Key Points
Question
How does RO948 F 18 positron emission tomographic scanning discriminate between Alzheimer disease and other neurodegenerative disorders in comparison with magnetic resonance imaging and cerebrospinal fluid measures?
Findings
In this diagnostic study including 613 patients from the Swedish BioFINDER-2 clinical trial, standard uptake value ratios of RO948 F 18 were higher in patients with Alzheimer disease dementia compared with cognitively unimpaired controls and patients with other neurodegenerative disorders; furthermore, RO948 F 18 outperformed magnetic resonance imaging and cerebrospinal fluid measures. Generally, tau positron emission tomographic positivity was confined to amyloid β–positive cases or MAPT R406W mutation carriers in this cohort; in patients with semantic variant primary progressive aphasia, RO948 F 18 retention was lower than that for flortaucipir F 18.
Meaning
These findings suggest that RO948 F 18 has a high specificity for Alzheimer disease–type tau and highlight its potential as a diagnostic marker in the workup of patients treated in memory clinics.
Abstract
Importance
The diagnostic performance of second-generation tau positron emission tomographic (PET) tracers is not yet known.
Objective
To examine the novel tau PET tracer RO948 F 18 ([18F]RO948) performance in discriminating Alzheimer disease (AD) from non-AD neurodegenerative disorders.
Design, Setting, and Participants
In this diagnostic study, 613 participants in the Swedish BioFINDER-2 study were consecutively enrolled in a prospective cross-sectional study from September 4, 2017, to August 28, 2019. Participants included 257 cognitively unimpaired controls, 154 patients with mild cognitive impairment, 100 patients with AD dementia, and 102 with non-AD neurodegenerative disorders. Evaluation included a comparison of tau PET tracer [18F]RO948 with magnetic resonance imaging (MRI) and cerebrospinal fluid and a head-to-head comparison between [18F]RO948 and flortaucipir F 18 ([18F]flortaucipir) in patients with semantic variant primary progressive aphasia (svPPA).
Exposures
[18F]RO948 (all patients) and [18F]flortaucipir (3 patients with svPPA) tau PET; MRI (hippocampal volume, composite temporal lobe cortical thickness, whole-brain cortical thickness) and cerebrospinal fluid measures (p-tau181 and amyloid Aβ42 and Aβ40 ratio[Aβ42/Aβ40], and Aβ42/p-tau181 ratio).
Main Outcomes and Measures
Standard uptake value ratios (SUVRs) in 4 predefined regions of interest (ROIs) reflecting Braak staging scheme for tau pathology and encompass I-II (entorhinal cortex), III-IV (inferior/middle temporal, fusiform gyrus, parahippocampal cortex, and amygdala), I-IV, and V-VI (widespread neocortical areas), area under the receiver operating characteristic curve (AUC) values, and subtraction images between [18F]RO948 and [18F]flortaucipir.
Results
Diagnostic groups among the 613 participants included cognitively unimpaired (mean [SD] age, 65.8 [12.1] years; 117 men [46%]), mild cognitive impairment (age, 70.8 [8.3] years; 82 men [53%]), AD dementia (age, 73.5 [6.7] years; 57 men [57%]), and non-AD disorders (age, 70.5 [8.6] years; 41 men [40%]). Retention of [18F]RO948 was higher in AD dementia compared with all other diagnostic groups. [18F]RO948 could distinguish patients with AD dementia from individuals without cognitive impairment and those with non-AD disorders, and the highest AUC was obtained using the I-IV ROI (AUC = 0.98; 95% CI, 0.96-0.99 for AD vs no cognitive impairment and AUC = 0.97; 95% CI, 0.95-0.99 for AD vs non-AD disorders), which outperformed MRI (highest AUC = 0.91 for AD vs no cognitive impairment using whole-brain thickness, and AUC = 0.80 for AD vs non-AD disorders using temporal lobe thickness) and cerebrospinal fluid measures (highest AUC = 0.94 for AD vs no cognitive impairment using Aβ42/p-tau181, and AUC = 0.93 for AD vs non-AD disorders using Aβ42/Aβ40). Generally, tau PET positivity using [18F]RO948 was observed only in Aβ-positive cases or in MAPT R406W mutation carriers. Retention of [18F]RO948 was not pronounced in patients with svPPA, and head-to-head comparison revealed lower temporal lobe uptake than with [18F]flortaucipir.
Conclusions and Relevance
In this study, elevated [18F]RO948 SUVRs were most often seen among Aβ-positive cases, which suggests that [18F]RO948 has high specificity for AD-type tau and highlights its potential as a diagnostic marker in the differential diagnosis of AD.
This diagnostic study examines the use of RO948 F 18 in tau positron emission tomographic imaging as a diagnostic marker for identification of Alzheimer disease compared with magnetic resonance imaging and cerebrospinal fluid measures.
Introduction
Alzheimer disease (AD) is characterized by deposition of amyloid β (Aβ) in senile plaques and hyperphosphorylated tau in neurofibrillary tangles,1 which consist mainly of paired helical filaments (PHFs) comprising a mixture of 3- to 4-repeat tau isoforms.2,3 Tau pathology is also seen in various non-AD neurodegenerative disorders, including progressive supranuclear palsy and other variants of frontotemporal lobar degeneration.4 Tau deposits in these disorders, however, differ from the AD-type PHFs consisting of 3- to 4 repeat tau isoforms.5 In AD, cortical neurofibrillary neurotangles are closely associated with neurodegeneration and onset of cognitive impairment.6
Tau-selective positron emission tomographic (PET) tracers have facilitated in vivo studies of tau pathology in neurodegenerative disorders.7 The most commonly studied tau PET tracer, flortaucipir F 18 ([18F]flortaucipir),8 discriminates AD from other neurodegenerative diseases and outperforms magnetic resonance imaging (MRI).9 However, [18F]flortaucipir has off-target binding in the basal ganglia, brainstem, and choroid plexus10 and has elevated retention in the semantic variant of primary progressive aphasia (svPPA),11 a condition most often associated with TDP-43 type C pathology.12,13 Other tau PET tracers include GTP1 F 18,14 MK6240 F 18,15,16 and RO948 F 18([18F]RO948).17 While structurally similar to [18F]flortaucipir, [18F]RO94818,19,20 has more rapid kinetics and less off-target binding in the basal ganglia.19,21 The diagnostic performance of these second-generation tau PET tracers is not known. To our knowledge, there have been no head-to-head comparisons with other imaging or cerebrospinal fluid (CSF) markers. We tested [18F]RO948 tau PET for discriminating AD from non-AD in patients with neurodegenerative disorders, in individuals with no cognitive impairment, and in comparison with established MRI and CSF markers. We also directly compared [18F]RO948 and [18F]flortaucipir in a subset of patients with svPPA to examine the specificity of both tracers.
Methods
Participants
In this diagnostic study, we included participants in the prospective and longitudinal Swedish BioFINDER-2 study, including individuals without cognitive impairment and patients with mild cognitive impairment (MCI), AD dementia, and non-AD neurodegenerative disorders.22 eMethods 1 in the Supplement presents the inclusion and exclusion criteria. Groups were established without the use of biomarkers, but cognitively unimpaired and MCI participants were subdivided based on Aβ status (CSF, Aβ42/Aβ40; cutoff <0.089, as defined in clinical practice at the Sahlgrenska University Hospital, Mölndal, Sweden). We included only patients with Aβ-positive AD dementia in accordance with the National Institute on Aging-Alzheimer Association research framework.23 In BioFINDER-2, Aβ PET is by design performed only in individuals without cognitive impairment and those with MCI; CSF Aβ42/Aβ40 was thus chosen to have a common measure of Aβ pathology across all enrollees. All participants gave written informed consent; no financial compensation was provided. Ethical approval was given by the Regional Ethical Committee in Lund, Sweden. Approval for PET imaging was obtained from the Swedish Medicines and Products Agency and the local radiation safety committee at Skåne University Hospital, Lund, Sweden. The present study was conducted from September 4, 2017, to August 28, 2019.
Image Acquisition and Processing
Full PET details are described elsewhere24 and included in eMethods 2 in the Supplement. PET using [18F]RO948 was performed on a digital scanner (Discovery MI; GE Healthcare) 70 to 90 minutes following injection (list-mode acquisition). This protocol was chosen over full-dynamic scanning to make PET scanning feasible for the large number of patients recruited into BioFINDER-2, with the acquisition window selected using previous [18F]RO948 work.19 In the patients with svPPA who underwent an additional scan with [18F]flortaucipir, data were acquired 80 to 100 minutes after injection using the same digital scanner.25 Standardized uptake value ratio (SUVR) images were created using the inferior cerebellar cortex as the reference region.26 We report findings uncorrected for partial volume error or corrected (geometric transfer matrix)27 (eTable 3, eTable 4, eFigure 5, and eFigure 6 in the Supplement). A high-resolution T1-weighted MRI was acquired (3T MAGNETOM Prisma; Siemens Healthineers) for PET image coregistration and template normalization.
Region-of-Interest Definition
To cover brain areas affected by neurofibrillary tangle pathology across the course of AD,28,29 4 FreeSurfer-based composite regions of interest (ROIs) were created. These ROIs were originally developed using [18F]flortaucipir28 to approximate the Braak staging scheme for tau pathology29 and encompass I-II (entorhinal cortex), III-IV (inferior/middle temporal, fusiform gyrus, parahippocampal cortex, and amygdala), I-IV,30,31 and V-VI (widespread neocortical areas) (eFigure 1 in the Supplement). Individual tau-imaging ROIs (ie, I-II, III, IV, V, and VI) were also investigated. To examine the [18F]RO948 signal in regions that are comparatively unaffected by neurofibrillary tangle pathology,29 primary sensory and motor cortex ROIs were included. For the head-to-head comparison between [18F]RO948 and [18F]flortaucipir in svPPA, owing to the focal nature of retention with [18F]flortaucipir in patients with svPPA (confined to the anterior temporal lobe, including the white matter), participant-specific ROIs were drawn encompassing voxels showing elevated SUVRs within the temporal lobes, including subcortical white matter (eMethods 3 in the Supplement).
Comparative Diagnostic Performance of [18F]RO948
For comparison with the [18F]RO948 SUVR, 3 predefined MRI markers were selected9: hippocampal volume (adjusted for intracranial volume), cortical thickness within a temporal meta-ROI encompassing regions susceptible to AD (mean thickness in the bilateral entorhinal, inferior/middle temporal, and fusiform cortices),32 and whole-brain cortical thickness. Furthermore, 3 CSF measures were included: p-tau181, Aβ42/Aβ40,33 and Aβ42/p-tau181.34 Cerebrospinal fluid p-tau181, Aβ42, and Aβ40 were quantified using enzyme-linked immunosorbent assays (Innotest, Fujirebio). Additional details can be found in eMethods 4 in the Supplement.
Statistical Analysis
Groups were compared using Kruskal-Wallis or Fisher exact tests. Thresholds for tau PET positivity within composite ROIs were defined using the mean SUVR within a given ROI plus 2.5 SDs among 17 Aβ-negative young controls aged 20 to 40 years (eTable 1 in the Supplement).35 The cutoffs were chosen to slightly favor specificity for AD pathology over sensitivity. Mean [18F]RO948 SUVR images and scatterplots for ROIs are shown in eFigure 2 in the Supplement for these participants. Group differences in the [18F]RO948 SUVR were compared across ROIs (analysis of variance and post hoc t tests) and voxelwise (pairwise t tests, P < .001, k≥100) using SPM, version 12 software (eFigure 3 in the Supplement). Familywise error–corrected maps are shown in eFigure 4 in the Supplement. Owing to the small number of patients with svPPA, [18F]RO948 and [18F]flortaucipir were compared using a voxelwise subtraction analysis as a complement to the ROI-based comparison. Receiver operating characteristic analyses were performed to generate area under the curve (AUC) values (AD dementia and Aβ-positive MCI vs no cognitive impairment and non-AD). Differences in AUCs between modalities (PET, MRI, and CSF) were evaluated using bootstrapping (n = 1000).36,37,38 In addition to AUC, sensitivity and specificity, likelihood ratios (positive and negative), and percentage of agreement between classifiers (ie, diagnosis and [18F]RO948 SUVR: true outcomes and all outcomes) are reported. All analyses were performed in R, version 3.5.3 (R Foundation). Significance was set at a 2-sided level of P < .05.
Results
Participants
A total of 613 participants, including 257 cognitively unimpaired controls (38% Aβ-positive), 154 with MCI (62% Aβ-positive), 100 with AD dementia (100% Aβ-positive), and 102 with a non-AD neurodegenerative disorder (41% Aβ-positive; behavioral variant frontotemporal dementia [n = 12], svPPA [n = 7], dementia with Lewy bodies [n = 25], progressive supranuclear palsy [n = 16], Parkinson disease with or without cognitive impairment [n = 26]), multiple system atrophy [n = 6], and vascular dementia [n = 10]) were included. Baseline characteristics are provided in Table 1 and in the eResults and eTable 2 in the Supplement. Partial volume error–corrected [18F]RO948 PET SUVR data are presented in eTable 3 and eTable 4 in the Supplement. Regarding the high percentage of APOE ε4 carriership among controls, the BioFINDER-2 study, by design, enrolls younger (age, 40-65 years) and older (age, 65-100 years) controls, with the aim to build 2 study populations with 50% APOE ε4 carriers in each. This design was intended to enrich for Aβ pathology to be able to study the very early (preclinical) stages of AD. Details on both [18F]RO948 and [18F]flortaucipir in patients with svPPA are included in eTable 5 in the Supplement.
Table 1. Participant Characteristics.
| Characteristic | Cognitively unimpaired controls (n = 257)a | MCI (n = 154) | AD dementia (n = 100) | Non-AD (n = 102)b |
|---|---|---|---|---|
| Age, mean (SD) [range], y | 65.8 (12.1) [41-89] | 70.8 (8.3) [47-94]c | 73.5 (6.7) [66-87]c,d,e | 70.5 (8.6) [36-87]c |
| Sex, No. (%) | ||||
| Men | 117 (46) | 82 (53) | 57 (57) | 41 (40)f,g |
| Women | 140 (54) | 72 (47) | 43 (43) | 61 (60) |
| Education, mean (SD), y | 12.7 (3.4) | 12.3 (3.5) | 12.2 (3.6) | 12.4 (3.5) |
| MMSE score, mean (SD) | 29 (1.15)h,i,j | 27.1 (2.1)h,j | 20 (4.3) | 25 (4.6)h |
| Aβ status, No. (%) | ||||
| Negative | 159 (62) | 58 (38) | 0 | 60 (59) |
| Positive | 98 (38) | 96 (62) | 100 (100) | 42 (41) |
| APOE ε4 status, No. (%) | ||||
| Negative | 136 (53) | 73 (47) | 32 (32) | 68 (67) |
| Positive | 121 (47) | 81 (53)d | 68 (68) | 34 (33) |
| RO948 F 18 SUVR within tau-imaging ROIs, mean (SD)k | ||||
| Braak stage I-II | 1.16 (0.22) | 1.36 (0.34)e,l | 2.02 (0.40)c,i,j | 1.21 (0.26) |
| Braak stage III-IV | 1.17 (0.15) | 1.30 (0.32)e,l | 2.18 (0.70)c,i,j | 1.20 (0.17) |
| Braak stage I-IV | 1.17 (0.15) | 1.31 (0.30)e,l | 2.19 (0.58)c,i,j | 1.19 (0.15) |
| Braak stage V-VI | 1.06 (0.10) | 1.08 (0.16) | 1.53 (0.21)c,i,j | 1.05 (0.11) |
Abbreviations: Aβ, amyloid β; AD, Alzheimer disease; MCI, mild cognitive impairment; MMSE; Mini-Mental State Examination; ROI, region of interest; SUVR, standard uptake value ratio.
Regarding the high percentage of APOE ε4 carriership among controls, by design, the BioFINDER-2 study enrolls younger (age, 40-65 years) and older (age, 65-100 years) controls, with the aim to build 2 study populations with 50% APOE ε4 carriers in each. This design was intended to enrich for Aβ pathologic characteristics to be able to study the very early (preclinical) stages of AD.
Neurodegenerative disorders other than AD.
Significantly higher than cognitively unimpaired controls, P < .001.
Significantly higher than MCI, P < .05.
Significantly higher than non-AD, P < .01.
Significantly higher than AD dementia, P < .05.
Significantly higher than cognitively unimpaired controls, P < .05.
Significantly higher than AD dementia, P < .001.
Significantly higher than MCI, P < .001.
Significantly higher than non-AD, P < .001.
Braak stage I-II, entorhinal cortex; III-IV, inferior/middle temporal, fusiform gyrus, parahippocampal cortex, and amygdala; I-IV; and V-VI, widespread neocortical.
Significantly higher than cognitively unimpaired controls, P < .01.
[18F]RO948 SUVRs Across Tau-Imaging Regions
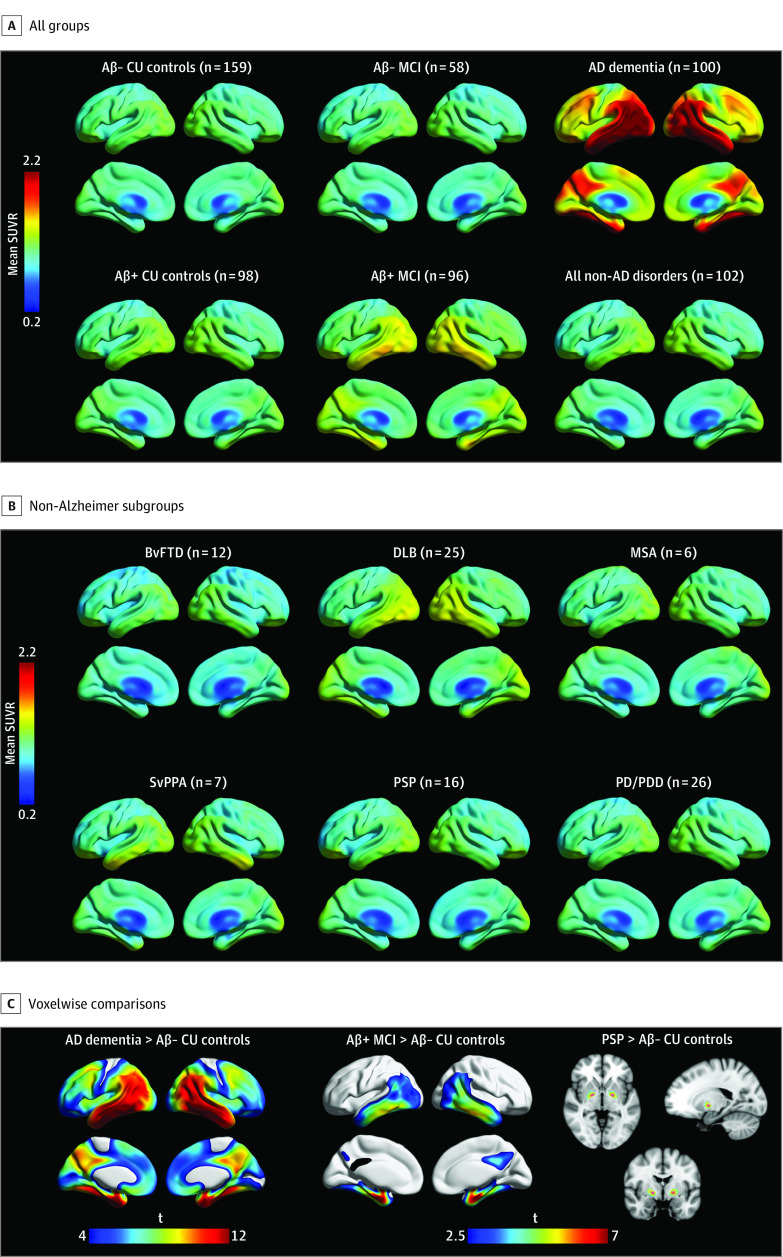
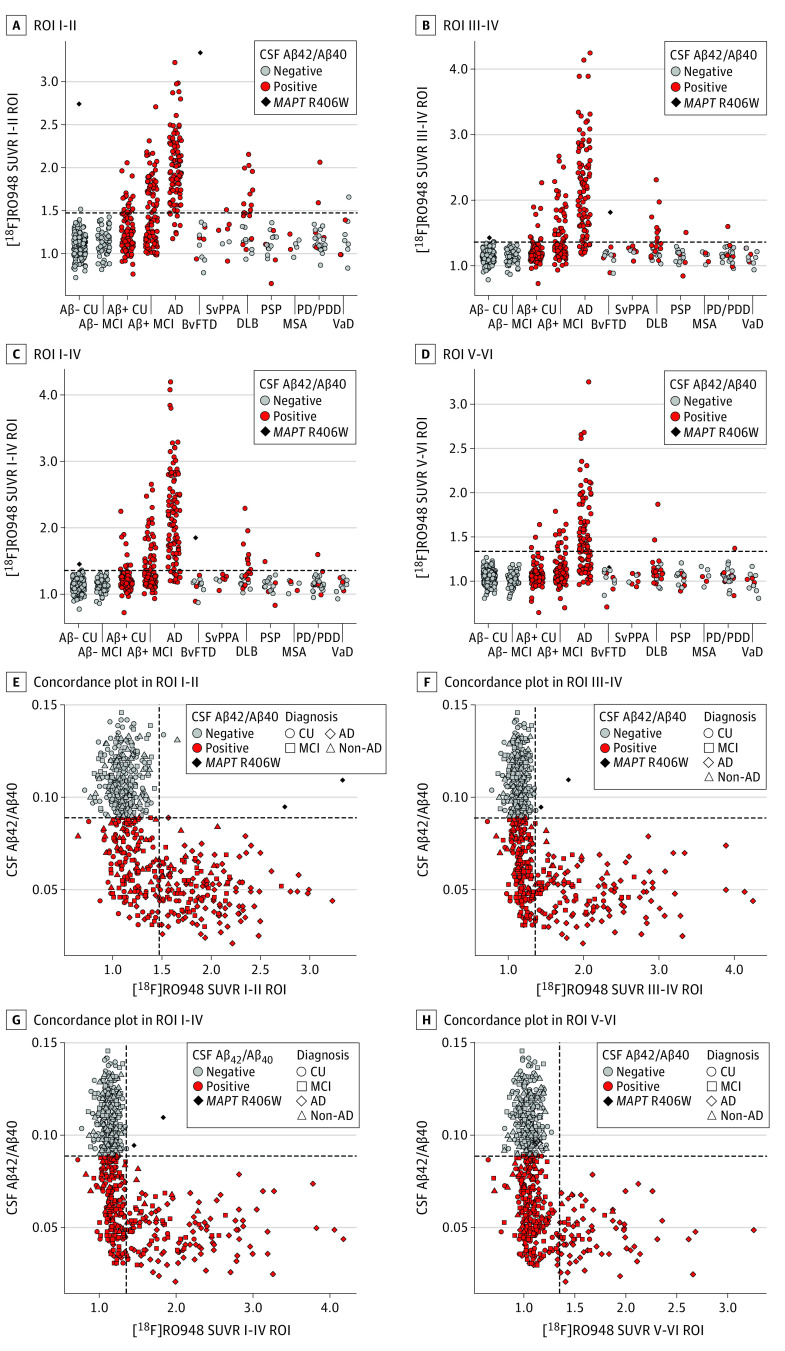
Figure 1A and B show mean [18F]RO948 SUVR images across diagnostic groups. Voxelwise comparisons are shown in Figure 1C and eFigure 3 in the Supplement. Familywise error–corrected results are shown in eFigure 4 in the Supplement. Compared with Aβ-negative cognitively unimpaired controls, [18F]RO948 retention was higher in patients with AD dementia (widespread), Aβ-positive MCI (temporal/parietal cortices), dementia with Lewy bodies (temporal/lateral parietal cortices), svPPA (temporal pole), and progressive supranuclear palsy (globus pallidus) (eFigure 3 in the Supplement). Figure 2A-D show [18F]RO948 SUVRs across all diagnostic groups for the 4 different tau-imaging ROIs (I-II, III-IV, I-IV, and V-VI). Irrespective of clinical diagnosis, tau PET positivity was almost exclusively observed in Aβ-positive individuals. Exceptions included MAPT R406W mutation carriers: one individual without cognitive impairment and one patient with vascular dementia with isolated tau PET positivity in the I-II ROI (entorhinal cortex). In contrast with the findings of previous studies with [18F]flortaucipir,11,39,40,41 no patient with svPPA was tau PET positive across the cortical ROIs (Figure 2). Results were essentially the same when using partial volume error–corrected [18F]RO948 SUVR data (eFigure 5 and eFigure 6 in the Supplement). The [18F]RO948 SUVRs using more liberal cutoffs and by age are shown in eFigure 7 and eFigure 8 in the Supplement, and [18F]RO948 SUVRs in primary sensory and motor cortices are shown in eFigure 9 in the Supplement.
Figure 1. Mean RO948 F 18 ([18F]RO948) Standardized Uptake Value Ratio (SUVR) Images Across Diagnostic Groups.
A, Cognitively unimpaired (CU) control individuals and patients with mild cognitive impairment (MCI) and Alzheimer disease (AD) dementia. AD dementia was greater than amyloid β (Aβ)-negative CU controls and Aβ-positive MCI. B, Non-AD subgroups compared with Aβ-negative CU controls. Aβ vs APOE ε4 status (% positive) by non-AD subgroup was as follows: behavioral variant of frontotemporal dementia (BvFTD) (50% vs 33%), dementia with Lewy bodies (DLB) (60% vs 52%), multiple system atrophy (MSA) (33% vs 33%), semantic variant primary progressive aphasia (svPPA) (71% vs 17%), progressive supranuclear palsy (PSP) (38% vs 31%), and Parkinson disease/Parkinson disease dementia (PD/PDD) (23% vs 27%). C, Voxelwise group differences in [18F]RO948 SUVRs. AD dementia, Aβ-positive MCI, and PSP are compared with Aβ-negative CU controls. A cluster threshold of 100 voxels was applied with no correction for multiple comparisons (P < .001). t indicates t value.
Figure 2. Mean RO948 F 18 ([18F]RO948) Standardized Uptake Value Ratios (SUVRs) Across Diagnostic Groups Within Tau-Imaging Regions of Interest (ROIs).
ROI groupings were I-II (A), III-IV (B), I-IV (C), and V-VI (D). Concordance plots are shown between the [18F]RO948 SUVR and cerebrospinal fluid (CSF) amyloid β42 and β40 (Aβ42/Aβ40) for ROIs I-II (E), III-IV (F), I-IV (G), and V-VI (H). The horizontal dashed lines indicate the cutoffs for tau positivity across the ROIs, defined using the mean plus 2.5 SDs in Aβ-negative young controls (I-II ROI>1.48; III-IV and I-IV ROIs >1.36; V-VI ROI>1.35). The vertical dashed line indicates the cutoff for Aβ-positivity (CSF Aβ42/Aβ40<0.089, as established in an independent population by the neurochemistry laboratory at the Sahlgrenska University Hospital, Mölndal, Sweden). Abbreviation expansions appear in caption to Figure 1. VaD indicates vascular dementia.
AD Dementia vs Non-AD Disorders
The [18F]RO948 SUVRs could distinguish AD dementia from non-AD disorders (Table 2). Group separation was best using the I-IV ROI, with an AUC of 0.97 (95% CI, 0.95-0.99), 91.3% (95% CI, 87.2%-94.9%) correctly classified, sensitivity of 91.9% (95% CI, 85.9%-97.0%), and specificity of 90.6% (95% CI, 84.4%-95.8%). Comparable findings were observed using the I-II and III-IV ROIs. While the V-VI ROI showed a similarly high AUC (0.92; 95% CI, 0.88-0.96), the percentage of correctly classified participants was lower (78.5%; 95% CI, 73.3%-83.6%) owing to a clearly lower sensitivity of 59.6% (95% CI, 50.5%-69.7%) but with only somewhat better specificity (97.9%; 95% CI, 94.8%-100%). Positivity in the V-VI ROI had a higher positive likelihood ratio (19.07; 95% CI, 6.19-58.77) compared with the other ROIs (range, 6.79-8.73). The diagnostic performance of [18F]RO948 when separating AD from different types of non-AD disorders was high except when distinguishing AD from dementia with Lewy bodies, where specificity was low (70.0%; 95% CI, 52.3%-87.0%) (eTable 6 in the Supplement). Considering that mixed dementia with Lewy bodies and AD are common,42 results obtained when the dementia with Lewy bodies group is subdivided by Aβ status are shown in eFigure 10 and eFigure 11 in the Supplement. Furthermore, the diagnostic performance of [18F]RO948 using SUVRs in individual tau-imaging ROIs is summarized in eTable 7 in the Supplement. Diagnostic performance for tau-imaging ROIs using lower cutoffs for [18F]RO948 SUVRs (mean [SD] +1.5 [2] in Aβ-negative young controls) are summarized in eTable 8 and eFigure 7 in the Supplement.
Table 2. Diagnostic Performance of RO948 F 18 PET in Distinguishing AD Dementia and Aβ-Positive Mild Cognitive Impairment From Non-AD Neurodegenerative Disorders.
| Tau-imaging ROI | Cutoffa | Performance (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| AUC | Agreement | Sensitivity | Specificity | Likelihood ratio | |||
| Positive | Negative | ||||||
| AD dementia (n = 100) vs cognitively unimpaired controls (n = 257)b | |||||||
| Braak stage I-II | >1.48 | 0.97 (0.95-0.98) | 92.3 (89.2-94.8) | 91.9 (85.9-97.0) | 92.4 (88.9-95.4) | 11.31 (7.40-17.28) | 0.09 (0.05-0.17) |
| Braak stage III-IV | >1.36 | 0.97 (0.95-0.99) | 92.5 (89.9-95.3) | 85.9 (78.8-91.9) | 95.1 (92.4-97.7) | 15.26 (9.13-25.53) | 0.14 (0.08-0.23) |
| Braak stage I-IV | >1.36 | 0.98 (0.96-0.99) | 93.9 (91.4-96.4) | 91.0 (84.9-96.0) | 95.1 (92.4-97.3) | 17.20 (10.10-29.31) | 0.10 (0.05-0.18) |
| Braak stage V-VI | >1.35 | 0.91 (0.87-0.95) | 87.9 (85.1-90.6) | 59.6 (49.9-69.7) | 98.5 (97.0-99.6) | 48.87 (15.69-152.24) | 0.41 (0.32-0.52) |
| AD dementia (n = 100) vs non-AD disorders (n = 102)b,c | |||||||
| Braak stage I-II | >1.48 | 0.96 (0.93-0.99) | 90.3 (86.1-94.4) | 92.9 (87.9-97.0) | 87.5 (80.0-93.8) | 6.79 (4.08-11.29) | 0.09 (0.05-0.18) |
| Braak stage III-IV | >1.36 | 0.96 (0.94-0.98) | 88.7 (84.2-92.9) | 86.9 (79.8-92.9) | 90.6 (84.4-95.8) | 8.34 (4.62-15.07) | 0.15 (0.09-0.24) |
| Braak stage I-IV | >1.36 | 0.97 (0.95-0.99) | 91.3 (87.2-94.9) | 91.9 (85.9-97.0) | 90.6 (84.4-95.8) | 8.73 (4.84-15.74) | 0.10 (0.05-0.19) |
| Braak stage V-VI | >1.35 | 0.92 (0.88-0.96) | 78.5 (73.3-83.6) | 59.6 (50.5-69.7) | 97.9 (94.8-100) | 19.07 (6.19-58.77) | 0.42 (0.33-0.53) |
| Aβ-positive MCI (n = 96) vs cognitively unimpaired controls (n = 257)b | |||||||
| Braak stage I-II | >1.48 | 0.78 (0.72-0.84) | 80.6 (77.3-83.9) | 46.9 (37.5-56.3) | 92.6 (89.6-95.6) | 5.77 (3.60-9.23) | 0.58 (0.48-0.70) |
| Braak stage III-IV | >1.36 | 0.77 (0.72-0.83) | 79.8 (76.5-82.8) | 36.5 (27.1-45.8) | 95.2 (92.6-97.4) | 6.41 (3.61-11.36) | 0.67 (0.58-0.79) |
| Braak stage I-IV | >1.36 | 0.80 (0.75-0.85) | 80.1 (76.8-83.1) | 37.5 (28.1-47.0) | 95.2 (92.6-97.8) | 12.22 (7.06-21.17) | 0.37 (0.28-0.49) |
| Braak stage V-VI | >1.35 | 0.59 (0.52-0.66) | 76.2 (74.3-78.1) | 13.0 (6.3-18.8) | 99.0 (97.0-100) | 11.06 (3.22-37.95) | 0.88 (0.81-0.95) |
| Aβ-positive MCI (n = 96) vs non-AD disorders (n = 102)b,c | |||||||
| Braak stage I-II | >1.48 | 0.72 (0.65-0.79) | 68.0 (61.4-73.6) | 47.0 (37.1-57.3) | 87.1 (80.2-94.1) | 3.46 (2.00-5.99) | 0.61 (0.50-0.75) |
| Braak stage III-IV | >1.36 | 0.71 (0.64-0.78) | 64.0 (58.4-70.0) | 36.5 (27.1-45.8) | 90.1 (84.2-95.1) | 3.50 (1.84-6.66) | 0.71 (0.60-0.84) |
| Braak stage I-IV | >1.36 | 0.73 (0.66-0.80) | 65.0 (59.0-70.1) | 37.5 (28.1-46.9) | 90.1 (84.2-95.1) | 6.20 (3.39-11.35) | 0.40 (0.30-0.52) |
| Braak stage V-VI | >1.35 | 0.59 (0.53-0.69) | 56.0 (52.3-60.0) | 13.0 (6.3-19.8) | 97.0 (94.1-100) | 4.33 (1.28-14.72) | 0.89 (0.82-0.97) |
Abbreviations: Aβ, amyloid β; AD, Alzheimer disease; AUC, area under the curve; MCI, mild cognitive impairment; PET, positron emission tomographic; ROI, region of interest; SUVR, standardized uptake value ratio.
Thresholds for tau PET positivity within composite ROIs were established using the mean SUVR within a given ROI plus 2.5 SDs among 17 Aβ-negative young (age, 20-40) control individuals.
Braak stage I-II, entorhinal cortex; III-IV, inferior/middle temporal, fusiform gyrus, parahippocampal cortex, and amygdala; I-IV; and V-VI, widespread neocortical.
Neurodegenerative disorders other than AD.
Aβ-Positive MCI vs Non-AD Disorders
Compared to the AD dementia group, the discriminative ability of the [18F]RO948 SUVR across ROIs was, as expected, lower for the separation of Aβ-positive MCI from the non-AD group (Table 2). Using the I-IV ROI, which provided the best separation for both contrasts, the AUC for Aβ-positive MCI vs the non-AD group was 0.73 (95% CI, 0.66-0.80), the percentage of correctly classified participants was 65.0% (95% CI, 59.0%-70.1%), sensitivity was 37.5% (95% CI, 28.1%-46.9%), and specificity was 90.1% (95% CI, 84.2%-95.1%).
[18F]RO948 vs MRI and CSF Markers
Figure 3A shows that the highest AUC was obtained using the I-IV ROI (0.98; 95% CI, 0.96-0.99), which outperformed MRI (highest AUC = 0.91 (95% CI, 0.86-0.93) using whole-brain cortical thickness) and CSF measures (highest AUC = 0.94, 95% CI, 0.91-0.96) using Aβ42/p-tau181. Figure 3B shows that AUCs for [18F]RO948 (I-II ROI: 0.96; 95% CI, 0.93-0.99; I-IV ROI: 0.97; 95% CI, 0.95-0.99; and V-VI ROI: 0.92; 95% CI, 0.88-0.96) were significantly higher than those for MRI (AUC hippocampal volume: 0.65; 95% CI, 0.58-0.73; temporal lobe thickness: 0.80; 95% CI, 0.73-0.86; and whole-brain thickness: 0.67; 95% CI, 0.60-0.75; P < .001 for all comparisons). The AUCs were also significantly higher than the CSF AD biomarkers (p-tau181: 0.92; 95% CI, 0.88-0.95; P < .05; Aβ42/Aβ40: 0.93; 95% CI, 0.89-0.96; P < .05; and Aβ42/p-tau181: 0.92; 95% CI, 0.88-0.97; P < .05 compared with all [18F]RO948 ROIs) for distinguishing AD dementia from non-AD. When comparing patients with AD dementia with both controls with no cognitive impairment and patients with non-AD disorders, [18F]RO948 SUVR measures also carried higher specificity (Table 2; eTable 9 in the Supplement). In participants with AD dementia vs controls with no cognitive impairment, the [18F]RO948 SUVR within the I-IV ROI had a specificity of 95.1% (95% CI, 92.4%-97.3%) compared with 63.9% (95% CI, 58.2%-69.6%) for CSF. With Aβ42/Aβ40, similar findings were seen for AD dementia vs non-AD with a specificity of the [18F]RO948 SUVR within the I-IV ROI of 90.6% (95% CI, 84.4%-95.8%) compared with 61.5% (95% CI, 52.1%-70.8%) for Aβ42/Aβ40. Similar findings also were obtained when looking at Aβ-positive MCI. For the contrast Aβ-positive MCI vs non-AD disorders, AUCs for [18F]RO948 were higher than for MRI (range, [18F]RO948 ROIs, 0.59-0.73; range, MRI, 0.51-0.59; P < .001 for all comparisons), but CSF ratio measures provided the best group separation (Aβ42/Aβ40, 0.89 [95% CI, 0.84-0.93]; Aβ42/p-tau181, 0.83 [95% CI, 0.79-0.88]). Cerebrospinal fluid p-tau181 by diagnostic group and its association with the [18F]RO948 SUVR in tau-imaging ROIs are shown in eFigure 12 and eFigure 13 in the Supplement. The III-IV region, omitted owing to its similar performance to I-IV, had the following AUC values: AD dementia vs cognitively unimpaired controls, AUC = 0.97 (95% CI, 0.95-0.99); AD dementia vs non-AD, AUC = 0.96 (95% CI, 0.94-0.98); Aβ-positive MCI vs cognitively unimpaired controls, AUC = 0.77 (95% CI, 0.72-0.83); and Aβ-positive MCI vs non-AD, AUC = 0.71 (95% CI, 0.64-0.78).
Figure 3. Plots From Receiver Operating Characteristic Analyses for RO948 F 18 ([18F]RO948) Tau Positron Emission Tomographic (PET) Tracers, Magnetic Resonance Imaging (MRI), and Cerebrospinal Fluid (CSF) Measures for Distinguishing Alzheimer Disease (AD) Dementia and Amyloid β (Aβ)-Positive Mild Cognitive Impairment (MCI) From Cognitively Unimpaired Controls and Non-AD Neurodegenerative Disorders.
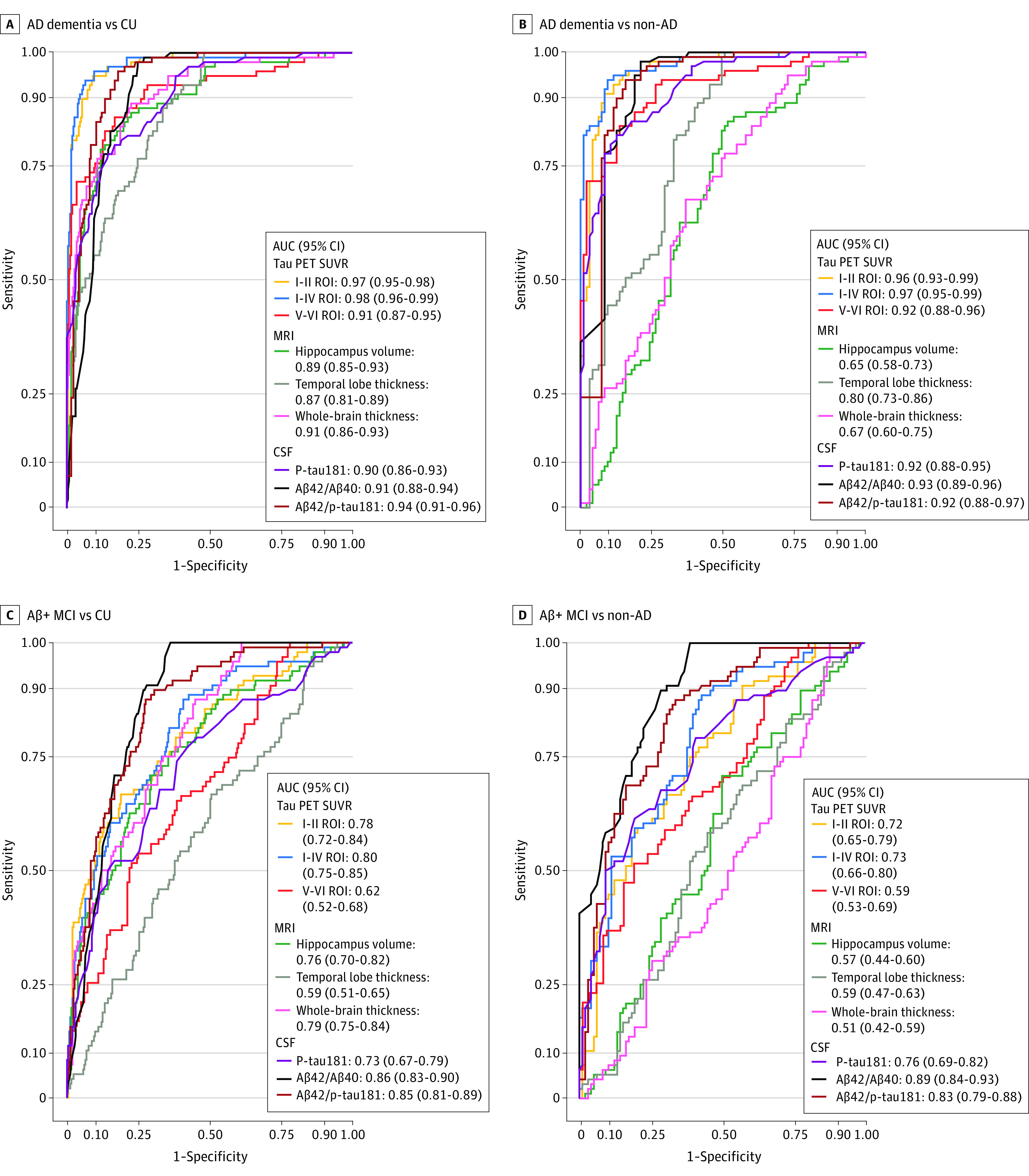
Receiver operating characteristic curves are shown for the following groups: AD dementia vs cognitively unimpaired (CU) controls (A) and non-AD disorders (B), and Aβ-positive MCI vs CU controls (C) and non-AD disorders (D). The III-IV region of interest, omitted owing to its similar performance to I-IV, had the following area under the curve (AUC) values: AD dementia vs cognitively unimpaired controls, AUC = 0.97 (95% CI, 0.95-0.99); AD dementia vs non-AD: AUC = 0.96 (95% CI, 0.94-0.98); Aβ-positive MCI vs cognitively unimpaired controls: AUC = 0.77 (95% CI, 0.72-0.83); and Aβ-positive MCI vs non-AD: AUC = 0.71 (95% CI, 0.65-0.78). ROI indicates region of interest; SUVR, standardized uptake value ratio.
[18F]RO948 and [18F]Flortaucipir SUVRs in svPPA
All 7 patients with svPPA exhibited low retention of [18F]RO948 (Figure 2). Visual inspection of [18F]RO948 and [18F]flortaucipir SUVR images in the 3 patients with svPPA who underwent PET studies with both tracers suggested higher retention of [18F]flortaucipir (eFigure 14A in the Supplement). This finding was confirmed by a voxelwise subtraction analysis that showed [18F]flortaucipir SUVRs to be higher than those for [18F]RO948 ([18F]flortaucipir − [18F]RO948), particularly within the anterior temporal lobe bilaterally (eFigure 14B in the Supplement). The reverse operation ([18F]R0948 − ([18F]flortaucipir) showed higher SUVR values for [18F]R0948 only within the retina and skull/meninges (eFigure 14C in the Supplement). Higher retention of [18F]flortaucipir was further supported by comparison of SUVR values within the anterior temporal lobe ROI (eTable 10 in the Supplement).
Retention of [18F]RO948 in the Skull/Meninges
Elevated retention of [18F]R0948 in the skull/meninges (confluent signal on visual inspection, with SUVR>2.5)24 was seen in 4.4% of BioFINDER-2 participants (eTable 11 in the Supplement). Furthermore, to assess the association between this off-target signal and diagnostic performance, receiver operating characteristic analyses were repeated, excluding participants with high SUVRs in the skull/meninges. Resulting AUC values did not differ significantly from those including all participants (eTable 11 in the Supplement).
Discussion
Overall findings with [18F]RO948 supported in vitro findings of high specificity of [18F]RO948 for AD-type neurofibrillary tangles.18,20 Within the I-II (entorhinal cortex) ROI, primary age-related tauopathy43 may account for the participants (1 with no cognitive impairment and 1 with vascular dementia) who showed tau PET positivity using [18F]RO948 (hypothetically, since primary age-related tauopathy cannot be diagnosed during life). Across limbic (III-IV and I-IV) and neocortical (V-VI) ROIs, tau positivity was seen in Aβ-positive cases and in 2 carriers of an MAPT R406W mutation associated with the formation of AD-like PHFs.44 In general, [18F]RO948 did not bind significantly in the included non-AD disorders. Some cortical signal was seen in dementia with Lewy bodies, but this signal appeared to be associated with Aβ-positive cases. Alzheimer disease is a frequent concomitant pathology in dementia with Lewy bodies,42,45 possibly owing to the cross-seeding of α-synuclein, Aβ, and tau pathologies.46,47
Imaging with Aβ PET and CSF ratios including Aβ42 shows high sensitivity to Aβ pathology, changing approximately 15 to 25 years before the onset of cognitive decline.48,49 Owing to the high prevalence of Aβ positivity in cognitively unimpaired, older, healthy individuals and in older persons with non-AD neurodegenerative diseases, Aβ biomarkers are generally used to rule out—as opposed to rule in—AD as the cause of cognitive impairment.50 Indeed, Aβ positivity has been shown to increase with age in most non-AD disorders, in which Aβ may be a secondary condition.45 In a recent study,9 [18F]flortaucipir showed greater discriminative accuracy in older populations compared with Aβ biomarkers. Our finding of higher specificity for [18F]RO948 than for CSF Aβ42/Aβ40, when distinguishing AD dementia from no cognitive impairment and non-AD diseases, is consistent with this study and earlier findings that tau PET positivity is associated with the onset of cognitive decline and dementia.51 Together, these findings appear to support the notion that tau PET is a valuable addition to Aβ biomarkers in the workup of early-onset dementia and as a stand-alone test in patients with late-onset dementia where Aβ pathology is frequent. A single tau PET scan may preclude the need for Aβ PET or CSF AD biomarkers, as well as fluorodeoxyglucose F 18 PET, in the diagnostic workup of amnestic dementia (eFigure 15 in the Supplement) given the finding that tau PET positivity was virtually confined to Aβ-positive cases and studies showing a high overlap between tau PET and hypometabolism.52,53
The diagnostic performance of [18F]RO948 was lower when differentiating Aβ-positive MCI from other conditions compared with AD dementia (Figure 1). Tau PET may thus have greater value for differential diagnosis of dementia as opposed to early disease where CSF biomarkers may have better sensitivity.54 Longitudinal studies are needed to address which biomarker (tau PET, Aβ PET, or CSF AD biomarkers) is best for estimation of progression to AD dementia in MCI.
Compared with [18F]RO948, MRI and CSF measures performed less well in distinguishing AD dementia from non-AD disorders. The CSF AD biomarkers, including CSF Aβ42, and the ratios including Aβ42, may plateau early in the course of the disease and best act as disease state markers, reflecting the intensity of the disease process.54 Tau PET and MRI-based atrophy measures, by contrast, change in a somewhat continuous fashion throughout the disease, reflecting disease progression.54 Tau accumulation and cortical atrophy are offset processes, however, with PHF tau thought to accumulate upstream from neurodegeneration.55,56 As such, the accumulation of tau aggregates may be the most dynamic of the 3 processes within the present cohort, with CSF markers already fully changed in participants without cognitive impairment but with Aβ pathology. While our findings are consistent with earlier work showing that tau PET outperformed MRI in differentiating AD from non-AD disorders,9 the present study is, to our knowledge, the first to report findings from a large number of individuals using a second-generation tau tracer and directly compare the diagnostic performance of tau PET, MRI, and CSF markers for AD vs other neurodegenerative disorders. The optimal diagnostic marker may depend on disease phase. Tau PET may prove best for the differentiation of early AD dementia from other neurodegenerative disorders, and CSF and Aβ PET may better aid differential diagnosis in preclinical and prodromal AD.
On the basis of postmortem studies, TDP-43 type C pathology is the primary substrate of svPPA in most patients, although AD and Pick disease have also been shown as causes.12,57,58,59 Several in vivo PET studies9,11,39,40 have identified increased retention of [18F]flortaucipir in svPPA despite autoradiographic studies showing minimal to no binding of [18F]flortaucipir to TDP-43 pathology.60,61,62 Off-target binding to a neurodegenerative process other than tau has been proposed as an explanation,11 as has binding to monoamine oxidase B expressed by reactive astrocytes,63 although in vivo findings are mixed with respect to this hypothesis.39,40,64,65 Although the lower signal seen with [18F]RO948 suggests a more favorable specificity profile compared with [18F]flortaucipir, this observation can only be considered preliminary owing to the lack of neuropathologic confirmation of underlying TDP-43 pathology in the included svPPA cases and the fact that an [18F]RO948 signal was seen, albeit at a low level. Although [18F]RO948 has been shown to have negligible retention in vivo in the basal ganglia, thalamus, and choroid plexus,21 retention in the skull/meninges—a feature seen in the subtraction analysis—has been reported21,24 and has also been shown to affect other tau tracers, such as MK6240 F 18.15,16 Although the cause of this off-target binding is unclear, the frequency of elevated [18F]RO948 SUVRs in the skull/meninges was relatively low and has been shown to be stable over time.24 In the present study, the diagnostic performance of [18F]RO948 did not improve after participants with high off-target binding were removed.
Strengths and Limitations
Strengths of this study include the large sample size, the use of a novel tau tracer with image acquisition performed in a single PET center, and the multiple imaging and fluid biomarkers used for comparisons of diagnostic performance. This study has limitations. One limitation was that clinical diagnosis was considered to be the reference standard, as autopsy data were not available. Because the clinical diagnosis of AD may be inaccurate,66 the standard used for comparison in the receiver operating characteristic analysis was suboptimal. Furthermore, the number of participants in several of the non-AD subgroups was small (eg, multiple system atrophy and vascular dementia). Findings in these groups should be considered preliminary. Cerebrospinal fluid Aβ42/Aβ40 vs Aβ PET was used to define Aβ status. This difference is unlikely to have biased group assignment (Aβ-positive/Aβ-negative) considering the high concordance with Aβ PET.67 Consensus is lacking regarding the optimal approach to determine cutoffs for tau PET SUVRs. Our approach was conservative and consistent with those used in comparable studies.35 The generalizability of our findings to additional second-generation tau tracers remains uncertain until similar studies are performed using these compounds.
Conclusions
The findings suggest that [18F]RO948 PET was able to discriminate AD dementia from other neurodegenerative diseases in a memory clinic setting and was superior to CSF p-tau181, Aβ42/Aβ40, Aβ42/p-tau181, and MRI measures. The results suggest that [18F]RO948 has high specificity for AD-type PHF tau pathology outside the medial temporal lobe that is seen only in the context of Aβ positivity and certain MAPT mutations.
eMethods 1. Inclusion and Exclusion Criteria for the Swedish BioFINDER 2 Study
eMethods 2. Additional Details About [18F]RO948 Preprocessing
eMethods 3. Temporal Regions of Interest in Semantic Variant Primary Progressive Aphasia
eMethods 4. Additional Details About CSF Procedures
eTable 1. Demographics of Young (Age 20-40) Aβ-Negative Controls Used to Set [18F]RO948 SUVR Cutoffs
eResults. Participant Characteristics
eTable 2. Demographics for Non-Alzheimer Disease Subgroups
eTable 3. Partial Volume Error Corrected [18F]RO948 SUVR Data for Cognitively Unimpaired Controls and Mild Cognitive Impairment, Alzheimer Disease Dementia and Non-Alzheimer Disease Disorder Patients
eTable 4. Partial Volume Error Corrected [18F]RO948 SUVR Data for Non-AD Subgroups
eTable 5. Demographics for svPPA Cases
eTable 6. Diagnostic Performance of [18F]RO948 SUVR Using the Tau-Imaging I-IV ROI for AD Dementia and Aβ-Positive MCI vs Non-AD Disorders
eTable 7. Diagnostic Performance of [18F]RO948 SUVR Using Individual Tau Imaging ROIs
eTable 8. AUC Values for [18F]RO948 SUVR Using Different Cutoffs
eTable 9. Diagnostic Performance of CSF Aβ42/Aβ40 for AD Dementia and Aβ Positive MCI vs Other Non-AD Disorders and CU Controls
eTable 10. [18F]Flortaucipir and [18F]RO948 SUVR Values in Temporal and Primary Somatosensory Cortex ROIs for Semantic Variant Primary Progressive Aphasia
eTable 11. Area Under the Receiver Operating Characteristic Curve Values for [18F]RO948 SUVR in Tau Imaging ROIs With and Without Subjects Showing High Skull/Meningeal Signal
eFigure 1. Tau PET Imaging Composite ROIs Approximating the Braak Post-Mortem Staging Scheme for Tau Pathology
eFigure 2. Mean [18F]RO948 Images and Scatterplots for the Young (Age 20-40) Aβ-Negative Controls Used to Set Cutoffs for [18F]RO948 SUVR Across Tau-Imaging ROIs
eFigure 3. Voxelwise Group Differences in [18F]RO948 SUVR
eFigure 4. Voxelwise Group Differences in [18F]RO948 SUVR Using Family Wise Error Corrected Data
eFigure 5. Partial Volume Corrected [18F]RO948 Standardized Uptake Values Ratios (SUVRs) Across Diagnostic Groups Within Tau-Imaging ROIs
eFigure 6. Concordance Plots Between Partial Volume Corrected [18F]RO948 Standardized Uptake Values Ratios (SUVRs) and CSF Aβ42/Aβ40
eFigure 7. [18F]RO948 SUVR Across Tau-Imaging ROIs Using Lower Cutoffs
eFigure 8. [18F]RO948 SUVR Across Tau-Imaging ROIs by Age (Above and Below 65)
eFigure 9. [18F]RO948 SUVR in Primary Somatosensory and Motor Cortices
eFigure 10. Mean [18F]RO948 Standardized Uptake Values Ratios (SUVR) Across Diagnostic Groups (DLB Subdivided by Aβ-Status) Within Tau-Imaging ROIs
eFigure 11. Plots From Receiver Operating Characteristic Analyses ([18F]RO948, MRI- and CSF-Measures) for Distinguishing AD Dementia and Aβ-Positive MCI Fm Non-AD Neurodegenerative Disorders
eFigure 12. CSF P-Tau181 Levels by Diagnostic Group
eFigure 13. CSF P-Tau181 Levels Across Tau-Imaging ROIs
eFigure 14. [18F]RO948 and [18F]Flortaucipir PET in Semantic Variant Primary Progressive Aphasia
eFigure 15. Decision Tree Outlining the Potential Clinical Utility of Tau-PET Imaging Across Different Dementia Disorders, Including Alzheimer Disease
References
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387-403. doi: 10.1016/S0140-6736(06)69113-7 [DOI] [PubMed] [Google Scholar]
- 2.Jellinger KA, Bancher C. Neuropathology of Alzheimer’s disease: a critical update. J Neural Transm Suppl. 1998;54:77-95. doi: 10.1007/978-3-7091-7508-8_8 [DOI] [PubMed] [Google Scholar]
- 3.Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7(8):656-664. doi: 10.2174/156720510793611592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121-1159. doi: 10.1146/annurev.neuro.24.1.1121 [DOI] [PubMed] [Google Scholar]
- 5.Goedert M. Tau filaments in neurodegenerative diseases. FEBS Lett. 2018;592(14):2383-2391. doi: 10.1002/1873-3468.13108 [DOI] [PubMed] [Google Scholar]
- 6.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C; Medical Research Council Cognitive Function and Ageing Study . Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302-2309. doi: 10.1056/NEJMoa0806142 [DOI] [PubMed] [Google Scholar]
- 7.Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol. 2015;14(1):114-124. doi: 10.1016/S1474-4422(14)70252-2 [DOI] [PubMed] [Google Scholar]
- 8.Schöll M, Maass A, Mattsson N, et al. Biomarkers for tau pathology. Mol Cell Neurosci. 2019;97:18-33. doi: 10.1016/j.mcn.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320(11):1151-1162. doi: 10.1001/jama.2018.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamura N, Harada R, Ishiki A, Kikuchi A, Nakamura T, Kudo Y. The development and validation of tau PET tracers: current status and future directions. Clin Transl Imaging. 2018;6(4):305-316. doi: 10.1007/s40336-018-0290-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith R, Santillo AF, Waldö ML, et al. 18F-flortaucipir in TDP-43 associated frontotemporal dementia. Sci Rep. 2019;9(1):6082. doi: 10.1038/s41598-019-42625-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann DMA, Snowden JS. Frontotemporal lobar degeneration: pathogenesis, pathology and pathways to phenotype. Brain Pathol. 2017;27(6):723-736. doi: 10.1111/bpa.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122(1):111-113. doi: 10.1007/s00401-011-0845-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanabria Bohórquez S, Marik J, Ogasawara A, et al. [18F]GTP1 (Genentech tau probe 1), a radioligand for detecting neurofibrillary tangle tau pathology in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2019;46(10):2077-2089. doi: 10.1007/s00259-019-04399-0 [DOI] [PubMed] [Google Scholar]
- 15.Pascoal TA, Shin M, Kang MS, et al. In vivo quantification of neurofibrillary tangles with [18F]MK-6240. Alzheimers Res Ther. 2018;10(1):74. doi: 10.1186/s13195-018-0402-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betthauser TJ, Cody KA, Zammit MD, et al. In vivo characterization and quantification of neurofibrillary tau PET radioligand 18F-MK-6240 in humans from Alzheimer disease dementia to young controls. J Nucl Med. 2019;60(1):93-99. doi: 10.2967/jnumed.118.209650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leuzy A, Chiotis K, Lemoine L, et al. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol Psychiatry. 2019;24(8):1112-1134. doi: 10.1038/s41380-018-0342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honer M, Gobbi L, Knust H, et al. Preclinical evaluation of 18F-RO6958948, 11C-RO6931643, and 11C-RO6924963 as novel PET radiotracers for imaging tau aggregates in Alzheimer disease. J Nucl Med. 2018;59(4):675-681. doi: 10.2967/jnumed.117.196741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong DF, Comley RA, Kuwabara H, et al. Characterization of 3 novel tau radiopharmaceuticals, 11C-RO-963, 11C-RO-643, and 18F-RO-948, in healthy controls and in Alzheimer subjects. J Nucl Med. 2018;59(12):1869-1876. doi: 10.2967/jnumed.118.209916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobbi LC, Knust H, Körner M, et al. Identification of three novel radiotracers for imaging aggregated tau in Alzheimer’s disease with positron emission tomography. J Med Chem. 2017;60(17):7350-7370. doi: 10.1021/acs.jmedchem.7b00632 [DOI] [PubMed] [Google Scholar]
- 21.Kuwabara H, Comley RA, Borroni E, et al. Evaluation of 18F-RO-948 PET for quantitative assessment of tau accumulation in the human brain. J Nucl Med. 2018;59(12):1877-1884. doi: 10.2967/jnumed.118.214437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Swedish BioFINDER 2 Study (BioFINDER2). ClinicalTrials.gov Identifier: NCT03174938. Updated April 18, 2018. Accessed February 1, 2020. https://clinicaltrials.gov/ct2/show/NCT03174938
- 23.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith R, Schöll M, Leuzy A, et al. Head-to-head comparison of tau positron emission tomography tracers [18F]flortaucipir and [18F]RO948. Eur J Nucl Med Mol Imaging. 2020;47(2):342-354. doi: 10.1007/s00259-019-04496-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn A, Schain M, Erlandsson M, et al. Modeling strategies for quantification of in vivo 18F-AV-1451 binding in patients with tau pathology. J Nucl Med. 2017;58(4):623-631. doi: 10.2967/jnumed.116.174508 [DOI] [PubMed] [Google Scholar]
- 26.Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief. 2017;15:648-657. doi: 10.1016/j.dib.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39(5):904-911. [PubMed] [Google Scholar]
- 28.Cho H, Choi JY, Hwang MS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80(2):247-258. doi: 10.1002/ana.24711 [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 30.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50-95 years: a cross-sectional study. Lancet Neurol. 2017;16(6):435-444. doi: 10.1016/S1474-4422(17)30077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13(3):205-216. doi: 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR Jr, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138(pt 12):3747-3759. doi: 10.1093/brain/awv283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janelidze S, Pannee J, Mikulskis A, et al. Concordance between different amyloid immunoassays and visual amyloid positron emission tomographic assessment. JAMA Neurol. 2017;74(12):1492-1501. doi: 10.1001/jamaneurol.2017.2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansson O, Seibyl J, Stomrud E, et al. ; Swedish BioFINDER Study Group; Alzheimer’s Disease Neuroimaging Initiative . CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470-1481. doi: 10.1016/j.jalz.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navitsky M, Kotari V, Southekal S, et al. Association between APOE isoform and 18-month tau accumulation. Presentation at: Alzheimer’s Association International Conference; July 16, 2019; Los Angeles, CA. [Google Scholar]
- 36.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839-843. doi: 10.1148/radiology.148.3.6878708 [DOI] [PubMed] [Google Scholar]
- 37.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29-36. doi: 10.1148/radiology.143.1.7063747 [DOI] [PubMed] [Google Scholar]
- 38.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bevan-Jones WR, Cope TE, Jones PS, et al. [18F]AV-1451 binding in vivo mirrors the expected distribution of TDP-43 pathology in the semantic variant of primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2018;89(10):1032-1037. doi: 10.1136/jnnp-2017-316402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makaretz SJ, Quimby M, Collins J, et al. Flortaucipir tau PET imaging in semantic variant primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2018;89(10):1024-1031. doi: 10.1136/jnnp-2017-316409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai RM, Bejanin A, Lesman-Segev O, et al. 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res Ther. 2019;11(1):13. doi: 10.1186/s13195-019-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257(1-2):80-87. doi: 10.1016/j.jns.2007.01.045 [DOI] [PubMed] [Google Scholar]
- 43.Jellinger KA, Alafuzoff I, Attems J, et al. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol. 2015;129(5):757-762. doi: 10.1007/s00401-015-1407-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed LA, Grabowski TJ, Schmidt ML, et al. Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol. 1997;42(4):564-572. doi: 10.1002/ana.410420406 [DOI] [PubMed] [Google Scholar]
- 45.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. ; Amyloid PET Study Group . Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939-1949. doi: 10.1001/jama.2015.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ono K, Takahashi R, Ikeda T, Yamada M. Cross-seeding effects of amyloid β-protein and α-synuclein. J Neurochem. 2012;122(5):883-890. doi: 10.1111/j.1471-4159.2012.07847.x [DOI] [PubMed] [Google Scholar]
- 47.Guo JL, Covell DJ, Daniels JP, et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154(1):103-117. doi: 10.1016/j.cell.2013.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bateman RJ, Xiong C, Benzinger TL, et al. ; Dominantly Inherited Alzheimer Network . Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804. doi: 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69(1):98-106. doi: 10.1001/archgenpsychiatry.2011.155 [DOI] [PubMed] [Google Scholar]
- 50.Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. J Nucl Med. 2013;54(7):1011-1013. doi: 10.2967/jnumed.113.127068 [DOI] [PubMed] [Google Scholar]
- 51.Quiroz YT, Sperling RA, Norton DJ, et al. Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol. 2018;75(5):548-556. doi: 10.1001/jamaneurol.2017.4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitwell JL, Graff-Radford J, Tosakulwong N, et al. Imaging correlations of tau, amyloid, metabolism, and atrophy in typical and atypical Alzheimer’s disease. Alzheimers Dement. 2018;14(8):1005-1014. doi: 10.1016/j.jalz.2018.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattsson N, Schöll M, Strandberg O, et al. 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer’s disease. EMBO Mol Med. 2017;9(9):1212-1223. doi: 10.15252/emmm.201707809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDade E, Bateman RJ. Tau positron emission tomography in autosomal dominant Alzheimer disease: small windows, big picture. JAMA Neurol. 2018;75(5):536-538. doi: 10.1001/jamaneurol.2017.4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chare L, Hodges JR, Leyton CE, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. 2014;85(8):865-870. doi: 10.1136/jnnp-2013-306948 [DOI] [PubMed] [Google Scholar]
- 58.Hodges JR, Mitchell J, Dawson K, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133(pt 1):300-306. doi: 10.1093/brain/awp248 [DOI] [PubMed] [Google Scholar]
- 59.Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Ann Neurol. 2018;84(5):729-740. doi: 10.1002/ana.25333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787-800. doi: 10.1002/ana.24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun. 2016;4(1):58. doi: 10.1186/s40478-016-0315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sander K, Lashley T, Gami P, et al. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 2016;12(11):1116-1124. doi: 10.1016/j.jalz.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 63.Miller ZA, Rankin KP, Graff-Radford NR, et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J Neurol Neurosurg Psychiatry. 2013;84(9):956-962. doi: 10.1136/jnnp-2012-304644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aldridge GM, Birnschein A, Denburg NL, Narayanan NS. Parkinson’s disease dementia and dementia with lewy bodies have similar neuropsychological profiles. Front Neurol. 2018;9:123. doi: 10.3389/fneur.2018.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen AK, Brooks DJ, Borghammer P. MAO-B inhibitors do not block in vivo flortaucipir([18F]-AV-1451) binding. Mol Imaging Biol. 2018;20(3):356-360. doi: 10.1007/s11307-017-1143-1 [DOI] [PubMed] [Google Scholar]
- 66.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297-309. doi: 10.1016/j.tips.2015.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Inclusion and Exclusion Criteria for the Swedish BioFINDER 2 Study
eMethods 2. Additional Details About [18F]RO948 Preprocessing
eMethods 3. Temporal Regions of Interest in Semantic Variant Primary Progressive Aphasia
eMethods 4. Additional Details About CSF Procedures
eTable 1. Demographics of Young (Age 20-40) Aβ-Negative Controls Used to Set [18F]RO948 SUVR Cutoffs
eResults. Participant Characteristics
eTable 2. Demographics for Non-Alzheimer Disease Subgroups
eTable 3. Partial Volume Error Corrected [18F]RO948 SUVR Data for Cognitively Unimpaired Controls and Mild Cognitive Impairment, Alzheimer Disease Dementia and Non-Alzheimer Disease Disorder Patients
eTable 4. Partial Volume Error Corrected [18F]RO948 SUVR Data for Non-AD Subgroups
eTable 5. Demographics for svPPA Cases
eTable 6. Diagnostic Performance of [18F]RO948 SUVR Using the Tau-Imaging I-IV ROI for AD Dementia and Aβ-Positive MCI vs Non-AD Disorders
eTable 7. Diagnostic Performance of [18F]RO948 SUVR Using Individual Tau Imaging ROIs
eTable 8. AUC Values for [18F]RO948 SUVR Using Different Cutoffs
eTable 9. Diagnostic Performance of CSF Aβ42/Aβ40 for AD Dementia and Aβ Positive MCI vs Other Non-AD Disorders and CU Controls
eTable 10. [18F]Flortaucipir and [18F]RO948 SUVR Values in Temporal and Primary Somatosensory Cortex ROIs for Semantic Variant Primary Progressive Aphasia
eTable 11. Area Under the Receiver Operating Characteristic Curve Values for [18F]RO948 SUVR in Tau Imaging ROIs With and Without Subjects Showing High Skull/Meningeal Signal
eFigure 1. Tau PET Imaging Composite ROIs Approximating the Braak Post-Mortem Staging Scheme for Tau Pathology
eFigure 2. Mean [18F]RO948 Images and Scatterplots for the Young (Age 20-40) Aβ-Negative Controls Used to Set Cutoffs for [18F]RO948 SUVR Across Tau-Imaging ROIs
eFigure 3. Voxelwise Group Differences in [18F]RO948 SUVR
eFigure 4. Voxelwise Group Differences in [18F]RO948 SUVR Using Family Wise Error Corrected Data
eFigure 5. Partial Volume Corrected [18F]RO948 Standardized Uptake Values Ratios (SUVRs) Across Diagnostic Groups Within Tau-Imaging ROIs
eFigure 6. Concordance Plots Between Partial Volume Corrected [18F]RO948 Standardized Uptake Values Ratios (SUVRs) and CSF Aβ42/Aβ40
eFigure 7. [18F]RO948 SUVR Across Tau-Imaging ROIs Using Lower Cutoffs
eFigure 8. [18F]RO948 SUVR Across Tau-Imaging ROIs by Age (Above and Below 65)
eFigure 9. [18F]RO948 SUVR in Primary Somatosensory and Motor Cortices
eFigure 10. Mean [18F]RO948 Standardized Uptake Values Ratios (SUVR) Across Diagnostic Groups (DLB Subdivided by Aβ-Status) Within Tau-Imaging ROIs
eFigure 11. Plots From Receiver Operating Characteristic Analyses ([18F]RO948, MRI- and CSF-Measures) for Distinguishing AD Dementia and Aβ-Positive MCI Fm Non-AD Neurodegenerative Disorders
eFigure 12. CSF P-Tau181 Levels by Diagnostic Group
eFigure 13. CSF P-Tau181 Levels Across Tau-Imaging ROIs
eFigure 14. [18F]RO948 and [18F]Flortaucipir PET in Semantic Variant Primary Progressive Aphasia
eFigure 15. Decision Tree Outlining the Potential Clinical Utility of Tau-PET Imaging Across Different Dementia Disorders, Including Alzheimer Disease