of women were of post-menopausal age (i.e. ≥55 years). Women were more obese than men, more likely to have never smoked, and less likely to have cardiovascular disease.
Table 1.
Characteristics of men and women, MESA-sleep cohort
| Men (N = 954) | Women (N = 1103) | P | |
|---|---|---|---|
| Race/Ethnicity, n (%) | 0.68 | ||
| White, Caucasian | 346 (36.3) | 397 (36.0) | |
| Chinese-American | 123 (12.9) | 127 (11.5) | |
| Black, African-American | 256 (26.8) | 317 (28.7) | |
| Hispanic | 229 (24.0) | 262 (23.8) | |
| Age, years ±SD | 68.6 ± 9.2 | 68.4 ± 9.1 | 0.64 |
| Waist circumference, cm ±SD | 101.2 ± 12.6 | 97.8 ± 15.7 | <0.001 |
| BMI, kg/m2 ±SD | 28.2 ± 4.6 | 29.0 ± 6.2 | 0.0008 |
| Estrogens, yes n (%) | 0 (0) | 65 (5.9) | <0.001 |
| Currently employed, n (%) | 219 (23.1) | 264 (24.1) | 0.59 |
| Current smoking status, n (%) | <0.000 | ||
| Never smoker | 437 (46.6) | 681 (62.3) | 1 |
| Former smoker | 432 (46.1) | 347 (31.8) | |
| Current smoker | 69 (7.4) | 65 (6.0) | |
| Comorbidities | |||
| CVD | 116 (12.2%) | 100 (9.1%) | 0.03 |
| Heart failure | 31 (3.3%) | 29 (2.6%) | 0.41 |
| COPD | 16 (1.7%) | 18 (1.6%) | 0.18 |
| Diabetes | 201 (21.1%) | 204 (18.5%) | 0.16 |
| Hypertension | 525 (55.1%) | 641 (58.2%) | 0.16 |
CVD = cardiovascular disease (i.e. history of myocardial infarction, coronary artery disease, angina, and/or stroke); COPD = chronic obstructive pulmonary disease.
Polysomnographic indices significantly differed between sexes (Table 2). Women demonstrated features suggestive of better sleep quality such as longer total sleep time, higher sleep efficiency, and lower arousal index (in both REM and NREM sleep) (p < 0.05). Women also had less lighter sleep (stages N1 and N2), and more N3 and REM sleep compared to men (p < 0.01). Applying the 4%-desaturation threshold to define events (AHI4P), AHI4P was on average 40% lower in women than men (by an average of 7 events/h). However, this was driven by differences in AHI4P in NREM sleep (AHI4P in REM sleep was similar for men and women). Moreover, when defining OSA using an AHI4P threshold of 15 event/h, prevalence was 37.8% and 17.1% for men and women, respectively, during NREM sleep; and 57.4% and 56.6% in men and women during REM sleep. Similar sex differences were observed when defining AHI on the basis of all apneas (regardless of desaturation) and hypopneas with 4%-desaturation.
Table 2.
Polysomnographic features of men and women
| Men (N = 954) | Women (N = 1103) | P | |
|---|---|---|---|
| Total sleep time (TST)a, min | 340.5 ± 79.53 | 376.50 ± 81.59 | <0.0001 |
| Sleep efficiencya, % | 73.51 ± 13.90 | 77.66 ± 12.76 | <0.0001 |
| REM latencya, min | 102.1 ± 74.31 | 114.7±77.39 | 0.0002 |
| N1a, %TST | 17.16 ± 10.47 | 12.00 ± 7.15 | <0.0001 |
| N2a, %TST | 58.69 ± 10.36 | 56.59±10.08 | <0.0001 |
| N3a, %TST | 7.08 ± 7.45 | 12.58 ± 9.46 | <0.0001 |
| REMa, %TST | 17.08 ± 6.60 | 18.82 ± 6.67 | <0.0001 |
| Arousal Index (AI)a, events/h | 25.42 ± 13.22 | 19.65 ± 10.24 | <0.0001 |
| AI-REMa, events/h | 19.76 ± 12.25 | 15.95 ± 11.04 | <0.0001 |
| AI-NREMa, events/h | 26.40 ± 14.13 | 20.39 ± 10.98 | <0.0001 |
| AHI4Pa, events/h | 17.57 ± 18.03 | 10.43 ± 13.47 | <0.0001 |
| REM-AHI4Pa, events/h | 24.14 ± 21.68 | 22.72 ± 22.53 | 0.15 |
| NREM-AHI4Pa, events/h | 16.75 ± 18.52 | 8.20 ± 13.02 | <0.0001 |
| AHI4P ≥ 15, n (%) | 392 (41.1%) | 240 (21.8%) | <0.0001 |
| REM-AHI4P ≥ 15, n (%) | 499 (57.4%) | 539 (56.6%) | 0.7504 |
| NREM-AHI4P ≥ 15, n (%) | 346 (37.8%) | 171 (17.1%) | <0.0001 |
| mAHI4Pa | 19.12 ± 18.76 | 11.12 ± 13.65 | <0.0001 |
| Overall ratio of obstructive apneas to hypopneasa | 0.70 ± 6.29 | 0.32 ± 2.29 | 0.08 |
| NREM ratio of obstructive apneas to hypopneasa | 0.75 ± 5.64 | 0.28 ± 2.24 | 0.0165 |
| REM ratio of obstructive apneas to hypopneasa | 0.54 ± 1.95 | 0.42 ± 1.71 | 0.1633 |
TST = total sleep time; AI = arousal index; AHI4P = apnea hypopnea index, all apneas and hypopneas, each associated with more than 4% desaturation; mAHI4P = medicare AHI, defined as all apneas (regardless of desaturation) plus hypopneas associated with more than 4% desaturation; ratio of obstructive apneas to hypopneas = all obstructive apneas divided by hypopneas with at least 3% desaturation.
aContinuous variables, mean ± SD, respectively.
Sex differences in apnea–hypopnea ratio
The ratio of apneas to hypopneas is correlated with airway collapsibility as measured by Pcrit [15], a surrogate of airway collapsibility. Men had a significantly higher proportion of obstructive apneas compared to hypopneas in NREM, with smaller and non-significant differences in REM sleep. (Table 2)
Sex differences in AHI by varying desaturations, arousals, sleep-state, and body position
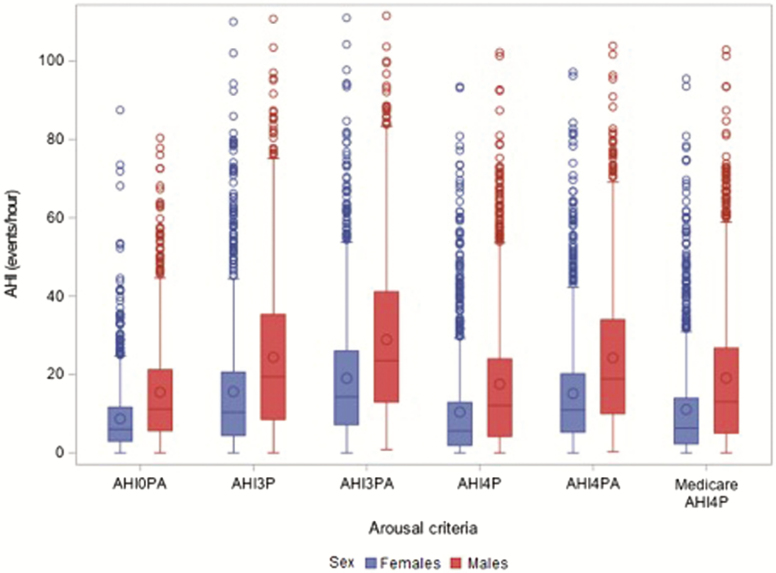
Figure 1 shows the unadjusted values for AHI in men and women as desaturation and arousal criteria for events vary. As expected, in both sexes, AHI levels increased as desaturation criteria decreased, and across desaturation criteria, increased with inclusion of arousal as an alternative qualifying metric.
Figure 1.
Variation in AHI by event definition in men and women. Shown are box whisper plots showing the upper and lower quartiles and median values for AHI0PA, AHI3P, AHI3PA, AHI4P, AHI4PA, and mAHI4P for women (blue) and men (red).
In adjusted models, the AHI4P in men was estimated to be 1.92 of that in women (Table 3). The inclusion of 3%-desaturations and arousals for respiratory events led to significantly smaller male–female differences in AHI compared to AHI4P. When including arousals (AHI4PA), AHI in men was estimated to be 1.81 that of women. A similar decrease in the sex difference in AHI was observed when considering a 3%-desaturation requirement regardless of arousals (1.82); and was further reduced to 1.72 with use of 3%-desaturation or arousal to capture events. As an alternative way of considering the impact of including events with a at least 3% or arousal to a definition that only included events with a at least 4% desaturation, estimated average AHI increased by 83.7% (from 10.4 to 19.1) in women but only by 64.2% (from 17.6 to 28.9) in men.
Table 3.
Variation in male/female ratio (95% CI) for alternative AHI definitions, adjusted for age, BMI, waist circumference, and smoking status
| AHI definitions: varying both apnea and hypopnea definitions, or position | M/F log AHI ratio; 95% CI |
|---|---|
| AHI4P | 1.92 (1.75, 2.10) |
| AHI4P-non-supine | 1.67 (1.51, 1.85) |
| AHI4P-supine | 1.99 (1.81, 2.19) |
| AHI4P-NREM | 2.49 (2.25, 2.76) |
| AHI4P-REM | 1.14 (1.04, 1.24) |
| AHI4PA | 1.81 (1.70, 1.93) |
| AHI3P | 1.82 (1.69, 1.96) |
| AHI3PA | 1.72 (1.62, 1.82) |
See online Data Supplement for full definitions of each AHI.
AHI4P = AHI that includes apneas and hypopneas associated with at least 4% oxygen desaturation.
AHI4P-non-supine; AHI4P-supine; AHI4P-NREM; AHI4P-REM = AHI that includes apneas and hypopneas associated with at least 4% oxygen desaturation during non-supine, supine, NREM, and REM sleep respectively.
AHI4PA = AHI that includes apneas and hypopneas associated with at least 4% oxygen desaturation or with an arousal.
AHI3P = AHI that includes apneas and hypopneas associated with at least 3% oxygen desaturation.
AHI3PA = AHI that includes apneas and hypopneas associated with at least 3% oxygen desaturation or with an arousal. Statistical assessments of change in the M/F ratios comparing different definitions were based on bootstrap analyses of the change in effect estimates of each M/F ratio: AHI3PA/AHI4P: 0.90 (95% CI: 0.85, 0.95); AHI3P/AHI3PA: 0.95 (0.91, 0.99); AHI3P/AHI4P: 0.95 (0.91, 0.99); AHI4P/AHI4PA: 1.06 (1.00, 1.12); AHI4P Supine/AHI4P non-supine: 1.19 (1.06, 1.32).
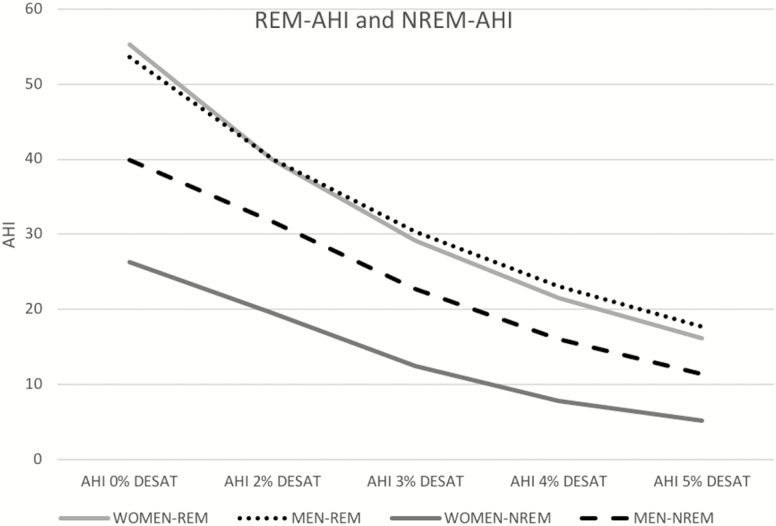
In NREM and REM sleep, the ratio of AHI4P in men compared to women was estimated to be 2.49 and 1.14, respectively (Table 3). Figure 2 further illustrates differences in AHI when varying desaturation criteria for identifying events for NREM and REM sleep separately, showing similar AHI levels in REM sleep in men and women for any AHI definition, but consistently higher AHI levels in men during NREM sleep.
Figure 2.
Displays how AHI varies as desaturation criteria for events change from 0% to 5% in REM and NREM sleep. For all desaturation criteria, AHI is higher in NREM in men than women. However, in REM sleep, mean AHI values are similar regardless of AHI definition (differences further quantified later).
In the non-supine position, AHI4P was 1.67 in men compared to women (Table 3). In the supine position the male/female ratio was significantly higher at 1.99.
Similar patterns were observed when the AHI was defined on the basis of all apneas (regardless of desaturation or arousal), and only varying hypopnea definitions (see mAHI variables; Supplementary Table S2).
Sex differences in OSA physiological endotypes
Table 4 shows the unadjusted differences in three key physiological endotypes derived in NREM sleep. Men had significantly greater LG, ArT, and upper airway collapsibility (i.e. lower Vpassive).
Table 4.
Sex differences in physiological endotypes during NREM sleep
| Men Mean (SD) | Women Mean (SD) | Difference Mean (SD) | P | |
|---|---|---|---|---|
| Loop gain (sensitivity) | N = 910 0.59 (0.17) | N = 1038 0.56 (0.17) | 0.03 (0.17) | 0.0002 |
| Arousal threshold, % eupnea | N = 901 125.8 (29.63) | N = 1030 114.0 (19.34) | 11.84 (24.68) | <0.0001 |
| Collapsibility (Vpassive), % eupnea | N = 916 90.95 (14.92) | N = 1052 95.77 (7.21) | −4.82 (11.46) | <0.0001 |
Loop gain (sensitivity of the ventilatory control system) is the magnitude of the ventilatory drive increase (e.g. overshoot) observed in response to a reduction in ventilation (e.g. apnea or hypopnea). The Arousal Threshold is the median calculated ventilatory drive that occurs immediately preceding scored EEG arousals from sleep, expressed as percentage of ventilatory drive at eupnea. Collapsibility (Vpassive) is the median level of ventilation during normal ventilatory drive, expressed as percentage of eupneic ventilation, where lower Vpassive represents greater collapsibility.
Consideration of individual physiological endotypes significantly narrowed the sex differences for each AHI measure (bootstrap p-values for change in sex effect on AHI when considering each endotype compared to baseline models, all <0.05), with the greatest change attributed to ArT (Table 5 and Supplementary Table S3). Together, LG and collapsibility (i.e. complementary physiological risk factors of ventilatory control and anatomy; Model 5), explained approximately 21% of the relative sex difference in AHI4P, 20% in AHI4P in non-supine position, 16% in supine position, 24% in NREM sleep, and eliminated any evidence of excess AHI4P in REM sleep. In NREM, these phenotypes explained up to 30% of the relative sex differences in AHI4P. Similar changes in relative sex differences for AHI3P were observed after accounting for physiological endotypes.
Table 5.
Men/women ratio (95% confidence intervals) of log AHI4P, accounting for endotypes
| AHI definition | Baseline modela | Model 2 Baseline + Loop gain | Model 3 Baseline + Vpassive | Model 4 Baseline + arousal threshold | Model 5 Baseline + loop gain + Vpassive |
|---|---|---|---|---|---|
| AHI4P | 1.92 (1.75, 2.10) | 1.74 (1.59, 1.91) | 1.59 (1.45, 1.74) | 1.41 (1.29, 1.54) | 1.52 (1.39, 1.67) |
| AHI4P-non-supine | 1.67 (1.51, 1.85) | 1.51 (1.36, 1.68) | 1.39 (1.26, 1.55) | 1.30 (1.17, 1.45) | 1.34 (1.21, 1.49) |
| AHI4P-supine | 1.99 (1.81, 2.19) | 1.86 (1.68, 2.04) | 1.73 (1.57, 1.90) | 1.57 (1.43, 1.72) | 1.68 (1.52, 1.85) |
| AHI4P-NREM | 2.49 (2.25, 2.76) | 2.22 (2.01, 2.47) | 1.99 (1.80, 2.20) | 1.72 (1.57, 1.89) | 1.88 (1.70, 2.08) |
| AHI4P-REM | 1.14 (1.04, 1.24) | 1.08 (0.99, 1.19) | 1.03 (0.94, 1.13) | 0.97 (0.88, 1.07) | 1.02 (0.92, 1.11) |
See Data Supplement for full definitions of each AHI.
aAHI measures were log transformed and the baseline and each model were adjusted for age, BMI, waist circumference, and smoking status. Models 2–5 also include adjustment for the physiological phenotypes. Each value represents the ratio of the log-transformed AHI in men/women and 95% confidence interval for this ratio. For any given AHI definition (column 1) adjusting for any endotype(s) (models 2–5) significantly decreased the M/F ratio. See Supplementary Table S3 for the effect estimates and 95% confidence intervals comparing pairs of models, providing evaluation of the statistical difference between models.
Discussion
This is the first study to quantify the influences of variations in desaturations, arousals, sleep-state, and body position on AHI levels in men and women as well as to evaluate sex differences in OSA physiological endotypes in a large, diverse community population. Our findings suggest that: (1) There are significant sex differences in AHI values across a broad range of definitions in NREM sleep in both supine and non-supine sleep. Incorporating more liberal desaturation criteria or arousals narrowed the differences in AHI between sexes, suggesting stricter desaturation criteria contribute to under-estimation of OSA in women compared to men; (2) Large sex differences for AHI were observed in NREM but not REM sleep and were partially explained by sex differences in mechanistic endotypes related to Vpassive, LG, and ArT, suggesting that neuromuscular compensatory mechanisms, rather than simple anatomy (which would manifest in all sleep states), distinguish propensity for OSA in men; (3) The marked sex differences in AHI in NREM but not REM sleep support the likelihood of fundamentally different mechanisms that underlie OSA during NREM and REM sleep. The mechanisms that provide compensation in women during NREM sleep do not substantively protect women from airway collapse during REM. These findings underscore the need to consider state-specific measures of OSA as well as sex differences when disentangling the heterogeneity of this complex disorder.
Changing event definitions
We investigated the impact of different event definitions on relative AHI levels in men and women. Scoring guidelines that require 4%-desaturation for event recognition are criticized because of potential under-estimation of events that result in less desaturation or result in arousal without desaturation. Historically, the latter events were labeled “respiratory effort related arousals,” which is reported to be more common in women than men [16]. Although recent AASM guidelines allow hypopneas to be scored on the basis of a 3% desaturation or an arousal, there is considerable controversy over optimal definitions. Currently, U.S. Medicare recognizes only the AHI4P definition. We found that the AHI3PA compared to the AHI4P definition resulted in a larger relative increase in women than men. Thus, defining respiratory events using lower saturation levels and including arousals will increase the relative proportion of women classified with OSA. It is possible that a higher impact on changing definition would be observed in a younger population.
Supine and non-supine AHI
Supine versus non-supine positioning resulted in relatively higher AHI levels in men compared to women. This suggests that men have less neuromuscular compensation when the airway undergoes narrowing due to gravitational and positional influences.
State-specific sex differences
The finding of state-specific sex differences in AHI are consistent with clinic-based studies demonstrating a propensity for REM-OSA in women [17]. O’Connor’s study looked at REM:NREM-AHI ratio more than 2 to define REM-OSA, and similarly concluded that OSA is less severe in women because of milder OSA during NREM sleep [17]. Building on this, we also found that while NREM-AHI levels were approximately twofold higher in men than women, OSA prevalence and AHI severity during REM sleep were strikingly similar between sexes, regardless of how AHI was defined. Furthermore, our study showed the ratio of obstructive apneas to hypopneas (a measure of airway collapsibility) was higher in men only during NREM sleep, and comparable between sexes in REM sleep. Although there is debate whether REM-OSA is a unique clinical entity, the observed sex differences in AHI in NREM but not in REM sleep suggest that mechanisms for OSA differ by sleep-state. A recent genome-wide association study identified genetic variants for OSA when state-specific and sex-specific traits were examined, suggesting that molecular mechanisms for OSA differ by both state and sex [18]. Mechanisms for REM-OSA include cholinergic-mediated inhibition of the hypoglossal neural output resulting in suppression of genioglossus muscle tone and increased propensity for upper airway collapse [19]. Chemosensitivity likely plays a lesser role in stabilizing breathing in REM compared to NREM sleep. Our results highlight the importance of state-dependent mechanisms for OSA, with a likely shared mechanism between men and women during REM sleep.
The relatively high REM-AHI in women is of clinical significance given growing evidence that REM-OSA is independently associated with adverse outcomes, including prevalent and incident hypertension [20], carotid intimal thickening [21], and cardiovascular disease [22]. Although current guidelines do not directly address the REM-AHI level for treating OSA, our data suggest that women may be disproportionately under-treated for OSA based on the total AHI, which predominantly reflects NREM-AHI. Under-treatment of OSA may therefore contribute to cardiovascular disease in women.
Contribution of endotypes in NREM sleep
Each endotype differed significantly between the sexes and explained 11% (LG), 20% (Vpassive) and 30% (ArT) of the sex ratio of age, obesity, and smoking-adjusted AHI-NREM levels. It is likely other factors such as dilator sensory-motor function and more specific aspects of airway structure and function vary by sex and influence AHI. However, a 15%–30% difference in AHI is important, and is comparable to the effect of a 5%–10% weight reduction [23].
The higher LG and greater airway collapsibility in NREM sleep observed in men is consistent with small laboratory studies using invasive measurements and imaging that showed greater ventilatory instability and anatomic risk for OSA in men [24, 25]. We also found that women had a lower ArT, and differences in ArT explained a significant proportion of the lower NREM-AHI in women. The role of arousals in OSA pathogenesis is complex. In certain individuals, arousals may promote sleep apnea propagation by causing ventilatory overshoot and unstable breathing in those predisposed (e.g. with high LG) [26]. Arousals may also prevent ventilatory-drive related accumulation of pharyngeal dilator muscle activity that could improve pharyngeal patency in some individuals [27]. However, a low ArT has been associated with lower AHI and more hypopneas relative to apneas [28], the phenotype we observed in women. A protective role of a low ArT may be attributed to more effective post-arousal airway muscle tone that counters the destabilizing influence of a large ventilatory overshoot [25, 29]. A lower ArT may also favor partial airway obstruction and prevent significant oxygen desaturation [30]. A higher ArT, observed in men with more severe OSA, may also reflect a response to chronic sleep disruption and hypoxemia [31]. Thus, the role of arousals in the pathogenesis of OSA is complex, and there are likely other features of arousals that impact AHI, such as arousal intensity [32], post-arousal ventilatory overshoot [25], and timing of arousal in relation to obstructive events [33], which may vary between men and women.
Strengths and limitations
There are several strengths to the study. We studied a large community-based ethnically diverse sample, allowing for generalizability. Participants underwent standardized PSG scored using rigorous protocols by research technologists blinded to other data. We employed a novel method for non-invasively characterizing endotypic traits to measure mechanistic pathways for upper airway obstruction. Use of a flexible data system allowed us to explore AHI levels across different desaturation and arousal criteria. Rigorous statistical methods for quantifying sex differences were used that accounted for the distributional characteristics of our outcome variables.
There are some study limitations. Participants were older and therefore sex differences in premenopausal women are not addressed. Mechanistic endotypes were estimated from PSG using transformed nasal pressure signals as a substitute for gold standard measures of ventilation; the method assumes that ventilatory drive is equal to observed ventilation for all breaths that are not part of an obstructive event. Despite these assumptions, non-invasive estimates of these traits have been shown to correlate strongly with more invasive methods [6]. Endotypic polysomnographic measures were derived in OSA patients using an AHI definition with 3% desaturation or arousal, and therefore, the accuracy and applicability of these measures to an AHI standard or to a population different from which these measures were derived need to be considered [4, 6, 7]. Finally, traits likely vary with sleep state, body position, and within a night, and future studies are needed to determine how best to summarize polysomnographic results from multiple states and positions across the night.
Conclusion and clinical implications
There are several clinically significant implications of this study. First, definitions that use a 3% or arousal event definition and that highlight REM-AHI levels may be particularly helpful in identifying OSA in women. Second, new treatments may need to target different mechanisms in REM versus NREM sleep and suggest the possibility that women and men will respond to some treatments differently. Considering these sex differences is critical when enrolling participants in new trials testing pharmacological and other advanced treatments of OSA, and sex-specific analyses likely will be needed. Third, we estimate that approximately 10%–30% of the sex differences in AHI levels in NREM could be explained by our mechanistic traits. Targeting these mechanisms may provide clinically significant improvements in NREM-AHI. Finally, our study begins to lay a foundation for sleep precision medicine, highlighting the roles of state-specific and sex-specific differences as an initial focus for better addressing the heterogeneity of OSA.
Supplementary Material
Acknowledgments
The authors thank the investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding
This research was supported by National Institutes of Health [grants R35HL135818, R01HL098433]; and by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
Conflict of interest statement: C.W. reports consulting and personal fees from Jazz Pharmaceuticals and EBSCO Health Industries, outside of the submitted work. A.A. serves as consultant for Somnifix, outside of the submitted work. D.W. reports employment at Philips Respironics, and consulting or personal fees from Animed and Night Balance, outside of the submitted work. A.W. reports personal fees from Nox, Bayer, Somnifix, Apnimed, Galvani, Cambridge Sound, outside of the submitted work. S.R. received grant and consulting support from Jazz Pharmaceuticals outside of the submitted work. M.R., S.S., and T.S. have no conflicts of interest to report.
References
- 1. Redline S, et al. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149(3 Pt 1):722–726. [DOI] [PubMed] [Google Scholar]
- 2. Muraja-Murro A, et al. Adjustment of apnea–hypopnea index with severity of obstruction events enhances detection of sleep apnea patients with the highest risk of severe health consequences. Sleep Breath. 2014;18(3):641–647. [DOI] [PubMed] [Google Scholar]
- 3. Acosta-Castro P, et al. REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohort. Eur Respir J. 2018;52(2):1702484. [DOI] [PubMed] [Google Scholar]
- 4. Azarbarzin A, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the osteoporotic fractures in men study and the sleep heart health study. Eur Heart J. 2019;40(14):1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butler MP, et al. Apnea–hypopnea event duration predicts mortality in men and women in the sleep heart health study. Am J Respir Crit Care Med. 2019;199(7):903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sands SA, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(9):1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sands SA, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep. 2018;41(1). doi:10.1093/sleep/zsx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landry SA, et al. Ventilatory control sensitivity in patients with obstructive sleep apnea is sleep stage dependent. Sleep. 2018;41(5). doi:10.1093/sleep/zsy040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bild DE, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 10. Chen X, et al. Racial/Ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azarbarzin A, et al. Estimation of pharyngeal collapsibility during sleep by peak inspiratory airflow. Sleep. 2017;40(1). doi:10.1093/sleep/zsw005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terrill PI, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45(2):408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wellman A, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985). 2013;114(7):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Efron B, et al. An Introduction to the Bootstrap. Chapter 13: Confidence Intervals Based on Bootstrap Percentiles. Chapman & Hall/CRC: Taylor and Francis; 1994. [Google Scholar]
- 15. Gleadhill IC, et al. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143(6):1300–1303. [DOI] [PubMed] [Google Scholar]
- 16. Stoohs RA, et al. Differences in clinical features of upper airway resistance syndrome, primary snoring, and obstructive sleep apnea/hypopnea syndrome. Sleep Med. 2008;9(2):121–128. [DOI] [PubMed] [Google Scholar]
- 17. O’Connor C, et al. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1465–1472. [DOI] [PubMed] [Google Scholar]
- 18. Chen H, et al. Multiethnic meta-analysis identifies RAI1 as a possible obstructive sleep apnea-related quantitative trait locus in men. Am J Respir Cell Mol Biol. 2018;58(3):391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McSharry DG, et al. Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep. 2014;37(3):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mokhlesi B, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ljunggren M, Lindberg E, Franklin KA, et al. Obstructive sleep apnea during rapid eye movement sleep is associated with early signs of atherosclerosis in women. Sleep. 2018;41(7). doi:10.1093/sleep/zsy099 [DOI] [PubMed] [Google Scholar]
- 22. Aurora RN, et al. Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med. 2018;197(5):653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peppard PE, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. [DOI] [PubMed] [Google Scholar]
- 24. Jordan AS, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol (1985). 2005;99(5):2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jordan AS, et al. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168(12):1512–1519. [DOI] [PubMed] [Google Scholar]
- 26. Jordan AS, et al. Physiology of arousal in obstructive sleep apnea and potential impacts for sedative treatment. Am J Respir Crit Care Med. 2017;196(7):814–821. [DOI] [PubMed] [Google Scholar]
- 27. Younes M, et al. Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J Appl Physiol (1985). 2012;112(2):249–258. [DOI] [PubMed] [Google Scholar]
- 28. Azarbarzin A, et al. Relationship between arousal intensity and heart rate response to arousal. Sleep. 2014;37(4):645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jordan AS, et al. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184(10):1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guilleminault C, et al. Upper airway sleep-disordered breathing in women. Ann Intern Med. 1995;122(7):493–501. [DOI] [PubMed] [Google Scholar]
- 31. Eckert DJ, et al. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985). 2014;116(3):302–313. [DOI] [PubMed] [Google Scholar]
- 32. Amatoury J, et al. Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep. 2016;39(12):2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169(5):623–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.