Abstract
Study Objectives
This study evaluated differences in upper airway, soft tissues and craniofacial structures between Asians from China and Europeans from Iceland with OSA using three-dimensional magnetic resonance imaging (MRI).
Methods
Airway sizes, soft tissue volumes, and craniofacial dimensions were compared between Icelandic (N = 108) and Chinese (N = 57) patients with oxygen desaturation index (ODI) ≥ 10 events/h matched for age, gender, and ODI. Mixed effects models adjusting for height or BMI and residual differences in age and ODI were utilized.
Results
In our matched sample, compared to Icelandic OSA patients, Chinese patients had smaller BMI (p < 0.0001) and neck circumference (p = 0.011). In covariate adjusted analyses, Chinese showed smaller retropalatal airway size (p ≤ 0.002), and smaller combined soft tissues, tongue, fat pads, and pterygoid (all p ≤ 0.0001), but male Chinese demonstrated a larger soft palate volume (p ≤ 0.001). For craniofacial dimensions, Chinese demonstrated bigger ANB angle (p ≤ 0.0196), differently shaped mandibles, including shorter corpus length (p < 0.0001) but longer ramus length (p < 0.0001), and a wider (p < 0.0001) and shallower (p ≤ 0.0001) maxilla.
Conclusions
Compared to Icelandic patients of similar age, gender and ODI, Chinese patients had smaller retropalatal airway and combined soft tissue, but bigger soft palate volume (in males), and differently shaped mandible and maxilla with more bony restrictions. Results support an ethnic difference in upper airway anatomy related to OSA, which may inform targeted therapies.
Keywords: obstructive sleep apnea, ethnicity, upper airway structure, three-dimensional magnetic resonance imaging
Statement of Significance.
This study used three-dimensional magnetic resonance imaging to compare upper airway anatomic risk factors for obstructive sleep apnea between Asians from China and Europeans from Iceland with similar age, gender, and disease severity. The results indicate that Icelandic patients have larger combined soft tissue volume, while Chinese patients have larger soft palate volume (in males), as well as smaller retropalatal airway areas and more restricted mandibular and maxillary bone structures. These results confirm and extend evidence of ethnic differences in the upper airway anatomy related to obstructive sleep apnea, and may help to inform targeted treatment approaches.
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by repeated episodes of airway collapse during sleep. Obesity, enlarged upper airway soft tissues, craniofacial abnormalities and their interactions play key roles in anatomical risk for OSA [1–3]. Using three-dimensional magnetic resonance imaging (MRI), upper airway soft tissue volumes have been shown to be larger in patients with OSA [1, 4], which may reduce airway caliber, increasing the propensity for airway collapse during sleep. In addition, alterations in the craniofacial skeleton have been implicated as risk factors for OSA. Previous cephalometric studies showed that individuals with OSA have smaller, retroposed mandibles (retrognathia), an inferiorly positioned hyoid bone, narrow posterior airway spaces, and retroposition of the maxilla [5–7]. Based on three-dimensional MRI, studies found that smaller mandibular length and depth in men and greater hyoid-to-nasion and supramentale-to-hyoid distances in both men and women were associated with increased risk of OSA [7].
Ethnicity influences OSA risk factors. Although Chinese patients are less obese than Caucasians, the prevalence of OSA is similar in the two populations [8]. A study using two-dimensional cephalometry concluded that Chinese patients had more craniofacial bony restriction and Caucasians were more obese when matched on the apnea–hypopnea index (AHI) [9]. This suggests distinct, ethnic-specific OSA etiologies in the two groups.
Through three-dimensional MRI, quantitative comparisons of upper airway anatomy can be performed [1, 7]. However, there are a limited number of interethnic studies simultaneously evaluating airway, soft tissues, and craniofacial structures in OSA patients. Thus, the present study examined differences in upper airway anatomy between Asian patients with OSA from China and European patients with OSA from Iceland with the same age, gender, and OSA severity. We hypothesized that Chinese would have smaller airway sizes, smaller soft tissue volumes and greater craniofacial restriction compared to Icelandic patients. Our findings elucidate the distinct anatomic factors between OSA patients in the two ethnic groups and provide further understanding of possible ethnic-specific pathogenesis and treatment of OSA.
Methods
See Supplementary material for details.
Study subjects
Analyses were performed in Icelandic and Chinese patients from clinical sleep centers in each country with data on age, gender, BMI and oxygen desaturation index (ODI). All subjects studied had ODI ≥10 events/h. The Institutional Review Boards at Landspitali University Hospital, Iceland, and Shanxi Tongcoal General Hospital, China, approved the project. Written informed consent was obtained from all participants.
Icelandic patients were from the Icelandic Sleep Apnea Cohort (ISAC); described elsewhere [10–12]. A total of 616 Icelandic patients with ODI ≥10 events/h and available phenotype data were eligible for inclusion. Chinese patients with symptoms suggestive of OSA were recruited from the sleep center in Shanxi Tongcoal General Hospital, China. A total of 103 Chinese patients were recruited, of whom 72 had an ODI ≥10 events/h and available phenotype data for inclusion.
Sleep studies
All patients from Iceland were diagnosed with a portable monitor, as previously described [10–12]. Scoring was started 30 min into the recording and ended 5 min before the study recording was completed to avoid issues related to potential periods of wakefulness. In Chinese patients, in-laboratory polysomnography (PSG) was performed according to the recommendation of the American Academy of Sleep Medicine [13]. To obtain an equivalent ODI to that from portable monitoring in Iceland, ODI from the PSG was recalculated as the number of oxygen desaturations at least 4% per hour of total analysis time, defined using the same approach as on portable monitors in Iceland (e.g. starting 30 min after lights-off and ending 5 min before lights-on).
Magnetic resonance imaging
Three-dimensional upper airway MRI was performed in both samples using the same protocol, as described previously [1, 7, 14, 15]. MRI analysis to quantify upper airway (Figure S1), volumetric (Figure S2), and craniofacial (Figures S3–S9) measurements was performed at the University of Pennsylvania, as previously described [1, 7, 14, 15].
Statistical analysis
To control for covariate differences, Icelandic and Chinese patients were matched with respect to age (±2 years), gender, and ODI (±5 events/h) using SAS 9.4 (SAS Institute, Cary, NC) [16, 17]. Given the larger pool of Icelandic patients, to increase statistical power we allowed up to a 2:1 Icelandic:Chinese patient matching; Chinese patients with only 1 available match in Iceland were retained as 1:1 matched pairs (see details on statistical power and precision in Online Supplement). Continuous and categorical data were compared between Icelandic and Chinese participants using linear or logistic mixed models, respectively, controlling for matched set as a random effect. Primary analyses were adjusted for residual differences in age and ODI after matching, and height. To evaluate the effect of obesity, the secondary analyses were adjusted for age, ODI, and BMI. To control for multiple MRI measurements being compared, while recognizing that different relationships can be expected for specific groups of MRI parameters, we defined sub-domains of airway, soft tissue and craniofacial measures and utilize domain-specific Bonferroni corrections, as in prior studies [15, 18]. To evaluate the influence of gender on ethnic differences, we tested for a gender-by-ethnicity interaction; any interaction with p < 0.10 was considered suggestive and differences between Icelandic and Chinese patients were then assessed separately within males and females.
Results
Patient demographics
One-hundred sixty-five patients with OSA (108 Icelandic, 57 Chinese) were included in the age, gender, and ODI matched analysis set (51 Icelandic:Chinese pairs matched 2:1 and 6 pairs matched 1:1). Given matching, there were no differences in age (p = 0.941), gender (p = 0.989) or ODI (p = 0.684) between Icelandic and Chinese patients (Table 1); participants were on average middle-aged with moderate to severe OSA and a majority were male. Icelandic patients were more obese than Chinese based on BMI (p < 0.0001) and neck circumference (p = 0.011), taller (p < 0.0001), and heavier (p < 0.0001).
Table 1.
Patient demographics matched for age, sex, and ODI
| Measure* | Icelandic | Chinese | p |
|---|---|---|---|
| N † | 108 | 57 | — |
| Age (years) | 50.85 ± 8.77 | 50.35 ± 8.83 | 0.9406 |
| Male, N (%) | 84 (77.78) | 44 (77.19) | 0.9888 |
| Height (cm) | 177.1 ± 8.62 | 168.5 ± 6.65 | <0.0001 |
| Weight (kg) | 103.0 ± 17.09 | 78.29 ± 10.28 | <0.0001 |
| BMI (kg/m2) | 32.81 ± 4.83 | 27.71 ± 3.50 | <0.0001 |
| Neck circumference (cm) | 42.48 ± 3.84 | 41.18 ± 2.87 | 0.0107 |
| ODI (events/h) | 37.45 ± 18.87 | 38.13 ± 20.02 | 0.6840 |
AHI, apnea–hypopnea index, BMI, body mass index, ODI, oxygen desaturation index. *Estimates presented as mean ± SD for continuous variables or N (percent) for categorical;
† N = 51 2:1 Icelandic: Chinese matched sets and N = 6 1:1 matched pairs. Significant p-values in bold.
Upper airway caliber
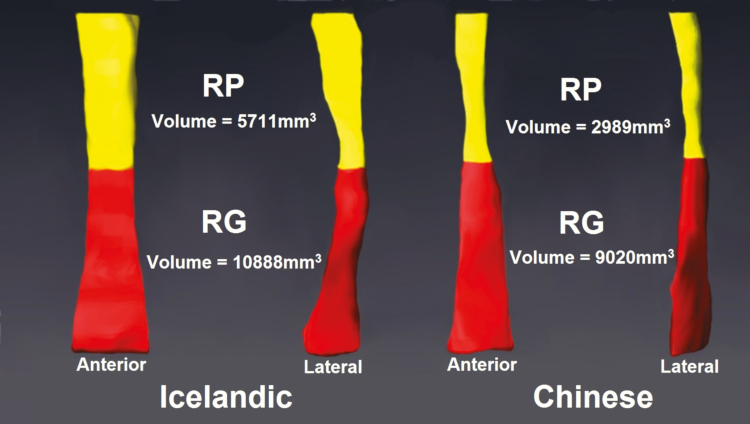
Comparisons of upper airway caliber between Icelandic and Chinese patients are presented in Table 2 (adjusted) and Supplementary Table S1 (unadjusted). Adjusting for age, ODI, and height, Chinese patients had significantly smaller total airway volume (p = 0.008) and cross-sectional area (p = 0.012), and smaller retropalatal (RP) airway (volume [p < 0.0001], mean [p < 0.0001] and minimum [p = 0.002] cross-sectional area, minimum anteroposterior [AP] distance [p = 0.0004], and minimum lateral distance [p < 0.0001]). There were no differences in the size of the retroglossal (RG) airway (Table 2). Adjusting for BMI instead of height, Chinese patients had significantly smaller total airway sizes (all p < 0.0001), smaller RP airway sizes for all measures (all p < 0.002), and smaller RG airway volume (p = 0.0004). Thus, data indicate robust differences in airway size, with a smaller retropalatal airway and total airway volume in Chinese (Figure 1).
Table 2.
Adjusted comparison of upper airway caliber between Icelandic and Chinese patients matched for age, gender, and ODI
| Domain | Variable | Model 1: age, ODI, height Adjusted mean (95% CI) | p | Model 2: age, ODI, BMI Adjusted mean (95% CI) | p | ||
|---|---|---|---|---|---|---|---|
| Icelandic | Chinese | Icelandic | Chinese | ||||
| Total Airway * | Length (mm) | 72.83 (71.64, 74.02) | 71.48 (69.76, 73.19) | 0.2306 | 74.56 (72.97, 76.16) | 67.99 (65.90, 70.08) | <0.0001 |
| Volume (mm3) | 13384 (12526, 14242) | 11315 (10082, 12549) | 0.0075 | 14177 (13238, 15116) | 9655 (8374, 10937) | <0.0001 | |
| Cross-sectional area (mm2) | 182.3 (171.1, 193.4) | 157.1 (141.1, 173.1) | 0.0120 | 189.4 (177.9, 200.8) | 142.5 (126.5, 158.5) | <0.0001 | |
| RP airway † | Volume (mm3) | 5057 (4667, 5447) | 3391 (2843, 3939) | <0.0001 | 5337 (4925, 5749) | 2839 (2277, 3401) | <0.0001 |
| Min. cross-sectional area (mm2) | 64.49 (58.36, 70.61) | 46.32 (37.55, 55.10) | 0.0018 | 67.39 (61.29, 73.49) | 40.87 (32.14, 49.60) | <0.0001 | |
| Mean cross-sectional area (mm2) | 141.3 (132.0, 150.6) | 90.61 (77.23, 104.0) | <0.0001 | 147.4 (137.9, 157.0) | 78.59 (65.07, 92.10) | <0.0001 | |
| Min. AP distance (mm) | 6.77 (6.38, 7.17) | 5.54 (4.99, 6.08) | 0.0004 | 6.72 (6.32, 7.11) | 5.64 (5.10, 6.18) | 0.0017 | |
| Min. lateral distance (mm) | 13.76 (12.77, 14.75) | 8.86 (7.49, 10.22) | <0.0001 | 14.35 (13.39, 15.32) | 7.77 (6.45, 9.08) | <0.0001 | |
| RG Airway † | Volume (mm3) | 8314 (7698, 8930) | 7917 (7009, 8825) | 0.4934 | 8834 (8168, 9500) | 6803 (5864, 7742) | 0.0004 |
| Min. cross-sectional area (mm2) | 136.3 (123.3, 149.2) | 136.2 (117.9, 154.5) | 0.9962 | 140.4 (127.4, 153.5) | 128.4 (110.1, 146.6) | 0.3083 | |
| Mean cross-sectional area (mm2) | 219.5 (202.8, 236.3) | 237.1 (212.7, 261.4) | 0.2521 | 226.8 (209.7, 244.0) | 221.9 (197.5, 246.3) | 0.7441 | |
| Min. AP distance (mm) | 11.59 (10.77, 12.40) | 10.27 (9.14, 11.40) | 0.0722 | 11.47 (10.66, 12.27) | 10.48 (9.37, 11.59) | 0.1679 | |
| Min. lateral distance (mm) | 18.23 (16.83, 19.63) | 18.37 (16.41, 20.32) | 0.9154 | 18.43 (17.04, 19.82) | 17.99 (16.07, 19.92) | 0.7268 |
Significant p-values are given in bold.
AP, anteroposterior, BMI, body mass index, CI, confidence interval, ODI, oxygen desaturation index, RG, retroglossal, RP, retropalatal.
*Bonferroni corrected significance level: p < 0.0167 (equals 0.05/3).
†Bonferroni corrected significance level: p < 0.01 (equals 0.05/5).
Figure 1.
Three-dimensional reconstruction of the upper airway (anterior and lateral views), separated into retropalatal (RP, yellow) and retroglossal (RG, red) regions in representative age, gender, and ODI matched Icelandic and Chinese patients. The Icelandic patient (left), was a 63.5 years-old male with a BMI of 33.0 kg/m2 and ODI of 59.4 events/h. The Chinese patient (right), was a 65.0 years-old male with BMI of 25.5 kg/m2 and ODI of 57.9 events/h. As observed in our analyses, the Chinese patient has a smaller airway than the Icelandic patient in the RP region, but not the RG region.
Upper airway soft tissues
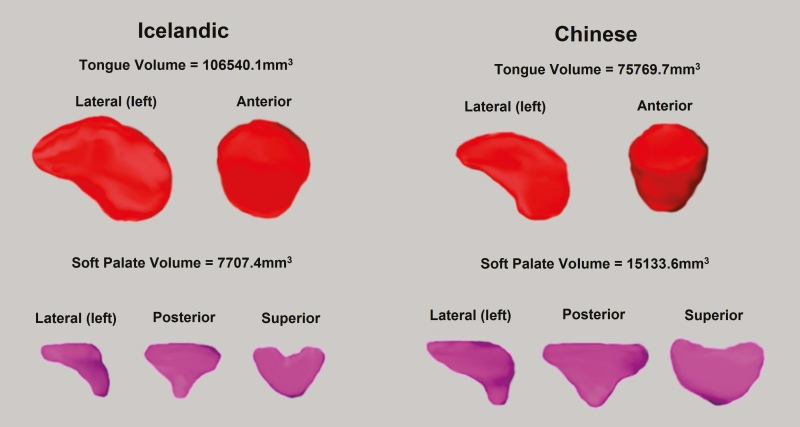
Analyses comparing soft tissue volumes between Chinese and Icelandic patients are shown in Table 3 (adjusted) and Supplementary Table S2 (unadjusted). Adjusting for age, ODI, and height, Icelandic patients had larger combined soft tissue (p < 0.0001), tongues (all p < 0.0001), fat pad (p < 0.0001), and pterygoid (p = 0.0003) volumes. However, Chinese patients demonstrated larger soft palate volume (p = 0.0001). There were no significant differences in lateral wall volumes between the two ethnic groups. Results were similar controlling for BMI instead of height, except the soft palate difference was non-significant; this may be due to effect modification by gender (described below). Overall, results indicate soft tissue anatomic differences between the two racial groups, with enlargement of tongue, fat pads, and pterygoid volumes in Icelandic patients (Figure 2).
Table 3.
Adjusted comparisons of soft tissue volumes between Icelandic and Chinese patients matched for age, gender, and ODI
| Domain | Variable | Model 1: age, ODI, height Adjusted mean (95% CI) | P | Model 2: age, ODI, BMI Adjusted mean (95% CI) | P | ||
|---|---|---|---|---|---|---|---|
| Icelandic | Chinese | Icelandic | Chinese | ||||
| Total volumes* | Combined soft tissue (mm3) | 202871 (198010, 207733) | 184037 (177128, 190947) | <0.0001 | 203682 (197796, 209569) | 180454 (172970, 187939) | <0.0001 |
| Tongue (mm3) | 132661 (129488, 135835) | 116183(111650, 120715) | <0.0001 | 132929 (129306, 136553) | 114702 (109950, 119453) | <0.0001 | |
| Soft palate (mm3) | 9810 (9297, 10323) | 11652 (10920, 12383) | 0.0001 | 10224 (9630, 10817) | 10797 (10020, 11574) | 0.2079 | |
| Lateral walls (mm3) | 28103 (26801, 29406) | 29481 (27605, 31358) | 0.2429 | 28723 (27293, 30153) | 28083 (26085, 30081) | 0.6024 | |
| Fat pads (mm3) | 8629 (8175, 9082) | 6352 (5681, 7022) | <0.0001 | 8541 (8089, 8994) | 6522 (5863, 7181) | <0.0001 | |
| Epiglottis (mm3) | 1461 (1343, 1580) | 1436 (1261, 1612) | 0.8214 | 1491 (1367, 1615) | 1369 (1191, 1548) | 0.2711 | |
| Pterygoid (mm3) | 22275 (21257, 23294) | 19218 (17812, 20624) | 0.0003 | 22241 (21116, 23366) | 19102 (17668, 20536) | 0.0001 | |
| Partial volumes ‡ | Genioglossus (mm3) | 101302 (99118, 103486) | 91576 (88436, 94715) | <0.0001 | 101664 (99212, 104115) | 90383 (87055, 93710) | <0.0001 |
| Other tongue (mm3) | 31328 (29932, 32724) | 24812 (22774, 26850) | <0.0001 | 31395 (29866, 32925) | 24395 (22313, 26477) | <0.0001 | |
| RP lateral walls (mm3) | 14563 (13653, 15473) | 15235 (13927, 16543) | 0.4197 | 14695(13751, 15639) | 14929 (13599, 16259) | 0.7798 | |
| RG lateral walls (mm3) | 13567 (12806, 14329) | 14195 (13063, 15327) | 0.3936 | 14082 (13262, 14901) | 13153 (11933, 14372) | 0.2411 |
Significant p-values are given in bold.
AP, anteroposterior, CI, confidence interval, ODI, oxygen desaturation index, RG, retroglossal, RP, retropalatal.
*Bonferroni corrected significance level: p < 0.0071 (equals 0.05/7).
‡Bonferroni corrected significance level: p < 0.0125 (equals 0.05/4).
Figure 2.
Three-dimensional reconstruction of tongue [genioglossus muscle] and soft palate volumes in representative age, gender, and ODI matched Icelandic and Chinese patients. The Icelandic patient (left), is a 26 years-old male with BMI of 35.2 kg/m2 and ODI of 10.8 events/h. The Chinese patient (right) is a 25 years-old male with BMI of 23.8 kg/m2 and ODI of 13.6 events/h. Reflecting our results, the Chinese patient had a smaller tongue but larger soft palate than the Icelandic patient.
Craniofacial dimensions
We compared craniofacial dimensions between the two ethnic groups, including adjusted (Table 4) and unadjusted (Supplementary Tables S3) analyses of craniofacial angles, mandibular measurements, maxillary measurements, hyoid distances, craniofacial heights, and craniofacial volume and areas.
Table 4.
Adjusted comparisons of craniofacial angles between Icelandic and Chinese patients matched for age, gender, and ODI
| Variable | Model 1: age, ODI, height Adjusted mean (95% CI) | p | Model 2: age, ODI, BMI Adjusted mean (95% CI) | p | ||
|---|---|---|---|---|---|---|
| Icelandic | Chinese | Icelandic | Chinese | |||
| SNA angle (°) | 84.49 (83.57, 85.41) | 85.99 (84.66, 87.32) | 0.0825 | 84.74 (83.82, 85.66) | 85.50 (84.19, 86.81) | 0.3686 |
| SNB angle (°) | 81.29 (80.40, 82.19) | 81.34 (80.04, 82.64) | 0.9537 | 81.37 (80.48, 82.26) | 81.19 (79.89, 82.48) | 0.8256 |
| ANB angle (°) | 3.18 (2.62, 3.74) | 4.97 (4.15, 5.78) | 0.0009 | 3.38 (2.83, 3.92) | 4.60 (3.80, 5.40) | 0.0196 |
| Saddle angle (°) | 125.7 (124.4, 126.9) | 125.7 (123.9, 127.5) | 0.9940 | 124.5 (123.2, 125.7) | 128.0 (126.2, 129.8) | 0.0023 |
| ACB:HP (°) | 12.89 (11.68, 14.10) | 10.39 (8.624, 12.15) | 0.0314 | 11.79 (10.71, 12.88) | 12.50 (10.93, 14.07) | 0.4939 |
Bonferroni corrected significance level: p < 0.01 (equals 0.05/5). Significant p-values are given in bold.
ACB, anterior cranial base, ANB, Subspinale–Nasion–Supramentale (the difference between SNA and SNB), BMI, body mass index, CI, confidence interval, HP, horizontal plane, ODI, oxygen desaturation index, SNA, Sella (S)–Nasion (N)–Subspinale (A), SNB, Sella (S)–Nasion (N)–Supramentale (B).
Craniofacial angles
Adjusting for age, ODI, and height (Table 4), Chinese patients had a significantly bigger ANB angle (Subspinale–Nasion–Supramentale; p = 0.001) and nominally smaller ACB:HP (anterior cranial base and horizontal plane; p = 0.031) compared to Icelandic patients (Supplementary Figure S10). Adjusting for BMI instead of height, the differences in ANB angle became nominal (p = 0.020), and Chinese showed significantly larger saddle angle (p = 0.002) than Icelandic patients. Results imply Chinese patients have a more retrognathic mandible in reference to the maxilla, but Icelandic patients have a normal anteroposterior relationship between the maxilla and mandible.
Mandibular measurements
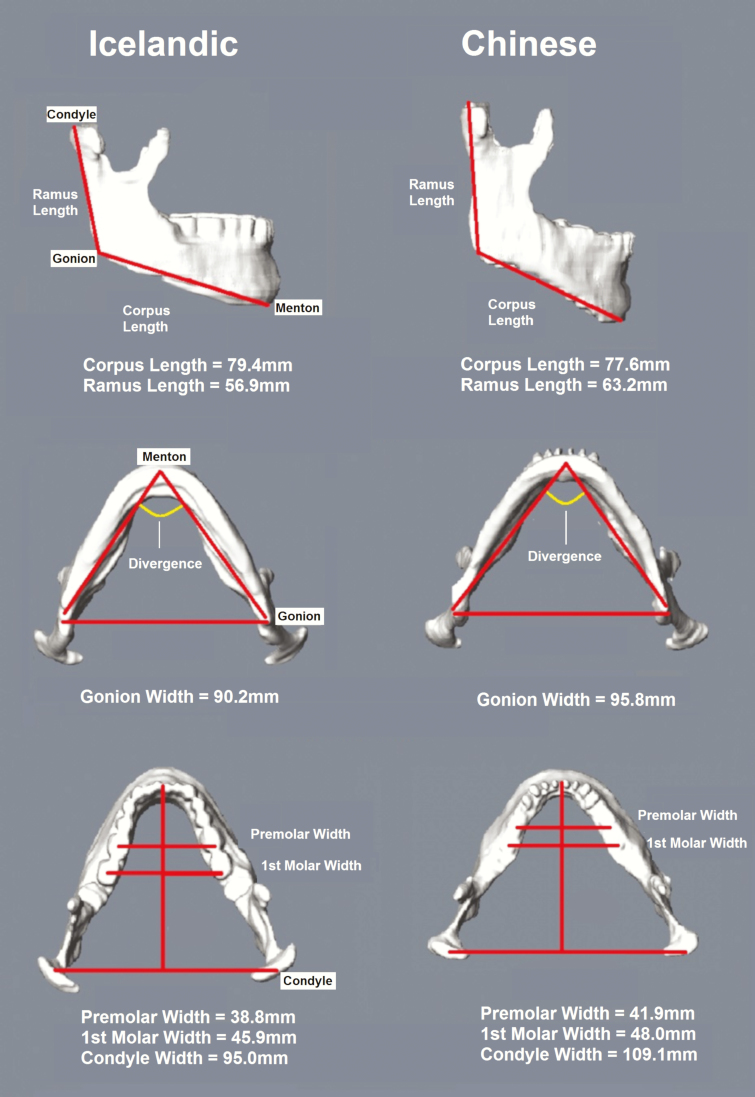
Chinese and Icelandic patients had differently shaped mandibles (Figure 3). Adjusting for age, ODI and either height or BMI (Table 5), we found significant differences in mandibular lengths (all p ≤ 0.0001) and widths (all p < 0.0001). The difference in divergence was nominal (p ≤ 0.016). Chinese patients showed similar mandibular depth, larger mandibular ramus and total lengths, greater mandibular widths, but smaller mandibular corpus length compared to Icelandic patients.
Figure 3.
Three-dimensional reconstruction of the mandible in representative age, gender and ODI matched Icelandic and Chinese patients. The Icelandic patient (left) is a 59 years-old female with a BMI of 26.0 kg/m2 and ODI of 28.8 events/h. The Chinese patient (right) is a 58 years-old female with BMI of 26.0 kg/m2 and ODI of 31.1 events/h. As in our results, the Chinese patient has a longer ramus length, shorter corpus length, and greater mandibular widths than the Icelandic patient.
Table 5.
Adjusted comparisons of mandibular measurements between Icelandic and Chinese patients matched for age, gender and ODI
| Variable | Model 1: age, ODI, height Adjusted mean (95% CI) | P | Model 2: age, ODI, BMI Adjusted mean (95% CI) | P | ||
|---|---|---|---|---|---|---|
| Icelandic | Chinese | Icelandic | Chinese | |||
| Depth (mm) | 76.29 (75.03, 77.55) | 75.26 (73.44, 77.08) | 0.3907 | 76.23 (74.98, 77.48) | 75.38 (73.57, 77.19) | 0.4752 |
| Length corpus (mm) | 93.80 (92.79, 94.82) | 80.57 (79.09, 82.05) | <0.0001 | 94.16 (93.10, 95.22) | 79.89 (78.35, 81.42) | <0.0001 |
| Length ramus (mm) | 45.23 (43.87, 46.60) | 67.48 (65.52, 69.43) | <0.0001 | 46.15 (44.80, 47.50) | 65.72 (63.80, 67.63) | <0.0001 |
| Total length (mm) | 139.1 (137.5, 140.6) | 148.1 (145.9, 150.3) | <0.0001 | 140.3 (138.6, 142.0) | 145.8 (143.5, 148.1) | 0.0001 |
| Width second premolar (mm) | 38.57 (37.95, 39.18) | 41.46 (40.61, 42.31) | <0.0001 | 38.51 (37.89, 39.13) | 41.54 (40.71, 42.37) | <0.0001 |
| Width first molar (mm) | 44.77 (44.07, 45.47) | 48.62 (47.67, 49.57) | <0.0001 | 44.73 (44.03, 45.43) | 48.65 (47.72, 49.58) | <0.0001 |
| Width gonion (mm) | 90.05 (88.94, 91.16) | 98.78 (97.17, 100.4) | <0.0001 | 90.51 (89.36, 91.67) | 97.86 (96.22, 99.50) | <0.0001 |
| Width condyle (mm) | 102.6 (101.4, 103.7) | 110.9 (109.3, 112.6) | <0.0001 | 103.0 (101.8, 104.2) | 110.0 (108.4, 111.7) | <0.0001 |
| Divergence (°) | 72.12 (71.04, 73.20) | 69.70 (68.23, 71.18) | 0.0076 | 72.03 (70.95, 73.12) | 69.88 (68.42, 71.34) | 0.0159 |
Significant p-values are given in bold.
BMI, body mass index, CI, confidence interval, ODI, oxygen desaturation index.
Bonferroni corrected significance level: p < 0.0056 (equals 0.05/9).
Maxillary measurements
Similarly, we observed differently shaped maxilla between Chinese and Icelandic patients (Supplementary Figure S11). Adjusting for age, ODI, and either height or BMI (Table 6), Chinese demonstrated a wider maxilla (all p < 0.0001) with shallower maxillary unit depth (p ≤ 0.0001) and larger maxillary divergence (p ≤ 0.011).
Table 6.
Adjusted Comparisons of Maxillary measurements between Icelandic and Chinese Patients Matched for Age, Gender and ODI
| Variable | Model 1: age, ODI, height Adjusted mean (95% CI) | p | Model 2: age, ODI, BMI Adjusted mean (95% CI) | p | ||
|---|---|---|---|---|---|---|
| Icelandic | Chinese | Icelandic | Chinese | |||
| Depth (mm) | 47.64 (46.88, 48.39) | 44.83 (43.76, 45.89) | 0.0001 | 47.68 (46.90, 48.46) | 44.69 (43.63, 45.75) | <0.0001 |
| Divergence (mm) | 55.41 (54.27, 56.55) | 58.83 (57.20, 60.45) | 0.0011 | 55.68 (54.57, 56.80) | 58.29 (56.70, 59.89) | 0.0114 |
| Width second premolar (mm) | 40.55 (39.94, 41.16) | 44.64 (43.80, 45.47) | <0.0001 | 40.38 (39.77, 41.00) | 44.93 (44.12, 45.74) | <0.0001 |
| Width first molar (mm) | 45.18 (44.59, 45.78) | 49.52 (48.69, 50.34) | <0.0001 | 45.14 (44.56, 45.72) | 49.59 (48.79, 50.40) | <0.0001 |
BMI, body mass index, CI, confidence interval, ODI, oxygen desaturation index. Bonferroni corrected significance level: p < 0.0125 (equals 0.05/4).
Hyoid distances
Adjusting for age, ODI, and height (Table 7), Chinese had significantly longer hyoid-to-C3 (p = 0.006) and hyoid-to-sella (p < 0.0001) distances, and shorter retropogonion-to-C3 distance (p < 0.0001) than Icelandic patients. Adjusting for BMI instead of height, only the difference in retropogonion-to-C3 distance remained significant (p = 0.008); the larger hyoid-to-C3 distance in Chinese became nominal (p = 0.035). Thus, results suggest ethnic associations in hyoid-related measures may be driven by differences in obesity between the two ethnic groups.
Table 7.
Adjusted comparisons of hyoid distances between Icelandic and Chinese patients matched for age, gender and ODI
| Variable | Model 1: age, ODI, height Adjusted mean (95% CI) | p | Model 2: age, ODI, BMI Adjusted mean (95% CI) | p | ||
|---|---|---|---|---|---|---|
| Icelandic | Chinese | Icelandic | Chinese | |||
| Hyoid to retropogonion (mm) | 42.92 (41.69, 44.15) | 40.71 (38.89, 42.52) | 0.0636 | 42.76 (41.54, 43.98) | 41.01 (39.24, 42.78) | 0.1292 |
| Hyoid to C3 (mm) | 36.94 (35.94, 37.93) | 39.46 (38.03, 40.89) | 0.0064 | 37.12 (36.06, 38.19) | 39.03 (37.57, 40.48) | 0.0350 |
| Hyoid to sella (mm) | 119.3 (117.6, 121.0) | 129.3 (126.8, 131.7) | <0.0001 | 122.4 (120.1, 124.6) | 123.1 (120.1, 126.1) | 0.6854 |
| Retropogonion to C3 (mm) | 72.29 (70.80, 73.79) | 65.34 (63.14, 67.54) | <0.0001 | 71.08 (69.67, 72.49) | 67.69 (65.69, 69.70) | 0.0080 |
Significant p-values are given in bold. Bonferroni corrected significance level: p < 0.0125 (equals 0.05/4).
BMI, body mass index, C3, the 3rd cervical vertebrae, CI, confidence interval, ODI, oxygen desaturation index.
Craniofacial heights
Adjusting for age, ODI, and height (Table 8), Chinese generally had larger upper (p = 0.001) and anterior (p = 0.001) facial height than Icelandic patients. All differences were non-significant when adjusting for BMI instead of height, suggesting obesity may play a role in accounting for these differences.
Table 8.
Adjusted comparisons of craniofacial heights between Icelandic and Chinese patients matched for age, gender and ODI
| Variable | Model 1: age, ODI, height Adjusted mean (95% CI) | p | Model 2: age, ODI, BMI Adjusted mean (95% CI) | p | ||
|---|---|---|---|---|---|---|
| Icelandic | Chinese | Icelandic | Chinese | |||
| Upper facial height (UFH) (mm) | 48.40 (47.66, 49.14) | 50.76 (49.68, 51.83) | 0.0009 | 48.85 (48.07, 49.62) | 49.87 (48.77, 50.98) | 0.1511 |
| Lower facial height (LFH) (mm) | 72.31 (71.21, 73.41) | 73.90 (72.29, 75.50) | 0.1337 | 73.01 (71.88, 74.13) | 72.55 (70.92, 74.19) | 0.6716 |
| Anterior facial height (AFH) (mm) | 120.9 (119.5, 122.4) | 125.4 (123.3, 127.6) | 0.0013 | 122.0 (120.5, 123.5) | 123.4 (121.2, 125.6) | 0.3177 |
| UFH/AFH | 0.401 (0.395, 0.406) | 0.405 (0.397, 0.413) | 0.4102 | 0.401 (0.396, 0.407) | 0.404 (0.396, 0.412) | 0.5421 |
| PNS to anterior arch atlas (mm) | 33.14 (32.26, 34.02) | 33.22 (31.93, 34.52) | 0.9239 | 32.66 (31.82, 33.50) | 34.17 (32.93, 35.40) | 0.0633 |
Bonferroni corrected significance level: p < 0.01 (equals 0.05/5). Significant p-values are given in bold.
BMI, body mass index, CI, confidence interval, ODI, oxygen desaturation index, PNS, posterior nasal spine.
Craniofacial volume and areas
When adjusting for age, ODI, and height (Table 9), Chinese showed bigger oropharyngeal area (p = 0.006) than Icelandic patients, and a nominally larger naso-oropharyngeal area (p = 0.034). Adjusting for BMI instead of height, these differences became nonsignificant; however, a nominal difference was seen in nasopharyngeal area (p = 0.014). Thus, there is suggestive, but inconsistent evidence of differences in naso-oropharyngeal areas. Interestingly, while differently shaped mandibles were observed, the overall intramandibular volume was not significantly different between Chinese and Icelandic patients adjusting for age, ODI, and either height (p = 0.225) or BMI (p = 0.065).
Table 9.
Adjusted comparisons of craniofacial volume and areas between Icelandic and Chinese patients matched for age, gender and ODI
| Variable | Model 1: age, ODI, height Adjusted mean (95% CI) | P | Model 2: age, ODI, BMI Adjusted mean (95% CI) | P | ||
|---|---|---|---|---|---|---|
| Icelandic | Chinese | Icelandic | Chinese | |||
| Intramandibular volume (mm3) | 181984 (176470, 187498) | 175890 (167810, 183970) | 0.2249 | 182813(176867, 188759) | 173659 (165358, 181960) | 0.0654 |
| Nasooropharyngeal area (mm2) | 8339 (8153, 8525) | 8711 (8442, 8981) | 0.0344 | 8499 (8295, 8703) | 8399 (8112, 8686) | 0.5861 |
| Oropharyngeal area (mm2) | 5876 (5740, 6011) | 6224 (6029, 6420) | 0.0062 | 5974 (5828, 6120) | 6032 (5826, 6239) | 0.6600 |
| Nasopharyngeal area (mm2) | 2465 (2399, 2531) | 2487 (2390, 2583) | 0.7317 | 2526 (2456, 2597) | 2367 (2266, 2467) | 0.0140 |
Bonferroni corrected significance level: p < 0.0125 (equals 0.05/4). Nasopharyngeal area: Nasion-anterior Nasal Spine-Basion-Nasion (Na-ANS-Ba-Na); oropharyngeal area: anterior Nasal Spine-Menton-Third cervical vertebrae-Basion-Anterior Nasal Spine (Na-ANS-Me-C3-Ba-Na); Naso-oropharyngeal area: Na-ANS-Me-C3-Ba-Na. Significant p-values are given in bold.
BMI, body mass index, CI, confidence interval, ODI, oxygen desaturation index.
Gender-specific ethnic differences
To assess whether stratification by gender was required to accurately capture ethnic differences, we tested for gender-by-ethnicity interactions for all measurements; suggestive interactions (p < 0.10) were observed in adjusted (Table 10) or unadjusted (Supplementary Table S4) analyses. Adjusting for age, ODI, and height, gender-specific ethnic differences were identified for RP minimum anteroposterior (AP) distance (p = 0.020), soft palate volume (p = 0.006), mandibular length corpus (p = 0.046) and ramus (p = 0.014), mandibular width at the first molar (p = 0.071), mandibular divergence (p = 0.098) and hyoid-to-sella distance (p = 0.019). Results were similar when adjusting for BMI instead of height: the gender effect on hyoid-to-sella distance became nonsignificant, but a borderline interaction on maxillary depth emerged (p = 0.099). Ethnic differences in mandibular lengths and width were significant in both genders; larger differences in length were seen in males and a larger difference in width was seen in females. Interestingly, the smaller RP minimal AP distance (p ≤ 0.002), larger soft palate volume (p ≤ 0.016) and smaller mandibular divergence (p ≤ 0.012) in Chinese compared to Icelandic patients was primarily seen in males, with smaller, nonsignificant differences in females (although the female sample is small).
Table 10.
Upper airway measurements with evidence for racial differences modified by gender
| Variable* | Inter-action P-value | Male | P | Female | P | ||
|---|---|---|---|---|---|---|---|
| Icelandic N = 84 | Chinese N = 44 | Icelandic N = 24 | Chinese N = 13 | ||||
| Model 1: age, ODI, height adjusted mean (95% CI) | |||||||
| RP min. AP distance (mm) | 0.0204 | 7.18 (6.72, 7.65) | 5.36 (4.70, 6.01) | <0.0001 | 5.69 (4.87, 6.51) | 5.60 (4.53, 6.66) | 0.8830 |
| Soft palate volume (mm3) | 0.0058 | 10709 (10146, 11271) | 12272 (11438, 13106) | 0.0051 | 7870 (7340, 8400) | 7174 (6430, 7918) | 0.1305 |
| Mandibular length corpus (mm) | 0.0460 | 95.99 (94.79, 97.19) | 80.83 (79.03, 82.63) | <0.0001 | 87.70 (85.73, 89.68) | 77.23 (74.41, 80.04) | <0.0001 |
| Mandibular length ramus (mm) | 0.0142 | 45.50 (43.91, 47.10) | 68.54 (66.30, 70.78) | <0.0001 | 45.26 (42.23, 48.28) | 62.51 (58.20, 66.82) | <0.0001 |
| Mandibular width first molar (mm) | 0.0709 | 45.46 (44.70, 46.23) | 48.66 (47.57, 49.75) | <0.0001 | 43.08 (41.08, 45.07) | 46.65 (44.43, 48.86) | 0.0060 |
| Mandibular divergence (°) | 0.0977 | 72.01 (70.77, 73.24) | 68.59 (66.82, 70.35) | 0.0025 | 72.37 (69.88, 74.85) | 73.46 (70.43, 76.48) | 0.4993 |
| Maxillary depth (mm) | 0.1343 | — | — | — | — | — | — |
| Hyoid to sella (mm) | 0.0192 | 123.5 (121.6, 125.3) | 131.8 (129.0, 134.6) | <0.0001 | 107.7 (104.6, 110.7) | 116.1 (111.8, 120.5) | 0.0035 |
| Model 2: age, ODI, BMI adjusted mean (95% CI) | |||||||
| RP min. AP distance (mm) | 0.0252 | 6.96 (6.51, 7.42) | 5.76 (5.14, 6.38) | 0.0024 | 5.73 (4.92, 6.53) | 5.53 (4.50, 6.56) | 0.7446 |
| Soft palate volume (mm3) | 0.0069 | 10799 (10244, 11354) | 12099 (11285, 12913) | 0.0159 | 7910 (7354, 8467) | 7108 (6360, 7855) | 0.0682 |
| Mandibular length corpus (mm) | 0.0414 | 95.73 (94.60, 96.87) | 81.33 (79.66, 83.00) | <0.0001 | 87.97 (85.99, 89.96) | 76.73 (73.93, 79.52) | <0.0001 |
| Mandibular length ramus (mm) | 0.0194 | 46.10 (44.56, 47.64) | 67.37 (65.24, 69.51) | <0.0001 | 45.98 (43.07, 48.88) | 61.19 (57.10, 65.27) | <0.0001 |
| Mandibular width first molar (mm) | 0.0872 | 45.26 (44.55, 45.97) | 49.02 (48.04, 50.00) | <0.0001 | 42.33 (40.43, 44.24) | 47.78 (45.60, 49.95) | <0.0001 |
| Mandibular divergence (°) | 0.0999 | 71.77 (70.55, 72.98) | 69.05 (67.35, 70.76) | 0.0118 | 72.88 (70.41, 75.34) | 72.53 (69.59, 75.48) | 0.8206 |
| Maxillary depth (mm) | 0.0992 | 48.03 (47.24, 48.83) | 46.45 (45.30, 47.61) | 0.0391 | 45.76 (44.53, 46.99) | 40.41 (38.76, 42.06) | <0.0001 |
| Hyoid to sella (mm) | 0.1061 | — | — | — | — | — | — |
Significant p-values are given in bold.
AP, anteroposterior, BMI, body mass index, CI, confidence interval, ODI, oxygen desaturation index, RP, retropalatal.
*Upper airway measurements with suggestive evidence of a gender-by-race interaction in either Model 1 or Model 2. .
Discussion
This is the first study to use three-dimensional MRI to explore differences in airway sizes, soft tissue volumes and craniofacial structures between Asian patients from China and European patients from Iceland with similar OSA severity. Results support ethnic-related differences in upper airway anatomy in subjects with OSA. Chinese patients demonstrated narrower airways, particularly in the retropalatal region. Icelandic patients had larger tongue, parapharyngeal fat pads, pterygoid, and combined soft tissue volumes. However, larger soft palate volume was observed in male Chinese patients. Chinese patients demonstrated characteristics consistent with more bony restrictions, including larger ANB angle, shorter mandibular length corpus and shorter maxillary depth in both male and females. Moreover, differently shaped mandible and maxilla were observed between Chinese and Icelandic patients. Ultimately, understanding ethnicity-specific differences in OSA-related anatomy can play an important role in improving disease identification and treatment in these populations.
Different anatomy in Chinese and Icelandic patients with OSA
Airway dimensions
While airway obstruction during sleep occurs at multiple levels in OSA patients, studies demonstrate particular importance of the retropalatal airway [19–21]. A prior study shows that the minimum retropalatal airway area was significantly smaller in OSA patients than normal subjects [4]. Utilizing dynamic sleep MRI, patients with OSA had retropalatal airway collapse during sleep [20]. This propensity to collapse in the retropalatal region was supported by data showing a smaller retropalatal airway, but not retroglossal airway, in normal subjects [21]. The present study supports ethnic differences in patients with OSA in the retropalatal region, which was significantly smaller in Chinese than Icelandic patients. Given the association between smaller retropalatal airway and OSA, this suggests retropalatal airway size may be more important in OSA etiology among Chinese patients. In contrast, no robust differences were found in the retroglossal airway. Although the specific causes may vary, the observation of a larger soft palate and smaller mandibular divergence in male Chinese patients may play a role in the observed smaller RP airway minimal AP distance in males.
Soft tissue volumes
Studies have demonstrated enlargement of upper airway soft tissues in OSA patients [1]. In our prior research, increased tongue, lateral pharyngeal wall, and combined soft tissue volumes were important OSA risk factors [1].
In the present study, after matching on age, gender, and ODI and controlling for height or BMI, Icelandic patients demonstrated larger tongues, parapharyngeal fat pads, pterygoids, and combined soft tissue volumes than Chinese. Thus, the differences are not likely explained by greater height or obesity in the Icelandic patients, although there may be differences in regional fat deposition. In contrast, among males, Chinese patients showed larger soft palate volumes than Icelandic patients. The finding of larger soft tissues among Icelandic patients is compatible with previous findings of greater size of the tongue in Caucasian patients compared to Chinese at similar AHI [9]. However, our observation of larger soft palate in male Chinese patients is novel. While this result seemingly contrasts with prior results showing a longer soft palate in Caucasian than Chinese patients [9], this previous study was not able to measure volume, highlighting the novelty of the MRI approach used here. Moreover, despite the difference in overall soft tissue volume, the lateral wall volume was not different between two ethnic groups. This could suggest that the lateral walls, which have previously been shown to be an important OSA risk factor [1], play a similar role in determining OSA risk, independent of ethnicity-specific etiologies. Determining this definitively would require study of controls in each population. Ultimately, results indicate that a number of soft tissue volumes differ between the two ethnic groups studied here. Given that larger soft tissues are seen in Icelandic patients, and increased soft tissue volumes have been previously demonstrated to be a risk factor for OSA [1], our data suggest that soft tissues could be more important in OSA etiology among Caucasians.
Differences in upper airway soft tissues may come from distinct fat distributions between Icelandic and Chinese patients. Chinese adults were shown to have a greater proportion of fat, especially in the trunk region, than Caucasians with similar BMI [22]. Similarly, a recent study found ethnic differences in the propensity to store fat intra-abdominally [23]. Moreover, studies have demonstrated that upper airway soft tissue sizes are in part genetically determined [24, 25]. Thus, future studies should examine the distributions of fat in upper airway structures and the role of genetic factors. It is feasible that more fat is deposited in the soft palate of male Chinese patients or that ethnic differences in genetic factors determining soft tissue size exist.
Craniofacial structures
Certain craniofacial characteristics have been implicated in OSA. Shorter mandibular length and smaller mandibular depth in men, greater hyoid-to-nasion and supramentale-to-hyoid distances in men and women are independent risk factors [7]. A previous two-dimensional cephalometric study found that Chinese patients had bigger SNA and ANB angles and shorter cranial base, midface length, maxilla, and mandible compared to Caucasians with similar AHI [9].
Our study confirms and extends these prior results [9]. Chinese patients with OSA had more craniofacial bony restriction including larger ANB angle, shorter mandibular corpus length and shorter maxilla depth when compared to Icelandic patients. Using three-dimensional MRI, we also demonstrate differently shaped mandible and maxilla between Chinese and Icelandic patients. Chinese had shorter mandibular corpus length, but longer mandibular ramus and total length. These significant, but opposite differences in length may account for the observation of no significant difference in intramandibular volumes between the two ethnic groups. Our study is the first to compare the 3D intramandibular volume between patients from different ethnicities. Mandibular depth which had been also demonstrated as an independent risk factor of OSA in a previous study [7] was not found to be significantly different between two ethnic groups. As found previously, we observed a gender-specific effect on ethnic differences in mandibular measures [26, 27]. Mandibular divergence was smaller in male Chinese patients. While other differences in mandibular measures were generally significant in both genders, differences in mandibular length were greater in males, while differences in width were greater in females. Chinese also had differently shaped maxilla, with shorter depth and greater width compared to Icelandic patients. The potential role of differences in mandibular and maxillary shape on OSA physiology and airway collapsibility remains to be determined. Evaluating this through dynamic or sleep MRI in association with physiological phenotypes [28] is an important direction for studies in these ethnic groups.
As with soft tissues, craniofacial differences could be driven by genetics. Previous studies have demonstrated familial aggregation of craniofacial structures [18, 24, 29, 30], many of which differ between Chinese and Icelandic patients in our study. Genome-wide significant associations for facial morphology have also been observed [31]. Future studies should address the genetic factors that control craniofacial dimensions in these ethnic groups.
Study limitations
There are several limitations for discussion. First, patients were initially diagnosed with OSA using PSG in China, but portable monitors in Iceland. To account for this difference in diagnostic testing, we recalculated the ODI from PSG in Chinese patients so that it was equivalent to that derived from portable monitoring in Iceland. In particular, in both samples the ODI was calculated as the number of desaturations ≥4% per hour of total analysis time, determined as the total recording time beginning 30 min after the start of the study and stopping 5 min prior to the end of the study. The ODI has been demonstrated to be a reliable measure of disease severity in patients with OSA [32].
Second, our study compared Icelandic and Chinese patients with at least moderate OSA (defined as an ODI ≥ 10 events/h); healthy controls and patients with mild OSA were not recruited. Therefore, results are generalizable only to patients with moderate or severe disease. In addition, while data inform ethnic differences in important intermediate OSA anatomic risk factors, the lack of controls means that we cannot directly evaluate the ethnic-specific impact of these factors on the risk of developing OSA. Structural risk factors for OSA have has been shown previously [33, 34]. However, when our results are combined with existing literature, they do implicate specific variables as potentially more or less important in ethnic-specific OSA etiology. Ultimately, future research including controls and patients with mild OSA among the ethnic groups studied here, as well as upper airway analysis, should be conducted to confirm ethnic-specific associations with OSA risk and severity. While there is utility in analyses that include noncases, there are also important benefits of understanding ethnic differences among cases with OSA. Prior studies have used the same design to illustrate ethnic differences in anatomy using cephalometry [9] and physiological measures related to OSA [35]. Results of the present study using three-dimensional MRI can be combined with the prior study by Lee et al. [9] to better understand ethnic-specific differences in upper airway anatomy among cases with similar OSA severity. Importantly, these differences can be leveraged to help to target specific personalized treatments (e.g. mandibular device vs CPAP therapy).
In addition to these limitations, this manuscript represents a secondary analysis of a large, multicenter prospective study in Iceland, combined with a recently recruited sample of Chinese patients from one clinical sleep center in China. The imaging techniques were performed using identical methods and covariate matching was leveraged to increase power and reduce the selection bias. Despite this, it is possible that differences in the upper airway anatomy found here are in part explained by differences in clinical referral patterns between Iceland and our single center in China. Future studies including patients from multiple centers in China are needed to understand differences on a more national scale. Similarly, our analyses focused specifically on patients from China and Iceland, and thus may not be generalizable to all OSA patients of Asian or European ancestry. Finally, a large majority of patients were male, reflecting the expected gender distribution of OSA; while we evaluated the influence of gender on ethnic differences using interaction tests, future studies should recruit more females to robustly assess gender differences and increase generalizability.
Study strengths
Strengths of this study include the robust analysis methods and relatively large sample of patients from two ethnic groups, matched on important clinical factors. In addition, we utilized state-of-the-art three-dimensional MRI techniques to quantify novel measures of upper airway size, soft tissue volumes and craniofacial structures.
Conclusions
In summary, we used three-dimensional MRI to examine differences in upper airway size, soft tissue volumes and craniofacial dimensions between Asian patients from China and European patients from Iceland with similar age, gender and OSA severity. Results are compatible with previous studies [9], and extend these findings by quantifying anatomy with three-dimensional imaging. Chinese patients showed smaller airway size, mainly in the retropalatal region. While Icelandic patients have greater combined volumes of the measured soft tissues in both males and females, our results provide new evidence of a larger soft palate in male Chinese patients. Finally, we found differences in the overall shapes of the mandible and maxilla between the two ethnic groups, and Chinese patients demonstrated evidence of more bony restrictions. Differences in upper airway anatomy should inform therapies and future studies on the relative efficacy of different treatments (e.g. positive airway pressure vs. oral appliances). Ultimately, our results further the understanding of the ethnicity-specific anatomical characteristics among OSA patients, which can inform potential personalized treatment of OSA within these populations.
Supplementary Material
Acknowledgment
The authors thank Yi Sun for scoring the sleep studies. International Sleep Research Training Program.
Funding
This work was supported by National Institutes of Health (grant P01 HL094307).
Conflict of interest statement. Dr. Pack is the John L. Miclot Professor of Medicine at the University of Pennsylvania. Dr. Schwab reports grants from NIH during the conduct of the study. Dr. Xu was supported by Young Elite Scientists Sponsorship Program of China Association for Science and Technology. Other authors reported no conflict of interest.
Authors’ contribution
Conception and design: A.I.P., T.G. and R.J.S. Data acquisition: Q.L., Z.W., J.W. Analysis and interpretation: A.W., B.S., L.X., B.T.K., B.B., S.J, A.I.P., R.J.S. Drafting of the manuscript: L.X., B.T.K., A.W., A.IP., T.G. and R.J.S. Revision: L.X., B.T.K., A.I.P and R.J.S.
References
- 1. Schwab RJ, et al. . Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522–530. [DOI] [PubMed] [Google Scholar]
- 2. Watanabe T, et al. . Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165(2):260–265. [DOI] [PubMed] [Google Scholar]
- 3. Ferguson KA, et al. . The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest. 1995;108(2):375–381. [DOI] [PubMed] [Google Scholar]
- 4. Schwab RJ, et al. . Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1673–1689. [DOI] [PubMed] [Google Scholar]
- 5. Lowe AA, et al. . Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 1995;107(6):589–595. [DOI] [PubMed] [Google Scholar]
- 6. Nelson S, et al. . Contribution of craniofacial risk factors in increasing apneic activity among obese and nonobese habitual snorers. Chest. 1997;111(1):154–162. [DOI] [PubMed] [Google Scholar]
- 7. Chi L, et al. . Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur Respir J. 2011;38(2):348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ip MS, et al. . A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119(1):62–69. [DOI] [PubMed] [Google Scholar]
- 9. Richard WW, et al. . Differences in craniofacial structures and obesity in caucasian and chinese patients with obstructive sleep apnea. Sleep. 2010;33(8):1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnardottir ES, et al. . The role of obesity, different fat compartments and sleep apnea severity in circulating leptin levels: the Icelandic Sleep Apnea Cohort study. Int J Obes (Lond). 2013;37(6):835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnardottir ES, et al. . Nocturnal sweating--a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013;3(5):e002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnardottir ES, et al. . The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep. 2012;35(7):921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kushida CA, et al. . Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. [DOI] [PubMed] [Google Scholar]
- 14. Sutherland K, et al. . Facial phenotyping by quantitative photography reflects craniofacial morphology measured on magnetic resonance imaging in Icelandic sleep apnea patients. Sleep. 2014;37(5):959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwab RJ, et al. . Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am J Respir Crit Care Med. 2015;191(11):1295–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bergstralh EJ, et al. . Software for optimal matching in observational studies. Epidemiology. 1996;7(3):331–332. [PubMed] [Google Scholar]
- 17. Rosenbaum PR. Optimal matching for observational studies. J Am Statist Assoc. 1989;84(408):1024–1032. [Google Scholar]
- 18. Chi L, et al. . Heritability of craniofacial structures in normal subjects and patients with sleep apnea. Sleep. 2014;37(10):1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badr MS, et al. . Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol (1985). 1995;78(5):1806–1815. [DOI] [PubMed] [Google Scholar]
- 20. Huon LK, et al. . Dynamic upper airway collapse observed from sleep MRI: BMI-matched severe and mild OSA patients. Eur Arch Otorhinolaryngol. 2016;273(11):4021–4026. [DOI] [PubMed] [Google Scholar]
- 21. Trudo FJ, et al. . State-related changes in upper airway caliber and surrounding soft-tissue structures in normal subjects. Am J Respir Crit Care Med. 1998;158(4):1259–1270. [DOI] [PubMed] [Google Scholar]
- 22. He W, et al. . Greater abdominal fat accumulation is associated with higher metabolic risk in Chinese than in white people: an ethnicity study. PLoS One. 2013;8(3):e58688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim U, et al. . Propensity for intra-abdominal and hepatic adiposity varies among ethnic groups. Gastroenterology. 2019;156(4):966–975.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathur R, et al. . Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med. 1995;122(3):174–178. [DOI] [PubMed] [Google Scholar]
- 25. Schwab RJ, et al. . Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173(4):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radia S, et al. . Relationship between maxillary central incisor proportions and facial proportions. J Prosthet Dent. 2016;115(6):741–748. [DOI] [PubMed] [Google Scholar]
- 27. Rosas A, et al. . Sexual dimorphism in the Atapuerca-SH hominids: the evidence from the mandibles. J Hum Evol. 2002;42(4):451–474. [DOI] [PubMed] [Google Scholar]
- 28. Eckert DJ, et al. . Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guilleminault C, et al. . Familial aggregates in obstructive sleep apnea syndrome. Chest. 1995;107(6):1545–1551. [DOI] [PubMed] [Google Scholar]
- 30. Savoye I, et al. . A genetic study of anteroposterior and vertical facial proportions using model-fitting. Angle Orthod. 1998;68(5):467–470. [DOI] [PubMed] [Google Scholar]
- 31. Shaffer JR, et al. . Genome-wide association study reveals multiple loci influencing normal human facial morphology. PLoS Genet. 2016;12(8):e1006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fietze I, et al. . Night-to-night variation of the oxygen desaturation index in sleep apnoea syndrome. Eur Respir J. 2004;24(6):987–993. [DOI] [PubMed] [Google Scholar]
- 33. Hui DS, et al. . Cephalometric assessment of craniofacial morphology in Chinese patients with obstructive sleep apnoea. Respir Med. 2003;97(6):640–646. [DOI] [PubMed] [Google Scholar]
- 34. Hoekema A, et al. . Craniofacial morphology and obstructive sleep apnoea: a cephalometric analysis. J Oral Rehabil. 2003;30(7):690–696. [DOI] [PubMed] [Google Scholar]
- 35. O’Driscoll DM, et al. . The physiological phenotype of obstructive sleep apnea differs between Caucasian and Chinese patients. Sleep. 2019;42(11). doi:10.1093/sleep/zsz186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.