Abstract
For almost half a century, cardiac transplant has been the only long-term treatment for patients with end-stage heart failure. Implantable left ventricular assist devices (LVADs) have emerged as a new treatment option for advanced heart failure as destination therapy for patients either too old or not suitable for transplant. A meta-analysis presenting head-to-head comparisons of cardiac transplant versus LVAD as destination therapy (LVAD-DT) found no difference in 1-year mortality rates between LVAD-DT and cardiac transplant (OR 1.49; 95% CI [0.48–4.66]; I2=82.8%). Moreover, a recent subanalysis from the Interagency Registry for Mechanically Assisted Circulatory Support found similar outcomes after LVAD-DT implantation in both transplant and non-transplant centres. The time is right for LVAD-DT in non-transplant centres, provided multidisciplinary heart failure teams and expertise are in place.
Keywords: Cardiac transplant, left ventricular assist device, destination therapy, outcomes
“The time is always right to do what is right.”
– Martin Luther King Jr (1929–1968)
Heart failure (HF) has increased at a fast pace during the 21st century to become the main cause of morbidity and mortality for patients with cardiovascular disorders in developed nations, leading to increasing healthcare costs and declining quality of life. The expected prevalence of HF was ~25 million in 2011, and was anticipated to rise to >40 million worldwide in 2018, according to market research reports and industry analysis. Data from the National Heart, Lung and Blood Institute indicate that HF incidence is ~21 per 1,000 people aged >65 years.[1] The total costs for HF are projected to reach $69.7 billion by 2030, an increase of 127% from 2012.[2]
In Catalonia, the most recent prevalence data indicate that HF affects 155,883 patients (out of 7.5 million inhabitants).[3] Over the past three decades, research has led to better management of HF using drugs, new devices and a more holistic approach in multidisciplinary HF clinics. This has led to improvements in survival, yet a percentage of patients with progressive HF continue to require cardiac transplant or mechanical circulatory support to prolong life.[4]
Approximately 50% of HF patients have reduced ejection fraction, and 10% of these patients experience refractory HF symptoms (New York Heart Association functional class IIIb to IV, stage D). Cardiac transplant is currently, and has been for the past 50 years, the preferred long-term treatment for eligible patients with end-stage advanced HF. Nevertheless, the availability of donor hearts is limited and not all patients are eligible for cardiac transplant.
In Catalonia, the number of patients with advanced HF is probably ~500–1000; however, cardiac transplant is only recommended for a limited number of eligible patients aged <70 years. The transplant rate in Catalonia has ranged between 55 and 70 hearts per year during the past two decades. These numbers illustrate the paucity of donor hearts for transplant, and the fact that most candidates ultimately do not receive a compatible graft. Implantable left ventricular assist devices (LVADs), which fully or partly support the left ventricle, are an alternative therapy for patients with end-stage advanced HF. A long-term LVAD is used as a bridge to transplant (BTT) while patients await a suitable heart, or as permanent destination therapy (DT) that provides both life prolongation and proper quality of life.
Cardiac Transplant: Pros and Cons
Despite recent advances in mechanical circulatory support, cardiac transplant remains the treatment of choice for patients with advanced HF. The short- and long-term outcomes following cardiac transplant are remarkable, with a median survival of 10.7 years.[5] For transplant patients, there is a marked improvement in survival, quality of life and functional status.[6] During the past decades, there has been continuous improvement in morbidity and mortality, despite older and higher-risk recipients receiving transplants. However, graft failure, rejection and infection remain significant causes of morbidity and mortality, precluding better short- and long-term outcomes. The highest incidence of mortality occurs in the first 6 months post-transplant, with the perioperative hospitalisation period having the highest risk of death. After the first year, the mortality rate decreases to 3–4% per year.[6]
However, mid- to long-term mortality continues to be affected by progressive cardiac allograft vasculopathy, late graft failure, rejection, infectious complications and issues due to chronic immunosuppression, including malignancy. The ultimate goals of preventing rejection and finding alternatives to immunosuppression remain elusive. In addition, chronic kidney disease is common after heart transplant and is associated with increased mortality. Furthermore, up to 39% of cardiac transplant recipients will develop diabetes after transplant. The major factor limiting cardiac transplant has been the insufficient donor supply, which is currently limited to approximately 4,000 hearts annually worldwide.
Destination Therapy with Left Ventricular Assist Devices: Pros and Cons
LVADs have revolutionised the management of patients with advanced HF, providing an alternative to cardiac transplant. LVADs were initially implanted as a BTT to reduce the high mortality rates among patients who were awaiting donor hearts. However, the paucity of donor organs, along with the substantial increases in the comorbidities and the age of the HF population, have led to LVADs being used as a DT for advanced HF.
LVADs can be broadly classified as either pulsatile flow/positive displacement or continuous flow/rotary systems. Continuous flow systems have several advantages over pulsatile flow pumps, including a more compact size and improved surfaces, as well as reduced surgical trauma and thrombotic complications. Continuous flow pumps can be further classified into centrifugal and axial flow pumps. Centrifugal flow pumps are smaller than the axial pumps available, and have a tubular configuration that allows them to be implanted faster and even less invasively; therefore, they are probably more cost-effective.[7] Third-generation implantable continuous-flow LVADs, incorporating improved pump technologies, have improved pump performance and patient healthcare.
The recent Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3) trial demonstrated that implantation of a fully magnetically levitated centrifugal-flow pump (HeartMate 3) was associated with better outcomes at 6 months than an axial-flow pump (HeartMate II), primarily because of the lower rate of reoperation for pump malfunction.[8]
The use of a LVAD as DT (LVAD-DT) was approved by the US Food and Drug Administration in 2010; since then, LVAD-DT implantations have rapidly increased. The proportion of patients allocated to LVAD-DT increased from 19.6% from 2008 to 2010, to 45.7% of all implants in 2014. The miniaturisation of devices, the evolution of device technology and improvements in the operative techniques, as well as better patient selection and complication management have led to a significant improvement in survival rates.
The 8th Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) report on >20,000 LVAD implantations from 2006 to 2016 found a 1-year survival rate of 81% and 2-year survival rate of 70%.[9] Nowadays, LVAD therapy constitutes an established treatment option for well-selected patients with advanced HF. As a result, the number of transplant and non-transplant centres integrating LVAD programs in their facilities is rapidly expanding, and a further increase in device implantations is anticipated in the near future.
Relevant recent literature demonstrates better event-free survival, symptoms and quality of life with LVAD-DT, as compared with optimal medical management.[10–12] The Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management (ROADMAP) study found that 12-month survival was greater for LVAD-DT versus optimal medical management (80 ± 4% versus 63 ± 5%; p=0.022) in patients with New York Heart Association Class IIIb/IV. Health-related quality of life and depression improved from baseline more significantly with LVADs than with optimal medical management.
Adverse events were higher in LVAD-DT patients, in the HeartMate II trial.[10] Starling et al. extended these findings up to 2 years of follow up.[11] More recently, the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry reported that survival was similar for medical and LVAD-DT in the overall cohort, which included the lower severity INTERMACS profiles 6 and 7, but survival was better with LVAD-DT among patients in INTERMACS profiles 4 and 5.[12]
Cardiac Transplant Versus Left Ventricular Assist Devices for Advanced Heart Failure
Theochari et al. performed a meta-analysis of the available studies presenting head-to-head comparisons of cardiac transplant versus LVAD-BTT or LVAD-DT for late (>6 months) all-cause mortality.[13] Eight studies were included that reported data on 7,957 patients. Seven studies compared cardiac transplant with LVAD-BTT, and five compared cardiac transplant with LVAD-DT, evaluating 1-year mortality. These studies found no difference in 1-year mortality rates between LVAD-BTT and cardiac transplant (OR 0.91; 95% CI [0.62–1.32]; I[2]=21.2%) or between LVAD-DT and cardiac transplant (OR 1.49; 95% CI [0.48–4.66]; I[2]=82.8%; Figure 1). Although complications with LVAD therapy are not uncommon, most are manageable, and current outcomes clearly support the use of a LVAD in advanced HF. Nevertheless, although there are certainly limitations to cardiac transplant, median survival at present is much better with transplant (~12 years) than LVAD (3–4 years).[14]
Figure 1: Forest Plot of the Odds Ratios for 1-year Mortality Between Cardiac Transplant and Left Ventricular Assist Device Destination Therapy.
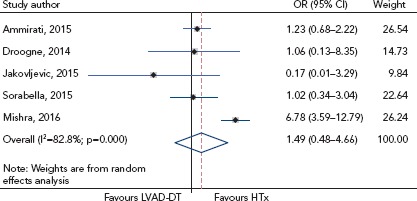
There was no difference in 1-year mortality rates between left ventricular assist device as destination therapy (LVAD-DT) and cardiac transplant (HTx) among the five studies. Source: Theochari et al. 2018.[13] Reproduced with permission from AME Publishing Company.
Destination Left Ventricular Assist Devices in Non-transplant Centres
Since the US Food and Drug Administration approval of LVAD-DT, the number of hospitals offering LVAD therapy has grown rapidly, with a rising number performed at centres without internal transplant programs.
Brinkley et al. sought to determine whether the outcomes after LVAD-DT implantation were similar at transplant and non-transplant centres.[15] The authors analysed all adult recipients of a primary, continuous-flow LVAD-DT between 2012 and 2014 from the INTERMACS registry. Subjects were classified according to their implanting centre as transplant (n=3,323) or non-transplant (n=260). Outcomes included overall survival, freedom from death or major adverse event, rates of individual adverse events, rehospitalisation and health-related quality of life. The 1-month (94.2%; 95% CI [95.0–93.4] versus 94.2%; 95% CI [97.1–91.4]) and 12-month (76.4%; 95% CI [77.9–74.8] versus 71.3%; 95% CI [77.4–65.2]) survival rates were similar at transplant versus non-transplant centres (HR 0.88; 95% CI [0.70–1.12]). The risk remained similar after adjustment for baseline characteristics (HR 0.88; 95% CI [0.69–1.12]). The rates for freedom from death or major adverse event at 12 months (29.0%; 95% CI [30.6–27.3] versus 29.8%; 95% CI [36.0–23.6]) were similar at transplant and non-transplant centres (adjusted HR 1.01; 95% CI [0.87–1.18]; Figure 2). The individual adverse event rates, rehospitalisation and post-implant health-related quality of life were also similar. The authors concluded that in a large, modern cohort of LVAD-DT recipients, outcomes after implantation were similar at transplant and non-transplant centres.[15]
Figure 2: Kaplan–Meier Analysis of Freedom from Death or Major Adverse Events at Transplant and Non-transplant Centres.
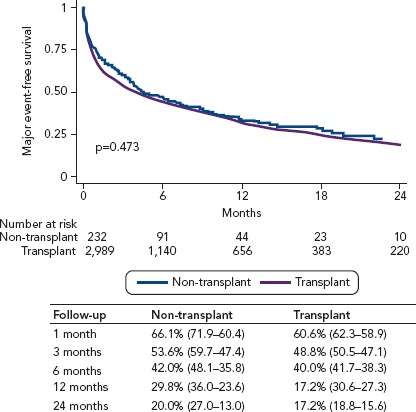
Major adverse events included death, stroke, major bleeding, pump exchange, device infection, device malfunction and right heart failure. Source: Brinkley et al. 2018.[15] Reproduced with permission from Wolters Kluwer Health.
Assuming the appropriate infrastructure is in place (described below), the findings of these studies should mitigate concerns regarding a broader extension of LVAD-DT, as the expanded access to this restorative and life-saving therapy at non-transplant centres has maintained good patient outcomes.
Characteristics of a Left Ventricular Assist Device Destination Therapy Program
In general, an LVAD-DT program is considered a challenging endeavour, yet it does not require the co-existence of a parallel in-hospital transplant program. The Essen Experience, recently reported by Papathanasiou and Luedike, provided the following advice.[16]
A LVAD-DT program should be part of a multidisciplinary HF clinic consisting (at least) of a cardiology and a cardiac surgery department with an adequate number of potential LVAD-DT candidates. Considering the multi-organ manifestations of advanced HF and the broad spectrum of non-surgical interventions indicated in this patient cohort, the candidate centre should perform interventional and surgical cardiac procedures, cardiac electronic device implantation, and intensive cardiovascular care. A dedicated outpatient clinic is part of the required infrastructure to provide high-quality, long-term care of ambulatory patients.
The physician leadership team is the core of the LVAD-DT program. An experienced HF cardiologist and a cardiac surgeon with expertise in mechanical circulatory support should supervise all aspects of device implementation, including patient selection, staff training, quality controls and cost-effectiveness.
A qualified team of surgeons, HF cardiologists and nurses familiar with the complexity of LVAD-DT should be organised. Staff training in special skills, and familiarity with the psychosocial, technical and pharmaceutical issues is of paramount importance for all parties. Rehabilitation physicians should be part of the caring team.
A transplant centre affiliation is necessary for patients who are or may become eligible for LVAD-BTT and should be offered transplant candidacy. Participation in a palliative care network or at least an on-site consulting service should be available for the end-of-life care of LVAD patients.
Conclusion
LVADs have transformed the treatment landscape of HF and are now adopted for long-term ambulatory support of patients with advanced disease. As the number of LVAD-DT implants is anticipated to rise, clinicians will need to integrate dedicated programs in their HF clinics and be actively involved in the care of patients on LVAD support. Indeed, all LVAD centres, regardless of their transplant capabilities, are required to have multidisciplinary HF teams to guide patient selection and assist in the long-term care of this unique population. The time is right for LVAD-DT in non-transplant centres.
References
- 1.Huffman MD, Berry JD, Ning H et al. Lifetime risk for heart failure among white and black Americans. J Am Coll Cardiol. 2013;61:1510–7. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW et al. Heart disease and stroke statistics – 2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Cainzos-Achirica M, Capdevila C, Vela E et al. Individual income, mortality and healthcare resource use in patients with chronic heart failure living in a universal healthcare system: a population-based study in Catalonia, Spain. Int J Cardiol. 2019;277:250–7. doi: 10.1016/j.ijcard.2018.10.099. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 5.Stehlik J, Edwards LB, Kucheryavaya AY et al. The Registry of the International Society for Heart and Lung Transplant: Twenty-eighth Adult Heart Transplant Report – 2011. J Heart Lung Transplant. 2011;30:1078–94. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 6.McCartney SL, Patel C, Del Rio JM. Long-term outcomes and management of the heart transplant recipient. Best Pract Res Clin Anaesthesiol. 2017;31:237–48. doi: 10.1016/j.bpa.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Olsen DB. The history of continuous-flow blood pumps. Artif Organs. 2000;24:401–4. doi: 10.1046/j.1525-1594.2000.06652.x. [DOI] [PubMed] [Google Scholar]
- 8.Mehra MR, Naka Y, Uriel N et al. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376:440–50. doi: 10.1056/NEJMoa1610426. [DOI] [PubMed] [Google Scholar]
- 9.Kirklin JK, Pagani FD, Kormos RL et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–6. doi: 10.1016/j.healun.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Estep JD, Starling RC, Horstmanshof DA et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP Study. J Am Coll Cardiol. 2015;66:1747–61. doi: 10.1016/j.jacc.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 11.Starling RC, Estep JD, Horstmanshof DA et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: the ROADMAP study 2-year results. JACC Heart Fail. 2017;5:518–27. doi: 10.1016/j.jchf.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Ambardekar AV, Kittleson MM, Palardy M et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant. 2019;38:408–17. doi: 10.1016/j.healun.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theochari CA, Michalopoulos G, Oikonomou EK et al. Heart transplant versus left ventricular assist devices as destination therapy or bridge to transplant for 1-year mortality: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2018;7:3. doi: 10.21037/acs.2017.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein DJ, Meyns B, Xie R et al. Third annual report from the ISHLT Mechanically Assisted Circulatory Support Registry: a comparison of centrifugal and axial continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2019;38:352–63. doi: 10.1016/j.healun.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Brinkley DM, DeNofrio D, Ruthazer R et al. Outcomes after continuous-flow left ventricular assist device implantation as destination therapy at transplant versus nontransplant centers. Circ Heart Fail. 2018;11:e004384. doi: 10.1161/CIRCHEARTFAILURE.117.004384. [DOI] [PubMed] [Google Scholar]
- 16.Papathanasiou M, Luedike P. The evolution of left ventricular assist devices. HeathManagement. 2019;19:70–2. [Google Scholar]


