Abstract
Amyloid beta (Aβ) depositions are more abundant in HIV-infected brains. The blood–brain barrier, with its backbone created by endothelial cells, is assumed to be a core player in Aβ homeostasis and may contribute to Aβ accumulation in the brain. Exposure to HIV increases shedding of extracellular vesicles (EVs) from human brain endothelial cells and alters EV-Aβ levels. EVs carrying various cargo molecules, including a complex set of proteins, can profoundly affect the biology of surrounding neurovascular unit cells. In the current study, we sought to examine how exposure to HIV, alone or together with Aβ, affects the surface and total proteomic landscape of brain endothelial EVs. By using this unbiased approach, we gained an unprecedented, high-resolution insight into these changes. Our data suggest that HIV and Aβ profoundly remodel the proteome of brain endothelial EVs, altering the pathway networks and functional interactions among proteins. These events may contribute to the EV-mediated amyloid pathology in the HIV-infected brain and may be relevant to HIV-1-associated neurocognitive disorders.
Keywords: HIV-1, amyloid beta, extracellular vesicles, blood–brain barrier
1. Introduction
HIV-infected brains tend to have enhanced amyloid beta (Aβ) deposition [1,2,3,4,5,6], mostly in the perivascular space [3,7,8,9]. Indeed, the blood–brain barrier (BBB) is thought to be a key player in the brain’s Aβ homeostasis [10]. It is now widely accepted that extracellular vesicles (EVs) may also be important in Aβ pathology [11,12,13,14,15,16,17]. Our earlier work has shown that HIV can increase the release of brain endothelial EVs and alter EV-Aβ levels. Moreover, brain endothelial cell-derived EVs can transfer Aβ to other cells of the neurovascular unit [18]. EVs carry specific cargo molecules, including a complex set of proteins, which can be transferred to the neighboring cells and affect their biology. Some of these proteins are on the EV surface. The surface proteins may allow for selective EV uptake by the recipient cells, like in the case of receptor-mediated endocytosis. Total proteomics can give detailed information on the EV protein cargo overall. Surface proteomics could indicate the “address” of a targeted delivery, while total proteomics would represent the delivered “package.”
In this work, we investigated how exposure to HIV, alone and together with Aβ, impacts the surface and total proteomic landscape of EVs from human brain microvascular endothelial cells (HBMEC-EVs). By using this unbiased strategy, we obtained a complex, high-resolution insight into these changes.
2. Results
2.1. Extracellular Vesicles from Human Brain Microvascular Endothelial Cells (HBMEC-EVs) Are Enriched with the Major EV Markers
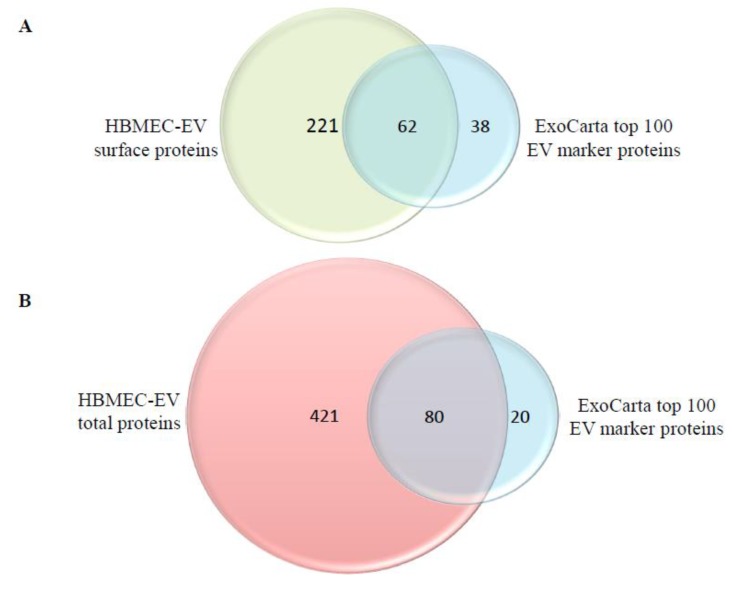
At first, we examined whether proteins that are frequently identified in EVs/exosomes from various sources can be found in our isolated HBMEC-EVs. Based on the ExoCarta EV proteomics database from different human cell types that have been isolated using different approaches [19,20], we compiled the list of 100 marker proteins that are most often present on EVs (Table 1). The surface HBMEC-EV proteome, which contained a total of 283 identified proteins, included 62 of the top 100 ExoCarta EV markers (Figure 1A, Table 1). In addition, the total HBMEC-EV proteome, which contained 501 identified proteins, included 80 of such markers (Figure 1B, Table 1). These results demonstrate that our HBMEC-EV isolation was highly enriched with known EV markers.
Table 1.
List of the top 100 ExoCarta proteins present in the brain endothelial extracellular vesicle (EV) surface (S) and total (T) proteome. Bold, top 100 ExoCarta proteins present in S or T; bold and red, proteins present in both S and T.
| Gene Symbol | Detected in S | Detected in T | |
|---|---|---|---|
| 1 | CD9 | − | + |
| 2 | HSPA8 | + | + |
| 3 | PDCD6IP | + | + |
| 4 | GAPDH | + | + |
| 5 | ACTB | + | + |
| 6 | ANXA2 | + | + |
| 7 | CD63 | − | + |
| 8 | SDCBP | + | + |
| 9 | ENO1 | + | + |
| 10 | HSP90AA1 | + | + |
| 11 | TSG101 | − | + |
| 12 | PKM | + | + |
| 13 | LDHA | + | + |
| 14 | EEF1A1 | + | + |
| 15 | YWHAZ | + | + |
| 16 | PGK1 | + | + |
| 17 | EEF2 | + | + |
| 18 | ALDOA | + | + |
| 19 | HSP90AB1 | + | + |
| 20 | ANXA5 | + | + |
| 21 | FASN | + | + |
| 22 | YWHAE | + | + |
| 23 | CLTC | + | + |
| 24 | CD81 | − | + |
| 25 | ALB | + | + |
| 26 | VCP | + | + |
| 27 | TPI1 | + | + |
| 28 | PPIA | + | + |
| 29 | MSN | + | + |
| 30 | CFL1 | + | + |
| 31 | PRDX1 | + | + |
| 32 | PFN1 | + | + |
| 33 | RAP1B | + | + |
| 34 | ITGB1 | + | + |
| 35 | HSPA5 | + | + |
| 36 | SLC3A2 | − | + |
| 37 | HIST1H4A | + | + |
| 38 | GNB2 | − | − |
| 39 | ATP1A1 | − | + |
| 40 | YWHAQ | + | + |
| 41 | FLOT1 | − | − |
| 42 | FLNA | + | + |
| 43 | CLIC1 | + | + |
| 44 | CDC42 | + | + |
| 45 | CCT2 | + | + |
| 46 | A2M | + | + |
| 47 | YWHAG | + | + |
| 48 | TUBA1B | + | + |
| 49 | RAC1 | − | + |
| 50 | LGALS3BP | + | + |
| 51 | HSPA1A | + | + |
| 52 | GNAI2 | + | + |
| 53 | ANXA1 | + | + |
| 54 | RHOA | − | − |
| 55 | MFGE8 | − | + |
| 56 | PRDX2 | + | − |
| 57 | GDI2 | + | + |
| 58 | EHD4 | − | + |
| 59 | ACTN4 | + | + |
| 60 | YWHAB | − | − |
| 61 | RAB7A | − | + |
| 62 | LDHB | + | + |
| 63 | GNAS | − | − |
| 64 | TFRC | − | − |
| 65 | RAB5C | − | + |
| 66 | ARF1 | − | − |
| 67 | ANXA6 | + | + |
| 68 | ANXA11 | − | + |
| 69 | ACTG1 | − | − |
| 70 | KPNB1 | + | + |
| 71 | EZR | − | + |
| 72 | ANXA4 | − | − |
| 73 | ACLY | + | + |
| 74 | TUBA1C | − | − |
| 75 | RAB14 | − | + |
| 76 | HIST2H4A | − | − |
| 77 | GNB1 | + | + |
| 78 | UBA1 | + | + |
| 79 | THBS1 | + | + |
| 80 | RAN | + | + |
| 81 | RAB5A | − | − |
| 82 | PTGFRN | + | + |
| 83 | CCT5 | + | + |
| 84 | CCT3 | − | + |
| 85 | BSG | − | + |
| 86 | AHCY | + | + |
| 87 | RAB5B | − | − |
| 88 | RAB1A | − | + |
| 89 | LAMP2 | − | + |
| 90 | ITGA6 | − | − |
| 91 | HIST1H4B | − | − |
| 92 | GSN | + | + |
| 93 | FN1 | + | + |
| 94 | YWHAH | − | + |
| 95 | TUBA1A | + | − |
| 96 | TKT | − | − |
| 97 | TCP1 | + | + |
| 98 | STOM | − | + |
| 99 | SLC16A1 | − | − |
| 100 | RAB8A | − | − |
Figure 1.
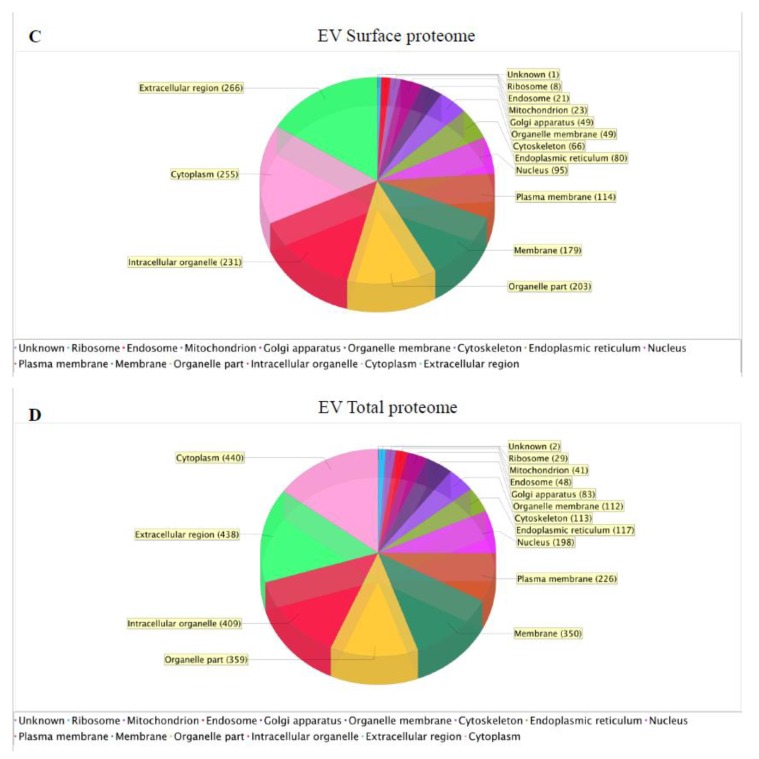
Extracellular vesicle (EV)-specific markers in the surface and total proteomes of human brain microvascular endothelial cells (HBMEC)-derived EVs. Venn diagram showing the overlap between the HBMEC-EV surface proteome (283 proteins) (A) or the HBMEC-EV total proteome (501 proteins) (B) and the top 100 EV marker proteins from ExoCarta. Cellular component enrichment of the identified surface (C) and total (D) EV proteomes. The identified EV proteins were enriched for cellular component using the Scaffold software.
2.2. Cellular Component Enrichment of the Identified Surface and Total EV Proteins
Using the Scaffold software, we next evaluated the HBMEC-EV proteins according to their known cellular localization. This approach may indicate the parent cellular compartment origin of the identified HBMEC-EV proteins. The majority of the HBMEC-EV surface proteins were extracellular region proteins, followed by cytoplasmic, intracellular organelle, membrane, nuclear, endoplasmic reticulum, cytoskeleton, Golgi, mitochondrial, endosomal, ribosomal proteins, and one unknown protein (Figure 1C). For the total HBMEC-EV proteome, the majority of proteins were cytoplasmic and extracellular region proteins (Figure 1D).
2.3. HIV and Aβ Exposure Results in Unique HBMEC-EV Proteome Signatures
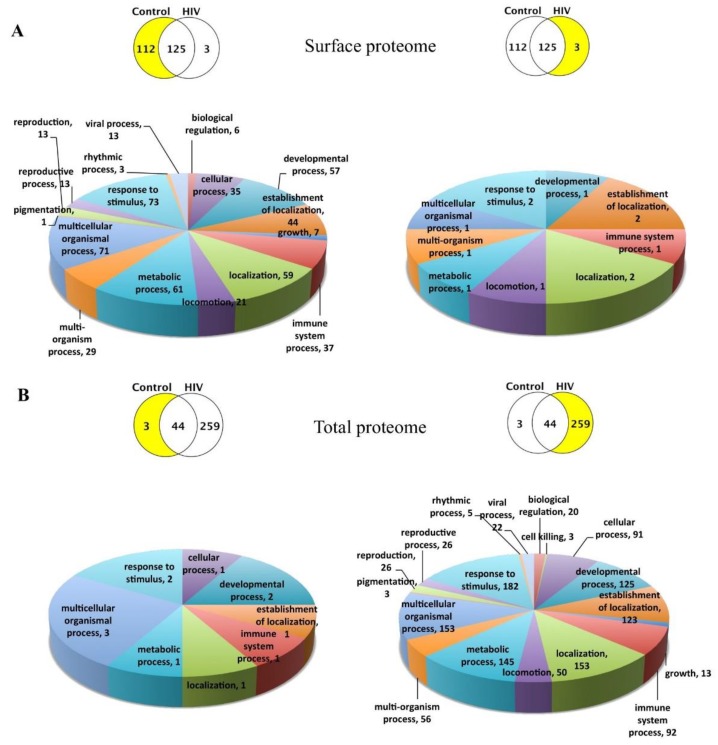
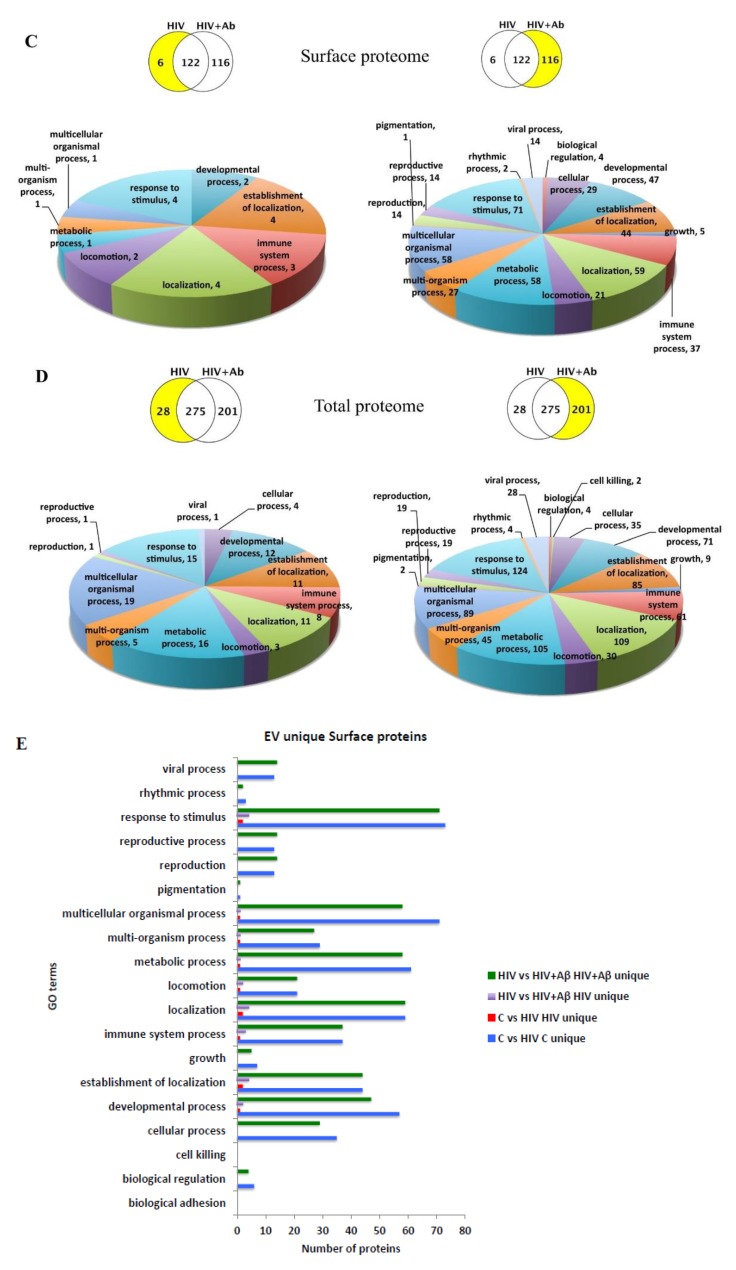
We next focused on the unique proteins induced by the exposure to HIV and Aβ. Comparison of the control vs. HIV surface HBMEC-EV proteomes identified 112 unique proteins in the control and three unique proteins in the HIV group (Figure 2A). By contrast, a similar comparison for the total proteome identified only three unique proteins in the control and as many as 259 unique proteins in the HIV group (Figure 2B). Comparison of the surface proteome between the HIV vs. HIV+Aβ groups identified six unique proteins in the HIV group and 116 unique proteins in the HIV+Aβ group (Figure 2C). Finally, analysis of the total proteome revealed 28 unique proteins in the HIV group and 201 unique proteins in the HIV+Aβ group (Figure 2D). A list of these unique proteins is provided in Table 2 and Table 3 for the surface and total proteomes, respectively.
Figure 2.
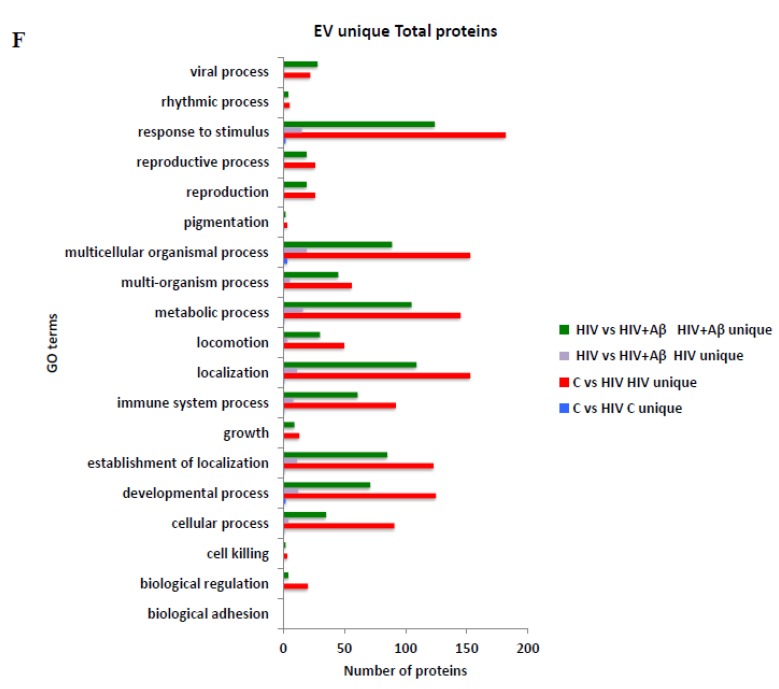
Enrichment for biological processes of the identified unique EV proteins. Scaffold software was used to enrich for the main biological processes for the identified unique EV proteins. The upper Venn diagrams show the compared groups with the number of their unique and shared proteins. The lower pie charts depict the enriched biological processes corresponding to the unique lists highlighted in yellow. The number of proteins in a particular biological process category is also provided. (A) Surface proteome, control vs. HIV. (B) Total proteome, control vs. HIV. (C) Surface proteome, HIV vs. HIV+ amyloid beta (Aβ). (D) Total proteome, HIV vs. HIV+Aβ. Combined graph for the biological processes in the EV unique surface (E) and total (F) proteomes. The number of unique proteins corresponding to the main biological processes in the different comparisons is illustrated on the graph.
Table 2.
List of the unique proteins in the EV surface proteome.
| Control vs. HIV | HIV vs. HIV+Aβ | ||||||
|---|---|---|---|---|---|---|---|
| Control Unique | HIV Unique | HIV Unique | HIV+Aβ Unique | ||||
| 1433E | GPC6 | TGM1 | TITIN DYH8 IGHG2 |
TITIN NIN DYH8 CAP1 ARPC4 IGHG2 |
1433E | GPC1 | TCPA |
| 1433G | GSTP1 | TIG1 | 1433G | GPC6 | TCPB | ||
| 1433T | IGL1 | TIMP3 | 1A34 | GSTA5 | TCPE | ||
| 1A34 | ITA3 | TPM4 | 1B15 | GSTP1 | TCPH | ||
| 5NTD | ITAV | TRFE | 5NTD | HMCN1 | TCPZ | ||
| 6PGD | ITB1 | TSP4 | ACLY | IGL1 | TIG1 | ||
| ACLY | K2C6B | UBB | ACTC | IMB1 | TPM4 | ||
| ACTC | LAMA1 | UGPA | ADA10 | ITA3 | TRFE | ||
| ALDOA | LCAT | URP2 | AL9A1 | ITA5 | TSP4 | ||
| AMPN | LDHA | VINC | ALDOA | ITAV | UBA1 | ||
| AMY1 | LDHB | WDR1 | AMY1 | ITB1 | UBB | ||
| ANXA1 | LOXL2 | WNT5A | ANXA1 | LDHA | UGPA | ||
| APOA4 | LRC17 | ARF3 | LDHB | URP2 | |||
| ARF3 | LRP1 | ARP2 | LOXL2 | VINC | |||
| ARGI1 | LTBP1 | ARP3 | LRC17 | WDR1 | |||
| ARP2 | MIME | ASPM | LRP1 | WNT5A | |||
| ARP3 | MMP2 | ATL1 | LTBP1 | ||||
| ATS13 | MPRI | ATX2 | MIME | ||||
| C1S | MYL6 | B4GA1 | MMP2 | ||||
| CASPE | NID2 | C1S | NID2 | ||||
| CCD80 | P3H1 | CAZA1 | P3H1 | ||||
| CFAH | PAI1 | CCD80 | PAI1 | ||||
| CHIA | PCOC1 | CDC42 | PDC6I | ||||
| CLIC1 | PDC6I | CHIA | PDIA3 | ||||
| CO4A2 | PDIA3 | CHSS2 | PGK1 | ||||
| CO5A2 | PGK1 | CISY | PGM1 | ||||
| CO7 | PLEC | CLIC1 | PLEC | ||||
| CO7A1 | PLOD3 | CLUS | PLOD3 | ||||
| COBA1 | PPIA | CO4A2 | PPIA | ||||
| COF1 | PRDX4 | CO5A2 | PRDX2 | ||||
| COFA1 | PYGB | CO7A1 | PRDX6 | ||||
| COMP | RAB1B | COBA1 | PUR6 | ||||
| EF1G | RACK1 | COF1 | PYGB | ||||
| ENOB | RAP1B | COFA1 | PYGL | ||||
| EXT1 | RLA0 | COMP | RACK1 | ||||
| EXT2 | RS16 | EF1G | RAP1B | ||||
| F13A | S10A9 | EXT1 | RGN | ||||
| FA11 | SDCB1 | F13A | RIMB1 | ||||
| FAS | SEPR | FA11 | RL12 | ||||
| FBLN1 | SPB12 | FAS | S10A9 | ||||
| FBN1 | SPR1B | FBLN1 | SDCB1 | ||||
| FBN2 | SPR2E | FBN1 | SERA | ||||
| FLNB | SRCRL | FBN2 | SERPH | ||||
| FLNC | SRPX2 | FLNB | SPR2E | ||||
| FPRP | SULF1 | FLNC | SRCRL | ||||
| FRIH | SULF2 | FPRP | SRPX2 | ||||
| FSCN1 | SYTC | FRIH | SULF1 | ||||
| GAS6 | TAGL2 | FRIL | SULF2 | ||||
| GNAI2 | TBA1A | FSCN1 | SYTC | ||||
| GPC1 | TCPD | GDIB | TAGL2 | ||||
Table 3.
List of the unique proteins in the EV total proteome.
| Control vs. HIV | ||||||
|---|---|---|---|---|---|---|
| Control Unique | HIV Unique | |||||
| ACTC MYH1 TAU |
1433T | CD81 | GDIB | MVP | S10AB | URP2 |
| 1433Z | CD82 | GELS | MYH9 | SAHH | VIME | |
| 1A24 | CLH1 | GGCT | MYL6 | SCRB2 | VINC | |
| 5NTD | CLIC1 | GNAI2 | MYOF | SDC4 | VPS35 | |
| 6PGD | CO1A2 | GPC6 | NID1 | SDCB1 | VTNC | |
| A4 | CO3A1 | GRP78 | NID2 | SEPR | VWF | |
| ACLY | CO4A1 | GSLG1 | NNMT | SERPH | WDR1 | |
| ACTN1 | CO4A2 | GTR1 | OLFL3 | SND1 | WNT5A | |
| ACTN4 | CO5 | H31 | PAI1 | SNED1 | ZA2G | |
| ADA10 | CO5A1 | H4 | PDC6I | SPTB2 | ||
| AEBP1 | CO5A2 | HEP2 | PDIA3 | SPTN1 | ||
| AGRIN | CO6A2 | HS90A | PFKAP | SRCRL | ||
| AHNK | CO7A1 | HS90B | PGK1 | SRGN | ||
| ALDOA | CO9 | HSP7C | PGS1 | SRPX | ||
| AMPN | COEA1 | HTRA1 | PGS2 | SRPX2 | ||
| AMY1 | COFA1 | IF4A1 | PKP1 | SULF1 | ||
| ANT3 | COIA1 | IGHA1 | PLEC | SULF2 | ||
| ANX11 | COMP | ITA3 | PLMN | SYDC | ||
| ANXA1 | CYTA | ITA4 | PLOD1 | SYTC | ||
| ANXA5 | DPYL2 | ITA5 | PLOD3 | TAGL2 | ||
| ANXA6 | DYHC1 | ITAV | PLS1 | TBA1B | ||
| AP2A1 | EF1A1 | ITB1 | PLS3 | TBB4B | ||
| AP2M1 | EF2 | ITIH1 | PPIB | TCPA | ||
| APLP2 | EHD1 | ITIH3 | PRC2A | TCPB | ||
| APOA4 | EHD2 | ITIH4 | PRDX1 | TCPD | ||
| APOB | EMIL1 | KLK7 | PRDX6 | TCPH | ||
| APOE | ENOA | KPYM | PROF1 | TCPQ | ||
| ARF3 | ENPL | LAMA1 | PSB5 | TCPZ | ||
| ARF4 | EXT1 | LAMA2 | PTX3 | TENA | ||
| ARGI1 | EXT2 | LAMA4 | PXDN | TERA | ||
| ARP2 | F13A | LAMA5 | RAB5C | TGM1 | ||
| ARPC2 | FA5 | LAMP1 | RAB7A | TGM2 | ||
| AT1A1 | FAS | LAMP2 | RAC1 | TGM3 | ||
| ATL1 | FAT1 | LDHA | RACK1 | THBG | ||
| ATS12 | FBLN1 | LDHB | RAN | THRB | ||
| ATS13 | FBN1 | LEG1 | RAP1B | THY1 | ||
| ATX2 | FBX50 | LORI | RB11A | TIG1 | ||
| B4GA1 | FETA | LOXL2 | RHOC | TITIN | ||
| BGH3 | FIBB | LRC17 | RIMB1 | TLN1 | ||
| BMP1 | FILA | LRP1 | RL10A | TPIS | ||
| C1QT3 | FLNA | LTBP1 | RL12 | TPM4 | ||
| C1S | FLNB | LTBP2 | RL13A | TRFE | ||
| CAP1 | FLNC | LYSC | RL27 | TRFL | ||
| CASPE | FPRP | MAMC2 | RL6 | TSN14 | ||
| CATA | FSCN1 | MARCS | RS16 | TSP 1 | ||
| CCD80 | G3P | MFGM | RS3 | TSP 2 | ||
| CD151 | GALK1 | MMP2 | RS4X | TSP 3 | ||
| CD44 | GAS6 | MOES | RS8 | TTYH3 | ||
| CD59 | GBB1 | MOT4 | RSSA | UBA1 | ||
| CD63 | GBG12 | MRC2 | S10A9 | UBB | ||
| HIV vs. HIV+Aβ | ||||||
| HIV unique | HIV+Aβ unique | |||||
| AHNK | 1433E | CO8B | MIME | RL3 | ||
| ARGI1 | 1433F | COF1 | MOB1B | RL7 | ||
| ATX2 | 1433G | COPB2 | MPRI | RL7A | ||
| B4GA1 | 1B40 | COR1A | MRP | RLA0 | ||
| CASPE | 2AAA | COR1C | MXRA5 | RS11 | ||
| CATA | 4F2 | CTL1 | MYH16 | RS18 | ||
| CYTA | ACTC | CTL2 | NDKA | RS2 | ||
| FBX50 | AK1A1 | CTND1 | NEP | RS20 | ||
| FILA | AL9A1 | CYFP1 | NIBL1 | RS25 | ||
| GGCT | ALS | DHX9 | NOTC3 | RS3A | ||
| HORN | ANGL2 | DSG4 | NRP1 | RS9 | ||
| IGHA1 | ANGL4 | DX39B | OLM2B | RTN4 | ||
| K1C13 | ANM1 | ECM1 | P3H1 | RUVB1 | ||
| KLK7 | ANR31 | ECM2 | PAMR1 | SC23A | ||
| LORI | AP1G1 | EEA1 | PARVB | SCUB3 | ||
| LYSC | AP2B1 | EF1G | PCOC1 | SEM3C | ||
| MYOF | APOM | EGLN | PDIA1 | SEP11 | ||
| PLS1 | ARF6 | EHD4 | PDIA6 | SEPT2 | ||
| PRC2A | ARP3 | EIF3A | PDLI5 | SERA | ||
| RIMB1 | ARPC4 | EZRI | PGFRB | SLIT2 | ||
| RL27 | ASSY | FA10 | PGM1 | STOM | ||
| S10A9 | AT1B3 | FA11 | PIP | SVEP1 | ||
| SNED1 | ATPA | FBN2 | PLOD2 | SYFB | ||
| SPB12 | ATPB | FIBG | PP1B | SYHC | ||
| TGM1 | ATS7 | FRIH | PPIA | SYK | ||
| TGM3 | B4GT5 | G6PD | PRS23 | SYRC | ||
| TITIN | BASI | G6PI | PRS8 | SYSC | ||
| ZA2G | BASP1 | GANAB | PSA3 | TARSH | ||
| BGAL | H13 | PSA6 | TBB2A | |||
| C1R | H2A1 | PSD11 | TBB5 | |||
| C1TC | H2B1K | PSD12 | TCPE | |||
| CAD23 | HGFL | PSD13 | TCPG | |||
| CALR | HHIP | PSMD1 | TGFB1 | |||
| CAND1 | HMCN1 | PSMD2 | TICN1 | |||
| CAPZB | HNRPK | PSMD3 | TIE1 | |||
| CAV1 | IGSF8 | PUR6 | TIMP3 | |||
| CAZA1 | ILK | PYGB | TS101 | |||
| CBPN | IMB1 | PYGL | TSN6 | |||
| CCBE1 | IPO5 | QSOX1 | TSN9 | |||
| CD9 | IPO7 | RAB10 | TSP4 | |||
| CDC42 | IQGA1 | RAB14 | UACA | |||
| CEMIP | KCRM | RAB1A | UGDH | |||
| CFAH | KR101 | RAB2A | VAT1 | |||
| CHIA | KR111 | RALA | VDAC1 | |||
| CHSS2 | KRA11 | RELN | VDAC2 | |||
| CISY | LAMB2 | RGN | VGFR1 | |||
| CLUS | LIS1 | RL14 | XPO1 | |||
| CNTN1 | LMNA | RL18 | XPO2 | |||
| CO7 | LRC15 | RL18A | XPP1 | |||
| CO8A | LUM | RL22 | XRCC6 | |||
| XYLT1 | ||||||
2.4. Functional Enrichment of the Unique HBMEC-EV Proteins
We next grouped these unique protein signatures into the biological process categories of the Scaffold software. Overall, 19 main categories were established, and the number of unique proteins mapping to these categories is illustrated in Figure 2, separately for the surface (A and C) and the total proteome (B and D). Note that individual proteins could map to more than one category; on the other hand, not all categories have been identified for all comparisons. This is consistent with the fact that selected group comparisons identified only a limited number of unique proteins that mapped to a limited number of categories. The number of unique proteins corresponding to the main biological process categories in the combined comparisons is illustrated on the bar graphs in Figure 2E for the surface proteome and Figure 2F for the total proteome. The majority of both surface and total unique proteins were mapped to “response to stimulus,” ”multicellular organismal process,” ”metabolic process,” and “localization” categories.
Next, we evaluated the unique proteins in the control vs. HIV and in the HIV vs. HIV+Aβ comparisons using STRING for functional enrichment in the biological processes and the Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways. In addition, we enriched these analyses for cellular components and PMID publications.
The results of these analyses for the EV surface proteome unique proteins in the control group in the control vs. HIV comparison are listed in Table 4 and Supplementary Table S1A. In addition, Supplementary Table S1B lists the enrichment for cellular components. The observed gene count (Obs), background gene count (Bgr), false discovery rate (FDR), and matched proteins are also included in these tables. The three unique proteins identified when comparing the surface proteome in the HIV group to the control group are dynein heavy chain 8, axonemal (DNAH8), titin (TTN), and immunoglobulin heavy constant gamma 2 (IGHG2). According to the description in the STRING or GeneCards database, DNAH8 is a force-generating protein of the respiratory cilia and is also involved in sperm motility. In addition, DNAH8 is highly expressed in prostate cancer [21]. Titin appears to be a key component of the vertebrate striated muscles [22]. IGHG2 may take part in antigen binding and the regulation of actin dynamics. It was linked to severe respiratory syncytial virus infection [23]. Overall, very limited or no data were found for the different enrichment analyses in STRING regarding these three proteins.
Table 4.
Biological processes, KEGG pathways, and PMIDs for the EV surface unique proteins in the control group for the control vs. HIV comparison.
|
Gene Ontology (GO) Terms for Biological Processes
10 Most Significant Results per FDR (for all GO Terms, See Supplemental Table S1A) | |||||
| Term description | Obs | Bgr | FDR | Matching proteins in the network | |
| Extracellular structure organization | 16 | 339 | 2.01 × 10−10 | APOA4,COMP,FBLN1,FBN1,FBN2,GAS6,LAMA1,LCAT,LOXL2,MMP2,NID2,PLOD3,PRDX4,SERPINE1,SULF1,SULF2 | |
| Extracellular matrix organization | 14 | 296 | 4.25 × 10−9 | COMP,FBLN1,FBN1,FBN2,GAS6,LAMA1,LOXL2,MMP2,NID2,PLOD3,PRDX4,SERPINE1,SULF1,SULF2 | |
| Organonitrogen compound metabolic process | 42 | 5281 | 3.69 × 10 -6 | ACLY,AICDA,ALDOA,ANXA1,APOA4,C1S,CHIA,EEF1G,EXT1,EXT2,F13A1,FBLN1,FBN1,GAS6,GNB2L1,GPC1,GPC6,GSTP1,IGF2R,IGLL1,KRT1,LCAT,LDHA,LDHB,LEPRE1,LOXL2,LRP1,LTBP1,MMP2,MSRB1,PDIA3,PGD,PGK1,PLOD3,PPIA,PRDX4,RAB1B,SULF1,SULF2,TGM1,UBB,WNT5A | |
| Immune response | 22 | 1560 | 6.48 × 10−6 | ACLY,ACTR3,AICDA,ALDOA,ANXA1,APOA4,C1S,CHIA,FAS,FLNB,GAS6,GSTP1,IGF2R,IGLL1,KRT1,LRP1,MSRB1,PPIA,PRDX4,PYGB,RAP1B,WNT5A | |
| Vesicle-mediated transport | 23 | 1699 | 6.48 × 10−6 | ACLY,ACTR3,ALDOA,ANXA1,ARF3,F13A1,FERMT3,GAS6,GSTP1,IGF2R,IGLL1,KRT1,LOXL2,LRP1,PPIA,PRDX4,PYGB,RAB1B,RAP1B,SERPINE1,TIMP3,UBB,WDR1 | |
| Regulated exocytosis | 15 | 691 | 6.48 × 10−6 | ACLY,ALDOA,F13A1,FERMT3,GAS6,GSTP1,IGF2R,KRT1,PPIA,PRDX4,PYGB,RAP1B,SERPINE1,TIMP3,WDR1 | |
| Positive regulation of biological process | 42 | 5459 | 6.48 × 10−6 | ACLY,ACTC1,ACTR3,AICDA,ANXA1,APOA4,C1S,CHIA,CLIC1,FAS,FBLN1,FBN1,FBN2,FERMT3,FSCN1,GAS6,GNAI2,GNB2L1,GPC1,GSTP1,IGF2R,IGLL1,KRT1,LDHA,LEPRE1,LOXL2,LRP1,MMP2,PDIA3,PPIA,RAB1B,RAP1B,SERPINE1,SRPX2,SULF1,SULF2,TGM1,THBS4,TIMP3,UBB,WDR1,WNT5A | |
| Anatomical structure development | 40 | 5085 | 6.48 × 10−6 | ACTC1,AICDA,ANXA1,APOA4,COMP,EXT1,EXT2,FAS,FBLN1,FBN1,FBN2,FERMT3,FLNB,FLNC,FSCN1,GAS6,GNB2L1,GPC1,GSTP1,IGF2R,KRT1,LDHA,LEPRE1,LOXL2,LRP1,LTBP1,MMP2,MYL6,PGK1,PLOD3,PRDX4,RAP1B,SERPINE1,SRPX2,SULF1,SULF2,TGM1,UBB,WDR1,WNT5A | |
| Response to stimulus | 51 | 7824 | 6.48 × 10−6 | ACLY,ACTC1,ACTR3,AICDA,ALDOA,ANXA1,APOA4,C1S,CHIA,CLIC1,EEF1G,EXT1,EXT2,F13A1,FAS,FBLN1,FBN1,FERMT3,FLNB,FSCN1,GAS6,GNAI2,GNB2L1,GPC1,GPC6,GPRC5A,GSTP1,IGF2R,IGLL1,KRT1,LAMA1,LDHA,LOXL2,LRP1,LTBP1,MMP2,MSRB1,PDIA3,PGK1,PLOD3,PPIA,PRDX4,PYGB,RAP1B,SERPINE1,SULF1,SULF2,THBS4,TIMP3,UBB,WNT5A | |
| Positive regulation of cellular process | 39 | 4898 | 7.40 × 10−6 | ACLY,ACTR3,AICDA,ANXA1,APOA4,CHIA,CLIC1,FAS,FBLN1,FBN1,FBN2,FERMT3,FSCN1,GAS6,GNAI2,GNB2L1,GPC1,GSTP1,IGF2R,IGLL1,LDHA,LEPRE1,LOXL2,LRP1,MMP2,PDIA3,PPIA,RAB1B,RAP1B,SERPINE1,SRPX2,SULF1,SULF2,TGM1,THBS4,TIMP3,UBB,WDR1,WNT5A | |
| KEGG Pathways | |||||
| Term Description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Proteoglycans in cancer | 7 | 195 | 0.00093 | FAS,FLNB,FLNC,GPC1,MMP2,TIMP3,WNT5A | |
| Focal adhesion | 6 | 197 | 0.0053 | COMP,FLNB,FLNC,LAMA1,RAP1B,THBS4 | |
| Glycolysis / Gluconeogenesis | 4 | 68 | 0.0054 | ALDOA,LDHA,LDHB,PGK1 | |
| HIF-1 signaling pathway | 4 | 98 | 0.0155 | ALDOA,LDHA,PGK1,SERPINE1 | |
| Cholesterol metabolism | 3 | 48 | 0.0195 | APOA4,LCAT,LRP1 | |
| Malaria | 3 | 47 | 0.0195 | COMP,LRP1,THBS4 | |
| 10 Most Significant PMID Publications per FDR | |||||
| Term ID | Term Description | Obs | Bgr | FDR | Matching Proteins in the Network |
| PMID:21654676 | (2011) D-glucuronyl C5-epimerase suppresses small-cell lung cancer cell proliferation in vitro and tumour growth in vivo. | 8 | 62 | 1.79 × 10−5 | EXT1,EXT2,FAS,GPC1,GPC6,MMP2,SERPINE1,TIMP3 |
| PMID:22393382 | (2012) In vitro phenotypic, genomic and proteomic characterization of a cytokine-resistant murine Beta-TC3 cell line. | 7 | 42 | 2.32 × 10−5 | ALDOA,FAS,GSTP1,LDHA,LDHB,PDIA3,PRDX4 |
| PMID:25829250 | (2015) Insights into the key roles of proteoglycans in breast cancer biology and translational medicine. | 10 | 156 | 2.32 × 10−5 | EXT1,FBLN1,FBN1,GPC1,GPC6,MMP2,SULF1,SULF2,TIMP3,WNT5A |
| PMID:26779482 | (2015) The Extracellular Matrix in Bronchopulmonary Dysplasia: Target and Source. | 7 | 41 | 2.32 × 10−5 | FBLN1,FBN1,FBN2,LOXL2,LTBP1,PLOD3,SULF2 |
| PMID:23143224 | (2013) The biology of the extracellular matrix: Novel insights. | 6 | 28 | 5.53 × 10−5 | COMP,FBN1,FBN2,LTBP1,MMP2,TIMP3 |
| PMID:24223867 | (2013) Lactate-modulated induction of THBS-1 activates transforming growth factor (TGF)-beta2 and migration of glioma cells in vitro. | 6 | 31 | 7.90 × 10−5 | COMP,LDHA,LDHB,MMP2,SERPINE1,THBS4 |
| PMID:26076122 | (2015) Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: Mechanisms and mysteries. | 6 | 31 | 7.90 × 10−5 | EXT1,EXT2,GPC1,GPC6,SULF1,SULF2 |
| PMID:20236620 | (2010) Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. | 6 | 33 | 8.27 × 10−5 | FBLN1,FBN1,FBN2,LOXL2,LTBP1,TIMP3 |
| PMID:20140087 | (2010) Comprehensive identification and modified-site mapping of S-nitrosylated targets in prostate epithelial cells. | 8 | 103 | 8.31 × 10−5 | ALDOA,ANXA1,CLIC1,FLNB,FLNC,PDIA3,PGK1,PLEC |
| PMID:27513329 | (2016) Differential Expression Pattern of THBS1 and THBS2 in Lung Cancer: Clinical Outcome and a Systematic-Analysis of Microarray Databases. | 7 | 65 | 8.31 × 10−5 | COMP,FBLN1,FBN1,MMP2,NID2,SULF1,THBS4 |
Next, we analyzed the EV surface proteome unique lists for the HIV vs. HIV+Aβ comparison in order to dissect the effect of exogenous EV-Aβ cargo in the context of HIV. In this analysis, six unique proteins were identified in the HIV group, namely, TTN, ninein (NIN), DNAH8, adenylyl cyclase-associated protein 1 (CAP1), actin-related protein 2/3 complex subunit 4 (ARPC4), and IGHG2. For these unique proteins, all enriched biological processes are shown in Table 5. No KEGG pathways were enriched; however, several PMID publications were found by textmining (Table 5). Cellular localization of these enriched proteins to only a few categories was found, namely, “cytoskeletal part” (ARPC4, CAP1, DNAH8, NIN, TTN), “actin cytoskeleton” (ARPC4, CAP1, TTN), “supramolecular fiber” (DNAH8, NIN, TTN), “microtubule” (DNAH8, NIN), “ciliary part” (DNAH8, NIN), and “cytoplasmic region” (CAP1, DNAH8).
Table 5.
Biological processes and PMIDs for the EV surface unique proteins in the HIV group for the HIV vs. HIV+Aβ comparison.
| Gene Ontology (GO) Terms for Biological Processes | |||||
| Term Description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Cytoskeleton organization | 5 | 953 | 8.35 × 10−5 | ARPC4,CAP1,DNAH8,NIN,TTN | |
| Supramolecular fiber organization | 4 | 383 | 0.00011 | ARPC4,CAP1,NIN,TTN | |
| Actin filament organization | 3 | 200 | 0.0011 | ARPC4,CAP1,TTN | |
| Cellular protein-containing complex assembly | 4 | 832 | 0.0012 | ARPC4,DNAH8,NIN,TTN | |
| Actin polymerization or depolymerization | 2 | 43 | 0.0031 | ARPC4,CAP1 | |
| Protein polymerization | 2 | 83 | 0.0058 | ARPC4,NIN | |
| Localization | 5 | 5233 | 0.0296 | ARPC4,CAP1,DNAH8,NIN,TTN | |
| PMID Publications | |||||
| Term ID | Term Description | Obs | Bgr | FDR | Matching Proteins in the Network |
| PMID:21050039 | (2010) Titin A-band-specific monoclonal antibody Tit1 5H1.1. Cellular Titin as a centriolar protein in non-muscle cells. | 2 | 2 | 0.0016 | NIN,TTN |
| PMID:22985877 | (2012) Epitope of titin A-band-specific monoclonal antibody Tit1 5 H1.1 is highly conserved in several Fn3 domains of the titin molecule. Centriole staining in human, mouse and zebrafish cells. | 2 | 6 | 0.0037 | NIN,TTN |
| PMID:26655833 | (2016) The centrosome is an actin-organizing centre. | 2 | 12 | 0.0081 | ARPC4,NIN |
| PMID:27094867 | (2016) Mutations in human C2CD3 cause skeletal dysplasia and provide new insights into phenotypic and cellular consequences of altered C2CD3 function. | 2 | 27 | 0.027 | NIN,TTN |
| PMID:29255378 | (2017) The human, F-actin-based cytoskeleton as a mutagen sensor. | 2 | 35 | 0.0353 | DNAH8,TTN |
For the unique proteins in the HIV+Aβ group in this comparison, the enriched biological processes, KEGG pathways, and PMID publications are presented in Table 6 and Supplementary Table S2A. The enrichment for cellular components is included in Supplementary Table S2B.
Table 6.
Biological processes, KEGG pathways, and PMIDs for the EV surface unique proteins in the HIV+Aβ group for the HIV vs. HIV+Aβ comparison.
|
Gene ontology (GO) Terms for Biological Processes
10 Most Significant Results per FDR (for All GO Terms, See Supplementary Table S2A) | |||||
| Term Description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Immune effector process | 18 | 927 | 9.47 × 10−6 | ACLY,ACTR3,AICDA,ALDOA,C1S,CDC42,GSTP,IGLL1,KPNB1,KRT1,LRP1,PGM1,PPIA,PRDX6,PYGB,PYGL,RAP1B,WDR1 | |
| Leukocyte-mediated immunity | 15 | 632 | 9.47 × 10−6 | ACLY,AICDA,ALDOA,C1S,GSTP1,IGLL1,KPNB1,KRT1,PGM1,PPIA,PRDX6,PYGB,PYGL,RAP1B,WDR1 | |
| Vesicle-mediated transport | 23 | 1699 | 9.70 × 10−6 | ACLY,ACTR3,ALDOA,ANXA1,ARF3,CDC42,F13A1,FERMT3,GSTP1,IGLL1,KPNB1,KRT1,LOXL2,LRP1,PGM1,PPIA,PRDX6,PYGB,PYGL,RAP1B,SERPINE1,UBB,WDR1 | |
| Extracellular matrix organization | 11 | 296 | 9.70 × 10−6 | COMP,FBLN1,FBN1,FBN2,LOXL2,MMP2,NID2,PLOD3,SERPINE1,SULF1,SULF2 | |
| Regulated exocytosis | 15 | 691 | 1.15 × 10−5 | ACLY,ALDOA,F13A1,FERMT3,GSTP1,KPNB1,KRT1,PGM1,PPIA,PRDX6,PYGB,PYGL,RAP1B,SERPINE1,WDR1 | |
| Response to stimulus | 51 | 7824 | 1.20 × 10−5 | ACLY,ACTC1,ACTR3,AICDA,ALDOA,ANXA1,C1S,CDC42,CHIA,CLIC1,EEF1G,EXT1,F13A1,FAS,FBLN1,FBN1,FERMT3,FLNB,FSCN1,GNB2L1,GPC1,GPC6,GPRC5A,GSTP1,HMCN1,IGLL1,KPNB1,KRT1,LDHA,LOXL2,LRP1,LTBP1,MMP2,PDIA3,PGK1,PGM1,PHGDH,PLOD3,PPIA,PRDX2,PRDX6,PYGB,PYGL,RAP1B,SERPINE1,SULF1,SULF2,THBS4,UBA1,UBB,WNT5A | |
| Negative regulation of cellular response to growth factor stimulus | 8 | 137 | 1.59 × 10−5 | FBN1,FBN2,GPC1,LTBP1,SULF1,SULF2,UBB,WNT5A | |
| Immune system process | 26 | 2370 | 2.26 × 10−5 | ACLY,ACTR3,AICDA,ALDOA,ANXA1,C1S,CDC42,CHIA,FAS,FLNB,GPC1,GSTP1,IGLL1,KPNB1,KRT1,LRP1,PDIA3,PGM1,PPIA,PRDX6,PYGB,PYGL,RAP1B,UBB,WDR1,WNT5A | |
| Carbohydrate metabolic process | 12 | 457 | 2.26 × 10−5 | ALDOA,AMY1B,CHIA,EXT1,FBN1,LDHA,LDHB,PGK1,PGM1,PYGB,PYGL,RGN | |
| Immune response | 21 | 1560 | 2.26 × 10−5 | ACLY,ACTR3,AICDA,ALDOA,ANXA1,C1S,CHIA,FAS,FLNB,GSTP1,IGLL1,KPNB1,KRT1,LRP1,PGM1,PPIA,PRDX6,PYGB,PYGL,RAP1B,WNT5A | |
| KEGG Pathways | |||||
| Term Description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Glycolysis / Gluconeogenesis | 5 | 68 | 0.00077 | ALDOA,LDHA,LDHB,PGK1,PGM1 | |
| Proteoglycans in cancer | 7 | 195 | 0.00077 | CDC42,FAS,FLNB,FLNC,GPC1,MMP2,WNT5A | |
| Focal adhesion | 6 | 197 | 0.0035 | CDC42,COMP,FLNB,FLNC,RAP1B,THBS4 | |
| Pentose phosphate pathway | 3 | 30 | 0.0068 | ALDOA,PGM1,RGN | |
| Starch and sucrose metabolism | 3 | 33 | 0.0071 | PGM1,PYGB,PYGL | |
| Metabolic pathways | 13 | 1250 | 0.0095 | ACLY,ALDOA,CHIA,EXT1,LDHA,LDHB,PGK1,PGM1,PHGDH,PRDX6,PYGB,PYGL,RGN | |
| HIF-1 signaling pathway | 4 | 98 | 0.0095 | ALDOA,LDHA,PGK1,SERPINE1 | |
| Glucagon signaling pathway | 4 | 100 | 0.0095 | LDHA,LDHB,PYGB,PYGL | |
| Malaria | 3 | 47 | 0.0104 | COMP,LRP1,THBS4 | |
| Carbon metabolism | 4 | 116 | 0.011 | ALDOA,PGK1,PHGDH,RGN | |
| Fluid shear stress and atherosclerosis | 4 | 133 | 0.0164 | GPC1,GSTA5,GSTP1,MMP2 | |
| Biosynthesis of amino acids | 3 | 72 | 0.0233 | ALDOA,PGK1,PHGDH | |
| Platinum drug resistance | 3 | 70 | 0.0233 | FAS,GSTA5,GSTP1 | |
| Necroptosis | 4 | 155 | 0.0233 | FAS,PPIA,PYGB,PYGL | |
| Complement and coagulation cascades | 3 | 78 | 0.0251 | C1S,F13A1,SERPINE1 | |
| Salmonella infection | 3 | 84 | 0.0288 | CDC42,FLNB,FLNC | |
| MAPK signaling pathway | 5 | 293 | 0.0307 | CDC42,FAS,FLNB,FLNC,RAP1B | |
| AGE-RAGE signaling pathway in diabetic complications | 3 | 98 | 0.0388 | CDC42,MMP2,SERPINE1 | |
| Human papillomavirus infection | 5 | 317 | 0.0388 | CDC42,COMP,FAS,THBS4,WNT5A | |
| Propanoate metabolism | 2 | 32 | 0.0405 | LDHA,LDHB | |
| Leukocyte transendothelial migration | 3 | 112 | 0.0476 | CDC42,MMP2,RAP1B | |
| Primary immunodeficiency | 2 | 37 | 0.0481 | AICDA,IGLL1 | |
| 10 Most Significant PMID Publications per FDR | |||||
| Term ID | Term Description | Obs | Bgr | FDR | Matching Proteins in the Network |
| PMID:23823696 | (2013) Isobaric Tagging-Based Quantification for Proteomic Analysis: A Comparative Study of Spared and Affected Muscles from mdx Mice at the Early Phase of Dystrophy. | 8 | 42 | 1.26 × 10−6 | ACLY,ALDOA,ANXA1,EEF1G,LDHB,PGM1,PPIA,PRDX2 |
| PMID:29250190 | (2017) Role of exosomes in hepatocellular carcinoma cell mobility alteration. | 7 | 34 | 8.40 × 10−6 | ANXA1,CLIC1,FBLN1,LRP1,PPIA,PYGB,PYGL |
| PMID:20140087 | (2010) Comprehensive identification and modified-site mapping of S-nitrosylated targets in prostate epithelial cells. | 9 | 103 | 9.47 × 10−6 | ALDOA,ANXA1,CLIC1,FLNB,FLNC,KPNB1,PDIA3,PGK1,PLEC |
| PMID:29360750 | (2018) Proteomic Analysis of Secretomes of Oncolytic Herpes Simplex Virus-Infected Squamous Cell Carcinoma Cells. | 7 | 37 | 9.47 × 10−6 | ACLY,ANXA1,FBN1,FLNC,FSCN1,MMP2,PRDX2 |
| PMID:26779482 | (2015) The Extracellular Matrix in Bronchopulmonary Dysplasia: Target and Source. | 7 | 41 | 1.08 × 10−5 | FBLN1,FBN1,FBN2,LOXL2,LTBP1,PLOD3,SULF2 |
| PMID:24142637 | (2013) Gastric autoantigenic proteins in Helicobacter pylori infection. | 7 | 50 | 2.96 × 10−5 | ACTR3,GSTP1,LDHB,PDIA3,PRDX2,PRDX6,WDR1 |
| PMID:26184160 | (2015) A Review: Proteomics in Nasopharyngeal Carcinoma. | 8 | 83 | 2.96 × 10−5 | ANXA1,CLIC1,KRT1,MMP2,PPIA,PRDX2,PRDX6,SERPINE1 |
| PMID:26918450 | (2016) A nuclear-directed human pancreatic ribonuclease (PE5) targets the metabolic phenotype of cancer cells. | 8 | 89 | 3.71 × 10−5 | ACLY,CLIC1,GPC1,GPC6,LDHA,PGM1,PHGDH,WNT5A |
| PMID:24223867 | (2013) Lactate-modulated induction of THBS-1 activates transforming growth factor (TGF)-beta2 and migration of glioma cells in vitro. | 6 | 31 | 5.98 × 10−5 | COMP,LDHA,LDHB,MMP2,SERPINE1,THBS4 |
| PMID:20236620 | (2010) Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. | 6 | 33 | 7.46 × 10−5 | FBLN1,FBN1,FBN2,HMCN1,LOXL2,LTBP1 |
Next, we analyzed the EV total proteome unique lists for the control vs. HIV comparison. For the unique proteins in the control group, no gene ontology (GO) terms were found for biological processes. Similarly, no KEGG Pathways were enriched, likely because only three unique proteins were identified in this group and comparison. The cellular localization of these proteins is presented in Supplementary Table S3. In addition, the first 10 PMID publications enriched are shown in Table 7. The total proteome revealed 259 unique proteins in the HIV group that mapped to a variety of GO terms for biological processes (Table 8 and Supplementary Table S4A). They were also enriched in several KEGG pathways (Table 8) and assigned to diverse cellular components, as listed in Supplementary Table S4B. Textmining resulted in an unbiased PubMed search with the 10 most significant publications listed in Table 8.
Table 7.
PMIDs for the EV total unique proteins in the control group for the control vs. HIV comparison.
| The 10 Most Significant PMID Publications According to FDR | |||||
| Term ID | Term Description | Obs | Bgr | FDR | Matching Proteins in the Network |
| PMID:19812696 | (2009) Cancer genomics identifies regulatory gene networks associated with the transition from dysplasia to advanced lung adenocarcinomas induced by c-Raf-1. | 3 | 154 | 0.0084 | ACTC1,MAPT,MYH1 |
| PMID:20587776 | (2010) Mathematical modeling of endocytic actin patch kinetics in fission yeast: disassembly requires release of actin filament fragments. | 2 | 12 | 0.0086 | ACTC1,MYH1 |
| PMID:25275480 | (2014) Urethral dysfunction in female mice with estrogen receptor Beta deficiency. | 2 | 10 | 0.0086 | ACTC1,MYH1 |
| PMID:22406440 | (2012) Deferiprone reduces amyloid-Beta and tau phosphorylation levels but not reactive oxygen species generation in hippocampus of rabbits fed a cholesterol-enriched diet. | 2 | 15 | 0.0088 | ACTC1,MAPT |
| PMID:10931867 | (2000) Distinct families of Z-line targeting modules in the COOH-terminal region of nebulin. | 2 | 25 | 0.0099 | ACTC1,MYH1 |
| PMID:11994316 | (2002) The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. | 2 | 26 | 0.0099 | ACTC1,MYH1 |
| PMID:14557251 | (2003) Skeletal myosin heavy chain function in cultured lung myofibroblasts. | 2 | 26 | 0.0099 | ACTC1,MYH1 |
| PMID:17908293 | (2007) Identification of genes differentially expressed during prenatal development of skeletal muscle in two pig breeds differing in muscularity. | 2 | 52 | 0.0099 | ACTC1,MYH1 |
| PMID:19291799 | (2009) Fast-twitch sarcomeric and glycolytic enzyme protein loss in inclusion body myositis. | 2 | 36 | 0.0099 | MAPT,MYH1 |
| PMID:19325835 | (2008) Myosin assembly, maintenance and degradation in muscle: Role of the chaperone UNC-45 in myosin thick filament dynamics. | 2 | 44 | 0.0099 | ACTC1,MYH1 |
Table 8.
Biological processes, KEGG pathways, and PMIDs for the EV total unique proteins in the HIV group for the control vs. HIV comparison.
| Gene Ontology (GO) Terms for Biological Processes 10 Most Significant Results per FDR (for All GO Terms, See Supplementary Table S4A) | |||||
| Term Description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Vesicle-mediated transport | 57 | 1699 | 1.02 × 10−18 | ACLY,ACTN1,ACTN4,ALDOA,ANXA1,ANXA11,ANXA5,AP2A1,AP2M1,APLP2,APOB,APOE,ARF3,ARF4,ARPC2,CAP1,CD44,CD59,CD63,CD81,EEF2,EHD1,EHD2,F13A1,FERMT3,FLNA,GAS6,ITIH3,ITIH4,KRT1,LAMP1,LAMP2,LOXL2,LRP1,MFGE8,MRC2,MVP,MYH9,PKP1,PRDX6,PTX3,RAB5C,RAB7A,RAC1,RAP1B,SERPINE1,SPTBN1,SRGN,SRPX,TGM2,THBS1,TLN1,TTN,UBB,VPS35,VWF,WDR1 | |
| Extracellular structure organization | 28 | 339 | 7.06 × 10−17 | AGRN,APOA4,APOB,APOE,BMP1,CD44,COMP,DCN,FBLN1,FBN1,GAS6,HTRA1,KLK7,LAMA1,LAMA2,LAMA4,LAMA5,LOXL2,MMP2,NID1,NID2,PLOD3,PXDN,SERPINE1,SULF1,SULF2,THBS1,VWF | |
| Platelet degranulation | 20 | 129 | 2.26 × 10−16 | ACTN1,ACTN4,ALDOA,ANXA5,APLP2,CD63,F13A1,FERMT3,FLNA,GAS6,ITIH3,ITIH4,LAMP2,SERPINE1,SRGN,THBS1,TLN1,TTN,VWF,WDR1 | |
| Regulated exocytosis | 35 | 691 | 1.19 × 10−15 | ACLY,ACTN1,ACTN4,ALDOA,ANXA5,APLP2,CAP1,CD44,CD59,CD63,EEF2,F13A1,FERMT3,FLNA,GAS6,ITIH3,ITIH4,KRT1,LAMP1,LAMP2,MVP,PKP1,PRDX6,PTX3,RAB5C,RAB7A,RAC1,RAP1B,SERPINE1,SRGN,THBS1,TLN1,TTN,VWF,WDR1 | |
| Extracellular matrix organization | 25 | 296 | 2.14 × 10−15 | AGRN,BMP1,CD44,COMP,DCN,FBLN1,FBN1,GAS6,HTRA1,KLK7,LAMA1,LAMA2,LAMA4,LAMA5,LOXL2,MMP2,NID1,NID2,PLOD3,PXDN,SERPINE1,SULF1,SULF2,THBS1,VWF | |
| Cellular component organization | 89 | 5163 | 2.93 × 10−14 | ACTN1,ACTN4,AGRN,ALDOA,ANXA1,ANXA6,AP2A1,AP2M1,APOA4,APOB,APOE,ARF4,ARPC2,ATL1,ATXN2,BMP1,CAP1,CD151,CD44,CD59,COMP,DCN,EHD1,EHD2,EXT1,FAS,FAT1,FBLN1,FBN1,FERMT3,FLNA,FLNB,FLNC,FSCN1,GAS6,GGCT,HIST1H4F,HTRA1,KLK7,KRT1,LAMA1,LAMA2,LAMA4,LAMA5,LAMP2,LOXL2,LTBP2,MFGE8,MMP2,MSRB1,MYH9,MYOF,NID1,NID2,PKP1,PLEC,PLOD3,PLS1,PLS3,PTGFRN,PXDN,RAB7A,RAC1,RAN,RHOC,SDC4,SEMG1,SERPINE1,SGCG,SLC25A6,SPAG1,SPTBN1,SRGN,SRPX,SULF1,SULF2,TGM1,TGM2,TGM3,THBS1,THY1,TLN1,TPM4,TTN,UBB,VPS35,VWF,WDR1,WNT5A | |
| Secretion by cell | 37 | 959 | 2.43 × 10−13 | ACLY,ACTN1,ACTN4,ALDOA,ANXA1,ANXA5,APLP2,CAP1,CD44,CD59,CD63,EEF2,F13A1,FERMT3,FLNA,GAS6,ITIH3,ITIH4,KRT1,LAMP1,LAMP2,LTBP2,MVP,PKP1,PRDX6,PTX3,RAB5C,RAB7A,RAC1,RAP1B,SERPINE1,SRGN,THBS1,TLN1,TTN,VWF,WDR1 | |
| Response to stimulus | 107 | 7824 | 6.96 × 10−12 | ACLY,ACTN4,AFP,AGRN,AHCY,AICDA,ALDOA,ANXA1,ANXA11,ANXA5,ANXA6,AP2A1,AP2M1,APLP2,APOA4,APOB,APOE,ARF4,ARPC2,AZGP1,C1S,CAP1,CD151,CD44,CD59,CD63,CD81,CD82,CLIC1,DCN,EEF2,EHD1,EHD2,EXT1,EXT2,F13A1,FAS,FBLN1,FBN1,FERMT3,FLNA,FLNB,FSCN1,GAS6,GGCT,GNAI2,GNB2L1,GPC6,GPRC5A,HIST1H4F,HSPA5,ITIH4,KRT1,LAMA1,LAMA2,LAMA5,LAMP1,LAMP2,LDHA,LOXL2,LRP1,LTBP1,LTBP2,MMP2,MRC2,MSRB1,MVP,MYH9,MYOF,NNMT,PDIA3,PGK1,PKP1,PLOD1,PLOD3,POLR3G,PRDX1,PRDX6,PTX3,PXDN,RAB5C,RAB7A,RAC1,RAN,RAP1B,RHOC,SDC4,SEMG1,SERPINE1,SLC25A6,SPTBN1,SRGN,SRPX,STK33,SULF1,SULF2,TGM2,THBS1,THRB,THY1,TLN1,TTN,UBA1,UBB,VPS35,VWF,WNT5A | |
| Localization | 83 | 5233 | 9.26 × 10−11 | ACLY,ACTN1,ACTN4,AGRN,ALDOA,ANXA1,ANXA11,ANXA5,ANXA6,AP2A1,AP2M1,APLP2,APOA4,APOB,APOE,ARF3,ARF4,ARPC2,ATXN2,AZGP1,CAP1,CD151,CD44,CD59,CD63,CD81,CLIC1,EEF2,EHD1,EHD2,F13A1,FAT1,FBN1,FERMT3,FLNA,FLNB,FSCN1,GAS6,GPC6,HSPA5,ITIH3,ITIH4,KRT1,LAMA5,LAMP1,LAMP2,LOXL2,LRP1,LTBP1,LTBP2,MFGE8,MRC2,MVP,MYH9,PKP1,PLOD3,PLS1,PRDX6,PTX3,RAB5C,RAB7A,RAC1,RAN,RAP1B,RHOC,SDC4,SERPINE1,SLC25A6,SPTBN1,SRGN,SRPX,SRPX2,TGM2,THBS1,THY1,TLN1,TTN,TTYH3,UBB,VPS35,VWF,WDR1,WNT5A | |
| Anatomical structure development | 80 | 5085 | 5.90 × 10−10 | ACTN1,AEBP1,AFP,AGRN,AICDA,ANXA1,AP2A1,APOA4,APOB,APOE,ARF4,ATL1,BMP1,C6orf58,CAP1,CD151,CD44,COMP,DCN,EEF2,EHD1,EXT1,EXT2,FAS,FAT1,FBLN1,FBN1,FERMT3,FLNA,FLNB,FLNC,FSCN1,GAS6,GNB2L1,HSPA5,HTRA1,KLK7,KRT1,LAMA2,LAMA5,LDHA,LOXL2,LRP1,LTBP1,MFGE8,MMP2,MYH9,MYL6,MYOF,NID1,NNMT,PGK1,PKP1,PLOD1,PLOD3,PLS3,PPIB,PRDX1,RAC1,RAP1B,RHOC,SDC4,SERPINE1,SGCG,SPTBN1,SRGN,SRPX2,SULF1,SULF2,TGM1,TGM2,TGM3,THBS1,THBS3,THRB,THY1,TTN,UBB,WDR1,WNT5A | |
| KEGG Pathways | |||||
| Term Description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Focal adhesion | 17 | 197 | 1.23 × 10−10 | ACTN1,ACTN4,COMP,FLNA,FLNB,FLNC,LAMA1,LAMA2,LAMA4,LAMA5,RAC1,RAP1B,THBS1,THBS2,THBS3,TLN1,VWF | |
| ECM-receptor interaction | 12 | 81 | 5.47 × 10−10 | AGRN,CD44,COMP,LAMA1,LAMA2,LAMA4,LAMA5,SDC4,THBS1,THBS2,THBS3,VWF | |
| Proteoglycans in cancer | 12 | 195 | 3.88 × 10−6 | CD44,CD63,DCN,FAS,FLNA,FLNB,FLNC,MMP2,RAC1,SDC4,THBS1,WNT5A | |
| Phagosome | 10 | 145 | 1.40 × 10−5 | COMP,LAMP1,LAMP2,MRC2,RAB5C,RAB7A,RAC1,THBS1,THBS2,THBS3 | |
| Amoebiasis | 8 | 94 | 4.01 × 10−5 | ACTN1,ACTN4,LAMA1,LAMA2,LAMA4,LAMA5,RAB5C,RAB7A | |
| Malaria | 6 | 47 | 8.88 × 10−5 | CD81,COMP,LRP1,THBS1,THBS2,THBS3 | |
| Salmonella infection | 7 | 84 | 0.00016 | ARPC2,FLNA,FLNB,FLNC,MYH9,RAB7A,RAC1 | |
| Endocytosis | 10 | 242 | 0.00054 | AP2A1,AP2M1,ARF3,ARPC2,EHD1,EHD2,RAB5C,RAB7A,UBB,VPS35 | |
| Leukocyte transendothelial migration | 7 | 112 | 0.0007 | ACTN1,ACTN4,GNAI2,MMP2,RAC1,RAP1B,THY1 | |
| Human papillomavirus infection | 11 | 317 | 0.00079 | COMP,FAS,LAMA1,LAMA2,LAMA4,LAMA5,THBS1,THBS2,THBS3,VWF,WNT5A | |
| PI3K-Akt signaling pathway | 10 | 348 | 0.0069 | COMP,LAMA1,LAMA2,LAMA4,LAMA5,RAC1,THBS1,THBS2,THBS3,VWF | |
| Complement and coagulation cascades | 5 | 78 | 0.0069 | C1S,CD59,F13A1,SERPINE1,VWF | |
| Cholesterol metabolism | 4 | 48 | 0.0088 | APOA4,APOB,APOE,LRP1 | |
| Toxoplasmosis | 5 | 109 | 0.0226 | GNAI2,LAMA1,LAMA2,LAMA4,LAMA5 | |
| Glycolysis / Gluconeogenesis | 4 | 68 | 0.0259 | ALDOA,LDHA,LDHB,PGK1 | |
| p53 signaling pathway | 4 | 68 | 0.0259 | CD82,FAS,SERPINE1,THBS1 | |
| Platelet activation | 5 | 123 | 0.0308 | FERMT3,GNAI2,RAP1B,TLN1,VWF | |
| 10 Most Significant PMID Publications per FDR | |||||
| Term ID | Term Description | Obs | Bgr | FDR | Matching Proteins in the Network |
| PMID:29250190 | (2017) Role of exosomes in hepatocellular carcinoma cell mobility alteration. | 17 | 34 | 1.84 × 10−18 | ACTN1,ANXA1,ANXA11,ANXA5,ANXA6,APOB,APOE,CAP1,CLIC1,FBLN1,FLNA,ITIH4,LRP1,MFGE8,NID1,RAN,TLN1 |
| PMID:24009881 | (2012) Quantitative proteomics of extracellular vesicles derived from human primary and metastatic colorectal cancer cells. | 21 | 161 | 9.74 × 10−14 | AHCY,ANXA1,ANXA11,ANXA5,ANXA6,ARF3,ARPC2,CD44,CD63,CD81,FSCN1,KRT1,LAMP1,MFGE8,MYH9,MYL6,PGK1,PTGFRN,RAB5C,RAB7A,VPS35 |
| PMID:19948009 | (2009) Proteomic analysis of blastema formation in regenerating axolotl limbs. | 22 | 221 | 1.76 × 10−12 | ANXA1,ANXA11,ANXA5,ANXA6,DCN,EEF2,FBN1,FLNB,GNB2L1,MVP,MYH9,MYL6,MYOF,PDIA3,PLS3,PRDX1,PXDN,RAN,SND1,TTN,UBA1,WNT5A |
| PMID:24392111 | (2014) Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? | 16 | 79 | 1.87 × 10−12 | ALDOA,ANXA5,CD44,CD63,CD81,CD82,EEF2,FLNC,LAMP1,LAMP2,LDHA,MYOF,PGK1,TLN1,TTN,VPS35 |
| PMID:27605433 | (2016) Secreted primary human malignant mesothelioma exosome signature reflects oncogenic cargo. | 17 | 107 | 5.36 × 10−12 | ACLY,ANXA1,ANXA6,CD44,CD63,CD81,CD82,FAT1,GNB2L1,LAMA1,LAMP1,MFGE8,MMP2,PLS3,SULF1,THBS1,VPS35 |
| PMID:22897585 | (2012) Rat mammary extracellular matrix composition and response to ibuprofen treatment during postpartum involution by differential GeLC-MSMS analysis. | 13 | 42 | 1.24 × 10−11 | AGRN,ANXA1,ANXA11,ANXA5,ANXA6,CD44,DCN,FBN1,LAMA1,LAMA2,LAMA4,LAMA5,VWF |
| PMID:27770278 | (2017) Comprehensive proteome profiling of glioblastoma-derived extracellular vesicles identifies markers for more aggressive disease. | 14 | 63 | 3.75 × 10−11 | ACTN4,ANXA1,CCT6A,CD44,EHD1,HSPA5,LAMA4,MMP2,MVP,MYH9,RAB5C,RAB7A,UBA1,VPS35 |
| PMID:22159717 | (2012) The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. | 14 | 64 | 3.97 × 10−11 | AGRN,ANXA1,ANXA11,ANXA5,ANXA6,DCN,FBN1,LOXL2,LTBP2,NID1,NID2,SRPX,THBS1,VWF |
| PMID:25201077 | (2015) Proteomics of apheresis platelet supernatants during routine storage: Gender-related differences. | 16 | 106 | 5.20 × 10−-11 | ACTN1,APOB,APOE,ARPC2,C1S,FERMT3,FLNA,ITIH4,LDHA,MMP2,MYL6,PRDX6,SRGN,THBS1,TLN1,VWF |
| PMID:28071719 | (2017) Quantitative proteomic profiling of the extracellular matrix of pancreatic islets during the angiogenic switch and insulinoma progression. | 13 | 54 | 1.20 × 10−10 | ANXA1,ANXA11,ANXA5,ANXA6,DCN,FBN1,LAMA1,LAMA2,LAMA4,LAMA5,NID1,NID2,THBS2 |
Finally, we analyzed the list of the unique proteins present in the total HBMEC-EV proteome in the HIV and HIV+Aβ groups. The unique proteins in the HIV group in this comparison mapped to only one GO term for biological processes, namely, “cell envelope organization,” presented in Table 9. No KEGG pathways and no cellular components were enriched for this group. The first 10 textmined PMID citations are presented in Table 9. The unique proteins in the HIV+Aβ group were enriched to several biological processes, KEGG pathways, and PMID publications (Table 10 and Supplementary Table S5A). Supplementary Table S5B lists the enrichments for the cellular component in this group.
Table 9.
Biological processes and PMIDs for the EV total unique proteins in the HIV group for the HIV vs. HIV+Aβ comparison.
| Gene ontology (GO) Terms for Biological Processes | |||||
| Term Description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Cell envelope organization | 2 | 3 | 0.0017 | TGM1,TGM3 | |
| 10 Most Significant PMID Publications per FDR | |||||
| Term ID | Term Description | Obs | Bgr | FDR | Matching Proteins in the Network |
| PMID:22329734 | (2012) Expression profile of cornified envelope structural proteins and keratinocyte differentiation-regulating proteins during skin barrier repair. | 3 | 14 | 0.0016 | KLK7,TGM1,TGM3 |
| PMID:11093806 | (2000) Transglutaminase-3, an esophageal cancer-related gene. | 2 | 2 | 0.0136 | TGM1,TGM3 |
| PMID:11562168 | (2001) Crystallization and preliminary X-ray analysis of human transglutaminase 3 from zymogen to active form. | 2 | 2 | 0.0136 | TGM1,TGM3 |
| PMID:11980702 | (2002) Three-dimensional structure of the human transglutaminase 3 enzyme: binding of calcium ions changes structure for activation. | 2 | 2 | 0.0136 | TGM1,TGM3 |
| PMID:12850301 | (2003) Analysis of epidermal-type transglutaminase (transglutaminase 3) in human stratified epithelia and cultured keratinocytes using monoclonal antibodies. | 2 | 3 | 0.0136 | TGM1,TGM3 |
| PMID:14508061 | (2003) A model for the reaction mechanism of the transglutaminase 3 enzyme. | 2 | 2 | 0.0136 | TGM1,TGM3 |
| PMID:14645372 | (2004) Structural basis for the coordinated regulation of transglutaminase 3 by guanine nucleotides and calciummagnesium. | 2 | 2 | 0.0136 | TGM1,TGM3 |
| PMID:14987256 | (2004) Identification of calcium-inducible genes in primary keratinocytes using suppression-subtractive hybridization. | 2 | 8 | 0.0136 | KLK7,TGM1 |
| PMID:15084592 | (2004) Crystal structure of transglutaminase 3 in complex with GMP: structural basis for nucleotide specificity. | 2 | 2 | 0.0136 | TGM1,TGM3 |
| PMID:15172109 | (2004) Transglutaminase activity and transglutaminase mRNA transcripts in gerbil brain ischemia. | 2 | 3 | 0.0136 | TGM1,TGM3 |
Table 10.
Biological processes, KEGG pathways, and PMIDs for the EV total unique proteins in the HIV+Aβ group for the HIV vs. HIV+Aβ comparison.
|
Gene Ontology (GO) Terms for Biological Processes
10 Most Significant Results per FDR (for All GO Terms, See Supplementary Table S5A) | |||||
| Term Description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Vesicle-mediated transport | 41 | 1699 | 2.01 × 10−13 | ACTR3,AP1G1,AP2B1,ARF6,ARPC4,CALR,CAND1,CAPZB,CAV1,CD9,CDC42,COPB2,ECM1,EEA1,EHD4,IGF2R,KPNB1,MME,NME1,PDIA6,PGM1,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,RAB1A,RAB2A,RALA,SLC44A2,SOD1,STOM,SYK,TGFB1,TIMP3,VAT1,XRCC6 | |
| Localization | 66 | 5233 | 4.37 × 10−11 | ACTR3,AP1G1,AP2B1,APOM,ARF6,ARPC4,CALR,CAND1,CAPZB,CAV1,CD9,CDC42,COPB2,CSE1L,DHX9,ECM1,EEA1,EHD4,FBN2,IGF2R,IGSF8,ILK,IPO5,IPO7,KPNB1,LMNA,MME,NME1,NRP1,PAFAH1B1,PDIA6,PGM1,PIP,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,RAB1A,RAB2A,RALA,RELN,RNF128,RPL14,RTN4,SLC3A2,SLC44A1,SLC44A2,SLIT2,SOD1,SPOCK1,STOM,SYK,TGFB1,THBS4,TIMP3,VAT1,VDAC1,VDAC2,WLS,XPO1,XRCC6 | |
| Secretion | 30 | 1070 | 8.12 × 10−11 | CAND1,CAV1,CD9,ECM1,IGF2R,KPNB1,MME,NME1,PAFAH1B1,PGM1,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,RAB1A,RALA,SLC44A2,SOD1,STOM,SYK,TGFB1,TIMP3,VAT1,WLS,XRCC6 | |
| Transport | 57 | 4130 | 1.80 × 10−10 | ACTR3,AP1G1,AP2B1,APOM,ARF6,ARPC4,CALR,CAND1,CAPZB,CAV1,CD9,CDC42,COPB2,CSE1L,DHX9,ECM1,EEA1,EHD4,IGF2R,IPO5,IPO7,KPNB1,LMNA,MME,NME1,NRP1,PAFAH1B1,PDIA6,PGM1,PIP,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,RAB1A,RAB2A,RALA,RPL14,SLC3A2,SLC44A1,SLC44A2,SOD1,STOM,SYK,TGFB1,TIMP3,VAT1,VDAC1,VDAC2,WLS,XPO1,XRCC6 | |
| Secretion by cell | 28 | 959 | 1.80 × 10−10 | CAND1,CD9,ECM1,IGF2R,KPNB1,MME,PAFAH1B1,PGM1,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,RAB1A,RALA,SLC44A2,SOD1,STOM,SYK,TGFB1,TIMP3,VAT1,WLS,XRCC6 | |
| Regulated exocytosis | 24 | 691 | 2.67 × 10−10 | CAND1,CD9,ECM1,IGF2R,KPNB1,MME,PGM1,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,SLC44A2,SOD1,STOM,SYK,TGFB1,TIMP3,VAT1,XRCC6 | |
| Exocytosis | 25 | 774 | 3.25 × 10−10 | CAND1,CD9,ECM1,IGF2R,KPNB1,MME,PGM1,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,RALA,SLC44A2,SOD1,STOM,SYK,TGFB1,TIMP3,VAT1,XRCC6 | |
| Neutrophil activation involved in immune response | 19 | 489 | 1.06 × 10−8 | CAND1,IGF2R,KPNB1,MME,PGM1,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,SLC44A2,STOM,SYK,VAT1,XRCC6 | |
| Myeloid leukocyte activation | 20 | 574 | 1.48 × 10−8 | CAND1,IGF2R,KPNB1,MME,PGM1,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,SLC44A2,STOM,SYK,TGFB1,VAT1,XRCC6 | |
| Neutrophil degranulation | 18 | 485 | 4.95 × 10−8 | CAND1,IGF2R,KPNB1,MME,PGM1,PPIA,PSMD1,PSMD2,PSMD3,PYGB,PYGL,QSOX1,RAB10,RAB14,SLC44A2,STOM,VAT1,XRCC6 | |
| KEGG Pathways | |||||
| Term description | Obs | Bgr | FDR | Matching Proteins in the Network | |
| Endocytosis | 10 | 242 | 0.00016 | AP2B1,ARF6,ARPC4,CAPZB,CAV1,CDC42,EEA1,EHD4,IGF2R,RAB10 | |
| Focal adhesion | 8 | 197 | 0.0012 | CAV1,CDC42,ILK,LAMB2,PARVB,PPP1CB,RELN,THBS4 | |
| Bacterial invasion of epithelial cells | 5 | 72 | 0.003 | ARPC4,CAV1,CDC42,ILK,SEPT2 | |
| Pentose phosphate pathway | 3 | 30 | 0.0278 | G6PD,PGM1,RGN | |
| Starch and sucrose metabolism | 3 | 33 | 0.0278 | PGM1,PYGB,PYGL | |
| Proteoglycans in cancer | 6 | 195 | 0.0278 | CAV1,CDC42,LUM,PPP1CB,TGFB1,TIMP3 | |
| Proteasome | 3 | 43 | 0.0347 | PSMD1,PSMD2,PSMD3 | |
| Necroptosis | 5 | 155 | 0.0347 | PPIA,PYGB,PYGL,VDAC1,VDAC2 | |
| Fc gamma R-mediated phagocytosis | 4 | 89 | 0.0347 | ARF6,ARPC4,CDC42,SYK | |
| Amino sugar and nucleotide sugar metabolism | 3 | 48 | 0.0396 | CHIA,PGM1,UGDH | |
| HTLV-I infection | 6 | 250 | 0.0396 | CALR,NRP1,TGFB1,VDAC1,VDAC2,XPO1 | |
| 10 Most Significant PMID Publications per FDR | |||||
| Term ID | Term Description | Obs | Bgr | FDR | Matching Proteins in the Network |
| PMID:11149929 | (2001) The phagosome proteome: insight into phagosome functions. | 9 | 47 | 3.12 × 10−6 | ARF6,CALR,DFFA,P4HB,RAB10,RAB14,RAB2A,STOM,VDAC1 |
| PMID:17892558 | (2007) Quantifying raft proteins in neonatal mouse brain by ‘tube-gel’ protein digestion label-free shotgun proteomics. | 10 | 83 | 6.99 × 10−6 | ACTC1,BASP1,CAV1,CNTN1,RAB10,RAB14,RAB1A,RAB2A,SLC3A2,VDAC1 |
| PMID:22578496 | (2012) Harnessing the power of the endosome to regulate neural development. | 7 | 35 | 0.00014 | ARF6,EEA1,EHD4,NRP1,RAB14,RTN4,WLS |
| PMID:24009881 | (2012) Quantitative proteomics of extracellular vesicles derived from human primary and metastatic colorectal cancer cells. | 11 | 161 | 0.00014 | ACTR3,CAPZB,CD9,EHD4,ILK,RAB10,RALA,SLC3A2,SLC44A1,SYK,UGDH |
| PMID:27770278 | (2017) Comprehensive proteome profiling of glioblastoma-derived extracellular vesicles identifies markers for more aggressive disease. | 8 | 63 | 0.00016 | ACTR3,CALR,ECM1,IGF2R,IPO5,PSMD2,RAB10TGFB1 |
| PMID:26205348 | (2015) Fluoxetine increases plasticity and modulates the proteomic profile in the adult mouse visual cortex. | 6 | 22 | 0.00023 | AP1G1,CDC42,NME1,SOD1,VDAC1,VDAC2 |
| PMID:20140087 | (2010) Comprehensive identification and modified-site mapping of S-nitrosylated targets in prostate epithelial cells. | 9 | 103 | 0.00024 | DHX9,HNRNPK,KPNB1,LMNA,P4HB,PDIA6,RTN4,VDAC1,VDAC2 |
| PMID:27549615 | (2016) Genome-wide association study to identify potential genetic modifiers in a canine model for Duchenne muscular dystrophy. | 6 | 23 | 0.00024 | LMNA,PAMR1,PPIA,PSMD2,SLIT2,THBS4 |
| PMID:23170974 | (2012) Integrated miRNA, mRNA and protein expression analysis reveals the role of post-transcriptional regulation in controlling CHO cell growth rate. | 6 | 27 | 0.00044 | HNRNPK,RAB10,RAB14,RAB1A,RAB2A,RPL14 |
| PMID:24505448 | (2014) Characterisation of four LIM protein-encoding genes involved in infection-related development and pathogenicity by the rice blast fungus Magnaporthe oryzae. | 6 | 28 | 0.00047 | CDC42,ILK,LMNA,PHGDH,RAB2A,XRCC6 |
2.5. Analysis of Unique Protein Interactions
We also explored in STRING whether these unique proteins have functional interactions among each other. The statistical background assumed for this enrichment analysis was the whole human genome. We filtered our search for established interactions only for the input proteins, for the highest confidence (over 0.900), and for a static map without the protein structures. In the obtained interaction maps, different nodes are connected with colored lines depending on the functional association type. The results imply that the identified proteins have more interactions among themselves than what would be expected for a random set of proteins of similar size, drawn from the genome. Such enrichments indicate that the proteins are, at least partially, biologically connected as a group and may contribute jointly to shared functions.
The interactions of the 112 unique surface proteins in the control group as compared to the HIV group are illustrated in Figure 3A. The HIV group in this comparison had only three unique surface proteins (DNAH8, TTN, and IGHG2). Being present on the EV surface, these proteins may be prone to interact with their potential functional partners beyond the EV surface. Therefore, we examined their possible interactions not only with each other but with other proteins as well. The STRING program identified predicted functional partners for DNAH8 and TTN, and the top five candidates that were predicted with the highest confidence, as well as their interacting networks, are illustrated in Figure 3B.
Figure 3.
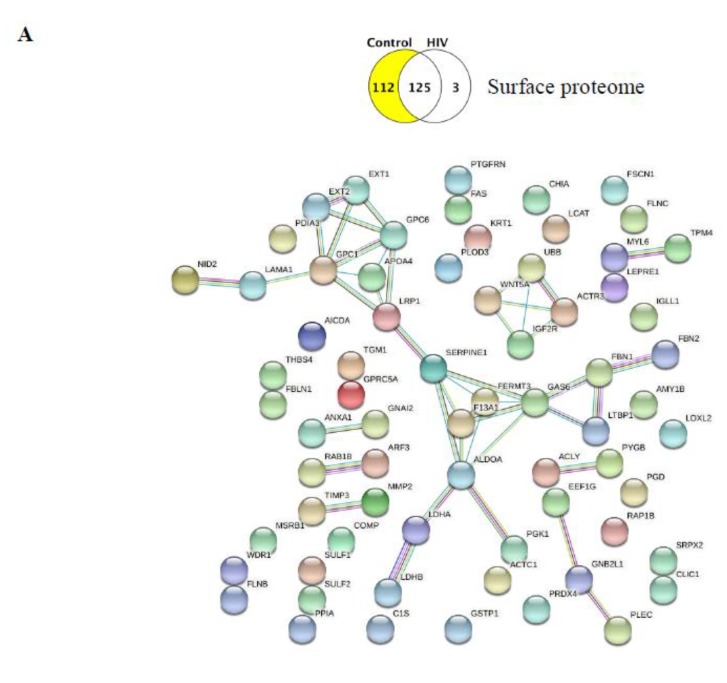
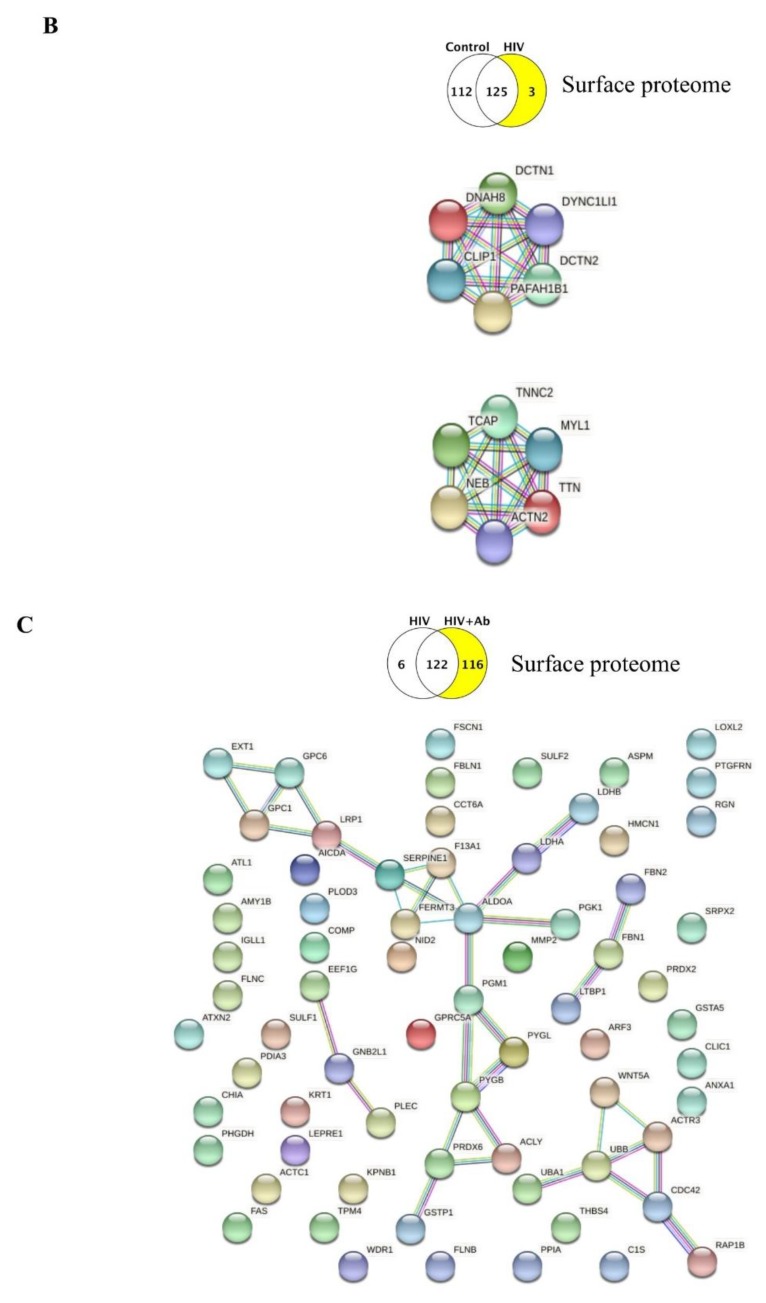
Protein–protein interactions between the identified unique proteins of the EV surface proteome. Venn diagrams illustrating the type of comparison and the number of identified unique proteins (highlighted). (A) Protein–protein interactions (PPI) (STRING) among the unique surface proteins in the control group. Only interactions with the highest confidence are shown with a minimum required interaction score of 0.900 (PPI enrichment p-value: 6.59 × 10−7; the network has significantly more interactions than expected). Known interactions: From curated databases (turquoise), experimentally determined (pink); predicted interactions: Gene neighborhood (green), gene fusions (red), gene co-occurrence (blue); other interactions: Textmining (light green), co-expression (black), protein homology (purple). (B) No interactions with highest confidence were identified in STRING among the three unique proteins identified in the HIV group. Predicted functional partners of dynein heavy chain 8, axonemal (DNAH8) (upper map) and titin (TTN) (lower map). Only the first shell of five interactions with the highest confidence is shown. Color code of the interaction lines as described in (A). (C) Protein–protein interactions among the unique proteins in the HIV+Aβ group. Only interactions with the highest confidence are shown (PPI enrichment p-value: 0.00158; the network has significantly more interactions than expected). Color code of the interaction lines as described in (A).
Next, we evaluated the unique surface protein list in the HIV vs. HIV+Aβ group. No protein–protein interactions were found for the six proteins uniquely expressed in the HIV group. By contrast, the HIV+Aβ unique surface proteins had several complex interactions, as illustrated in Figure 3C.
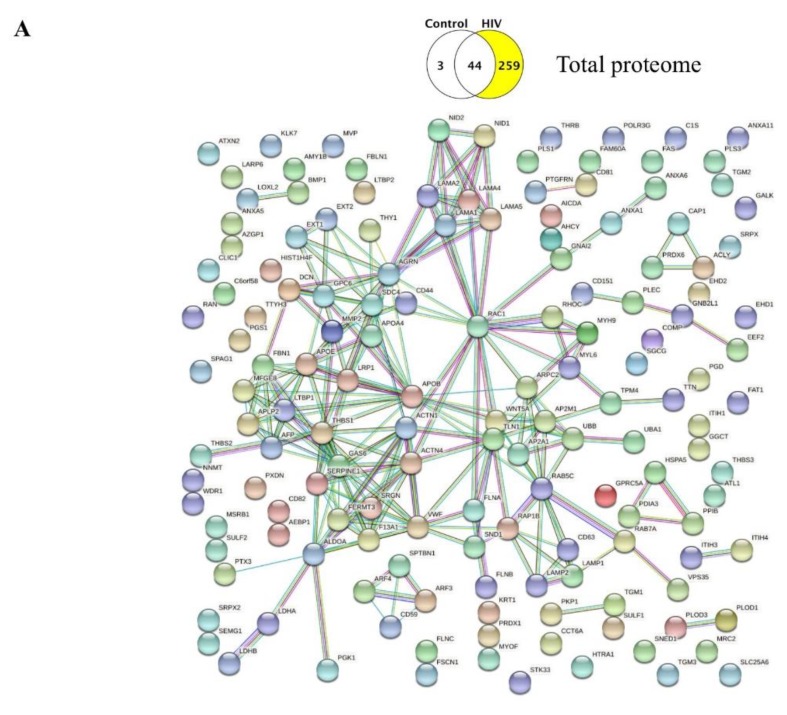
Finally, we analyzed the interactions between the unique proteins present in the total HBMEC-EV proteome. No interactions were found in the control group as compared to the HIV group; however, the elaborate interaction map for the total unique proteins in the HIV group is presented in Figure 4A. For the HIV vs. HIV+Aβ comparison, the HIV group exhibited 28 unique proteins without any identified interactions. In contrast, the unique proteins in the HIV+Aβ group showed a complicated interaction network, as illustrated in Figure 4B.
Figure 4.
Protein–protein interactions in the identified unique proteins of the EV total proteome. Venn diagrams illustrating the type of comparison and the number of identified unique proteins (highlighted). (A) Protein–protein interactions among the unique proteins in the HIV group. Only interactions with the highest confidence are shown (PPI enrichment p-value: 1.0 × 10−16; the network has significantly more interactions than expected). (B) Protein–protein interactions among the unique proteins in the HIV+Aβ group. Only interactions with the highest confidence are shown (PPI enrichment p-value: 1.45 × 10−7; the network has significantly more interactions than expected). Color code of the interaction lines as described in Figure 3A.
3. Discussion
In the current study, we evaluated HBMEC-EV surface and total proteome changes evoked by HIV-1 alone and together with Aβ. We limited our analyses to the unique lists of proteins identified in the treatment groups; thus, we did not include the shared protein lists and the complex changes in the up- and down-regulated proteins. In addition, we specifically focused on the unique proteins in the control vs. HIV and in the HIV vs. HIV+Aβ group comparisons. The identified proteins were mapped to different gene ontology (GO) terms for biological processes, KEGG pathways, and Cell Components. We also explored the protein–protein interactions among the identified unique proteins.
Overall, the surface proteome control vs. HIV comparison indicated that the functions of the identified unique proteins ranged from diverse biological processes in the control (mainly “extracellular matrix organization,” “metabolic processes,” “vesicle-mediated transport,” “exocytosis”) and KEGG pathways (mainly “proteoglycans in cancer,” “focal adhesion,” “carbohydrate and cholesterol metabolism,” “HIF-1 signaling pathway”) to few or no distinct biological processes in the HIV group (Figure 2A and Table 4). The latter phenomenon was likely due to the limited number of proteins (namely, DNAH8, TTN, IGHG2) that were unique in the HIV-1 group when compared to the HBMEC-EV surface proteome of the controls. Nevertheless, we found several potential functional partners for DNAH8, such as platelet-activating factor acetylhydrolase IB subunit alpha (PAFAH1B1), dynactin subunit 1 (DCTN1), dynactin subunit 2 (DCTN2), CAP-Gly domain-containing linker protein 1 (CLIP1), and cytoplasmic dynein 1 light intermediate chain 1 (DYNC1LI1). Similarly, we identified several predicted functional partners for TTN, namely, nebulin (NEB), telethonin (TCAP), troponin C, skeletal muscle (TNNC2), myosin light chain 1/3, skeletal muscle isoform (MYL1), and alpha-actinin-2 (ACTN2) (Figure 3B). Thus, these few unique surface EV proteins in the HIV group may engage primarily with proteins of actin cytoskeleton/microtubule remodeling and vesicle-mediated transport.
The control EV proteome exhibited more than a hundred unique proteins; thus, it appears that after HIV-1 exposure of the parent cells, the EV surface proteome almost completely “blended” into the control proteome. This relative lack of surface HBMEC-EV protein signature in the HIV group is particularly striking in light of our previous findings where the exposure of HBMEC to HIV results in increased EV shedding [18] and the fact that EVs are involved in spreading HIV infection to the neighboring cells. However, it is possible that the localization of some proteins could alter from the EV surface to the vesicle lumen, resulting in a highly enriched total but not surface proteome. Indeed, comparison of the total proteome revealed a highly diverse number of 259 unique proteins in the HIV group as compared to the control that mapped to a variety of biological processes and KEGG pathways. The most prominent enrichment among the biological processes category was “vesicle-mediated transport,” followed by “extracellular structure organization.” In addition, mapping these unique proteins to “exocytosis” and “secretion by cell” categories points to processes that may be involved in EV release and EV transport (Figure 2B and Table 8). Likewise, the KEGG pathways were also diverse, from “focal adhesion” and “endothelial cell medium (ECM)-receptor interaction” to “proteoglycans in cancer,” different infections, “endocytosis,” “cholesterol metabolism,” and “glycolysis/gluconeogenesis” (Table 8). Thus, the total EV proteome in the HIV group, with a large number of unique proteins, may suggest that the rich, unique cargo is somewhat “hidden” within the EVs with a surface proteome that was barely altered. This notion is supported by the observations that the HIV group in the HIV versus HIV+Aβ group surface proteome comparison also exhibited only six unique proteins (Figure 2C). On the other hand, the relative lack of unique EV surface protein signatures may facilitate EV internalization and, thus, HIV transmission to other cells.
In addition to the effects of HIV-1, we explored the impact of Aβ on the HBMEC-EV proteome in the context of HIV-1. It was reported that increased brain Aβ induced profound proteome remodeling in multiple cell types, altering brain molecular pathways in an Alzheimer’s disease (AD) mouse model [24]. Another brain proteomic study using a different AD mouse model with amyloid and neurofibrillary tangle pathologies indicated age-dependent immune responses and synaptic dysfunctions. It was proposed that these changes were evoked by the advancing Aβ pathology in the brain [25], further demonstrating the importance of proteomic analyses in studies on the mechanisms of amyloid pathology.
Comparison of surface proteomes of EVs derived from HBMEC exposed to HIV alone vs. HIV+Aβ revealed profound changes, as demonstrated by 116 unique proteins in the HIV+Aβ group (Figure 2C). Aβ, acting on a HIV background, appeared to shift biological processes from mainly actin cytoskeleton organization (Table 5) to immune responses, extracellular matrix organization, and carbohydrate metabolic processes. In addition, enrichment of the “vesicle-mediated transport” and “exocytosis” also pointed to processes involved in EV release and EV transport (Figure 2C and Table 6). The KEGG pathways changed from a “blended” profile in the HIV group to a very diverse profile in the HIV+Aβ group, pointing mainly to the carbohydrate metabolic processes, “focal adhesion,” different infections, and signaling pathways as demonstrated by HIF-1, MAPK, and AGE-RAGE enrichment (Table 6). Regarding these signaling pathways, we have shown before the involvement of the RAGE pathway in the HIV-induced Aβ accumulation in HBMEC [26].
The HIV vs. HIV+Aβ comparison for the total proteome indicated substantial remodeling in the HIV+Aβ with 201 unique proteins as compared to 28 of such proteins in the HIV group. Consistent with HIV+Aβ-mediated EV release [18], the biological processes changed from “cell envelope organization” (Table 9) to mainly “vesicle-mediated transport,” “exocytosis,” and immune responses (Figure 2D and Table 10). The KEGG pathways also shifted to a diverse profile. “Endocytosis” was the most significant, followed by “focal adhesion” and “bacterial invasion of epithelial cells.” Several proteins were part of the carbohydrate metabolic pathways, such as the “pentose phosphate pathway,” “starch and sucrose metabolism,” and “proteoglycans in cancer” (Table 10).
Surprisingly, surface and total proteome analysis across different groups did not find any Aβ species in EVs, not even in samples that were isolated from Aβ-exposed HBMEC. This lack of Aβ identification could be related to technical issues, such as aggregation of Aβ, its insolubility, and possibly indigestibility by trypsin. The tryptic peptide used to quantify β-amyloid, LVFFAEDVGSNK, corresponding to amino acids 688–699, maps to all species of Aβ and full-length APP [27] and has been identified in the human CSF proteome [28]. In our study, no peptides mapping to the Aβ-generating region of APP were identified, even though APP was identified on the surface proteome. Similar obstacles were described in another proteomic study, in which Aβ was not identified in human AD brains. However, Aβ was detected by dot blot and ELISA from the same samples [29], supporting the notion that the lack of Aβ detection in the proteome was likely due to technical limitations.
Our previous studies demonstrated that treatment of HBMEC with Aβ could enrich EVs with this peptide, which can then be carried and delivered to different cells of the neurovascular unit [18,30]. In support of these findings, literature reports described Aβ as being present on the EV surface. For example, neuron-derived EVs accelerated Aβ fibril formation from monomeric Aβ, and this process was inhibited by cleavage of glycosphingolipid (GSL) glycans by endoglycoceramidase (EGCase) [31]. The same group also demonstrated that EV GSL-glycans were critical for Aβ binding in vitro and in vivo [15]. GSLs are found mainly in lipid rafts in the outer layer plasma membrane with their glycans facing outside; however, they are more abundant in EVs than in the parent cells [15]. Besides GSL, EVs were shown to bind Aβ through the prion protein (PrP) [14], a glycosylphosphatidylinositol-anchored protein in the outer leaflet of the neuron and neuron-derived EV membrane [32].
Some of the unique proteins identified in our HBMEC-derived EVs exhibit a substantial overlap with proteins detected by label-free proteomics in Aβ-enriched extracts from human AD brains [29], suggesting the relevance of EV proteins to Aβ pathology. The examples include ANXA5, FGB, LAMA5, and VIM found both in the total proteome of EVs in the HIV group and in Aβ-enriched extracts from human AD brains [29]. In addition, specific types of tubulins, such as TUBA1B and TUBB4B, were present, although they did not change in AD brains. Among the unique proteins in the HIV+Aβ group’s total proteome, FGG and HIST1H2BK, as well as tubulins TUBB and TUBB2A, were also enriched in extracts from AD brains [29]. In addition, HIST1H2BK has been one of the unique proteins in the EV total proteome from the Aβ group. In contrast, RNF213 was not identified in any of our EV samples, although it was unique to the AD brain samples and also found within the amyloid plaques [29]. One explanation for this phenomenon could be that RNF213 in the AD brain might not originate from brain endothelial cells.
Analysis for predicted significant functional interactions among the unique proteins produced several elaborate interaction maps (Figure 3 and Figure 4). It is striking to notice that several proteins on these maps act like “hubs” or centers by having a substantial number of connections to other proteins. Such “hubs” for the surface proteomes were SERPINE1 (PAI-1), GPC1, FERMT3 (Figure 3A), and ALDOA (Figure 3C). The most complex functional interaction maps were obtained for the total proteomes due to the high number of unique proteins. The identified “hubs” were RAC1, GAS6, SERPINE1, AGRN, APOB, and RAB5C (Figure 4A), as well as CDC42 and RAB1A (Figure 4B). Among these proteins, endothelial AGRN (agrin) was shown to be implicated in the brain Aβ pathology. For example, deletion of the Agrn gene from endothelial cells resulted in significantly increased Aβ levels in the mouse brain; however, overexpression of Agrn restored brain Aβ levels [33]. SERPINE1 (PAI-1) and GPC1 (glypican-1) may be additional important players in the Aβ pathology [34,35]. Indeed, GPC1, a heparan sulfate proteoglycan, localized mainly in detergent-insoluble, GSL-rich membrane domains, was shown to bind fibrillar Aβ in the human brain [36], further suggesting that protein “hubs” identified in the present study may be involved in EV-mediated Aβ pathology.
In summary, our results provide information, with an unprecedented resolution, on the brain endothelial surface and total EV proteome changes after HIV and Aβ exposure of the parent cells. The analyses identified protein–protein interaction networks, biological processes, pathways, and cellular localization. Overall, the obtained results factor for a better understanding of HBMEC-EV protein landscape changes induced by HIV and Aβ and their contribution to the HIV-associated Aβ pathology in the brain.
4. Materials and Methods
4.1. Cell Cultures
Primary human brain microvascular endothelial cells (HBMEC) used in the study were purchased from ScienCell Research laboratories (Carlsbad, CA, USA). HBMEC were isolated from human brain and cryopreserved at passage one. HBMEC were characterized by immunofluorescence with antibodies specific to vWF/Factor VIII and CD31 (PECAM). Cells were cultured on bovine plasma fibronectin (ScienCell)-coated dishes in endothelial cell medium (ECM). Specifically, 500 mL of basal ECM medium was supplemented with 25 mL of exosome-depleted fetal bovine serum (Exo-FBS; System Biosciences, Mountain View, CA, USA), 5 mL of endothelial cell growth supplement (ECGS, ScienCell), and 5 mL of penicillin/streptomycin solution (P/S, ScienCell). We initiated two separate cultures on 100 mm cell culture dishes to reduce the number of passages and subcultured the cells twice at the 1:4 ratio. This resulted in 32 confluent cultures, with the average cell number at the end of experiment of 9.065 × 107 cells/dish. Sixteen confluent cultures were used for EV surface proteomics, and 16 for EV total proteomics. The treatment groups were: 1) Control exposed to vehicle, 2) Aβ alone, 3) HIV alone, 4) HIV plus Aβ, with four samples/group.
4.2. HIV Infection and Aβ Treatment
HIV-1 stock was generated using human embryonic kidney (HEK) 293T cells (ATCC, Manassas, VA, USA) transfected with pYK-JRCSF plasmid containing full-length proviral DNA. Throughout the study, HBMEC were exposed to HIV particles at the p24 level of 30 ng/mL as previously reported [37]. Treatment was terminated by removing the cell culture media for EV isolation.
Aβ (1–40) was purchased from Anaspec (San Jose, CA, USA) and dissolved in PBS. Freshly solubilized Aβ solutions without pre-aggregation were used for experiments as such a form of Aβ was demonstrated to induce proinflammatory reactions in isolated rat brain microvessels [38]. Cells were treated with Aβ (1–40) at the concentration of 100 nM for 48 h in complete medium. Although uptake of Aβ by the BBB occurs rapidly [39], we terminated the treatment at 48 h to allow more EV to be secreted into the culture medium. Confluent HBMEC were exposed to HIV-1 or/and Aβ (1–40) for 48 h.
4.3. EV Isolation
EV isolation was performed using ExoQuick-TC precipitation solution (System Biosciences) from conditioned culture media according to the manufacturer’s specifications. Briefly, 10 mL culture media from confluent HBMEC cultures was centrifuged at 3000 g for 15 min to remove cells and debris, and then mixed thoroughly with 2 mL of Exo-Quick precipitation solution and incubated overnight at 4 °C. The next day, samples were centrifuged at 1500 g for 30 min, and the supernatants were removed and centrifuged again at 1500 g for 5 min. The EV pellets were stored at –80 °C and used for proteomics analysis. Separate EV samples were prepared for EV surface and total proteomics.
4.4. Proteomics
Sample Preparation. Each sample was processed by SDS-PAGE using a 10% Bis Tris NuPage mini-gel (Invitrogen, Waltham, MA, USA) in the MES buffer system. The migration windows (1 cm lane) were excised and processed by in-gel digestion with trypsin using a ProGest robot (DigiLab) with the following protocol: The samples were washed with 25 mM ammonium bicarbonate followed by acetonitrile, reduced with 10 mM dithiothreitol at 60 °C, followed by alkylation with 50 mM iodoacetamide at room temperature, digested with trypsin (Promega, Madison, WI, USA) at 37 °C for 4 h, and quenched with formic acid. The supernatants were then analyzed directly without further processing.
Mass Spectrometry. Half of each digested sample was analyzed by nano LC-MS/MS with a Waters NanoAcquity HPLC system interfaced to a ThermoFisher Q Exactive. Peptides were loaded on a trapping column and eluted over a 75 μm analytical column at 350 nL/min; both columns were packed with Luna C18 resin (Phenomenex, Torrance, CA, USA). The mass spectrometer was operated in data-dependent mode, with the Orbitrap operating at 70,000 FWHM and 17,500 FWHM for MS and MS/MS respectively. The fifteen most abundant ions were selected for MS/MS.
Data Processing. Data were searched using Mascot (Matrix Science, London, UK; version 2.6.0) with the following parameters: Enzyme: Trypsin/P; Databases: SwissProt Human (concatenated forward and reverse plus common contaminants); fixed modifications: Carbamidomethyl (C); variable modifications: Acetyl (N-term), deamidation (N,Q), oxidation (M), Pyro-Glu (N-term Q); mass values: Monoisotopic; peptide mass tolerance: 10 ppm; fragment mass tolerance: 0.02 Da; max missed cleavages: 2. Mascot DAT files were parsed into Scaffold (Proteome Software, version Scaffold 4.8.7, Proteome Software Inc., Portland, OR, USA) for validation, filtering, and to create a non-redundant list per sample. Data were filtered using a 1% protein and peptide FDR and required at least two unique peptides per protein. Protein probabilities were assigned by the Protein Prophet algorithm [40]. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Proteins were annotated with GO terms from NCBI (downloaded on Sep 6, 2018) [41]. The normalized spectral abundance factor (NSAF) calculation contains the conversion to the spectral abundance factor (SAF) and subsequent normalized spectral abundance factor (NSAF). This was based on the equation: NSAF = (SpC/MW)/Σ(SpC/MW)N, where SpC = spectral counts, MW = protein molecular weight in kDa, and N = total number of proteins. NSAF values can be used to approximate the relative abundance of proteins within a given sample and the relative abundance of a given protein between samples. The different treatment groups were compared using the t-test, and p < 0.05 was considered significant.
4.5. ExoCarta Database Search and Functional Enrichment Analysis
The list of the top 100 proteins most often identified in EVs was composed based on the ExoCarta EV proteomics database from different human cell types [19]. Enrichment in molecular functions of the identified EV proteins was analyzed using the Scaffold Proteome Software and STRING [42]. A gene ontology analysis study was carried out with the proteomic profiles obtained to identify overrepresentation profiles. Gene ontology was investigated at the levels of the biological process, KEGG pathways, and cell component. Textmining in STRING provided the most relevant publications for a particular enrichment. Kyoto Encyclopedia of Genes and Genomes (KEGG) established pathway maps representing molecular interactions, reactions, and relation networks for Metabolism, Genetic Information Processing, Environmental Information Processing, Cellular Processes, Organismal Systems, Human Diseases and Drug Development. KEGG PATHWAY is the reference database for pathway mapping in KEGG Mapper.
Abbreviations
| Aβ | amyloid beta |
| AD | Alzheimer’s disease |
| BBB | Blood–brain barrier |
| ECGS | Endothelial cell growth supplement |
| EV | Extracellular vesicle |
| ELISA | Enzyme-linked immunosorbent assay |
| HAND | HIV-associated neurocognitive disorders; |
| HBMEC | Human brain microvascular endothelial cells |
| HEK cells | Human embryonic kidney cells |
| HIV | Human immunodeficiency virus type 1 |
| PBS | Phosphate buffered saline |
| PECAM | Platelet endothelial cell adhesion molecule |
| RAGE | Receptor for advanced glycation end products |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/8/2741/s1.
Author Contributions
Conceptualization, I.E.A. and M.T.; Formal analysis, I.E.A. and B.B.S.; Funding acquisition, M.T.; Investigation, I.E.A. and B.B.S.; Supervision, I.E.A. and M.T.; Visualization, I.E.A.; Writing—original draft, I.E.A.; Writing—review & editing, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the Florida Department of Health grant 8AZ24 and the National Institutes of Health (NIH), grants MH072567, MH098891, HL126559, DA039576, DA040537, DA044579, and DA047157. We acknowledge support from the Miami Center for AIDS Research (CFAR) at the University of Miami Miller School of Medicine, which is funded by a grant (P30AI073961) from the National Institutes of Health (NIH). We would like to thank Joshua Zahner, undergraduate student at the University of Miami, for his help in sorting the unique protein lists.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Esiri M.M., Biddolph S.C., Morris C.S. Prevalence of Alzheimer plaques in AIDS. J. Neurol. Neurosurg. Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rempel H.C., Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. Aids. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- 3.Xu J., Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: A foreseeable medical challenge in post-HAART era. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2009;4:200–212. doi: 10.1007/s11481-008-9136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brew B.J., Crowe S.M., Landay A., Cysique L.A., Guillemin G. Neurodegeneration and ageing in the HAART era. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2009;4:163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 5.Achim C.L., Adame A., Dumaop W., Everall I.P., Masliah E., Neurobehavioral Research C. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulliam L. HIV regulation of amyloid beta production. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2009;4:213–217. doi: 10.1007/s11481-009-9151-9. [DOI] [PubMed] [Google Scholar]
- 7.Green D.A., Masliah E., Vinters H.V., Beizai P., Moore D.J., Achim C.L. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. Aids. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 8.Soontornniyomkij V., Moore D.J., Gouaux B., Soontornniyomkij B., Tatro E.T., Umlauf A., Masliah E., Levine A.J., Singer E.J., Vinters H.V., et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. Aids. 2012;26:2327–2335. doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinbrink F., Evers S., Buerke B., Young P., Arendt G., Koutsilieri E., Reichelt D., Lohmann H., Husstedt I.W., German Competence Network H.A. Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur. J. Neurol. 2013;20:420–428. doi: 10.1111/ene.12006. [DOI] [PubMed] [Google Scholar]
- 10.Deane R., Zlokovic B.V. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 11.Vella L.J., Sharples R.A., Nisbet R.M., Cappai R., Hill A.F. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur. Biophys. J. EBJ. 2008;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- 12.Kalani A., Tyagi A., Tyagi N. Exosomes: Mediators of neurodegeneration, neuroprotection and therapeutics. Mol. Neurobiol. 2014;49:590–600. doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A., Pulliam L. Exosomes as mediators of neuroinflammation. J. Neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An K., Klyubin I., Kim Y., Jung J.H., Mably A.J., O’Dowd S.T., Lynch T., Kanmert D., Lemere C.A., Finan G.M., et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol. Brain. 2013;6:47. doi: 10.1186/1756-6606-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuyama K., Sun H., Sakai S., Mitsutake S., Okada M., Tahara H., Furukawa J., Fujitani N., Shinohara Y., Igarashi Y. Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J. Biol. Chem. 2014;289:24488–24498. doi: 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuyama K., Sun H., Usuki S., Sakai S., Hanamatsu H., Mioka T., Kimura N., Okada M., Tahara H., Furukawa J., et al. A potential function for neuronal exosomes: Sequestering intracerebral amyloid-beta peptide. FEBS Lett. 2015;589:84–88. doi: 10.1016/j.febslet.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Dinkins M.B., Dasgupta S., Wang G., Zhu G., He Q., Kong J.N., Bieberich E. The 5XFAD Mouse Model of Alzheimer’s Disease Exhibits an Age-Dependent Increase in Anti-Ceramide IgG and Exogenous Administration of Ceramide Further Increases Anti-Ceramide Titers and Amyloid Plaque Burden. J. Alzheimer’s Dis. JAD. 2015;46:55–61. doi: 10.3233/JAD-150088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andras I.E., Leda A., Contreras M.G., Bertrand L., Park M., Skowronska M., Toborek M. Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology. Mol. Cell. Neurosci. 2017;79:12–22. doi: 10.1016/j.mcn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keerthikumar S., Chisanga D., Ariyaratne D., Al Saffar H., Anand S., Zhao K., Samuel M., Pathan M., Jois M., Chilamkurti N., et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathivanan S., Fahner C.J., Reid G.E., Simpson R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Ledet R.J., Imberg-Kazdan K., Logan S.K., Garabedian M.J. Dynein axonemal heavy chain 8 promotes androgen receptor activity and associates with prostate cancer progression. Oncotarget. 2016;7:49268–49280. doi: 10.18632/oncotarget.10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauveau C., Rowell J., Ferreiro A. A rising titan: TTN review and mutation update. Hum. Mutat. 2014;35:1046–1059. doi: 10.1002/humu.22611. [DOI] [PubMed] [Google Scholar]
- 23.Aurivillius M., Oymar K., Oxelius V.A. Immunoglobulin heavy G2 chain (IGHG2) gene restriction in the development of severe respiratory syncytial virus infection. Acta Paediatr. 2005;94:414–418. doi: 10.1080/08035250410023656. [DOI] [PubMed] [Google Scholar]
- 24.Savas J.N., Wang Y.Z., DeNardo L.A., Martinez-Bartolome S., McClatchy D.B., Hark T.J., Shanks N.F., Cozzolino K.A., Lavallee-Adam M., Smukowski S.N., et al. Amyloid Accumulation Drives Proteome-wide Alterations in Mouse Models of Alzheimer’s Disease-like Pathology. Cell Rep. 2017;21:2614–2627. doi: 10.1016/j.celrep.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D.K., Park J., Han D., Yang J., Kim A., Woo J., Kim Y., Mook-Jung I. Molecular and functional signatures in a novel Alzheimer’s disease mouse model assessed by quantitative proteomics. Mol. Neurodegener. 2018;13:2. doi: 10.1186/s13024-017-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andras I.E., Eum S.Y., Huang W., Zhong Y., Hennig B., Toborek M. HIV-1-induced amyloid beta accumulation in brain endothelial cells is attenuated by simvastatin. Mol. Cell. Neurosci. 2010;43:232–243. doi: 10.1016/j.mcn.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L., Petyuk V.A., Tasaki S., Boyle P.A., Gaiteri C., Schneider J.A., De Jager P.L., Bennett D.A. Association of Cortical beta-Amyloid Protein in the Absence of Insoluble Deposits With Alzheimer Disease. JAMA Neurol. 2019;76:818–826. doi: 10.1001/jamaneurol.2019.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macron C., Lane L., Nunez Galindo A., Dayon L. Deep Dive on the Proteome of Human Cerebrospinal Fluid: A Valuable Data Resource for Biomarker Discovery and Missing Protein Identification. J. Proteome Res. 2018;17:4113–4126. doi: 10.1021/acs.jproteome.8b00300. [DOI] [PubMed] [Google Scholar]
- 29.Pedrero-Prieto C.M., Flores-Cuadrado A., Saiz-Sanchez D., Ubeda-Banon I., Frontinan-Rubio J., Alcain F.J., Mateos-Hernandez L., de la Fuente J., Duran-Prado M., Villar M., et al. Human amyloid-beta enriched extracts: Evaluation of in vitro and in vivo internalization and molecular characterization. Alzheimer’s Res. Ther. 2019;11:56. doi: 10.1186/s13195-019-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andras I.E., Toborek M. Extracellular vesicles of the blood-brain barrier. Tissue Barriers. 2016;4:e1131804. doi: 10.1080/21688370.2015.1131804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuyama K., Sun H., Mitsutake S., Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J. Biol. Chem. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauren J., Gimbel D.A., Nygaard H.B., Gilbert J.W., Strittmatter S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauch S.M., Huen K., Miller M.C., Chaudry H., Lau M., Sanes J.R., Johanson C.E., Stopa E.G., Burgess R.W. Changes in brain beta-amyloid deposition and aquaporin 4 levels in response to altered agrin expression in mice. J. Neuropathol. Exp. Neurol. 2011;70:1124–1137. doi: 10.1097/NEN.0b013e31823b0b12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R.M., van Groen T., Katre A., Cao D., Kadisha I., Ballinger C., Wang L., Carroll S.L., Li L. Knockout of plasminogen activator inhibitor 1 gene reduces amyloid beta peptide burden in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2011;32:1079–1089. doi: 10.1016/j.neurobiolaging.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bi Oh S., Suh N., Kim I., Lee J.Y. Impacts of aging and amyloid-beta deposition on plasminogen activators and plasminogen activator inhibitor-1 in the Tg2576 mouse model of Alzheimer’s disease. Brain Res. 2015;1597:159–167. doi: 10.1016/j.brainres.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe N., Araki W., Chui D.H., Makifuchi T., Ihara Y., Tabira T. Glypican-1 as an Abeta binding HSPG in the human brain: Its localization in DIG domains and possible roles in the pathogenesis of Alzheimer’s disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004;18:1013–1015. doi: 10.1096/fj.03-1040fje. [DOI] [PubMed] [Google Scholar]
- 37.Andras I.E., Toborek M. HIV-1 stimulates nuclear entry of amyloid beta via dynamin dependent EEA1 and TGF-beta/Smad signaling. Exp. Cell Res. 2014;323:66–76. doi: 10.1016/j.yexcr.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paris D., Townsend K.P., Obregon D.F., Humphrey J., Mullan M. Pro-inflammatory effect of freshly solubilized beta-amyloid peptides in the brain. Prostaglandins Other Lipid Mediat. 2002;70:1–12. doi: 10.1016/S0090-6980(02)00111-9. [DOI] [PubMed] [Google Scholar]
- 39.Yamada K., Hashimoto T., Yabuki C., Nagae Y., Tachikawa M., Strickland D.K., Liu Q., Bu G., Basak J.M., Holtzman D.M., et al. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J. Biol. Chem. 2008;283:34554–34562. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nesvizhskii A.I., Keller A., Kolker E., Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 41.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.