Abstract
The bovine immune system is known for its unusual traits relating to immunoglobulin and antiviral responses. Peptidylarginine deiminases (PADs) are phylogenetically conserved enzymes that cause post-translational deimination, contributing to protein moonlighting in health and disease. PADs also regulate extracellular vesicle (EV) release, forming a critical part of cellular communication. As PAD-mediated mechanisms in bovine immunology and physiology remain to be investigated, this study profiled deimination signatures in serum and serum-EVs in Bos taurus. Bos EVs were poly-dispersed in a 70–500 nm size range and showed differences in deiminated protein cargo, compared with whole sera. Key immune, metabolic and gene regulatory proteins were identified to be post-translationally deiminated with some overlapping hits in sera and EVs (e.g., immunoglobulins), while some were unique to either serum or serum-EVs (e.g., histones). Protein–protein interaction network analysis of deiminated proteins revealed KEGG pathways common for serum and serum-EVs, including complement and coagulation cascades, viral infection (enveloped viruses), viral myocarditis, bacterial and parasitic infections, autoimmune disease, immunodeficiency intestinal IgA production, B-cell receptor signalling, natural killer cell mediated cytotoxicity, platelet activation and hematopoiesis, alongside metabolic pathways including ferroptosis, vitamin digestion and absorption, cholesterol metabolism and mineral absorption. KEGG pathways specific to EVs related to HIF-1 signalling, oestrogen signalling and biosynthesis of amino acids. KEGG pathways specific for serum only, related to Epstein–Barr virus infection, transcription mis-regulation in cancer, bladder cancer, Rap1 signalling pathway, calcium signalling pathway and ECM-receptor interaction. This indicates differences in physiological and pathological pathways for deiminated proteins in serum-EVs, compared with serum. Our findings may shed light on pathways underlying a number of pathological and anti-pathogenic (viral, bacterial, parasitic) pathways, with putative translatable value to human pathologies, zoonotic diseases and development of therapies for infections, including anti-viral therapies.
Keywords: peptidylarginine deiminases (PADs), protein deimination, bovine (Bos taurus), extracellular vesicles (EVs), immunity, metabolism, anti-viral
1. Introduction
Cattle are mammalian ruminants of the genus Bos, comprised of domesticated and wild cattle with five main extant (living) species [1]. The lifespan of Bos is 18-25 years in the wild and as cattle are valuable livestock that form an important part of food security, bovine research is important for livestock management. Furthermore cows fall under a group of long-lived mammals that display considerable cancer resistance [2]. With considerably long life spans and unusual immunological characteristics cows may hold information for molecular pathways underlying such physiological traits. The bovine immune system has received considerable attention in the medical field due to its unique immunoglobulin traits, including exceptional ability to reach recessed viral epitopes on enveloped viruses. Therefore, a particular research focus has been on their unusual ultralong CDR3H “cattlebodies”, which are being developed for immunotherapy, including against retroviral infections such as HIV [3,4,5].
Peptidylarginine deiminases (PADs) are phylogenetically conserved calcium-dependent enzymes which cause an irreversible post-translational conversion of arginine to citrulline in target proteins. This modification causes structural, and sometimes functional, changes of target cytoskeletal, cytoplasmic, mitochondrial and nuclear proteins, including loss or gain of function or denaturation. Deimination can furthermore cause the generation of neo-epitopes and affect gene regulation [6,7,8,9,10,11]. This post-translational modification is most effective on beta-sheets and disordered proteins [7] and can also facilitate protein moonlighting, where one polypeptide can exhibit multifaceted functions that are physiologically relevant. As this is an evolutionarily acquired phenomenon, moonlighting facilitated by post-translational changes, such as deimination, may contribute to protein’s diverse and conserved functions throughout phylogeny [12,13]. PADs are identified throughout phylogeny from bacteria to mammals. In mammals, five tissue specific PAD isozymes with deimination activity are described, three in chicken and alligator, one in bony and cartilaginous fish [6,14,15,16,17], and PAD homologues (arginine deiminases, ADI) in parasites [18], fungi [19] and bacteria [20,21]. While in Bos taurus five PAD isozymes have been reported: PAD1 (NP_001094742.1), PAD2 (NP_001098922.1), PAD3 (XP_010800991.1), PAD4 (NP_001179102.1) and PAD6 (XP_002685843.1), few studies, besides assessment of cattle PAD ability to deiminate human myelin basic protein [22] and inhibitory effects of paclitaxel on PAD activity in bovine brain extract [23], have hitherto been carried out on PAD protein function, or on putative physiological relevance for PAD-mediated post-translational deimination in cattle.
PADs play important roles in a range of pathologies, including chronic, autoimmune and neurodegenerative diseases, as well as in cancer [9,10,11,24]. PADs also play roles in hypoxia and CNS regeneration [25,26,27,28,29], and PAD-mediated mechanisms have been related to ageing [30,31]. Importantly, PADs have also been implicated in infection, including sepsis, endotoxemia [32,33,34,35,36,37,38,39], in antibiotic resistance [21] and other anti-pathogenic responses, including anti-viral ones [37,40,41]. Roles for anti-viral responses via PAD-mediated neutrophil extracellular trap formation (NETosis) have furthermore been identified in cattle respiratory syncytial virus disease, via the detection of deiminated/citrullinated histone H3 [42]. Roles for PADs in tissue remodeling and immunity have also recently been described [15,16,43]. PADs have furthermore been identified as phylogenetically conserved key regulators of cellular extracellular vesicle (EV) release [21,44,45,46]. EV-mediated cellular communication is a phylogenetically conserved phenomenon [47], with EVs transferring cargo proteins and genetic material characteristic of the cells of origin [11,48,49,50,51]. As EV cargo is comprised of a large range of proteins, enzymes and genetic material, and as EVs can easily be isolated from a range of body fluids, including serum and plasma, EV signatures can be useful biomarkers [52,53].
While work on EVs has largely focused on human pathologies [53], EVs are gaining increasing interest also in veterinary medicine, including in cattle [47,54,55,56,57]. Studies on EVs in cattle have been in relation to host-pathogen interactions [58,59,60,61], including in bovine respiratory disease [62,63] and in response to infection [64,65], as well as for export of viral proteins [66,67,68]. EV research in cattle has furthermore been in relation to the estrous cycle, fertility and reproduction [56,69,70,71,72,73,74], as well as for roles during embryonic development [75,76,77,78]. Bovine milk EVs have been assessed for biological activities [79,80,81,82,83], for the application of milk EVs as safe drug delivery vehicles [84,85,86], as well as assessment of viral transfer via milk to calves [87]. Bovine milk EVs have also been investigated as an anti-inflammatory treatment in autoimmune disease [88,89] and in necrotizing enterocolitis [90]. Cattle EVs have furthermore been investigated in relation to neurological disease, including bovine spongiform encephalopathy (BSE) [54].
A recent body of comparative studies with respect to EVs and EV cargo has been performed in a range of taxa throughout the phylogenetic tree, including with a particular emphasis on deiminated protein cargo by our group [17,91,92,93,94,95,96,97,98,99] Hitherto though, no such evaluation of post-translational protein cargo in cattle serum or serum-EVs has been carried out and therefore warrants further exploration. In the current study, post-translationally deiminated protein signatures were assessed in serum and serum-EVs of Bos taurus. We report for the first time post-translational deimination of key immune, metabolic and nuclear proteins in cattle, and identify differences in KEGG pathways enriched for deiminated proteins in serum-EVs compared to whole serum. Our findings provide novel insight into the unusual immunological traits of cattle, including a new angle on their unusual anti-viral activity. Our findings further current understanding of protein moonlighting via deimination in physiological and immunological pathways, underlying anti-pathogenic responses throughout phylogeny.
2. Results
2.1. Characterisation of Bovine Serum-EVs
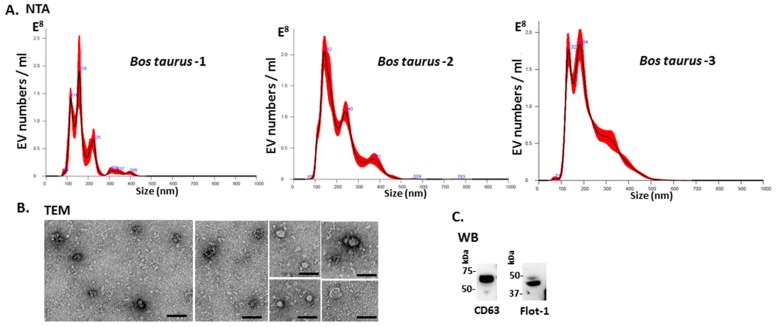
Bos taurus serum-EVs were assessed by nanoparticle tracking analysis (NTA) for particle numbers and size distribution using the NanoSight NS300 system, revealing a poly-dispersed population of EVs in the size range of 70–500 nm (Figure 1A). Further characterization was performed by western blotting using the EV-specific markers CD63 and Flot-1 (Figure 1B), which showed positive at protein band sizes corresponding to what is observed for these EV markers in other taxa, including in human [17,46,91,92,93,96,97,98,99], and by transmission electron microscopy (TEM), confirming typical EV morphology (Figure 1C). Some variation was observed between the three individuals with respect to EV yield, ranging from 1.72 × 1010 to 2.46 × 1010 per mL, and modal EV size, which fell in the range of 146–165 nm.
Figure 1.
Extracellular vesicle profiling in bovine serum. (A) Nanoparticle tracking analysis shows a size distribution of plasma-EVs from Bos taurus in the size range of 70 to 500 nm, with main peaks at approximately 120–240 nm. (B) Transmission electron microscopy (TEM) analysis of bovine serum-derived EVs shows typical EV morphology; scale bar is 50 nm in all figures. (C) Western blotting analysis confirms that bovine EVs are positive for the phylogenetically conserved EV-specific markers CD63 and Flot-1, showing positive at expected molecular weight size corresponding to what is observed in other taxa (kDa = kilodaltons).
2.2. PAD Protein Homologues and Deiminated Proteins in Bovine Serum and Serum-EVs
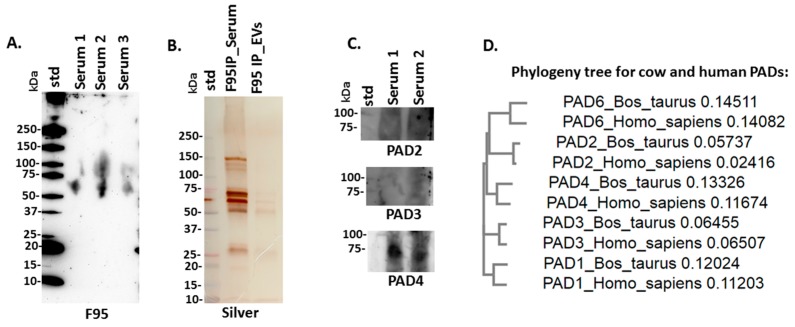
For assessment of total deiminated proteins present in serum, F95-positive proteins were detected by western blotting in the size range of mainly 50–150 kDa (Figure 2A). Following immunoprecipitation for F95-enrichment of deiminated proteins in serum and serum-EVs, silverstaining revealed F95-enriched protein bands between 15–150 kDa in serum and in EVs protein bands were observed mainly in the size range of 50–150 kDa (Figure 2B). For assessment of bovine PAD protein homologues, anti-human PAD-isozyme specific antibodies were used for western blotting, identifying a positive protein band at 70–75 kDa mainly cross-reacting with anti-human PAD4 antibody in bovine serum (Figure 2C). A neighbor-joining phylogeny tree was furthermore constructed for bovine PADs (PAD1, NP_001094742.1; PAD2, NP_001098922.1; PAD3, XP_010800991.1; PAD4, NP_001179102.1 and PAD6, XP_002685843.1) compared with human PADs (PAD1, NP_037490.2; PAD2, NP_031391.2; PAD3, NP_057317.2; PAD4, NP_036519.2 and PAD6, NP_997304.3), using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/), showing that the individual bovine and human PAD isoforms clustered together (Figure 2D).
Figure 2.
Deiminated proteins and peptidylarginine deiminases (PADs) in bovine serum. (A) Total deiminated proteins were identified in bovine serum using the pan-deimination specific F95 antibody. (B) F95-enriched IP fraction from bovine serum and serum-extracellular vesicles (EVs), shown by silver-staining. (C) Immunodetection of PAD homologues in bovine sera by western blotting, using anti-human PAD2, PAD3 and PAD4 antibodies. (D) A neighbor-joining phylogeny tree for bovine and human PAD isozymes.
2.3. LC-MS/MS Analysis of Deiminated Proteins in Bovine Serum and Serum-EVs
Protein identification of deiminated proteins in bovine serum and serum-EVs was carried out using F95-enrichment and LC-MS/MS analysis, searching for species-specific protein hits using the Bos taurus protein database. In serum, 118 species-specific deiminated protein hits were identified and 31 were specific to total serum only (Table 1; see detailed analysis for all hits in Supplementary Table S1). In serum-EVs, 179 species-specific deiminated protein hits were identified and 90 were specific to EVs only (Table 2; see detailed analysis for all hits in Supplementary Table S2).
Table 1.
Deiminated proteins in bovine serum (Bos taurus), as identified by F95-enrichment and LC-MS/MS analysis. Deiminated proteins from serum were isolated by immunoprecipitation using the pan-deimination F95 antibody. The resulting F95-enriched eluate was then analysed by LC-MS/MS and peak list files submitted to Mascot. Bos taurus species-specific peptide sequence hits are listed, showing number of sequences for protein hits and total score. Blue highlighted rows indicate protein hits identified in whole sera only. Uncharacterised proteins based on LC-MS/MS search were further confirmed in STRING, based on the protein symbol, and protein identification from STRING, where retrieved, is shown in brackets ([]). For further full LC-MS/MS analysis of all protein hits see Supplementary Table S1.
| Protein Name | Symbol | Sequences | Total Score (p < 0.05) 1 |
|---|---|---|---|
| Serotransferrin | G3X6N3 | 76 | 5712 |
| Serotransferrin | Q29443 | 76 | 5711 |
| Complement factor H | Q28085 | 77 | 5123 |
| Alpha-2-macroglobulin | Q7SIH1 | 77 | 5079 |
| Serum albumin | A0A140T897 | 59 | 3944 |
| Uncharacterised protein [complement factor H] | F1MC45 | 54 | 3745 |
| Complement C3 | Q2UVX4 | 68 | 3483 |
| Uncharacterised protein [ceruloplasmin] | F1N076 | 44 | 2712 |
| Embryo-specific fibronectin 1 | B8Y9S9 | 37 | 2263 |
| Hemopexin | Q3SZV7 | 30 | 2019 |
| Uncharacterised protein [complement C4A] | E1BH06 | 34 | 1997 |
| C4b-binding protein alpha chain | Q28065 | 33 | 1841 |
| Kininogen-1 | A0A140T8C8 | 23 | 1505 |
| Kininogen-1 | P01044 | 22 | 1449 |
| Histidine-rich glycoprotein | F1MKS5 | 26 | 1432 |
| Uncharacterised protein (uncharacterised) | F1MJK3 | 26 | 1291 |
| Kininogen-2 | P01045 | 19 | 1271 |
| Uncharacterised protein (uncharacterised) | G3X7F3 | 16 | 1259 |
| Uncharacterised protein (uncharacterised) | G3N0V0 | 19 | 1186 |
| Apolipoprotein A-I preproprotein | V6F9A2 | 19 | 1122 |
| Uncharacterised protein (uncharacterised) | F1MVK1 | 17 | 953 |
| SERPIND1 protein | A6QPP2 | 16 | 865 |
| Uncharacterised protein (uncharacterised) | F1MZ96 | 13 | 837 |
| Uncharacterised protein [Bos taurus immunoglobulin lambda-like polypeptide 1 (IGLL1)] | F1MLW7 | 10 | 815 |
| Uncharacterised protein [Bos taurus immunoglobulin lambda-like polypeptide 1 (IGLL1)] | F1MCF8 | 10 | 796 |
| Uncharacterised protein [Immunoglobulin heavy constant mu] | G5E5T5 | 12 | 784 |
| Uncharacterised protein [Immunoglobulin heavy constant mu] | G5E513 | 10 | 732 |
| Uncharacterised protein (uncharacterised) | G3N0S9 | 12 | 648 |
| Histidine-rich glycoprotein | P33433 | 12 | 623 |
| Alpha-2-antiplasmin | P28800 | 11 | 531 |
| Uncharacterised protein (uncharacterised) | F1MLW8 | 5 | 434 |
| Hemoglobin subunit beta | P02070 | 6 | 422 |
| Vitamin D-binding protein | F1N5M2 | 9 | 420 |
| Alpha-1-antiproteinase | P34955 | 8 | 418 |
| Adiponectin | Q3Y5Z3 | 6 | 411 |
| Uncharacterised protein (uncharacterised) | F1MW79 | 7 | 403 |
| ECM1 protein | A5PJT7 | 8 | 395 |
| Complement C1q subcomponent subunit B | Q2KIV9 | 8 | 390 |
| Plasma kallikrein | Q2KJ63 | 8 | 385 |
| Inter-alpha-trypsin inhibitor heavy chain H4 | F1MMD7 | 6 | 367 |
| C1QC protein | Q1RMH5 | 5 | 347 |
| Uncharacterised protein [Serpin A3-5; Serine protease inhibitor] | G8JKW7 | 6 | 338 |
| Apolipoprotein A-IV | F1N3Q7 | 7 | 338 |
| Inter-alpha-trypsin inhibitor heavy chain H2 | F1MNW4 | 6 | 338 |
| Selenoprotein P | P49907 | 6 | 323 |
| Plasminogen | E1B726 | 6 | 302 |
| Complement C1q subcomponent subunit A | Q5E9E3 | 5 | 300 |
| Uncharacterised protein [Bos taurus insulin-like growth factor binding protein, acid labile subunit (IGFALS)] | F1MJZ4 | 6 | 277 |
| Thrombospondin-1 | F1N3A1 | 7 | 273 |
| Serpin A3-3 | G3N1U4 | 5 | 271 |
| Protein HP-20 homolog | Q2KIT0 | 5 | 269 |
| Uncharacterised protein (uncharacterised) | G5E604 | 4 | 234 |
| Inter-alpha-trypsin inhibitor heavy chain H1 | Q0VCM5 | 4 | 234 |
| Uncharacterised protein (uncharacterised) | E1B805 | 5 | 232 |
| Uncharacterised protein (uncharacterised) | G3MXD9 | 3 | 209 |
| Uncharacterised protein [Bos taurus immunoglobulin lambda-like polypeptide 1 (IGLL1)] | G3N2D7 | 3 | 204 |
| Keratin, type II cytoskeletal 7 | Q29S21 | 4 | 199 |
| Keratin, type I cytoskeletal 14 | F1MC11 | 4 | 199 |
| Uncharacterised protein [Heparan sulfate proteoglycan 2] | F1MER7 | 4 | 195 |
| Alpha-2-HS-glycoprotein | cRAPR1|FETUA_BOVIN | 4 | 190 |
| Uncharacterised protein (uncharacterised) | G3N1H5 | 2 | 187 |
| Serpin A3-8 | A0A0A0MP89 | 3 | 184 |
| Complement factor B | P81187 | 4 | 183 |
| Protein AMBP | F1MMK9 | 5 | 181 |
| Serpin A3-2 | A2I7M9 | 4 | 180 |
| Antithrombin-III | F1MSZ6 | 5 | 176 |
| Paraoxonase 1 | Q2KIW1 | 4 | 174 |
| Gelsolin | F1MJH1 | 3 | 171 |
| Uncharacterised protein (uncharacterised) | G3N3Q3 | 2 | 168 |
| Complement C5a anaphylatoxin | F1MY85 | 3 | 166 |
| Uncharacterised protein (uncharacterised) | G5E5H2 | 3 | 162 |
| Serpin A3-7 | A0A0A0MP92 | 4 | 160 |
| C1QTNF3 protein | A7MB82 | 3 | 160 |
| Uncharacterised protein [Bos taurus serotransferrin-like] | E1BI82 | 4 | 157 |
| Uncharacterised protein [Apolipoprotein B] | E1BNR0 | 4 | 150 |
| Primary amine oxidase, liver isozyme | Q29437 | 3 | 137 |
| Coagulation factor XI | F1MUT4 | 2 | 127 |
| Hepatocyte growth factor-like protein | E1BDW7 | 2 | 121 |
| Fibrinogen beta chain | F1MAV0 | 3 | 108 |
| Immunoglobulin J chain | Q3SYR8 | 2 | 107 |
| Uncharacterised protein (uncharacterised) | G3MXB5 | 2 | 100 |
| Uncharacterised protein [CD5 molecule-like] | F1N514 | 2 | 94 |
| Uncharacterised protein (uncharacterised) | G3MXG6 | 2 | 94 |
| Complement component C9 | Q3MHN2 | 2 | 87 |
| Complement component C6 | F1MM86 | 3 | 86 |
| Uncharacterised protein (uncharacterised) | G5E5V1 | 2 | 86 |
| Hemoglobin subunit alpha | P01966 | 2 | 85 |
| Uncharacterised protein (uncharacterised) | G3N028 | 2 | 75 |
| Fibrinogen alpha chain | A5PJE3 | 1 | 74 |
| Vitronectin | Q3ZBS7 | 2 | 73 |
| Alpha-1B-glycoprotein | Q2KJF1 | 1 | 66 |
| Coagulation factor XII | F1MTT3 | 1 | 65 |
| Uncharacterised protein (uncharacterised) | F1MSF0 | 2 | 63 |
| Prothrombin | P00735 | 1 | 61 |
| Uncharacterised protein [Nidogen 1] | F1MWN3 | 1 | 57 |
| Protein HP-25 homolog 2 | Q2KIU3 | 1 | 57 |
| Clusterin | F1MWI1 | 1 | 55 |
| Contactin-1 | F1MVI0 | 1 | 54 |
| Polymeric immunoglobulin receptor | F1MR22 | 2 | 51 |
| Uncharacterised protein [Sperm associated antigen 9] | F1MZ69 | 2 | 51 |
| Uncharacterised protein [Ankyrin repeat domain 11] | E1BAT5 | 2 | 51 |
| Beta-2-glycoprotein 1 | A0A140T843 | 1 | 50 |
| Carboxypeptidase N catalytic chain | Q2KJ83 | 1 | 49 |
| Uncharacterised protein [complement component 8, alpha polypeptide (C8A)] | F1MX87 | 1 | 48 |
| Fibrinogen gamma-B chain | F1MGU7 | 1 | 48 |
| Uncharacterised protein [ADAM metallopeptidase with thrombospondin type 1 motif, 13] | F1MVP0 | 1 | 47 |
| Uncharacterised protein [Talin 1] | F1MDH3 | 1 | 46 |
| Complement C1s subcomponent | Q0VCX1 | 1 | 46 |
| Uncharacterised protein [CL43—Collectin-43] | F1MFY6 | 1 | 45 |
| Complement component C7 | F1N045 | 1 | 44 |
| CLEC11A protein | A5D7L1 | 1 | 43 |
| Uncharacterised protein [KCTD12—Potassium channel tetramerization domain containing 12] | G3N3D4 | 1 | 43 |
| Uncharacterised protein [BRIP1—BRCA1 interacting protein C-terminal helicase 1] | E1BNG9 | 1 | 39 |
| Cadherin-1 | F1N619 | 1 | 39 |
| Ubiquitin carboxyl-terminal hydrolase isozyme L5 | Q9XSJ0 | 1 | 38 |
| CPN2 protein | A6QP30 | 1 | 37 |
| Acyl-coenzyme A thioesterase THEM4 | A1A4L1 | 1 | 37 |
| Uncharacterised protein [SLAMF9—SLAM family member 9] | E1BNF9 | 1 | 36 |
| Uncharacterised protein [MED12; Mediator complex subunit 12] | F1MZ95 | 1 | 35 |
| Transthyretin | O46375 | 1 | 33 |
| Voltage-dependent L-type calcium channel subunit beta-3 | Q9MZL3 | 1 | 33 |
1 Ions score is −10*Log(P), where P is the probability that the observed match is a random event. Individual ions scores > 31 indicated identity or extensive homology (p < 0.05). Protein scores were derived from ions scores as a non-probabilistic basis for ranking protein hits.
Table 2.
Deiminated proteins in serum-EVs of cow (Bos taurus) as identified by F95-enrichment. Deiminated proteins from serum-EVs were isolated by immunoprecipitation using the pan-deimination F95 antibody. The resulting F95-enriched eluate was then analysed by LC-MS/MS and peak list files submitted to Mascot. Bos taurus species-specific peptide sequence hits are listed, showing number of sequences for protein hits and total score. Rows highlighted in pink indicate protein hits identified in serum-EVs only. Uncharacterised proteins based on LC-MS/MS search were further confirmed in STRING, based on the protein symbol, and protein identification from STRING, where retrieved, is shown in brackets ([]).For further full LC-MS/MS analysis of all protein hits see Supplementary Table S2.
| Protein Name | Symbol | Sequences | Total Score (p < 0.05) 1 |
|---|---|---|---|
| Serotransferrin | G3X6N3 | 75 | 4958 |
| Serotransferrin | Q29443 | 74 | 4941 |
| Complement factor H | Q28085 | 85 | 4700 |
| Alpha-2-macroglobulin | Q7SIH1 | 77 | 4633 |
| Serum albumin | A0A140T897 | 76 | 4604 |
| Complement C3 | Q2UVX4 | 83 | 4258 |
| Uncharacterised protein (CFH—Complement factor H) | F1MC45 | 61 | 3469 |
| Fibronectin | G5E5A9 | 56 | 2492 |
| Uncharacterised protein (Ceruloplasmin) | F1N076 | 37 | 1994 |
| Uncharacterised protein (uncharacterised) | F1MJK3 | 38 | 1870 |
| Uncharacterised protein (C4A—Complement C4) | E1BH06 | 35 | 1840 |
| C4b-binding protein alpha chain | Q28065 | 36 | 1761 |
| Keratin, type II cytoskeletal 5 | M0QVZ6 | 35 | 1750 |
| Hemopexin | Q3SZV7 | 30 | 1594 |
| Keratin, type I cytoskeletal 14 | F1MC11 | 29 | 1592 |
| Uncharacterised protein (uncharacterised) | G3N0V0 | 25 | 1542 |
| Histidine-rich glycoprotein | F1MKS5 | 26 | 1407 |
| Uncharacterised protein (keratin 33A (KRT33A)) | F1MXG6 | 22 | 1311 |
| KRT33A protein | A5PJJ1 | 22 | 1306 |
| Keratin 31 | Q148I8 | 22 | 1291 |
| Uncharacterised protein (uncharacterised) | G3X7F3 | 20 | 1288 |
| Keratin, type II cuticular Hb1 | Q148H4 | 25 | 1225 |
| Uncharacterised protein (keratin 34 (KRT34)) | F1MSA6 | 21 | 1209 |
| Kininogen-1 | A0A140T8C8 | 21 | 1188 |
| Kininogen-1 | P01044 | 21 | 1164 |
| Uncharacterised protein (Desmoplakin) | E1BKT9 | 27 | 1156 |
| Uncharacterised protein (keratin 86 (KRT86)) | E1B898 | 22 | 1104 |
| Apolipoprotein A-I preproprotein | V6F9A2 | 22 | 1101 |
| Uncharacterised protein (uncharacterised) | F1MVK1 | 20 | 1066 |
| Uncharacterised protein (KRT6A—Keratin, type II cytoskeletal 59 kDa, component IV) | M0QVY0 | 21 | 1049 |
| Kininogen-2 | P01045 | 19 | 1042 |
| Uncharacterised protein (KRT3—Keratin, type II cytoskeletal 68 kDa, component IB) | G3MXL3 | 21 | 1034 |
| Junction plakoglobin | Q8SPJ1 | 21 | 987 |
| Keratin, type II cytoskeletal | Q08D91 | 19 | 971 |
| Uncharacterised protein (Immunoglobulin heavy constant mu) | G5E5T5 | 16 | 938 |
| Keratin, type I cytoskeletal 17 | A0A140T867 | 17 | 890 |
| Uncharacterised protein (IGLL1—immunoglobulin lambda-like polypeptide 1) | F1MCF8 | 12 | 886 |
| Uncharacterised protein (uncharacterised) | F1MH40 | 13 | 872 |
| Uncharacterised protein (uncharacterised) | F1MZ96 | 14 | 863 |
| Keratin 10 (Epidermolytic hyperkeratosis; keratosis palmaris et plantaris) | A6QNZ7 | 15 | 854 |
| Uncharacterised protein (uncharacterised) | F1MLW7 | 10 | 849 |
| Complement C4 | P01030 | 16 | 826 |
| Uncharacterised protein (Immunoglobulin heavy constant mu) | G5E513 | 15 | 810 |
| KRT15 protein | Q17QL7 | 13 | 734 |
| Uncharacterised protein (uncharacterised) | G3N0S9 | 14 | 631 |
| Keratin, type I cytoskeletal 19 | P08728 | 12 | 625 |
| KRT4 protein | A4IFP2 | 12 | 623 |
| SERPIND1 protein | A6QPP2 | 12 | 606 |
| Uncharacterised protein (uncharacterised) | F1MLW8 | 7 | 588 |
| Histidine-rich glycoprotein | P33433 | 9 | 566 |
| Inter-alpha-trypsin inhibitor heavy chain H2 | F1MNW4 | 12 | 554 |
| Inter-alpha-trypsin inhibitor heavy chain H4 | F1MMD7 | 10 | 521 |
| Vitamin D-binding protein | F1N5M2 | 12 | 517 |
| Antithrombin-III | F1MSZ6 | 11 | 492 |
| Plasminogen | P06868 | 14 | 485 |
| Apolipoprotein A-IV | Q32PJ2 | 11 | 475 |
| Serpin A3-3 | G3N1U4 | 9 | 442 |
| Complement factor B | P81187 | 9 | 429 |
| C1QC protein | Q1RMH5 | 8 | 428 |
| Complement C1q subcomponent subunit B | Q2KIV9 | 7 | 413 |
| Uncharacterised protein (serotransferrin-like) | E1BI82 | 8 | 411 |
| Uncharacterised protein (KRT16—Keratin 16) | G3X7W8 | 9 | 407 |
| Complement C1q subcomponent subunit A | Q5E9E3 | 7 | 401 |
| Serpin A3-4 | A2I7N0 | 9 | 383 |
| Alpha-1B-glycoprotein | Q2KJF1 | 7 | 381 |
| Uncharacterised protein (uncharacterised) | G3N3Q3 | 5 | 367 |
| Uncharacterised protein (Serpin A3-5) | G8JKW7 | 7 | 366 |
| Keratin, type II cytoskeletal 78 | A6QNX5 | 7 | 356 |
| Primary amine oxidase, liver isozyme | Q29437 | 8 | 352 |
| Serpin A3-2 | A2I7M9 | 7 | 333 |
| Uncharacterised protein (uncharacterised) | F1MW79 | 6 | 330 |
| Uncharacterised protein (uncharacterised) | G3N1H5 | 5 | 325 |
| Uncharacterised protein (KRT77—Keratin 77) | G3MYU2 | 6 | 310 |
| Uncharacterised protein (uncharacterised) | E1B805 | 7 | 309 |
| Uncharacterised protein (uncharacterised) | G3MXG6 | 4 | 305 |
| Uncharacterised protein (uncharacterised) | G3MXD9 | 5 | 300 |
| Uncharacterised protein (uncharacterised) | G3N2P6 | 5 | 292 |
| Uncharacterised protein (insulin-like growth factor binding protein, acid labile subunit (IGFALS)) | F1MJZ4 | 5 | 286 |
| Adiponectin | Q3Y5Z3 | 5 | 282 |
| Alpha-2-antiplasmin | P28800 | 7 | 271 |
| Protein AMBP | F1MMK9 | 6 | 268 |
| Vitronectin | Q3ZBS7 | 5 | 264 |
| Uncharacterised protein (uncharacterised) | G5E604 | 5 | 254 |
| Desmoglein-1 | F1MIW8 | 7 | 252 |
| Fibrinogen gamma-B chain | F1MGU7 | 5 | 252 |
| Uncharacterised protein (uncharacterised) | G3MY71 | 5 | 251 |
| Annexin A2 | P04272 | 5 | 244 |
| Fibrinogen alpha chain | A5PJE3 | 5 | 243 |
| Uncharacterised protein (uncharacterised) | G3MWT1 | 3 | 241 |
| Glyceraldehyde-3-phosphate dehydrogenase | P10096 | 6 | 238 |
| Serpin A3-7 | A0A0A0MP92 | 5 | 218 |
| Plakophilin-1 | Q28161 | 4 | 216 |
| Inter-alpha-trypsin inhibitor heavy chain H1 | Q0VCM5 | 5 | 213 |
| Hemoglobin subunit beta | P02070 | 4 | 212 |
| Alpha-1-antiproteinase | P34955 | 4 | 209 |
| Keratin, type II | A0JND2 | 4 | 198 |
| Actin, cytoplasmic 1 | F1MRD0 | 5 | 197 |
| Keratin, type I cytoskeletal 28 | Q148H6 | 4 | 194 |
| Thrombospondin-1 | F1N3A1 | 6 | 192 |
| Keratin, type I cytoskeletal 20 | F1MPK1 | 4 | 191 |
| ECM1 protein | A5PJT7 | 5 | 188 |
| Uncharacterised protein (Bos taurus immunoglobulin lambda-like polypeptide 1 (IGLL1)) | G3N2D7 | 3 | 186 |
| Selenoprotein P | P49907 | 5 | 184 |
| Uncharacterised protein (uncharacterised) | A0A0A0MPA0 | 4 | 176 |
| Gelsolin | F1MJH1 | 4 | 175 |
| Lactotransferrin | P24627 | 4 | 169 |
| Fibrinogen beta chain | F1MAV0 | 5 | 162 |
| Uncharacterised protein (uncharacterised) | G5E5H2 | 3 | 156 |
| Protein HP-20 homolog | Q2KIT0 | 3 | 151 |
| Uncharacterised protein (TGM1—Transglutaminase 1) | F1MBB7 | 2 | 143 |
| Actin, gamma-enteric smooth muscle | F1MKC4 | 4 | 143 |
| Uncharacterised protein (CD5L—CD5 molecule-like) | F1N514 | 4 | 131 |
| Complement component C7 | F1N045 | 4 | 128 |
| Uncharacterised protein (APOB—Apolipoprotein B) | E1BNR0 | 4 | 119 |
| Arginase-1 | Q2KJ64 | 3 | 119 |
| Alpha-S1-casein | CASA1_BOVIN | 2 | 115 |
| Uncharacterised protein (uncharacterised) | G3N028 | 2 | 114 |
| Alpha-2-HS-glycoprotein | cRAPR1|FETUA_BOVIN | 2 | 106 |
| Paraoxonase 1 | Q2KIW1 | 3 | 106 |
| Beta-2-glycoprotein 1 | A0A140T843 | 2 | 97 |
| Uncharacterised protein (uncharacterised) | G5E5V1 | 2 | 89 |
| Uncharacterised protein (Histone H2B family) | E1B7N8 | 2 | 88 |
| Complement component 1, r subcomponent | A5D9E9 | 3 | 83 |
| Histone H2A | A0A0A0MP90 | 2 | 80 |
| Uncharacterised protein (SLK-STE20-like kinase) | G3X696 | 3 | 80 |
| Prothrombin | P00735 | 2 | 79 |
| Peroxiredoxin-2 | Q9BGI3 | 3 | 79 |
| Uncharacterised protein (uncharacterised) | G3MZE0 | 1 | 69 |
| Uncharacterised protein (complement component 8, alpha polypeptide (C8A)) | F1MX87 | 1 | 69 |
| Uncharacterised protein (C1orf68—Chromosome 1 open reading frame 68) | G3N3D3 | 2 | 68 |
| Complement C5a anaphylatoxin | F1MY85 | 2 | 63 |
| Uncharacterised protein (complement factor I (CFI)) | F1N4M7 | 1 | 61 |
| Uncharacterised protein (armadillo repeat gene deleted in velocardiofacial syndrome (ARVCF)) | E1BPV1 | 2 | 59 |
| Uncharacterised protein (NEK10-Uncharacterised protein; NIMA-related kinase 10) | E1BHZ1 | 2 | 58 |
| Keratin associated protein 13-1 | A1A4M9 | 2 | 57 |
| Uncharacterised protein (uncharacterised) | F1MSF0 | 2 | 57 |
| Histone H4 | E1B9M9 | 1 | 56 |
| Gamma-glutamylcyclotransferase | Q32LE4 | 1 | 55 |
| Uncharacterised protein (JAKMIP2—Janus kinase and microtubule interacting protein 2) | G5E551 | 2 | 54 |
| TOX high mobility group box family member 4 | Q0P5K4 | 1 | 53 |
| Uncharacterised protein (TBC1D32—TBC1 domain family, member 32) | F1N7V1 | 2 | 51 |
| Proteasome subunit alpha type-6 | Q2YDE4 | 1 | 50 |
| Uncharacterised protein (KIAA1009 ortholog) | F1MZ01 | 2 | 50 |
| Uncharacterised protein (MYO5B—Myosin VB) | F1MMQ6 | 2 | 50 |
| Uncharacterised protein (Phospholipid phosphatase related 4) | F1MJ26 | 2 | 49 |
| Uncharacterised protein (MGA—MGA, MAX dimerization protein) | E1BKB7 | 2 | 49 |
| Uncharacterised protein (GOLGB1—Golgin B1) | E1BKZ5 | 2 | 47 |
| Histone H3 | E1BGN3 | 1 | 46 |
| Uncharacterised protein (CROCC—Ciliary rootlet coiled-coil, rootletin) | E1BBS9 | 3 | 46 |
| Protein FAM149B1 | A0JNF3 | 2 | 45 |
| Alpha-enolase | F1MB08 | 2 | 45 |
| Uncharacterised protein (UBR4—Ubiquitin protein ligase E3 component n-recognin 4) | E1BHT5 | 3 | 45 |
| Complement component C9 | Q3MHN2 | 1 | 44 |
| Uncharacterised protein (DOCK8—Bos taurus dedicator of cytokinesis 8 (DOCK8)) | E1BNA6 | 2 | 44 |
| Uncharacterised protein (KPRP—Keratinocyte proline-rich protein) | E1BLN6 | 1 | 44 |
| Uncharacterised protein (CCDC14—Coiled-coil domain containing 14) | F1MS02 | 2 | 43 |
| Uncharacterised protein (Strawberry notch homolog 1(Drosophila)) | E1BMP8 | 3 | 42 |
| DNA-directed RNA polymerase subunit beta | A5PJW8 | 1 | 42 |
| Uncharacterised protein (CRYGN-Crystallin, gamma N) | E1BDQ1 | 1 | 42 |
| Flotillin-1 | Q08DN8 | 1 | 41 |
| Uncharacterised protein (SMG6—Uncharacterised protein; Bos taurus smg-6 homolog, nonsense mediated mRNA decay factor (C. elegans) (SMG6)) | E1BFK4 | 2 | 41 |
| C1QTNF3 protein | A7MB82 | 1 | 41 |
| Uncharacterised protein (Protocadherin gamma subfamily B, 1) | F1MCA2 | 1 | 40 |
| Uncharacterised protein (Death-associated protein kinase 1) | F1MRL0 | 1 | 39 |
| Acyl-coenzyme A thioesterase THEM4 | A1A4L1 | 1 | 37 |
| Uncharacterised protein (Centrosomal protein 104kDa) | E1BND2 | 1 | 37 |
| C8G protein | A8YXZ2 | 1 | 36 |
| Uncharacterised protein (SERPINB12—Serpin family B member 12) | E1BDF5 | 1 | 36 |
| Uncharacterised protein (SLAMF9 SLAM family member 9) | E1BNF9 | 1 | 36 |
| Complement C1s subcomponent | Q0VCX1 | 1 | 36 |
| Uncharacterised protein (methyltransferase like 3 (METTL3)) | F1MX80 | 1 | 35 |
| Uncharacterised protein (SPAG9—Sperm associated antigen 9) | F1MZ69 | 1 | 35 |
| Adenosylhomocysteinase | Q3MHL4 | 1 | 35 |
| Uncharacterised protein (HIVEP3—Human immunodeficiency virus type I enhancer binding protein 3) | F1MBK6 | 1 | 34 |
| Coagulation factor V | F1N0I3 | 1 | 33 |
| Uncharacterised protein (VPREB1—Uncharacterised protein) | F1N160 | 1 | 33 |
| Uncharacterised protein (SERPING1) | E1BMJ0 | 1 | 33 |
| PPARD protein | A4IFL4 | 32 | |
| Uncharacterised protein | F1MZ93 | 1 | 32 |
| Uncharacterised protein (NCBP1—Nuclear cap binding protein subunit 1) | E1BMM0 | 1 | 32 |
| Transthyretin | O46375 | 1 | 32 |
| Uncharacterised protein (Cryptochrome-1) | F1MXB2 | 1 | 32 |
| Tubulin alpha chain | F1MNF8 | 1 | 31 |
1 Ions score is −10*Log(P), where P is the probability that the observed match is a random event. Individual ions scores > 31 indicated identity or extensive homology (p < 0.05). Protein scores were derived from ions scores as a non-probabilistic basis for ranking protein hits.
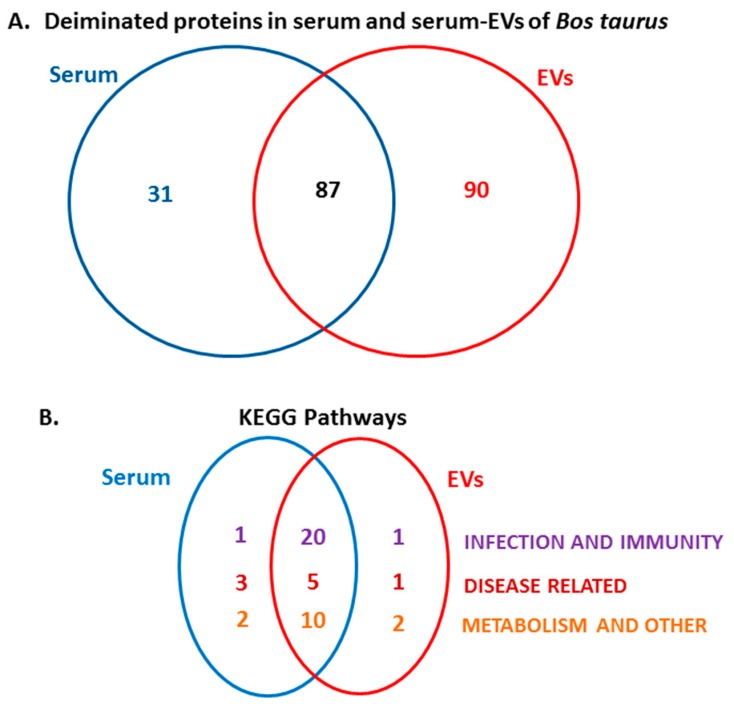
The Venn diagram in Figure 3 represents common and specific deimination hits in serum and serum-EVs. Overall 87 deiminated protein hits were common to serum and serum-EVs, while 31 hits were identified to be deiminated in serum only and 90 hits were identified to be deiminated in EVs only (Figure 3A). Following KEGG pathway analysis for these deiminated protein hits, a number of common KEGG pathways enriched in deiminated proteins were identified in serum and serum EVs, while differences were observed in KEGG pathways relating to infection, immunity, disease and metabolism, that were specific to serum or serum-EVs only, respectively (Figure 3B).
Figure 3.
Deiminated proteins identified in bovine serum and serum-EVs. (A) Species specific hits identified for deiminated proteins in bovine serum (Table 1) and serum-EVs (Table 2) respectively, as well as number of overlapping hits are presented. (B) KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways identified to be enriched in deiminated proteins in bovine serum and serum-EVs respectively, as well as number of overlapping KEGG pathways, are presented. For specific KEGG pathways presented in the Venn diagram, see the protein–protein interaction networks in Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8.
2.4. Protein–Protein Interaction Network Identification of Deiminated Proteins in Bovine Serum and Serum-EVs
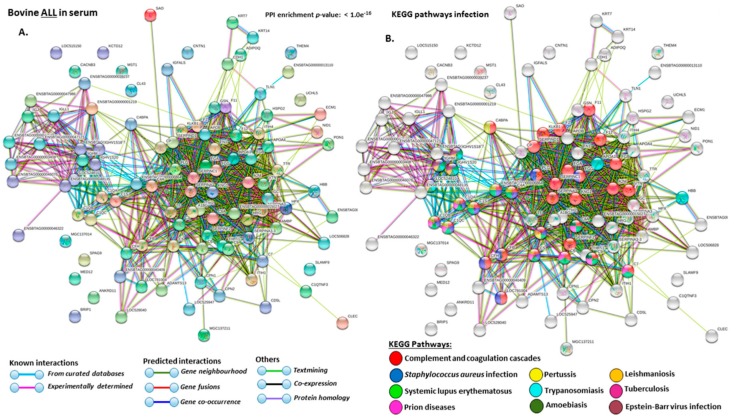
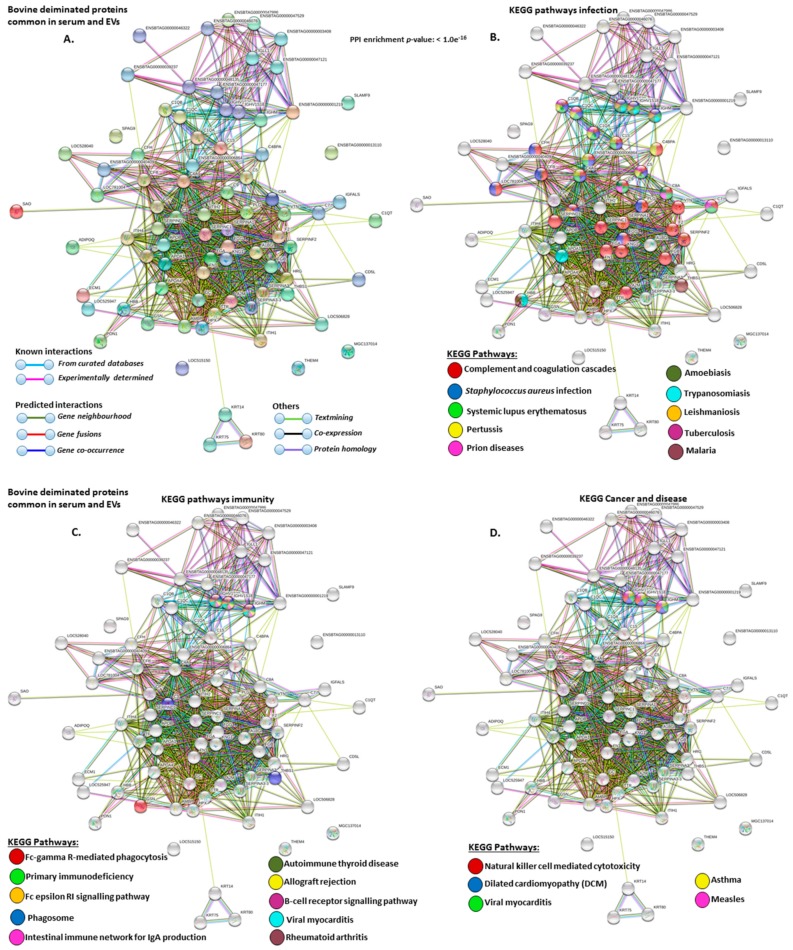
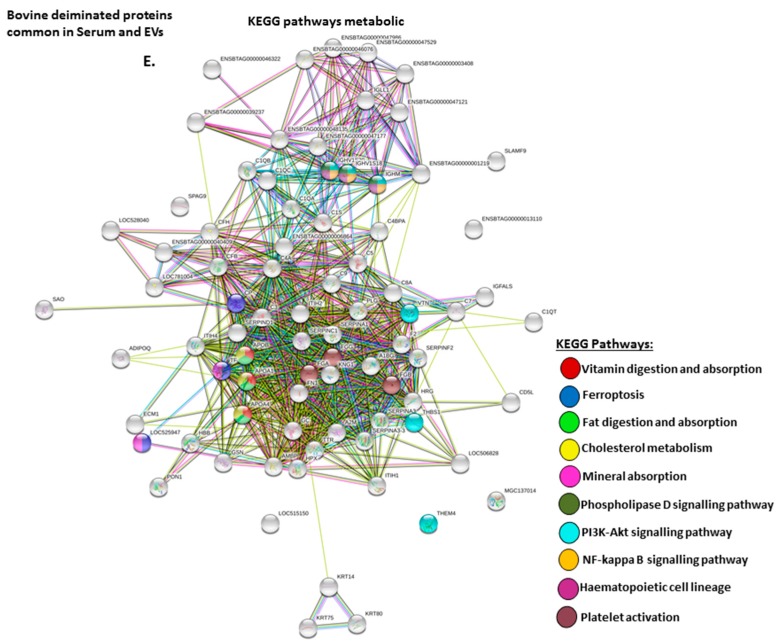
For the prediction of protein–protein interaction networks of the deimination candidate proteins, the protein ID lists for serum and serum-EVs respectively, were submitted to STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) analysis (https://string-db.org/) and analyzed for KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways (Figure 3B and Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8). Protein interaction networks were based on known and predicted interactions and represent all deiminated proteins identified in serum (Figure 4), all deiminated proteins identified in EVs (Figure 5) as well as deiminated proteins identified in serum only (Figure 6) or in EVs only (Figure 7), or common deimination candidates in EVs and serum (Figure 8). The PPI enrichment p-value for all deiminated proteins identified in bovine serum (based on protein identifier sequences) was found to be p < 1.0 × 10−16 and for all deiminated proteins identified in the serum-derived EVs, the PPI enrichment p-value was also found to be p < 1.0 × 10−16 (Figure 4 and Figure 5). For deiminated proteins identified in serum only (but not EVs) the PPI enrichment p-value was also p < 1.0 × 10−16 (Figure 6), while for deiminated proteins identified specifically in EVs, the PPI enrichment p-value was p = 4.83 × 10−8 (Figure 7). For common proteins found deiminated in serum and in serum-EVs, the PPI enrichment p-value was p < 1.0 × 10−16 (Figure 8). This indicates that in all cases, the identified protein networks have significantly more interactions than what would be expected for a random set of proteins of similar size, based on information drawn from the genome.
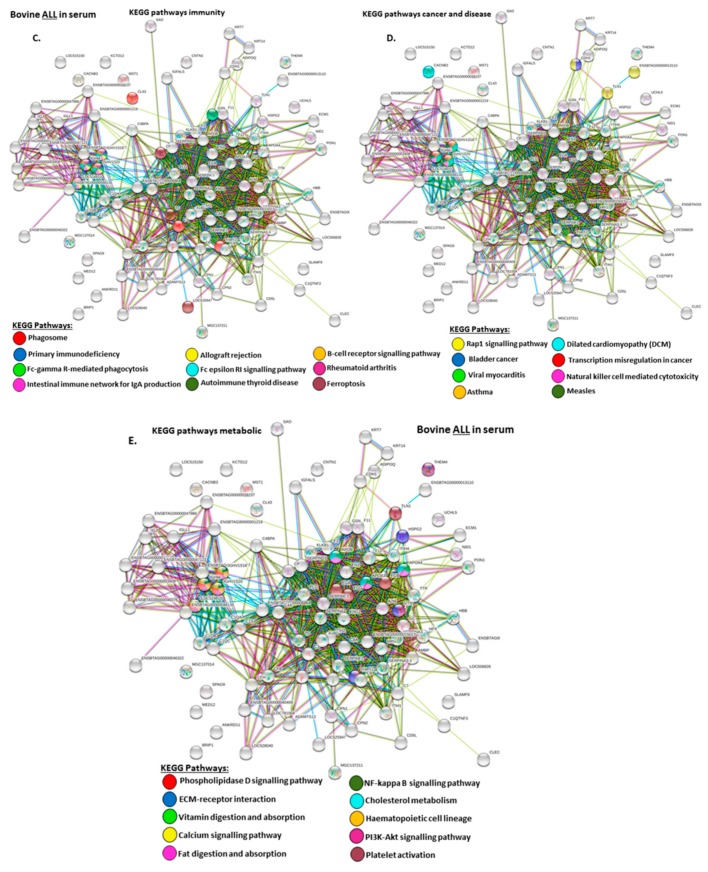
Figure 4.
Protein–protein interaction networks show all deiminated proteins which were identified in bovine serum. Protein–protein interactions were reconstructed based on both known and predicted interactions in serum of Bos taurus, using STRING analysis. (A) Query proteins are indicated by the coloured nodes represent and first shell of interactors. (B) KEGG pathways relating to the identified proteins and reported in STRING and relating to infection are highlighted (see colour code included in the figure). (C). KEGG pathways relating to the identified proteins and reported in STRING are highlighted for immunity (see colour code included in the figure). (D). KEGG pathways relating to cancer and disease for deiminated proteins identified are highlighted (see colour code included in the figure). (E). KEEG pathways relating to metabolism for deiminated proteins identified are highlighted (see the colour code included in the figure). The coloured lines highlight which protein interactions are identified through known interactions (this refers to curated databases, experimentally determined), through predicted interactions (this refers to gene neighborhood, gene fusion, gene co-occurrence) or through co-expression, text mining or protein homology (the colour key for connective lines is included in the figure).
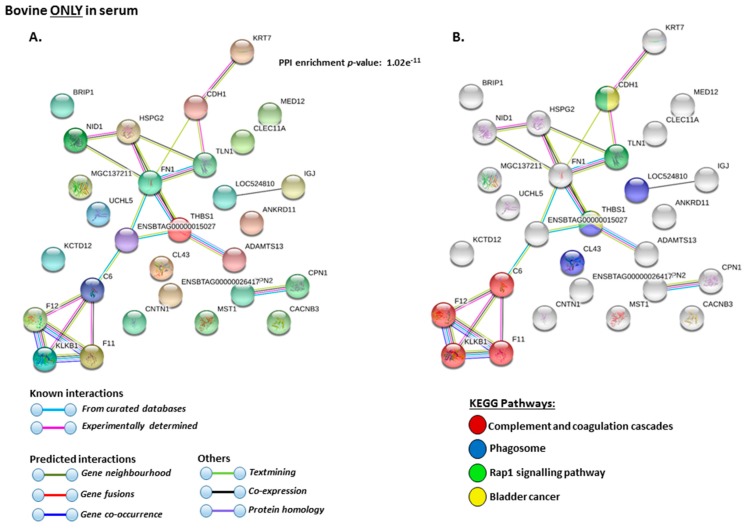
Figure 5.
Protein–protein interaction networks of deiminated protein candidates identified in bovine serum only (not identified in EVs). Protein–protein interactions were reconstructed based on both known and predicted interactions by STRING analysis. (A) Query proteins are indicated by the coloured nodes and represent the first shell of interactors. (B) KEGG pathways reported in STRING and related to the identified proteins are highlighted (see the colour code included in the figure). The coloured lines highlight which protein interactions are identified through known interactions (this refers to curated databases, experimentally determined), through predicted interactions (this refers to gene neighborhood, gene fusion, gene co-occurrence) or through co-expression, text mining or protein homology (the colour key for connective lines is included in the figure).
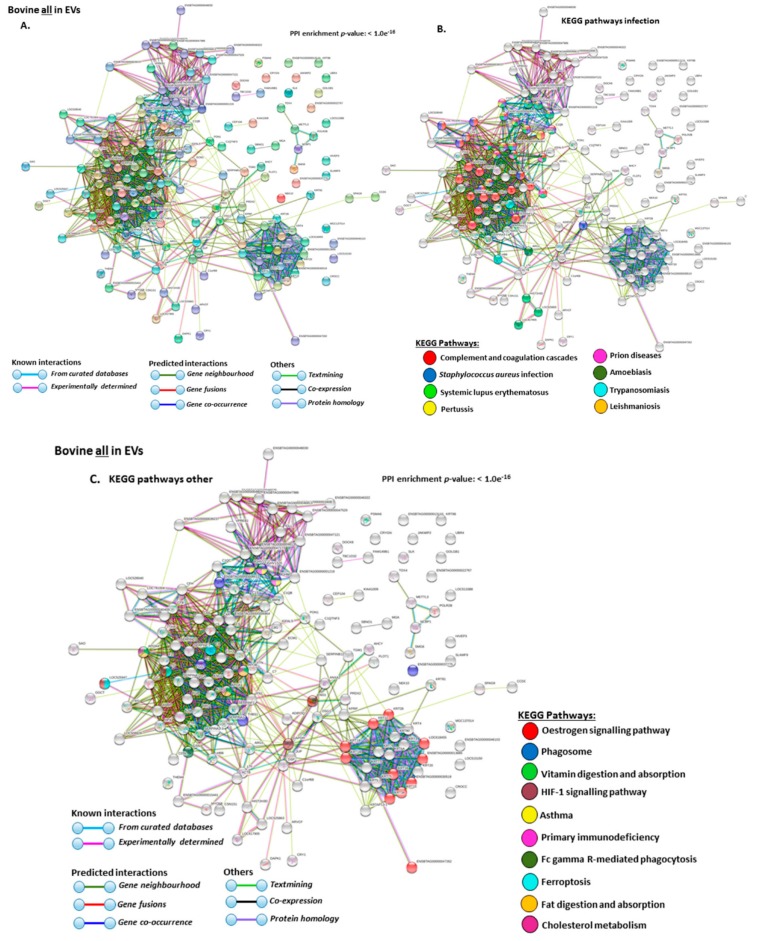
Figure 6.
Protein–protein interaction networks of all deiminated proteins identified in serum-EVs of Bos taurus. Protein–protein interactions were reconstructed based on both known and predicted interactions by STRING analysis. (A) Query proteins are indicated by the coloured nodes and represent the first shell of interactors. (B) KEGG pathways reported in STRING and relating to infection are highlighted (see colour code included in the figure). (C) KEGG pathways relating to the identified proteins and reported in STRING are highlighted for other pathways (see colour code included in the figure). The coloured lines highlight which protein interactions are identified through known interactions (this refers to curated databases, experimentally determined), through predicted interactions (this refers to gene neighborhood, gene fusion, gene co-occurrence) or through co-expression, text mining or protein homology (the colour key for connective lines is included in the figure).
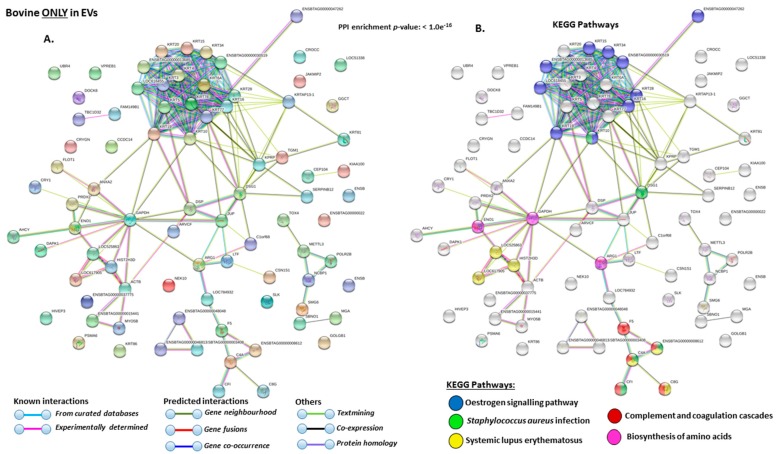
Figure 7.
Protein–protein interaction networks of deiminated protein candidates identified in bovine serum-EVs only (not identified in total serum). Protein–protein interactions were reconstructed based on both known and predicted interactions by STRING analysis. (A) Query proteins are indicated by the coloured nodes and represent the first shell of interactors. (B) KEEG pathways relating to the identified deiminated proteins and reported in STRING are highlighted (see colour code included in the figure). The coloured lines highlight which protein interactions are identified through known interactions (this refers to curated databases, experimentally determined), through predicted interactions (this refers to gene neighborhood, gene fusion, gene co-occurrence) or through co-expression, text mining or protein homology (the colour key for connective lines is included in the figure).
Figure 8.
Protein–protein interaction networks for common deiminated protein candidates identified in bovine serum and serum-EVs (excluding serum-specific or EV specific candidates). Protein–protein interactions were reconstructed based on both known and predicted interactions by STRING analysis. (A) Query proteins are indicated by the coloured nodes and represent the first shell of interactors. (B) KEGG pathways for to the identified deiminated proteins and reported in STRING and relating to infection are highlighted (see colour code included in the figure). (C) KEGG pathways relating to the identified proteins and reported in STRING are highlighted for immunity (see colour code included in the figure). (D) KEGG pathways relating to cancer and disease for deiminated proteins identified are highlighted for (see colour code included in the figure). (E) KEEG pathways relating to metabolism for deiminated proteins identified are highlighted (see colour code included in the figure). The coloured lines highlight which protein interactions are identified through known interactions (this refers to curated databases, experimentally determined), through predicted interactions (this refers to gene neighborhood, gene fusion, gene co-occurrence) or through co-expression, text mining or protein homology (the colour key for connective lines is included in the figure).
The KEEG pathways related to all deiminated proteins identified as deiminated in whole serum are shown in Figure 4A–E. For KEGG pathways relating to infection these belonged to: “Complement and coagulation cascades”, “S. aureus infection”, “systemic lupus erythematous”, “prion diseases”, “pertussis”, “trypanosomiasis”, “amoebiasis”, “leishmaniosis”, “tuberculosis”, “Epstein–Barr virus infection” (Figure 4B); For KEGG pathways relating to immunity these related to: “phagosome”, “primary immunodeficiency”, “FC-gamma R-mediated phagocytosis”, “intestinal immune network for IgA production”, “allograft rejection”, “Fc epsilon RI signalling pathway”, “autoimmune thyroid disease”, “B-cell receptor signalling pathway”, “rheumatoid arthritis”, “ferroptosis” (Figure 4C); For KEGG pathways relating to cancer and disease these related to: “transcription misregulation in cancer”, “bladder cancer”, “viral myocarditis”, “Rap1 signalling pathway”, “natural killer cell mediated cytotoxicity”, “measles”, “dilated cardiomyopathy”, “asthma” (Figure 4D); For KEGG metabolic pathways these related to: “phospholipidase D signalling pathway”; “ECM-receptor interaction”; “vitamin digestion and absorpition”, “calcium signalling pathway”, “fat digestion and absorption”, “NF-kappa B signalling pathway”, “cholesterol catabolism”, “hematopoietic cell lineage”, “PI3K-Akt signalling pathway” and “platelet activation”(Figure 4E).
The KEEG pathways related to deiminated proteins identified as deiminated in whole serum only are shown in Figure 5A,B. These were: “complement and coagulation cascades”, “phagosome”, “Rap1 signalling pathway” and “bladder cancer”.
The KEEG pathways related to all deiminated proteins identified in EVs are shown in Figure 6A–C. For KEGG pathways relating to infection these belonged to: “complement and coagulation cascades”, “S. aureus infection”, “systemic lupus erythematous”, “pertussis”, “prion diseases”, “amoebiasis”, “trypanosomiasis”, “leishmaniosis” (Figure 6B); Other KEGG pathways identified were: “oestrogen signalling pathway”, “phagosome” “vitamin digestion and absorption”, “asthma”, “primary immunodeficiency”, “Fc gamma R-mediated phagocytosis”, “ferroptosis”, “ fat digestion and absorption”, “cholesterol metabolism”, “HIF-1 signalling pathway”.
The KEEG pathways related to deiminated proteins identified as deiminated in EVs only are shown in Figure 7A,B. These were: “oestrogen signalling pathway”, “S. aureus infection”, “complement and coagulation cascades”, “biosynthesis of amino acids”.
Deiminated proteins that were common to serum and serum-EVs were furthermore analyzed for KEGG pathways (Figure 8A–E). Pathways identified for infection were: “complement and coagulation cascades”, “S. aureus infection”, “systemic lupus erythematous”, “pertussis”, “prion diseases”, “amoebiasis”, “trypanosomiasis”, “leishmaniosis”, “tuberculosis”, “malaria” (Figure 8B); KEGG pathways for immunity (and diseases) were: “Fc gamma R-mediated phagocytosis”, “primary immunodeficiency”, “Fc epsilon RI signalling pathway”, “intestinal immune network for IgA production”, “autoimmune thyroid disease”, “allograft rejection”, B-cell receptor signalling pathway”, “phagosome”, ”viral myocarditis”, rheumatoid arthritis (Figure 8C); KEGG pathways for cancer and disease were: “natural killer cell mediated cytotoxicity”, “dilated cardiomyopathy (DCM)”, “viral myocarditis”, “asthma”, “measles” (Figure 8D); KEGG metabolic pathways for common deiminated proteins in serum and serum-EVs were: “Vitamin digestion and absorption”, “ferroptosis”, “fat digestion and absorption”, “cholesterol metabolism”, “mineral absorption”, “phospholipase D signalling pathway”, PI3K-Akt signalling pathway”, NF-kappa B signalling pathway”, “hematopoietic cell lineage” and “platelet activation” (Figure 8E).
3. Discussion
The current study is the first to profile deiminated protein signatures in serum and serum-EVs of cattle, using Bos taurus as a model species. F95-enrichment for deiminated proteins from serum and serum-EVs revealed a range of immunological, metabolic and gene regulatory proteins as candidates for this post-translational modification, therefore indicating hitherto under-recognized modes for protein-moonlighting of these proteins in bovine immunity and physiology. Few studies have hitherto assessed roles for PADs and deimination in cattle [22,23,42], while a range of studies have been carried out on EVs in relation to cattle immunity, fertility and development [54,57,60,61,63,65,68,74,78,83,85,88,90]. Export of post-translationally modified proteins, such as deiminated proteins in the current study, has not been assessed before in cattle serum.
PAD proteins were assessed in bovine serum via cross-reaction to antibodies raised against human PAD2, PAD3 and PAD4 isozymes, with the strongest cross-reaction found with anti-human PAD4 at a predicted size of 70–75 kDa for PAD proteins. Protein sequence alignment of cow and human PADs showed that the individual isozymes clustered together between the two species, therefore the lack of cross-reaction with anti-human PAD2 and PAD3 antibodies may possibly be explained by differences in folding of the PADs, but will remain to be further investigated. It must be noted that neither anti-human PAD1 nor PAD6 antibodies were tested against bovine sera in the current study. The anti-human antibodies have previously been shown to cross-react with PADs from diverse taxa, with PAD2, which is considered the phylogenetically most conserved isoform, predominantly cross-reacting best with other species, including bony and cartilaginous fish, birds, mole-rat, alligator, camelids, cetaceans and pinnipeds [15,16,17,92,93,96,97,98,99].
A number of bovine species-specific deiminated protein candidates were identified in both serum and serum-EVs, using F95-enrichment in tandem with LC-MS/MS analysis. This analysis revealed some key metabolic and immune related proteins, with 87 characterised common deiminated proteins in serum and EVs, while 31 characterised deiminated protein hits were specific for serum and 90 characterised deiminated protein hits were specific for serum-EVs. Overall, the hit for deiminated proteins was higher in the serum-EVs, with a total hit of 179, compared to 118 in serum. Upon assessment of protein–protein interaction networks using STRING analysis, the PPI enrichment p-value for all deiminated proteins identified in bovine serum and serum-EVs, as well as for deiminated proteins identified either in serum or EVs only, indicated that the identified protein networks have significantly more interactions than expected for a random set of proteins of similar size, drawn from the genome, and that the proteins are at least partially biologically connected, as a group (Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8). When assessing KEGG pathways for deiminated proteins relating to infection and immunity, diseases and metabolism, a number of pathways overlapped between serum and serum-EVs, while KEGG pathways only found in EVs related to HIF-1 signalling, oestrogen signalling and biosynthesis of amino acids. KEGG pathways specific for serum only related to Epstein–Barr virus infection, transcription misregulation in cancer, bladder cancer, Rap1 signalling pathway, calcium signalling pathway, ECM-receptor interaction. This indicates differences in cell-communication via export of deiminated proteins in serum-EVs (Figure 3B).
KEGG pathways in common with both serum and serum-EVs related to a number of immunological pathways including the complement and coagulation cascade, bacterial infection, parasitic infection and importantly viral infections, as well as viral myocarditis. The role for deimination of immunoglobulins may be of considerable importance, particularly in relation to enveloped viruses as identified in the current study via deiminated KEGG pathways highlighted for Epstein–Barr virus in cow serum. Deimination in this KEGG pathway has also recently been identified in aggressive glioblastoma cells [100], and the relationship between viral infection cancers still remains to be fully understood [101,102]. In the cow, a link between viral infections and cancer has also been made [103,104,105]. Furthermore, oncolytic viruses from cow, such as bovine herpesvirus type 1, have been considered for application as a human broad spectrum cancer therapeutic [106]. The cow is known for its unusual immune responses and is a model organism for studying neutralizing antibodies against viral infections, including HIV [3,4,5]. Understanding of immune responses against enveloped viruses is of pivotal importance as indeed coronaviruses (CoVs) including severe acute respiratory syndrome (SARS) and COVID-19, also fall under enveloped viruses. CoVs are enveloped, positive-stranded RNA viruses with a nucleocapsid, and the structure of the envelope has a crucial role in virus pathogenicity as it promotes viral assembly and release [107]. While a range of studies has focused on modelling the envelope proteins, post-translational deimination has not yet been investigated in relation to SARS and CoVs, while it has been previously identified to play roles in rhinovirus infection [41]. Therefore, the roles for post-translational modifications via deimination may play some roles in structural interactions with the virus, particularly as cow immunoglobulins were here identified as deimination candidates, both in serum as well as in serum-EVs, and cow antibodies with an extended knob structure formed from the third complementarity determining region of the heavy chain are known to bind bovine and human pathogens and be capable of remarkably broad viral neutralization [3,4,5,108]. Post-translational deimination of cow Ig’s, which are reported in the current study for the first time, have yet to be further explored. Roles for PADs in anti-viral responses have previously been identified via the generation of NETosis [37], which can be PAD-mediated and is also a recognized mechanism in anti-pathogenic functions in cattle [109]. Deimination has also been identified to modulate chemokines in anti-HIV responses [40] and higher levels of anti-cyclic citrullinated peptide antibodies have been found sera of HIV patients [110]. Higher amounts of cyclic citrullinated peptides in sera have indeed been related to infectious diseases including a number of viral, bacterial and parasitic infections [111]. The ability of PAD homologues in bacteria, for example bacterial arginine deiminase in Mycoplasma arginini, a Gram-negative bacterium, has furthermore been shown to act as an effective anti-viral agent against HIV [112]. Interestingly, a recent comparison of transcriptomics data between healthy and SARS-CoV-2 infected patients’ lung biopsies (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA615032), reveals a 6-fold downregulation of PAD4 mRNA level, assessing 17 SARS-CoV-2 versus 106 healthy individuals. This highlights importance for PADs in virus host-pathogen interactions, including in COVID-19. Only PAD4 was reported in this COVID-19 patient cohort. Importantly, as PAD4 is involved in gene regulation and also considered one key-driver of NETosis [37], reduced PAD4 levels, observed in these COVID-19 patients, may contribute to less defences against viral infection due to gene regulatory changes, changes in deimination of immune related proteins, or impaired NETosis in these individuals. Whether the virus manipulates PAD4 expression, as an immune evasion mechanism, or whether individuals with lower PAD4 are more prone to SARS-CoV-2 infection, remains to be further investigated. Myocarditis is furthermore one of the hallmarks of COVID-19 [113] and roles for deimination/citrullination have previously been identified in a murine model of coxsackievirus B3-induced viral myocarditis [114]. As viral myocarditis was here identified as a KEGG pathway for deiminated proteins in cow sera and serum-EVs, to what extent PADs and deimination could be involved in viral induced myocarditis, including in relation to COVID-19, may be of considerable interest and remains to be further investigated.
The complement and coagulation cascades were identified as a common KEGG immune pathway enriched in deiminated proteins in cow serum and serum-EVs. The complement system bridges innate and adaptive immunity, has roles in clearing apoptotic and necrotic cells and is in the front line for clearing invading pathogens [115,116,117,118,119]. Dysregulation of the complement system is furthermore associated with a range of pathologies [120]. In the current study a range of proteins from the complement cascade were identified as deiminated in cow serum and serum EVs, with complement components identified both in serum and serum-EVs including C1q, C3, C4A, C5a, C7, C8, C9 factor B, Factor H, C4-binding protein; while complement components found deiminated only in whole serum were C6, clusterin, and collectin-43 (CL43). C3 is the central component of the complement cascade and has furthermore been implicated in tissue remodeling during teleost ontogeny [121,122,123,124] as well as in regeneration [125]. A range of complement proteins have recently been identified by our group to be deiminated in a range of taxa throughout the phylogenetic tree including teleost and cartilaginous fish, camelids, cetaceans, pinnipeds, camelids and peglagic seabirds [16,17,91,92,93,94,95,96,98,99]. The bovine complement system [126] has been widely studied, including in immune defences against bovine anaplasmosis [127], in relation to heat-stress in dairy cows [128], susceptibility to bacterial infection relating to copper-deficiency [129]. Roles for modulation of complement regulation in relation to poxviral and pestiviral infections have been identified [130,131,132], including immune evasion by bovine herpesvirus 1 (BHV-1) [133], while and roles for antibody-dependent complement-mediated killing of mycoplasma have recently been revealed [134]. The finding of deiminated complement components highlight hitherto understudied roles for post-translational deimination in the known diversity of complement function throughout phylogeny [135,136,137,138,139]. Furthermore, immune evasion of bacteria (Gingivalis) by deimination of the host’s C5a has previously been identified [20] and indeed C5a was here identified as a deimination candidate in cow serum. Our findings suggest that protein deimination may play hitherto unidentified roles in the known unusual anti-pathogenic functions of cow serum, including via EV-transport, also contributing to complement function in homeostatic processes.
Alpha-2-macroglobulin was found to be deiminated in cow serum and serum-EVs. It forms part of the innate immune system and clears active proteases from tissue fluids [140]. In cow plasma-EVs, alpha-2-macroglobulin has been identified [55], but the current study is the first report of deiminated bovine alpha-2-macroglobulin. Alpha-2-macroglobulin is related to the other thioester-containing proteins, complement proteins C3, C4 and C5, and is a phylogenetically conserved protein from arthropods to mammals [115,141,142]. The deimination of alpha-2-macroglobulin has recently been identified by our group in teleost fish, camelid, alligator and birds [15,92,93,99]. In the cow, alpha-2-macroglobulin has been widely studied and has amongst other binding affinities to TGF-beta and a range of cytokines [143] While alpha-2-macroglobulin is a known glycoprotein, less is known about deimination and such post-translational modification may facilitate protein moonlighting contribute both to its conserved immunological, as well as multifaceted functions in a range of taxa.
Serotransferrin was identified as a major deimination candidate in the current study both in cow serum and serum-EVs. It is an iron-binding protein with multifaceted functions in development and immunity [144,145]. Serotransferrin is found both in plasma and mucosal tissue and has important roles in anti-pathogenic responses across phylogeny, including in cattle, by withdrawing iron from pathogens including bacteria, fungi and viruses [146,147,148,149]. Furthermore, some viruses, including zoonitic ones, have been identified to utilize human transferrin receptor for viral entry and infection [150,151]. In chronic disease, for example lung diseases, the dysregulation of iron homeostasis can facilitate viral respiratory infections and secondary bacterial infections [152]. Deimination of serotransferrin has previously been identified by our group in teleost serum and mucosa [15,16]. Therefore, its deimination identified here in cow indicates that serotransferrin is deiminated in several taxa and this may be of considerable importance in its immune responses to a range of pathogens throughout phylogeny, as well as with respect to a range of human diseases and infections.
Intestinal immune network for IgA production was identified as deiminated for proteins common in serum and serum-EVs. The gut associated lymphoid system in the intestinal mucosa produces the highest level of antibody-secreting (IgA) plasma cells in the body [153,154]. It has been established that IgA cells play roles beyond antibody production, including having monocytic potential and antimicrobial properties via rapid secretion of cytokines as well as acting as regulators of inflammation [153]. IgA production in the gut mucosa plays major roles in maintaining the integrity of the mucosa and is therefore imperative for the host’s homeostasis and survival [154]. IgA is also produced in the BALT of the respiratory mucosal system [154] and is therefore important in defences against respiratory infections, including viral infections and this has also been identified in cattle bovine respiratory syncytial virus [155]. Deimination has been related to mucosal immunity in teleost fish, including in response to bacterial immune challenge and in EVs mediated mucosal immunity [15,16]. Roles for deimination in the regulation of intestinal immune networks for IgA production identified here may be of considerable interest and remain to be further investigated.
Various immunoglobulin (Ig) proteins and Ig superfamily members were identified here to be deiminated in cow serum and serum-EVs, confirming that Ig’s can be exported via EVs. Ig’s identified both in serum and serum-EVs were immunoglobulin lambda-like and immunoglobulin heavy chain constant mu. Ig’s identified in addition as deiminated in serum only, were the immunoglobulin J (joining) chain responsible for dimeric IgA and pentameric IgM and the polymeric immunoglobulin receptor (pIgR) that facilitates IgA’s translocation from the lamina propria through intestinal epithelia to the lumen. This is the first report of deiminated Ig’s in bovine serum and serum-EVs. Previous studies have identified that cow plasma EVs contain immunoglobulin J chain [55], but this did not come up as a deimination candidate in EVs, only in whole serum in the current study, and therefore may not be exported in EVs in the deiminated form. We have previously confirmed post-translational deimination if Ig’s in several other taxa, including shark, camelid, alligator and birds [17,92,93,99], as well as in teleost fish [15,16], and furthermore reported EV-mediated transport of Ig’s in shark and camelid [17,92]. Ig’s are key molecules in adaptive immunity and studied in diverse taxa. Post-translational deimination of Ig’s and roles in Ig functions remain to be further investigated but have been related to the IgG Fc region in bronchiectasis and RA [156]. Current understanding of Ig diversity throughout the phylogenetic tree is still incomplete [157,158,159,160,161,162]. Therefore, our identification of deimination of Ig’s in diverse taxa, including in bovine Ig’s in the current study, highlights a novel phylogenetically conserved concept of the diversification of Ig’s via this post-translational change. Our reported findings may furthermore shed some light on the unusual immune responses in bovine sera, relating to their immunoglobulins.
Serpins (serine proteases) were identified both in serum and serum-EVs, with both common candidates in serum and EVs as well as some specific serpin targets deiminated in serum or EVs. Serpins have multifaceted roles, ranging from protease inhibition to transport of hormones as well as regulation of chromatin organization [163]. Anti-viral roles for serine proteases have been identified in the airway, and serine proteases have been identified as drug targets for respiratory diseases, including virus infections [164,165]. Bovine serpins are cross-class inhibitors and shown to be strongly active against trypsin, as well as regulating caspases, therefore playing important roles in control of apoptosis in mammals [166]. In cow, serine proteases are for example involved in defences against Babesia bovis, a tick-borne and major apicomplexan pathogen in the cattle industry worldwide [167]. Serpins are identified in a range of taxa and for example serpin based peptides from alligator have been assessed as antimicrobials against multi-drug resistant pathogens [168]. Interestingly, serpins have been identified to be deiminated in alligator, and furthermore in humans, where deimination has been related to the modulation of protease activity and downstream effects on serpin-regulated pathways in rheumatoid arthritis [169]. As deimination of serpin pathways seems therefore a conserved phenomenon in a range of taxa, including in cattle as observed here, such post-translational regulation via deimination may contribute to its various functions, relating to immunity and anti-pathogenic defences, as well as also autoimmune diseases, and this remains to be further investigated.
Fat digestion and absorption was identified as a KEGG pathway in both cow serum and serum-EVs alongside adiponectin as a specific target. The adipocytokine signalling pathway has key regulatory roles in metabolism and glucose regulation [170,171,172,173] and is linked to a range of pathologies, including type II diabetes and insulin resistance [174], to myopathies [175] and cancer [176]. Adiponectin has also been linked to longevity [177] and to regenerative functions [178]. In the cow, adiponectin is a known glycoprotein and has been assessed in bovine tissues as well as body fluids [179] and amongst other been identified as a pro-survival signal in ER stress in the mammary gland [180]. Adiponectin has recently been identified as a deimination candidate in several taxa with unusual metabolism, including camelids, the naked mole-rat, orca and alligator [92,93,96,98]. A range of apolipoproteins was furthermore identified to be deiminated in the current study in cow serum and serum-EVs. In cattle, apolipoprotein B-100, apolipoprotein A-I and apolipoprotein C-III have previously been identified as biomarkers for fatty liver and related diseases [181]. In other taxa, apolipoproteins have also been found to display antimicrobial activity against a range of pathogenic bacteria [182,183,184,185] as well as against viral (HIV) replication [186]. Various apolipoproteins have recently been identified as deimination protein candidates by our group in several taxa [15,16,92,93,99]. The deimination of apolipoproteins therefore needs further investigation in relation to their multifaceted phylogenic immune and metabolic functions.
Selenoprotein P (Sepp1) was identified to be deiminated in whole serum and serum-EVs. It is a plasma glycoprotein, mainly secreted from liver but also other tissues and contains most of the selenium in mammalian plasma [187,188,189]. In the cow roles for selenoproteins in mammary gland physiology and in milk formation have been identified [190] and roles for responses to oxidative stress in bovine arterial endothelial cells have also been suggested [191]. It has antioxidant properties [188] and serves in homeostasis and distribution of selenium [189]. Roles for selenoproteins in the regulation of the contraction and relaxation of bronchiolar smooth muscle have furthermore been identified [192]. Sepp1 is known to be glycosylated, and recently it has been identified as a deimination candidate in cetaceans, pinnipeds and alligators [93,96,97]. The contribution of deimination in the functional diversity and conserved functions of Sepp1 throughout phylogeny will remain to be further investigated.
KEGG pathways for ECM-receptor interactions were identified to be enriched in deiminated proteins in whole cow serum only. ECM-receptor interactions play both direct and indirect roles in controlling multifaceted cellular activities, such as cell differentiation, migration and proliferation as well as cell adhesion and apoptosis [193]. ECM-receptor interaction KEGG pathways have been identified in cancer [193] and enrichment for this pathway has been reported mesenchymal stem cell EVs [194]. Regulation of ECM-receptor interactions via post-translational deimination has recently been identified by our group via enrichment of deiminated proteins in KEGG pathways for ECM-receptor interactions in the fin whale, a long-lived cancer-resistant animal [96], in the alligator, an animal with unusual antibacterial and anti-viral responses [93], in the wandering albatross (Diomedea exulans), also an unusually long-lived bird for an avian species [99], as well as in aggressive brain cancer cells [100]. In the cow, ECM-receptor interactions are amongst other related to depot-specific adipogenesis [195] and found to be over-represented in specific cattle breeds [196]. ECM-receptor interaction pathway has furthermore been identified in Holstein cattle in relation to adaptive immune responses following viral vaccination [197] and in intra-mammary infection with Streptococcus agalactiae [198]. Therefore, deimination in this pathway may be of significant importance in anti-bacterial and anti-viral responses. Deimination in ECM-receptor interaction pathway is here described for the first time in cow and may play roles in the multifaceted functions of this pathway.
KEGG pathways for calcium signalling pathway were here identified to be enriched in deiminated proteins in whole bovine serum only. Calcium is a key modulator in a range of immunological, metabolic and developmental functions [199,200,201,202]. Calcium signalling is linked to a wide range of physiological and pathological processes [203,204,205], is a key driver for post-translational deimination [206,207,208] and plays an important role in the regulation of EVs [11,48]. In cattle, calcium is linked to many immunological pathways including inflammatory diseases [209], virus replication [210], parasitic infection [211], wound healing [212], reproduction [213,214] and adrenal function [215]. Interestingly, calcium regulation has been shown to differ between Holstein and Jersey cows, including via serotonergic stimulation of the calcium pathway [216]. The identification in the current study of enrichment in deiminated proteins in the calcium signalling pathway in cow serum indicates a regulatory function in multiple key pathways of physiological and pathological processes via this post-translational modification.
RAP1 (Ras-related protein 1) signalling pathway was identified as enriched in deiminated proteins in whole bovine serum. It belongs to a superfamily of small GTPases, with pleiotrophic regulatory functions in cellular processes, such as nuclear transport, cell cycle progression, vesicle trafficking, cell adhesion and cytoskeletal rearrangement [217]. Rap1 is an inflammatory regulator, is highly expressed in platelets and is a key molecule for platelet activation and adhesion in injury [218,219]. In mammals, Rap1 is a phylogenetically conserved telomere-interacting protein and promotes endothelial barrier function and angiogenesis [220,221], as well as being involved in cancer cell invasion and metastasis [222]. Rap1 also has roles in oxidative stress and metabolism, and besides cancer it is linked to metabolic and cardiac disorders, including cardiomyopathy [217] as well as Kabuki syndrome (characterised by congenital anomalities and developmental delay) [223]. Rap1 has also been linked to bone growth, including in bovine chondrocyte models [224]. Rap1 has been identified to undergo post-translational modifications by phosphorylation, geranylgeranylation or guanine nucleotide exchange factors [225], but deimination in this pathway has not been identified before, to our knowledge. To what extent deimination contributes to the multifaceted functions of Rap1 signalling in health and disease may therefore be of considerable interest.
Transcription misregulation in cancer and bladder cancer KEGG pathways were identified as enriched in deiminated proteins in bovine serum. Cows belong to a group of long-lived mammals that display cancer resistance and are considered a translationally valuable model for human cancers [2], including for breast cancer as they have been reported to be resistant to mammary cancer [226]. Cattle do generate bladder tumors and these have been studied in relation to various molecular mechanisms as well as in relation to viral infections, including papilloma and leukemia viruses, also in a comparative context to human cancer [103,227,228,229]. Bovine leukemia virus has furthermore been linked to telomerase upregulation and tumor induction in cattle [230]. PADs and deimination have been widely studied in a range of cancers, including via epigenetic regulation [9,10,11,100,231,232,233,234] but this is the first identification of post-translational regulation of such pathways via deimination in cattle.
HIF-1 (hypoxia inducible factor) signalling KEGG pathways were enriched in deiminated proteins in serum-EVs only. HIF-1 regulates oxygen homeostasis and is the key factor mediating the mammalian hypoxic response [235]. HIF-1 has roles in inflammation, cancer, angiogenesis and cardiovascular disease [236,237]. Furthermore, roles for HIF have been identified in bacterial infections [235] and a link between pathogenic agents and cancer has been identified, as viruses, bacteria and parasites may deregulate HIF-1 signalling associated cancer cells [238]. In cows, HIF-1 has for example been found to play roles in immune responses against Theileria annulata, a protozoan parasite of major economically importance for the cattle industry [239]. HIF-1 has also been identified to be important in angiogenesis and the maintenance of capillary structures for final follicle maturation in the cow ovary [240]. Furthermore, HIF-1 has recently been identified as a genomic marker differing between cattle breeds, including highland-adapted cattle [241]. Interestingly, enrichment in deiminated proteins in KEGG HIF-1 signalling pathways has previously been identified in EVs only, in some species with unusual immune and metabolic function including the naked mole-rat and deep-diving whales, both of which are animal models of hypoxia-tolerance and cancer-resistance [96,98]. Furthermore, deimination of HIF-1 regulation were recently identified in aggressive brain cancer (glioblastoma) cells [100]. To what extent deimination regulates HIF-1 signalling in relation to cancer, inflammation and infectious disease, including in cattle, remains to be further investigated.
KEGG pathways for biosynthesis of amino acids were identified as deiminated in serum-EVs only. This may be of considerable interest for comparative metabolic studies, particularly as amino acid assessment for mammalian metabolism and for research into ageing and disease has received some attention [242]. In dairy cows, these pathways are studies as they are of importance for understanding of optimization of diets and increased efficiency of microbial protein synthesis [243]. Enrichment for deiminated proteins in this KEGG pathway has previously been identified in alligator serum-EVs [93] as well as in cetacean sera [96] by our group, while mechanisms for deimination in this pathway remain to be elucidated.
The presence of deiminated histones H2A, H2B, H3 and H4 was identified in serum-EVs only, but not in whole bovine serum. Interestingly, a similar EV-mediated export of deiminated histones, as observed in cow serum-EVs in the current study, was recently identified in the naked mole-rat, an animal with unusual immunological and metabolic traits [98], as well as in alligator, also an animal with unusual antimicrobial, including anti-viral responses [93]. Deiminated histone H3 is commonly used as an indicator for neutrophil extracellular trap formation (NETosis) [31,244], which has been related to PAD4 and implicated in anti-viral responses in cattle respiratory syncytial virus disease [42], in response to parasitic infections in cattle [245,246,247] as well as in response to certain antibiotics [248]. Interestingly, in crocodilians, extracellular histones H2A and H4 have been identified to act as inhibitors of viral (HIV) infection in vitro [249], although roles for post-translational deimination were not assessed. Anti-microbial effects for histones have furthermore been observed in teleost fish mucosal immunity for H2A [250] as well as for deiminated histone H3 [15]. Histone H3 deimination has furthermore been linked to inflammatory and hypoxic responses during CNS regeneration in avian and murine models [25,26]. Histone deimination is also a known epigenetic mechanism in cancer [11,231,233]. The multifaceted functions of histones in immunity, including via post-translational regulation, such as deimination identified here, remains to be further investigated in cattle, including in relation to possible roles in anti-viral and other anti-pathogenic or disease related responses.
The present study highlights novel aspects of protein moonlighting in immunity and metabolism of cattle, via post-translational deimination, including via EV-mediated transport. Our findings indicate differences in physiological and pathological pathways for deiminated proteins in serum-EVs, compared with whole serum, and may shed light on pathways underlying a number of pathological and anti-pathogenic (viral, bacterial, parasitic) pathways, with putative translatable value to human pathologies, zoonotic diseases and development of therapies for infections, including anti-viral therapies. While these data fall short of directing immediate clinical intervention for human disease, they do mandate further study of deimination in future work of broader scope and pre-clinical focus. For example, deimination will need further exploration as an additional diversification mechanism of ultralong CDR3 “cattlebodies” that have shown remarkable reach for broad viral neutralization. Also, importance for PADs in host-pathogen interactions, including in viral infections and in relation to COVID-19, may be of considerable importance and needs to be further investigated.
4. Materials and Methods
4.1. Serum Sampling from Cow
Blood was collected from the jugular vein of three healthy 5 months old Holstein breed steer and serum was prepared following clotting of blood for 2 h at room temperature (RT), by centrifugation for five minutes at 200× g. Sample collection was conducted under Texas A&M Institutional Animal Care and Use Protocol # 2015-078. Serum was aliquoted and kept at –80 °C until used. These samples were excess from a distinct study protocol and thus no animals needed to be purchased or euthanized for the study described here, consistent with the ethical principle of replacement, reduction and refinement.
4.2. Isolation of Extracellular Vesicles and Nanoparticle Tracking Analysis (NTA)
Serum-EVs were isolated from serum of individual animals (n = 3), using sequential centrifugation and ultracentrifugation in accordance to previously established protocols [17,46,98] and according to the recommendations of MISEV2018 (the minimal information for studies of extracellular vesicles 2018; [251]). For each individual EV preparation, 100 μL of bovine serum were diluted 1:5 in Dulbecco’s PBS (DPBS, ultrafiltered using a 0.22 μm filter, before use) and then centrifuged at 4000× g for 30 min at 4 °C, to ensure the removal of aggregates and apoptotic bodies. Thereafter the supernatants were collected and centrifuged further, using ultracentrifugation at 100,000× g for 1 h at 4 °C. The EV-enriched pellets were resuspended in 1 mL DPBS and ultracentrifuged again at 100,000× g for 1 h at 4 °C. The resulting washed EV pellets were then resuspended in 100 µL DPBS and frozen at –80 °C until further use. For EV size distribution profiles and EV quantification, NTA analysis was carried out using the NanoSight NS300 system (Malvern Panalytical, Malvern, UK), which analyses particle size based on Brownian motion. The EV samples were diluted 1/100 in DPBS (10 μL of EV preparation diluted in 990 μL of DPBS) and applied to the NanoSight using a syringe pump to ensure continuous flow of the sample. For each sample, five 60 second videos were recorded, keeping the number of particles per frame in-between 40 to 60. Replicate histograms were generated from the videos, using the NanoSight software 3.0 (Malvern), representing mean and confidence intervals of the 5 recordings for each sample.
4.3. Transmission Electron Microscopy (TEM)
A pool of EVs, isolated from serum of the three individual animals as described above, was used for morphological analysis using TEM. Following isolation, the EVs were resuspended in 100 mM sodium cacodylate buffer (pH 7.4) and a drop (~3–5 μL) of the suspension was placed onto a grid with previously glow discharged carbon support film. After the suspension had partly dried, the EVs were fixed by placing the grid onto a drop of a fixative solution (2.5% glutaraldehyde in 100 mM sodium cacodylate buffer (pH 7.0)) for 1 min at room temperature and washed afterwards by touching the grid to the surface of three drops of distilled water. Excess water was removed by touching the grid to a filter paper. Next, the EVs were stained with 2% aqueous Uranyl Acetate (Sigma-Aldrich) for 1 min, the excess stain was removed by touching the grid edge to a filter paper and the grid was let to dry. Imaging of EVs was performed using a JEOL JEM 1400 transmission electron microscope (JEOL, Tokyo, Japan) operated at 80 kV at a magnification of 30,000× to 60,000×. Digital images were recorded using an AMT XR60 CCD camera (Deben UK Limited, Bury Saint Edmunds, UK).
4.4. Isolation of Deiminated Proteins in Serum and EVs Using F95-enrichment
Immunoprecipitation and isolation of deiminated proteins in serum and serum-derived EVs was carried out using the Catch and Release®v2.0 immunoprecipitation kit (Merck, UK) in conjunction with the F95 pan-deimination antibody (MABN328, Merck), which has been developed against a deca-citrullinated peptide and specifically detects proteins modified by citrullination [252]. Bovine serum pools of the three individual animals (3 × 25 μL) were used for F95-enrichment from whole serum, while for EVs, total protein was first extracted from the pool of EVs derived from 3 animals (EV pellets derived from 100 μL serum per animal), using RIPA+ buffer (Sigma, UK). Following application of RIPA+ buffer, the EVs were incubated on ice for 2 h followed by centrifugation at 16,000× g for 30 min to collect the protein containing supernatant. Thereafter, immunoprecipitation (F95-enrichment) was carried out overnight on a rotating platform at 4 °C. The F95 bound proteins were eluted using denaturing elution buffer (Merck), according to the manufacturer’s instructions (Merck) and diluted 1:1 in Laemmli sample buffer. The F95-enriched eluates from whole serum and serum -EVs were then analyzed by SDS-PAGE, followed by silver staining, Western blotting or LC-MS/MS.
4.5. Western Blotting Analysis
Bovine serum and serum-EVs were diluted 1:1 in denaturing 2× Laemmli sample buffer (containing 5% beta-mercaptoethanol, BioRad, Kidlington, UK) and boiled for 5 min at 100 °C. Proteins were separated by SDS-PAGE using 4%-20% gradient TGX gels (BioRad UK). Western blotting was carried out using the Trans-Blot® SD semi-dry transfer cell (BioRad, UK); even transfer was assessed by staining the membranes with PonceauS (Sigma, UK). Blocking was performed for 1 h at room temperature using 5% bovine serum albumin (BSA; Sigma, UK), in Tris buffered saline (TBS) containing 0.1% Tween20 (BioRad, UK; TBS-T). Following blocking the membranes were incubated overnight at 4 °C on a shaking platform with the primary antibodies, which were diluted in TBS-T. For detection of deiminated/citrullinated proteins, the F95 pan-deimination antibody was used (MABN328, Merck, 1/1000). For detection of PAD proteins in bovine serum, the following anti-human PAD antibodies were used: anti-PAD2 (ab50257, Abcam, Cambridge, 1/1000); anti-PAD3 (ab50246, Abcam, 1/1000) and anti-PAD4 (ab50247, Abcam, 1/1000), and have previously been shown to cross-react with PAD homologues in a range of taxa [15,16,17,25,26,92,93,96,97,98,99]. EV isolates were blotted against two EV-specific markers: CD63 (ab216130, 1/1000) and Flotillin-1 (Flot-1, ab41927, 1/2000), for characterisation of EVs. After primary antibody incubation the membranes were washed for 3 × 10 min in TBS-T at RT and incubated for 1 h, at RT with HRP-conjugated secondary antibodies (anti-rabbit IgG (BioRad) or anti-mouse IgM (BioRad) respectively, diluted 1/3000 in TBS-T). The membranes were then washed in TBS-T for 5 × 10 min and positive proteins bands were visualized digitally, using enhanced chemiluminescence (ECL, GE Healthcare, Amersham Pl, Little Chalfont, UK) and the UVP BioDoc-ITTM System (Thermo Fischer Scientific, Hemel Hempstead, UK).
4.6. Silver Staining
F95-enriched protein eluates from bovine serum and serum-EVs were silver stained following SDS-PAGE (using 4–20% gradient TGX gels, BioRad, UK) under reducing conditions, using the BioRad Silver Stain Plus Kit (1610449, BioRad, UK), according to the manufacturer’s instructions (BioRad).
4.7. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis of Deiminated Protein Candidates
F95-enriched eluates from bovine serum and serum-EVs respectively, were analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS), according to previously described methods [93,99]. For LC-MS/MS analysis, the F95-enriched eluates were run 0.5 cm into a 12% TGX gel (BioRad, UK), the band cut out, trypsin digested and subjected to proteomic analysis on a Dionex Ultimate 3000 RSLC nanoUPLC (Thermo Fisher Scientific Inc, Waltham, MA, USA.) system and a QExactive Orbitrap mass spectrometer (Thermo Fisher Scientific Inc, Waltham, MA, USA) according to previously described methods [93,99]. In brief, peptides were separated by reverse-phase chromatography and a Thermo Scientific reverse-phase nano Easy-spray column (Thermo Scientific PepMap C18, 2 µm particle size, 100A pore size, 75 µm i.d. × 50 cm length). First, a pre-column (Thermo Scientific PepMap 100 C18, 5 µm particle size, 100A pore size, 300 µm i.d. × 5 mm length) was used for loading the peptides from the Ultimate 3000 autosampler, in the presence of 0.1% formic acid for 3 min (flow rate 10 µL/min). Therafter, peptides were eluted from the pre-column onto the analytical column (solvent A = water + 0.1% formic acid; solvent B = 80% acetonitrile, 20% water + 0.1% formic acid). A linear gradient of 2–40% B was applied for 30 min. An easy-Spray source (Thermo Fischer Scientific Inc., Waltham, MA, USA) as used to spray the LC eluant into the mass spectrometer. The m/z values of the eluting ions were measured using an Orbitrap mass analyzer. The resolution was set at 70,000 and scanning was performed at m/z 380–1500. Fragment ions were automatically isolated and generated based on data dependent scans (top 20) in the HCD collision cell by higher energy collisional dissociation (HCD, NCE:25%). The resulting fragment ions were measured using the Orbitrap analyzer, set at a resolution of 17500. Ions that were singly charged, or with unassigned charge states, were excluded from selection for MS/MS. Furthermore, a dynamic exclusion window of 20 seconds was applied. The Protein Discoverer (version 2.1., Thermo Scientific) was used to process the data post-run and the MS/MS data was thereafter converted to mgf files. For identification of deiminated protein hits, the files were then submitted to the Mascot search algorithm (Matrix Science, London, UK) and searched against the UniProt Bos taurus_ 20170607 database (24148 sequences; 40594 residues) and Bos_primigenius primigenius_20191209 database (54 sequences; 14783 residues), as well as a common contaminant sequences (123 sequences; 40594 residues). Fragment and peptide mass tolerances and were set at 0.1 Da and 20 ppm, respectively. The peptide cut-off score was set at 31 and significance at p < 0.05 (carried out by Cambridge Proteomics, Cambridge, UK).
4.8. Protein–Protein Interaction Network Analysis
For the identification and prediction of putative protein–protein interaction networks for deiminated proteins identified in bovine serum and serum-EVs, STRING analysis (Search Tool for the Retrieval of Interacting Genes/Proteins; https://string-db.org/) was used. Protein networks were built based on the protein IDs and using the function of “search multiple proteins” in STRING, choosing “Bos taurus” for the species database. For analysis, “medium conficence” and “basic settings” were selected and the colour lines connecting nodes indicated the following evidence-based interactions for the network edges: “known interactions”, which are based on curated databases or experimentally determined interactions; “predicted interactions”, which are based on protein homology, co-expression, gene fusion, gene neighborhood, gene co-occurrence, or established by text mining.
4.9. Phylogenetic Comparison of Bos PADs with Human PADs
A neighbor-joining phylogeny tree was constructed using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) based on sequence alignment of amino acid sequences for bovine PADs 1-6 (PAD1, NP_001094742.1; PAD2, NP_001098922.1; PAD3, XP_010800991.1; PAD4, NP_001179102.1 and PAD6, XP_002685843.1) compared with human PADs 1-6 (PAD1, NP_037490.2; PAD2, NP_031391.2; PAD3, NP_057317.2; PAD4, NP_036519.2 and PAD6, NP_997304.3).
4.10. Statistical Analysis
The histograms and graphs were prepared using the Nanosight 3.0 software (Malvern Panalytical) and GraphPad Prism version 7 (GraphPad Software, San Diego, CA, USA). NTA curves represent mean and standard error of mean (SEM), indicated by confidence intervals. STRING analysis (https://string-db.org/) was used for prediction of protein–protein interaction networks. Significance was considered as p ≤ 0.05.
5. Conclusions
In the current study we report for the first time deimination profiles of serum and serum-EVs of Bos taurus. Post-translational deimination of key immune, metabolic and nuclear proteins was identified and these related to KEGG pathways for a number of immunological pathways with relevance to viral, bacterial and parasitic infections, to chronic and autoimmune diseases, as well as a range of metabolic pathways. Our findings highlight novel aspects of protein moonlighting in immunity and metabolism of cattle, via post-translational deimination, including via EV-mediated transport.
While these data fall short of directing immediate clinical intervention for human disease, they do mandate further study of deimination as an additional diversification mechanism of ultralong CDR3 “cattlebodies” that have shown remarkable reach for broad viral neutralization. This study limitation of this comparative biochemical basic science must be addressed in future work of broader scope and pre-clinical focus. Furthermore, transcriptomics data revealing downregulation in PAD4 mRNA levels in SARS-CoV-2 infected patient’ lung biopsies, compared to healthy controls (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA615032), indicates importance for PADs in host-virus interactions in COVID-19 and will need to be further explored.
EVs research in comparative immunology and veterinary sciences is a rapidly growing field and while EVs have been previously assessed in cattle, this study is the first to characterise deiminated protein signatures in bovine serum and serum-EVs. The identification of post-translational deimination and EV-mediated communication in bovine immunity and physiology, revealed here, contributes to current understanding of protein moonlighting functions and EV-mediated communication in cattle, providing novel insight into their unusual immune systems and physiological traits. Our findings may shed light on pathways underlying a number of pathological and anti-pathogenic, including anti-viral, pathways, with putative translatable value to human pathologies, zoonotic diseases and development of therapies for infections, including anti-viral therapies.
Acknowledgments
The authors would like to thank Yagnesh Umrania and Michael Deery at the Cambridge Centre for Proteomics for the LC–MS/MS analysis. Thanks are due to The Guy Foundation for funding the purchase of equipment utilized in this work.
Abbreviations
| BSA | Bovine serum albumin |
| CD63 | CD63 antigen; granulophysin; lysosomal-associated membrane protein 3 |
| CoV | Coronavirus |
| COVID-19 | Coronavirus disease 2019 |
| ECL | Enhanced chemiluminescence |
| ECM | Extracellular matrix |
| EVs | Extracellular vesicles |
| F95 | Pan-deimination/citrullination antibody |
| FBS | Fetal bovine serum |
| Flot-1 | Flotillin-1 |
| HIF | Hypoxia inducible factor |
| Ig | Immunoglobulin |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LC-MS/MS | Liquid chromatography mass spectrometry |
| NETosis | Neutrophil extracellular trap formation |
| NTA | Nanoparticle tracking analysis |
| PAD | Peptidylarginine deiminase |
| RAP1 | Ras-related protein 1 |
| SARS | Severe acute respiratory syndrome |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TBS | Tris buffered saline |
| TEM | Transmission electron microscopy |
| WB | Western blotting |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/8/2861/s1, Table S1. Full LC-MS/MS analysis of F95-enriched proteins in bovine serum. Supplementary Table S2. Full LC-MS/MS analysis of F95-enriched proteins in bovine serum-EVs.
Author Contributions
Conceptualization, S.L.; methodology, I.K. and S.L.; validation, M.F.C. and S.L.; formal analysis, S.L.; investigation, M.F.C., and S.L.; resources, M.F.C., I.K. and S.L.; data curation, S.L.; writing—original draft preparation, S.L.; writing—review and editing, M.F.C. and S.L.; visualization, I.K., and S.L.; supervision, S.L.; project administration, S.L.; funding acquisition, M.F.C. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded in part by a University of Westminster start-up grant to SL and U.S. National Science Foundation grant IOS 1656870 to M.F.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Grubb P.B. In: Mammal Species of the World. A Taxonomic and Geographic Reference. 3rd ed. Wilson D.E., Reeder D.M., editors. Johns Hopkins University Press; Baltimore, MD, USA: 2005. online edition. [Google Scholar]
- 2.Seluanov A., Gladyshev V.N., Vijg J., Gorbunova V. Mechanisms of cancer resistance in long-lived mammals. Nat. Rev. Cancer. 2018;18:433–441. doi: 10.1038/s41568-018-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sok D., Le K.M., Vadnais M., Saye-Francisco K.L., Jardine J.G., Torres J.L., Berndsen Z.T., Kong L., Stanfield R., Ruiz J., et al. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature. 2017;548:108–111. doi: 10.1038/nature23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanfield R.L., Haakenson J., Deiss T.C., Criscitiello M.F., Wilson I.A., Smider V.V. The Unusual Genetics and Biochemistry of Bovine Immunoglobulins. Adv. Immunol. 2018;137:135–164. doi: 10.1016/bs.ai.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deiss T.C., Vadnais M., Wang F., Chen P.L., Torkamani A., Mwangi W., Lefranc M.P., Criscitiello M.F., Smider V.V. Immunogenetic factors driving formation of ultralong VH CDR3 in Bos taurus antibodies. Cell. Mol. Immunol. 2019;16:53–64. doi: 10.1038/cmi.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vossenaar E.R., Zendman A.J., van Venrooij W.J., Pruijn G.J. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 7.György B., Toth E., Tarcsa E., Falus A., Buzas E.I. Citrullination: A posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006;38:1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Bicker K.L., Thompson P.R. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers. 2013;99:155–163. doi: 10.1002/bip.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S., Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim. Biophys. Acta. 2013;1829:1126–1135. doi: 10.1016/j.bbagrm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witalison E.E., Thompson P.R., Hofseth L.J. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr. Drug Targets. 2015;16:700–710. doi: 10.2174/1389450116666150202160954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange S., Gallagher M., Kholia S., Kosgodage U.S., Hristova M., Hardy J., Inal J.M. Peptidylarginine Deiminases-Roles in Cancer and Neurodegeneration and Possible Avenues for Therapeutic Intervention via Modulation of Exosome and Microvesicle (EMV) Release? Int. J. Mol. Sci. 2017;18:1196. doi: 10.3390/ijms18061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson B., Martin A.C. Protein moonlighting: A new factor in biology and medicine. Biochem. Soc. Trans. 2014;42:1671–1678. doi: 10.1042/BST20140273. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey C.J. Protein moonlighting: What is it, and why is it important? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373:20160523. doi: 10.1098/rstb.2016.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebl A., Köllner B., Anders E., Wimmers K., Goldammer T. Peptidylarginine deiminase gene is differentially expressed in freshwater and brackish water rainbow trout. Mol. Biol. Rep. 2010;37:2333–2339. doi: 10.1007/s11033-009-9738-5. [DOI] [PubMed] [Google Scholar]
- 15.Magnadottir B., Hayes P., Hristova M., Bragason B.T., Nicholas A.P., Dodds A.W., Guðmundsdóttir S., Lange S. Post-translational Protein Deimination in Cod (Gadus morhua L.) Ontogeny–Novel Roles in Tissue Remodelling and Mucosal Immune Defences? Dev. Comp. Immunol. 2018;87:157–170. doi: 10.1016/j.dci.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Magnadottir B., Bragason B.T., Bricknell I.R., Bowden T., Nicholas A.P., Hristova M., Guðmundsdóttir S., Dodds A.W., Lange S. Peptidylarginine Deiminase and Deiminated Proteins are detected throughout Early Halibut Ontogeny-Complement Components C3 and C4 are post-translationally Deiminated in Halibut (Hippoglossus hippoglossus L.) Dev. Comp Immunol. 2019;92:1–19. doi: 10.1016/j.dci.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Criscitiello M.F., Kraev I., Lange S. Deiminated proteins in extracellular vesicles and plasma of nurse shark (Ginglymostoma cirratum)—Novel insights into shark immunity. Fish Shellfish Immunol. 2019;92:249–255. doi: 10.1016/j.fsi.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Gavinho B., Rossi I.V., Evans-Osses I., Lange S., Ramirez M.I. Peptidylarginine deiminase inhibition abolishes the production of large extracellular vesicles from Giardia intestinalis, affecting host-pathogen interactions by hindering adhesion to host cells. BioRxiv. 2019:586438. doi: 10.1101/586438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sayed A.S.A., Shindia A.A., AbouZaid A.A., Yassin A.M., Ali G.S., Sitohy M.Z. Biochemical characterization of peptidylarginine deiminase-like orthologs from thermotolerant Emericella dentata and Aspergillus nidulans. Enzyme Microb Technol. 2019;124:41–53. doi: 10.1016/j.enzmictec.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Bielecka E., Scavenius C., Kantyka T., Jusko M., Mizgalska D., Szmigielski B., Potempa B., Enghild J.J., Prossnitz E.R., Blom A.M., et al. Peptidyl arginine deiminase from Porphyromonas gingivalis abolishes anaphylatoxin C5a activity. J. Biol. Chem. 2014;289:32481–32487. doi: 10.1074/jbc.C114.617142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosgodage U.S., Matewele P., Mastroianni G., Kraev I., Brotherton D., Awamaria B., Nicholas A.P., Lange S., Inal J.M. Peptidylarginine Deiminase Inhibitors Reduce Bacterial Membrane Vesicle Release and Sensitize Bacteria to Antibiotic Treatment. Front. Cell. Infect. Microbiol. 2019;9:227. doi: 10.3389/fcimb.2019.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamensa J.W., Moscarello M.A. Deimination of human myelin basic protein by a peptidylarginine deiminase from bovine brain. J. Neurochem. 1993;61:987–996. doi: 10.1111/j.1471-4159.1993.tb03612.x. [DOI] [PubMed] [Google Scholar]
- 23.Pritzker L.B., Moscarello M.A. A novel microtubule independent effect of paclitaxel: The inhibition of peptidylarginine deiminase from bovine brain. Biochim. Biophys. Acta. 1998;1388:154–160. doi: 10.1016/S0167-4838(98)00175-7. [DOI] [PubMed] [Google Scholar]
- 24.Mohanan S., Cherrington B.D., Horibata S., McElwee J.L., Thompson P.R., Coonrod S.A. Potential role of peptidylarginine deiminase enzymes and protein citrullination in cancer pathogenesis. Biochem. Res. Int. 2012;2012:895343. doi: 10.1155/2012/895343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange S., Gögel S., Leung K.Y., Vernay B., Nicholas A.P., Causey C.P., Thompson P.R., Greene N.D., Ferretti P. Protein deiminases: New players in the developmentally regulated loss of neural regenerative ability. Dev. Biol. 2011;355:205–214. doi: 10.1016/j.ydbio.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange S., Rocha-Ferreira E., Thei L., Mawjee P., Bennett K., Thompson P.R., Subramanian V., Nicholas A.P., Peebles D., Hristova M., et al. Peptidylarginine deiminases: Novel drug targets for prevention of neuronal damage following hypoxic ischemic insult (HI) in neonates. J. Neurochem. 2014;130:555–562. doi: 10.1111/jnc.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange S. Peptidylarginine Deiminases as Drug Targets in Neonatal Hypoxic-Ischemic Encephalopathy. Front. Neurol. 2016;7:22. doi: 10.3389/fneur.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sase T., Arito M., Onodera H., Omoteyama K., Kurokawa M.S., Kagami Y., Ishigami A., Tanaka Y., Kato T. Hypoxia-induced production of peptidylarginine deiminases and citrullinated proteins in malignant glioma cells. Biochem. Biophys. Res. Commun. 2017;482:50–56. doi: 10.1016/j.bbrc.2016.10.154. [DOI] [PubMed] [Google Scholar]
- 29.Yu R., Li C., Sun L., Jian L., Ma Z., Zhao J., Liu X. Hypoxia induces production of citrullinated proteins in human fibroblast-like synoviocytes through regulating HIF1α. Scand. J. Immunol. 2018;87:e12654. doi: 10.1111/sji.12654. [DOI] [PubMed] [Google Scholar]
- 30.Ding D., Enriquez-Algeciras M., Bhattacharya S.K., Bonilha V.L. Protein Deimination in Aging and Age-Related Diseases with Ocular Manifestations. In: Nicholas A., Bhattacharya S., Thompson P., editors. Protein Deimination in Human Health and Disease. Springer; Cham, Switzerland: 2017. [Google Scholar]
- 31.Wong S.L., Wagner D.D. Peptidylarginine deiminase 4: A nuclear button triggering neutrophil extracellular traps in inflammatory diseases and aging. FASEB J. 2018;32:6358–6370. doi: 10.1096/fj.201800691R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan B., Alam H.B., Chong W., Mobley J., Liu B., Deng Q., Liang Y., Wang Y., Chen E., Wang T., et al. CitH3: A reliable blood biomarker for diagnosis and treatment of endotoxic shock. Sci. Rep. 2017;7:8972. doi: 10.1038/s41598-017-09337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biron B.M., Chung C.S., Chen Y., Wilson Z., Fallon E.A., Reichner J.S., Ayala A. PAD4 deficiency leads to decreased organ dysfunction and improved survival in a dual insult model of hemorrhagic shock and sepsis. J. Immunol. 2018;200:1817–1828. doi: 10.4049/jimmunol.1700639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claushuis T.A.M., van der Donk L.E.H., Luitse A.L., van Veen H.A., van der Wel N.N., van Vught L.A., Roelofs J.J.T.H., de Boer O.J., Lankelma J.M., Boon L., et al. Role of Peptidylarginine Deiminase 4 in Neutrophil Extracellular Trap Formation and Host Defense during Klebsiella pneumoniae-Induced Pneumonia-Derived Sepsis. J. Immunol. 2018;201:1241–1252. doi: 10.4049/jimmunol.1800314. [DOI] [PubMed] [Google Scholar]
- 35.Costa N.A., Gut A.L., Azevedo P.S., Polegato B.F., Magalhães E.S., Ishikawa L.L.W., Bruder R.C.S., Silva E.A.D., Gonçalves R.B., Tanni S.E., et al. Peptidylarginine deiminase 4 concentration, but not PADI4 polymorphisms, is associated with ICU mortality in septic shock patients. J. Cell Mol. Med. 2018;22:4732–4737. doi: 10.1111/jcmm.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Y., Pan B., Alam H.B., Deng Q., Wang Y., Chen E., Liu B., Tian Y., Williams A.M., Duan X., et al. Inhibition of peptidylarginine deiminase alleviates LPS-induced pulmonary dysfunction and improves survival in a mouse model of lethal endotoxemia. Eur. J. Pharmacol. 2018;833:432–440. doi: 10.1016/j.ejphar.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muraro S.P., De Souza G.F., Gallo S.W., Da Silva B.K., De Oliveira S.D., Vinolo M.A.R., Saraiva E.M., Porto B.N. Respiratory Syncytial Virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci. Rep. 2018;8:14166. doi: 10.1038/s41598-018-32576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stobernack T., du Teil Espina M., Mulder L.M., Palma Medina L.M., Piebenga D.R., Gabarrini G., Zhao X., Janssen K.M.J., Hulzebos J., Brouwer E., et al. A Secreted Bacterial Peptidylarginine Deiminase Can Neutralize Human Innate Immune Defenses. MBio. 2018;9:e01704-18. doi: 10.1128/mBio.01704-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha P., Yeoh B.S., Xiao X., Golonka R.M., Singh V., Wang Y., Vijay-Kumar M. PAD4-dependent NETs generation are indispensable for intestinal clearance of Citrobacter rodentium. Mucosal Immunol. 2019;12:761–771. doi: 10.1038/s41385-019-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Struyf S., Noppen S., Loos T., Mortier A., Gouwy M., Verbeke H., Huskens D., Luangsay S., Parmentier M., Geboes K., et al. Citrullination of CXCL12 differentially reduces CXCR4 and CXCR7 binding with loss of inflammatory and anti-HIV-1 activity via CXCR4. J. Immunol. 2009;182:666–674. doi: 10.4049/jimmunol.182.1.666. [DOI] [PubMed] [Google Scholar]
- 41.Casanova V., Sousa F.H., Shakamuri P., Svoboda P., Buch C., D’Acremont M., Christophorou M.A., Pohl J., Stevens C., Barlow P.G. Citrullination Alters the Antiviral and Immunomodulatory Activities of the Human Cathelicidin LL-37 during Rhinovirus Infection. Front. Immunol. 2020;11:85. doi: 10.3389/fimmu.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortjens B., de Boer O.J., de Jong R., Antonis A.F., Sabogal Piñeros Y.S., Lutter R., van Woensel J.B., Bem R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016;238:401–411. doi: 10.1002/path.4660. [DOI] [PubMed] [Google Scholar]
- 43.Magnadottir B., Hayes P., Gísladóttir B., Bragason B.Þ., Hristova M., Nicholas A.P., Guðmundsdóttir S., Lange S. Pentraxins CRP-I and CRP-II are post-translationally deiminated and differ in tissue specificity in cod (Gadus morhua L.) ontogeny. Dev. Comp. Immunol. 2018;87:1–11. doi: 10.1016/j.dci.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Kholia S., Jorfi S., Thompson P.R., Causey C.P., Nicholas A.P., Inal J.M., Lange S. A Novel Role for Peptidylarginine Deiminases (PADs) in Microvesicle Release: A Therapeutic Potential for PAD Inhibitors to Sensitize Prostate Cancer Cells to Chemotherapy. J. Extracell. Vesicles. 2015;4:26192. doi: 10.3402/jev.v4.26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosgodage U.S., Trindade R.P., Thompson P.T., Inal J.M., Lange S. Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy. Int. J. Mol. Sci. 2017;18:1007. doi: 10.3390/ijms18051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kosgodage U.S., Onganer P.U., Maclatchy A., Nicholas A.P., Inal J.M., Lange S. Peptidylarginine Deiminases Post-translationally Deiminate Prohibitin and Modulate Extracellular Vesicle Release and miRNAs 21 and 126 in Glioblastoma Multiforme. Int. J. Mol. Sci. 2018;20:103. doi: 10.3390/ijms20010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawson C., Kovacs D., Finding E., Ulfelder E., Luis-Fuentes V. Extracellular Vesicles: Evolutionarily Conserved Mediators of Intercellular Communication. Yale J. Biol. Med. 2017;90:481–491. [PMC free article] [PubMed] [Google Scholar]
- 48.Inal J.M., Ansa-Addo E.A., Lange S. Interplay of host-pathogen microvesicles and their role in infectious disease. Biochem. Soc. Trans. 2013;41:258–262. doi: 10.1042/BST20120257. [DOI] [PubMed] [Google Scholar]
- 49.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 50.Turchinovich A., Drapkina O., Tonevitsky A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front. Immunol. 2019;10:202. doi: 10.3389/fimmu.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vagner T., Chin A., Mariscal J., Bannykh S., Engman D.M., Di Vizio D. Protein Composition Reflects Extracellular Vesicle Heterogeneity. Proteomics. 2019;19:e1800167. doi: 10.1002/pmic.201800167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Słomka A., Urban S.K., Lukacs-Kornek V., Żekanowska E., Kornek M. Large Extracellular Vesicles: Have We Found the Holy Grail of Inflammation? Front. Immunol. 2018;9:2723. doi: 10.3389/fimmu.2018.02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coleman B.M., Hill A.F. Extracellular vesicles--Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin. Cell Dev. Biol. 2015;40:89–96. doi: 10.1016/j.semcdb.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Koh Y.Q., Peiris H.N., Vaswani K., Meier S., Burke C.R., Macdonald K.A., Roche J.R., Almughlliq F., Arachchige B.J., Reed S., et al. Characterization of exosomes from body fluids of dairy cows. J. Anim. Sci. 2017;95:3893–3904. doi: 10.2527/jas2017.1727. [DOI] [PubMed] [Google Scholar]
- 56.Gatien J., Mermillod P., Tsikis G., Bernardi O., Janati Idrissi S., Uzbekov R., Le Bourhis D., Salvetti P., Almiñana C., Saint-Dizier M. Metabolomic Profile of Oviductal Extracellular Vesicles across the Estrous Cycle in Cattle. Int. J. Mol. Sci. 2019;20:6339. doi: 10.3390/ijms20246339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinlapadeelerdkul T., Sonoda H., Uchida K., Kitahara G., Ikeda M. Release of urinary aquaporin-2-bearing extracellular vesicles is decreased in pregnant Japanese Black cattle. J. Vet. Med. Sci. 2019;81:1609–1615. doi: 10.1292/jvms.19-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huson K.M., Morphew R.M., Allen N.R., Hegarty M.J., Worgan H.J., Girdwood S.E., Jones E.L., Phillips H.C., Vickers M., Swain M., et al. Polyomic tools for an emerging livestock parasite, the rumen fluke Calicophoron daubneyi; identifying shifts in rumen functionality. Parasites Vectors. 2018;11:617. doi: 10.1186/s13071-018-3225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S., Gong P., Tai L., Li X., Wang X., Zhao C., Zhang X., Yang Z., Yang J., Li J., et al. Extracellular Vesicles Secreted by Neospora caninum Are Recognized by Toll-Like Receptor 2 and Modulate Host Cell Innate Immunity Through the MAPK Signaling Pathway. Front. Immunol. 2018;9:1633. doi: 10.3389/fimmu.2018.01633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tartaglia N.R., Breyne K., Meyer E., Cauty C., Jardin J., Chrétien D., Dupont A., Demeyere K., Berkova N., Azevedo V., et al. Staphylococcus aureus Extracellular Vesicles Elicit an Immunostimulatory Response in vivo on the Murine Mammary Gland. Front. Cell. Infect. Microbiol. 2018;8:277. doi: 10.3389/fcimb.2018.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gillan V., Simpson D.M., Kinnaird J., Maitland K., Shiels B., Devaney E. Characterisation of infection associated microRNA and protein cargo in extracellular vesicles of Theileria annulata infected leukocytes. Cell. Microbiol. 2019;21:e12969. doi: 10.1111/cmi.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramírez Rico G., Martínez-Castillo M., González-Ruíz C., Luna-Castro S., de la Garza M. Mannheimia haemolytica A2 secretes different proteases into the culture medium and in outer membrane vesicles. Microb. Pathog. 2017;113:276–281. doi: 10.1016/j.micpath.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 63.Confer A.W., Ayalew S. Mannheimia haemolytica in bovine respiratory disease: Immunogens, potential immunogens, and vaccines. Anim. Health Res. Rev. 2018;19:79–99. doi: 10.1017/S1466252318000142. [DOI] [PubMed] [Google Scholar]
- 64.Sun J., Aswath K., Schroeder S.G., Lippolis J.D., Reinhardt T.A., Sonstegard T.S. MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genom. 2015;16:806. doi: 10.1186/s12864-015-2044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Almughlliq F.B., Koh Y.Q., Peiris H.N., Vaswani K., McDougall S., Graham E.M., Burke C.R., Arachchige B.J., Reed S., Mitchell M.D. Proteomic content of circulating exosomes in dairy cows with or without uterine infection. Theriogenology. 2018;114:173–179. doi: 10.1016/j.theriogenology.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 66.De Gassart A., Trentin B., Martin M., Hocquellet A., Bette-Bobillo P., Mamoun R., Vidal M. Exosomal sorting of the cytoplasmic domain of bovine leukemia virus TM Env protein. Cell Biol. Int. 2009;33:36–48. doi: 10.1016/j.cellbi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Hutchinson E.C., Charles P.D., Hester S.S., Thomas B., Trudgian D., Martínez-Alonso M., Fodor E. Conserved and host-specific features of influenza virion architecture. Nat. Commun. 2014;5:4816. doi: 10.1038/ncomms5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahsan N.A., Sampey G.C., Lepene B., Akpamagbo Y., Barclay R.A., Iordanskiy S., Hakami R.M., Kashanchi F. Presence of Viral RNA and Proteins in Exosomes from Cellular Clones Resistant to Rift Valley Fever Virus Infection. Front. Microbiol. 2016;7:139. doi: 10.3389/fmicb.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitchell M.D., Scholz-Romero K., Reed S., Peiris H.N., Koh Y.Q., Meier S., Walker C.G., Burke C.R., Roche J.R., Rice G., et al. Plasma exosome profiles from dairy cows with divergent fertility phenotypes. J. Dairy Sci. 2016;99:7590–7601. doi: 10.3168/jds.2016-11060. [DOI] [PubMed] [Google Scholar]
- 70.Hung W.T., Navakanitworakul R., Khan T., Zhang P., Davis J.S., McGinnis L.K., Christenson L.K. Stage-specific follicular extracellular vesicle uptake and regulation of bovine granulosa cell proliferation. Biol. Reprod. 2017;97:644–655. doi: 10.1093/biolre/iox106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Almiñana C., Tsikis G., Labas V., Uzbekov R., da Silveira J.C., Bauersachs S., Mermillod P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018;19:622. doi: 10.1186/s12864-018-4982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura K., Kusama K., Ideta A., Kimura K., Hori M., Imakawa K. Effects of miR-98 in intrauterine extracellular vesicles on maternal immune regulation during the peri-implantation period in cattle. Sci. Rep. 2019;9:20330. doi: 10.1038/s41598-019-56879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao G., Guo S., Jiang K., Zhang T., Wu H., Qiu C., Deng G. MiRNA profiling of plasma-derived exosomes from dairy cows during gestation. Theriogenology. 2019;130:89–98. doi: 10.1016/j.theriogenology.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 74.de Ávila A.C.F.C.M., Bridi A., Andrade G.M., Del Collado M., Sangalli J.R., Nociti R.P., da Silva Junior W.A., Bastien A., Robert C., Meirelles F.V., et al. Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation†. Biol Reprod. 2020;102:362–375. doi: 10.1093/biolre/ioz177. [DOI] [PubMed] [Google Scholar]
- 75.Andrade G.M., Bomfim M.M., Del Collado M., Meirelles F.V., Perecin F., da Silveira J.C. Oxygen tension modulates extracellular vesicles and its miRNA contents in bovine embryo culture medium. Mol. Reprod. Dev. 2019;86:1067–1080. doi: 10.1002/mrd.23223. [DOI] [PubMed] [Google Scholar]
- 76.Giacomini E., Alleva E., Fornelli G., Quartucci A., Privitera L., Vanni V.S., Viganò P. Embryonic extracellular vesicles as informers to the immune cells at the maternal-fetal interface. Clin. Exp. Immunol. 2019;198:15–23. doi: 10.1111/cei.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mellisho E.A., Briones M.A., Velásquez A.E., Cabezas J., Castro F.O., Rodríguez-Álvarez L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction. 2019;158:477–492. doi: 10.1530/REP-19-0233. [DOI] [PubMed] [Google Scholar]
- 78.Bauersachs S., Mermillod P., Almiñana C. The Oviductal Extracellular Vesicles’ RNA Cargo Regulates the Bovine Embryonic Transcriptome. Int. J. Mol. Sci. 2020;21:1303. doi: 10.3390/ijms21041303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blans K., Hansen M.S., Sørensen L.V., Hvam M.L., Howard K.A., Möller A., Wiking L., Larsen L.B., Rasmussen J.T. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles. 2017;6:1294340. doi: 10.1080/20013078.2017.1294340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zempleni J., Aguilar-Lozano A., Sadri M., Sukreet S., Manca S., Wu D., Zhou F., Mutai E. Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants. J. Nutr. 2017;147:3–10. doi: 10.3945/jn.116.238949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nordgren T.M., Heires A.J., Zempleni J., Swanson B.J., Wichman C., Romberger D.J. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J. Nutr. Biochem. 2019;64:110–120. doi: 10.1016/j.jnutbio.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Melnik B.C., Schmitz G. Exosomes of pasteurized milk: Potential pathogens of Western diseases. J. Transl. Med. 2019;17:3. doi: 10.1186/s12967-018-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benmoussa A., Laugier J., Beauparlant C.J., Lambert M., Droit A., Provost P. Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. J. Dairy Sci. 2020;103:16–29. doi: 10.3168/jds.2019-16880. [DOI] [PubMed] [Google Scholar]
- 84.Munagala R., Aqil F., Jeyabalan J., Gupta R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aqil F., Munagala R., Jeyabalan J., Agrawal A.K., Kyakulaga A.H., Wilcher S.A., Gupta R.C. Milk exosomes—Natural nanoparticles for siRNA delivery. Cancer Lett. 2019;449:186–195. doi: 10.1016/j.canlet.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 86.Matsuda A., Moirangthem A., Angom R.S., Ishiguro K., Driscoll J., Yan I.K., Mukhopadhyay D., Patel T. Safety of bovine milk derived extracellular vesicles used for delivery of RNA therapeutics in zebrafish and mice. J. Appl. Toxicol. 2019 doi: 10.1002/jat.3938. [DOI] [PubMed] [Google Scholar]
- 87.Yamada T., Shigemura H., Ishiguro N., Inoshima Y. Cell Infectivity in relation to bovine leukemia virus gp51 and p24 in bovine milk exosomes. PLoS ONE. 2013;8:e77359. doi: 10.1371/journal.pone.0077359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arntz O.J., Pieters B.C., Oliveira M.C., Broeren M.G., Bennink M.B., de Vries M., van Lent P.L., Koenders M.I., van den Berg W.B., van der Kraan P.M., et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015;59:1701–1712. doi: 10.1002/mnfr.201500222. [DOI] [PubMed] [Google Scholar]
- 89.Mokarizadeh A., Hassanzadeh K., Abdi M., Soraya H., Faryabi M.R., Mohammadi E., Ahmadi A. Transdermal delivery of bovine milk vesicles in patients with multiple sclerosis: A novel strategy to induce MOG-specific tolerance. Med. Hypotheses. 2015;85:141–144. doi: 10.1016/j.mehy.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 90.Li B., Hock A., Wu R.Y., Minich A., Botts S.R., Lee C., Antounians L., Miyake H., Koike Y., Chen Y., et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE. 2019;14:e0211431. doi: 10.1371/journal.pone.0211431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lange S., Kraev I., Magnadóttir B., Dodds A.W. Complement component C4-like protein in Atlantic cod (Gadus morhua L.)—Detection in ontogeny and identification of post-translational deimination in serum and extracellular vesicles. Dev. Comp. Immunol. 2019;101:103437. doi: 10.1016/j.dci.2019.103437. [DOI] [PubMed] [Google Scholar]
- 92.Criscitiello M.F., Kraev I., Lange S. Deiminated proteins in extracellular vesicles and serum of llama (Lama glama)-Novel insights into camelid immunity. Mol. Immunol. 2020;117:37–53. doi: 10.1016/j.molimm.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Criscitiello M.F., Kraev I., Lange S. Deimination Protein Profiles in Alligator mississippiensis Reveal Plasma and Extracellular Vesicle-specific Signatures Relating to Immunity, Metabolic Function and Gene Regulation. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Magnadottir B., Kraev I., Guðmundsdóttir S., Dodds A.W., Lange S. Extracellular vesicles from cod (Gadus morhua L.) mucus contain innate immune factors and deiminated protein cargo. Dev. Comp. Immunol. 2019;99:103397. doi: 10.1016/j.dci.2019.103397. [DOI] [PubMed] [Google Scholar]
- 95.Magnadottir B., Uysal-Onganer P., Kraev I., Dodds A.W., Gudmundsdottir S., Lange S. Extracellular vesicles, deiminated protein cargo and microRNAs are novel serum biomarkers for environmental rearing temperature in Atlantic cod (Gadus morhua L.) Aquacult. Rep. 2020;16:100245. doi: 10.1016/j.aqrep.2019.100245. [DOI] [Google Scholar]
- 96.Magnadottir B., Uysal-Onganer P., Kraev I., Svansson V., Hayes P., Lange S. Deiminated Proteins and Extracellular Vesicles—Novel Serum Biomarkers in Whales and Orca. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020;34:100676. doi: 10.1016/j.cbd.2020.100676. [DOI] [PubMed] [Google Scholar]
- 97.Magnadóttir B., Uysal-Onganer P., Kraev I., Svansson V., Skírnisson K., Lange S. Deiminated proteins and extracellular vesicles as novel biomarkers in pinnipeds: Grey seal (Halichoerus gryptus) and harbour seal (Phoca vitulina) Biochimie. 2020;171–172:79–90. doi: 10.1016/j.biochi.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 98.Pamenter M.E., Uysal-Onganer P., Huynh K.W., Kraev I., Lange S. Post-translational Deimination of Immunological and Metabolic Protein Markers in Plasma and Extracellular Vesicles of Naked Mole-Rat (Heterocephalus glaber) Int. J. Mol. Sci. 2019;20:5378. doi: 10.3390/ijms20215378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phillips R.A., Kraev I., Lange S. Protein Deimination and Extracellular Vesicle Profiles in Antarctic Seabirds. Biology. 2020;9:15. doi: 10.3390/biology9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uysal-Onganer P., MacLatchy A., Mahmoud R., Kraev I., Thompson P.R., Inal J., Lange S. Peptidylarginine deiminase isozyme-specific PAD2, PAD3 and PAD4 inhibitors differentially modulate extracellular vesicle signatures and cell invasion in two glioblastoma multiforme cell lines. Int. J. Mol. Sci. 2020;21:1495. doi: 10.3390/ijms21041495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akhtar S., Vranic S., Cyprian F.S., Al Moustafa A.E. Epstein-Barr Virus in Gliomas: Cause, Association, or Artifact? Front. Oncol. 2018;8:123. doi: 10.3389/fonc.2018.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Limam S., Missaoui N., Mestiri S., Yacoubi M.T., Krifa H., Selmi B., Mokni M. Epstein-Barr virus infection in gliomas. Curr. Res. Transl. Med. 2019;67:129–133. doi: 10.1016/j.retram.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 103.Knottenbelt D.C. Cancer-blame it all on viruses! Bladder tumours in cattle and sarcoids in horses may help us understand the relationship between some cancers and viruses. Vet. J. 2007;174:456–459. doi: 10.1016/j.tvjl.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 104.Borzacchiello G., Roperto F. Bovine papillomaviruses, papillomas and cancer in cattle. Vet. Res. 2008;39:45. doi: 10.1051/vetres:2008022. [DOI] [PubMed] [Google Scholar]
- 105.Martinez Cuesta L., Lendez P.A., Nieto Farias M.V., Dolcini G.L., Ceriani M.C. Can Bovine Leukemia Virus Be Related to Human Breast Cancer? A Review of the Evidence. J. Mammary Gland Biol. Neoplasia. 2018;23:101–107. doi: 10.1007/s10911-018-9397-z. [DOI] [PubMed] [Google Scholar]
- 106.Cuddington B.P., Mossman K.L. Oncolytic bovine herpesvirus type 1 as a broad spectrum cancer therapeutic. Curr. Opin. Virol. 2015;13:11–16. doi: 10.1016/j.coviro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 107.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2020. [(accessed on 6 April 2020)]. Features, Evaluation and Treatment Coronavirus (COVID-19) [Updated 2020 Mar 20] Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/ [Google Scholar]
- 108.Wang F., Ekiert D.C., Ahmad I., Yu W., Zhang Y., Bazirgan O., Torkamani A., Raudsepp T., Mwangi W., Criscitiello M.F., et al. Reshaping antibody diversity. Cell. 2013;153:1379–1393. doi: 10.1016/j.cell.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hellenbrand K.M., Forsythe K.M., Rivera-Rivas J.J., Czuprynski C.J., Aulik N.A. Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb. Pathog. 2013;54:67–75. doi: 10.1016/j.micpath.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Méric de Bellefon L., Épée H., Langhendries J.P., De Wit S., Corazza F., Di Romana S. Increase in the prevalence of anti-cyclic citrullinated peptide antibodies in the serum of 185 patients infected with Human Immunodeficiency Virus. Joint Bone Spine. 2015;82:467–468. doi: 10.1016/j.jbspin.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 111.Lima I., Santiago M. Antibodies against cyclic citrullinated peptides in infectious diseases--a systematic review. Clin. Rheumatol. 2010;29:1345–1351. doi: 10.1007/s10067-010-1544-x. [DOI] [PubMed] [Google Scholar]
- 112.Kubo M., Nishitsuji H., Kurihara K., Hayashi T., Masuda T., Kannagi M. Suppression of human immunodeficiency virus type 1 replication by arginine deiminase of Mycoplasma arginini. J. Gen. Virol. 2006;87 Pt 6:1589–1593. doi: 10.1099/vir.0.81549-0. [DOI] [PubMed] [Google Scholar]
- 113.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., Brown T.S., Nigoghossian C., Zidar D.A., Haythe J., et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the Coronavirus Disease 2019 (COVID-19) Pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. pii: S0735-1097(20)34637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mikami S., Kawashima S., Kanazawa K., Hirata K., Katayama Y., Hotta H., Hayashi Y., Ito H., Yokoyama M. Expression of nitric oxide synthase in a murine model of viral myocarditis induced by coxsackievirus B3. Biochem. Biophys. Res. Commun. 1996;220:983–989. doi: 10.1006/bbrc.1996.0519. [DOI] [PubMed] [Google Scholar]
- 115.Dodds A.W., Law S.K. The phylogeny and evolution of the thioester bond-containing proteins C3, C4 and alpha 2-macroglobulin. Immunol. Rev. 1998;166:15–26. doi: 10.1111/j.1600-065X.1998.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 116.Fishelson Z., Attali G., Mevorach D. Complement and apoptosis. Mol. Immunol. 2001;38:207–219. doi: 10.1016/S0161-5890(01)00055-4. [DOI] [PubMed] [Google Scholar]
- 117.Hart S.P., Smith J.R., Dransfield I. Phagocytosis of opsonized apoptotic cells: Roles for ‘old-fashioned’ receptors for antibody and complement. Clin. Exp. Immunol. 2004;135:181–185. doi: 10.1111/j.1365-2249.2003.02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carroll M.V., Sim R.B. Complement in health and disease. Adv. Drug Deliv. Rev. 2011;63:965–975. doi: 10.1016/j.addr.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 119.Morgan B.P., Walters D., Serna M., Bubeck D. Terminal complexes of the complement system: New structural insights and their relevance to function. Immunol. Rev. 2016;274:141–151. doi: 10.1111/imr.12461. [DOI] [PubMed] [Google Scholar]
- 120.Schröder-Braunstein J., Kirschfink M. Complement deficiencies and dysregulation: Pathophysiological consequences, modern analysis, and clinical management. Mol. Immunol. 2019;114:299–311. doi: 10.1016/j.molimm.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 121.Lange S., Bambir S., Dodds A.W., Magnadottir B. The ontogeny of complement component C3 in Atlantic Cod (Gadus morhua L.)—An immunohistochemical study. Fish Shellfish Immunol. 2004;16:359–367. doi: 10.1016/j.fsi.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 122.Lange S., Bambir S., Dodds A.W., Magnadottir B. An immunohistochemical study on complement component C3 in juvenile Atlantic halibut (Hippoglossus hippoglossus L.) Dev. Comp. Immunol. 2004;28:593–601. doi: 10.1016/j.dci.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 123.Lange S., Dodds A.W., Gudmundsdóttir S., Bambir S.H., Magnadottir B. The ontogenic transcription of complement component C3 and Apolipoprotein A-I tRNA in Atlantic cod (Gadus morhua L.)—A role in development and homeostasis? Dev. Comp. Immunol. 2005;29:1065–1077. doi: 10.1016/j.dci.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 124.Lange S., Bambir S.H., Dodds A.W., Bowden T., Bricknell I., Espelid S., Magnadottir B. Complement component C3 transcription in Atlantic halibut (Hippoglossus hippoglossus L.) larvae. Fish Shellfish Immunol. 2006;20:285–294. doi: 10.1016/j.fsi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 125.Kimura Y., Madhavan M., Call M.K., Santiago W., Tsonis P.A., Lambris J.D., Del Rio-Tsonis K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J. Immunol. 2003;170:2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- 126.Linscott W.D., Triglia R.P. The bovine complement system. Adv. Exp. Med. Biol. 1981;137:413–430. [PubMed] [Google Scholar]
- 127.Soler-Rodríguez A.M., Romano E., Aranguren Y., Soyano A. A new hemolytic assay for bovine serum complement and its application during experimental bovine anaplasmosis. Vet. Immunol. Immunopathol. 1990;24:347–360. doi: 10.1016/0165-2427(90)90005-D. [DOI] [PubMed] [Google Scholar]
- 128.Min L., Cheng J., Zhao S., Tian H., Zhang Y., Li S., Yang H., Zheng N., Wang J. Plasma-based proteomics reveals immune response, complement and coagulation cascades pathway shifts in heat-stressed lactating dairy cows. J. Proteomics. 2016;146:99–108. doi: 10.1016/j.jprot.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 129.Postma G.C., Minatel L., Olivares R.W., Schapira A., Dallorso M.E., Carfagnini J.C. Bactericidal activity of lachrymal secretion and complement system in copper deficient bovines. Biol. Trace Elem. Res. 2013;153:178–183. doi: 10.1007/s12011-013-9664-1. [DOI] [PubMed] [Google Scholar]
- 130.Yadav V.N., Pyaram K., Ahmad M., Sahu A. Species selectivity in poxviral complement regulators is dictated by the charge reversal in the central complement control protein modules. J. Immunol. 2012;189:1431–1439. doi: 10.4049/jimmunol.1200946. [DOI] [PubMed] [Google Scholar]
- 131.Ostachuk A. Bovine viral diarrhea virus structural protein E2 as a complement regulatory protein. Arch. Virol. 2016;161:1769–1782. doi: 10.1007/s00705-016-2835-6. [DOI] [PubMed] [Google Scholar]
- 132.Liu C., Liu Y., Liang L., Cui S., Zhang Y. RNA-Seq based transcriptome analysis during bovine viral diarrhoea virus (BVDV) infection. BMC Genom. 2019;20:774. doi: 10.1186/s12864-019-6120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Levings R.L., Roth J.A. Immunity to bovine herpesvirus 1: I. Viral lifecycle and innate immunity. Anim. Health Res. Rev. 2013;14:88–102. doi: 10.1017/S1466252313000042. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y.K., Li X., Zhao H.R., Jiang F., Wang Z.H., Wu W.X. Antibodies Specific to Membrane Proteins Are Effective in Complement-Mediated Killing of Mycoplasma bovis. Infect. Immun. 2019;87:e00740-19. doi: 10.1128/IAI.00740-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boshra H., Li J., Sunyer J.O. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006;20:239–262. doi: 10.1016/j.fsi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 136.Sunyer J.O., Lambris J.D. Evolution and diversity of the complement system of poikilothermic vertebrates. Immunol. Rev. 1998;166:39–57. doi: 10.1111/j.1600-065X.1998.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 137.Nakao M., Kato-Unoki Y., Nakahara M., Mutsuro J., Somamoto T. Diversified components of the bony fish complement system: More genes for robuster innate defense? Adv. Exp. Med. Biol. 2006;586:121–138. doi: 10.1007/0-387-34134-X_9. [DOI] [PubMed] [Google Scholar]
- 138.Nakao M., Tsujikura M., Ichiki S., Vo T.K., Somamoto T. The complement system in teleost fish: Progress of post-homolog-hunting researches. Dev. Comp. Immunol. 2011;35:1296–1308. doi: 10.1016/j.dci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 139.Forn-Cuní G., Reis E.S., Dios S., Posada D., Lambris J.D., Figueras A., Novoa B. The evolution and appearance of C3 duplications in fish originate an exclusive teleost c3 gene form with anti-inflammatory activity. PLoS ONE. 2014;9:e99673. doi: 10.1371/journal.pone.0099673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Armstrong P.B., Quigley J.P. Alpha2-macroglobulin: An evolutionarily conserved arm of the innate immune system. Dev. Comp. Immunol. 1999;23:375–390. doi: 10.1016/S0145-305X(99)00018-X. [DOI] [PubMed] [Google Scholar]
- 141.Davies S.G., Sim R.B. Intramolecular general acid catalysis in the binding reactions of alpha 2-macroglobulin and complement components C3 and C4. Biosci. Rep. 1981;1:461–468. doi: 10.1007/BF01121579. [DOI] [PubMed] [Google Scholar]
- 142.Sottrup-Jensen L., Stepanik T.M., Kristensen T., Lønblad P.B., Jones C.M., Wierzbicki D.M., Magnusson S., Domdey H., Wetsel R.A., Lundwall A., et al. Common evolutionary origin of alpha 2-macroglobulin and complement components C3 and C4. Proc. Natl. Acad. Sci. USA. 1985;82:9–13. doi: 10.1073/pnas.82.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feige J.J., Negoescu A., Keramidas M., Souchelnitskiy S., Chambaz E.M. Alpha 2-macroglobulin: A binding protein for transforming growth factor-beta and various cytokines. Horm. Res. Paediatr. 1996;45:227–232. doi: 10.1159/000184793. [DOI] [PubMed] [Google Scholar]
- 144.Gomme P.T., McCann K.B., Bertolini J. Transferrin: Structure, function and potential therapeutic actions. Drug Discov. Today. 2005;10:267–273. doi: 10.1016/S1359-6446(04)03333-1. [DOI] [PubMed] [Google Scholar]
- 145.Kawabata H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019;133:46–54. doi: 10.1016/j.freeradbiomed.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 146.Bezkorovainy A. Antimicrobial properties of iron-binding proteins. Adv. Exp. Med. Biol. 1981;135:139–154. doi: 10.1007/978-1-4615-9200-6_8. [DOI] [PubMed] [Google Scholar]
- 147.Ratledge C., Dover L.G. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 148.Rice J.A., Carrasco-Medina L., Hodgins D.C., Shewen P.E. Mannheimia haemolytica and bovine respiratory disease. Anim. Health Res. Rev. 2007;8:117–128. doi: 10.1017/S1466252307001375. [DOI] [PubMed] [Google Scholar]
- 149.Barber M.F., Elde N.C. Buried Treasure: Evolutionary Perspectives on Microbial Iron Piracy. Trends Genet. 2015;31:627–636. doi: 10.1016/j.tig.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sarute N., Ross S.R. New World Arenavirus Biology. Annu. Rev. Virol. 2017;4:141–158. doi: 10.1146/annurev-virology-101416-042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wessling-Resnick M. Crossing the Iron Gate: Why and How Transferrin Receptors Mediate Viral Entry. Annu. Rev. Nutr. 2018;38:431–458. doi: 10.1146/annurev-nutr-082117-051749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hendricks M.R., Lashua L.P., Fischer D.K., Flitter B.A., Eichinger K.M., Durbin J.E., Sarkar S.N., Coyne C.B., Empey K.M., Bomberger J.M. Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proc. Natl. Acad. Sci. USA. 2016;113:1642–1647. doi: 10.1073/pnas.1516979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gommerman J.L., Rojas O.L., Fritz J.H. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes. 2014;5:262–652. doi: 10.4161/19490976.2014.969977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chase C., Kaushik R.S. Mucosal Immune System of Cattle: All Immune Responses Begin Here. Vet. Clin. N. Am. Food Anim. Pract. 2019;35:431–451. doi: 10.1016/j.cvfa.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kolb E.A., Buterbaugh R.E., Rinehart C.L., Ensley D., Perry G.A., Abdelsalam K.W., Chase C.C.L. Protection against bovine respiratory syncytial virus in calves vaccinated with adjuvanted modified live vaccine administered in the face of maternal antibody. Vaccine. 2020;38:298–308. doi: 10.1016/j.vaccine.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 156.Hutchinson D., Clarke A., Heesom K., Murphy D., Eggleton P. Carbamylation/citrullination of IgG Fc in bronchiectasis, established RA with bronchiectasis and RA smokers: A potential risk factor for disease. ERJ Open Res. 2017;3:00018–02017. doi: 10.1183/23120541.00018-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lundqvist M.L., Middleton D.L., Radford C., Warr G.W., Magor K.E. Immunoglobulins of the non-galliform birds: Antibody expression and repertoire in the duck. Dev. Comp. Immunol. 2006;30:93–100. doi: 10.1016/j.dci.2005.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de los Rios M., Criscitiello M.F., Smider V.V. Structural and genetic diversity in antibody repertoires from diverse species. Curr. Opin. Struct. Biol. 2015;33:27–41. doi: 10.1016/j.sbi.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Z., Takizawa F., Casadei E., Shibasaki Y., Ding Y., Sauters T.J.C., Yu Y., Salinas I., Sunyer J.O. Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution. Sci. Immunol. 2020;5:eaay3254. doi: 10.1126/sciimmunol.aay3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Akula S., Hellman L. The Appearance and Diversification of Receptors for IgM during Vertebrate Evolution. Curr. Top. Microbiol. Immunol. 2017;408:1–23. doi: 10.1007/82_2017_22. [DOI] [PubMed] [Google Scholar]
- 161.Zhang X., Calvert R.A., Sutton B.J., Doré K.A. IgY: A key isotype in antibody evolution. Biol. Rev. Camb. Philos. Soc. 2017;92:2144–2156. doi: 10.1111/brv.12325. [DOI] [PubMed] [Google Scholar]
- 162.Magadan S., Krasnov A., Hadi-Saljoqi S., Afanasyev S., Mondot S., Lallias D., Castro R., Salinas I., Sunyer O., Hansen J., et al. Standardized IMGT® Nomenclature of Salmonidae IGH Genes, the Paradigm of Atlantic Salmon and Rainbow Trout: From Genomics to Repertoires. Front. Immunol. 2019;10:2541. doi: 10.3389/fimmu.2019.02541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ragg H. The role of serpins in the surveillance of the secretory pathway. Cell. Mol. Life Sci. 2007;64:2763–2770. doi: 10.1007/s00018-007-7157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laporte M., Naesens L. Airway proteases: An emerging drug target for influenza and other respiratory virus infections. Curr. Opin. Virol. 2017;24:16–24. doi: 10.1016/j.coviro.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Menou A., Duitman J., Flajolet P., Sallenave J.M., Mailleux A.A., Crestani B. Human airway trypsin-like protease, a serine protease involved in respiratory diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L657–L668. doi: 10.1152/ajplung.00509.2016. [DOI] [PubMed] [Google Scholar]
- 166.Gagaoua M., Hafid K., Boudida Y., Becila S., Ouali A., Picard B., Boudjellal A., Sentandreu M.A. Caspases and Thrombin Activity Regulation by Specific Serpin Inhibitors in Bovine Skeletal Muscle. Appl. Biochem. Biotechnol. 2015;177:279–303. doi: 10.1007/s12010-015-1762-4. [DOI] [PubMed] [Google Scholar]
- 167.Florin-Christensen M., Schnittger L., Dominguez M., Mesplet M., Rodríguez A., Ferreri L., Asenzo G., Wilkowsky S., Farber M., Echaide I., et al. Search for Babesia bovis vaccine candidates. Parassitologia. 2007;49(Suppl. 1):9–12. [PubMed] [Google Scholar]
- 168.Barksdale S.M., Hrifko E.J., Chung E.M., van Hoek M.L. Peptides from American alligator plasma are antimicrobial against multi-drug resistant bacterial pathogens including Acinetobacter baumannii. BMC Microbiol. 2016;16:189. doi: 10.1186/s12866-016-0799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tilvawala R., Nguyen S.H., Maurais A.J., Nemmara V.V., Nagar M., Salinger A.J., Nagpal S., Weerapana E., Thompson P.R. The Rheumatoid Arthritis-Associated Citrullinome. Cell. Chem. Biol. 2018;25:691–704.e6. doi: 10.1016/j.chembiol.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 171.Kadowaki T., Yamauchi T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 172.Almabouada F., Diaz-Ruiz A., Rabanal-Ruiz Y., Peinado J.R., Vazquez-Martinez R., Malagon M.M. Adiponectin receptors form homomers and heteromers exhibiting distinct ligand binding and intracellular signaling properties. J. Biol. Chem. 2013;288:3112–3125. doi: 10.1074/jbc.M112.404624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fiaschi T. Mechanisms of Adiponectin Action. Int. J. Mol. Sci. 2019;20:2894. doi: 10.3390/ijms20122894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Frankenberg A.D.V., Reis A.F., Gerchman F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arch. Endocrinol. Metab. 2017;61:614–622. doi: 10.1590/2359-3997000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gamberi T., Magherini F., Fiaschi T. Adiponectin in Myopathies. Int. J. Mol. Sci. 2019;20:1544. doi: 10.3390/ijms20071544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Parida S., Siddharth S., Sharma D. Adiponectin, obesity, and cancer: Clash of the bigwigs in health and disease. Int. J. Mol. Sci. 2019;20:2519. doi: 10.3390/ijms20102519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen Y.L., Tao J., Zhao P.J., Tang W., Xu J.P., Zhang K.Q., Zou C.G. Adiponectin receptor PAQR-2 signaling senses low temperature to promote C. elegans longevity by regulating autophagy. Nat. Commun. 2019;10:2602. doi: 10.1038/s41467-019-10475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fiaschi T., Magherini F., Gamberi T., Modesti P.A., Modesti A. Adiponectin as a tissue regenerating hormone: More than a metabolic function. Cell. Mol. Life Sci. 2014;71:1917–1925. doi: 10.1007/s00018-013-1537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sauerwein H., Häußler S. Endogenous and exogenous factors influencing the concentrations of adiponectin in body fluids and tissues in the bovine. Domest. Anim. Endocrinol. 2016;56:S33–S43. doi: 10.1016/j.domaniend.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 180.Jeong W., Bae H., Lim W., Bazer F.W., Song G. Adiponectin: A prosurvival and proproliferation signal that increases bovine mammary epithelial cell numbers and protects them from endoplasmic reticulum stress responses. J. Anim. Sci. 2017;95:5278–5289. doi: 10.2527/jas2017.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Katoh N. Relevance of apolipoproteins in the development of fatty liver and fatty liver-related peripartum diseases in dairy cows. J. Vet. Med. Sci. 2002;64:293–307. doi: 10.1292/jvms.64.293. [DOI] [PubMed] [Google Scholar]
- 182.Biedzka-Sarek M., Metso J., Kateifides A., Meri T., Jokiranta T.S., Muszyński A., Radziejewska-Lebrecht J., Zannis V., Skurnik M., Jauhiainen M. Apolipoprotein A-I exerts bactericidal activity against Yersinia enterocolitica Serotype O:3. J. Biol. Chem. 2011;286:38211–38219. doi: 10.1074/jbc.M111.249482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sigel S., Bunk S., Meergans T., Doninger B., Stich K., Stulnig T., Derfler K., Hoffmann J., Deininger S., von Aulock S., et al. Apolipoprotein B100 is a suppressor of Staphylococcus aureus-induced innate immune responses in humans and mice. Eur. J. Immunol. 2012;42:2983–2989. doi: 10.1002/eji.201242564. [DOI] [PubMed] [Google Scholar]
- 184.Johnston L.D., Brown G., Gauthier D., Reece K., Kator H., Van Veld P. Apolipoprotein A-I from striped bass (Morone saxatilis) demonstrates antibacterial activity in vitro. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;151:167–175. doi: 10.1016/j.cbpb.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 185.Wang C.Q., Yang C.S., Yang Y., Pan F., He L.Y., Wang A.M. An apolipoprotein E mimetic peptide with activities against multidrug-resistant bacteria and immunomodulatory effects. J. Pept. Sci. 2013;19:745–750. doi: 10.1002/psc.2570. [DOI] [PubMed] [Google Scholar]
- 186.Ghimire D., Rai M., Gaur R. Novel host restriction factors implicated in HIV-1 replication. J. Gen. Virol. 2018;99:435–446. doi: 10.1099/jgv.0.001026. [DOI] [PubMed] [Google Scholar]
- 187.Awadeh F.T., Abdelrahman M.M., Kincaid R.L., Finley J.W. Effect of selenium supplements on the distribution of selenium among serum proteins in cattle. J. Dairy Sci. 1998;81:1089–1094. doi: 10.3168/jds.S0022-0302(98)75670-X. [DOI] [PubMed] [Google Scholar]
- 188.Mostert V. Selenoprotein P: Properties, functions, and regulation. Arch. Biochem. Biophys. 2000;376:433–438. doi: 10.1006/abbi.2000.1735. [DOI] [PubMed] [Google Scholar]
- 189.Burk R.F., Hill K.E. Selenoprotein P-expression, functions, and roles in mammals. Biochim. Biophys. Acta. 2009;1790:1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bruzelius K., Hoac T., Sundler R., Onning G., Akesson B. Occurrence of selenoprotein enzyme activities and mRNA in bovine mammary tissue. J. Dairy Sci. 2007;90:918–927. doi: 10.3168/jds.S0022-0302(07)71575-8. [DOI] [PubMed] [Google Scholar]
- 191.Hara S., Shoji Y., Sakurai A., Yuasa K., Himeno S., Imura N. Effects of selenium deficiency on expression of selenoproteins in bovine arterial endothelial cells. Biol. Pharm. Bull. 2001;24:754–759. doi: 10.1248/bpb.24.754. [DOI] [PubMed] [Google Scholar]
- 192.Ogawa S.K., Shin M.C., Hirashima M., Akaike N., Ito Y. Effects of selenoprotein P on the contraction and relaxation of the airway smooth muscle. Gen. Physiol. Biophys. 2013;32:47–54. doi: 10.4149/gpb_2013012. [DOI] [PubMed] [Google Scholar]
- 193.Bao Y., Wang L., Shi L., Yun F., Liu X., Chen Y., Chen C., Ren Y., Jia Y. Transcriptome profiling revealed multiple genes and ECM-receptor interaction pathways that may be associated with breast cancer. Cell Mol. Biol. Lett. 2019;24:38. doi: 10.1186/s11658-019-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mardpour S., Hamidieh A.A., Taleahmad S., Sharifzad F., Taghikhani A., Baharvand H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J. Cell. Physiol. 2019;234:8249–8258. doi: 10.1002/jcp.27669. [DOI] [PubMed] [Google Scholar]
- 195.Lee H.J., Jang M., Kim H., Kwak W., Park W., Hwang J.Y., Lee C.K., Jang G.W., Park M.N., Kim H.C., et al. Comparative Transcriptome Analysis of Adipose Tissues Reveals that ECM-Receptor Interaction Is Involved in the Depot-Specific Adipogenesis in Cattle. PLoS ONE. 2013;8:e66267. doi: 10.1371/journal.pone.0066267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stafuzza N.B., Zerlotini A., Lobo F.P., Yamagishi M.E., Chud T.C., Caetano A.R., Munari D.P., Garrick D.J., Machado M.A., Martins M.F., et al. Single nucleotide variants and InDels identified from whole-genome re-sequencing of Guzerat, Gyr, Girolando and Holstein cattle breeds. PLoS ONE. 2017;12:e0173954. doi: 10.1371/journal.pone.0173954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lopez B.I., Santiago K.G., Lee D., Ha S., Seo K. RNA Sequencing (RNA-Seq) Based Transcriptome Analysis in Immune Response of Holstein Cattle to Killed Vaccine against Bovine Viral Diarrhea Virus Type I. Animals. 2020;10:344. doi: 10.3390/ani10020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang H., Jiang H., Fan Y., Chen Z., Li M., Mao Y., Karrow N.A., Loor J.J., Moore S., Yang Z. Transcriptomics and iTRAQ-Proteomics Analyses of Bovine Mammary Tissue with Streptococcus agalactiae-Induced Mastitis. J. Agric. Food Chem. 2018;66:11188–11196. doi: 10.1021/acs.jafc.8b02386. [DOI] [PubMed] [Google Scholar]
- 199.Marchi S., Patergnani S., Missiroli S., Morciano G., Rimessi A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 200.Paupe V., Prudent J. New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem. Biophys. Res. Commun. 2018;500:75–86. doi: 10.1016/j.bbrc.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.King M.M., Kayastha B.B., Franklin M.J., Patrauchan M.A. Calcium Regulation of Bacterial Virulence. Adv. Exp. Med. Biol. 2020;1131:827–855. doi: 10.1007/978-3-030-12457-1_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Puri B.K. Calcium Signaling and Gene Expression. Adv. Exp. Med. Biol. 2020;1131:537–545. doi: 10.1007/978-3-030-12457-1_22. [DOI] [PubMed] [Google Scholar]
- 203.Granatiero V., De Stefani D., Rizzuto R. Mitochondrial Calcium Handling in Physiology and Disease. Adv. Exp. Med. Biol. 2017;982:25–47. doi: 10.1007/978-3-319-55330-6_2. [DOI] [PubMed] [Google Scholar]
- 204.Mustaly-Kalimi S., Littlefield A.M., Stutzmann G.E. Calcium Signaling Deficits in Glia and Autophagic Pathways Contributing to Neurodegenerative Disease. Antioxid. Redox Signal. 2018;29:1158–1175. doi: 10.1089/ars.2017.7266. [DOI] [PubMed] [Google Scholar]
- 205.Gorvin C.M. Molecular and clinical insights from studies of calcium-sensing receptor mutations. J. Mol. Endocrinol. 2019;63:R1–R16. doi: 10.1530/JME-19-0104. [DOI] [PubMed] [Google Scholar]
- 206.Alghamdi M., Alasmari D., Assiri A., Mattar E., Aljaddawi A.A., Alattas S.G., Redwan E.M. An Overview of the Intrinsic Role of Citrullination in Autoimmune Disorders. J. Immunol. Res. 2019;2019:7592851. doi: 10.1155/2019/7592851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mondal S., Thompson P.R. Protein Arginine Deiminases (PADs): Biochemistry and Chemical Biology of Protein Citrullination. Acc. Chem. Res. 2019;52:818–832. doi: 10.1021/acs.accounts.9b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Méchin M.C., Takahara H., Simon M. Deimination and Peptidylarginine Deiminases in Skin Physiology and Diseases. Int. J. Mol. Sci. 2020;21:566. doi: 10.3390/ijms21020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Burgos R.A., Conejeros I., Hidalgo M.A., Werling D., Hermosilla C. Calcium influx, a new potential therapeutic target in the control of neutrophil-dependent inflammatory diseases in bovines. Vet. Immunol. Immunopathol. 2011;143:1–10. doi: 10.1016/j.vetimm.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 210.Zhu L., Huang L., Zhu Y., Ding X., Zhu G. Calcium signaling involved in bovine herpesvirus 1 replication in MDBK cells. Acta Virol. 2017;61:487–491. doi: 10.4149/av_2017_412. [DOI] [PubMed] [Google Scholar]
- 211.Wadhawan M., Tiwari S., Sharma S., Rathaur S. Identification and characterization of a novel prolyl oligopeptidase in filarial parasite Setaria cervi. Biochem. Biophys. Res. Commun. 2018;495:2235–2241. doi: 10.1016/j.bbrc.2017.12.093. [DOI] [PubMed] [Google Scholar]
- 212.Justet C., Chifflet S., Hernandez J.A. Calcium Oscillatory Behavior and Its Possible Role during Wound Healing in Bovine Corneal Endothelial Cells in Culture. Biomed. Res. Int. 2019;2019:8647121. doi: 10.1155/2019/8647121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wright M.F., Bowdridge E., McDermott E.L., Richardson S., Scheidler J., Syed Q., Bush T., Inskeep E.K., Flores J.A. Mechanisms of intracellular calcium homeostasis in developing and mature bovine corpora lutea. Biol. Reprod. 2014;90:55. doi: 10.1095/biolreprod.113.113662. [DOI] [PubMed] [Google Scholar]
- 214.Rezende F.M., Dietsch G.O., Peñagaricano F. Genetic dissection of bull fertility in US Jersey dairy cattle. Anim. Genet. 2018;49:393–402. doi: 10.1111/age.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Félix-Martínez G.J., Gil A., Segura J., Villanueva J., Gutíerrez L.M. Modeling the influence of co-localized intracellular calcium stores on the secretory response of bovine chromaffin cells. Comput. Biol. Med. 2018;100:165–175. doi: 10.1016/j.compbiomed.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 216.Hernandez L.L. TRIENNIAL LACTATION SYMPOSIUM/BOLFA: Serotonin and the regulation of calcium transport in dairy cows. J. Anim. Sci. 2017;95:5711–5719. doi: 10.2527/jas2017.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shah S., Brock E.J., Ji K., Mattingly R.R. Ras and Rap1: A tale of two GTPases. Semin. Cancer Biol. 2019;54:29–39. doi: 10.1016/j.semcancer.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Greenwood J., Steinman L., Zamvil S.S. Statin therapy in autoimmunity: From protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006;5:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stefanini L., Bergmeier W. RAP GTPases and platelet integrin signaling. Platelets. 2019;30:41–47. doi: 10.1080/09537104.2018.1476681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cai Y., Kandula V., Kosuru R., Ye X., Irwin M.G., Xia Z. Decoding telomere protein Rap1: Its telomeric and nontelomeric functions and potential implications in diabetic cardiomyopathy. Cell Cycle. 2017;16:1765–1773. doi: 10.1080/15384101.2017.1371886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chrzanowska-Wodnicka M. Rap1 in endothelial biology. Curr. Opin. Hematol. 2017;24:248–255. doi: 10.1097/MOH.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang Y.L., Wang R.C., Cheng K., Ring B.Z., Su L. Roles of Rap1 signaling in tumor cell migration and invasion. Cancer Biol. Med. 2017;14:90–99. doi: 10.20892/j.issn.2095-3941.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bögershausen N., Tsai I.C., Pohl E., Kiper P.Ö., Beleggia F., Percin E.F., Keupp K., Matchan A., Milz E., Alanay Y., et al. RAP1-mediated MEK/ERK pathway defects in Kabuki syndrome. J. Clin. Investig. 2015;125:3585–3599. doi: 10.1172/JCI80102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hutchison M.R., White P.C. Prostacyclin regulates bone growth via the Epac/Rap1 pathway. Endocrinology. 2015;156:499–510. doi: 10.1210/en.2014-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jaśkiewicz A., Pająk B., Orzechowski A. The Many Faces of Rap1 GTPase. Int. J. Mol. Sci. 2018;19:2848. doi: 10.3390/ijms19102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Munson L., Moresco A. Comparative pathology of mammary gland cancers in domestic and wild animals. Breast Dis. 2007;28:7–21. doi: 10.3233/BD-2007-28102. [DOI] [PubMed] [Google Scholar]
- 227.Munday J.S. Bovine and human papillomaviruses: A comparative review. Vet. Pathol. 2014;51:1063–1075. doi: 10.1177/0300985814537837. [DOI] [PubMed] [Google Scholar]
- 228.Roperto S., Munday J.S., Corrado F., Goria M., Roperto F. Detection of bovine papillomavirus type 14 DNA sequences in urinary bladder tumors in cattle. Vet. Microbiol. 2016;190:1–4. doi: 10.1016/j.vetmic.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 229.Peng H., Wu C., Li J., Li C., Chen Z., Pei Z., Tao L., Gong Y., Pan Y., Bai H., et al. Detection and genomic characterization of Bovine papillomavirus isolated from Chinese native cattle. Transbound. Emerg. Dis. 2019;66:2197–2203. doi: 10.1111/tbed.13285. [DOI] [PubMed] [Google Scholar]
- 230.Hemmatzadeh F., Keyvanfar H., Hasan N.H., Niap F., Bani Hassan E., Hematzade A., Ebrahimie E., McWhorter A., Ignjatovic J. Interaction between Bovine leukemia virus (BLV) infection and age on telomerase misregulation. Vet. Res. Commun. 2015;39:97–103. doi: 10.1007/s11259-015-9629-2. [DOI] [PubMed] [Google Scholar]
- 231.Fuhrmann J., Thompson P.R. Protein Arginine Methylation and Citrullination in Epigenetic Regulation. ACS Chem. Biol. 2016;11:654–668. doi: 10.1021/acschembio.5b00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yuzhalin A.E. Citrullination in cancer. Cancer Res. 2019;79:1274–1284. doi: 10.1158/0008-5472.CAN-18-2797. [DOI] [PubMed] [Google Scholar]
- 233.Beato M., Sharma P. Peptidyl Arginine Deiminase 2 (PADI2)-Mediated Arginine Citrullination Modulates Transcription in Cancer. Int. J. Mol. Sci. 2020;21:1351. doi: 10.3390/ijms21041351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Brentville V.A., Vankemmelbeke M., Metheringham R.L., Durrant L.G. Post-translational modifications such as citrullination are excellent targets for cancer therapy. Semin. Immunol. 2020;47:101393. doi: 10.1016/j.smim.2020.101393. [DOI] [PubMed] [Google Scholar]
- 235.Devraj G., Beerlage C., Brüne B., Kempf V.A. Hypoxia and HIF-1 activation in bacterial infections. Microbes Infect. 2017;19:144–156. doi: 10.1016/j.micinf.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 236.Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer. 2016;138:1058–1066. doi: 10.1002/ijc.29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pezzuto A., Carico E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review. Curr. Mol. Med. 2018;18:343–351. doi: 10.2174/1566524018666181109121849. [DOI] [PubMed] [Google Scholar]
- 238.Zhu C., Zhu Q., Wang C., Zhang L., Wei F., Cai Q. Hostile takeover: Manipulation of HIF-1 signaling in pathogen-associated cancers (Review) Int. J. Oncol. 2016;49:1269–1276. doi: 10.3892/ijo.2016.3633. [DOI] [PubMed] [Google Scholar]
- 239.Metheni M., Lombès A., Bouillaud F., Batteux F., Langsley G. HIF-1α induction, proliferation and glycolysis of Theileria-infected leukocytes. Cell Microbiol. 2015;17:467–472. doi: 10.1111/cmi.12421. [DOI] [PubMed] [Google Scholar]
- 240.Berisha B., Schams D., Rodler D., Pfaffl M.W. Angiogenesis in the Ovary—The Most Important Regulatory Event for Follicle and Corpus Luteum Development and Function in Cow—An Overview. Anat. Histol. Embryol. 2016;45:124–130. doi: 10.1111/ahe.12180. [DOI] [PubMed] [Google Scholar]
- 241.Iqbal N., Liu X., Yang T., Huang Z., Hanif Q., Asif M., Khan Q.M., Mansoor S. Genomic variants identified from whole-genome resequencing of indicine cattle breeds from Pakistan. PLoS ONE. 2019;14:e0215065. doi: 10.1371/journal.pone.0215065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fry B., Carter J.F. Stable carbon isotope diagnostics of mammalian metabolism, a high-resolution isotomics approach using amino acid carboxyl groups. PLoS ONE. 2019;14:e0224297. doi: 10.1371/journal.pone.0224297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Schwab C.G., Broderick G.A. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 2017;100:10094–10112. doi: 10.3168/jds.2017-13320. [DOI] [PubMed] [Google Scholar]
- 244.Hamam H.J., Palaniyar N. Post-Translational Modifications in NETosis and NETs-Mediated Diseases. Biomolecules. 2019;9:369. doi: 10.3390/biom9080369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yildiz K., Gokpinar S., Gazyagci A.N., Babur C., Sursal N., Azkur A.K. Role of NETs in the difference in host susceptibility to Toxoplasma gondii between sheep and cattle. Vet. Immunol. Immunopathol. 2017;189:1–10. doi: 10.1016/j.vetimm.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 246.Villagra-Blanco R., Silva L.M.R., Muñoz-Caro T., Yang Z., Li J., Gärtner U., Taubert A., Zhang X., Hermosilla C. Bovine Polymorphonuclear Neutrophils Cast Neutrophil Extracellular Traps against the Abortive Parasite Neospora caninum. Front. Immunol. 2017;8:606. doi: 10.3389/fimmu.2017.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mendez J., Sun D., Tuo W., Xiao Z. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite Ostertagia ostertagi. Sci. Rep. 2018;8:17598. doi: 10.1038/s41598-018-36070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jerjomiceva N., Seri H., Völlger L., Wang Y., Zeitouni N., Naim H.Y., von Köckritz-Blickwede M. Enrofloxacin enhances the formation of neutrophil extracellular traps in bovine granulocytes. J. Innate Immun. 2014;6:706–712. doi: 10.1159/000358881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kozlowski H.N., Lai E.T., Havugimana P.C., White C., Emili A., Sakac D., Binnington B., Neschadim A., McCarthy S.D., Branch D.R. Extracellular histones identified in crocodile blood inhibit in-vitro HIV-1 infection. AIDS. 2016;30:2043–2052. doi: 10.1097/QAD.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 250.Fernandes J.M., Kemp G.D., Molle M.G., Smith V.J. Anti-microbial properties of histone H2A from skin secretions of rainbow trout, Oncorhynchus mykiss. Biochem. J. 2002;368 Pt 2:611–620. doi: 10.1042/bj20020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nicholas A.P., Whitaker J.N. Preparation of a monoclonal antibody to citrullinated epitopes: Its characterization and some applications to immunohistochemistry in human brain. Glia. 2002;37:328–336. doi: 10.1002/glia.10039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.